Abstract
Influenza viruses can take on two distinct morphologies: filamentous or spherical. While the functional significance of each virion type is unclear, filaments are generally observed in low-passage-number isolates, while an exclusively spherical morphology is seen in strains grown extensively in laboratory substrates. Previous studies have shown that filamentous morphology is lost upon passage in eggs. The fact that the filamentous morphology is maintained in nature but not in the laboratory suggests that filaments provide an advantage in the host that is not necessary for growth in laboratory substrates. To test this hypothesis and identify naturally occurring mutations that alter morphology, we examined the effect of serial adaptation in eggs, MDCK cells, and guinea pigs. Two filamentous strains, A/Netherlands/602/2009 (H1N1) and A/Georgia/M5081/2012 (H1N1), were passaged in eggs and MDCK cells. Conversely, the spherical laboratory strain A/Puerto Rico/8/1934 (H1N1) was passaged in guinea pigs. We found that although passage in eggs and MDCK cells can lead to a loss of filaments, an exclusively spherical morphology is not required for highly efficient growth in either substrate. We did, however, identify two point mutations in the matrix of egg passage 10 isolates that confer spherical morphology and increased growth in eggs. In contrast, serial passage in guinea pigs resulted in the selection of filament-forming variants. Sequencing revealed point mutations to the PR8 matrix that, when introduced individually, yielded filaments. These findings suggest a functional role for filaments in the infected host and expand the breadth of mutations known to affect influenza virus shape.
INTRODUCTION
Influenza A virus is an enveloped, negative-sense, RNA virus with an eight-segmented genome (1). In humans, these viruses cause widespread seasonal epidemics of respiratory disease, as well as occasional pandemics, the most recent of which occurred in 2009 (2).
Early research found that influenza virus is pleomorphic, forming both filamentous and spherical virions (3). Filaments are generally found in primary or low-passage-number isolates, and their formation by influenza viruses of both avian and human origin has been reported (4–6). These filaments are of various lengths but can reach up to 30 μm in size (7). While populations of spherical virions measuring about 100 nm in diameter are also observed in primary isolates, laboratory-adapted strains, such as A/Puerto Rico/8/1934 (H1N1) (PR8) and A/WSN/1933 (H1N1) (WSN), are made up exclusively of spherical and ovoid particles (7).
Early studies revealed that filamentous strains gradually become spherical following repeated passage in embryonated chicken eggs (ECEs) (8, 9). Whether this conversion also occurs upon passage in MDCK cells is unclear, as we were unable to find data on this point reported in the literature. More recently, reverse genetics-based mapping studies demonstrated that the M1 protein is a major genetic determinant of virion morphology (10, 11). Additionally, it has been shown that the cytoplasmic tails of the M2 proton channel, hemagglutinin (HA), and neuraminidase (NA) proteins influence the morphology of influenza virus (12, 13).
The fact that filaments are maintained in nature but lost upon passage in ECEs implies that filaments confer a selective advantage in the infected host that is not necessary for growth in the laboratory. While several studies have identified amino acid changes that mediate the switch from a filamentous to spherical morphology and vice versa, these mutations were identified via the artificial methods of alanine scanning (14) and substitution of differing amino acids from strains of the opposite morphology (10, 11). To our knowledge, naturally arising mutations that impact virion morphology have not been reported.
We aimed to evaluate the hypothesis that filamentous virion morphology confers a selective advantage in vivo, while viruses with exclusively spherical morphology are more fit in laboratory substrates. Since introduction of artificial mutations that are known to change virion morphology (10, 11, 14) was found to lead to general attenuation (data not shown), we undertook serial passage experiments aimed at identifying naturally occurring mutations that alter morphology. The passage experiments themselves offered insight into the interplay between virion morphology and viral fitness by revealing which morphologies are selected for in ECEs, MDCK cells, and guinea pigs. When two human strains with mixed filamentous and spherical morphology were passaged in ECEs or MDCK cells, we found that while filaments were not always maintained, an exclusively spherical morphology was not necessary for increased growth in laboratory substrates. Conversely, serial passage of the spherical PR8 virus in guinea pigs led to the emergence of filamentous virions, suggesting that filaments confer a selective advantage in the infected animal host. Through sequencing of egg- and guinea pig-passaged viruses, respectively, we identified point mutations in the M1 matrix protein that convert the NL602 virus to a spherical morphology or cause the PR8 virus to form filaments. The resultant mutant viruses were then characterized in ECEs, MDCK cells, and guinea pigs.
MATERIALS AND METHODS
Viruses.
rNL602wt, rPR8wt, rNL602 M1 T169I, rNL602 M1 Q198K, rPR8 M1 N87S, rPR8 M1 N92S, rPR8 M1 R101G, and rPR8 M1 S157C viruses were generated using reverse genetics essentially as previously described (15, 16). In brief, rNL602-based viruses were recovered by 8 (pHW) plasmid transfection of 293T cells and subsequent coculture with MDCK cells. rPR8-based viruses were recovered by 8 (pDZ) plasmid transfection of 293T cells and subsequent injection of transfected cells and culture medium into 9- to 11-day-old embryonated chicken's eggs. rNL602 virus and mutants thereof were grown in MDCK cells, and rPR8 virus and mutants were grown in 9- to 11-day-old embryonated chicken's eggs. A/Georgia/M5081/2012 (H1N1) (M5081), A/Georgia/F32551/2012 (H1N1) (F32551), and A/Georgia/T51700/2012 (H1N1) (T51700) viruses were isolated from distinct clinical specimens obtained from the microbiology laboratory of Children's Healthcare of Atlanta. F32551 and T51700 viruses were isolated through direct inoculation of differentiated human tracheobronchial epithelial (HTBE) cells. In the case of M5081 virus, a plaque assay in MDCK cells was performed with the nasal swab material, a single plaque was isolated, and this plaque material was amplified in HTBE cells to generate a working stock.
