Abstract
Yearly vaccination with the trivalent inactivated influenza vaccine (TIV) is recommended, since current vaccines induce little cross neutralization to divergent influenza strains. Whether the TIV can induce antibody-dependent cellular cytotoxicity (ADCC) responses that can cross-recognize divergent influenza virus strains is unknown. We immunized 6 influenza-naive pigtail macaques twice with the 2011–2012 season TIV and then challenged the macaques, along with 12 control macaques, serially with H1N1 and H3N2 viruses. We measured ADCC responses in plasma to a panel of H1 and H3 hemagglutinin (HA) proteins and influenza virus-specific CD8 T cell (CTL) responses using a sensitive major histocompatibility complex (MHC) tetramer reagent. The TIV was weakly immunogenic and, although binding antibodies were detected by enzyme-linked immunosorbent assay (ELISA), did not induce detectable influenza virus-specific ADCC or CTL responses. The H1N1 challenge elicited robust ADCC to both homologous and heterologous H1 HA proteins, but not influenza virus HA proteins from different subtypes (H2 to H7). There was no anamnestic influenza virus-specific ADCC or CTL response in vaccinated animals. The subsequent H3N2 challenge did not induce or boost ADCC either to H1 HA proteins or to divergent H3 proteins but did boost CTL responses. ADCC or CTL responses were not induced by TIV vaccination in influenza-naive macaques. There was a marked difference in the ability of infection compared to that of vaccination to induce cross-reactive ADCC and CTL responses. Improved vaccination strategies are needed to induce broad-based ADCC immunity to influenza.
INTRODUCTION
Influenza epidemics and pandemics cause significant human morbidity and mortality worldwide. The burden of seasonal influenza virus infections is partially reduced through seasonal vaccination with trivalent inactivated influenza vaccine (TIV), which is generally formulated annually with H1N1, H3N2, and type B influenza virus strains. In any given influenza season, the TIV has moderate efficacy, and was 56% effective in the 2012 season (1, 2). The standard TIV contains 15 μg of hemagglutinin (HA) proteins from 3 influenza virus strains, is typically unadjuvanted, and is administered intramuscularly as a single dose. The TIV is thought to act by inducing or boosting neutralizing antibodies to the influenza virus surface HA glycoproteins. However, vaccine-induced neutralizing antibodies to influenza virus are highly strain specific, and there are intense efforts to improve influenza vaccines to induce broad cross-reactive immunity to divergent influenza virus strains (3).
Seasonal TIVs have been mainly investigated for their ability to induce antibodies capable of neutralizing influenza virus. However, influenza virus-specific antibodies induced by TIV vaccination may have other, nonneutralizing activities, including complement-mediated lysis (4, 5), phagocytosis (6, 7), and antibody-dependent cellular cytotoxicity (ADCC) (8–11). We speculate that these nonneutralizing antibodies have greater cross-reactivity than antibodies capable of neutralization alone. We have previously shown that influenza virus-specific ADCC-mediating antibodies to divergent influenza virus strains are present in healthy individuals in the absence of any neutralizing antibodies (12, 13). These ADCC-mediating antibodies may not target the same antigenic sites as previously described for influenza virus-specific neutralizing antibodies (14, 15). In particular, antibodies capable of mediating ADCC bind to whole virus or antigens on the surfaces of virus-infected cells, allowing effector cells, such as natural killer (NK) cells, to then bind to the antibody Fc region via their CD16 (FcγRIII) receptors (12, 13). This leads to both the killing of the influenza virus-infected cell and release of proinflammatory cytokines, including gamma interferon (IFN-γ). Previous studies on ADCC to influenza virus were performed in the late 1970s to early 1980s using chromium-51 release assays (8–11). Recently, we developed novel flow cytometry-based assays to study influenza virus-specific ADCC and have shown that ADCC-mediating antibodies to divergent influenza virus strains are induced by influenza virus infection (12). Further, we have found that subjects older than 45 years of age commonly possessed cross-reactive ADCC-mediating antibodies to the 2009 swine origin H1N1 pandemic [A(H1N1)pdm09] virus prior to 2009 that may have contributed to the partial protection from severe A(H1N1)pdm09 infection within this age group (13).
It is not clear if standard TIV vaccination results in the induction of ADCC-mediating antibodies and, if ADCC-mediating antibodies are induced, how cross-reactive they are. On one hand, the narrow efficacy of TIV vaccination in humans suggests the level of cross-reactive ADCC-mediating antibodies may be either minimal or ineffective (16, 17). On the other hand, induction of binding antibodies frequently leads to a subset of antibodies that mediate ADCC. Further, there is evidence of limited cross-reactive immunity induced by TIV vaccination in humans (18).
The ubiquitous exposure of adult humans to influenza virus results in a level of background cross-reactive ADCC that makes evaluating the ability of the TIV to induce influenza virus-specific ADCC-mediating antibodies difficult (12). Studies of ADCC in mouse and ferret models are difficult due to the lack of immunological reagents and established ADCC assays. We recently studied influenza virus-specific ADCC in rhesus macaques serially infected with seasonal H1N1 and pandemic H1N1 influenza viruses (19). We found that a seasonal H1N1 infection resulted in cross-reactive ADCC-mediating antibodies to A(H1N1)pdm09 virus and that these ADCC antibodies rapidly rose following subsequent A(H1N1)pdm09 virus challenge. We reasoned that pigtail macaques might also be a useful animal model for studying whether the TIV primes or induces ADCC immunity to influenza virus.
MATERIALS AND METHODS
Macaque vaccination and infection strategy.
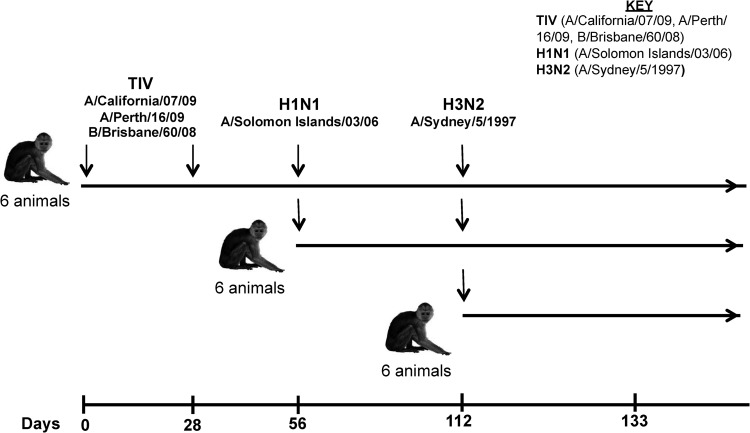
Eighteen influenza-naive young male adult pigtail macaques (Macaca nemestrina) were studied as approved by the Commonwealth Scientific and Industrial Research Organization (CSIRO) Animal Health Animal Ethics Committee. Prior to any procedure, the animals were anesthetized intramuscularly with ketamine. Six naive macaques were vaccinated intramuscularly in the quadriceps muscle twice, 28 days apart, with the standard 2012 TIV (0.5 ml; Vaxigrip; Sanofi Pasteur) containing HA proteins from A/California/07/2009 (H1N1), A/Perth/16/2009 (H3N2), and B/Brisbane/60/2008 (type B) (Fig. 1). Twenty-eight days after the final vaccination, the 6 vaccinated macaques and 6 additional naive macaques were inoculated with a total of 4.6 × 106 PFU of A/Solomon Islands/3/2006 (H1N1) virus via the larynx, tonsils, and conjunctivae, as described previously (19–21) (Fig. 1). Fifty-six days after the H1N1 infection, these 12 H1N1-infected macaques plus an additional 6 naive macaques were inoculated with A/Sydney/5/1997 (H3N2) as described above. Virus levels in nasal and pharyngeal secretions were assessed for the presence of influenza virus RNA using a reverse transcription (RT)-PCR assay targeting the influenza virus matrix genome segment, as previously described (10). Hemagglutination inhibition (HI) assays were performed as previously described (22).
Fig 1.
Timeline of TIV vaccination and H1N1/H3N2 challenge in pigtail macaques.
Influenza virus antigens and influenza viruses.
Mammalian-cell expressed recombinant HA and NP proteins were purchased from Sinobiologicals (Shanghai, China). Madin-Darby Canine Kidney (MDCK) cell-grown influenza viruses antigenically distinct from 2012 TIV strains were used to inoculate the animals: A/Solomon Islands/3/2006 (H1N1) and A/Sydney/5/1997 (H3N2). Viral titers were determined by plaque assay as previously described (19, 20).
Influenza virus-specific enzyme-linked immunosorbent assay (ELISA).
Polyvinyl plates (Thermofisher Scientific, Scoresby, Victoria, Australia) were coated overnight at 4°C with 1 μg/ml of purified influenza virus (either A/California/07/2009 or A/Perth/16/2009). The plates were blocked with phosphate-buffered saline (PBS) plus 10% bovine serum albumin (BSA) (Sigma, St. Louis, MO) for 1 h at 37°C. The plates were washed 4 times with PBS plus 0.01% Tween 20 (PBST) and incubated with preabsorbed heat-inactivated heparin anticoagulated plasma at half-log dilutions in PBS plus 5% BSA and 0.01% Tween 20 (starting at 1:100 dilution). Samples were preabsorbed by incubating them on a separate 96-well polyvinyl plate for 2 h at 37°C. The plates were washed 6 times with PBST and incubated with a rabbit anti-macaque IgG (Sigma, St. Louis, MO) for 2 h at room temperature. Subsequently, the plates were washed 6 times with PBST, incubated with TrueBlue solution (KLP, Gaithersburg, MD), and allowed to develop for 5 min. Color development was stopped with 0.1 M HCl and read on a microplate reader immediately at 450 nm with a reference of 540 nm. Antibody titers were expressed as the reciprocal of the dilution giving an optical density (OD) reading four times that of the background.
NK cell activation ADCC assay.
