Abstract
Chikungunya virus (CHIKV) is a reemerging mosquito-borne pathogen that causes incapacitating disease in humans characterized by intense joint pain that can persist for weeks, months, or even years. Although there is some evidence of persistent CHIKV infection in humans suffering from chronic rheumatologic disease symptoms, little is known about chronic disease pathogenesis, and no specific therapies exist for acute or chronic CHIKV disease. To investigate mechanisms of chronic CHIKV-induced disease, we utilized a mouse model and defined the duration of CHIKV infection in tissues and the associated histopathological changes. Although CHIKV RNA was readily detectable in a variety of tissues very early after infection, CHIKV RNA persisted specifically in joint-associated tissues for at least 16 weeks. Inoculation of Rag1−/− mice, which lack T and B cells, resulted in higher viral levels in a variety of tissues, suggesting that adaptive immunity controls the tissue specificity and persistence of CHIKV infection. The presence of CHIKV RNA in tissues of wild-type and Rag1−/− mice was associated with histopathological evidence of synovitis, arthritis, and tendonitis; thus, CHIKV-induced persistent arthritis is not mediated primarily by adaptive immune responses. Finally, we show that prophylactic administration of CHIKV-specific monoclonal antibodies prevented the establishment of CHIKV persistence, whereas therapeutic administration had tissue-specific efficacy. These findings suggest that chronic musculoskeletal tissue pathology is caused by persistent CHIKV infection and controlled by adaptive immune responses. Our results have significant implications for the development of strategies to mitigate the disease burden associated with CHIKV infection in humans.
INTRODUCTION
Chikungunya virus (CHIKV) is a mosquito-transmitted, positive-sense, single-stranded RNA virus in the Alphavirus genus of the Togaviridae family (1–3). CHIKV was first isolated from a patient during an outbreak of febrile disease and acute crippling joint pains in the southern province of Tanzania in 1952 and 1953 (4). Until 2004, CHIKV was known to cause debilitating rheumatologic disease in many parts of Sub-Saharan Africa and Asia (5). However, since 2004, CHIKV has caused a series of epidemics, which began in Kenya, spread to islands in the Indian Ocean and India, and now occur in Southeast Asia and the Pacific Region (6). These more recent outbreaks have resulted in millions of disease cases, and imported CHIKV infections have been reported in nearly 40 countries, including the United States, Brazil, Japan, and multiple European countries (7). In addition, CHIKV has adapted to new mosquito vectors (7), which has resulted in autochthonous transmission for the first time in several locations, including Italy, France, New Caledonia, Papua New Guinea, and Yemen (8, 9). This expanded epidemiology prompted the Pan American Health Organization and the Centers for Disease Control and Prevention to release a preparedness guide that anticipates CHIKV epidemics in the Americas (10).
The clinical manifestations following CHIKV infection include a sudden onset of fever, rash, intense pain in peripheral joints, myalgia, and impaired ambulation (11). This acute stage lasts for 1 to 2 weeks and is typically followed by defervescence and convalescence. However, in a subset of people infected with CHIKV, some disease signs and symptoms, such as joint swelling, joint stiffness, arthralgia, and tendonitis/tenosynovitis, can last for months to years and often occur in a relapsing/fluctuating manner (12–17). Chronic joint pain is not exclusive to CHIKV among alphavirus family members and also is caused by related viruses, including Sindbis (SINV), Ross River, o'nyong-nyong, and Mayaro viruses (1). Similar to CHIKV infections, the cause of persistent joint disease by these other alphaviruses is unclear; however, there is little evidence for the development of autoimmunity in individuals experiencing chronic disease (1, 11). Thus, an unresolved question in the field is whether chronic musculoskeletal disease is associated with or caused by persistent CHIKV infection. Several studies have detected persistence of CHIKV-specific immunoglobulin M (IgM) in humans, which is suggestive of, although by no means conclusive for, the persistence of viral antigens (12, 13, 18–20); however, the persistence of CHIKV-specific IgM has not yet been associated with persistent arthralgia or joint pathology (13). Additionally, immunohistochemical analysis of synovial and muscle tissues from patients with chronic disease revealed CHIKV antigen in perivascular macrophages and muscle satellite cells as well as extensive inflammation (12, 21). More recently, CHIKV RNA and antigens were detected up to 90 days postinoculation (dpi) in the spleen, lymph nodes, liver, and muscle tissue of infected macaques (22). Although CHIKV was detected in macaques inoculated with a range of virus doses, only those receiving the highest doses of virus developed musculoskeletal disease (22).