Serial passage.
Both rNL602wt and M5081wt viruses were passaged blindly 10 times in embryonated chicken eggs and in MDCK cells. For the egg passages, six replicate passages were run for each virus. The initial inoculum was 250 PFU/egg. In subsequent passages, eggs were inoculated with 100 μl undiluted allantoic fluid from the previous passage. Inoculated eggs were incubated at 37°C for 48 h and then at 4°C overnight prior to collection of allantoic fluid.
For the MDCK cell passage, three replicate passages were run for each virus. Initially, cells were infected at a multiplicity of infection (MOI) of 0.05 PFU/cell. In subsequent passages, cells were inoculated with a 1:10 dilution of cell culture supernatant from the previous passage. Cells were incubated for 48 h at 33°C, after which the cell culture supernatant was collected. To test whether the use of undiluted allantoic fluid or culture supernatant obscured the emergence of spherical variants at low levels, we performed an additional three passages in each substrate, with each virus lineage, performing a 10−3 dilution prior to passage. The resultant passage 13 (P13) populations of virus showed morphology similar to their P10 counterparts (data not shown).
rPR8wt virus was passaged 12 times in guinea pigs. At each passage, a single guinea pig was infected intranasally with 104 PFU of virus in 300 μl of phosphate-buffered saline (PBS). Nasal wash samples were collected in PBS at 4 days postinfection, the titers were determined via plaque assay in MDCK cells, and then they were used as the inoculum for the next passage. If the titer was too low to permit inoculation with 104 PFU, undiluted nasal wash was used.
M segment sequencing.
Viral RNA was extracted from rNL602 EP10 plaque clones or rPR8 guinea pig P12 nasal wash fluid using the QIAamp viral RNA mini kit (Qiagen) according to the manufacturer's instructions. cDNA of the M segment was generated using Transcriptor reverse transcriptase (Roche) and a universal forward primer for the M segment (17). The resulting product was then amplified using the Expand high-fidelity PCR system (Roche) and universal forward and reverse primers for the M segment (17). PCR products were then extracted from agarose gel slices using the QIAquick gel extraction kit (Qiagen) and sequenced directly (Genewiz).
Transmission electron microscopy (TEM).
For imaging of virions, MDCK cells were infected at an MOI of 5 PFU/cell. At 16 h postinfection, cells were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer for 2 to 3 h at room temperature or overnight at 4°C. If, due to low stock titers, an MOI of 5 PFU/cell could not be achieved, cells were infected at the highest possible MOI and incubated for 24 h prior to fixing. Cells were then embedded in Eponate 12 resin, cut into 80-nm sections, and stained with 5% uranyl acetate and 2% lead citrate at the Emory Robert P. Apkarian Integrated Electron Microscopy Core. After sample preparation, grids were imaged at 75 kV using a Hitachi H-7500 transmission electron microscope.
TEM particle counts.
Virions within TEM fields were counted at a magnification of ×40,000. For each virus, between 47 and 207 virions were counted. Filaments were defined as being equal to or greater than 300 nm in length. Virions shorter than 300 nm in length were defined as spheres. Empty virions were additionally counted as spheres, although such particles could represent a cross-section through a filamentous virion. From these counts, the percentage of spherical and filamentous virions was calculated. The difference in proportions test was used to determine if the proportion of virions that were filamentous was significantly different from that of the wild type. Results were considered significant if P < 0.05.
Growth curves.
Growth curves in MDCK cells were performed in triplicate using an MOI of 0.001 PFU/cell. Tissue culture supernatant was collected at 1, 6, 12, 24, 48, and 72 h postinfection. Virus titer was determined via plaque assay in MDCK cells. Growth curves in 9- to 11-day-old embryonated chicken eggs were performed using three eggs/virus/time point. Each egg was infected with 250 PFU of virus. At each time point (1, 12, 24, 48, and 72 h postinfection), eggs were placed at 4°C to halt virus growth. Allantoic fluid was collected the next day from chilled eggs, and virus titer was quantified via plaque assay in MDCK cells.
Growth experiments in guinea pigs.
Female Hartley strain guinea pigs weighing 300 to 350 g were obtained from Charles River Laboratories. Four animals were infected intranasally with 1,000 PFU of each virus in 300 μl of PBS. Nasal washes were collected in PBS on days 2, 4, 6, and 8 postinfection, and virus titer was quantified via plaque assay in MDCK cells.
Transmission experiments in guinea pigs.
Four guinea pigs were infected intranasally with 1,000 PFU of each virus in 300 μl of PBS. Animals were then housed in environmental chambers (Caron model 6040) kept at constant temperature (10°C) and relative humidity (20%). Twenty-four hours postinfection, an uninfected guinea pig was cohoused with each infected guinea pig. Nasal washes were collected in PBS on days 2, 4, 6, and 8 postinfection, and virus titer was quantified via plaque assay in MDCK cells. All animal experiments were carried out according to the guidelines and with the approval of the Emory Institutional Animal Care and Use Committee (IACUC).
RESULTS
Human clinical influenza virus isolates produce filamentous virions.
