Abstract
Type I interferon (IFN-I) inhibits the replication of different viruses. However, the effect of IFN-I on the human T-lymphotropic virus type 1 (HTLV-1) viral cycle is controversial. Here, we investigated the consequences of IFN-α addition for different steps of HTLV-1 and HTLV-2 infection. We first show that alpha interferon (IFN-α) efficiently impairs HTLV-1 and HTLV-2 de novo infection in a T cell line and in primary lymphocytes. Using pseudotyped viruses expressing HTLV-1 envelope, we then show that cell-free infection is insensitive to IFN-α, demonstrating that the cytokine does not affect the early stages of the viral cycle. In contrast, intracellular levels of Gag, Env, or Tax protein are affected by IFN-α treatment in T cells, primary lymphocytes, or 293T cells transfected with HTLV-1 or HTLV-2 molecular clones, demonstrating that IFN-α acts during the late stages of infection. We show that IFN-α does not affect Tax-mediated transcription and acts at a posttranscriptional level. Using either small interfering RNA (siRNA) directed against PKR or a PKR inhibitor, we demonstrate that PKR, whose expression is induced by interferon, plays a major role in IFN-α-induced HTLV-1/2 inhibition. These results indicate that IFN-α has a strong repressive effect on the HTLV-1 and HTLV-2 viral cycle during de novo infection of cells that are natural targets of the viruses.
INTRODUCTION
Human T-lymphotropic virus type 1 (HTLV-1) infects 5 to 10 million people worldwide (1). In 2 to 5% of infected individuals, HTLV-1 causes either adult T-cell leukemia/lymphoma (ATLL) or a neurodegenerative disorder called HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (2–5). Interestingly, despite a high percentage of similarity in its genomic organization with HTLV-1, HTLV-2 has been associated with lymphocytosis and with rare cases of HAM/TSP (6), but not with leukemia (7–9), and the molecular determinants that would explain those differences are the subject of numerous investigations (for a recent review, see reference 10).
Innate immunity plays a critical role in the host response to a microbial infection. The interferon (IFN) family includes three classes, i.e., type I (IFN-I, including alpha interferon [IFN-α] and IFN-β), type II (IFN-γ), and IFN-λ molecules. IFN-I is rapidly induced following viral infections (11). Binding of IFN-Is to their receptors (IFNAR1/IFNAR2) initiates the Janus kinases-signal transducers and activators of transcription (JAK-STAT) intracellular signaling pathway, leading to transcription activation of IFN-stimulated genes (ISGs) that are responsible for the antiviral, antiproliferative, and immunoregulatory responses (12).
ISGs target different steps of the viral life cycle (13, 14). As an example, simian tripartite interaction motif 5α (TRIM-5α) targets incoming human immunodeficiency virus type 1 (HIV-1) particles; apolipoprotein B mRNA-editing catalytic polypeptide-like 3G (APOBEC3G) edits the HIV-1 genome during reverse transcription (RT) in the absence of Vif; 2′-5′ oligoadenylate synthetase and RNase L are responsible for mRNA degradation in cases of dengue virus, chikungunya virus, or hepatitis C virus (HCV) infection; double-stranded RNA (ds-RNA)-activated serine/threonine protein kinase (PKR) prevents viral mRNA translation in cells infected with hepatitis B virus, HCV, or HIV; and tetherin prevents HIV-1 particle release in cells infected with HIV-1 that does not encode the Vpu viral protein.
A study demonstrated that ultracentrifuged HTLV-1 particles induce IFN-I secretion after their incubation with plasmacytoid dendritic cells (15). In addition, an inverse correlation was described between the HTLV-1 proviral load (PVL) (i.e., the number of integrated copies of HTLV-1 expressed as a proportion of peripheral blood mononuclear cells [PBMCs]) and endogenous IFN-α secretion in ATLL patients (16), providing a rationale for IFN-α therapy in HTLV-1-infected individuals. Indeed, therapeutic treatments using IFN-α and IFN-β, alone or in combination with other molecules, such as azidothymidine (AZT), have been performed in ATLL patients (17–22) or TSP/HAM patients (23–28). The most remarkable effects were observed in chronic and smoldering ATLL patients treated with IFN-AZT combined chemotherapy, where sustained and complete remission was reached and maintained after 14 years of observation in some patients (29). The same therapeutic combination also improved the survival time of acute ATLL patients, who eventually relapsed (29).
However, IFN-α effects on the HTLV-1 cycle in vitro are controversial. It was shown that HTLV-1 gag mRNA decreased when HTLV-1-immortalized (interleukin 2 [IL-2]-dependent) T cells were cocultured with human 293T or murine NIH 3T3 nonlymphoid stromal cells (30). This effect was abolished when a polyclonal neutralizing antibody against IFN-β (but not against IFN-α) was added, indicating that IFN-β produced by stromal cells could inhibit virus production. Consistent with those data, HTLV-1 expression was restored when HTLV-1-infected cells were separated from IFN-producing stromal cells. Finally, using a murine model, the authors concluded that the decrease in HTLV-1 expression in vivo was linked to the IRF-7-dependent pathway (30). In contrast, other reports showed that IFN-α treatment of HTLV-1-transformed cells does not lead to any significant reduction in virus expression (31–33), suggesting that the infected cells, which chronically produce viral proteins and do not require IL-2 for their growth, are insensitive to IFN-I.
IFN-α treatment of 293T cells transfected with an HTLV-1 molecular clone inhibited virus assembly and release (34). Subsequent reports showed that ectopically overexpressed tetherin (which can be induced by IFN) prevents the release of HTLV-1 virus-like particles (expressing only gag/pol) or HTLV-1 particles from 293T-transfected cells (35, 36). Importantly, those reports also showed that tetherin decreases only cell-free transmission of HTLV-1 and does not impact cell-cell transmission, which is the main route of HTLV-1 transmission (35, 37, 38). The experiments, however, did not address whether other steps of HTLV-1 infection were sensitive to IFN-I.
The effects of exogenous IFN-I addition on the HTLV-1 cycle are therefore debated (for recent reviews, see references 39 and 40) and have never been investigated for HTLV-2. In addition, most reports have been performed using transfected epithelial cells, which do not represent target cells in vivo and do not allow the study of the early steps of infection. Here, we used different infection settings to show that IFN-α-treated T cells are refractory to primary HTLV infection and that IFN-α targets the late stages of the viral cycle. We demonstrate that IFN-α inhibits viral protein expression through PKR activation, leading to a decrease of viral protein synthesis.
MATERIALS AND METHODS
Cell culture.
293T and 293T-LTR-GFP (41) cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Gibco, Life Technologies) and 100 μg/ml penicillin-streptomycin (Gibco, Life Technologies). Jurkat, Jurkat-LTR-luc (42), and HTLV-1-infected (C91-PL and C8166) and HTLV-2-infected (C19) T cell lines and peripheral blood lymphocytes (PBLs) were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (Gibco, Life Technologies) and 100 μg/ml penicillin-streptomycin (Gibco, Life Technologies). PBLs were purified from the blood of healthy donors and were stimulated with phytohemagglutinin (PHA) (1 μg/ml; Sigma) and IL-2 (150 U/ml; Miltenyi Biotec) for 3 days. All cell lines were grown at 37°C in 5% CO2. 293T-LTR-GFP and Jurkat-LTR-luc cells are stably transfected with a plasmid encoding green florescent protein (GFP) or luciferase (luc), respectively, under the control of the HTLV-1 long terminal repeat (LTR) promoter.
Plasmids.
The HTLV-1 proviral DNA clone (pACH) was previously described (43). The HTLV-2 proviral DNA clone (pH6neo) and the SV2Neo plasmids (44, 45) were provided by P. Green. The pCMVHT1-M (46) and the pCRU5-HT1GFPLuc (35) plasmids were provided by D. Derse. The pSG5M-Tax1, pSG5M-Tax2, and HTLV-1– or HTLV-2–LTR–luciferase plasmids were previously described (47).
RNA extraction and real-time RT-PCR.
