Abstract
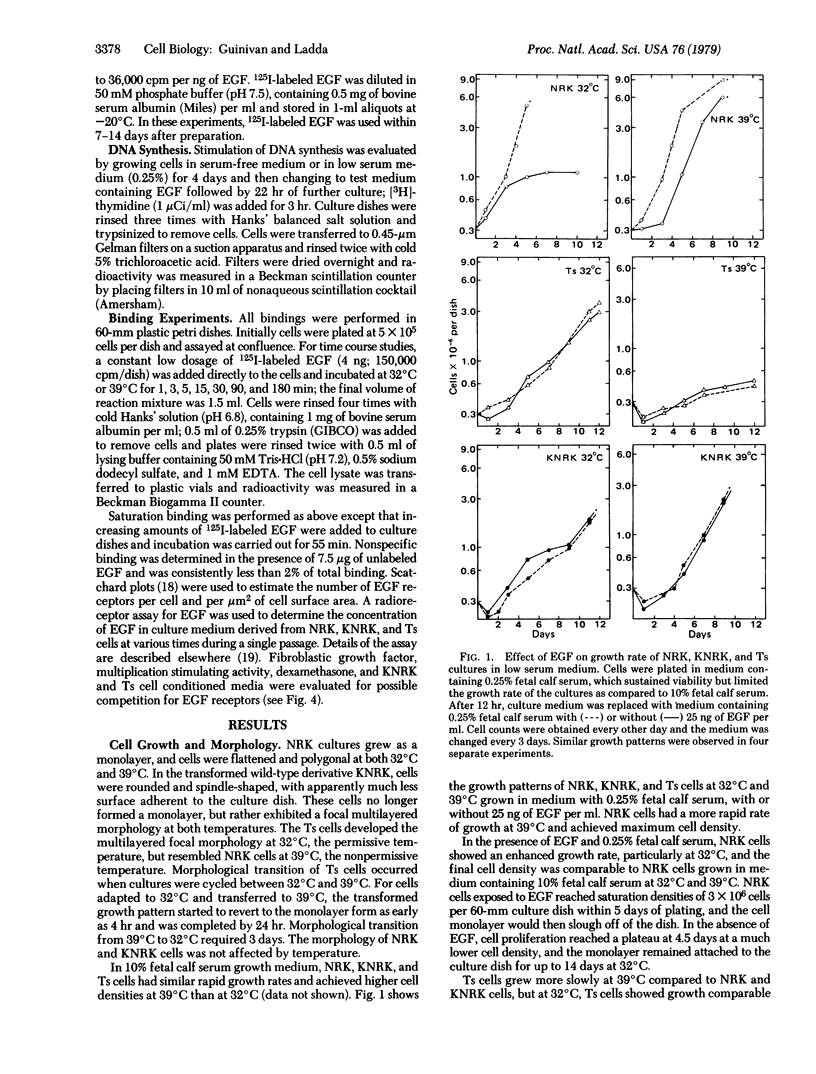
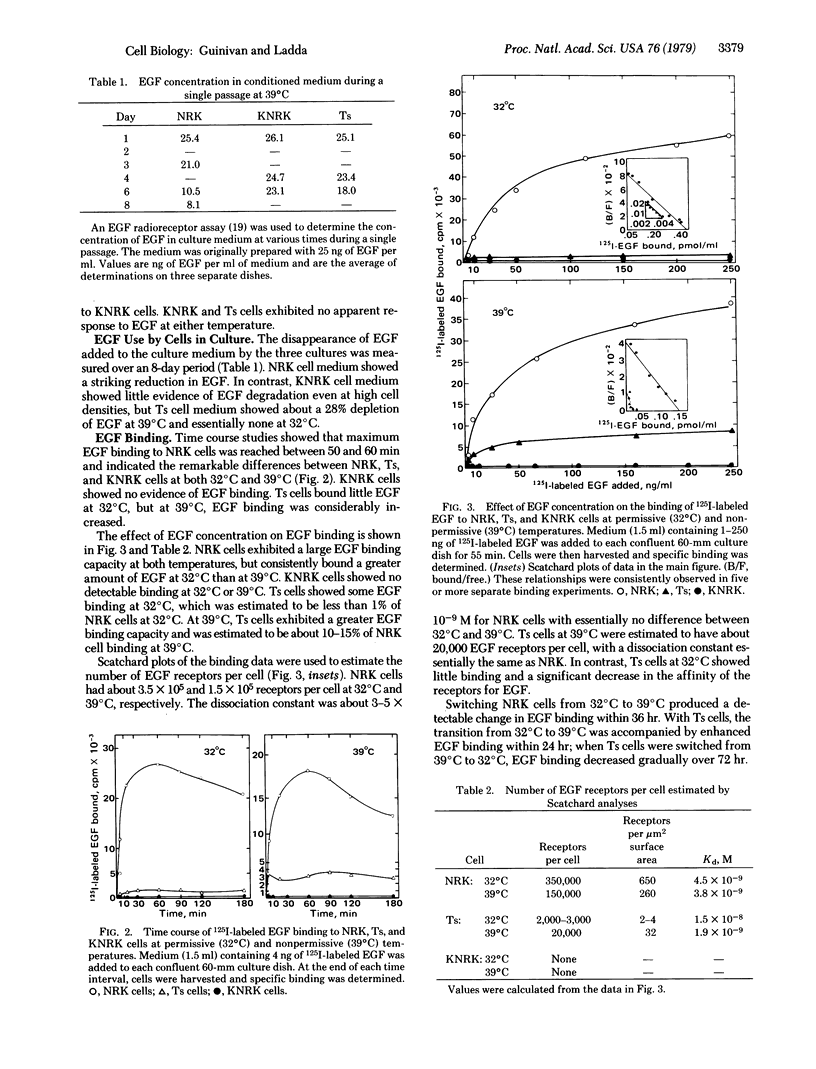
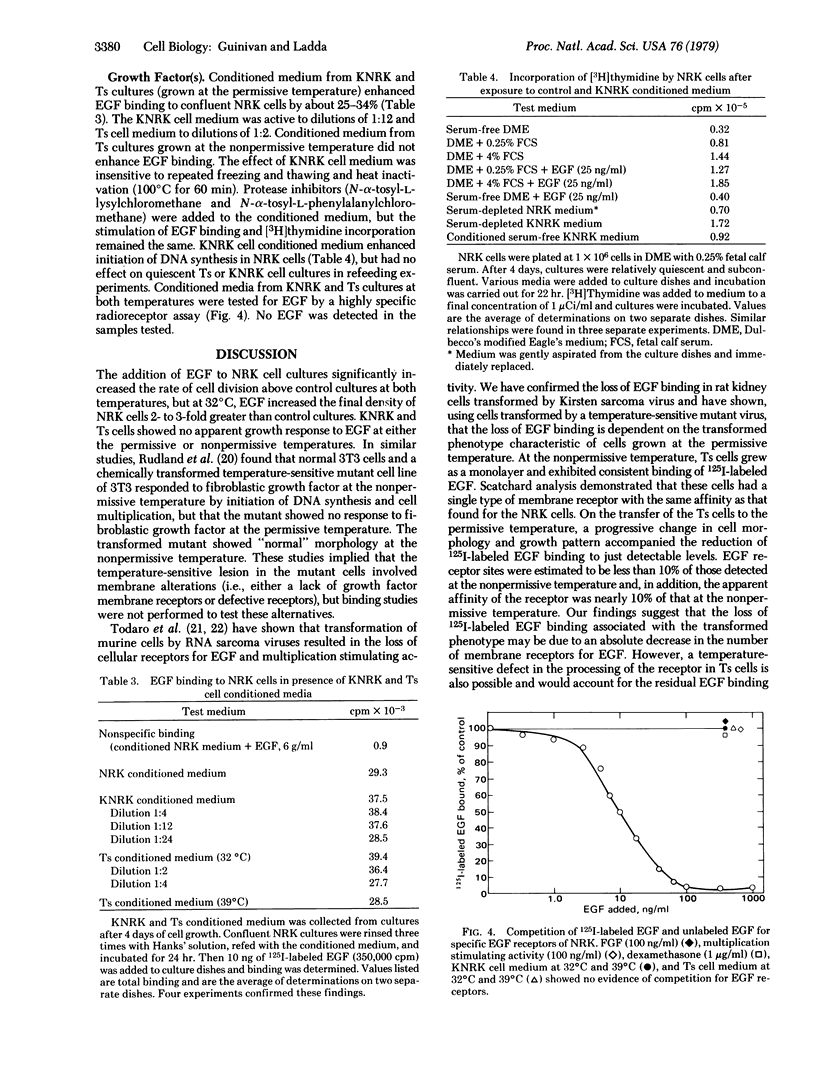
Normal rat kidney (NRK) cells infected with a temperature-sensitive mutant of Kirsten sarcoma virus (Ts cells) exhibited normal monolayer morphology identical to that observed for uninfected cells (NRK cells) at the nonpermissive temperature, 39 degrees C, but grew as multilayered foci resembling NRK cells transformed by the wild-type virus (KNRK cells) at 32 degrees C, the permissive temperature. NRK cell division was stimulated by epidermal growth factor (EGF), and these cells showed high levels of EGF receptors, as determined by 125I-labeled EGF binding. KNRK cells were unresponsive to EGF and no EGF receptors were detectable. Ts cells also were unresponsive to EGF at both temperatures, but exhibited just detectable EGF binding at 32 degrees C and 10-15% of NRK cell binding at 39 degrees C. Use of EGF added to the culture medium by these cells paralleled the receptor levels. Crossfeeding experiments among NRK, KNRK, and Ts cultures indicated that Ts cells at the permissive temperature and KNRK cells at both temperatures produced a heat-stable substance(s) which stimulated DNA synthesis in NRK cells independent of the presence of serum or of EGF. Conditioned medium from the transformed cultures also significantly enhanced EGF binding to NRK cells. These studies demonstrated a correlation between the transformed phenotype and the receptor levels of a potent cell mitogen, EGF, which was readily reversible in the Ts cultures. In addition, cultures expressing the transformed phenotype produced material that did not compete for the EGF receptor but did enhance EGF binding, in contrast to other reports involving sarcoma virus-transformed cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962 May;237:1555–1562. