Abstract
Some cdt genes are located within the genome of inducible or cryptic bacteriophages, but there is little information about the mechanisms of cdt transfer because of the reduced number of inducible Cdt phages described. In this study, a new self-inducible Myoviridae Cdt phage (ΦAA91) was isolated from a nonclinical O157:H7 Shiga toxin-producing Escherichia coli strain and was used to lysogenize a cdt-negative strain of Shigella sonnei. We found that the phage induced from S. sonnei (ΦAA91-ss) was not identical to the original phage. ΦAA91-ss was used to infect a collection of 57 bacterial strains, was infectious in 59.6% of the strains, and was able to lysogenize 22.8% of them. The complete sequence of ΦAA91-ss showed a 33,628-bp genome with characteristics of a P2-like phage with the cdt operon located near the cosR site. We found an IS21 element composed of two open reading frames inserted within the cox gene of the phage, causing gene truncation. Truncation of cox does not affect lytic induction but could contribute to phage recombination and generation of lysogens. The IS21 element was not present in the ΦAA91 phage from E. coli, but it was incorporated into the phage genome after its transduction in Shigella. This study shows empirically the evolution of temperate bacteriophages carrying virulence genes after infecting a new host and the generation of a phage population with better lysogenic abilities that would ultimately lead to the emergence of new pathogenic strains.
INTRODUCTION
Cytolethal distending toxin (Cdt) belongs to the AB2 type of toxins that block the G2 and early M phases during mitosis (1, 2). As a result, the cells do not divide, but since they continue to grow, they may distend up to five times their normal size before disintegration. Cdt is a virulence factor that benefits bacterial survival and enhances microbial pathogenicity (2). Cdts have an important role in the in vivo pathogenesis of Cdt-producing bacteria, causing severe complications, as demonstrated in mouse models (3).
The Cdt operon comprises three adjacent genes (cdtA, cdtB, and cdtC) that must be expressed for Cdt to initiate cellular toxicity. CdtB encodes the catalytic subunit, homologous to the mammalian DNase I, and CdtA and CdtC are binding proteins that deliver CdtB into the eukaryotic cells, with cytotoxic effects (3, 4). The complete Cdt operon has been reported in several Gram-negative bacteria, including Escherichia coli, Shigella spp., Campylobacter spp., Aggregatibacter actinomycetemcomitans, Haemophilus ducreyi, Helicobacter spp., and Vibrio (4–8). The Cdt subunits of a given genus show various degrees of similarity to the Cdt proteins of other genera.
The cdt operon is generally described as being located on the chromosome of Cdt-producing bacteria. However, in E. coli, five variants of Cdt have been described with different locations. Some variants are encoded by chromosomal genes (Cdt-II) (8) or by a pVir plasmid (Cdt-III) (9), and others are flanked by prophage genes, mostly bacteriophage P2 and bacteriophage lambda sequences (Cdt-I, Cdt-IV, and Cdt-V). A few studies have suggested that cdt genes in E. coli are located within prophages that could be defective (10–13) or within inducible bacteriophages (14, 15). Analyses of cdt flanking regions suggest that the cdt-I and cdt-IV genes have been acquired from a common ancestor by phage transduction and then evolved in their bacterial hosts (11).
Most available information about Cdt-producing strains pertains to clinical isolates. Recently, environmental strains harboring Cdt-V-encoding phages were described (14). Moreover, Cdt phages were detected in fecally polluted waters (14, 16) and showed high persistence under different disinfection processes and under natural inactivation conditions (16). Therefore, these phages may be interesting candidates for the mobilization of cdt genes in the environment. As with other virulence genes, the spread of cdt can lead to the emergence of new pathogenic strains or strains with increased virulence (17).
There is a clear relationship between Cdt production and pathogenic serotypes of Shiga toxin-producing E. coli (STEC) (10, 18, 19). While several studies have been conducted for other E. coli phages, including Stx phages, the relative frequency of the different Cdt phages in relevant pathotypes is not well defined. The information required about the mechanisms of induction of Cdt phages, their host range for lytic infection, and their ability to generate lysogens and to transduce cdt still is not available for Cdt phages. The limited number of inducible Cdt phages described so far represents an additional challenge for studying and understanding the role of these phages in cdt mobilization.
This study focuses on a new Cdt-V phage isolated from an E. coli O157:H7 strain from urban raw wastewater. We characterize its capacity for induction, infection, and transduction, and we present the complete phage genome sequence. Our study has revealed that the phage, during the lysogenization of a Shigella strain, has evolved and acquired new genes (an insertion element composed of two genes). We discuss how this genetic acquisition could influence the abilities of the phage to generate new lysogens and transduce the toxin gene.
MATERIALS AND METHODS
Bacterial strains, serotyping, bacteriophages, and media.
The Cdt phage in this study, named ΦAA91, was detected in a Cdt-positive E. coli strain of serotype O157:H7 strain 91 isolated from urban raw wastewater. This phage was induced from the wild-type strain as described below and used to lysogenize a collection of strains used as hosts for lytic infection and transduction (Table 1). A lysogen of the phage ΦAA91 in Shigella sonnei strain 866 was obtained. To avoid confusion, here the phage induced from the S. sonnei lysogen is called ΦAA91-ss. Luria-Bertani (LB) broth or LB agar was used to culture the bacteria. When necessary, media were supplemented with ampicillin (100 μg/ml) (Sigma-Aldrich, Steinheim, Germany).
Table 1.
Host range of phage ΦAA91-ss in a collection of cdt-negative strainsa
| Host | Strain or serotype | Originb | stx variant | Lysis | Transduction |
|---|---|---|---|---|---|
| E. coli | C600 | ATCC 23724 | − | − | |
| E. coli | DH5α | Invitrogen | − | − | |
| E. coli | O157:H7 | ATCC 43888 | ++ | − | |
| E. coli | WG5 | ATCC 700078 | ++ | + | |
| Salmonella Typhimurium | WG49 | ATCC 700730 | − | − | |
| S. sonnei | 866 | Clinical | ++ | + | |
| S. sonnei | 30673 | Clinical | + | − | |
| S. sonnei | 30674 | Clinical | ++ | + | |
| S. sonnei | 30676 | Clinical | ++ | + | |
| S. sonnei | 30679 | Clinical | ++ | + | |
| S. sonnei | 30682 | Clinical | ++ | + | |
| S. flexneri | 805-F | Clinical | + | − | |
| E. coli | O111 | Clinical | + | − | |
| E. coli | O26 | Clinical | − | − | |
| E. coli | O26 | Clinical | − | − | |
| E. coli | O157:H7 | Clinical | stx2a, stx2c | − | − |
| E. coli | O4:H4 | Clinical | stx2a | ++ | − |
| E. coli | O15:H16 | Clinical | stx2a | − | − |
| E. coli | O174:H- | Clinical | stx2c | ++ | − |
| E. coli | O104:H4 | Clinical | stx2a | − | − |
| E. coli | O146:H21 | H | stx2d | − | − |
| E. coli | O90:H- | M | stx2d | − | − |
| E. coli | O171:H2 | C | stx2c | − | − |
| E. coli | O171:H2 | C | stx2c | − | − |
| E. coli | O181:H49 | C | stx2a | ++ | − |
| E. coli | O181:H20 | C | stx2c | + | − |
| E. coli | O2:H25 | C | stx2g | + | − |
| E. coli | O136:H1 | C | stx2g | + | + |
| E. coli | O90:H- | H | stx2d | − | − |
| E. coli | ONT:H2 | C | stx2c, stx2d | + | − |
| E. coli | O2:H25 | C | stx2g | − | − |
| E. coli | O1:H20 | C | stx2a | + | − |
| E. coli | O8:H31 | C | stx2g | − | − |
| E. coli | O1:H20 | C | stx2a | + | − |
| E. coli | O157:H- | C | stx2c | + | − |
| E. coli | O22:H8 | C | stx2c | + | + |
| E. coli | O166:H21 | H | stx2c | − | − |
| E. coli | O157:H- | C | stx2c | − | − |
| E. coli | O156:H8 | C | stx2c | + | + |
| E. coli | O1:H20 | C | stx2a | − | − |
| E. coli | O171:H2 | C | stx2a, stx2c | + | − |
| E. coli | O76:H2 | C | stx2c | ++ | − |
| E. coli | O113:H21 | C | stx2a | + | + |
| E. coli | O100:H- | P | stx2e | + | − |
| E. coli | O171:H2 | C | stx2c | − | − |
| E. coli | O28:H28 | C | stx2a | + | + |
| E. coli | O8:H9 | H | stx2e | − | − |
| E. coli | O181:H49 | C | stx2a | + | − |
| E. coli | O171:H2 | C | stx2c | − | − |
| E. coli | ONT:H- | C | stx2c | + | − |
| E. coli | O2:H25 | M | stx2g | + | − |
| E. coli | ONT:H- | M | stx2e | − | − |
| E. coli | O22:H8 | C | stx2c | + | + |
| E. coli | O157:H7 | C | stx2c | + | − |
| E. coli | O157:H7 | C | stx2c | + | − |
| E. coli | O22:H8 | H | stx2c | ++ | + |
| E. coli | O1:H20 | H | stx2a | − | − |
Lytic infection ability was determined by observation of lysis visualized directly on the plate and after hybridization with the cdtB probe: +, weak lytic spot; ++, strong lytic spot; −, negative. Transduction ability was evaluated by PCR on colonies obtained after infection with the Cdt phage.
