Abstract
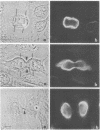
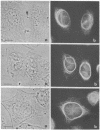
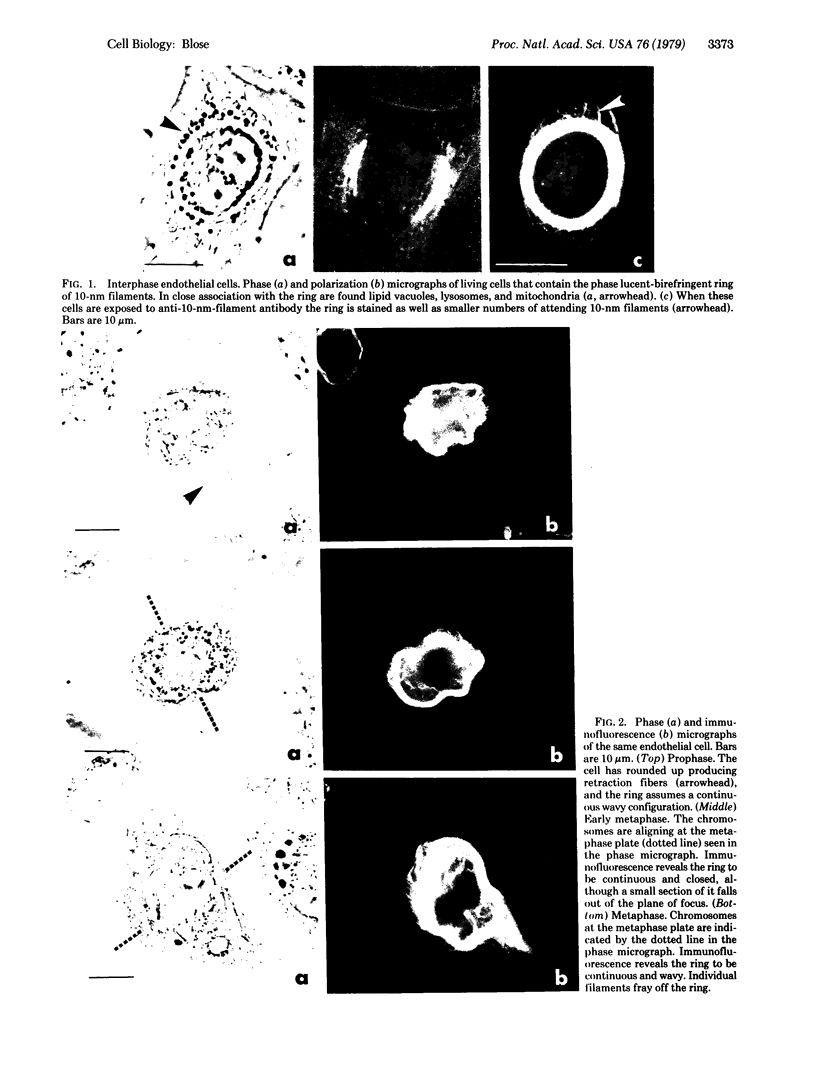
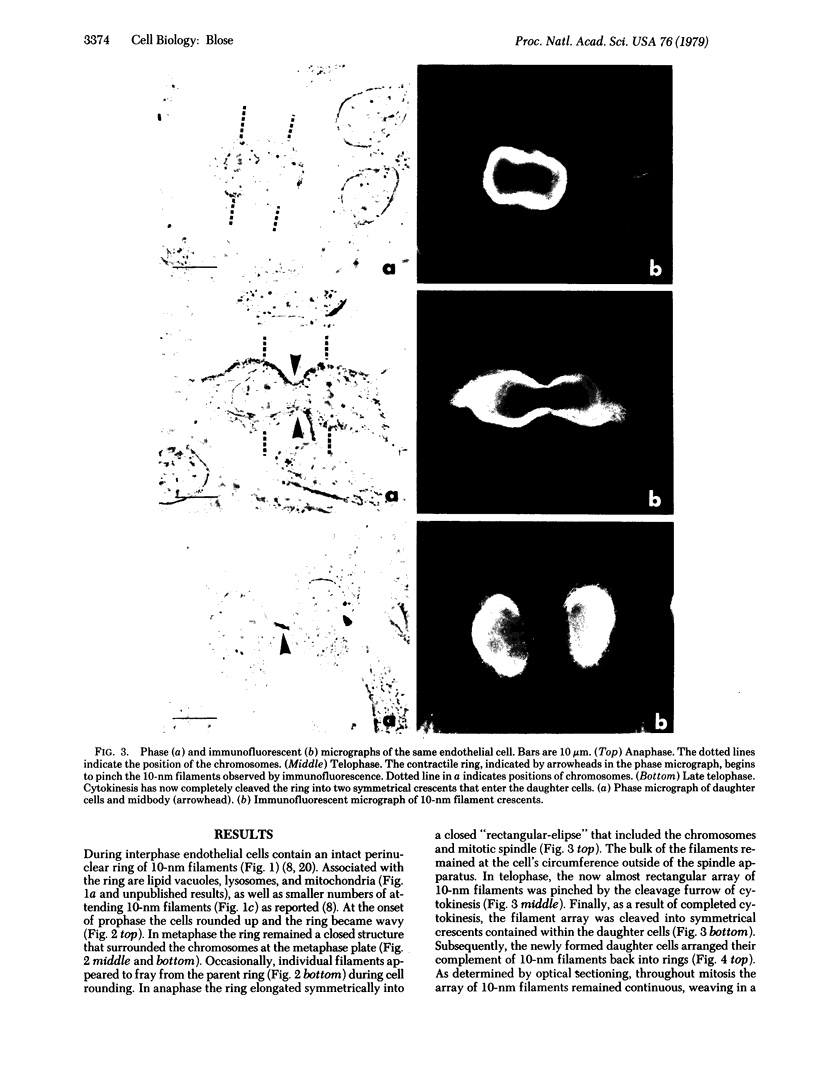
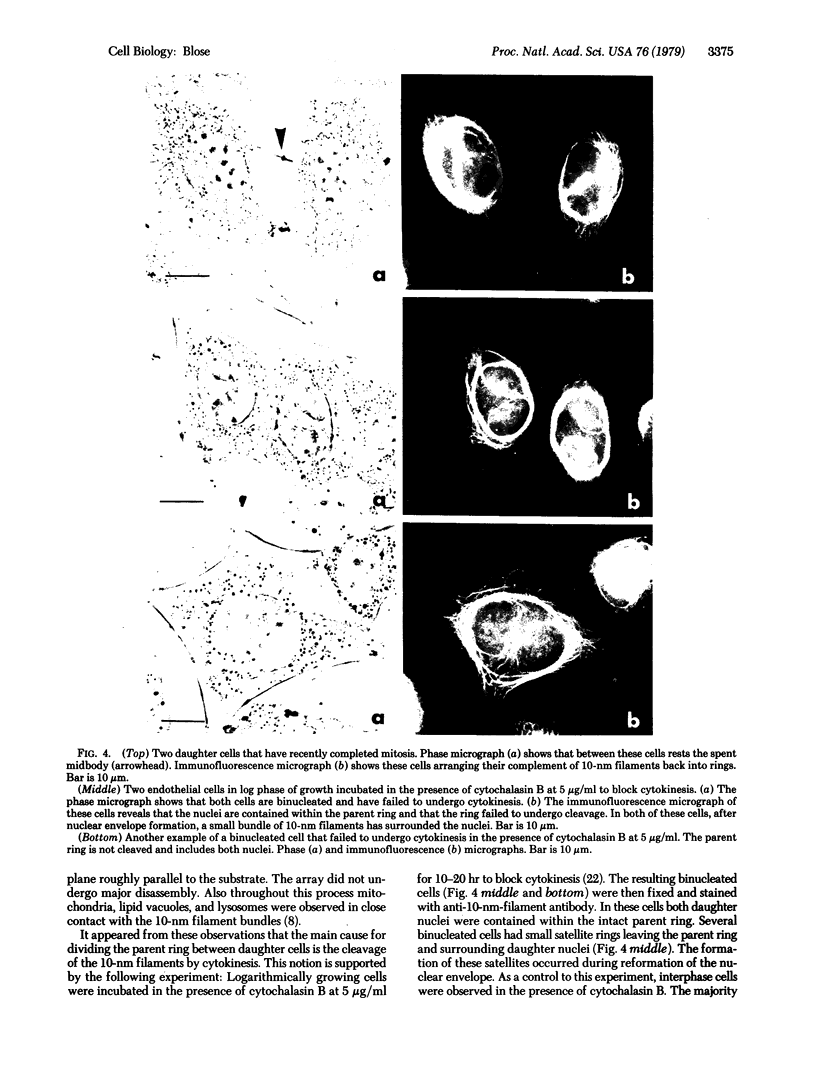
By indirect immunofluorescence the behavior of the 10-nm filaments was studied at various stages of mitosis in guinea pig vascular endothelial cells. Interphase cells contain a ring of 10-nm filaments that encircles the nucleus and is maintained in a plane parallel to the substrate. During prophase and metaphase the cells round up and the 10-nm filament ring becomes wavy though still a closed structure. As anaphase progresses the ring then elongates into a rectangle that contains the spindle apparatus and chromosomes. In late telophase, cytokinesis cleaves the 10-nm filaments into crescents at the site of the contractile ring. These crescents then close into rings in the daughter cells. If cytokinesis is inhibited with 5 microgram of cytochalasin B per ml, then cleavage of the 10-nm filaments is blocked and the daughter nuclei remain surrounded by the parent ring. At no point during mitosis does the array of 10-nm filaments undergo major disassembly. These results indicate that, in contrast to the other major cytoplasmic structures, ventral microfilament bundles and cytoplasmic microtubules, which disassemble and reassemble during mitosis, 10-nm filaments remain intact throughout this process. The possibility is discussed that these filaments may function in transport of organelles and structural proteins, and provide the daughter cells with topological information about placement and assembly of these elements within the microtrabecular lattice.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht-Buehler G. Daughter 3T3 cells. Are they mirror images of each other? J Cell Biol. 1977 Mar;72(3):595–603. doi: 10.1083/jcb.72.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberfeld P., Ericsson J. L., Perlmann P., Raftell M. Increased occurrence of cytoplasmic filaments in in vitro propagated rat liver epithelial cells. Exp Cell Res. 1965 Aug;39(1):301–305. doi: 10.1016/0014-4827(65)90034-0. [DOI] [PubMed] [Google Scholar]