To confirm that low-passage-number human influenza viruses, and particularly the strains that we were working with, have a filamentous morphology, thin-section transmission electron microscopy (TEM) was performed. MDCK cells infected with each of the following five strains was examined: (i) the A/Netherlands/602/2009 (H1N1) (NL602) virus biological isolate, grown in MDCK cells (passage 3); (ii) the recombinant A/Netherlands/602/2009 (H1N1) (rNL602) virus, generated by reverse genetics and amplified in MDCK cells (18); (iii) A/Georgia/M5081/2012 (H1N1) (M5081) virus, which was isolated from a nasal swab specimen passaged once in MDCK cells and again in differentiated human tracheobronchial epithelial (HTBE) cells; (iv) A/Georgia/T51700/2012 (H1N1) (T51700) virus, isolated through direct inoculation of HTBE cells; and (v) A/Georgia/F32551/2012 (H1N1) (F32551) virus, also isolated in HTBE cells. Our results (Fig. 1A to E) indicate that each of these human viruses produced virions of filamentous morphology, confirming that this property of clinical isolates is not unique to those strains previously described by others (6, 19).
Fig 1.

Low-passage-number human influenza virus isolates have filamentous morphology. Transmission electron micrographs are shown at a magnification of 60,000× for NL602wt (biological isolate) (A), rNL602wt (recombinant) (B), M5081wt (C), T51700wt (D), and F32551wt (E).
Spherical morphology is not required for increased growth in embryonated chicken eggs.
To investigate the effect of adaptation to ECEs on the morphology of low-passage-number human influenza virus isolates, we passaged rNL602wt virus blindly a total of 10 times in this substrate. Passage was performed in six parallel replicates, which are referred to as “lines” here. Provided all six lines persisted after passage 10 (P10), only three were picked for further analysis. For the purposes of this analysis, we defined spheres as being virions less than 300 nm in length. Filaments were defined as being equal to or greater than 300 nm in length.
In the case of rNL602, virus was recovered from all six lines after P10. Those with the highest viral titers, lines 2, 5, and 6, were chosen for further analysis. Virion morphology compared to the NL602wt virus was assessed using thin-section TEM of MDCK cells infected at high MOI. We found that all three of the P10 lines were comparable in morphology to the wild-type virus, showing both short and long filaments, with a small population of spheres (Fig. 2A to H and Q). This result was surprising, as previous studies have reported conversion to a predominantly spherical morphology in some strains in 10 passages or fewer (9).
Fig 2.
Ten egg passages led to loss of filaments for M5081 but not rNL602 virus. Transmission electron micrographs are shown of rNL602wt and M5081wt viruses compared to egg passage 10 (EP10) lines. All images at a magnification of 40,000× for rNL602wt (A and B), rNL602 EP10 line 2 (C and D), rNL602 EP10 line 5 (E and F), rNL602 EP10 line 6 (G and H), M5081wt (I and J), M5081 EP10 line 2 (K and L), M5081 EP10 line 4 (M and N), and M5081 EP10 line 5 (O and P). (Q) Particle count of rNL602wt compared to EP10 lines. (R) Particle counts of M5081wt compared to EP10 lines. An asterisk indicates that the proportion of filamentous virions was significantly different from that of the wt virus (P < 0.05 by difference in proportions test).
To investigate the possibility that the passage number required for morphology change is strain dependent, we also passaged a clinical strain, M5081, 10 times in ECEs. Prior to passage, M5081 virus was confirmed to be filamentous in nature by TEM, with many short filaments and occasional long filaments (Fig. 1C). Only 3/6 lines were recovered after P10: lines 2, 4, and 5. Upon thin-section TEM analysis, we found that, within all three EP10 populations, most virions were spherical in morphology (Fig. 2I to P and R). Thus, in the case of M5081 virus, serial passage in ECEs did appear to have an effect on virion morphology, suggesting that the rate of morphology change in response to passage is strain specific.
We next compared the growth of the wild-type and passaged strains of both rNL602 and M5081 viruses in ECEs. We found that both rNL602- and M5081-passaged viruses had marked growth advantages over the corresponding wild-type viruses, showing more rapid growth kinetics and higher viral titers (Fig. 3). These results show that conversion to a spherical morphology is not required for increased growth in ECEs, because although the morphology of M5081 virus appeared to be affected by passage, the rNL602-passaged lines still remained highly filamentous in morphology.
Fig 3.

EP10 lines show improved growth in embryonated chicken eggs. Eggs were infected with 250 PFU of the indicated viruses. (A) rNL602wt versus rNL602 EP10 lines; (B) M5081wt versus M5081 EP10 lines.
Spherical morphology is not required for increased growth in MDCK cells.
We also wanted to assess the effect of virus passage in MDCK cells, another common laboratory substrate for influenza virus. As with the ECEs, we passaged both rNL602wt and M5081wt viruses blindly 10 times in MDCK cells. Passage was done in 6-well plates with three parallel replicates (i.e., lines) for each virus.
Following 10 passages, the morphology of each virus line was compared to that of the wild type using thin-section TEM of infected MDCK cells. We found that, in the case of the rNL602 strains, 2/3 P10 lines remained highly filamentous in morphology, with filaments of various lengths present on the surface of infected cells (Fig. 4A to H and Q). In the case of M5081 virus, we found that line 3 consisted of virions that were mostly spheres or short filaments. No long filaments were observed. Lines 1 and 2, however, retained a morphology that was very similar to that of the wild type (Fig. 4I to P and R).
Fig 4.