H, strain isolated form raw urban wastewater (human fecal pollution); C, strain isolated from cattle wastewater; P, strain isolated from poultry wastewater; M, strain isolated from mixed animal wastewater.
PCR studies.
PCRs were performed using a GeneAmp PCR system 2400 (Perkin-Elmer, PE Applied Biosystems, Barcelona, Spain) with the oligonucleotides listed in Table 2. Screening for cdt was performed by multiplex cdt PCR (Table 2), and then the identity of the cdt variants was analyzed with the specific oligonucleotides. Purified DNA was diluted 1:20 in double-distilled water. The PCR product was analyzed by gel electrophoresis, and bands were visualized by ethidium bromide staining. When necessary, PCR products were purified using a PCR purification kit (Qiagen Inc., Valencia, CA).
Table 2.
Oligonucleotides used in this studya
| PCR target and primer | Sequence | Size (bp) | Reference or source |
|---|---|---|---|
| Multiplex cdt | |||
| CDT-up1 | GAAAGTAAATGGAATATAAATGTCCG | 466 | 10 |
| CDT-up2 | GAAAATAAATGGAACACACATGTCCG | 10 | |
| CDT-lp1 | AAATCACCAAGAATCATCCAGTTA | 10 | |
| CDT-lp2 | AAATCTCCTGCAATCATCCAGTTA | 10 | |
| qPCR cdt | |||
| qcdtB-F | AGGCCGATGAAGTGTTTGTTCTT | 69 | 14 |
| qcdtB-R | CAATCCGTATGCCAAGCAATGG | 14 | |
| qcdt-probe | 6-FAM-CCGCCCACCTTGCCTT- MGBNFQ | 14 | |
| cdt-VA | |||
| c338f | AGCATTAAATAAAAGCACGA | 1329 | 52 |
| c2135r r | TACTTGCTGTGGTCTGCTAT | 52 | |
| cdt-VB | |||
| c1309f | AGCACCCGCAGTATCTTTGA | 1363 | 52 |
| c2166r | AGCCTCTTTTATCGTCTGGA | 52 | |
| cdt-VC | |||
| P105 | GTCAACGAACATTAGATTAT | 748 | 52 |
| c2767r | ATGGTCATGCTTTGTTATAT | 52 | |
| Integration site left | |||
| attP1 | ACAGCGATACATCGTGAAGC | 654 | This study |
| attB1 | TCATCGGTGTTGGAGATATCA | This study | |
| Integration site right | |||
| attP2 | GCAATATAATCGCACTGCAA | 585 | This study |
| attB2 | GAGACAATGCATCGCCTTGT | This study | |
| cox | |||
| Cox up | ATGGAAGTCAATGACTATGT | 273 | This study |
| Cox lp | TCACAACCCCATCCACAAAAG | This study | |
| IS21 within cox | |||
| IS21rev | TGCACCGTTGCCCGGCAACC | This study | |
| IS21fw | AGGCATCAGCGCGGTATGGA | This study | |
| IS21 | |||
| IS21up | GGTTGCCGGGCAACGGTGCA | 949 | This study |
| IS21lp | TCCATACCGCGCTGATGCCT | This study | |
| stx2 | |||
| stx2-a | GCGGTTTTATTTGCATTAGC | 115 | 53 |
| stx2-b | TCCCGTCAACCTTCACTGTA | 53 | |
| stx2c | |||
| stx2c-a | GCGGTTTTATTTGCATTAGT | 124 | 53 |
| stx2c-b | AGTACTCTTTTCCGGCCACT | 53 | |
| stx2d | |||
| stx2d-a | GGTAAAATTGAGTTCTCTAAGTAT | 175 | 53 |
| stx2d-b | CAGCAAATCCTGAACCTGACG | 53 | |
| stx2e | |||
| stx2e-a | ATGAAGTGTATATTGTTAAAGTGGA | 303 | 53 |
| stx2e-b | AGCCACATATAAATTATTTCGT | 53 | |
| stx2g | |||
| stx2g-a | GTTATATTTCTGTGGATATC | 572 | 54 |
| stx2g-b | GAATAACCGCTACAGTA | 54 |
MGBNFQ, minor groove binder and nonfluorescent quencher.
Real-time qPCR.
Quantification of cdt-V gene by real-time quantitative PCR (qPCR) was performed as previously described (14). A pGEM-T Easy vector containing a 466-bp fragment of cdtB obtained with conventional PCR primers Cdt-up1/Cdt-lp1 (Table 2) was used for the standard curve (14) to quantify the number of cdt gene copies in phage DNA by qPCR.
The qPCR assay for cdt was a custom TaqMan (Applied Biosystems, Spain) set of primers and probe. The forward qcdtB-UP/LP and qcdt probe with a 6-carboxyfluorescein (FAM) reporter and a nonfluorescent quencher (NFQ) were used under standard conditions in a Step One reverse transcription-PCR (RT-PCR) system (Applied Biosystems, Spain). cdtB genes were amplified in a 20-μl reaction mixture using TaqMan environmental real-time PCR master mix 2.0 (Applied Biosystems, Spain). The reaction mixture contained 9 μl of the DNA sample or quantified plasmid DNA. All samples were run in triplicate, along with the standards, positive and negative controls, and the number of genome copies (GC) was the average from the three replicates. A 1:10,000 dilution of positive bacterial DNA was used as a positive control.
Controls were performed to rule out the presence of bacterial or nonencapsidated DNA. After DNase treatment but before desencapsidation, the samples were used as templates for qPCR of cdt. Negative results confirmed that all nonviral DNA was eliminated from the sample and that only genes in phage DNA would be detected.
Isolation of temperate Cdt bacteriophages and preparation of phage lysates.
A 10-ml culture of wild-type strain O157:H7 or of the lysogen of Cdt phage in S. sonnei 866 were grown from single colonies in LB broth at 37°C until the exponential growth phase, determined by an optical density at 600 nm (OD600) of 0.3 measured using a spectrophotometer (Spectronic 501; Milton Roy, Belgium). For induction experiments, noninduced cultures and cultures induced with mitomycin C (0.5 μg/ml), ciprofloxacin (0.4, 1, or 4 μg/ml), or EDTA (20 mM, pH 7.2) were incubated overnight at 37°C under aerobic conditions with agitation (180 rpm) in the dark. The cultures were centrifuged at 4,000 × g for 10 min, and the supernatants were filtered through low-protein-binding 0.22-μm-pore-size membrane filters (Millex-GP, Millipore, Bedford, MA).
To evaluate the number of PFU of each phage suspension, each suspension was 10-fold serially diluted. One 1 ml of each dilution was mixed with 1 ml of the host strain (E. coli WG5) and 2.5 ml of LB soft agar, poured onto LB agar plates, and incubated as described above.
For electron microscopy and for infectivity studies, phage ΦAA91 (from the O157:H7 wild-type strain) and ΦAA91-ss (from S. sonnei 866 lysogen) were isolated from single plaques of lysis obtained from E. coli WG5. Plaques isolated from 5 to 10 wells were removed with a wire loop, and each was suspended in 500 μl of LB. Suspensions were filtered through low-protein-binding 0.22-μm-pore-size membrane filters (Millex-GP, Millipore, Bedford, MA).
Isolation of phage DNA.