To investigate the basis of chronic CHIKV disease, we used a recently described mouse model in which the major disease signs (arthritis, synovitis, and tenosynovitis) during the acute stage were consistent with acute CHIKV disease in humans (23). Utilizing this model, we found that CHIKV RNA was cleared from visceral tissues of wild-type (WT) mice; however, CHIKV RNA persisted in joint-associated tissues to at least 16 weeks postinoculation (wpi). Rag1−/− mice, which lack mature B and T lymphocytes, sustained elevated levels of CHIKV RNA in joint-associated tissues, persistence of CHIKV RNA in muscle, and persistent viremia, suggesting that adaptive immune responses control persistent CHIKV infection. The persistence of CHIKV RNA in joint-associated tissues was associated with histopathological evidence of arthritis, synovitis, and tendonitis. Prophylactic administration of a combination of two highly neutralizing monoclonal antibodies (MAbs; CHK-166 and CHK-152) (24) prevented Rag1−/− mice from developing persistent CHIKV infection. Therapeutic administration of these MAbs at late times postinfection had tissue-specific efficacy in clearing CHIKV. Taken together, our findings suggest that chronic CHIKV musculoskeletal damage may be due to joint tissue-specific persistence of CHIKV infection. Our findings with Rag1−/− mice suggest that while the adaptive immune system is necessary for CHIKV clearance from muscle tissue and circulation, it cannot clear the virus from joint-associated tissues. This work establishes a small-animal model of chronic CHIKV infection that can be utilized to investigate molecular mechanisms of chronic disease pathogenesis as well as evaluate candidate therapies to mitigate persistent infection and joint pathology.
MATERIALS AND METHODS
Viruses.
The SL15649 strain of CHIKV (GenBank accession no. GU189061) was isolated from a serum sample collected from a febrile patient in Sri Lanka in 2006. This virus was passaged twice in Vero cells prior to generation of an infectious cDNA clone (23). Virus stocks were generated as previously described (23, 25). Stock virus titers were quantified by plaque assay on BHK-21 cells as previously described (23, 25). CHIKV strains 37997 and PO731460 were gifts of Ann Powers (Centers for Disease Control and Prevention, Fort Collins, CO). The PO731460 strain (GenBank accession no. HM045788) was isolated from a human patient in India in 1973 and passaged twice in Vero cells (6). The 37997 strain (GenBank accession no. AY726732) was isolated from Aedes furcifer mosquitoes in Senegal in 1983. This virus was passaged once in Aedes pseudoscutellaris (AP-61) cells and twice in Vero cells (26). Stock PO731460 and 37997 viruses were produced after a single passage in BHK-21 cells as previously described (27).
Mouse experiments.