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Carpenter G. Human epidermal growth factor: isolation and chemical and biological properties. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1317–1321. doi: 10.1073/pnas.72.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M., Miyakawa T., Fox C. F., Pruss R. M., Aharonov A., Herschman H. R. Specific radiolabeling of a cell surface receptor for epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2790–2794. doi: 10.1073/pnas.74.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature. 1974 May 10;249(453):123–127. doi: 10.1038/249123a0. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J. S. Mitogenic effect of fibroblast growth factor on early passage cultures of human and murine fibroblasts. J Cell Biol. 1975 Aug;66(2):451–457. doi: 10.1083/jcb.66.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Epidermal growth factor: receptors in human fibroblasts and modulation of action by cholera toxin. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2964–2968. doi: 10.1073/pnas.70.10.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladda R. L., Bullock L. P., Gianopoulos T., McCormick L. Radioreceptor assay for epidermal growth factor. Anal Biochem. 1979 Mar;93(2):286–294. doi: 10.1016/s0003-2697(79)80153-0. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. The cell surface and fibroblast proliferation some current research trends. Biochim Biophys Acta. 1975 Jul 11;417(2):153–172. doi: 10.1016/0304-419x(75)90003-7. [DOI] [PubMed] [Google Scholar]
- Paul D., Lipton A., Klinger I. Serum factor requirements of normal and simian virus 40-transformed 3T3 mouse fibroplasts. Proc Natl Acad Sci U S A. 1971 Mar;68(3):645–652. doi: 10.1073/pnas.68.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudland P. S., Eckhart W., Gospodarowicz D., Seifert W. Cell transformation mutants are not susceptible to growth activation by fibroblast growth factor at permissive temperatures. Nature. 1974 Jul 26;250(464):337–339. doi: 10.1038/250337a0. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Scolnick E. M., Stephenson J. R., Aaronson S. A. Isolation of temperature-sensitive mutants of murine sarcoma virus. J Virol. 1972 Oct;10(4):653–657. doi: 10.1128/jvi.10.4.653-657.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert W. E., Rudland P. S. Possible involvement of cyclic GMP in growth control of cultured mouse cells. Nature. 1974 Mar 8;248(5444):138–140. doi: 10.1038/248138a0. [DOI] [PubMed] [Google Scholar]
- Stoker M. G. Role of diffusion boundary layer in contact inhibition of growth. Nature. 1973 Nov 23;246(5430):200–203. doi: 10.1038/246200a0. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Stimulation by serum of multiplication of stationary chicken cells. J Cell Physiol. 1971 Oct;78(2):161–170. doi: 10.1002/jcp.1040780202. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E., Cohen S. Transformation by murine and feline sarcoma viruses specifically blocks binding of epidermal growth factor to cells. Nature. 1976 Nov 4;264(5581):26–31. doi: 10.1038/264026a0. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E., Nissley S. P., Rechler M. M. MSA and EGF receptors on sarcoma virus transformed cells and human fibrosarcoma cells in culture. Nature. 1977 Jun 9;267(5611):526–528. doi: 10.1038/267526a0. [DOI] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]