Ten serial passages in MDCK cells led to a loss of filaments for two of six lineages. Transmission electron micrographs are shown of rNL602wt and M5081wt viruses compared to MDCK passage 10 (CKP10) lines. All images are at a magnification of 40,000×. (A and B) rNL602wt; (C and D) rNL602 CKP10 line 1; (E and F) rNL602 CKP10 line 2; (G and H) rNL602 CKP10 line 3; (I and J) M5081wt; (K and L) M5081 CKP10 line 1; (M and N) M5081 CKP10 line 2; (O and P) M5081 CKP10 line 3. (Q) Particle count of rNL602wt compared to CKP10 lines. (R) Particle counts of M5081wt compared to CKP10 lines. An asterisk indicates that the proportion of filamentous virions was significantly different from that of the wt virus (P < 0.05 by difference in proportions test).
We next compared the growth of the P10 lines to that of the wild type in MDCK cells for both rNL602 and M5081 viruses. We found that, in the case of the rNL602 strain, the P10 lines displayed a modest growth advantage compared to the wild type, showing an approximately 10-fold increase at 48 h (Fig. 5A). The M5081 virus P10 lines exhibited a larger growth advantage (∼1,000-fold increase at 48 h) compared to the M5081wt virus (Fig. 5B). The differences between the rNL602 and the M5081 growth comparisons appear to be due to the more efficient growth in MDCK cells of the rNL602wt virus than the M5081wt virus. The rNL602wt virus may exhibit some adaptation to MDCK cells due to the fact that this stock was generated in MDCK cells, while the M5081 virus was grown in HTBE cells. As passaging only appeared to have an effect on the virion morphology of 2/6 P10 lines, the results show that, as was found in ECEs, a conversion to a spherical morphology is not necessary for increased growth in MDCK cells.
Fig 5.

CKP10 lines show improved growth in MDCK cells. Cells were infected at an MOI of 0.001 PFU/cell. (A) rNL602wt versus rNL602 CKP10 lines; (B) M5081wt versus M5081 CKP10 lines.
rNL602 M1 T169I and Q198K confer a spherical morphology and a growth advantage in ECEs.
Partial genome sequencing of plaque clones isolated from rNL602 EP10 lines revealed three mutations, two in the M1 matrix protein (T169I and Q198K) and one in the M2 ion channel protein (E70K). The M segments of six plaque clones were sequenced per line, and each mutation was identified in a single clone. The M1 T169I and M1 Q198K mutations were identified in separate clones of rNL602 EP10 line 2. The M2 E70K mutation was identified in a clone of line 6. Since the M segment gene products have been implicated previously in determining morphology, we wished to test whether these three amino acid changes would alter virion morphology. Therefore, we introduced each mutation into the M segment of rNL602wt virus using reverse genetics to generate three mutant viruses. When we examined these viruses using thin-section TEM of infected MDCK cells, we found that the M1 T169I virus produced spherical virions as well as short filaments. The majority of virions produced by the M1 Q198K mutant were also spherical, with very few filaments present. Lastly, the E70K mutant was found to be highly filamentous in morphology, similar to the rNL602wt virus; thus, it was excluded from further study (Fig. 6).
Fig 6.

T169I and Q198K mutations in the rNL602 M1 protein result in a predominantly spherical morphology. Transmission electron micrographs are shown of rNL602wt and mutant viruses. All images are at a magnification of 60,000×. (A and B) rNL602wt; (C and D) rNL602 M1 T169I; (E and F) rNL602 M1 Q198K; (G and H) rNL602 M2 E70K. (I) Particle counts of rNL602wt compared to mutant viruses. An asterisk indicates that the proportion of filamentous virions was significantly different from that of the wild-type virus (P < 0.05 by difference in proportions test).
We next compared the growth of the M1 T169I and Q198K mutants to that of rNL602wt virus in ECEs, MDCK cells, and guinea pigs. We found that both mutant viruses had a growth advantage in ECEs over the rNL602wt virus. In addition to higher peak titers, the mutant viruses displayed kinetics of growth similar to those of the EP10 lines (Fig. 7A). However, when growth of the wild-type virus in MDCK cells was compared to that of the mutant viruses, it was observed that both the M1 T169I and Q198K mutants had a mild growth defect in this substrate (Fig. 7B). A growth defect was also observed when the growth of the mutant viruses was compared to the growth of the wild type in guinea pigs (Fig. 7C). These observations suggest that both mutations are egg-specific adaptations. Thus, despite the finding that a spherical morphology is not necessary for increased growth in laboratory substrates, mutations arising naturally over the course of passage were found to convert the virus to the spherical phenotype and lead to a growth advantage in ECEs.
Fig 7.

Compared to the wild-type virus, rNL602 M1 T169I and M1 Q198K mutant viruses show improved growth in eggs but inferior growth in MDCK cells and guinea pigs. (A) Growth in eggs. Eggs were infected with 250 PFU of the indicated viruses. (B) Growth in MDCK cells. Cells were infected at an MOI of 0.001 PFU/cell. (C) Shedding from guinea pig nasal passages. Guinea pigs were infected intranasally with 1,000 PFU of the indicated viruses. Nasal washes were taken on days 2, 4, 6, and 8, and virus titer was quantified via plaque assay in MDCK cells.
Additionally, we wished to compare the transmission of the rNL602 mutant viruses to that of the wild type using a guinea pig contact transmission model. We found that the wild-type virus showed transmission to 3/4 contacts by day 4 and all contacts by day 6. In contrast, transmission of the mutant viruses was not detected until day 6. By day 8, all three viruses had reached 100% transmission (Fig. 8). The observed delay in transmission of the mutant viruses correlated with their delayed growth in guinea pigs, suggesting that the M1 T169I and Q198K mutations altered growth kinetics, and this effect, in turn, influenced the rate of transmission.