RNA was extracted using the RNA easy extract kit (Qiagen) according to the manufacturer's instructions and resuspended in 30 μl of water. Before reverse transcription, 500 ng of RNA was treated with 10 U of RNase-free DNase I (Qiagen) for 20 min at 27°C and then for 15 min at 60°C. Reverse transcription was then performed using the iScript cDNA synthesis kit (Bio-Rad) following the manufacturer's instructions. Quantitative PCR (qPCR) was performed using FastStart Universal SYBR green Master (Roche) on a StepOnePlus thermocycler (Applied Biosystems). Samples were incubated for 10 min at 95°C; then, 40 cycles were performed (10 s at 95°C and 30 s at 60°C), and melting-curve analysis was performed between 60°C and 95°C. cDNA samples were amplified with Mx1 primers (5′-AGCCACTGGACTGACGACTTG-3′ [forward] and 5′-AAATCACCACGGCTAACGGATAAG-3′ [reverse]). GAPDH (glyceraldehyde-3-phosphate dehydrogenase) primers (5′-AGCCACATCGCTCAGACAC-3′ [forward] and 5′-GCCCAATACGACCAAATCC-3′ [reverse]) were used for normalization (48).
Cell-to-cell infection.
Jurkat cells (106) or PBLs (5 × 105) were transfected with 5 μg of HTLV-1– or HTLV-2–LTR–luciferase plasmids using the Neon Transfection System (Invitrogen) following the manufacturer's instructions. The cells were then treated with various amounts (0 to 5,000 U/ml) of IFN-α2a (Tebu-Bio) for 24 h prior to coculture with HTLV-1-infected (C91-PL) or HTLV-2-infected (C19) cells (3:1 ratio). Prior to coculture, C91-PL or C19 cells were irradiated (77 Gy) from a 137Cs source (CIS BIO international; IBL 637) at 1.28 Gy/min. After 24 h of coculture, reporter activities were assayed using the luciferase reporter assay system (Promega). Luciferase activity was normalized by protein concentration as determined by the Bradford method (Bio-Rad).
(i) AZT treatment.
Jurkat cells or PBLs were treated with 50 μM AZT (Sigma) 24 h and 3 h before coculture with HTLV-infected and irradiated cells.
(ii) Serum treatment.
Jurkat cells were incubated 3 h before coculture in the presence of sera (1:1,000) obtained either from an HTLV-negative blood donor or from a HAM/TSP patient.
Cell-free infection.
293T cells (6 × 106) were seeded onto 100-mm dishes. Twenty-four hours later, the cells were transfected with 2 μg of the pCMVHT1-M packaging plasmid and 6 μg of the pCRU5-HT1GFPLuc reporter plasmid using the Polyfect reagent (Qiagen) following the manufacturer's instructions. Forty-eight hours posttransfection, supernatants were collected and filtered through a 0.45-μm filter. Five hundred microliters of filtered supernatant in the presence of Polybrene (8 μg/ml) was added to 106 Jurkat cells that had been treated or not with 1,000 U/ml of IFN-α2a for 24 h. Luciferase activity was measured 48 h postinfection (luciferase assay system; Promega). Luciferase activity was normalized by protein concentration as determined by the Bradford method (Bio-Rad).
Transfections with Tax-encoding plasmids.
293T cells (3 × 105) were seeded onto 6-well plates. The following day, 2 μg of a plasmid encoding Tax1 or Tax2 and 250 ng of a plasmid carrying the firefly luciferase gene under the control of the viral HTLV LTR (HTLV-1–LTR–luc or HTLV-2–luc) were transfected (PolyFect; Qiagen). The transfections were carried out in the presence of a Renilla luciferase vector (phRG-TK; 10 ng) in order to normalize for the transfection efficiency. The cells were then treated with increasing amounts (0 to 1,000 U/ml) of IFN-α. Luciferase activity was assayed 24 h posttransfection using the Dual-Luciferase Reporter Assay System (Promega).
Jurkat-LTR-luciferase cells (106) were transfected with 5 μg of a plasmid encoding Tax1 or Tax2 using the Neon Transfection System (Invitrogen) following the manufacturer's instructions. Cells were treated or not with 500 U/ml IFN-α2a. Twenty-four hours later, reporter activities were assayed using the luciferase reporter assay system (Promega). Luciferase activity was normalized by protein concentration as determined by the Bradford method (Bio-Rad).
Transfections with HTLV molecular clones.
Jurkat cells (106) or PBLs (5 105) were transfected with 2.5 μg of pACH (HTLV-1), pH6neo (HTLV-2), or SV2Neo (control) and 2.5 μg of the HTLV-1– or HTLV-2–LTR–luciferase plasmid using the Neon Transfection System (Invitrogen). Cells were treated or not with 1,000 U/ml of IFN-α2a. Forty-eight hours later, reporter activities were assayed using the luciferase reporter assay system (Promega). Luciferase activity was normalized by protein concentration as determined by the Bradford method (Bio-Rad).
293T cells (3 × 106) were seeded onto 100-mm dishes. The following day, 8 μg of pACH (HTLV-1), pH6neo (HTLV-2), or SV2Neo (backbone) plasmids was transfected with PolyFect reagent (Qiagen) following the manufacturer's instructions. The cells were immediately incubated in the presence of IFN-α2a (0 to 1,000 U/ml) for 48 h.
Fluorescence microscopy.
293T-LTR-GFP cells were seeded at a concentration of 3 × 105 cells per well onto 6-well plates. The following day, 2 μg of pACH (HTLV-1), pH6neo (HTLV-2), or SV2Neo backbone plasmids was transfected with PolyFect reagent (Qiagen). Two days after transfection, transfected 293T-LTR-GFP cells treated with 0 to 1,000 U/ml of IFN-α were analyzed with an AMG Evos fl Digital Inverted Fluorescence Microscope to visualize GFP fluorescence.
PKR inhibition. (i) C16 treatment.
Jurkat cells were transfected with pACH (HTLV-1), pH6neo (HTLV-2), or SV2Neo (backbone) and with the HTLV-1– or HTLV-2–LTR–luciferase plasmid as described above. One hour posttransfection, the cells were incubated in the presence of 50 nM imidazolo-oxindole C16 compound (PKR inhibitor; Sigma) resuspended in dimethyl sulfoxide (DMSO) or in the presence of DMSO alone (control). Two hours later, cells were treated or not with 1,000 U/ml of IFN-α2a. Forty-eight hours later, reporter activities were assayed using the luciferase reporter assay system (Promega). Luciferase activity was normalized by protein concentration as determined by the Bradford method (Bio-Rad).
(ii) PKR siRNA transfection.
293T cells were seeded at a concentration of 3 × 105 cells per well onto 6-well plates. The following day, 20 nM PKR small interfering RNA (siRNA) (On-Targetplus Smart pool EIF2AK2; Fermentas) or control siRNA (On-Targetplus Nontargeting Pool; Fermentas) were transfected (HiPerfect reagent; Qiagen) following the manufacturer's instructions. Twelve hours posttransfection, 1.2 μg of pACH or PH6neo plasmid or a plasmid encoding GFP, together with 20 nM siRNA, was transfected (Attracten; Qiagen) following the manufacturer's instructions. Cells were then treated or not with 100 U/ml of IFN-α2a for 48 h.
Immunoblot analyses.
Cells were washed with PBS, lysed (50 nM Tris-HCl, pH 7.4, 150 nM NaCl, 5 mM EDTA, 0.5% Nonidet-P-40, 0.2 mM Na3VO4, 50 mM NaF, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride) in the presence of protease inhibitors (Complete; Roche Applied Science) and incubated on ice. Cell debris was pelleted by centrifugation, and the protein concentration was determined by the Bradford method (Bio-Rad). Sixty micrograms of the proteins was loaded onto 4 to 12% NuPAGE gels (Novex; Invitrogen), subjected to electrophoresis at 150 V, and transferred onto a polyvinylidene difluoride (PVDF) membrane (Immobilon-P; Millipore). The membranes were blocked in a 5% milk-PBS-0.05% Tween 20 solution and then incubated overnight with the primary antibody, anti-PKR 71/10; dilution, 1:500) (49), anti-phospho-PKR (Epitomics 1120-1; dilution, 1:2000), anti-Tax-1-specific (Tab 172; dilution, 1:4,000), anti-Tax-2 (GP3738; dilution, 1:4,000) (50), anti-HTLV-1/2 p24 (Zeptometrix 75/4.21.11; 2.5 μg/ml; dilution. 1:400), or anti-β-actin clone AC74 (Sigma; dilution, 1:40,000). The next day, the membranes were washed and incubated either with anti-rabbit or with anti-mouse horseradish peroxidase-conjugated secondary antibodies and developed using an ECL Plus reagent kit (GE Healthcare).
RESULTS
IFN-α prevents HTLV infection and/or expression only in de novo-exposed T cells.