We recently described a novel ADCC assay that measures antibody-mediated NK cell activation (23), developed as modifications of human immunodeficiency virus (HIV)-specific ADCC assays (24, 25). This influenza virus ADCC assay was subsequently adapted to study macaque samples. Wells of a 96-well ELISA plate (Nunc, Rochester, NY) were coated overnight at 4°C with 600 ng/well of purified influenza virus protein in 1× PBS (HyClone, Logan, UT). The wells were washed repeatedly with 1× PBS and incubated with preabsorbed heat-inactivated heparin anti-coagulated plasma samples for 2 h at 37°C. The plasma samples were preabsorbed by incubation on a separate 96-well ELISA plate (Nunc, Rochester, NY) for 2 h at 37°C. The plates were washed repeatedly with 1× PBS, and then 106 freshly isolated naive pigtail macaque peripheral blood mononuclear cells (PBMCs) were added to each well. PBMCs isolated from naive healthy macaques with Ficoll-Paque (GE Healthcare, Madison, WI) were washed and resuspended in R10 medium (RPMI 1640 supplemented with 10% fetal calf serum, penicillin, streptomycin, and l-glutamine; HyClone, Logan, UT). In addition, anti-human CD107a-APC-H7 (H4A3 clone; BD Bioscience, San Jose, CA), 5 μg/ml of brefeldin A (Sigma, St. Louis, MO), and 5 μg/ml monensin (Golgi Stop; BD Bioscience, San Jose, CA) were added to each well and incubated for 5 h at 37°C with 5% CO2. The cells were then incubated with the surface antibodies anti-CD3 Pacific Blue (SP34-2 clone; BD Bioscience, San Jose, CA), anti-CD14 phycoerythrin (PE)-Cy7 (M5E2 clone; BD Bioscience, San Jose, CA), and anti-NKG2A allophycocyanin (APC) (clone Z199; Beckman Coulter, Brea, CA) for 30 min at room temperature in the dark. The cells were then fixed with 1% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) for 10 min and permeabilized using 1× FACS Permeabilizing Solution 2 for 10 min (BD Bioscience, San Jose, CA). Finally, the cells were incubated at room temperature for 1 h with anti-IFN-γ AF700 (B27 clone; BD Bioscience). The cells were fixed with 1% paraformaldehyde and acquired on an LSRII flow cytometer (BD Bioscience, San Jose, CA), with at least 200,000 lymphocyte events collected. Samples were analyzed using FlowJo Version 9.2 (TreeStar, Ashland, OR).
Influenza virus RA9 MHC tetramer staining of PBMCs.
To sensitively study CD8 T cell immunity to influenza virus, we utilized a recently described major histocompatibility complex class I (MHC-I) tetramer for the influenza virus RA9 CD8 T cell (CTL) epitope within the influenza virus nucleoprotein (NP) that is presented by the pigtail macaque Mane-A*084 MHC-I allele (19). All 18 macaques studied expressed the Mane-A*084 MHC-I allele, as assessed by Roche 454 deep sequencing and previously reported (26). The Mane-A*084-RA9 tetramer reagent was produced using the JA5 plasmids expressing Mane-A*084 and monkey β2M, as previously described (27). Thawed PBMCs were stained with anti-CD3 Pacific Blue (SP34-4), anti-CD8 PerCP (SK1), and the Mane-A*084-RA9-APC tetramer for 40 min at room temperature in the dark and subsequently washed and fixed with 10% formaldehyde solution. Samples were acquired on an LSRII flow cytometer (BD Bioscience, San Jose, CA), with at least 200,000 lymphocyte events collected. Samples were analyzed using FlowJo Version 9.2 (TreeStar, Ashland, OR).
Statistical analyses.
Statistical analyses used Prism GraphPad v6 (GraphPad Software, San Diego, CA). Data were analyzed by the Friedman test, followed by a separate Wilcoxon matched-pairs signed-rank test (Fig. 2B, D, and F, and 3C and D) and a separate Mann-Whitney U test (Fig. 4A and B and 5A and B) and Kruskal-Wallis tests followed by separate Mann-Whitney U tests (Fig. 6A, B, C, and D). The magnitude of the CTL response was assessed by the time-weighted-average level of RA9 epitope-specific CD8 T cells, calculated as the area under the curve of the time course in Fig. 6C divided by the corresponding time interval.
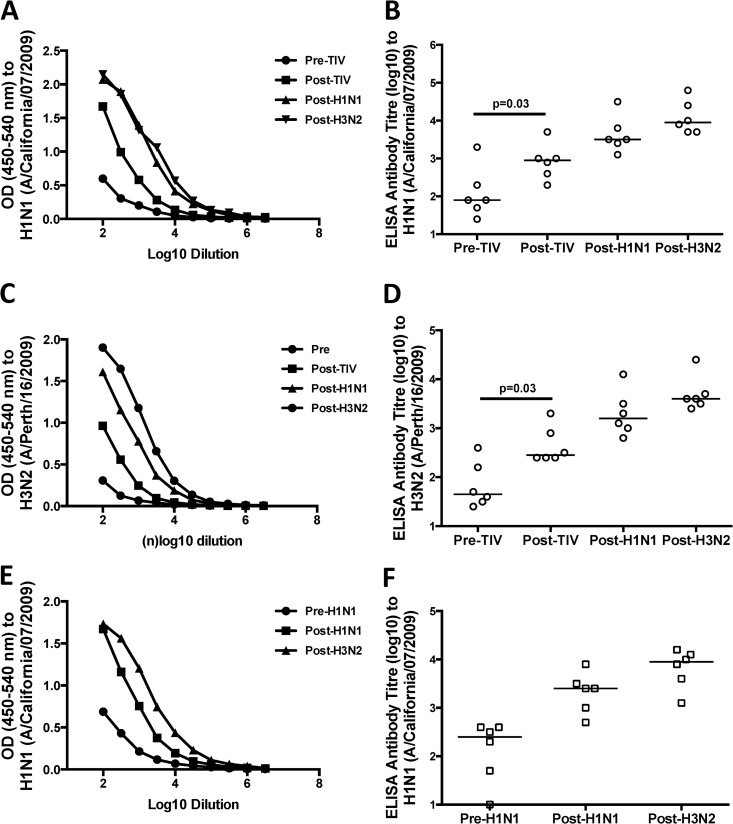
Fig 2.
Influenza virus-specific binding antibodies following vaccination and infection of macaques. (A to D) We used either purified H1N1 (A/California/07/2009) (A and B) or H3N2 (A/Perth/16/2009) (C and D) virus to measure binding antibodies in plasma samples taken from 6 macaques prior to immunization (Pre-TIV), 4 weeks postimmunization with two doses of TIV (Post-TIV), 4 weeks post-H1N1 challenge (Post-H1N1), and 4 weeks post-H3N2 challenge (Post-H3N2) by ELISA. (E and F) We used purified H1N1 (A/California/07/2009) to measure binding antibodies in plasma samples taken from 6 macaques prior to H1N1 (Pre-H1N1) challenge, post-H1N1 challenge (Post-H1N1), and post-H3N2 challenge (Post-H3N2) by ELISA. Plasma samples were all titrated via half-log dilutions, with a starting dilution of 1:100. The antibody titers are given as the reciprocal of the dilution giving an OD reading four times background. Antibody titers were compared for animals between time points, and statistical analysis was performed by Friedman test, followed by a separate Wilcoxon test (B, D, and F). The horizontal lines represent the median values of groups.
Fig 3.
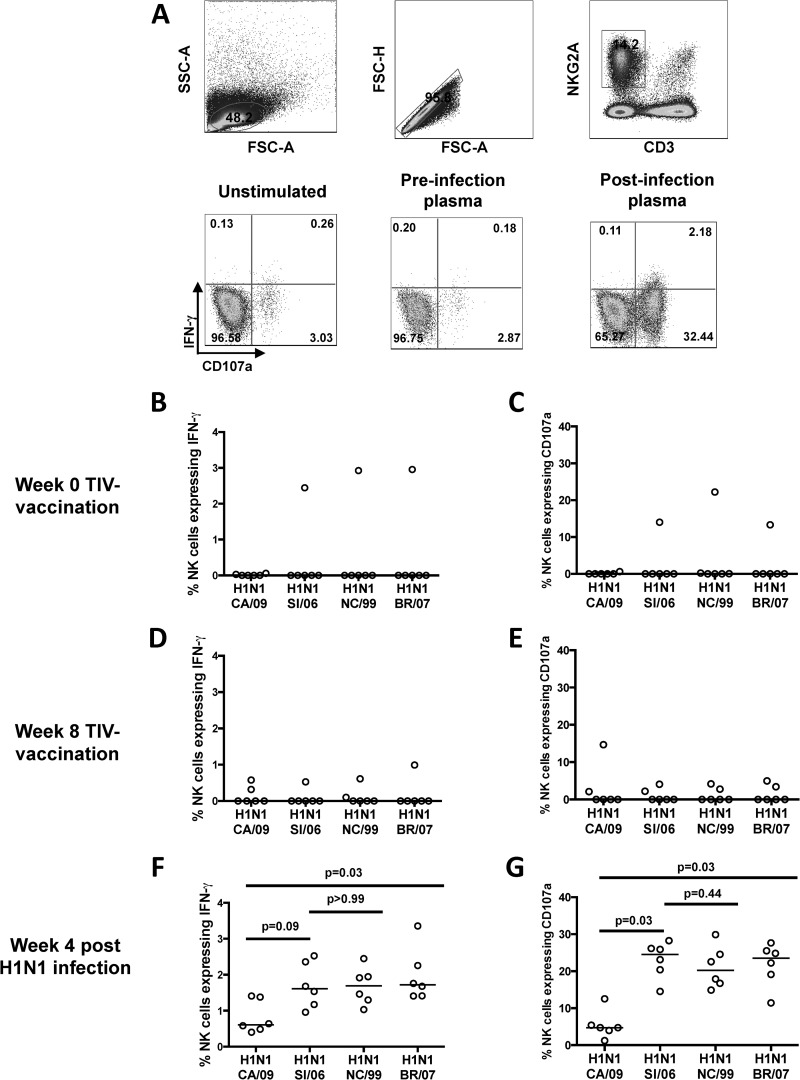
H1-specific ADCC after vaccination and H1N1 challenge. (A) Flow cytometry plots of NK cell activation ADCC assays. Gating strategy is shown in the upper plots. The lower plots show representative examples of antibody-mediated activation of CD3− NKG2A+ NK cells expressing IFN-γ and CD107a following exposure of healthy donor macaque PBMCs to either (i) no H1 protein (unstimulated) with post-H1N1 infection plasma, (ii) H1 protein and plasma postvaccination/preinfection, or (iii) H1 protein and post-H1N1 infection plasma. (B to G) Frequencies of NK cells expressing either IFN-γ or CD107a in the presence of plasma from 6 macaques prevaccination (B and C), 4 weeks after 2 TIV vaccinations (D and E), and 4 weeks after H1N1 infection (F and G). ADCC responses to the recombinant HA proteins from H1N1 influenza viruses A/California/7/2009 (CA/09), A/Solomon Islands/03/2006 (SI/06), A/New Caledonia/20/1999 (NC/99), and A/Brisbane/59/2007 (BR/07) were measured. The horizontal lines represent the median values of groups. Statistical analysis was performed by Friedman test, followed by a separate Wilcoxon matched-pairs signed-rank test. All samples were corrected for background based on their responses to wells containing plasma but with no plate-bound antigen.