- Blose S. H., Chacko S. Rings of intermediate (100 A) filament bundles in the perinuclear region of vascular endothelial cells. Their mobilization by colcemid and mitosis. J Cell Biol. 1976 Aug;70(2 Pt 1):459–466. doi: 10.1083/jcb.70.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blose S. H., Shelanski M. L., Chacko S. Localization of bovine brain filament antibody on intermediate (100 A) filaments in guinea pig vascular endothelial cells and chick cardiac muscle cells. Proc Natl Acad Sci U S A. 1977 Feb;74(2):662–665. doi: 10.1073/pnas.74.2.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke P. A filamentous cytoskeleton in vertebrate smooth muscle fibers. J Cell Biol. 1976 Mar;68(3):539–556. doi: 10.1083/jcb.68.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE PETRIS S., KARLSBAD G., PERNIS B. Filamentous structures in the cytoplasm of normal mononuclear phagocytes. J Ultrastruct Res. 1962 Aug;7:39–55. doi: 10.1016/s0022-5320(62)80025-2. [DOI] [PubMed] [Google Scholar]
- Dayton W. R., Goll D. E., Zeece M. G., Robson R. M., Reville W. J. A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Purification from porcine muscle. Biochemistry. 1976 May 18;15(10):2150–2158. doi: 10.1021/bi00655a019. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Fujiwara K., Pollard T. D. Fluorescent antibody localization of myosin in the cytoplasm, cleavage furrow, and mitotic spindle of human cells. J Cell Biol. 1976 Dec;71(3):848–875. doi: 10.1083/jcb.71.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K., Porter M. E., Pollard T. D. Alpha-actinin localization in the cleavage furrow during cytokinesis. J Cell Biol. 1978 Oct;79(1):268–275. doi: 10.1083/jcb.79.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. D., Follett E. A. Birefringent filamentous organelle in BHK-21 cells and its possible role in cell spreading and motility. Science. 1970 Jul 17;169(3942):286–288. doi: 10.1126/science.169.3942.286. [DOI] [PubMed] [Google Scholar]