The phage suspensions were treated with DNase (100 U/ml; Sigma-Aldrich, Spain). After digestion, DNase was heat inactivated (10 min at 80°C). Phage DNA was isolated from the phage lysates by proteinase K digestion and phenol-chloroform extraction as described previously (20). Purified DNA was eluted in a final volume of 50 μl of Tris-EDTA buffer (10 mM Tris-Cl, pH 7.5, 1 mM EDTA) and evaluated by agarose (0.8%) gel electrophoresis. The bands were viewed following ethidium bromide staining. The concentration and purity of the DNA extracted was determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Thermo Scientific, Wilmington, DE).
Preparation of DIG-labeled cdtB-specific gene probe.
A 466-bp fragment corresponding to cdtB resulting from amplification with primers Cdt-up1/Cdt-lp1 (Table 2) was labeled with digoxigenin (DIG) by incorporating DIG-11-deoxyuridine-triphosphate (Roche Diagnostics, Barcelona, Spain) during PCR as described previously (21) and was used as a probe. The probe was used for hybridization of phages and bacteria by plaque and colony blot assays as previously described (14). Hybridization was performed at 64°C according to standard procedures (20). Stringent hybridization was achieved with the DIG-DNA labeling and detection kit (Roche Diagnostics, Barcelona, Spain) according to the manufacturer's instructions.
Infectivity of Cdt phages.
To evaluate the ability of phages ΦAA91 and ΦAA91-ss to infect bacteria, a 10-μl drop of phage suspension, prepared from a single plaque as described above, was spotted onto a monolayer of the host strains tested (Table 1). The monolayer was prepared with 1 ml of log-phase culture (OD600 of 0.3) of each host strain, mixed with 2.5 ml of LB soft agar (LB broth with 0.7% agar), poured onto LB agar plates, and incubated at 37°C overnight. After incubation, the plaques were transferred to a nylon membrane (Hybond N+; Amersham Pharmacia Biotech, Spain) and hybridized with the cdtB-specific probe.
Generation of cdt lysogens.
The bacterial growth from the area of lysis generated by a drop of phage suspension on the agar monolayer of the different host strains (Table 1) was harvested in 1 ml of Ringer 1/4, 10-fold diluted, plated on LB agar, and incubated at 37°C for 18 h. Colonies obtained were evaluated for the presence of cdt by colony hybridization assay (14) using the DIG-labeled cdtB probe and confirmed by PCR.
Electron microscopy.
Phages ΦAA91 and ΦAA91-ss were propagated from a single plaque of lysis obtained from E. coli WG5 as described above. The phage suspensions obtained were 100-fold concentrated by means of protein concentrations (100-kDa Amicon Ultra centrifugal filter units; Millipore, Bedford, MA). Both phage suspensions were DNase treated as described above and purified by CsCl centrifugation (20). The easily visible gray band in which the bacteriophages were expected (20, 22), corresponding to a density of 1.45 ± 0.02 g/ml, was collected and dialyzed to remove the CsCl. A drop of phage suspension was deposited on copper grids with carbon-coated Formvar films and stained with 2% KOH phosphotungstic acid (pH 7.2) for 2.5 min. Samples were examined in a Jeol 1010 transmission electron microscope (JEOL USA, Inc.) operating at 80 kV.
Sequencing of Cdt phage DNA.
The ΦAA91-ss phage was purified from an induced culture of the S. sonnei lysogen. Phages were purified as described above and concentrated by protein concentrators (Amicon Ultra centrifugal filter units; Millipore, Bedford, MA). The phages present were further purified by CsCl centrifugation as described above. Contaminating bacterial DNA and RNA were removed by additional treatment with DNase (0.3 μg/ml) and RNase (0.3 μg/ml), and the mixtures were incubated at 37°C for 1 h. Phage DNA was extracted by proteinase K digestion and phenol-chloroform precipitation to a final concentration of 1 μg/μl. The purity and integrity of the DNA were confirmed by a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Thermo Scientific, Wilmington, DE) and 0.8% agarose gel electrophoresis.
Genome sequencing was performed using an Ion Torrent personal genome machine (PGM) (Life Technologies). Libraries were generated using 0.5 μg of the genomic DNA and an Ion Xpress Plus fragment library kit comprising the Ion Shear chemistry according to the user guide. After dilution of each library, 4.5 × 108 molecules were used as the templates for clonal amplification on Ion Sphere particles during the emulsion PCR according to the Ion Xpress template 200 kit manual (Life Technologies). The amplification was loaded onto an Ion 316 chip and subsequently sequenced using 105 sequencing cycles according to the Ion Sequencing 200 kit user guide. Approximately 105 sequencing cycles resulted in an average reading length of 182 nucleotides.
Assembly of the sequence was accomplished using CLC Genomic Workbench 6 software. The phage DNA-predicted open reading frames (ORFs) were identified using the GeneMark gene prediction program (http://exon.gatech.edu) (23) and ORF Finder (www.ncbi.nlm.nih.gov). These predictions were annotated by comparison to the nonredundant database using BLASTN from the NCBI (www.ncbi.nlm.nih.gov) and the European Bioinformatics Institute (UniProtKB/Swiss-Prot). MAUVE software (24) was used to compare ΦAA91-ss and other P2 and Cdt phages.
Nucleotide sequence accession number.
The nucleotide sequence of the phage ΦAA91-ss genome of this study was submitted to the GenBank database library and assigned accession number KF322032.
RESULTS
Detection and isolation of Cdt phage ΦAA91.
Bacteriophage ΦAA91 was isolated from the cdt-positive O157:H7 E. coli STEC strain that also harbors an inducible Stx phage. In order to characterize the Cdt phage without interference from the Stx phage, it was necessary to mobilize this phage to another susceptible host (14). For this, we used S. sonnei strain 866, which did not show the presence of inducible phages under the conditions tested. Following the common procedures for temperate phage induction, phage ΦAA91 was isolated after mitomycin C induction of an LB culture of the wild-type strain and purified. Dilutions of the phage suspension were plated on an agar monolayer of the E. coli WG5 strain. Lytic plaques formed by phage ΦAA91 on S. sonnei were clearly lytic and measured 1.2 ± 0.2 mm in diameter.
Assays for the generation of ΦAA91 lysogens in several E. coli and Shigella strains (Table 1) were unsuccessful, except for one lysogen obtained with S. sonnei 866. The cdt-positive S. sonnei 866 lysogen was confirmed by PCR. The lysogen was subcultured three times to confirm its stability, and our observations indicated that no losses of the phage were observed after subcultivation. Sequencing of the cdt variant confirmed that this phage was carrying the cdt-V variant of the gene.
Morphological characterization of phages ΦAA91 and ΦAA91-ss.
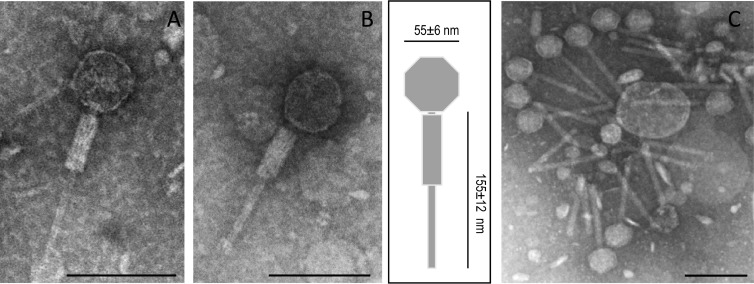
The morphology of phage ΦAA91 obtained from O157:H7 strains and of phage ΦAA91-ss obtained from S. sonnei lysogen were examined using a phage suspension obtained after propagating a single lytic plaque and CsCl purification. Both phages had identical Myoviridae morphology (22), with a capsid measuring 55 ± 6 nm in diameter and a contractile tail of 155 ± 12 nm (Fig. 1).
Fig 1.
Electron micrographs of phage ΦAA91 (A) and phage ΦAA91-ss (B), corresponding to the Myoviridae morphological type. (A and B) Single phage showing the tail with contracted sheath and diagram indicating the size of capsid and tail (in nm). (C) Group of phage ΦAA91-ss attached to particles showing the tails noncontracted. Bar, 100 nm.
Induction of phage ΦAA91-ss.