Fig 4.
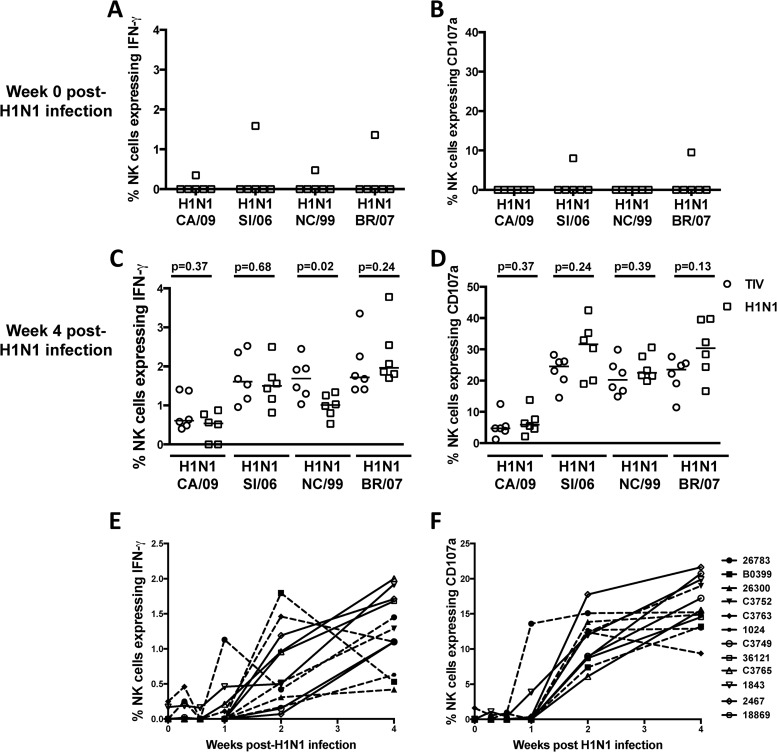
ADCC to H1 protein in unvaccinated macaques versus vaccinated macaques after H1N1 challenge. (A and B) Frequencies of NK cells expressing IFN-γ and CD107a in response to plasma from 6 control unvaccinated macaques prior to H1N1 infection. (C and D) Comparison of the frequencies of NK cells expressing IFN-γ and CD107a in response to plasma from either 6 control unvaccinated macaques (squares) or 6 vaccinated macaques (circles) 4 weeks after H1N1 infection. (E and F). Comparison of the frequencies of NK cells expressing IFN-γ and CD107a toward recombinant HA protein from A/Solomon Islands/3/2006 in the presence of plasma from either 6 control unvaccinated macaques (open symbols) or 6 vaccinated macaques (solid symbols) at days 0, 2, 4, 8, 15, and 29 post-H1N1 infection. ADCC responses to the recombinant HA proteins from H1N1 influenza viruses A/California/7/2009 (CA/09), A/Solomon Islands/3/2006 (SI/06), A/New Caledonia/20/1999 (NC/99), and A/Brisbane/59/2007 (BR/07) were measured. The horizontal lines represent the median values of groups. Statistical analysis was performed by separate Mann-Whitney U tests. All samples were corrected for background based on their responses to wells containing plasma but with no plate-bound antigen.
Fig 5.
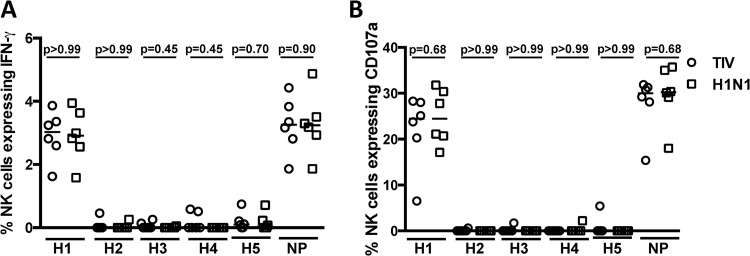
Lack of ADCC cross-recognition of other HA subtypes after H1N1 infection of macaques. Comparison of the frequencies of NK cells expressing IFN-γ (A) and CD107a (B) in response to plasma from either 6 unvaccinated macaques (squares) or 6 vaccinated macaques (circles) 4 weeks after H1N1 infection. ADCC responses to the recombinant HA proteins from H1N1 (A/California/7/2009), H2N2 (A/Japan/305/1957), H3N2 (A/Brisbane/10/2007), H4N6 (A/Swine/Ontario/01911-1/1999), H5N1 (A/Vietnam/1203/2004), and recombinant NP (A/Puerto Rico/8/1934) were measured. The horizontal lines represent the median values of groups. Statistical analysis was performed by separate Mann-Whitney U tests. All samples were corrected for background based on their responses to wells containing plasma but with no plate-bound antigen.
Fig 6.
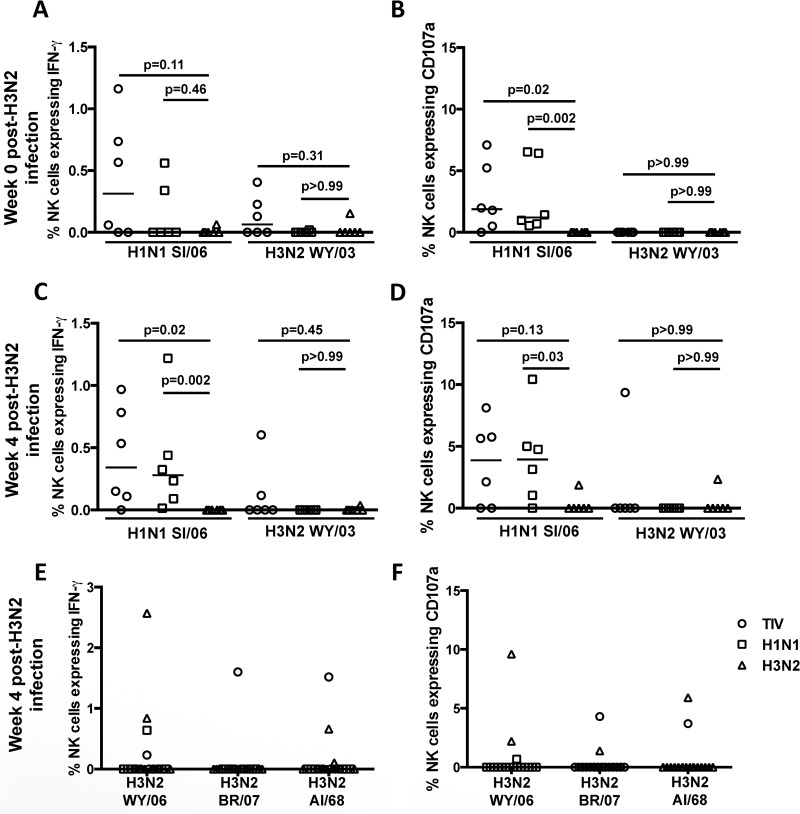
ADCC responses to H3N2 HA proteins in unvaccinated macaques compared to vaccinated H1N1-infected macaques and H1N1-infected macaques after H3N2 challenge. (A and B) Frequencies of NK cells expressing IFN-γ and CD107a in response to plasma from 6 unvaccinated/H1N1 challenge macaques (circles), 6 H1N1-infected macaques (squares), and 6 naive control macaques (triangles) prior to H3N2 challenge. (C and D) Frequencies of NK cells expressing IFN-γ and CD107a in response to plasma from 6 unvaccinated/H1N1 challenge macaques (circles), 6 H1N1-infected macaques (squares), and 6 naive control macaques (triangles) 4 weeks after H3N2 infection. (E and F) Frequencies of NK cells expressing IFN-γ and CD107a in the presence of plasma from 6 unvaccinated/H1N1 challenge macaques (circles), 6 H1N1-infected macaques (squares), and 6 naive control macaques (triangles) to recombinant HA proteins from seasonal H3N2 strains A/Wyoming/03/2003 (WY/06), A/Aichi/2/1968 (AI/68), and A/Brisbane/10/2007 (BR/07) 4 weeks after H3N2 infection. ADCC responses to the recombinant HA proteins from seasonal H3N2 and H1N1 influenza viruses A/Solomon Islands/03/2006 (SI/06) and A/Wyoming/03/2003 were also measured. The horizontal lines represent the median values of groups. Statistical analysis was performed by 2 separate Kruskal-Wallis tests, followed by separate Mann-Whitney U tests. All samples were corrected for background based on their responses to wells containing plasma but with no plate-bound antigen.
RESULTS
Outcome of the macaque influenza vaccination and challenge study.