- Gordon W. E., 3rd, Bushnell A., Burridge K. Characterization of the intermediate (10 nm) filaments of cultured cells using an autoimmune rabbit antiserum. Cell. 1978 Feb;13(2):249–261. doi: 10.1016/0092-8674(78)90194-0. [DOI] [PubMed] [Google Scholar]
- Herman I. M., Pollard T. D. Comparison of purified anti-actin and fluorescent-heavy meromyosin staining patterns in dividing cells. J Cell Biol. 1979 Mar;80(3):509–520. doi: 10.1083/jcb.80.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard B. D., Lazarides E. Copurification of actin and desmin from chicken smooth muscle and their copolymerization in vitro to intermediate filaments. J Cell Biol. 1979 Jan;80(1):166–182. doi: 10.1083/jcb.80.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. 10 nm filaments in normal and transformed cells. Cell. 1978 Jan;13(1):151–163. doi: 10.1016/0092-8674(78)90146-0. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J Cell Biol. 1968 Sep;38(3):538–555. doi: 10.1083/jcb.38.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimbow K., Fitzpatrick T. B. Changes in distribution pattern of cytoplasmic filaments in human melanocytes during ultraviolet-mediated melanin pigmentation. The role of the 100-Angstrom filaments in the elongation of melanocytic dendrites and in the movement and transfer of melanosomes. J Cell Biol. 1975 May;65(2):481–488. doi: 10.1083/jcb.65.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A. M., Chacko S. Myofibril organisation and mitosis in cultured cardiac muscle cells. Dev Biol. 1976 Feb;48(2):421–430. doi: 10.1016/0012-1606(76)90103-2. [DOI] [PubMed] [Google Scholar]