C57BL/6J WT mice (stock number 000664) and congenic Rag1−/− mice (stock number 002216) were obtained from the Jackson Laboratory and bred in specific-pathogen-free facilities at the University of Colorado. Animal husbandry and experiments were performed in accordance and with approval of the University of Colorado School of Medicine Institutional Animal Care and Use Committee guidelines. All mouse infection studies were performed in an animal biosafety level 3 laboratory. Three-week-old mice were used for all studies. Mice were inoculated in the left rear footpad with 103 PFU of virus in diluent (phosphate-buffered saline [PBS] supplemented with 1% fetal bovine serum [FBS]) in a volume of 10 μl. Mock-infected animals received diluent alone. Mice were monitored for disease signs and weighed at 24-hour intervals. On the termination day of each experiment, mice were sedated with isoflurane and euthanized by thoracotomy and exsanguination, blood was collected, and mice were perfused by intracardiac injection of 1× PBS or 4% paraformaldehyde, depending on the experiment. PBS-perfused tissues were removed by dissection and homogenized in TRIzol reagent (Life Technologies) for RNA isolation or PBS-1% FBS for tissue titers using a MagNA Lyser (Roche). For prophylaxis studies, MAbs (200 μg each of CHK-152 and CHK-166 [24] or 400 μg of WNV E16 [anti-West Nile virus E protein MAb] [28]) were administered by intraperitoneal (i.p.) inoculation on days −1 and +3 as previously described (24). For therapeutic studies, MAbs were administered on days +21 and +25.
Real-time RT-qPCR.
RNA was isolated using a PureLink RNA minikit (Life Technologies), and the amounts of CHIKV positive-strand RNA present in tissues were quantified as previously described (25). Briefly, a CHIKV-specific primer (CHIKV1036, 5′-ggcagtatcgtgaattcgatgcCGTGTCGGTAGTCTTGCACAT-3′; lowercase letters indicate that this region of the primer sequence is not complementary to the CHIKV sequence) was used to prime reverse transcription. CHIKV sequence-specific forward (CHIKV874, 5′-AAAGGGCAAACTCAGCTTCAC-3′) and reverse (CHIKV961, 5′-GCCTGGGCTCATCGTTATTC-3′) primers were used with an internal TaqMan probe (CHIKV899, 5′-6-carboxyfluorescein [FAM]-CGCTGTGATACAGTGGTTTCGTGTG-MGB-3′) that amplified a region in the nsP1 gene for quantitative PCR on a LightCycler 480 (Roche). Samples from mock-infected mice served to ensure assay specificity. For absolute quantification of CHIKV RNA, a standard curve was generated: 10-fold dilutions from 108 to 100 copies of CHIKV positive-strand genomic RNA, synthesized in vitro, were spiked into RNA from BHK-21 cells, and reverse transcription (RT) and quantitative PCR (qPCR) were performed in an identical manner. No template controls were run in parallel. To quantify CHIKV RNA from multiple CHIKV genotypes, a modified RT-qPCR assay was designed. In this assay, the first-strand cDNA reaction was primed with 250 ng of random primers (Life Technologies). A CHIKV sequence-specific forward primer (CHIKV2411, 5′-AGAGACCAGTCGACGTGTTGTAC-3′) and a CHIKV sequence-specific reverse primer (CHIKV2676, 5′-GTGCGCATTTTGCCTTCGTA-3′) were used in conjunction with a CHIKV sequence-specific TaqMan probe (CHIKV2579, 5′-FAM-ATCTGCACCCAAGTGTACCA-MGB-3′). A random primed cDNA standard curve was generated as described above. To quantify the CHIKV subgenomic 26S mRNA, random primed cDNA was used as a template for CHIKV sequence-specific forward (CHIKV10239, 5′-CGGCGTCTACCCATTTATGT-3′) and CHIKV sequence-specific reverse (CHIKV10363, 5′-CCCTGTATGCTGATGCAAATTC-3′) primers and a CHIKV sequence-specific TaqMan probe (CHIKV10290, 5′-FAM-AAACACGCAGTTGAGCGAAGCAC-MGB-3′) that amplified a region in the E1 gene. The nsP1 gene copy number was determined in parallel with random primed cDNA and the primers/probe described above. The data were expressed as a ratio of the E1 gene copy number divided by the nsP1 gene copy number.
Viral plaque assays.
Serial 10-fold dilutions of virus-containing samples were adsorbed on BHK-21 cells for 1 h at 37°C, followed by an overlay with 0.5% immunodiffusion agarose (MP Biomedicals) in medium for 38 to 40 h. Plaques were visualized by neutral red staining (Sigma). Plaque numbers were enumerated to determine the number of PFU/ml of culture supernatant and mouse serum or PFU/g of tissue.