ADCC responses induced by influenza virus infection could be effective in controlling disease, but it is unclear whether the current standard TIV can induce ADCC responses that cross-react with influenza virus strains not found within the vaccine. We vaccinated 6 influenza-naive macaques twice with the 2012 season TIV and then challenged the macaques serially with seasonal H1N1 (A/Solomon Islands/03/2006) and H3N2 (A/Sydney/05/1997) influenza viruses, using unmatched influenza virus strains not present within the 2012 TIV (Fig. 1). The TIV was well tolerated, as expected, but was poorly immunogenic. To assess seroconversion following TIV vaccination of macaques, all 6 animals were tested by ELISA for binding antibodies to the H1N1 (A/California/07/2009) and H3N2 (A/Perth/16/2009) virus strains found in the TIV. Half-log titration of plasma samples from animals prior to and following two immunizations with TIV suggested a marginal increase in binding antibodies to H1N1 and H3N2 viruses (Fig. 2A and C). The levels of binding antibodies to both H1N1 and H3N2 influenza virus strains were significantly higher 4 weeks following immunization with TIV (Wilcoxon test; P > 0.05) (Fig. 2B and C, respectively).
To evaluate neutralizing-antibody responses induced by the TIV, we assessed HI titers. Only one animal had an HI titer of ≥40 to the A(H1N1)pdm09 virus strain A/California/07/2009 present in the 2012 TIV 4 weeks after the second immunization (Table 1, week 8 time point). There was no detectable HI (<10) to another 3 nonpandemic H1N1 strains tested (A/Solomon Islands/3/2006, A/New Caledonia/20/1999, and A/Brisbane/59/2007) (Table 1) or to a panel of 4 H3N2 strains tested (A/Sydney/5/1997, A/Wyoming/3/2003, A/Brisbane/10/2007, and A/Aichi/2/1968) (data not shown).
Table 1.
Influenza virus HI titers after TIV vaccination and after H1N1 challenge and peak viral RNA levels
| Group | Animal ID | HI titera |
Peak viral RNA (log10 mean copies/ml)b |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1N1 CA/09 |
H1N1 SI/06 |
H1N1 NC/99 |
H1N1 BR/07 |
H3N2 SY/97 |
||||||||||||||
| W0 | W8 | W12 | W0 | W8 | W12 | W0 | W8 | W12 | W0 | W8 | W12 | W0 | W8 | W12 | Nasal | Pharyngeal | ||
| TIV vaccinated | 26783 | <10 | <10 | <10 | <10 | <10 | 40 | <10 | <10 | 40 | <10 | <10 | 40 | <10 | <10 | <10 | 7.11 | 7.09 |
| B0399 | <10 | 40 | <10 | <10 | <10 | 40 | <10 | <10 | 40 | <10 | <10 | 80 | <10 | <10 | <10 | 6.47 | 4.53 | |
| 26300 | <10 | <10 | <10 | <10 | <10 | 40 | <10 | <10 | 40 | <10 | <10 | 80 | <10 | <10 | <10 | 6.77 | 3.96 | |
| C3752 | <10 | <10 | <10 | <10 | <10 | 40 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 6.63 | 6.68 | |
| C3763 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 7.66 | 5.35 | |
| 1024 | <10 | <10 | <10 | <10 | <10 | 40 | <10 | <10 | 40 | <10 | <10 | 40 | <10 | <10 | <10 | 6.49 | 4.93 | |
| Unvaccinated | C3749 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 6.89 | 5.37 | |||||
| 36121 | <10 | <10 | <10 | 40 | <10 | 40 | <10 | 40 | <10 | <10 | 5.41 | 5.29 | ||||||
| C3765 | <10 | <10 | <10 | 40 | <10 | <10 | <10 | 40 | <10 | <10 | 6.74 | 4.84 | ||||||
| 1843 | <10 | <10 | <10 | 40 | <10 | <10 | <10 | 80 | <10 | <10 | 6.62 | 6.58 | ||||||
| 2467 | <10 | <10 | <10 | 40 | <10 | >10 | <10 | 40 | <10 | <10 | 7.34 | 6.24 | ||||||
| 18869 | <10 | <10 | <10 | 160 | <10 | 80 | <10 | 320 | <10 | <10 | 6.55 | 7.12 | ||||||
HI assay against H1N1 (A/California/07/2009, A/Solomon Islands/03/2006, A/New Caledonia/20/1999, or A/Brisbane/59/2007) or H3N2 (A/Sydney/5/1997) virus using sera from macaques either at week 0 (W0), week 8 (week 0 post-H1N1 infection), or week 12 (week 4 post-H1N1 infection) postvaccination. Inhibition of agglutination was assessed using 2-fold serum dilutions between 10 and 1,280. Detectable HI titers (≥40) are shown in boldface.
Serial swabs were taken on days 0, 2, 3, 4, and 8, and viral RNA levels were determined by real-time PCR.
The 6 vaccinated macaques, along with 6 naive controls, were subsequently challenged with an antigenically distinct H1N1 influenza virus (A/Solomon Islands/3/2006) via the nose and larynx 4 weeks after the final vaccination. As well as performing HI assays on plasma, we obtained nasal and pharyngeal swabs 2, 3, 4, and 8 days after challenge and determined the peak viral RNA levels by quantitative RT-PCR (Table 1). By 4 weeks after the H1N1 challenge, 10 of the 12 challenged macaques developed detectable HI titers (≥40) to the H1N1 A/Solomon Islands/03/2006 challenge strain. Additionally, there was substantial cross-reactive HI to both A/New Caledonia/20/1999 and A/Brisbane/59/2007 H1N1 viruses, but not to the A/California/7/2009 virus present in the 2012 TIV or any of the H3N2 strains tested (Table 2). There was no difference in HI antibody levels between TIV-vaccinated animals and naive controls challenged with the A/Solomon Islands/3/2006 virus. Viral RNA was recovered from nasal and pharyngeal swabs of all animals infected; however, there was no difference in peak viral RNA levels between TIV-vaccinated and naive control animals (Mann-Whitney U test; P = 0.79 and P = 0.47 for nasal and pharyngeal peak RNA levels, respectively) (Table 1). All 12 animals were tested for binding antibodies toward H1N1 (A/California/07/2009) and H3N2 (A/Perth/16/2009) viruses, and there was an increase in the level of binding antibodies following the H1N1 challenge (Fig. 2).
Table 2.
Influenza virus HI titers pre- and post-H3N2 challenge and peak viral RNA levels
| Group | Animal ID | HI titer before and after H3N2 challengea |
Peak viral RNA (log10 mean copies/ml)b |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H3N2 SY/97 |
H3N2 WY/03 |
H3N2 BR/07 |
H3N2 AI/68 |
H1N1 SI/06 |
|||||||||
| W15 | W19 | W15 | W19 | W15 | W19 | W15 | W19 | W15 | W19 | Nasal | Pharyngeal | ||
| TIV vaccinated and H1N1 infected | 26783 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 40 | <10 | − | 3.97 |
| B0399 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 80 | 80 | − | 5.50 | |
| 26300 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | − | 1.17 | |
| C3752 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 40 | <10 | − | 2.07 | |
| C3763 | <10 | 20 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 5.58 | 2.31 | |
| 1024 | <10 | 40 | <10 | <10 | <10 | <10 | <10 | <10 | 80 | 40 | 4.01 | 2.03 | |
| H1N1 infected only | C3749 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | − | 1.32 |
| 36121 | <10 | 20 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 5.88 | 2.84 | |
| C3765 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 3.56 | − | |
| 1843 | <10 | 40 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 40 | − | 6.25 | |
| 2467 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 3.58 | − | |
| 18869 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 80 | 40 | 5.60 | − | |
| Naive | 16570 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | − | − |
| 26301 | <10 | 320 | <10 | 40 | <10 | <10 | <10 | <10 | <10 | <10 | 6.56 | 7.21 | |
| 35377 | <10 | 20 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 5.18 | 6.59 | |
| 36271 | <10 | 40 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 4.35 | − | |
| 5798 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 5.03 | − | |
| C3770 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 4.34 | 6.02 | |
HI assay against H3N2 (A/Sydney/5/1997, A/Wyoming/03/2003, A/Brisbane/10/2007, or A/Aichi/02/1968) or H1N1 (A/Solomon Islands/03/2006) virus using sera from macaques at weeks 15 (W15) and 19, day 0, or day 28 post-H3N2 challenge. Inhibition of agglutination was assessed using 2-fold serum dilutions between 10 and 1,280. Detectable HI titers (≥40) are shown in boldface.
Serial swabs were taken on days 0, 2, 4, and 7, and viral RNA levels were determined by rRT-PCR. −, influenza matrix genome segment RNA was not detected in the rRT-PCR assay.
To further assess the impact of vaccination followed by a heterologous influenza virus infection on influenza immunity, we subsequently challenged all 12 H1N1-infected macaques with a seasonal H3N2 (A/Sydney/5/1997) influenza virus strain, along with 6 naive controls, 8 weeks after the H1N1 challenge. HI titers to H1N1 viruses were reduced by the time of the H3N2 challenge and were not boosted by H3N2 challenge. Further, HI antibodies to the H3N2 challenge strain (A/Sydney/5/1997) were detectable in only 7 of the 18 animals (HI titer, ≥20), and no cross-reactive HI antibodies to all 3 of the other H3N2 strains tested (A/Wyoming/03/2003, A/Brisbane/10/2007, and A/Aichi/02/1968) were observed in any animal. There was no marked difference in HI titers to the A/Sydney/5/1997 strain between the 6 TIV-vaccinated animals, the 6 animals that received only the H1N1 challenge, and the 6 naive controls (Kruskal-Wallis test; P = 0.73). At least low levels of viral RNA were recovered from either nasal or pharyngeal swabs of 17/18 animals challenged with H3N2 virus, although the peak levels were generally much lower than those recovered from H1N1-challenged animals (Table 1). There was no difference in peak viral RNA levels between the 6 TIV-vaccinated animals, the 6 animals that received only the H1N1 challenge, and the 6 naive controls animals (Kruskal-Wallis; P = 0.24 and P = 0.61 for nasal and pharyngeal swabs, respectively) (Table 2).