- Reddy M. K., Etlinger J. D., Rabinowitz M., Fischman D. A., Zak R. Removal of Z-lines and alpha-actinin from isolated myofibrils by a calcium-activated neutral protease. J Biol Chem. 1975 Jun 10;250(11):4278–4284. [PubMed] [Google Scholar]
- Sanger J. W. Changing patterns of actin localization during cell division. Proc Natl Acad Sci U S A. 1975 May;72(5):1913–1916. doi: 10.1073/pnas.72.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W. Intracellular localization of actin with fluorescently labelled heavy meromyosin. Cell Tissue Res. 1975 Aug 27;161(4):431–434. doi: 10.1007/BF00224134. [DOI] [PubMed] [Google Scholar]
- Sanger J. W. Presence of actin during chromosomal movement. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2451–2455. doi: 10.1073/pnas.72.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W., Sanger J. M. The cytoskeleton and cell division. Methods Achiev Exp Pathol. 1979;8:110–142. [PubMed] [Google Scholar]
- Schroeder T. E. Actin in dividing cells: contractile ring filaments bind heavy meromyosin. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1688–1692. doi: 10.1073/pnas.70.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starger J. M., Brown W. E., Goldman A. E., Goldman R. D. Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J Cell Biol. 1978 Jul;78(1):93–109. doi: 10.1083/jcb.78.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Goldman R. D. Functions of cytoplasmic fibers in intracellular movements in BHK-21 cells. J Cell Biol. 1978 Dec;79(3):708–726. doi: 10.1083/jcb.79.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman L., Krebs E. G. Identification of two protease inhibitors from bovine cardiac muscle. J Biol Chem. 1978 Sep 10;253(17):5888–5891. [PubMed] [Google Scholar]
- Wuerker R. B., Kirkpatrick J. B. Neuronal microtubules, neurofilaments, and microfilaments. Int Rev Cytol. 1972;33:45–75. doi: 10.1016/s0074-7696(08)61448-5. [DOI] [PubMed] [Google Scholar]