Histopathological analysis.
At specific times, mice were sacrificed and perfused by intracardiac injection of 4% paraformaldehyde, pH 7.3, and the indicated tissues were dissected and fixed in 4% paraformaldehyde, pH 7.3. Tissues were embedded in paraffin, and 5-μm sections were prepared. Tissue sections were stained with hematoxylin and eosin (H&E) and evaluated by light microscopy. Two anatomic pathologists blindly scored the presence, distribution, and severity of histological lesions. For all tissue changes, a scoring system was developed as follows: 0, absent; 1, minimal, less than 10% of tissue affected; 2, mild, 10 to 24% of tissue affected; 3, moderate, 25 to 39% of tissue affected; 4, marked, 40 to 59% of tissue affected; 5, severe, greater than 60% of tissue affected.
Statistical analysis.
All data were analyzed using GraphPad Prism 5 software. Data were evaluated for significant differences using either a two-tailed, unpaired t test with or without Welch's correction, a Mann-Whitney test, a one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test, or a two-way ANOVA followed by Bonferroni posttest analysis. A P value of <0.05 was considered statistically significant. All differences not indicated as significant had P values of >0.05.
RESULTS
Persistence of CHIKV is tissue specific.
To evaluate the duration of CHIKV infection in tissues, we utilized a WT C57BL/6 mouse model in which the major pathological findings during the acute stage of infection (arthritis, myositis, and tenosynovitis) were consistent with the disease in infected humans (23, 29). WT mice were inoculated subcutaneously in the left rear footpad with virus diluent only (mock) or 103 PFU of CHIKV strain SL15649. For many of the experiments, we monitored CHIKV infection in tissues using a highly sensitive and specific RT-qPCR assay. Following extensive intracardiac perfusion with PBS, positive-strand genomic CHIKV RNA burdens in the ankles and spleen at 3 dpi (n = 7) and 1 (n = 11), 2 (n = 8), 4 (n = 11), 6 (n = 7), 12 (n = 8), and 16 (n = 3) weeks postinoculation (wpi) were quantified by RT-qPCR with primers and probes complementary to sequences in the viral nsP1 gene coding region (Fig. 1A and Table 1). CHIKV RNA in tissues of mock-inoculated mice was below the limit of detection of this assay (data not shown), which confirmed the specificity of our measurements. The amount of CHIKV RNA in ankle-associated tissues of CHIKV-inoculated mice was highest at 3 dpi and declined during the first 2 to 4 weeks postinoculation. CHIKV RNA was detected in the left ankles of all mice, which is near the site of inoculation, for at least 16 wpi. CHIKV RNA also was detected in the right ankles of nearly all mice, a tissue distal to the site of inoculation, for at least 16 wpi. In addition to ankle-associated tissues, CHIKV RNA was measured at a low level in the spleen of WT mice (Fig. 1A and Table 1); however, levels in the spleen waned such that after 6 wpi, CHIKV RNA was undetectable. CHIKV RNA also persisted in the wrists of WT mice (Table 1), suggesting that CHIKV may establish persistent infections preferentially in joint-associated tissues.
Fig 1.