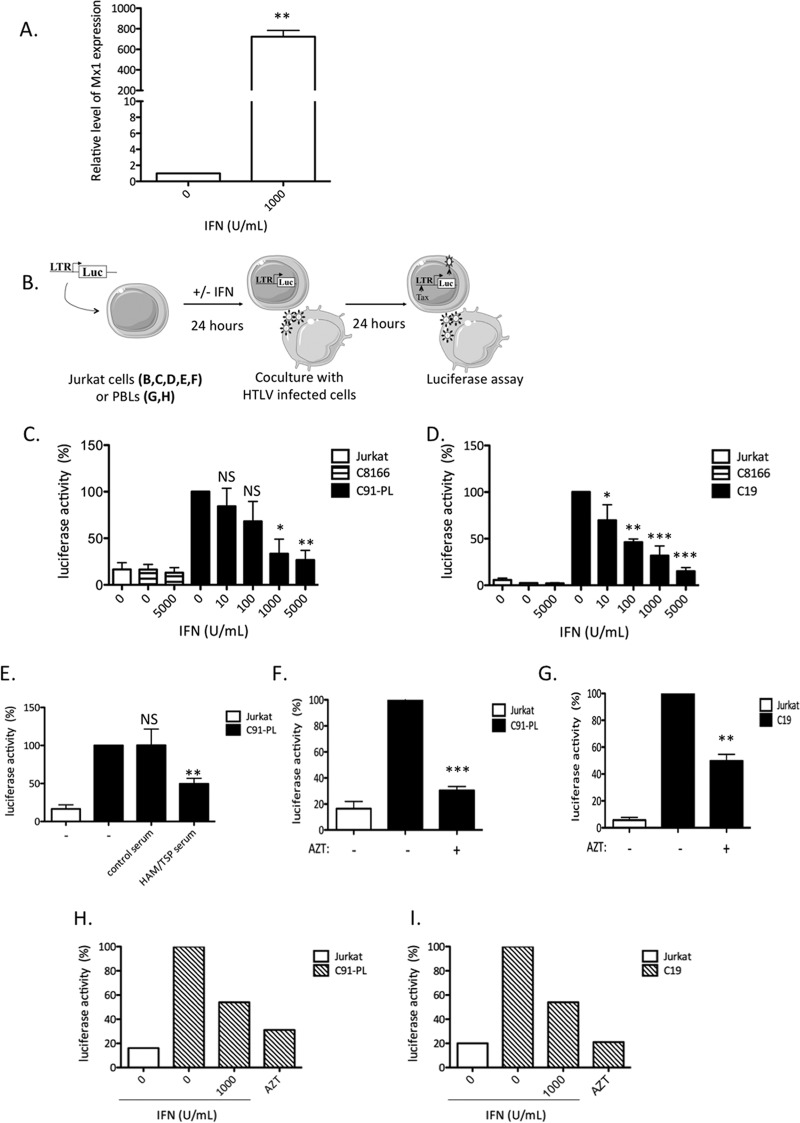
Chronically infected and transformed HTLV T-cell lines have previously been reported to be insensitive to IFN-α antiviral properties (31–33). However, IFN-α effects on de novo T-cell HTLV infection have not been investigated. We therefore used a coculture setting that allowed us to investigate HTLV-1/2 de novo infection in T cells in the presence or absence of type I interferon. First, we assessed Mx1 gene (a known interferon-inducible gene) expression in Jurkat cells following IFN-I treatment. A 700-fold increase in Mx1 mRNA was observed upon IFN-I addition (Fig. 1), demonstrating that IFN-α signaling is intact and promotes ISG expression in those cells. Jurkat target cells were then treated with IFN-α and then cocultured with gamma-irradiated C91-PL or C19 cells, used here as donor cells. In order to monitor de novo infection, target cells were transfected with an HTLV-1– or HTLV-2–LTR–luc reporter plasmid prior to coculture. In this system, LTR-dependent luciferase activity in target cells parallels viral expression driven from the LTR. Since Tax protein is necessary for LTR activation, levels of LTR-dependent luciferase activity indicate that viral entry, reverse transcription, proviral integration, de novo viral transcription, and posttranscriptional production of viral proteins have been completed, allowing at least the production of Tax (Fig. 1B).
Fig 1.
IFN-α treatment prevents HTLV-1 and HTLV-2 replication in T cells. (A) Jurkat cells were treated with 1,000 U/ml of IFN-α for 24 h, and Mx1 expression was determined by qRT-PCR. The values were normalized to GAPDH expression and compared to Mx1 expression in untreated cells, which was set to 1. The data are presented as the means and standard deviations (SD) from 3 independent experiments. The asterisks indicate statistically significant differences between treated and untreated cells (paired Student t test; **, P < 0.01). (B) Jurkat cells or PBLs were transfected with 5 μg of a plasmid carrying the luciferase gene under the control of the HTLV-1–LTR or HTLV-2–LTR and treated with IFN-α (0 to 5,000 U/ml) for 24 h. (C to I) Cells were then cocultured with irradiated HTLV-1 (C91-PL) (C, E, F, and H) or HTLV-2 (C19) (D, G, and I) or noninfected Jurkat or C8166 (Tax-expressing) T cells for 24 h. Tax expression was indirectly analyzed by a luciferase assay. Luciferase activity was normalized by protein concentration as determined by the Bradford method and calculated as the fold change compared to untreated cells arbitrarily set to 100%. (E) Three hours before coculture with irradiated C91-PL cells, Jurkat cells were incubated in the presence of serum (1:1,000) obtained either from a healthy blood donor or from a HAM/TSP patient. (F to I) Jurkat cells (F and G) or PBLs (H and I) were treated with AZT (50 μM) before coculture with C91-PL or C19 irradiated cells. (E to G) The data are means and SD from 3 independent experiments. The asterisks indicate statistically significant differences between treated and untreated cells (paired Student t test; *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, nonsignificant). (H and I) The data are representative of two different experiments obtained with two different blood donors.
Target cells cocultured either with C91-PL (Fig. 1C) or with C19 (Fig. 1D) donor cells showed a dose-dependent decrease in luciferase activity when treated with increasing amounts of IFN-α, indicating an IFN-α-induced decrease in viral expression. In order to determine whether donor cells could secrete Tax that would then activate the LTR-driven transcription in target cells independently of de novo infection, a similar experiment was performed with C8166 cells that synthesize Tax in larger amounts than C91-PL cells (data not shown) but do not produce any viral particles (51). In this case, luciferase activity was similar to background levels in the absence or presence of IFN-α (Fig. 1C and D, lanes 2 and 3). Similarly, background levels of luciferase activity were also measured when target cells were cocultured with noninfected Jurkat cells (Fig. 1C and D, lane 1).
To rule out the possibility that the luciferase signal is linked to passive diffusion of the Tax protein following membrane fusion, Jurkat target cells were also incubated with serum obtained from a healthy donor or from an HTLV-1 HAM/TSP patient (Fig. 1E) or with AZT, an inhibitor of reverse transcriptase (52) (Fig. 1F and G). AZT or HAM/TSP serum treatment led to a significant decrease in luciferase activity, whereas serum from healthy blood donors did not (Fig. 1E, F, and G). A similar experiment was also performed using PBLs obtained from healthy blood donors as target cells (Fig. 1H and I). The PBLs were transfected with an HTLV-luc reporter plasmid and treated with IFN-α or with AZT prior to coculture with HTLV-1 or HTLV-2 chronically infected cells. Both AZT and IFN-α induced a decrease in the luciferase activity (Fig. 1F and I). Altogether, these results demonstrate that luciferase signal is linked to de novo Tax synthesis and not to Tax transfer from HTLV-1/2-infected cells into target cells.
Finally, to rule out an indirect effect of IFN-α on C91-PL or C19 donor cells during coculture, IFN-α-pretreated target cells were washed before coculture with HTLV donor cells. The same dose-dependent decrease in luciferase activity was observed, confirming that IFN-α acts on target cells and not on donor cells (data not shown).
Altogether, these results demonstrate that productive infection of target cells is necessary for LTR activation and show that IFN-α pretreatment results in decreased viral expression in T cells exposed de novo to HTLVs.
IFN-α does not affect the first steps of the HTLV viral cycle.