Fig 8.

Compared to the wild-type virus, rNL602 M1 T169I and M1 Q198K mutant viruses show delayed growth and transmission in guinea pigs. Contact transmission of rNL602wt (A), rNL602 M1 T169I (B), and rNL602 M1 Q198K (C). Guinea pigs were infected intranasally with 1,000 PFU of the indicated viruses. At 24 h postinfection, an uninfected guinea pig was housed with an infected guinea pig. Guinea pigs were housed in environmental chambers at constant temperature (10°C) and relative humidity (20%). Nasal washes were taken on days 2, 4, 6, and 8, and virus titer was quantified via plaque assay in MDCK cells. Dashed lines represent initially infected animals. Solid lines represent exposed animals.
Serial passage in guinea pigs leads to emergence of filamentous virions.
In order to investigate the effects of serial passage in vivo on the morphology of a laboratory-adapted strain, we passaged rPR8wt virus 12 times in guinea pigs. Briefly, female Hartley guinea pigs were inoculated intranasally with 1 × 104 PFU of rPR8wt virus. At 4 days postinfection, nasal washes were collected. Following titration, 1 × 104 PFU of nasal wash was used for the next passage. In the event that this inoculum titer could not be attained, undiluted nasal wash was used instead.
After 12 passages, we assessed the morphology of the P12 virus using thin-section TEM of infected MDCK cells. While the rPR8wt-infected samples showed uniformly spherical virions (Fig. 9A), we observed the emergence of filamentous virions in the P12-infected samples (Fig. 9B to D). This emergence of filaments upon serial passage in guinea pigs indicates that a filamentous morphology is selected within an animal host, supporting our hypothesis that filaments play a functional role in vivo.
Fig 9.

Serial passage in guinea pigs led to the emergence of rPR8 virus variants with filamentous morphology. Transmission electron micrographs and growth of rPR8wt and rPR8 guinea pig passage 12 (P12) viruses. All TEM images are at a magnification of 60,000×. (A) rPR8wt virus; (B and D) rPR8 GP P12 virus. (E) Growth analysis in vivo. Guinea pigs were infected intranasally with 1,000 PFU of each virus. Nasal washes were taken on days 2, 4, 6, and 8, and virus titer was quantified via plaque assay in MDCK cells. An asterisk indicated that titers of the P12 virus were significantly greater than those of rPR8wt on day 2 (P = 0.022 by t test), while titers of rPR8wt were greater than those of P12 on day 4 (P = 0.035 by t test).
We next compared the growth of the P12 virus to that of the wild-type rPR8wt virus. We inoculated four guinea pigs intranasally with 1,000 PFU of either rPR8wt or P12 in 300 μl PBS. Nasal washes were taken at days 2, 4, 6, and 8 postinfection, and viral titer was determined via plaque assay on MDCK cells. We found that the P12 virus had a mild but statistically significant growth advantage at day 2 in guinea pigs compared to rPR8wt virus (Fig. 9E). These results, coupled with the emergence of filamentous virions in the P12 samples, suggest that filaments play a functional role within the infected host.
Mutations identified in the guinea pig P12 virus matrix protein result in robust filament formation.
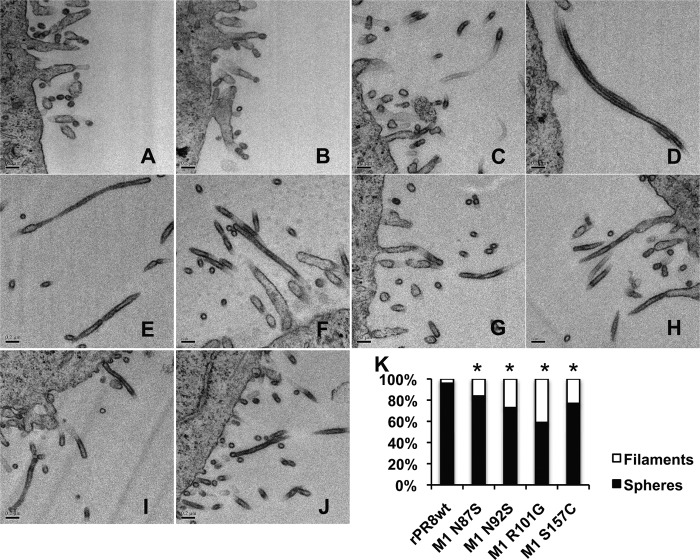
Through sequencing of the M segment of the guinea pig P12 virus pool, we identified seven nucleotide positions with two overlapping peaks, suggesting heterogeneity within the virus population at these sites. All seven were in the M1 matrix protein. We introduced each mutation individually into the cDNA of the PR8 M segment, and using reverse genetics, we were able to rescue mutant viruses from four of these plasmids. Examination of the four mutant viruses using thin-section TEM revealed that, compared to the wild type, all showed robust filament formation, producing significantly more filaments than rPR8wt (Fig. 10).
Fig 10.
Point mutations in the PR8 M1 protein result in robust filament formation. Transmission electron micrographs are shown of rPR8wt and mutant viruses. All images are at a magnification of 60,000×. (A and B) rPR8wt; (C and D) rPR8 M1 N87S; (E and F) rPR8 M1 N92S; (G and H) rPR8wt M1 R101G; (I and J) rPR8wt M1 S157C. (K) Particle counts of rPR8wt compared to mutant viruses. An asterisk indicates that the proportion of filamentous virions was significantly different from that of the wt virus (P < 0.05 by difference in proportions test).