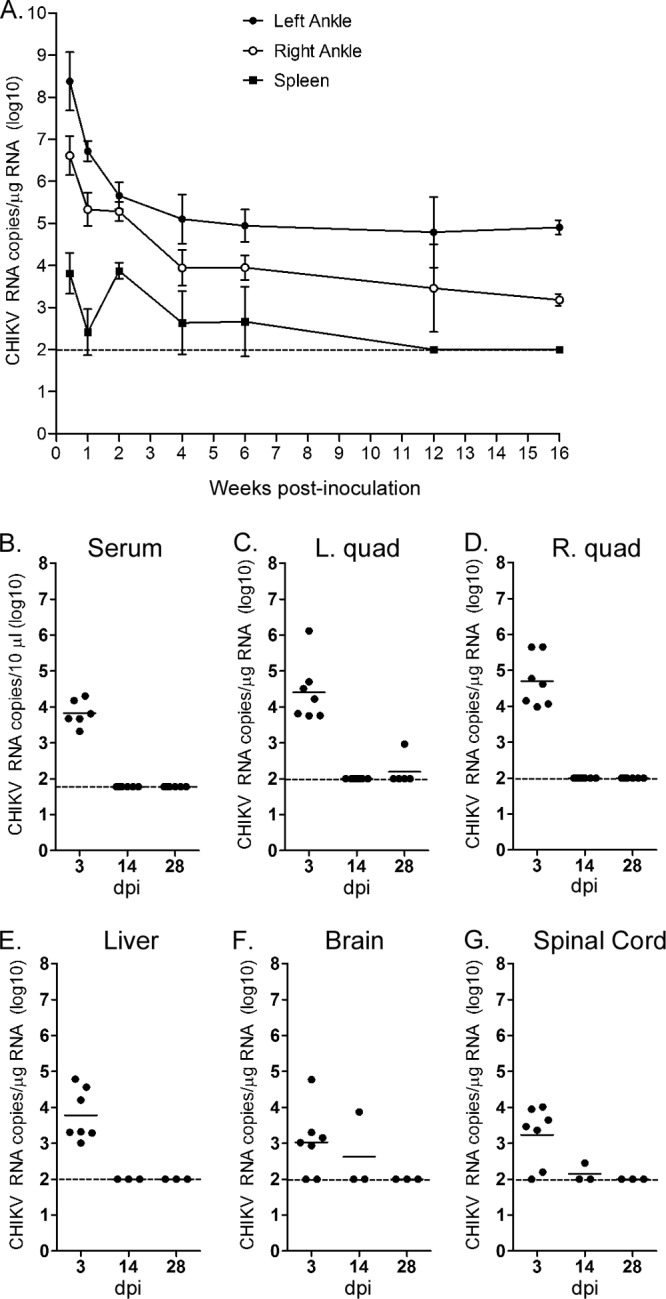
CHIKV RNA persists in joint-associated tissue and spleen of WT mice. Three-week-old WT C57BL/6 mice were mock inoculated (data not shown) or inoculated with 103 PFU of CHIKV by injection in the left rear footpad. Mice were sacrificed and perfused by intracardiac injection with PBS, and total RNA was isolated from the indicated tissues. (A) At 3 dpi (n = 7 mice) and 1 (n = 11), 2 (n = 8), 4 (n = 11), 6 (n = 7), 12 (n = 8), and 16 (n = 3) wpi, CHIKV RNA in the ankles and spleen was quantified by RT-qPCR. Each data point represents the arithmetic mean ± standard errors of the means (SEM), and the dashed line indicates the limit of detection. (B to G) At 3 (n = 7), 14 (n = 3 to 7), and 28 dpi (n = 3 to 5), CHIKV RNA in the serum (B), left quad (C), right quad (D), liver (E), brain (F), and spinal cord (G) was quantified by RT-qPCR. Horizontal lines indicate the means, and dashed lines indicate the limits of detection. Data shown are derived from 2 to 3 independent experiments, except data for 16 wpi, which were derived from a single experiment.
Table 1.