We next aimed to determine which steps of the viral cycle are targeted by IFN-α. To investigate the first steps of the HTLV viral cycle, i.e., viral entry and reverse transcription, we used a modified HTLV-1 genome in which a reporter cassette allowing expression of the luciferase gene was inserted downstream of a cytomegalovirus (CMV) promoter. This construct allows possible effects of IFN-α on integration, LTR-driven transcription, and/or posttranscriptional viral protein synthesis to be overcome. Viral particles pseudotyped with the HTLV-1 envelope were produced in 293T cells. Jurkat target cells were then pretreated with IFN-α or left untreated for 24 h before incubation with pseudotyped viruses. Expression of the transduced luciferase gene was then assessed. IFN-α treatment did not significantly alter luciferase activity in target cells (Fig. 2), indicating that neither entry nor reverse transcription is affected. Altogether, these results suggest that IFN-α inhibits a post-reverse transcription step of the HTLV viral cycle.
Fig 2.
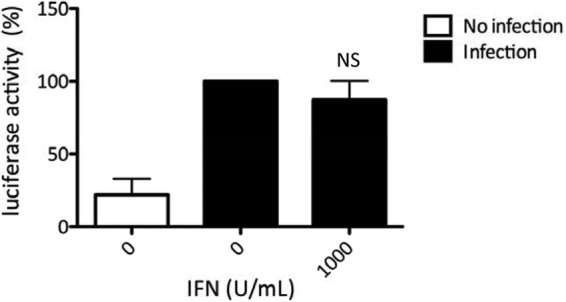
IFN-α treatment does not inhibit the early steps of the HTLV-1 viral cycle. Pseudotyped viral particles were produced after transfection of 6 × 106 293T cells with 2 μg of the pCMVHT1-M packaging plasmid and with 6 μg of a single-cycle reporter construct pCRU5-HT1GFPluc plasmid. Jurkat cells (106) were treated (1,000 U/ml IFN-α) or not for 24 h before infection with viral particles. Infection was assessed 48 h later with a luciferase assay. Luciferase activity values were normalized by protein concentration as determined by the Bradford method and calculated as the fold change compared to untreated cells arbitrarily set to 100%. The data are presented as the mean and SD from 3 independent experiments. NS, no statistically significant difference between treated and untreated cells (paired Student t test).
IFN-α treatment transcriptionally and/or posttranscriptionally inhibits HTLV expression.
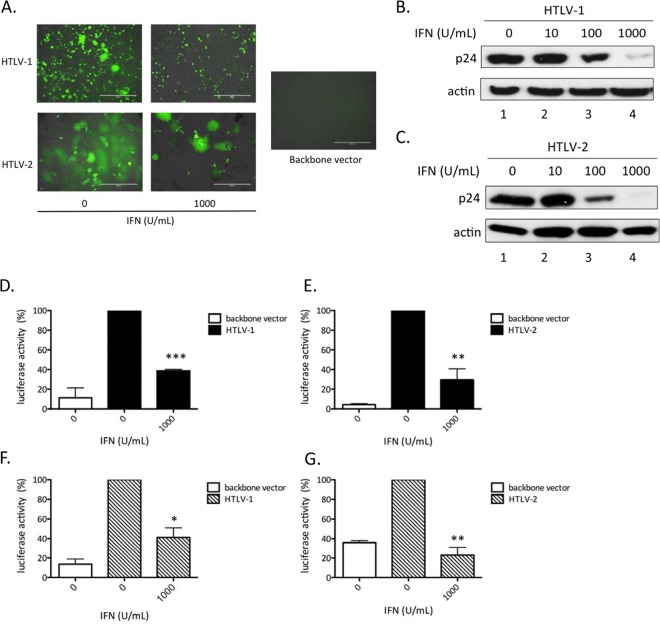
Since early steps of the HTLV cycle are not sensitive to IFN-α, we took advantage of the available HTLV-1 and HTLV-2 molecular clones. These plasmids allow the study of the viral steps that follow entry and reverse transcription. Target cells were transfected with the HTLV-1 (pACH) or HTLV-2 (pH6Neo) molecular clone and treated with increasing amounts of IFN-α (Fig. 3). Transfections were first performed in 293T target cells stably harboring an LTR-controlled GFP reporter gene whose expression parallels viral LTR-driven transcription. As additional and independent read-outs for viral expression, we also monitored formation of syncytia, indicative of Env expression, and intracellular p24gag expression. IFN-α treatment decreased the number of GFP-positive cells (Fig. 3A), indicating that IFN-α inhibited HTLV-1/2 LTR-driven transcription, either directly by altering Tax-mediated viral transcription or indirectly by decreasing Tax levels at posttranscriptional steps. IFN-α treatment also decreased the number of syncytia (Fig. 3A) and induced a dose-dependent decrease in intracellular p24gag protein levels (Fig. 3B and C), indicating that expression of both HTLV-1 and HTLV-2 Env and Gag proteins was altered. Consistent with those results, p19 levels in the supernatant also decreased (data not shown).
Fig 3.
IFN-α treatment inhibits HTLV-1 and HTLV-2 protein expression. (A) 293T-LTR-GFP cells (3 × 105) were transfected with 2 μg of the HTLV-1 (pACH) or HTLV-2 (pH6neo) molecular clone and treated for 48 h with IFN-α (0 to 1,000 U/ml). The cells were then analyzed using an AMG Evos fl Digital Inverted Fluorescence Microscope. Scale bars, 400 μm. (B and C) Western blot analyses (anti-gag p24 or anti-actin) were performed on 60 μg of proteins from whole-cell extracts obtained from cells transfected with the HTLV-1 (pACH) (B) or HTLV-2 (pH6neo) (C) molecular clone and treated with different doses of IFN-α. (D to G) Jurkat cells (106) (D and E) or PBLs (5 × 105) (F and G) were transfected with 2.5 μg of the HTLV-1 (pACH) (D and F) or HTLV-2 (pH6neo) (E and G) molecular clone, together with 2.5 μg of the HTLV-1 or HTLV-2 LTR reporter plasmid, and treated for 48 h with IFN-α (0 to 1,000 U/ml). Luciferase activity values were normalized by protein concentration as determined by the Bradford method and calculated as the fold change compared to untreated cells arbitrarily set to 100%. The data are presented as the means and SD from 3 independent experiments. The asterisks indicate statistically significant differences between treated and untreated cells (paired Student t test; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To confirm these results in T cell lines, Jurkat cells (Fig. 3D and E) or primary PBLs (Fig. 3F and G) were transfected with the HTLV-1 (pACH) or HTLV-2 (pH6Neo) molecular clone, together with the HTLV-1–LTR–luc (Fig. 3D and F) or HTLV-2–LTR–luc (Fig. 3E and G) reporter plasmid. Cells were then treated or not with IFN-α before luciferase assays were performed. As in 293T cells, a significant decrease in luciferase activity was observed in the presence of IFN-α.
Altogether, these results indicate that IFN-α treatment leads to decreased viral expression at a transcriptional or posttranscriptional stage.
IFN-α does not alter Tax-mediated transcription from the viral LTR.