We next compared the growth of the mutant viruses to the wild type in embryonated chicken eggs, MDCK cells, and guinea pigs. We found that in all three substrates, rPR8wt retained a growth advantage over most of the mutant viruses. The exception was the M1 N87S mutant, which grew comparably to rPR8wt in all three substrates (Fig. 11). As such, the N87S mutant was used in a contact transmission experiment in guinea pigs with rPR8wt and the PR8 GP P12 virus to determine if increased filament formation had an effect on virus transmission. Consistent with previously reported data, the rPR8wt virus did not transmit between contact guinea pigs (20). In contrast, the PR8 P12 virus transmitted to two of four contact guinea pigs (Fig. 12), indicating that mutations accumulated over the course of serial passage that promote transmission. Introduction of the N87S mutation alone, however, did not improve the transmissibility of the rPR8wt virus.
Fig 11.
Compared to the wild-type virus, rPR8 M1 mutant viruses show similar or inferior growth in eggs, MDCK cells, and guinea pigs. (A) Growth in eggs. Eggs were infected with 250 PFU of the indicated viruses. (B) Growth in MDCK cells. Cells were infected at an MOI of 0.001 PFU/cell. (C) Shedding from guinea pig nasal passages. Guinea pigs were infected intranasally with 1,000 PFU of the indicated viruses. Nasal washes were taken on days 2, 4, 6, and 8, and virus titer was quantified via plaque assay in MDCK cells.
Fig 12.

Compared to the rPR8wt virus, the rPR8 GP P12 virus transmits in guinea pigs while the rPR8 M1 N87S virus does not. Contact transmission results are shown for rPR8wt (A), rPR8 GP P12 (B), and rPR8 M1 N87S (C). Guinea pigs were infected intranasally with 1,000 PFU of the indicated viruses. At 24 h postinfection, an uninfected guinea pig was housed with an infected guinea pig. Animals were housed under controlled environmental conditions of 20% relative humidity and 10°C. Dashed lines represent initially infected animals. Solid lines represent exposed animals. Experiments shown in panels A and C were performed concurrently; the experiment shown in panel B was performed separately.
DISCUSSION
After the initial observation that serial passaging in ECEs had an effect on the morphology of influenza virus, little work has been done to elucidate why this phenomenon occurs or the mechanism behind it. Published observations showed the conversion of several strains of influenza virus to a predominantly spherical morphology by 10 passages in ECEs (9). Likewise, our results showed a shift in morphology favoring more spherical virions in all three EP10 lines of M5081. However, the rNL602 EP10 lines maintained a predominantly filamentous phenotype; this difference may be due to strain-specific requirements or the precise conditions of passaging. The idea of strain specificity is supported by the fact that some laboratory strains, such as A/Udorn/301/1972 (H3N2) and A/Victoria/3/1975 (H3N2), have retained their filamentous morphology despite being grown extensively in laboratory substrates (10, 11). To our knowledge, the effect of serial passage in MDCK cells on virion morphology has not been assessed. We found that 4 out of 6 of the CKP10 lines that were tested for both rNL602 and M5081 strains retained a highly filamentous morphology. The fact that all of the EP10 and CKP10 lines have a considerable growth advantage over their corresponding wild-type viruses, regardless of changes in morphology, indicates that a spherical morphology is not required for increased growth in laboratory substrates.
The presence of mutations that mediate a conversion to a spherical morphology within the populations of rNL602 EP10 strains suggests that some morphology transition took place, although the spherical variants were not readily detected by TEM. However, the fact that these mutant viruses retained a growth advantage in ECEs while showing attenuation in MDCK cells suggests that these mutations are adaptive specifically in eggs and not more generally in laboratory substrates. This finding agrees with our data indicating that 10 passages in MDCK cells brought about only a marginal change in morphology and in only one of the three rNL602 virus lineages. It should be noted, however, that although both the T169I and Q198K mutations confer a spherical morphology on the rNL602 virus and both improve growth in eggs, we have not demonstrated that these two effects are causally linked.
In contrast to the conversion from filamentous to spherical morphology through egg passage, we found that passage of a spherical, laboratory-adapted strain (rPR8) in an animal host led to the emergence of filamentous virions. The selection of filamentous variants in guinea pigs suggests that an elongated virion morphology confers a fitness advantage in vivo. Nevertheless, when point mutations identified within the rPR8 GP P12 virus population and leading to filament formation were introduced individually into the rPR8 virus, these mutations were found to attenuate the growth of the virus in guinea pigs. This result suggests that, to be advantageous, the individual changes to the M1 protein that we identified need to be coupled with complementary changes elsewhere in the genome. Similarly, although the rPR8 P12 virus population was observed to transmit to 2/4 contact guinea pigs, the filamentous mutant rPR8 M1 N87S did not transmit. This lack of transmission indicates that a filamentous morphology is not sufficient to confer a transmissible phenotype on the rPR8 virus, a finding that is not unexpected based on the multigenic nature of influenza virus transmission determinants (16, 20–28).