ADCC antibodies after vaccination and H1N1 challenge.
The H1N1 infection, but not vaccination with the A(H1N1)pdm09 strain in the TIV, induced cross-reactive antibodies that mediated HI activity to other antigenically similar H1N1 strains, but not to H3N2 strains. Since there were binding antibodies present following TIV immunization of macaques, it remained possible that a broader set of functional antibodies, such as ADCC-mediating antibodies, were induced by vaccination and/or infection. We assessed influenza virus-specific ADCC activity in plasma by utilizing a novel flow cytometry-based assay that measures the ability of HA-specific antibodies to activate healthy macaque donor CD3-NKG2A+ NK cells to express IFN-γ and the degranulation marker CD107a (LAMP-1) (Fig. 3A) (12, 13). Prior to vaccination, plasma antibodies from 1 of the 6 animals induced both NK cell IFN-γ and CD107a expression to 3 seasonal H1N1 strains (A/Solomon Islands/3/2006, A/New Caledonia/20/1999, and A/Brisbane/59/2007), but not to A/California/07/2009, despite the animal having no detectable HI antibodies to any of the strains (Fig. 3B and C). Indeed, this particular animal had higher levels of binding antibodies to A/California/07/2009 prior to vaccination (Fig. 2B). Four weeks after the 2nd vaccination, there was no significant rise in levels of ADCC antibody to A/California/07/2009 HA in the 6 animals (P = 0.50; Wilcoxon matched-pairs signed-rank test), with plasma from only 2 animals having any detectable capacity to activate NK cells (Fig. 3D and E). The one animal with baseline ADCC activity also had similar ADCC activity after vaccination, although the levels of ADCC-induced NK cell activation were reduced.
After the H1N1 challenge, HI antibodies to A/Solomon Islands/03/2006 were detected in 5/6 vaccinated animals (Table 1). Additionally, there was broad HI reactivity in 4 of these animals, with HI activity to two other seasonal H1N1 strains (A/New Caledonia/20/1999 and A/Brisbane/59/2007), but not the pandemic swine origin A/California/7/2009 strain. We detected HA-specific ADCC-mediating antibodies to the challenge strain (A/Solomon Islands/3/2006) and also the other 2 seasonal H1N1 strains tested (A/New Caledonia/20/1999 and A/Brisbane/59/2007) in all 6 vaccinated animals (Fig. 3F and G). The cross-reactive ADCC-mediating antibodies induced by infection with A/Solomon Islands/03/2006 were notable, with no difference in the levels of NK cell IFN-γ or CD107a expression to HA proteins from A/Solomon Islands/03/2006, A/New Caledonia/20/1999, or A/Brisbane/59/2007 (Friedman test [P = 0.03 and P = 0.002 for IFN-γ and CD107a, respectively], followed by separate Wilcoxon matched-pairs signed-rank tests [P > 0.05 for both IFN-γ and CD107a]) (Fig. 3F and G). In contrast, there was a more modest, though detectable, ADCC response to the swine origin A/California/7/2009 HA protein following the H1N1 challenge in all 6 animals; only 1 of these 6 animals had detectable HI antibodies to A/California/07/2009 following challenge (Fig. 3F and G). This suggests that H1N1 challenge with A/Solomon Islands/03/2006 produced broad reactivity to seasonal H1N1 strains and to a lesser extent the pandemic A/California/07/2009 strain.
No difference in infection-induced ADCC responses between naive and vaccinated macaques.
Although we did not detect significant HI or ADCC levels after TIV vaccination, all vaccinated animals had detectable low-level binding antibodies that may indicate possible vaccine priming and induction of low-level antibodies capable of mediating ADCC. These antibodies could potentially be enhanced following subsequent virus challenge in comparison to unvaccinated animals. We therefore evaluated influenza virus-specific ADCC responses in the 6 unvaccinated animals concurrently challenged with the H1N1 (A/Solomon Islands/03/2006) strain. Prior to infection, serum from 1 out of 6 animals had detectable ADCC activity to the HA proteins from H1N1 strains (Fig. 4A and B). Four weeks following the H1N1 infection, however, all 6 naive animals developed ADCC responses to all 4 H1N1 HA antigens tested (Fig. 4C and D). As noted previously, ADCC levels to HA proteins from the 3 seasonal H1N1 strains (A/Solomon Islands/3/2006, A/New Caledonia/20/1999, and A/Brisbane/59/2007) were generally higher than ADCC levels to the HA protein from A/California/04/2009. However, the ADCC levels against a range of HA proteins from H1N1 viruses in the vaccinated animals (open circles, Fig. 4C and D) were not significantly higher than ADCC in the naive animals (open squares, separate Mann-Whitney U-tests Fig. 4C and D), suggesting the vaccination did not prime an ADCC antibody response.
The difference in breadth of ADCC-inducing antibodies compared to the breadth of HI-inducing antibodies was also notable across all animals infected with the seasonal H1N1 (A/Solomon Islands/3/2006) virus. Combined, the 12 H1N1-infected animals all had clearly detectable ADCC to 3 distinct seasonal H1N1 viruses (Fig. 4C and D). Indeed, all 12 animals had clearly detectable ADCC to the A/California/7/2009 HA, but the same plasma did not induce HI activity. Further, ADCC to A/New Caledonia/20/1999 was detected in all 12 animals; however, HI-inducing antibodies were detectable in only 6/12 animals. The magnitude of ADCC-induced NK cell activation to HA protein from A/Solomon Islands/03/2006 did not correlate with the HI titer to this virus (R2 = 0.04; P = 0.54), highlighting the fact that these assays likely measure different subsets of antibodies.
The preceding experiments utilized plasma from vaccinated animals 4 weeks after the H1N1 challenge and found no difference in the magnitude of the ADCC response between vaccinated and control animals. However, this does not exclude a very early rise in vaccine-induced anamnestic ADCC antibody responses after H1N1 exposure. To evaluate this possibility, we studied serial serum samples at 2, 4, 8, 15, and 29 days after challenge for ADCC antibodies (Fig. 4E and F). There was no difference in the overall kinetics of induction of the influenza virus-specific ADCC response between vaccinated and control animals, with ADCC responses rising rapidly between days 8 and 15 post-H1N1 infection in most animals. Thus, 2 doses of the standard TIV did not prime ADCC responses in these influenza-naive macaques.
Breadth of ADCC response after H1N1 infection to heterotypic HA proteins.
Analyses of ADCC responses after the H1N1 infection demonstrated broad responses to HA proteins from several H1N1 strains. To further probe the breadth of ADCC induced by the H1N1 infection, we tested plasma 4 weeks after the H1N1 challenge for ADCC to H2 to H5 HA proteins, as well as to a conserved internal influenza virus A protein, NP (Fig. 5A and B). As expected, there was robust ADCC to the H1 HA protein (A/California/7/2009), but ADCC responses to the H2 to H5 proteins were negligible. There were no differences in the breadths of ADCC to non-H1 HA proteins between vaccinated and control animals (Mann-Whitney U test; P > 0.05). Interestingly, we found robust ADCC responses to the conserved internal influenza virus protein NP.
No increase in H1-cross-reactive ADCC antibodies after H3N2 challenge.
To assess the potential priming of ADCC responses to H3N2 viruses by an H1N1 infection, we rechallenged all 12 H1N1-infected animals, along with another 6 naive controls, with an H3N2 virus 2 months after the H1N1 infection. We assessed ADCC to both H1 and H3 HA proteins on the day of challenge and 4 weeks later. On the day of H3N2 challenge, ADCC responses to H1 protein from the 12 H1N1-infected animals were lower than we had observed earlier after the H1N1 infection (Fig. 6A and B). Sera from 3 H1N1-infected animals, previous recipients of the TIV, were able to weakly induce IFN-γ expression to the H3 protein from A/Wyoming/3/2003 prior to the H3N2 challenge, but this was not accompanied by concomitant CD107a expression. There were no significant ADCC responses detected in plasma from the 6 naive animals to either H1 or H3 protein prior to the H3N2 challenge (Fig. 6A and B).
Four weeks after the H3N2 challenge, ADCC responses to the H1 protein from A/Solomon Islands/3/2006 were not significantly different from the prechallenge levels in the 12 animals previously infected with H1N1 (Wilcoxon matched-pairs signed-rank test; P = 0.27) (data not shown), suggesting that the H3N2 infection did not boost H1-specific ADCC. Further, there was no H1-specific ADCC in the 6 naive H3N2-challenged macaques, and we observed ADCC to the H3 protein in only 3 of the 18 H3N2-challenged macaques (Fig. 6C and D).
The lack of ADCC responses to the H3 protein tested in our ADCC assay may have been in part due to our inability to source a precise match with the H3 protein present in the challenge virus. To determine whether ADCC responses to other H3 proteins were induced by the H3N2 challenge, we tested 2 additional H3 proteins (A/Brisbane/10/2007 and A/Aichi/2/1968) for ADCC-mediated NK cell activation. There were again only moderate ADCC responses to H3 proteins in only 3 animals at 4 weeks post-H3N2 challenge. Indeed, the 2 animals that had robust ADCC responses to A/Wyoming/03/2003 HA also demonstrated ADCC responses to A/Brisbane/10/2007 and A/Aichi/2/1968 HA proteins, suggesting that when ADCC was induced in animals, it was at least partially cross-reactive between H3 strains.
Induction of influenza virus-specific CTL responses by vaccination and challenge.