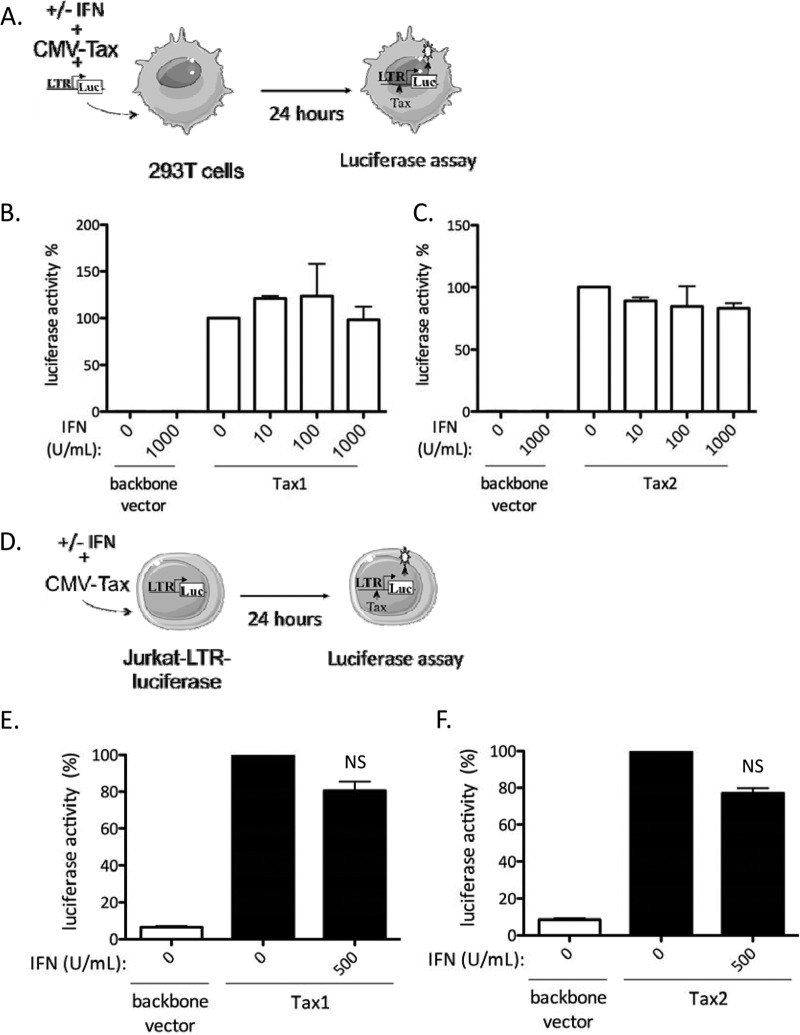
To assess whether IFN-α directly affects HTLV transcription, 293T cells were transfected with an HTLV-1– or HTLV-2–LTR–luc reporter construct, together with a CMV-dependent Tax expression plasmid, in the presence of increasing doses of IFN-α (Fig. 4A). Similar experiments were performed using Jurkat cells stably transfected with an HTLV-1 LTR construct (Fig. 4D), which can be activated either by HTLV-1 or by HTLV-2 Tax proteins (50). The CMV-dependent Tax expression plasmid ensures high levels of Tax. Hence, in these experimental settings, IFN-α should not affect Tax production. Interestingly, IFN-α treatment had no effect on Tax1-mediated (Fig. 4B and E) or Tax2-mediated (Fig. 4C and F) transcription, demonstrating that it did not prevent recruitment of the RNA Pol II machinery onto the LTR by Tax and suggesting that it affects posttranscriptional stages of viral expression.
Fig 4.
IFN-α does not prevent Tax-mediated viral transcription. (A to C) 293T cells (3 × 105) were transfected with 250 ng of HTLV-1–LTR–luc or HTLV-2–luc plasmids, 10 ng of a plasmid encoding Renilla luciferase, and 2 μg of a plasmid encoding HTLV-1 Tax (Tax1) (B), a plasmid encoding HTLV-2 Tax (Tax2) (C), a backbone vector. The cells were then treated with increasing amounts of IFN-α (0 to 1,000 U/ml), and 24 h after transfection, luciferase activity was measured and normalized. (D to F) Jurkat-LTR-luciferase cells (106) were transfected with 5 μg of Tax1 or Tax2 plasmids and treated for 24 h with 500 U/ml of IFN-α. Luciferase activity was measured and normalized by protein concentration using the Bradford method. Values were calculated as the fold change compared to untreated cells arbitrarily set to 100%. (B, C, E, and F) The data are presented as the means and SD from 2 independent experiments. NS, no significant difference between treated and untreated cells (paired Student t test).
IFN-α treatment inhibits posttranscriptional viral expression through PKR activation.
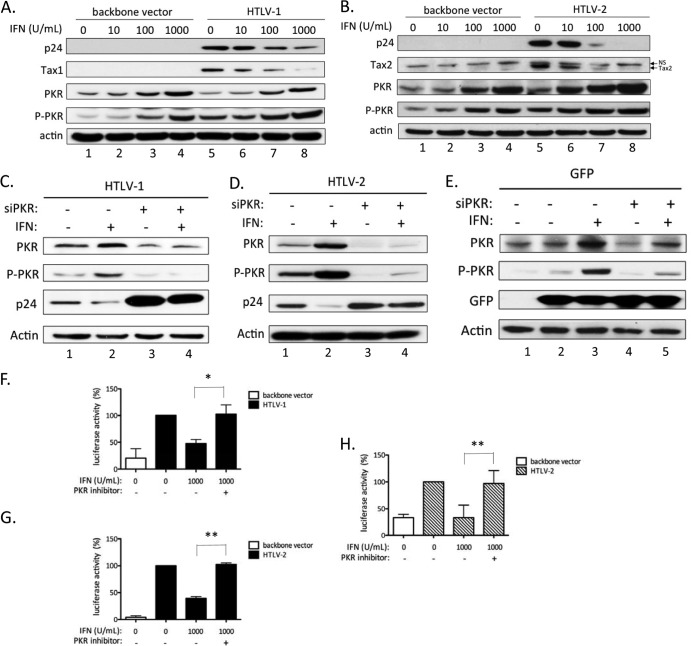
The PKR gene is an ISG that has been shown to inhibit viral mRNA translation from other viruses. We therefore sought to determine whether the inhibitory effects of IFN-α on HTLV-1/2 expression were linked to PKR activation. 293T cells were transfected with the HTLV-1 (pACH) or HTLV-2 (pH6Neo) molecular clone and treated with increasing amounts of IFN-α (Fig. 5). As seen in Fig. 3, Western blot analyses on cell lysates showed a decrease in intracellular p24gag and Tax levels upon IFN-α treatment (Fig. 5A and B). Interestingly, IFN-α treatment also led to a dose-dependent increase in both total PKR and activated PKR (phospho-PKR [P-PKR]) levels in HTLV-transfected cells, as well as in mock-transfected cells (Fig. 5A and B, compare lanes 1 to 4 to lanes 5 to 8). Thus, the IFN-α-induced decrease in viral expression correlates with the induction and activation of PKR.
Fig 5.
The IFN-α inhibitory effect is mediated through PKR activation. (A and B) 293T cells (3 × 106) were transfected with 8 μg of the HTLV-1 (pACH) (A) or HTLV-2 (pH6neo) (B) molecular clone or with a control plasmid (SV2Neo) and treated with increasing amounts of IFN-α (0 to 1,000 U/ml) for 48 h. Western blot analyses using anti-gag p24, anti-Tax1, anti-Tax2, anti-PKR, anti-P-PKR, and anti-actin were performed on 60 μg of proteins from whole-cell extracts obtained from transfected cells. (C, D, and E) 293T cells (3 × 105) were transfected with 20 nM siRNA directed against PKR (siPKR) or with 20 nM control siRNA. Twelve hours later, the cells were transfected with 20 nM the same siRNA, together with 1.2 μg of HTLV-1 (pACH) (C), HTLV-2 (pH6neo) (D), or a plasmid encoding GFP (E) and incubated or not with 100 U/ml of IFN-α. (F to H) Jurkat cells (106) (F and G) or primary lymphocytes (105) (H) were transfected with 2.5 μg of the pACH (F) or pH6neo (G and H) molecular clone, together with 2.5 μg of the HTLV-1 or HTLV-2 LTR reporter plasmid, and treated for 48 h with IFN-α (0 to 1,000 U/ml) in the presence or absence of a PKR inhibitor (C16; 100 nM). Luciferase activity values were normalized by protein concentration as determined by the Bradford method and calculated as the fold change compared to untreated cells set to 100%. The data are presented as the means and SD from 2 or 3 independent experiments. The asterisks indicate statistically significant differences between treated and untreated cells (paired Student t test; *, P < 0.05; **, P < 0.01).
To further test whether PKR is involved in inhibition of HTLV expression, PKR was silenced by siRNA before transfection of the HTLV-1 or HTLV-2 molecular clone and IFN-α treatment (Fig. 5C and D). In cells transfected with irrelevant siRNA, IFN-α led to decreased intracellular p24gag levels (Fig. 5C and D, lanes 1 to 2), concomitant with increased PKR and P-PKR levels. In contrast, cells transfected with PKR-specific siRNA displayed higher levels of p24gag protein in the absence of IFN-α treatment and remained insensitive to IFN-α treatment (Fig. 5C and D, compare lane 3 to lane 1 and lane 4 to lane 2). As a control, a plasmid encoding GFP was transfected. As expected, GFP expression was not affected by IFN-α either in the presence of control siRNA or when PKR-specific siRNA was transfected (Fig. 5E).
Jurkat cells were also transfected with the HTLV-1 (pACH) or HTLV-2 (pH6Neo) molecular clone and the HTLV-1– or HTLV-2–LTR–luc reporter plasmid (Fig. 5F and G). The Jurkat cells were then treated or not with IFN-α in the presence of C16, a specific PKR-inhibiting compound (53). A similar experiment was also performed using primary PBLs (Fig. 5H). Consistent with experiments performed with PKR siRNA, C16 compound prevented IFN-α from inhibiting luciferase activity both in Jurkat cells and in primary lymphocytes (Fig. 5F, G, and H).