The amino acids within M1 that we found to affect virion morphology differ, in most cases, from those described previously by others. For example, both Compans et al. and Elleman and Barclay found position 41 to be important; Bourmakina and Garcia-Sastre found positions 95 and 204 to determine morphology; and Burleigh et al. found position 102 to affect virion shape (10–12, 14). That the amino acids described here differ most likely is due to our approach: we have identified mutations arising naturally during serial passage, whereas previous research focused on amino acids differing between selected spherical (e.g., WSN and PR8) and filamentous (e.g., A/Udorn/301/1972 and A/Victoria/3/75) strains. Taken together, it appears that a number of different elements within the M1 protein can alter virion morphology, an observation that most likely will not be explained until the mechanism by which M1 directs the formation of filaments or spheres is revealed.
Overall, the mutations identified here occur at highly conserved sites within the M1 protein; nevertheless, a small number of natural isolates carrying the same or similar mutations were found in the NCBI database. Through M1 protein sequence alignment we found that, among human H1N1 strains isolated in the United States between 2008 and 2013, two isolates contained the Q198K mutation (A/Boston/685/2009 and A/Hawaii/07/2010). None of the mutations described here were identified in pre-2008 human H1N1 isolates or in avian H1N1 isolates for which M1 protein sequence data are available in the NCBI database. Interestingly, through alignment of the M1 sequence of swine H1N1 strains, we found some isolates containing the N87S (A/swine/Iowa/46519_1/2007), R101G (A/swine/Iowa/15/1930), S157C (A/swine/Iowa/H04YS2/2004, A/swine/Iowa/H03G1/2003, and A/swine/Iowa/H03LJ10/2003), and T169I (A/swine/Ontario/53518/03) mutations. Whether the mutations identified in the NCBI database affect morphology in the context of the particular strains carrying them is, of course, unknown.
Through characterization of paired spherical and filamentous viruses differing at a single amino acid (rPR8wt versus rPR8 M1 N87S and rNL602wt versus rNL602 M1 I169T or Q198K) in a guinea pig transmission model, we hoped to test whether filamentous morphology improves influenza virus transmission. While the spherical rNL602 viruses showed delayed transmission relative to the rNL602wt virus, the kinetics of transmission mirrored the kinetics of shedding, suggesting that the M1 mutations affected viral growth rather than transmission directly. Similarly, the rPR8 M1 N87S mutation, when introduced individually, led to attenuated growth in vivo, and no transmission was seen with this filament-producing variant. Taken together, our data do not suggest a role for virion morphology in determining transmission phenotype; however, our data do not exclude this possibility. The virus strains we are working with are highly transmissible (rNL602) and completely nontransmissible (rPR8) in the guinea pig model; thus, while changes in morphology are not sufficient to alter their respective transmission phenotypes, in the appropriate context, morphology may contribute to transmission. Our finding that filaments are selected in guinea pigs (even in the absence of transmission between hosts) clearly suggests that filamentous virions are functionally significant in vivo. The precise function(s) of filament production by influenza viruses will be pursued further in subsequent work.
ACKNOWLEDGMENTS
We are grateful to Ron Fouchier for the A/Netherlands/602/2009 (H1N1) virus and reverse genetics system. We also thank Hong Yi and Jeannette Taylor of the Robert P. Apkarian Integrated Electron Microscopy Core for TEM sample processing and Anshante Jones and Shamika Danzy for technical assistance.
This work was funded by the Centers for Excellence in Influenza Research and Surveillance (CEIRS), contract number HHSN266200700006C.
Footnotes
Published ahead of print 2 October 2013
REFERENCES
- 1.Palese P, Shaw ML. 2007. Orthomyxoviridae: the viruses and their replication, p 1647–1689 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 2.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM, Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 360:2605–2615 [DOI] [PubMed] [Google Scholar]
- 3.Chu CM, Dawson IM, Elford WJ. 1949. Filamentous forms associated with newly isolated influenza virus. Lancet i:602. [DOI] [PubMed] [Google Scholar]
- 4.Gubareva LV, Bethell R, Hart GJ, Murti KG, Penn CR, Webster RG. 1996. Characterization of mutants of influenza A virus selected with the neuraminidase inhibitor 4-guanidino-Neu5Ac2en. J. Virol. 70:1818–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shortridge KF, Zhou NN, Guan Y, Gao P, Ito T, Kawaoka Y, Kodihalli S, Krauss S, Markwell D, Murti KG, Norwood M, Senne D, Sims L, Takada A, Webster RG. 1998. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology 252:331–342 [DOI] [PubMed] [Google Scholar]
- 6.Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, Muramoto Y, Tamura D, Sakai-Tagawa Y, Noda T, Sakabe S, Imai M, Hatta Y, Watanabe S, Li C, Yamada S, Fujii K, Murakami S, Imai H, Kakugawa S, Ito M, Takano R, Iwatsuki-Horimoto K, Shimojima M, Horimoto T, Goto H, Takahashi K, Makino A, Ishigaki H, Nakayama M, Okamatsu M, Takahashi K, Warshauer D, Shult PA, Saito R, Suzuki H, Furuta Y, Yamashita M, Mitamura K, Nakano K, Nakamura M, Brockman-Schneider R, Mitamura H, Yamazaki M, Sugaya N, Suresh M, Ozawa M, Neumann G, Gern J, Kida H, Ogasawara K, Kawaoka Y. 2009. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosley VM, Wyckoff RW. 1946. Electron micrography of the virus of influenza. Nature 157:263. [DOI] [PubMed] [Google Scholar]