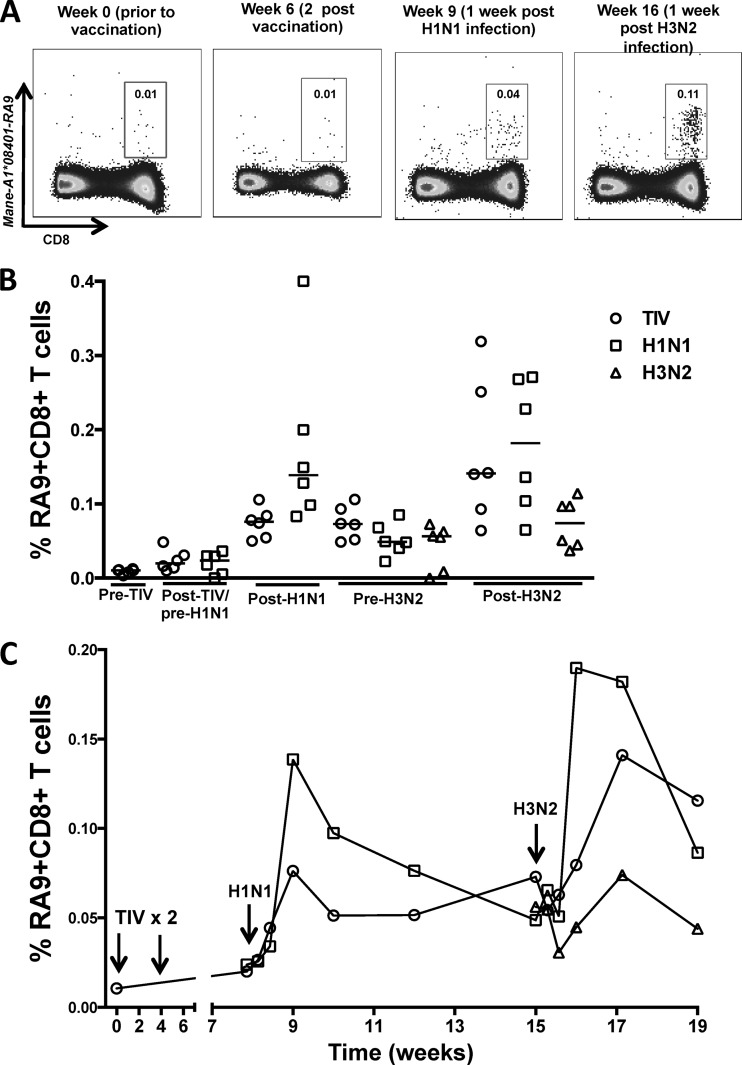
In addition to HA protein, TIVs contain internal proteins, such as NP, and it is possible a low level of vaccine-induced CTL priming to NP may be boosted upon subsequent infection with influenza virus. To determine whether the TIV vaccine primed influenza virus-specific CD8 T cells in macaques, we measured influenza virus-specific CD8 T cells prior to and following vaccination and H1N1/H3N2 infection using a sensitive MHC-I tetramer reagent. The tetramer presents the NP RA9 epitope bound to the Mane-A1*084 MHC-I protein. The vaccine and challenge influenza viruses used in this study have a conserved sequence across this NP epitope. NP-specific CD8 T cells were not detected above background levels following TIV vaccination (Fig. 7A). Following the H1N1 infection, NP-specific CD8 T cells were detected in both vaccinated animals and the unvaccinated controls, but there was no enhancement of the NP-specific CD8 T cells in the vaccinated animals (Fig. 7B). Indeed, the unvaccinated controls had marginally higher NP-specific CD8 T cells than the vaccinated animals (P = 0.0411 for the time-weighted average; Mann-Whitney test) (Fig. 6C). Following the H3N2 infection, there was a boost in CTL responses in the 12 animals previously infected with the H1N1 virus. The magnitude of the CTL response was greater in these 12 H1N1-primed animals than in naive animals challenged only with the H3N2 virus (P = 0.0002; Mann-Whitney test) (Fig. 7C). Further, the magnitude of the CTL response after the H3N2 infection of the 12 H1N1-infected macaques was greater than the CTL response detected after the initial H1N1 infection (P = 0.0068; Wilcoxon paired test) (Fig. 7C). This is consistent with the NP-specific CTL response induced by the H1N1 infection recognizing the same epitope during the H3N2 infection.
Fig 7.
Influenza virus-specific CD8 T cell responses over time following TIV vaccination and influenza virus infection of macaques. (A) Representative plots of Mane-A1*08401–RA9 tetramer-positive CD3− CD8+ T cells from a vaccinated H1N1- and H3N2-infected macaque (animal C3752) at week 0 prior to TIV vaccination, week 2 post-second TIV vaccination, week 1 post-H1N1 infection, and week 1 post-H3N2 infection. (B) Frequencies of RA9-specific T cells in 6 vaccinated H1N1-H3N2 macaques (TIV), 6 unvaccinated H1N1-H3N2 macaques (H1N1), and 6 H3N2 macaques (H3N2) at time points prior to TIV vaccination (Fig. 1 shows the groups), 4 weeks after the final TIV vaccination/pre-H1N1 infection, 1 week post-H1N1 infection (Post-H1N1), pre-H3N2 infection, and 2 weeks post-H3N2 infection (Post-H3N2). The horizontal lines represent the median values of groups. (C) Time course of median frequencies of RA9-specific CD8 T cells from 6 vaccinated H1N1-H3N2 macaques (TIV), 6 unvaccinated H1N1-H3N2 macaques (H1N1), and 6 H3N2 macaques (H3N2).
DISCUSSION
Generating robust immunity to diverse influenza virus subtypes is a major goal of influenza vaccinology. Inducing broad neutralizing antibodies to influenza virus has proven difficult, and there is considerable interest in alternate immune responses, such as ADCC-mediating antibodies and T cell responses, that may recognize diverse influenza virus strains (28, 29). Analyzing both ADCC and CTL responses in ferret and mouse models is difficult due to a lack of immunological reagents and/or established assays, and a background of multiple prior influenza virus exposures often confounds the study of influenza immunity in humans (12). We studied ADCC and CTL immunity following TIV vaccination and serial challenges with H1N1 and H3N2 influenza viruses in 18 pigtail macaques using novel ADCC assays and MHC-I tetramer reagents. We found that 2 doses of the TIV were poorly immunogenic in macaques and failed to elicit either ADCC or CD8 T cell responses. In contrast, infection with H1N1 induced robust ADCC and CTL responses that cross-recognized multiple H1 strains.
Our studies highlight the diverse immune responses induced by influenza virus infection compared to TIV vaccination. These studies confirm an urgent need for improved influenza vaccine strategies to broaden immunity against diverse influenza virus strains (30, 31). The TIV has variable immungenicity in humans, with a range of studies suggesting low to moderate immunogenicity of the vaccine at a single dose of 7.5 to 15 μg of HA protein (16, 17, 32, 33). Live attenuated influenza virus (LAIV) vaccines offer the potential to induce broader immunity (34, 35). LAIV vaccines show evidence of improved efficacy in children, although their efficacy in providing protection against seasonal influenza virus strains in adults is similar to that of standard TIV (36–38). The use of potent adjuvants combined with TIV may increase the magnitude and breadth of immunity to influenza (39). However, potent adjuvants may be accompanied by reduced tolerability. DNA vaccines, vector-based vaccines, virus-like particles, and prime-boost approaches are among a range of novel vaccination strategies to improve immunity to influenza, although the capacity of these new approaches to induce ADCC is not yet established (40–42).
Previous studies have indicated that influenza virus-specific CD8 T cells primed by influenza virus infection can be boosted with TIV or LAIV vaccines (43–45). In our study we investigated if CD8 T cell responses could be primed by TIV vaccination. No CTL responses were detected using a novel, sensitive MHC-I tetramer reagent. In contrast, H1N1 infection induced influenza virus-specific CD8 T cells, and H3N2 infection expanded the magnitude of the influenza virus-specific T cells. A background of previous influenza virus exposures may be required to generate boosting of both T cell and ADCC responses via vaccination.
Infection of pigtail macaques with the H3N2 strain we studied was more variable and resulted in a limited capacity to generate ADCC to diverse H3 strains. Further, the H3N2 challenge did not boost H1-specific ADCC responses. In addition, the H1N1 infection did not induce broad ADCC capable of recognizing H2 to H5 influenza virus subtypes. This suggests that cross-subtype ADCC immunity may be limited after 1 or 2 virus exposures and that multiple virus exposures may be required to raise more broadly reactive ADCC responses (12).
Antibodies that mediate HI activity and neutralization generally recognize highly variable antigenic regions on the globular head of HA and tend to be highly specific for a particular strain of influenza virus. We did not find an association between macaque HI antibodies and HA-specific ADCC antibodies, a finding we also observed in influenza virus-specific ADCC studies in humans (46). This suggests that ADCC and HI antibodies represent only a partially overlapping subset of antibodies. It will be of interest in future studies to map ADCC epitopes within HA to isolate more broadly cross-reactive antibodies that may recognize more conserved regions, as observed for rare broadly reactive neutralizing antibodies (47–49).
Our studies primarily focused on HA-specific ADCC responses, although we also detected ADCC responses to influenza virus NP. Previous studies have shown that passive transfer of NP-specific antibodies into mice provided protection from heterologous challenge (45, 50, 51). Unlike, other internal proteins, influenza virus NP is at least transiently expressed on the surfaces of virus-infected cells (52, 53). The mechanism by which these antibodies mediate their activity is unclear, with recent studies suggesting that antibody-mediated phagocytosis or complement-mediated lysis is unlikely to be the mechanism (54). Interestingly, in this study, we observed that NP-specific ADCC-mediating antibodies are generated following infection with H1N1 virus. This could potentially provide a mechanism by which NP-specific antibodies are mediating their protection. We have previously reported that the neuraminidase (NA) surface protein of influenza virus is also a target for ADCC responses in the sera of macaques infected with influenza virus, although the cross-reactivity of ADCC between different NA subtypes has not been studied (13). Several groups have studied ADCC responses to the relatively conserved M2 surface protein of influenza virus and have suggested a possible protective role for such antibodies in mouse models (55, 56).