These results demonstrate that PKR is a major effector of IFN-α inhibitory properties and indicate that IFN-α most likely inhibits HTLV expression through PKR-mediated inhibition of viral translation.
DISCUSSION
Interferon type I response allows cells to be protected against viruses. A recent report demonstrated that different viruses are targeted by specific sets of ISGs (54). The role of IFN-I in HTLV-1 pathogenesis, however, is controversial. The well-described cytostatic and antiviral properties of IFN-I first provided a strong rationale for treating HTLV-1-infected patients. A high PVL is one of the best predictors of HAM/TSP and ATLL, though HTLV persists and PVL is elevated in HAM/TSP patients in spite of an important cellular immune response against HTLV-1 antigens (55). A number of studies convincingly demonstrated that exogenous IFN-α, alone or in combination with other molecules, is particularly efficient for treating leukemic, smoldering, and chronic ATLL patients and significantly improves their survival, although the precise mechanism of action is still debated. It might involve an effect both on infected transformed cells that are poorly sensitive to IFN-I but respond to AZT if they have an intact p53 pathway and on noninfected cells present in the microenvironment that should become refractory to infection due to IFN-I (39, 56, 57). In contrast, IFN-α leads only to a minimal decrease in HTLV-1 PVL in HAM/TSP patients (27), suggesting that it cannot allow clearance of HTLV-1-infected cells, but rather, only transiently suppresses viral expression. This effect might be explained by partial or inefficient IFN-α antiviral activity on HAM/TSP patients' infected cells in vivo. Indeed, a recent transcriptomic study in which cells isolated from HAM/TSP patients were compared to those obtained from asymptomatic carriers or healthy controls suggested that a subset of IFN-inducible genes specifically contributes to HAM/TSP development rather than to the control of infection (58). Therefore, in HAM/TSP patients, some ISGs may promote inflammatory responses rather than immune responses able to control infected cells. Altogether, these results highlight the fact that the quality of IFN-α action varies according to the clinical status of HTLV-1-infected individuals.
Deciphering the cellular and molecular bases of IFN-α treatment efficacy in ATLL compared to HAM/TSP is important. A previous report demonstrated that AZT/IFN-α treatment does not have a direct cytotoxic effect in vitro on ex vivo ATLL cells (32). Using experimental settings that allowed us to study individual steps of the viral cycle in T cells, we report here that IFN-α treatment of uninfected T cells markedly inhibits HTLV-1/2 infection, as was also previously shown for HIV-1 (59). However, unlike HIV-1, this effect is not linked to a preintegration defect. In fact, we demonstrate that IFN-α affects both HTLV-1/2 protein expression and viral production. Consistent with a previous observation (34), we also observed a strong decrease in p19gag production in culture supernatant following IFN-α treatment (data not shown). Kinpara et al. also reported that IFN-β secretion by murine cells resulted in decreased p19gag in culture supernatant from IL-2-dependent (immortalized) HTLV-1-infected cells derived from different ATLL patients (30). Ilinskaya et al. recently demonstrated that the tetherin gene, a known ISG, strongly reduces cell-free infectivity of HTLV-1, but not cell-cell transmission (35). The fact that most transmission in our experimental system occurred through cell-cell contact (data not shown), therefore, excludes the possibility that tetherin plays a significant role in the IFN-α effects observed here and suggests that the decrease in viral production in the culture supernatant should have little effect on viral spread.
We demonstrated that IFN-α treatment promotes PKR phosphorylation. PKR is a kinase that is expressed in all tissues at a basal level and is induced by IFN-I (13). Active PKR is known to affect phosphorylation of eukaryotic initiation factor 2 (eIF2), which then suppresses mRNA translation (13). It would therefore be interesting to define whether HTLV-1/2 protein synthesis is affected through eIF2 phosphorylation, unlike a number of other viruses that evade this phenomenon (60, 61, 62).
Altogether, our experiments demonstrate that HTLV-1 and HTLV-2 are exquisitely sensitive to IFN-α. How can these data be reconciled with other studies demonstrating that HTLV-1-transformed or HTLV-1 Tax-expressing cells are insensitive to IFN-α and that Tax-1 blunts IFN signaling (63–67)? We hypothesize that Tax expression renders HTLV-infected cells poorly sensitive to IFN-α. Because incoming viral particles do not contain Tax, they do not alter IFN-α signaling in de novo-infected cells. Therefore, addition of the cytokine to target cells prior to infection activates transcription of ISGs (such as the PKR gene), which then impair completion of the HTLV cycle. If this model is correct, immediately treating ex vivo HTLV-1 patient cells (which do not originally express Tax) (68) with IFN-α should prevent viral expression, while the cells should become insensitive to the treatment a few hours after they are put into culture. Consistent with this hypothesis, Kinpara et al. reported that treating PBMCs obtained from a chronic ATLL patient with recombinant IFN-I strongly suppressed HTLV-1 p19 in the cell culture supernatant (30). AZT was previously shown to inhibit telomerase activity in HTLV-1-infected cells and to induce senescence (56). Since Tax expression is required for cell growth, this might also partly explain why treating infected cells that do not express or barely express Tax (the ATLL situation) with IFN-α/AZT allows their clearance.
In conclusion, the results presented here show that IFN-α inhibits HTLV infection in T lymphocytes exposed de novo to HTLV-1 or HTLV-2. Hence, IFN-α likely contributes to limiting viral spread to uninfected cells in asymptomatic carriers.
ACKNOWLEDGMENTS
R.M., C.J., and A.C. are supported by Ecole Normale Supérieure de Lyon. S.A.C. is supported by InCa. N.L.K. was supported by the Croucher Foundation. We acknowledge the support of ARC and InCa (Cancéropôle CLARA) and of La Ligue Contre le Cancer (Équipe Labelisée).
We thank P. Green and D. Derse for the gifts of different plasmids. We thank E. Meurs for the gift of the PKR antibody and M. I. Thoulouze for the gift of the Jurkat-LTR-luc cell line. We thank A. Gessain for the gift of TSP/HAM serum. We thank A. Cimarelli, T. Ohlmann, and the members of the Mahieux laboratory for their helpful suggestions. The figures were produced by Servier Medical Art.