CHIKV infection in tissues of infected animals
| Mouse strain | Tissue or seruma | No. of positive tissues/total number of tissues by day postinoculationb |
||||||
|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 14 | 28 | 42 | 84 | 112 | ||
| WT | Left ankle | 7/7 | 11/11 | 12/12 | 11/11 | 7/7 | 8/8 | 3/3 |
| Right ankle | 7/7 | 11/11 | 8/8 | 9/9 | 7/7 | 5/8 | 3/3 | |
| Left wrist | ND | ND | 5/5 | ND | ND | ND | 2/3 | |
| Right wrist | ND | ND | ND | ND | ND | ND | 1/3 | |
| Left quad | 7/7 | 1/11 | 0/8 | 1/6 | 0/3 | 0/5 | ND | |
| Right quad | 7/7 | 5/11 | 0/8 | 0/9 | 0/3 | 0/5 | ND | |
| Spleen | 7/7 | 6/11 | 4/7 | 6/11 | 3/7 | 0/8 | 0/3 | |
| Liver | 7/7 | ND | 0/3 | 0/3 | ND | ND | ND | |
| Brain | 5/7 | ND | 1/3 | 0/3 | ND | ND | ND | |
| Spinal cord | 6/7 | ND | 1/3 | 0/3 | ND | ND | ND | |
| Serum | 6/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | ND | |
| Rag1−/− | Left ankle | 6/6 | 6/6 | 9/9 | 6/6 | 7/7 | 5/5 | ND |
| Right ankle | 6/6 | 6/6 | 9/9 | 6/6 | 7/7 | 5/5 | ND | |
| Left quad | 6/6 | 6/6 | 9/9 | 6/6 | 7/7 | 5/5 | ND | |
| Right quad | 6/6 | 6/6 | 9/9 | 6/6 | 7/7 | 5/5 | ND | |
| Spleen | 6/6 | 1/6 | 0/9 | 0/6 | 0/7 | 0/5 | ND | |
| Liver | 6/6 | ND | 0/3 | 0/6 | 0/5 | ND | ND | |
| Brain | 6/6 | ND | ND | 2/6 | 0/5 | ND | ND | |
| Serum | 5/5 | 2/6 | 5/5 | 4/6 | 6/6 | 4/4 | ND | |
Tissues were analyzed for CHIKV positive-strand RNA via RT-qPCR. Limit of detection was 100 CHIKV RNA copies/μg of RNA. Serum was analyzed for infectious virus via direct plaque assays. Limit of detection was 50 PFU/ml of serum.
Data are expressed as the number of tissues that were CHIKV positive divided by the total number of tissues analyzed. Each tissue was from an independent mouse. ND, not done.
To evaluate further the tissue specificity of CHIKV RNA persistence, we quantified viral RNA levels in the serum (Fig. 1B), quadriceps muscles (Fig. 1C and D), liver (Fig. 1E), brain (Fig. 1F), and spinal cord (Fig. 1G) at 3, 14, and 28 dpi by RT-qPCR. CHIKV RNA was readily detected in these tissues at 3 dpi, a time point during the acute stage of infection. Consistent with the musculoskeletal tissue tropism of CHIKV and related arthritogenic alphaviruses (30, 31), the highest CHIKV RNA burdens during the acute stage (3 dpi) were present in joint-associated and skeletal muscle tissues (Fig. 1). In contrast to the ankles and spleen, CHIKV RNA was cleared rapidly from the serum, quadriceps muscles, liver, brain, and spinal cord of WT mice (Fig. 1 and Table 1). These findings indicate that persistence of CHIKV RNA following a subcutaneous inoculation is joint tissue specific.
CHIKV RNA persists in mice inoculated with East/Central/South African, West African, and Asian CHIKV strains.
Phylogenetic analyses have identified three genotypes of CHIKV strains: East/Central/South African (ECSA), West African, and Asian (6, 32). To determine if persistence of viral RNA in joint-associated tissues was specific to CHIKV strain SL15649, a member of the ECSA genotype, we tested two distantly related CHIKV strains: Asian strain PO731460 and West African strain 37997 (6). WT C57BL/6 mice were inoculated subcutaneously in the left rear footpad with 103 PFU of either CHIKV strain. Following extensive intracardiac perfusion with PBS, viral RNA levels in the left and right ankles at 4 wpi (n = 6) were quantified by RT-qPCR. As shown in Fig. 2, the amount of CHIKV RNA in ankle-associated tissues at 4 wpi in mice inoculated with strain 37997 or strain PO731460 was similar to or higher than that detected in ankle tissues of mice inoculated with strain SL15649. These data indicate that CHIKV strains from all described genotypes can establish persistent infections in murine joint tissue and, thus, this is not an unusual property of the recent ECSA epidemic strains.