Several aspects of our study warrant future exploration. First, background ADCC responses were observed in 1 of 6 of the influenza-naive macaques in the absence of HI antibodies. This may reflect prior exposure or cross-reactive responses initiated by another agent, although these responses were not boosted following the influenza virus infections. More intensive screening of sera from animals entering these studies may help reduce this issue in the future. Second, we did not detect ADCC to a series of H3N2 virus HA proteins, nor were we able to measure ADCC in the H3N2 strain in the vaccine due to a lack of availability of this HA protein. Future studies should more closely match all the vaccine antigens with the antigens used for in vitro testing. Third, although robust immune responses to influenza virus are observed in influenza virus-infected macaques, the infection is usually asymptomatic, suggesting such infections are partially attenuated in pigtail macaques. Indeed, the H3N2 challenge we studied successfully induced HI antibodies to A/Sydney/5/1997 in only 7 of 18 macaques, and higher levels of viral RNA were recovered from the nose and/or throat of only 9 of 18 macaques (>5 × 103 copies/ml). The limited infection of some macaques with this strain would be expected to attenuate immune responses. Future studies of H3N2 influenza virus strains that more reliably infect macaques should assist in further dissecting protective immunity to influenza. Fourth, TIV vaccination was of low immunogenicity in the influenza-naive macaques even after two doses of the human adult vaccine. Influenza-naive infants who have had limited to no exposure to influenza virus are usually given two doses of TIV to increase immunogenicity. Future studies could involve repeated vaccinations with larger doses to increase immunogenicity in macaques. Further, the assessment of LAIV and adjuvanted TIV preparations in macaques would potentially be another mechanism for increasing immunogenicity and a focus for future studies. Fifth, to clearly delineate the induction of new immune responses, we studied influenza-naive macaques. However, this would not capture the vaccine-induced boosting of immune responses primed by previous infections, as would be expected in humans. Adults are likely to have had many exposures to influenza virus throughout their lifetimes, generating a pool of influenza virus memory B cells that can expand upon vaccination. Indeed, recent antibody-sequencing studies after vaccination of humans with inactivated influenza virus showed that as humans age, fewer vaccine-elicited antibody responses are new IgM responses (57). Further studies of vaccines administered to both human populations and macaques previously infected with influenza virus are warranted. Sixth, although we sensitively detected CTL immunity with a novel MHC-I tetramer reagent, we did not study the breadth of CTL response or its functionality. We have previously observed marked differences in the functionality of influenza virus-specific CTL responses compared to simian immunodeficiency virus (SIV)-specific CTL responses in macaques (19). Further study of the quality and breadth of the influenza virus-specific CTL response may provide additional insights into protective immunity to influenza.
In summary, we found that TIV protein vaccination against influenza did not induce or prime ADCC or CTL responses. In contrast, infection with H1N1 influenza virus induced robust influenza virus-specific ADCC and CTL responses, and the ADCC responses recognized HA proteins from multiple H1N1 strains. We speculate that long-lived cross-reactive ADCC-mediating antibodies can be induced through vaccination, and this may provide a level of protection from antigenically distinct influenza viruses not currently present in vaccinated individuals.
ACKNOWLEDGMENTS
The work was supported by Australian National Health and Medical Research Council (NHMRC) awards 628331 and 510488. The Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health and Ageing.
Footnotes
Published ahead of print 9 October 2013
REFERENCES
- 1.Anonymous 2013. Interim adjusted estimates of seasonal influenza vaccine effectiveness—United States, February 2013. MMWR Morb. Mortal. Wkly. Rep. 62:119–123 [PMC free article] [PubMed] [Google Scholar]
- 2.Fielding JE, Grant KA, Garcia K, Kelly HA. 2011. Effectiveness of seasonal influenza vaccine against pandemic (H1N1) 2009 virus, Australia, 2010. Emerg. Infect. Dis. 17:1181–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rimmelzwaan GF, McElhaney JE. 2008. Correlates of protection: novel generations of influenza vaccines. Vaccine 26(Suppl 4):D41–D44 [DOI] [PubMed] [Google Scholar]
- 4.O'Brien KB, Morrison TE, Dundore DY, Heise MT, Schultz-Cherry S. 2011. A protective role for complement C3 protein during pandemic 2009 H1N1 and H5N1 influenza A virus infection. PLoS One 6:e17377. 10.1371/journal.pone.0017377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohta R, Torii Y, Imai M, Kimura H, Okada N, Ito Y. 2011. Serum concentrations of complement anaphylatoxins and proinflammatory mediators in patients with 2009 H1N1 influenza. Microbiol. Immunol. 55:191–198 [DOI] [PubMed] [Google Scholar]
- 6.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. 2001. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J. Immunol. 166:7381–7388 [DOI] [PubMed] [Google Scholar]
- 7.Laidlaw BJ, Decman V, Ali MA, Abt MC, Wolf AI, Monticelli LA, Mozdzanowska K, Angelosanto JM, Artis D, Erikson J, Wherry EJ. 2013. Cooperativity between CD8+ T cells, non-neutralizing antibodies, and alveolar macrophages is important for heterosubtypic influenza virus immunity. PLoS Pathog. 9:e1003207. 10.1371/journal.ppat.1003207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberg SB, Criswell BS, Six HR, Couch RB. 1978. Lymphocyte cytotoxicity to influenza virus-infected cells: response to vaccination and virus infection. Infect. Immun. 20:640–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto G, Wright PF, Karzon DT. 1983. Ability of human cord blood lymphocytes to mediate antibody-dependent cellular cytotoxicity against influenza virus-infected cells. Infect. Immun. 42:214–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto G, Wright PF, Karzon DT. 1983. Antibody-dependent cell-mediated cytotoxicity against influenza virus-infected cells. J. Infect. Dis. 148:785–794 [DOI] [PubMed] [Google Scholar]
- 11.Vella S, Rocchi G, Resta S, Marcelli M, De Felici A. 1980. Antibody reactive in antibody-dependent cell-mediated cytotoxicity following influenza virus vaccination. J. Med. Virol. 6:203–211 [DOI] [PubMed] [Google Scholar]
- 12.Jegaskanda S, Job ER, Kramski M, Laurie K, Isitman G, de Rose R, Winnall WR, Stratov I, Brooks AG, Reading PC, Kent SJ. 2013. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J. Immunol. 190:1837–1848 [DOI] [PubMed] [Google Scholar]
- 13.Jegaskanda S, Weinfurter JT, Friedrich TC, Kent SJ. 2013. Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. J. Virol. 87:5512–5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caton AJ, Brownlee GG, Yewdell JW, Gerhard W. 1982. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell 31:417–427 [DOI] [PubMed] [Google Scholar]
- 15.Wiley DC, Wilson IA, Skehel JJ. 1981. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature 289:373–378 [DOI] [PubMed] [Google Scholar]
- 16.Frey SE, Bernstein DI, Gerber MA, Keyserling HL, Munoz FM, Winokur PL, Turley CB, Rupp RE, Hill H, Wolff M, Noah DL, Ross AC, Cress G, Belshe RB. 2012. Safety and immune responses in children after concurrent or sequential 2009 H1N1 and 2009–2010 seasonal trivalent influenza vaccinations. J. Infect. Dis. 206:828–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li YP, Li W, Liang XF, Liu Y, Huang XC, Li CG, Li RC, Wang JZ, Wang HQ, Yin WD. 8 November 2012. Immunogenicity and safety of a 2009 pandemic influenza A (H1N1) monovalent vaccine in Chinese infants aged 6–35 months: a randomized, double-blind, controlled phase I clinical trial. Influenza Other Respi. Viruses [Epub ahead of print.] 10.1111/irv.12028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce MB, Belser JA, Gustin KM, Pappas C, Houser KV, Sun X, Maines TR, Pantin-Jackwood MJ, Katz JM, Tumpey TM. 2012. Seasonal trivalent inactivated influenza vaccine protects against 1918 Spanish influenza virus infection in ferrets. J. Virol. 86:7118–7125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jegaskanda S, Reece JC, De Rose R, Stambas J, Sullivan L, Brooks AG, Kent SJ, Sexton A. 2012. Comparison of influenza and SIV specific CD8 T cell responses in macaques. PLoS One 7:e32431. 10.1371/journal.pone.0032431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sexton A, De Rose R, Reece JC, Alcantara S, Loh L, Moffat JM, Laurie K, Hurt A, Doherty PC, Turner SJ, Kent SJ, Stambas J. 2009. Evaluation of recombinant influenza virus-simian immunodeficiency virus vaccines in macaques. J. Virol. 83:7619–7628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinfurter JT, Brunner K, Capuano SV, III, Li C, Broman KW, Kawaoka Y, Friedrich TC. 2011. Cross-reactive T cells are involved in rapid clearance of 2009 pandemic H1N1 influenza virus in nonhuman primates. PLoS Pathog. 7:e1002381. 10.1371/journal.ppat.1002381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McVernon J, Laurie K, Nolan T, Owen R, Irving D, Capper H, Hyland C, Faddy H, Carolan L, Barr I, Kelso A. 2010. Seroprevalence of 2009 pandemic influenza A(H1N1) virus in Australian blood donors, October-December 2009. Euro. Surveill. 15:pii:19678. [DOI] [PubMed] [Google Scholar]