Footnotes
Published ahead of print 2 October 2013
REFERENCES
- 1.Gessain A, Cassar O. 2012. Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol. 3:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous 1988. The third nation-wide study on adult T-cell leukemia/lymphoma (ATL) in Japan: characteristic patterns of HLA antigen and HTLV-I infection in ATL patients and their relatives. The T- and B-cell Malignancy Study Group. Int. J. Cancer 41:505–512 [DOI] [PubMed] [Google Scholar]
- 3.Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, de The G. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407–410 [DOI] [PubMed] [Google Scholar]
- 4.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i:1031–1032 [DOI] [PubMed] [Google Scholar]
- 5.Takatsuki T. 1977. Adult T-cell leukemia in Japan, p 73–77 In Seno S, Irino S. (ed), Topics in hematology. Excerpta Medica, Amsterdam, The Netherlands [Google Scholar]
- 6.Murphy EL, Fridey J, Smith JW, Engstrom J, Sacher RA, Miller K, Gibble J, Stevens J, Thomson R, Hansma D, Kaplan J, Khabbaz R, Nemo G. 1997. HTLV-associated myelopathy in a cohort of HTLV-I and HTLV-II-infected blood donors. The REDS investigators. Neurology 48:315–320 [DOI] [PubMed] [Google Scholar]
- 7.Bartman MT, Kaidarova Z, Hirschkorn D, Sacher RA, Fridey J, Garratty G, Gibble J, Smith JW, Newman B, Yeo AE, Murphy EL. 2008. Long-term increases in lymphocytes and platelets in human T-lymphotropic virus type II infection. Blood 112:3995–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feuer G, Green PL. 2005. Comparative biology of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2. Oncogene 24:5996–6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannian P, Green PL. 2010. Human T lymphotropic virus type 1 (HTLV-1): molecular biology and oncogenesis. Viruses 2:2037–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rende F, Cavallari I, Romanelli MG, Diani E, Bertazzoni U Ciminale V. 2012. Comparison of the genetic organization, expression strategies and oncogenic potential of HTLV-1 and HTLV-2. Leuk/ Res. Treatment 2012:876153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isaacs A, Lindenmann J. 1957. Virus interference. I. The interferon. Proc. R Soc. Lond. B Biol. Sci. 147:258–267 [PubMed] [Google Scholar]
- 12.de Weerd NA, Nguyen T. 2012. The interferons and their receptors—distribution and regulation. Immunol. Cell Biol. 90:483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadler AJ, Williams BR. 2008. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 8:559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang BX, Fish EN. 2012. The yin and yang of viruses and interferons. Trends Immunol. 33:190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colisson R, Barblu L, Gras C, Raynaud F, Hadj-Slimane R, Pique C, Hermine O, Lepelletier Y, Herbeuval JP. 2010. Free HTLV-1 induces TLR7-dependent innate immune response and TRAIL relocalization in killer plasmacytoid dendritic cells. Blood 115:2177–2185 [DOI] [PubMed] [Google Scholar]
- 16.Hishizawa M, Imada K, Kitawaki T, Ueda M, Kadowaki N, Uchiyama T. 2004. Depletion and impaired interferon-alpha-producing capacity of blood plasmacytoid dendritic cells in human T-cell leukaemia virus type I-infected individuals. Br. J. Haematol. 125:568–575 [DOI] [PubMed] [Google Scholar]
- 17.Gill PS, Harrington W, Jr, Kaplan MH, Ribeiro RC, Bennett JM, Liebman HA, Bernstein-Singer M, Espina BM, Cabral L, Allen S, Kornblau S, Pike MC, Levine AM. 1995. Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N. Engl. J. Med. 332:1744–1748 [DOI] [PubMed] [Google Scholar]
- 18.Hermine O, Allard I, Levy V, Arnulf B, Gessain A, Bazarbachi A. 2002. A prospective phase II clinical trial with the use of zidovudine and interferon-alpha in the acute and lymphoma forms of adult T-cell leukemia/lymphoma. Hematol. J. 3:276–282 [DOI] [PubMed] [Google Scholar]
- 19.Hermine O, Bouscary D, Gessain A, Turlure P, Leblond V, Franck N, Buzyn-Veil A, Rio B, Macintyre E, Dreyfus F, Bazarbachi A. 1995. Treatment of adult T-cell leukemia-lymphoma with zidovudine and interferon alfa. N. Engl. J. Med. 332:1749–1751 [DOI] [PubMed] [Google Scholar]
- 20.Matutes E, Taylor GP, Cavenagh J, Pagliuca A, Bareford D, Domingo A, Hamblin M, Kelsey S, Mir N, Reilly JT. 2001. Interferon alpha and zidovudine therapy in adult T-cell leukaemia lymphoma: response and outcome in 15 patients. Br. J. Haematol. 113:779–784 [DOI] [PubMed] [Google Scholar]
- 21.Ratner L, Harrington W, Feng X, Grant C, Jacobson S, Noy A, Sparano J, Lee J, Ambinder R, Campbell N, Lairmore M. 2009. Human T cell leukemia virus reactivation with progression of adult T-cell leukemia-lymphoma. PLoS One 4:e4420. 10.1371/journal.pone.0004420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White JD, Wharfe G, Stewart DM, Maher VE, Eicher D, Herring B, Derby M, Jackson-Booth PG, Marshall M, Lucy D, Jain A, Cranston B, Hanchard B, Lee CC, Top LE, Fleisher TA, Nelson DL, Waldmann TA. 2001. The combination of zidovudine and interferon alpha-2B in the treatment of adult T-cell leukemia/lymphoma. Leuk. Lymphoma 40:287–294 [DOI] [PubMed] [Google Scholar]
- 23.Arimura K, Nakagawa M, Izumo S, Usuku K, Itoyama Y, Kira J, Osame M. 2007. Safety and efficacy of interferon-alpha in 167 patients with human T-cell lymphotropic virus type 1-associated myelopathy. J. Neurovirol. 13:364–372 [DOI] [PubMed] [Google Scholar]
- 24.Izumo S, Goto I, Itoyama Y, Okajima T, Watanabe S, Kuroda Y, Araki S, Mori M, Nagataki S, Matsukura S, Akamine T, Nakagawa M, Yamamoto I, Osame M. 1996. Interferon-alpha is effective in HTLV-I-associated myelopathy: a multicenter, randomized, double-blind, controlled trial. Neurology 46:1016–1021 [DOI] [PubMed] [Google Scholar]
- 25.Kuroda Y, Kurohara K, Fujiyama F, Takashima H, Endo C, Matsui M, Neshige R, Kakigi R. 1992. Systemic interferon-alpha in the treatment of HTLV-I-associated myelopathy. Acta Neurol. Scand. 86:82–86 [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa M, Nakahara K, Maruyama Y, Kawabata M, Higuchi I, Kubota H, Izumo S, Arimura K, Osame M. 1996. Therapeutic trials in 200 patients with HTLV-I-associated myelopathy/tropical spastic paraparesis. J. Neurovirol. 2:345–355 [DOI] [PubMed] [Google Scholar]
- 27.Saito M, Nakagawa M, Kaseda S, Matsuzaki T, Jonosono M, Eiraku N, Kubota R, Takenouchi N, Nagai M, Furukawa Y, Usuku K, Izumo S, Osame M. 2004. Decreased human T lymphotropic virus type I (HTLV-I) provirus load and alteration in T cell phenotype after interferon-alpha therapy for HTLV-I-associated myelopathy/tropical spastic paraparesis. J. Infect. Dis. 189:29–40 [DOI] [PubMed] [Google Scholar]
- 28.Yamasaki K, Kira J, Koyanagi Y, Kawano Y, Miyano-Kurosaki N, Nakamura M, Baba E, Suzuki J, Yamamoto A, Yamamoto N, Kobayashi T. 1997. Long-term, high dose interferon-alpha treatment in HTLV-I-associated myelopathy/tropical spastic paraparesis: a combined clinical, virological and immunological study. J. Neurol. Sci. 147:135–144 [DOI] [PubMed] [Google Scholar]
- 29.Bazarbachi A, Plumelle Y, Carlos Ramos J, Tortevoye P, Otrock Z, Taylor G, Gessain A, Harrington W, Panelatti G, Hermine O. 2010. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J. Clin. Oncol. 28:4177–4183 [DOI] [PubMed] [Google Scholar]
- 30.Kinpara S, Hasegawa A, Utsunomiya A, Nishitsuji H, Furukawa H, Masuda T, Kannagi M. 2009. Stromal cell-mediated suppression of human T-cell leukemia virus type 1 expression in vitro and in vivo by type I interferon. J. Virol. 83:5101–5108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bazarbachi A, El-Sabban ME, Nasr R, Quignon F, Awaraji C, Kersual J, Dianoux L, Zermati Y, Haidar JH, Hermine O, de The H. 1999. Arsenic trioxide and interferon-alpha synergize to induce cell cycle arrest and apoptosis in human T-cell lymphotropic virus type I-transformed cells. Blood 93:278–283 [PubMed] [Google Scholar]
- 32.Bazarbachi A, Nasr R, El-Sabban ME, Mahe A, Mahieux R, Gessain A, Darwiche N, Dbaibo G, Kersual J, Zermati Y, Dianoux L, Chelbi-Alix MK, de The H, Hermine O. 2000. Evidence against a direct cytotoxic effect of alpha interferon and zidovudine in HTLV-I associated adult T cell leukemia/lymphoma. Leukemia 14:716–721 [DOI] [PubMed] [Google Scholar]
- 33.Mahieux R, Pise-Masison C, Gessain A, Brady JN, Olivier R, Perret E, Misteli T, Nicot C. 2001. Arsenic trioxide induces apoptosis in human T-cell leukemia virus type 1- and type 2-infected cells by a caspase-3-dependent mechanism involving Bcl-2 cleavage. Blood 98:3762–3769 [DOI] [PubMed] [Google Scholar]