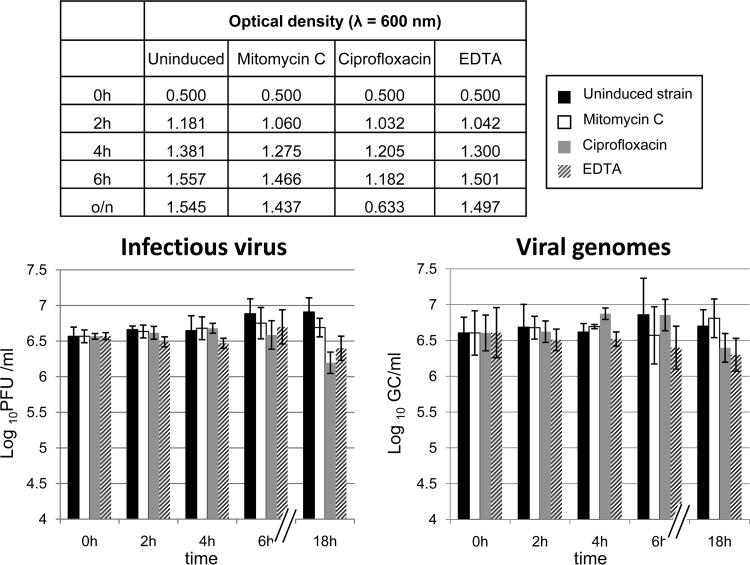
To determine whether cdt was located in the genome of an inducible prophage, infectious phages and viral genomes were counted without induction and after induction from S. sonnei lysogen with mitomycin C, ciprofloxacin, and EDTA at various times. Induction was also measured by a reduction of the OD600 of the bacterial culture, which indicates activation of the lytic cycle of induced prophages (Fig. 2).
Fig 2.
Induction experiments of phage ΦAA91-ss using mitomycin, ciprofloxacin, and EDTA. Monitoring of induction was performed by spectrophotometry at a wavelength of 600 nm (upper table), infectiveness on E. coli WG5 in log10 PFU/ml (chart on the left), and evaluation of phage genomes by qPCR in log10GC/ml (chart on the right).
The infectious particles or GC of the phage showed no significant increase (P > 0.05 by analysis of variance [ANOVA]) between the noninduced lysogenic strain culture and the culture treated with the inducing agents. After 18 h, a significantly (P < 0.05) lower OD600 was observed in the culture treated with ciprofloxacin (Fig. 2, upper table). However, this reduction was probably not due to the induction of ΦAA91-ss, as observed after evaluation of the number of infectious phages by plaque assay (Fig. 2, left) or after evaluation of cdt GC in phage DNA (Fig. 2, right).
These results indicated that phage ΦAA91-ss is not inducible through the previously described methods; rather, it appears to be self-inducible. Reduction of OD600 after ciprofloxacin exposure could be attributable to a decrease in the number of cells by the antimicrobial agent, since it was used at a high concentration. Lower concentrations of ciprofloxacin tested (0.4 and 1 μg/ml) did not show induction of ΦAA91-ss. Subsequent evaluation of the number of ΦAA91 particles from the wild-type E. coli O157:H7 strain confirmed that, as observed for the Shigella lysogen, the number of phages did not differ significantly between noninduced and induced cultures (data not shown).
Host range of phages ΦAA91 and ΦAA91-ss.
Each phage, isolated from a single plaque, was used to infect a collection of 57 strains, including one Salmonella strain, one Shigella flexneri strain, five S. sonnei strains, several E. coli laboratory strains, and wild-type STEC strains of different serotypes. Phages ΦAA91 and ΦAA91-ss had the same host range and caused clear lysis in 34 of the strains (Table 1). From these, phage ΦAA91 generated only one lysogen in S. sonnei 866. In contrast, phage ΦAA91-ss generated lysogens in 13 strains of different bacterial genera (Table 1).
Sequence of phage ΦAA91-ss.
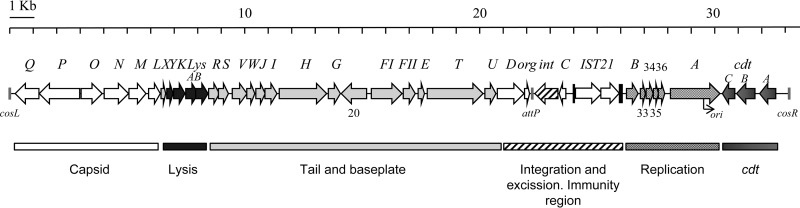
Phage ΦAA91-ss has a genome of 33,628 bp. Annotation of the phage genome showed multiple characteristics of P2 bacteriophages, although it also displayed some differences. The sequence is presented as a linear structure terminating with the cohesive ends (Table 3 and Fig. 3) and starting with cosL (left cohesive end). The first identified ORF is a capsid portal protein and is followed by a set of head synthesis proteins. Downstream of the capsid genes are the genes related to lysis (holin, lysozyme, and lysins A and B) (Table 3 and Fig. 3). The next set of genes corresponds to those proteins involved in tail and baseplate synthesis. Downstream are the genes involved in lysogeny (int, C, and expected cox). The phage attachment site (attP) (Fig. 3) is located between ogr (a positive regulatory factor required for P2 late gene transcription) (25) and the gene encoding integrase (int). The product of gene C is the repressor that regulates the lysogenic state. The next expected gene would be cox, encoding a multifunctional protein of 91 amino acids (26). However, in phage ΦAA91-ss, the cox gene is truncated by insertion of a long element, IS21, composed of two ORFs that encode a transposase and a transposition protein, respectively. We will devote more attention to this element in the next section. The sequence continues with ORFs identified as DNA replication proteins, particularly gene A, encoding gpA, which initiates replication by inducing a single-stranded cut at the origin of replication (ori) (5′-GCGCCTCGGAGTCCTGTCAA-3′) (Fig. 3) (27). Finally, we found the cdt operons (C, B, and A), which appeared downstream from gene A and upstream from cosR, at the right cohesive end of the phage genome.
Table 3.
Annotation of ORFs detected in the phage ΦAA91-ss genome
| ORF | Position (bp) | Size (bp) | Description | % Cover querya | % Identitya | Organism | NCBI protein accession no. |
|---|---|---|---|---|---|---|---|
| 1–19 | 19 | cosL; left cohesive end | |||||
| 1 | 189–1223 | 1,035 | Gene Q; capsid portal | 100 | 100 | E. coli prophage | WP_000038198.1 |
| 2 | 1223–2995 | 1,773 | Gene P; large terminase subunit | 100 | 100 | Yersinia phage | NP_839851.1 |
| 3 | 3169–4023 | 855 | Gene O; capsid scaffold | 100 | 99 | Prophage P2 in E. coli | YP_002391908.1 |
| 4 | 4082–5155 | 1,074 | Gene N; major capsid protein precursor | 100 | 100 | Prophage P2 in E. coli | WP_001248540.1 |
| 5 | 5159–5902 | 744 | Gene M; small terminase subunit | 100 | 100 | E. coli prophage | WP_000203437.1 |
| 6 | 6002–6511 | 510 | Gene L; capsid completion protein | 100 | 100 | E. coli prophage | YP_001746272.1 |
| 7 | 6511–6714 | 204 | Gene X; essential tail protein | 100 | 100 | P2-like prophage in E. coli | YP_005277372.1 |
| 8 | 6718–6999 | 282 | Gene Y; holin | 100 | 100 | Phage P2 | NP_046764.1 |
| 9 | 6999–7496 | 498 | Gene K; lysozyme | 100 | 100 | E. coli prophage | WP_000123122.1 |
| 10 | 7511–7936 | 426 | Lysin A | 100 | 100 | E. coli prophage | WP_000736580.1 |
| 11 | 7924–8349 | 426 | Lysin B | 100 | 100 | E. coli prophage | WP_000040681.1 |
| 12 | 8454–8924 | 471 | Gene R; essential tail completion protein | 100 | 100 | Phage P2 | WP_001367960.1 |
| 13 | 8917–9369 | 453 | Gene S; essential tail completion protein | 100 | 100 | Yersinia phage | NP_839862.1 |
| 14 | 9436–10071 | 636 | Gene V; baseplate protein | 100 | 100 | Phage P2 | WP_001093710.1 |
| 15 | 10068–10415 | 348 | Gene W; putative baseplate protein | 100 | 100 | E. coli prophage | YP_002391893.1 |
| 16 | 10420–11328 | 909 | Gene J; baseplate assembly | 100 | 99 | E. coli prophage | WP_001121491.1 |
| 17 | 11321–11851 | 531 | Gene I; baseplate assembly | 100 | 100 | Phage P2 | NP_046774.1 |
| 18 | 11862–13940 | 2,079 | Gene H; tail fiber | 100 | 100 | E. coli prophage | WP_000104689.1 |
| 19 | 13944–14471 | 528 | Gene G; putative tail fiber assembly | 100 | 100 | E. coli prophage | WP_001164103.1 |