- 23.Sorn S, Sok T, Ly S, Rith S, Tung N, Viari A, Gavotte L, Holl D, Seng H, Asgari N, Richner B, Laurent D, Chea N, Duong V, Toyoda T, Yasuda CY, Kitsutani P, Zhou P, Bing S, Deubel V, Donis R, Frutos R, Buchy P. 2013. Dynamic of H5N1 virus in Cambodia and emergence of a novel endemic sub-clade. Infect. Genet. Evol. 15:87–94 [DOI] [PubMed] [Google Scholar]
- 24.Chung AW, Isitman G, Navis M, Kramski M, Center RJ, Kent SJ, Stratov I. 2011. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc. Natl. Acad. Sci. U. S. A. 108:7505–7510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isitman G, Chung AW, Navis M, Kent SJ, Stratov I. 2011. Pol as a target for antibody dependent cellular cytotoxicity responses in HIV-1 infection. Virology 412:110–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez CS, Reece JC, Saepuloh U, De Rose R, Ishkandriati D, O'Connor DH, Wiseman RW, Kent SJ. 2011. Screening and confirmatory testing of MHC class I alleles in pig-tailed macaques. Immunogenetics 63:511–521 [DOI] [PubMed] [Google Scholar]
- 27.Smith MZ, Fernandez CS, Chung A, Dale CJ, De Rose R, Lin J, Brooks AG, Krebs KC, Watkins DI, O'Connor DH, Davenport MP, Kent SJ. 2005. The pigtail macaque MHC class I allele Mane-A*10 presents an immundominant SIV Gag epitope: identification, tetramer development and implications of immune escape and reversion. J. Med. Primatol. 34:282–293 [DOI] [PubMed] [Google Scholar]
- 28.Corti D, Lanzavecchia A. 2013. Broadly neutralizing antiviral antibodies. Annu. Rev. Immunol. 31:705–742 [DOI] [PubMed] [Google Scholar]
- 29.Rimmelzwaan GF, Fouchier RA, Osterhaus AD. 2007. Influenza virus-specific cytotoxic T lymphocytes: a correlate of protection and a basis for vaccine development. Curr. Opin. Biotechnol. 18:529–536 [DOI] [PubMed] [Google Scholar]
- 30.Baz M, Luke CJ, Cheng X, Jin H, Subbarao K. 28 May 2013. H5N1 vaccines in humans. Virus Res. [Epub ahead of print.] 10.1016/j.virusres.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodewes R, Fraaij PL, Kreijtz JH, Geelhoed-Mieras MM, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. 2012. Annual influenza vaccination affects the development of heterosubtypic immunity. Vaccine 30:7407–7410 [DOI] [PubMed] [Google Scholar]
- 32.Jackson LA, Chen WH, Stapleton JT, Dekker CL, Wald A, Brady RC, Edupuganti S, Winokur P, Mulligan MJ, Keyserling HL, Kotloff KL, Rouphael N, Noah DL, Hill H, Wolff MC. 2012. Immunogenicity and safety of varying dosages of a monovalent 2009 H1N1 influenza vaccine given with and without AS03 adjuvant system in healthy adults and older persons. J. Infect. Dis. 206:811–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langley JM, Reich D, Aggarwal N, Connor D, Lebel MH, Gupta A, Garfield H, Li P, Madan A, Vaughn DW. 2012. Randomized, multicenter trial of a single dose of AS03-adjuvanted or unadjuvanted H1N1 2009 pandemic influenza vaccine in children 6 months to <9 years of age: safety and immunogenicity. Pediatr. Infect. Dis. J. 31:848–858 [DOI] [PubMed] [Google Scholar]
- 34.Boonnak K, Paskel M, Matsuoka Y, Vogel L, Subbarao K. 2012. Evaluation of replication, immunogenicity and protective efficacy of a live attenuated cold-adapted pandemic H1N1 influenza virus vaccine in non-human primates. Vaccine 30:5603–5610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broadbent AJ, Subbarao K. 2011. Influenza virus vaccines: lessons from the 2009 H1N1 pandemic. Curr. Opin. Virol. 1:254–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ambrose CS, Wu X, Knuf M, Wutzler P. 2012. The efficacy of intranasal live attenuated influenza vaccine in children 2 through 17 years of age: a meta-analysis of 8 randomized controlled studies. Vaccine 30:886–892 [DOI] [PubMed] [Google Scholar]
- 37.Osterholm MT, Kelley NS, Sommer A, Belongia EA. 2012. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 12:36–44 [DOI] [PubMed] [Google Scholar]
- 38.Phillips CJ, Woolpert T, Sevick C, Faix D, Blair PJ, Crum-Cianflone NF. 2013. Comparison of the effectiveness of trivalent inactivated influenza vaccine and live, attenuated influenza vaccine in preventing influenza-like illness among US military service members, 2006–2009. Clin. Infect. Dis. 56:11–19 [DOI] [PubMed] [Google Scholar]
- 39.Nazareth I, Tavares F, Rosillon D, Haguinet F, Bauchau V. 5 February 2013. Safety of AS03-adjuvanted split-virion H1N1 (2009) pandemic influenza vaccine: a prospective cohort study. BMJ Open 3:pii:e001912. 10.1136/bmjopen-2012-001912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong L, Liu F, Fairman J, Hong DK, Lewis DB, Monath T, Warner JF, Belser JA, Patel J, Hancock K, Katz JM, Lu X. 2012. Cationic liposome-DNA complexes (CLDC) adjuvant enhances the immunogenicity and cross-protective efficacy of a pre-pandemic influenza A H5N1 vaccine in mice. Vaccine 30:254–264 [DOI] [PubMed] [Google Scholar]
- 41.Lillie PJ, Berthoud TK, Powell TJ, Lambe T, Mullarkey C, Spencer AJ, Hamill M, Peng Y, Blais ME, Duncan CJ, Sheehy SH, Havelock T, Faust SN, Williams RL, Gilbert A, Oxford J, Dong T, Hill AV, Gilbert SC. 2012. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin. Infect. Dis. 55:19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei CJ, Yassine HM, McTamney PM, Gall JG, Whittle JR, Boyington JC, Nabel GJ. 2012. Elicitation of broadly neutralizing influenza antibodies in animals with previous influenza exposure. Sci. Transl. Med. 4:147ra114. 10.1126/scitranslmed.3004273 [DOI] [PubMed] [Google Scholar]
- 43.Hoft DF, Babusis E, Worku S, Spencer CT, Lottenbach K, Truscott SM, Abate G, Sakala IG, Edwards KM, Creech CB, Gerber MA, Bernstein DI, Newman F, Graham I, Anderson EL, Belshe RB. 2011. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J. Infect. Dis. 204:845–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu JP, Phoon MC, Koh GC, Chen MI, Lee VJ, Wu Y, Xie ML, Cheong A, Leo YS, Chow VT. 2012. Comparison of neutralizing antibody and cell-mediated immune responses to pandemic H1N1 2009 influenza virus before and after H1N1 2009 influenza vaccination of elderly subjects and healthcare workers. Int. J. Infect. Dis. 16:e621–e627. 10.1016/j.ijid.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 45.Richards KA, Chaves FA, Alam S, Sant AJ. 2012. Trivalent inactivated influenza vaccines induce broad immunological reactivity to both internal virion components and influenza surface proteins. Vaccine 31:219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jegaskanda S, Laurie K, Amarasena T, Winnall W, Kramski M, De Rose R, Barr I, Brooks A, Reading PC, Kent SJ. 2013. Age-associated cross-reactive ADCC toward 2009-pandemic influenza. J. Infect. Dis. 208:1051–1061 [DOI] [PubMed] [Google Scholar]
- 47.Corti D, Suguitan AL, Jr, Pinna D, Silacci C, Fernandez-Rodriguez BM, Vanzetta F, Santos C, Luke CJ, Torres-Velez FJ, Temperton NJ, Weiss RA, Sallusto F, Subbarao K, Lanzavecchia A. 2010. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J. Clin. Invest. 120:1663–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, Lee JH, Metlagel Z, Bujny MV, Jongeneelen M, van der Vlugt R, Lamrani M, Korse HJ, Geelen E, Sahin O, Sieuwerts M, Brakenhoff JP, Vogels R, Li OT, Poon LL, Peiris M, Koudstaal W, Ward AB, Wilson IA, Goudsmit J, Friesen RH. 2012. Highly conserved protective epitopes on influenza B viruses. Science 337:1343–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lingwood D, McTamney PM, Yassine HM, Whittle JR, Guo X, Boyington JC, Wei CJ, Nabel GJ. 2012. Structural and genetic basis for development of broadly neutralizing influenza antibodies. Nature 489:566–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LaMere MW, Lam HT, Moquin A, Haynes L, Lund FE, Randall TD, Kaminski DA. 2011. Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus. J. Immunol. 186:4331–4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamere MW, Moquin A, Lee FE, Misra RS, Blair PJ, Haynes L, Randall TD, Lund FE, Kaminski DA. 2011. Regulation of antinucleoprotein IgG by systemic vaccination and its effect on influenza virus clearance. J. Virol. 85:5027–5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Virelizier JL, Allison AC, Oxford JS, Schild GC. 1977. Early presence of ribonucleoprotein antigen on surface of influenza virus-infected cells. Nature 266:52–54 [DOI] [PubMed] [Google Scholar]
- 53.Yewdell JW, Frank E, Gerhard W. 1981. Expression of influenza A virus internal antigens on the surface of infected P815 cells. J. Immunol. 126:1814–1819 [PubMed] [Google Scholar]
- 54.Bodewes R, Geelhoed-Mieras MM, Wrammert J, Ahmed R, Wilson PC, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. 2013. In vitro assessment of the immunological significance of a human monoclonal antibody directed to the influenza A virus nucleoprotein. Clin. Vaccine Immunol. 20:1333–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carmichael O, Schwarz C, Drucker D, Fletcher E, Harvey D, Beckett L, Jack CR, Jr, Weiner M, DeCarli C. 2010. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch. Neurol. 67:1370–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ranji SR, Shetty K, Posley KA, Lewis R, Sundaram V, Galvin CM, Winston LG. 2007. Closing the quality gap: a critical analysis of quality improvement strategies. Vol 6 Prevention of healthcare-associated infections. Agency for Healthcare Research and Quality, Rockville, MD: [PubMed] [Google Scholar]
- 57.Jiang N, He J, Weinstein JA, Penland L, Sasaki S, He XS, Dekker CL, Zheng NY, Huang M, Sullivan M, Wilson PC, Greenberg HB, Davis MM, Fisher DS, Quake SR. 2013. Lineage structure of the human antibody repertoire in response to influenza vaccination. Sci. Transl. Med. 5:171ra119. 10.1126/scitranslmed.3004794 [DOI] [PMC free article] [PubMed] [Google Scholar]