- 34.Feng X, Heyden NV, Ratner L. 2003. Alpha interferon inhibits human T-cell leukemia virus type 1 assembly by preventing Gag interaction with rafts. J. Virol. 77:13389–13395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ilinskaya A, Derse D, Hill S, Princler G, Heidecker G. 2013. Cell-cell transmission allows human T-lymphotropic virus 1 to circumvent tetherin restriction. Virology 436:201–209 [DOI] [PubMed] [Google Scholar]
- 36.Jouvenet N, Neil SJ, Zhadina M, Zang T, Kratovac Z, Lee Y, McNatt M, Hatziioannou T, Bieniasz PD. 2009. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 83:1837–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones KS, Lambert S, Bouttier M, Benit L, Ruscetti FW, Hermine O, Pique C. 2011. Molecular aspects of HTLV-1 entry: functional domains of the HTLV-1 surface subunit (SU) and their relationships to the entry receptors. Viruses 3:794–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pique C, Jones KS. 2012. Pathways of cell-cell transmission of HTLV-1. Front. Microbiol. 3:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Journo C, Mahieux R. 2011. HTLV-1 and innate immunity. Viruses 3:1374–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kannagi M, Hasegawa A, Takamori A, Kinpara S, Utsunomiya A. 2012. The roles of acquired and innate immunity in human T-cell leukemia virus type 1-mediated diseases. Front. Microbiol. 3:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delebecque F, Pramberger K, Prevost MC, Brahic M, Tangy F. 2002. A chimeric human T-cell lymphotropic virus type 1 with the envelope glycoprotein of Moloney murine leukemia virus is infectious for murine cells. J. Virol. 76:7883–7889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pais-Correia AM, Sachse M, Guadagnini S, Robbiati V, Lasserre R, Gessain A, Gout O, Alcover A, Thoulouze MI. 2010. Biofilm-like extracellular viral assemblies mediate HTLV-1 cell-to-cell transmission at virological synapses. Nat. Med. 16:83–89 [DOI] [PubMed] [Google Scholar]
- 43.Kimata JT, Wong FH, Wang JJ, Ratner L. 1994. Construction and characterization of infectious human T-cell leukemia virus type 1 molecular clones. Virology 204:656–664 [DOI] [PubMed] [Google Scholar]
- 44.Chen IS, McLaughlin J, Gasson JC, Clark SC, Golde DW. 1983. Molecular characterization of genome of a novel human T-cell leukaemia virus. Nature 305:502–505 [DOI] [PubMed] [Google Scholar]
- 45.Shimotohno K, Takahashi Y, Shimizu N, Gojobori T, Golde DW, Chen IS, Miwa M, Sugimura T. 1985. Complete nucleotide sequence of an infectious clone of human T-cell leukemia virus type II: an open reading frame for the protease gene. Proc. Natl. Acad. Sci. U. S. A. 82:3101–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Derse D, Hill SA, Lloyd PA, Chung H, Morse BA. 2001. Examining human T-lymphotropic virus type 1 infection and replication by cell-free infection with recombinant virus vectors. J. Virol. 75:8461–8468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Journo C, Bonnet A, Favre-Bonvin A, Turpin J, Vinera J, Cote E, Chevalier SA, Kfoury Y, Bazarbachi A, Pique C, Mahieux R. 2013. Human T cell leukemia virus type 2 tax-mediated NF-kappaB activation involves a mechanism independent of Tax conjugation to ubiquitin and SUMO. J. Virol. 87:1123–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathieu C, Guillaume V, Sabine A, Ong KC, Wong KT, Legras-Lachuer C, Horvat B. 2012. Lethal Nipah virus infection induces rapid overexpression of CXCL10. PLoS One 7:e32157. 10.1371/journal.pone.0032157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laurent AG, Krust B, Galabru J, Svab J, Hovanessian AG. 1985. Monoclonal antibodies to an interferon-induced Mr 68,000 protein and their use for the detection of double-stranded RNA-dependent protein kinase in human cells. Proc. Natl. Acad. Sci. U. S. A. 82:4341–4345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meertens L, Chevalier S, Weil R, Gessain A, Mahieux R. 2004. A 10-amino acid domain within human T-cell leukemia virus type 1 and type 2 tax protein sequences is responsible for their divergent subcellular distribution. J. Biol. Chem. 279:43307–43320 [DOI] [PubMed] [Google Scholar]
- 51.Bhat NK, Adachi Y, Samuel KP, Derse D. 1993. HTLV-1 gene expression by defective proviruses in an infected T-cell line. Virology 196:15–24 [DOI] [PubMed] [Google Scholar]
- 52.Macchi B, Faraoni I, Zhang J, Grelli S, Favalli C, Mastino A, Bonmassar E. 1997. AZT inhibits the transmission of human T cell leukaemia/lymphoma virus type I to adult peripheral blood mononuclear cells in vitro. J. Gen. Virol. 78:1007–1016 [DOI] [PubMed] [Google Scholar]
- 53.Nekhai S, Bottaro DP, Woldehawariat G, Spellerberg A, Petryshyn R. 2000. A cell-permeable peptide inhibits activation of PKR and enhances cell proliferation. Peptides 21:1449–1456 [DOI] [PubMed] [Google Scholar]
- 54.Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. 2011. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472:481–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cook LB, Elemans M, Rowan AG, Asquith B. 2013. HTLV-1: persistence and pathogenesis. Virology 435:131–140 [DOI] [PubMed] [Google Scholar]
- 56.Datta A, Bellon M, Sinha-Datta U, Bazarbachi A, Lepelletier Y, Canioni D, Waldmann TA, Hermine O, Nicot C. 2006. Persistent inhibition of telomerase reprograms adult T-cell leukemia to p53-dependent senescence. Blood 108:1021–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kinpara S, Kijiyama M, Takamori A, Hasegawa A, Sasada A, Masuda T, Tanaka Y, Utsunomiya A, Kannagi M. 2013. Interferon-alpha (IFN-alpha) suppresses HTLV-1 gene expression and cell cycling, while IFN-alpha combined with zidovudine induces p53 signaling and apoptosis in HTLV-1-infected cells. Retrovirology 10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tattermusch S, Skinner JA, Chaussabel D, Banchereau J, Berry MP, McNab FW, O'Garra A, Taylor GP, Bangham CR. 2012. Systems biology approaches reveal a specific interferon-inducible signature in HTLV-1 associated myelopathy. PLoS Pathog. 8:e1002480. 10.1371/journal.ppat.1002480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goujon C, Malim MH. 2010. Characterization of the alpha interferon-induced postentry block to HIV-1 infection in primary human macrophages and T cells. J. Virol. 84:9254–9266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garaigorta U, Chisari FV. 2009. Hepatitis C virus blocks interferon effector function by inducing protein kinase R phosphorylation. Cell Host Microbe 6:513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arnaud N, Dabo S, Maillard P, Budkowska A, Kalliampakou KI, Mavromara P, Garcin D, Hugon J, Gatignol A, Akazawa D, Wakita T, Meurs EF. 2010. Hepatitis C virus controls interferon production through PKR activation. PLoS One 5:e10575. 10.1371/journal.pone.0010575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rojas M, Arias CF, Lopez S. 2010. Protein kinase R is responsible for the phosphorylation of eIF2alpha in rotavirus infection. J. Virol. 84:10457–10466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Charoenthongtrakul S, Zhou Q, Shembade N, Harhaj NS, Harhaj EW. 2011. HTLV-I Tax inhibits innate antiviral signaling via NF-{kappa}B-dependent induction of SOCS1. J. Virol. 85:6955–6962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng X, Ratner L. 2008. Human T-cell leukemia virus type 1 blunts signaling by interferon alpha. Virology 374:210–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oliere S, Hernandez E, Lezin A, Arguello M, Douville R, Nguyen TL, Olindo S, Panelatti G, Kazanji M, Wilkinson P, Sekaly RP, Cesaire R, Hiscott J. 2010. HTLV-1 evades type I interferon antiviral signaling by inducing the suppressor of cytokine signaling 1 (SOCS1). PLoS Pathog. 6:e1001177. 10.1371/journal.ppat.1001177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith D, Buckle GJ, Hafler DA, Frank DA, Hollsberg P. 1999. HTLV-I-infected T cells evade the antiproliferative action of IFN-beta. Virology 257:314–321 [DOI] [PubMed] [Google Scholar]
- 67.Zhang J, Yamada O, Kawagishi K, Araki H, Yamaoka S, Hattori T, Shimotohno K. 2008. Human T-cell leukemia virus type 1 Tax modulates interferon-alpha signal transduction through competitive usage of the coactivator CBP/p300. Virology 379:306–313 [DOI] [PubMed] [Google Scholar]
- 68.Hanon E, Hall S, Taylor GP, Saito M, Davis R, Tanaka Y, Usuku K, Osame M, Weber JN, Bangham CR. 2000. Abundant tax protein expression in CD4+ T cells infected with human T-cell lymphotropic virus type I (HTLV-I) is prevented by cytotoxic T lymphocytes. Blood 95:1386–1392 [PubMed] [Google Scholar]