| 20 | 14571–15677 | 1,107 | Hypothetical protein | 100 | 100 | E. coli | WP_000382496.1 |
| 21 | 15979–17169 | 1,191 | Gene FI; major tail sheath | 100 | 100 | E. coli prophage | WP_001286731.1 |
| 22 | 17182–17700 | 519 | Gene FII; major tail tube | 100 | 100 | Phage P2 | NP_046779.1 |
| 23 | 17757–18032 | 276 | Gene E; tail protein | 100 | 100 | E. coli Mu-like prophage | WP_001031311.1 |
| 24 | 18177–20624 | 2,448 | Gene T; tail length determinator | 100 | 100 | E. coli prophage | WP_000069913.1 |
| 25 | 20639–21118 | 480 | Gene U; tail protein | 100 | 100 | Phage P2 | WP_000978923.1 |
| 26 | 21118–22281 | 1,164 | Gene D; phage late control | 100 | 100 | E. coli prophage | WP_001704948.1 |
| 27 | 22362–22580 | 219 | ogr; activator of late transcription; Att phage integration site | 100 | 100 | E. coli | WP_000280530.1 |
| 28 | 22853–23863 | 1,011 | Integrase | 100 | 100 | E. coli phage HP1 | WP_001336827.1 |
| 29 | 23903–24196 | 294 | Gene C; immunity control | 100 | 100 | E. coli prophage | YP_005280060.1 |
| 24349–24471 | 123 | Truncated cox gene | |||||
| 30 | 24553–25725 | 1,173 | IstA; IS21 transposase | 100 | 100 | S. sonnei | YP_309050.1 |
| 31 | 25725–26522 | 798 | IstB; IS21 transposition protein | 100 | 100 | S. sonnei | YP_309051.1 |
| 26581–26758 | 177 | Truncated cox gene | |||||
| 32 | 26922–27428 | 507 | Gene B; DNA replication protein | 100 | 100 | E. coli prophage | WP_000426197.1 |
| 33 | 27492–27716 | 225 | Hypothetical protein (similar to phage P2 ORF 80) | 100 | 100 | E. coli | YP_001746256.1 |
| 34 | 27716–28018 | 303 | Hypothetical protein (similar to phage P2 ORF 81) | 100 | 100 | E. coli | WP_001277965.1 |
| 35 | 28018–28242 | 225 | Zinc finger protein (similar to phage P2 ORF 82) | 100 | 100 | Phage P2 | NP_046793.1 |
| 36 | 28239–28514 | 276 | Replication initiation protein (similar to phage P2 ORF 83) | 100 | 100 | E. coli prophage | YP_852002.1 |
| 37 | 28504–30801 | 2,298 | Gene A; DNA replication | 100 | 100 | E. coli prophage | WP_000268595.1 |
| 38 | 30877–31422 | 546 | Cdt-V C operon | 100 | 100 | E. coli | WP_000825552.1 |
| 39 | 31437–32246 | 810 | Cdt-V B operon | 100 | 100 | E. coli Vir plasmid | YP_003034080.1 |
| 40 | 32243–33019 | 777 | Cdt-V A operon | 100 | 100 | E. coli | WP_001284793.1 |
| 33609–33628 | 19 | cosR; cohesive end |
Percentage of coverage and the closest identity to proteins as described in the NCBI databank.
Fig 3.
Genetic map of phage ΦAA91-ss. Relevant phage genes, identified by comparison to other P2-like phages, are indicated. Arrows show gene size, orientation, and context. Those ORFs without defined function are shown by ORF number. The scale (in kb) is indicated in the upper part of the figure. The origin of replication (ori), the cohesive ends (cosR and cosL), and the phage attachment site (attP) are indicated.
Determination of IS21 in the host strains.
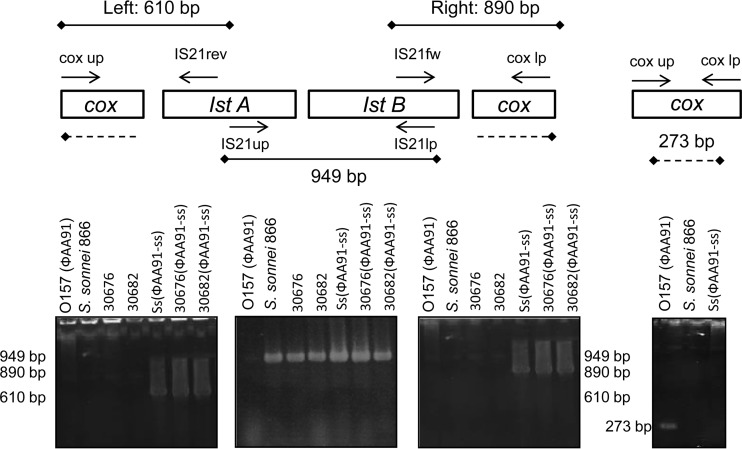
Many insertion elements have been identified in Shigella and E. coli located on a plasmid or in the chromosome. To determine whether the presence of IS21 in phage ΦAA91-ss was a product of rearrangements after lysogenizing the S. sonnei strain 866 or whether it was originally present in the phage in the wild-type O157:H7 E. coli strain, we used PCR to evaluate the fragment between the first part of cox and the IS21 element and the fragment comprising the last fragment of IS21 and the last cox fragment. In addition, we investigated whether the element is maintained in some S. sonnei strains of our collection in which the phage ΦAA91-ss generated new lysogens (Table 1). We amplified both segments using the primers described in Table 2 (Fig. 4). If the IS21 element is present within cox, amplification using the combination of primers cox-up and S21rev would result in an amplimer of 610 bp, while the combination of primers IS21fw and cox-lp would result in a fragment of 890 bp. Amplification of both regions confirmed the location of the IS21 element within cox in phage ΦAA91-ss from the S. sonnei 866 lysogen as well as from other S. sonnei lysogens (30676 and 30682; Table 1), as shown in Fig. 4. However, when looking at the original E. coli O157:H7 strain 91, where phage ΦAA91 was present, we found that cox appeared intact.
Fig 4.
Amplimers generated for detection of IS21 element in phages and host strains. Insertion of IS21 in cox was detected from its left and right side by the combination of IS21/cox primers. The element was detected in Shigella (results of S. sonnei strains 866, 30676, and 30682 are shown here) but not in the wild-type O157:H7 E. coli strain. A nontruncated cox gene was observed only in the wild-type O157:H7 E. coli strain.
PCR showed that the IS21 element was present in the S. sonnei strains but not in the wild-type O157:H7 strain, although phage ΦAA91 was present, confirming that incorporation of IS21 occurred after lysogenization in S. sonnei.
To evaluate the percentage of phages that could harbor the IS21 element, phage ΦAA91, induced from E. coli O157:H7 strain 91, and phage ΦAA91-ss, from S. sonnei 866 lysogen, were plated using E. coli WG5 as the host strain (negative for IS21). Plates containing between 20 and 50 plaques were used to isolate phages from the plaques of lysis. Phage DNA was extracted from the plaques, and the presence of IS21 within cox was evaluated by PCR amplification of the IS21 left and right insertion sites and those with intact cox gene by using primers cox-up/lp. The results of phage ΦAA91 induced from O157:H7 showed that 100% of plaques had the intact cox gene and that the IS21 element was absent. Results of plaques of ΦAA91-ss phages induced from S. sonnei 866 lysogen showed that 86.1% (±3.2%) of the plaques contained IS21 inserted within cox, while only 13.9% (±3.2%) presented intact cox and no IS21 element. The higher proportion of phages carrying IS21 is consistent with the sequence obtained. Phages induced from lysogens in S. sonnei strains 30676 and 30682 with phage ΦAA91-ss showed 100% of the plaques of lysis with the IS21 element and cox truncated, suggesting that lysogens were obtained by incorporation of ΦAA91-ss.
Determination of sequence similarity between ΦAA91 and ΦAA91-ss.
Using the ΦAA91-ss sequence as the template, we designed PCR primers that allowed the amplification of 30 consecutive fragments of ca. 1,200 bp each (data not shown). Each fragment amplified overlapped the previous and the following fragment, covering the whole length of the genome of phage ΦAA91-ss (33,628 bp). These primers were used to amplify the ΦAA91 DNA obtained from a single plaque, and ΦAA91-ss DNA was used as a control.