- 8.Kilbourne ED, Murphy JS. 1960. Genetic studies of influenza viruses. I. Viral morphology and growth capacity as exchangeable genetic traits. Rapid in ovo adapation of early passage Asian strain isolates by combination with PR8. J. Exp. Med. 111:387–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choppin PW, Murphy JS, Tamm I. 1960. Studies of two kinds of virus particles which comprise influenza A2 virus strains. III. Morphological characteristics: independence to morphological and functional traits. J. Exp. Med. 112:945–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourmakina SV, Garcia-Sastre A. 2003. Reverse genetics studies on the filamentous morphology of influenza A virus. J. Gen. Virol. 84:517–527 [DOI] [PubMed] [Google Scholar]
- 11.Elleman CJ, Barclay WS. 2004. The M1 matrix protein controls the filamentous phenotype of influenza A virus. Virology 321:144–153 [DOI] [PubMed] [Google Scholar]
- 12.Roberts PC, Lamb RA, Compans RW. 1998. The M1 and M2 proteins of influenza A virus are important determinants in filamentous particle formation. Virology 240:127–137 [DOI] [PubMed] [Google Scholar]
- 13.Jin H, Leser GP, Zhang J, Lamb RA. 1997. Influenza virus hemagglutinin and neuraminidase cytoplasmic tails control particle shape. EMBO J. 16:1236–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burleigh LM, Calder LJ, Skehel JJ, Steinhauer DA. 2005. Influenza A viruses with mutations in the M1 helix six domain display a wide variety of morphological phenotypes. J. Virol. 79:1262–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fodor E, Devenish L, Engelhardt OG, Palese P, Brownlee GG, Garcia-Sastre A. 1999. Rescue of influenza A virus from recombinant DNA. J. Virol. 73:9679–9682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steel J, Lowen AC, Mubareka S, Palese P. 2009. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathog. 5:e1000252. 10.1371/journal.ppat.1000252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. 2001. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 146:2275–2289 [DOI] [PubMed] [Google Scholar]
- 18.Herfst S, Chutinimitjul S, Ye J, de Wit E, Munster VJ, Schrauwen EJ, Bestebroer TM, Jonges M, Meijer A, Koopmans M, Rimmelzwaan GF, Osterhaus AD, Perez DR, Fouchier RA. 2010. Introduction of virulence markers in PB2 of pandemic swine-origin influenza virus does not result in enhanced virulence or transmission. J. Virol. 84:3752–3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldsmith CS, Metcalfe MG, Rollin DC, Shieh WJ, Paddock CD, Xu X, Zaki SR. 2011. Ultrastructural characterization of pandemic (H1N1) 2009 virus. Emerg. Infect. Dis. 17:2056–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou YY, Albrecht RA, Pica N, Lowen AC, Richt JA, Garcia-Sastre A, Palese P, Hai R. 2011. The M segment of the 2009 new pandemic H1N1 influenza virus is critical for its high transmission efficiency in the guinea pig model. J. Virol. 85:11235–11241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Y, Zhang Y, Shinya K, Deng G, Jiang Y, Li Z, Guan Y, Tian G, Li Y, Shi J, Liu L, Zeng X, Bu Z, Xia X, Kawaoka Y, Chen H. 2009. Identification of amino acids in HA and PB2 critical for the transmission of H5N1 avian influenza viruses in a mammalian host. PLoS Pathog. 5:e1000709. 10.1371/journal.ppat.1000709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakdawala SS, Lamirande EW, Suguitan AL, Wang W, Santos CP, Vogel L, Matsuoka Y, Lindsley WG, Jin H, Subbarao K. 2011. Eurasian-origin gene segments contribute to the transmissibility, aerosol release, and morphology of the 2009 pandemic H1N1 influenza virus. PLoS Pathog. 7:e1002443. 10.1371/journal.ppat.1002443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yen HL, Liang CH, Wu CY, Forrest HL, Ferguson A, Choy KT, Jones J, Wong DD, Cheung PP, Hsu CH, Li OT, Yuen KM, Chan RW, Poon LL, Chan MC, Nicholls JM, Krauss S, Wong CH, Guan Y, Webster RG, Webby RJ, Peiris M. 2011. Hemagglutinin-neuraminidase balance confers respiratory-droplet transmissibility of the pandemic H1N1 influenza virus in ferrets. Proc. Natl. Acad. Sci. U. S. A. 108:14264–14269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumpey TM, Maines TR, Van Hoeven N, Glaser L, Solorzano A, Pappas C, Cox NJ, Swayne DE, Palese P, Katz JM, Garcia-Sastre A. 2007. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science 315:655–659 [DOI] [PubMed] [Google Scholar]
- 25.Imai M, Watanabe T, Masato H, Das SC, Ozawa M, Shinya K, Zhong G, Hanson A, Katsura H, Watanabe S, Li C, Kawakami E, Yamada S, Kiso M, Suzuki Y, Maher EA, Neumann G, Kawaoka Y. 2012. Experimental adaptation of an influenza H5 HA confers resipratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486:420–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2012. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336:1534–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Hoeven N, Pappas C, Belser JA, Maines TR, Zeng H, Garcia Sastre A, Sasisekharan R, Katz JM, Tumpey TM. 2009. Human HA and polymerase subunit PB2 proteins confer transmission of an avian influenza virus through the air. Proc. Natl. Acad. Sci. U. S. A. 106:3366–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maines TR, Chen LM, Matsuoka Y, Chen H, Rowe T, Ortin J, Falcon A, Nguyen TH, Mai LE, Sedyaningsih QER, Harun S, Tumpey TM, Donis RO, Cox NJ, Subbarao K, Katz JM. 2006. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc. Natl. Acad. Sci. U. S. A. 103:12121–12126 [DOI] [PMC free article] [PubMed] [Google Scholar]