The sizes of the 30 fragments obtained were identical for both phages, except in three fragments (23, 24, and 25) (data not shown), in which at least one of the primers annealed with the IS21 fragment; therefore, no amplimers were obtained with phage ΦAA91 DNA, but they were obtained with ΦAA91-ss. Although minor variations in the sequence cannot be excluded, these results strongly indicated that compared to the genetic organization of the ΦAA91 genome, the ΦAA91-ss genome has been modified only in the cox gene by the incorporation of IS21.
Homology with other P2-like phages.
Comparison of the ΦAA91-ss phage genome to those of other P2 and Cdt phages showed different similarities. The most similar was the Enterobacteriaceae P2 phage carrying cdt-V (KC618326.1) (12); 79% of the sequence was 98% identical. The second most similar phage, with a similarity of 97% in 75% of its genome, was phage WPhi (AY135739.1) of E. coli. Finally, phage LC-413 (AY251033.1) of Yersinia showed 73% coverage with 96% similarity, and phage P2 (AF063097.1) of E. coli showed 69% coverage with 100% similarity in the covered sequence. Some bacterial genomes also showed similarity to the ΦAA91-ss phage genome. The most similar, with 84% of coverage with 100% identity, was S. sonnei strain 53G (HE616528.1). However, this sequence has not been annotated, hence it is not known whether this strain harbors a prophage. However, it does lack cdt, since the area of coverage did not include the cdt operon. A similarity of 99% in 83% of the sequence was observed for E. coli strain SMS-3-5 (CP000970.1), and 99% identity in 75% of the sequence was found with other E. coli W genomes (CP002185.1, CP002967.1, CP002516.1, and CP002970.1), all of them harboring a P2-like phage in which the cdt operon is absent. Other phages or bacterial strains showed lower coverage.
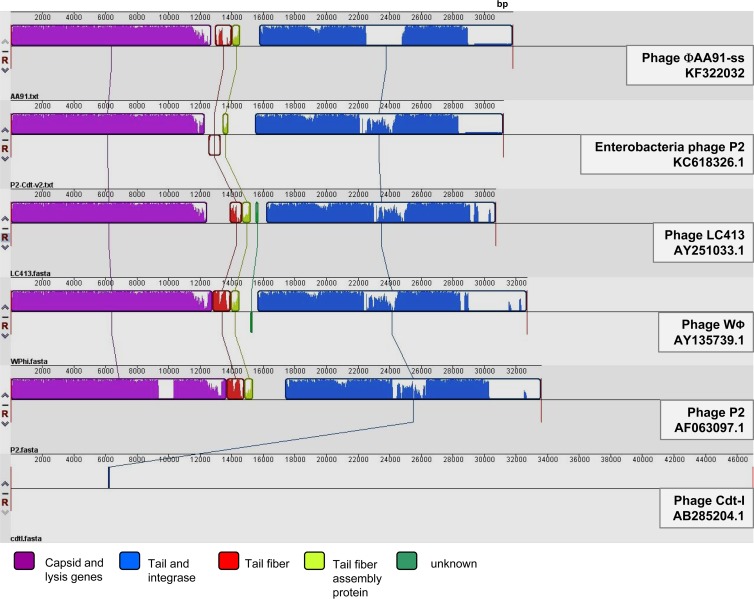
Progressive MAUVE analysis (24) (Fig. 5) allowed comparison of phage ΦAA91-ss to complete sequences of P2-like phages showing similarity and to the two Cdt phages in the databases: phage encoding Cdt-I (15) and a P2 cryptic prophage encoding Cdt-V (12) (accession number KC618326.1). The results show that despite the differences in their sequences, all phages shared regions in their genome's structure, with the exception of phage encoding Cdt-I, which has a completely different genome. In the remaining phages, two main structures were observed, one comprising capsid genes and the genes involved in lysis (holins and lysins) and a second set comprising genes involved in the phage tail and the integrases. Some other regions encoding tail fiber or tail assembly proteins presented greater degrees of variability than the previously mentioned regions (Fig. 5).
Fig 5.
MAUVE analysis comparison of Cdt phage ΦAA91-ss to P2-like phages (P2, LC413, and WΦ) and genomes of Cdt phages (P2-Cdt-V and Cdt-I).
Insertion site of phage ΦAA91-ss.
The att site of phage ΦAA91-ss is located between the ogr and int genes (Fig. 3). Its integration into the S. sonnei 866 lysogen was determined by designing primers attP1 and attP2 in both genes of phage DNA (Table 1) and combining these with primers attB1 and attB2 located in the fieF and cpxP genes, which were previously described as the insertion site of P2-like phages in E. coli W, E. coli K KO11FL (28), and S. sonnei 53G (accession number HE616528.1). Amplification of attP1/B1 and attB2/P2 showed amplimers of 654 and 585 bp, respectively, corresponding to the expected size and confirming that the phage was inserted into this bacterial site. The S. sonnei 866 strain used as a control did not show amplification at both sites. Moreover, ΦAA91-ss also is inserted in the same locus in lysogens of S. sonnei strains 30676 and 30682. Controls of bacterial strains without the phage were negative. Additionally, we obtained the same PCR results when testing phage ΦAA91 insertion in the E. coli O157:H7 wild-type strain. Thus, we concluded that ΦAA91 and ΦAA91-ss are inserted in the same locus in their respective host strains.
DISCUSSION
P2-like prophages seem to be quite common in E. coli but also seem to be distributed among other proteobacteria of the gamma subgroup (29). The genomes of phages HP1 and HP2 in Haemophilus influenzae (30), ΦCTX in Pseudomonas aeruginosa (31), and K139 in Vibrio cholerae (32) have all been sequenced and shown to be P2-like with respect to genome organization as well as nucleotide sequence. Phages PSP3 in Salmonella enterica serovar Potsdam (33) and SopEΦ in Salmonella enterica serovar Typhimurium (34) are also P2-like. Bacterial genome sequencing projects have revealed additional P2-like prophages, for example, Sp13 in the enterohemorrhagic Escherichia coli O157:H7 strain isolated during the Sakai outbreak in Japan (35).
In our previous study (14), cdt-V was found in an inducible Cdt bacteriophage. The presence of cdt in phages, although not always inducible, has been reported (11) and is supported by the P2 sequences (12) or lambdoid phage sequences (15) found in the flanking regions of cdt in several bacterial isolates. In this study, a new Cdt-V phage, named ΦAA91, was used to lysogenize S. sonnei strain 866, since this strain gave good results in studies with Cdt phages (14) and also had previously shown the capacity to generate lysogens of temperate Stx bacteriophages (21, 36). No lysogens of ΦAA91 were obtained with any of the other laboratory strains assayed. Since the phage induced from the S. sonnei strain was shown to be different from the phage induced from the E. coli wild type, it was renamed ΦAA91-ss.
Phages ΦAA91 and ΦAA91-ss are self-inducible, or at least lead to no increase in the number of infectious viruses or viral genome copies when using common phage-inducing agents (37–40). This can be attributed to the fact that these phages belong to the P2-like family of phages. In P2 phages, the product of gene C, which is responsible for lysogeny, cannot be cleaved by the RecA protease (41, 42). For this reason, this gene is not affected by UV or other inducing agents, causing derepression of the lysogenic state. Therefore, P2-like phages do not promote their lytic pathway when using one of the common inducing agents; rather, they maintain the same levels of induction in the absence of these agents. This lack of inducibility was previously observed for another phage harboring Cdt (phage Φ125) (14).
The remarkable difference between the genomes of phages ΦAA91 and ΦAA91-ss is the IS21 insertion element. Insertion elements are numerous in E. coli and Shigella genomes and are capable of causing many kinds of DNA rearrangements (43). Deletions as well as translocations and inversions are likely the result of the copious numbers of insertion sequence elements in bacterial chromosomes. This element is inserted within the cox gene, encoding Cox, which is responsible for inhibiting phage integration and activating phage DNA excision. In P2 phages, by leading to excision of P2 DNA out of the host chromosome, Cox is directly involved in the site-specific recombination event (44). Early expression of cox ensures that at least a few copies of the P2 genome are synthesized free of the host chromosome (44). However, a mutation in cox will produce a phage that is as efficient as or more efficient than the parental strain in the generation of lysogens. This phage would be able to recombine with higher frequency (5 to 20 times), at least in the att region pathway (45). Successful generation of lysogens with ΦAA91-ss in other hosts also could be attributable to its potentially higher recombination ability after cox truncation compared to the original phage, ΦAA91. This could not be properly assayed, since with the original phage, only one S. sonnei lysogen of ΦAA91 was obtained, and the phage induced from this strain (ΦAA91-ss) already carried IS21.
IS21 has not been found in the wild-type E. coli O157:H7 strain containing phage ΦAA91; therefore, it must have been incorporated into the phage genome during lysogenization of the Shigella strain in which this element is present and subsequently is maintained in the phage, since it was found in some lysogens generated a posteriori with phage ΦAA91-ss. The IS21 element has been reported to be located close to a cdt-III gene in a pVir plasmid in E. coli (46) but is present in a different position and with the opposite orientation. IS21 has also been found in P2-like phage ΦCTX in Pseudomonas aeruginosa (31) but without the cdt operon. The insertion of IS21 during the generation of Shigella lysogens provides experimental evidence of the evolution of phages after the lysogenization of new hosts, as widely reported, mostly by comparison of different phage genomes (47, 48).
The genes encoding Cdt were found inserted into a region of the phage (named TO) that has been identified as a hot spot for the insertion of foreign genes (49). The gene content of the TO region varies extensively among P2-like prophages found in E. coli (49, 50). A previous sequence of a prophage harboring the cdt operon indicates the same insertion site of the genes (12).
Phages ΦAA91 and ΦAA91-ss showed the morphology of Myoviridae and, like other P2-like phages, are transducing phages (51). In our previous studies with another Cdt-V phage, phage Φ125 (14), the phage showed Siphoviridae morphology, and no phages with Myoviridae morphology were observed. Siphoviridae morphology has also been reported for the Cdt-1Φ phage described by Asakura et al. (15). However, the cdt-V operon in phage Φ125 was flanked by a bacteriophage region showing homology with a fragment of a P2 phage. Similarly, homology with P2 in the flanking regions of the cdt-V cluster has been widely reported (11, 12, 18). Whether these previously reported phages belonged to the P2 family or were the product of recombination of these and other phages of the Siphoviridae group remains unknown. On the contrary, descriptions of the cdt-I and cdt-IV flanking sequences indicate that these are not always related to P2 phage genes (11, 15). In fact, variability among Cdt phages is not surprising considering the demonstrated ability of phages to interchange different fragments of diverse origin mediated by both homologous and nonhomologous recombination (48). The presence of P2-like phages in different genera (Vibrio, Salmonella, and E. coli, for example) is also coincident with the wide distribution of cdt genes in all of these genera.
The phage in this study is a good example that shows not only the ability of phages to generate new strains, in this case by the incorporation of cdt, but also the evolution of phages when moving from one strain to another, which can cause the generation of phage variants able to convert strains in a more successful way. It is of clinical importance to gain more insights into the in vivo dissemination of virulence factors by means of bacteriophages in enteric infections, particularly within the intestinal tract, where phages can spread through a wide range of hosts and cause the emergence of new pathogenic bacteria.
ACKNOWLEDGMENTS
This study was supported by the Generalitat de Catalunya (2009SGR1043), the Spanish Ministry of Education and Science (AGL2009-07576 and AGL2012-30880), the RecerCaixa program (Fundació Obra Social La Caixa), and the Xarxa de Referència en Biotecnologia (XRB). Anna Allué has received grant FI from the Generalitat de Catalunya, Catalonia, Spain.
Footnotes
Published ahead of print 9 October 2013
REFERENCES
- 1.Ohara M, Oswald E, Sugai M. 2004. Cytolethal distending toxin: a bacterial bullet targeted to nucleus. J. Biochem. 136:409–413 [DOI] [PubMed] [Google Scholar]
- 2.Oswald E, Nougayrède JP, Taieb F, Sugai M. 2005. Bacterial toxins that modulate host cell-cycle progression. Curr. Opin. Microbiol. 8:83–91 [DOI] [PubMed] [Google Scholar]
- 3.Ge Z, Schauer DB, Fox JG. 2008. In vivo virulence properties of bacterial cytolethal-distending toxin. Cell Microbiol. 10:1599–1607 [DOI] [PubMed] [Google Scholar]
- 4.Boesze-Battaglia K, Besack D, McKay T, Zekavat A, Otis L, Jordan-Sciutto K, Shenker BJ. 2006. Cholesterol-rich membrane microdomains mediate cell cycle arrest induced by Actinobacillus actinomycetemcomitans cytolethal-distending toxin. Cell Microbiol. 8:823–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith JL, Bayles DO. 2006. The contribution of cytolethal distending toxin to bacterial pathogenesis. Crit. Rev. Microbiol. 32:227–248 [DOI] [PubMed] [Google Scholar]
- 6.Pickett CL, Whitehouse CA. 1999. The cytolethal distending toxin family. Trends Microbiol. 7:292–297 [DOI] [PubMed] [Google Scholar]
- 7.Thelestam M, Frisan T. 2004. Cytolethal distending toxins. Rev. Physiol. Biochem. Pharmacol. 152:111–133 [DOI] [PubMed] [Google Scholar]
- 8.Okuda J, Kurazono H, Takeda Y. 1995. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb. Pathog. 18:167–172 [DOI] [PubMed] [Google Scholar]
- 9.Pérès SY, Marchès O, Daigle F, Nougayrède JP, Herault F, Tasca C, De Rycke J, Oswald E. 1997. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol. Microbiol. 24:1095–1107 [DOI] [PubMed] [Google Scholar]
- 10.Tóth I, Hérault F, Beutin L, Oswald E. 2003. Production of cytolethal distending toxins by pathogenic Escherichia coli strains isolated from human and animal sources: establishment of the existence of a new cdt variant (type IV). J. Clin. Microbiol. 41:4285–4291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tóth I, Nougayrède JP, Dobrindt U, Ledger TN, Boury M, Morabito S, Fujiwara T, Sugai M, Hacker J, Oswald E. 2009. Cytolethal distending toxin type I and type IV genes are framed with lambdoid prophage genes in extraintestinal pathogenic Escherichia coli. Infect. Immun. 77:492–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svab D, Horvath B, Maroti G, Dobrindt U, Toth I. 2013. Sequence variability of P2-like prophage genomes carrying the cytolethal distending toxin V operon in Escherichia coli O157. Appl. Environ. Microbiol. 79:4958–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bielaszewska M, Fell M, Greune L, Prager R, Fruth A, Tschäpe H, Schmidt MA, Karch H. 2004. Characterization of cytolethal distending toxin genes and expression in Shiga toxin-producing Escherichia coli strains of non-O157 serogroups. Infect. Immun. 72:1812–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allué-Guardia A, García-Aljaro C, Muniesa M. 2011. Bacteriophage-encoding cytolethal distending toxin type V gene induced from nonclinical Escherichia coli isolates. Infect. Immun. 79:3262–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asakura M, Hinenoya A, Alam MS, Shima K, Zahid SH, Shi L, Sugimoto N, Ghosh AN, Ramamurthy T, Faruque SM, Nair GB, Yamasaki S. 2007. An inducible lambdoid prophage encoding cytolethal distending toxin (Cdt-I) and a type III effector protein in enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 104:14483–14488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allué-Guardia A, Jofre J, Muniesa M. 2012. Stability and infectivity of cytolethal distending toxin type V gene-carrying bacteriophages in a water mesocosm and under different inactivation conditions. Appl. Environ. Microbiol. 78:5818–5823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cangelosi GA, Freitag NE, Buckley MR. 2004. From outside to inside. environmental microorganisms as human pathogens. American Academy of Microbiology report ASM, Washington, DC [Google Scholar]
- 18.Friedrich AW, Lu S, Bielaszewska M, Prager R, Bruns P, Xu JG, Tschäpe H, Karch H. 2006. Cytolethal distending toxin in Escherichia coli O157:H7: spectrum of conservation, structure, and endothelial toxicity. J. Clin. Microbiol. 44:1844–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bielaszewska M, Stoewe F, Fruth A, Zhang W, Prager R, Brockmeyer J, Mellmann A, Karch H, Friedrich AW. 2009. Shiga toxin, cytolethal distending toxin, and hemolysin repertoires in clinical Escherichia coli O91 isolates. J. Clin. Microbiol. 47:2061–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 21.Muniesa M, Blanco JE, De Simón M, Serra-Moreno R, Blanch AR, Jofre J. 2004. Diversity of stx2 converting bacteriophages induced from Shiga-toxin-producing Escherichia coli strains isolated from cattle. Microbiology 150:2959–2971 [DOI] [PubMed] [Google Scholar]
- 22.Franki RIB, Fauquet CM, Knudson DL, Brown F. 1991. Classification and nomenclature of viruses. Fifth report of the International Committee on Taxonomy of Viruses, suppl 2. Springer-Verlag, Vienna, Austria [Google Scholar]
- 23.Besemer J, Lomsadze A, Borodovsky M. 2001. GeneMarkS: a self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 29:2607–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darling AE, Mau B, Perna NT. 2010. ProgressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5:e11147. 10.1371/journal.pone.0011147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christie GE, Haggård-Ljungquist E, Feiwell R, Calendar R. 1986. Regulation of bacteriophage P2 late-gene expression: the ogr gene. Proc. Natl. Acad. Sci. U. S. A. 83:3238–3242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haggård-Ljungquist E, Kockum K, Bertani LE. 1987. DNA sequences of bacteriophage P2 early genes cox and B and their regulatory sites. Mol. Gen. Genet. 208:52–56 [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Haggård-Ljungquist E. 1994. Studies of bacteriophage P2 DNA replication: localization of the cleavage site of the A protein. Nucleic Acids Res. 22:5204–5210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner PC, Yomano LP, Jarboe LR, York SW, Baggett CL, Moritz BE, Zentz EB, Shanmugam KT, Ingram LO. 2012. Optical mapping and sequencing of the Escherichia coli KO11 genome reveal extensive chromosomal rearrangements, and multiple tandem copies of the Zymomonas mobilis pdc and adhB genes. J. Ind. Microbiol. Biotechnol. 39:629–639 [DOI] [PubMed] [Google Scholar]
- 29.Nilsson AS, Karlsson JL, Haggård-Ljungquist E. 2004. Site-specific recombination links the evolution of P2-like coliphages and pathogenic enterobacteria. Mol. Biol. Evol. 21:1–13 [DOI] [PubMed] [Google Scholar]
- 30.Esposito D, Fitzmaurice WP, Benjamin RC, Goodman SD, Waldman AS, Scocca JJ. 1996. The complete nucleotide sequence of bacteriophage HP1 DNA. Nucleic Acids Res. 24:2360–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakayama K, Kanaya S, Ohnishi M, Terawaki Y, Hayashi T. 1999. The complete nucleotide sequence of FCTX, a cytotoxin-converting phage of Pseudomonas aeruginosa: implications for phage evolution and horizontal transfer via bacteriophages. Mol. Microbiol. 31:399–419 [DOI] [PubMed] [Google Scholar]
- 32.Nesper J, Blass J, Fountoulakis M, Reidl J. 1999. Characterization of the major control region of Vibrio cholerae bacteriophage K139: immunity, exclusion, and integration. J. Bacteriol. 181:2902–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bullas LR, Mostaghimi AR, Arensdorf JJ, Rajadas PT, Zuccarelli AJ. 1991. Salmonella phage PSP3, another member of the P2-like phage group. Virology 185:918–921 [DOI] [PubMed] [Google Scholar]
- 34.Mirold S, Rabsch W, Tschäpe H, Hardt WD. 2001. Transfer of the Salmonella type III effector sopE between unrelated phage families. J. Mol. Biol. 312:7–16 [DOI] [PubMed] [Google Scholar]
- 35.Hayashi T, Makino K, Ohnishi M, Kurakowa K, Ishii K, Yokoyama K, Han CG, Ohtsubo E, Nakayama K, Murata T, Tanaka M, Tobe T, IIda T, Takami H, Honda T, Sasakawa C, Ogasawara N, Yasunaga T, Kuhara S, Shiba T, Hattori M, Shinagawa H. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 8:11–22 [DOI] [PubMed] [Google Scholar]
- 36.Serra-Moreno R, Jofre J, Muniesa M. 2007. Insertion site occupancy by stx2 bacteriophages depends on the locus availability of the host strain chromosome. J. Bacteriol. 189:6645–6654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imamovic L, Muniesa M. 2012. Characterizing RecA-independent induction of Shiga toxin2-encoding phages by EDTA treatment. PLoS One 7:e32393. 10.1371/journal.pone.0032393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livny J, Friedman DI. 2004. Characterizing spontaneous induction of Stx encoding phages using a selectable reporter system. Mol. Microbiol. 51:1691–1704 [DOI] [PubMed] [Google Scholar]
- 39.Fuchs S, Mühldorfer I, Donohue-Rolfe A, Kerényi M, Emödy L, Alexiev R, Nenkov P, Hacker J. 1999. Influence of RecA on in vivo virulence and Shiga toxin 2 production in Escherichia coli pathogens. Microb. Pathog. 27:13–23 [DOI] [PubMed] [Google Scholar]
- 40.Kimmitt PT, Harwood CR, Barer MR. 2000. Toxin gene expression by shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg. Infect. Dis. 6:458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lundqvist B, Bertani G. 1984. Immunity repressor of bacteriophage P2. Identification and DNA-binding activity. J. Mol. Biol. 178:629–651 [DOI] [PubMed] [Google Scholar]
- 42.Bertani LE, Six EW. 1988. The P2-like phages and their parasite, P4, p 73–143 In Calendar R. (ed), The cacteriophages, vol 2 Plenum Press, New York, NY [Google Scholar]
- 43.Yang F, Yang J, Zhang X, Chen L, Jiang Y, Yan Y, Tang X, Wang J, Xiong Z, Dong J, Xue Y, Zhu Y, Xu X, Sun L, Chen S, Nie H, Peng J, Xu J, Wang Y, Yuan Z, Wen Y, Yao Z, Shen Y, Qiang B, Hou Y, Yu J, Jin Q. 2005. Genome dynamics and diversity of Shigella species, the etiologic agents of bacillary dysentery. Nucleic Acids Res. 33:6445–6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saha S, Haggård-Ljungquist E, Nordström K. 1987. The cox protein of bacteriophage P2 inhibits the formation of the repressor protein and autoregulates the early operon. EMBO J. 6:3191–3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindahl G, Sunshine M. 1972. Excision-deficient mutants of bacteriophage P2. Virology 49:180–187 [DOI] [PubMed] [Google Scholar]
- 46.Johnson TJ, DebRoy C, Belton S, Williams ML, Lawrence M, Nolan LK, Thorsness JL. 2010. Pyrosequencing of the Vir plasmid of necrotoxigenic Escherichia coli. Vet. Microbiol. 144:100–109 [DOI] [PubMed] [Google Scholar]
- 47.Hendrix RW, Smith MCM, Burns RN, Ford ME, Hatfull GF. 1999. Evolutionary relationships among diverse bacteriophages and prophages: all the world's a phage. Proc. Natl. Acad. Sci. U. S. A. 96:2192–2197, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hendrix RW. 2003. Bacteriophage genomics. Curr. Opin. Microbiol. 6:506–511 [DOI] [PubMed] [Google Scholar]
- 49.Nilsson AS, Haggård-Ljungquist E. 2007. Evolution of P2-like phages and their impact on bacterial evolution. Res. Microbiol. 158:311–317 [DOI] [PubMed] [Google Scholar]
- 50.Odegrip R, Nilsson AS, Haggård-Ljungquist E. 2006. Identification of a gene encoding a functional reverse transcriptase within a highly variable locus in the P2-like coliphages. J. Bacteriol. 188:1643–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nilsson AS, Haggård-Ljungquist E. 2006. The P2-like bacteriophages, p 365–390 In Calendar R. (ed), The bacteriophages. ASM Press, Washington, DC [Google Scholar]
- 52.Janka A, Bielaszewska M, Dobrindt U, Greune L, Schmidt MA, Karch H. 2003. Cytolethal distending toxin gene cluster in enterohemorrhagic Escherichia coli O157:H- and O157:H7: characterization and evolutionary considerations. Infect. Immun. 71:3634–3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang G, Clark CG, Rodgers FG. 2002. Detection in Escherichia coli of the genes encoding the major virulence factors, the genes defining the O157:H7 serotype, and components of the type 2 Shiga toxin family by multiplex PCR. J. Clin. Microbiol. 40:3613–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leung PH, Peiris JS, Ng WW, Robins-Browne RM, Bettelheim KA, Yam WC. 2003. A newly discovered verotoxin variant, VT2g, produced by bovine verocytotoxigenic Escherichia coli. Appl. Environ. Microbiol. 69:7549–7553 [DOI] [PMC free article] [PubMed] [Google Scholar]