Fig 2.

Persistence of CHIKV RNA in joint-associated tissue is virus genotype independent. Three-week-old WT C57BL/6 mice were inoculated with 103 PFU of CHIKV strain 37997 or strain PO731460 by injection in the left rear footpad (n = 6 or 7/group). At 28 dpi, mice were sacrificed and perfused by intracardiac injection with PBS, and total RNA was isolated from the indicated tissues. CHIKV RNA in the left ankle (A) and right ankle (B) was quantified by RT-qPCR. Levels of CHIKV RNA from mice inoculated with 103 PFU of CHIKV strain SL15649 were quantified by the same assay for comparison. Horizontal lines indicate the means, and dashed lines indicate the limits of detection. *, P < 0.05; **, P < 0.01, as determined by one-way ANOVA followed by Tukey's multiple comparison test. Data shown are derived from two independent experiments.
Adaptive immunity controls persistence of CHIKV.
Chronic arthritis could be caused by persistent viral infection and/or persistent immunopathology associated with B and T cell immunity. To evaluate whether the adaptive immune response controls or contributes to CHIKV-induced joint disease and also impacts the tissue specificity of CHIKV persistence, Rag1−/− mice, which lack mature B and T lymphocytes (33), were inoculated subcutaneously in the left rear footpad with 103 PFU of CHIKV SL15649. The levels of CHIKV RNA in perfused tissues (ankles, quadriceps muscles, and spleen) at 3 dpi and 1, 2, 4, 6, and 12 wpi were quantified by RT-qPCR (Fig. 3A to E). The levels of CHIKV RNA were elevated in both the left ankle joint (11-fold [P < 0.001], 4-fold [P < 0.05], 12-fold [P < 0.05], 6-fold [P < 0.01], and 7-fold [P < 0.001] at 1, 2, 4, 6, and 12 wpi, respectively) and the right ankle joint (15-fold [P < 0.001], 32-fold [P < 0.001], 23-fold [P < 0.001], 26-fold [P < 0.001], and 404-fold [P < 0.001] at 3 dpi and 1, 4, 6, and 12 wpi, respectively) of Rag1−/− mice compared to those in WT mice (Fig. 3A and B). These data indicate that T and/or B cell immunity contributes to the control of CHIKV infection in joint tissues but is not sufficient to mediate complete clearance. CHIKV RNA persisted in quadriceps muscle tissues of Rag1−/− mice for at least 12 wpi but was cleared by 2 wpi from the same tissues of WT mice (Fig. 3C and D); thus, in contrast to the joint tissues, adaptive T and/or B cell immunity was sufficient to clear CHIKV from muscle tissues. Although the amounts of CHIKV RNA detected in the spleens of WT and Rag1−/− mice were similar at 3 dpi and 1 wpi, remarkably, viral RNA persisted in the spleen of WT mice for 6 wpi but fell below the limit of detection in the spleen of Rag1−/− mice at 2, 4, 6, and 12 wpi (Fig. 3E). These data suggest that the altered organization and/or cellularity of the spleen of Rag1−/− mice (34) prevents CHIKV persistence in this tissue; alternatively, although it has not been reported, CHIKV could have a limited tropism for subsets of B and T cells. We also quantified the amounts of infectious virus in the serum of CHIKV-inoculated WT and Rag1−/− mice via direct plaque assays. Infectious CHIKV was present in sera from both mouse strains at 3 dpi (Fig. 3F) but was not detected in sera of WT mice at any of the later time points evaluated. In contrast, Rag1−/− mice developed a persistent low level of viremia (Fig. 3F), which showed a pattern of fluctuation. A rapid decline in titer occurred between 3 and 7 dpi that was followed by a rise in titer between 1 and 2 wpi. Another drop in titer occurred at 4 wpi prior to the establishment of a steady-state level by 6 wpi. These data are consistent with recent studies showing that Rag2−/− mice and B cell-deficient mice developed a persistent viremia following CHIKV inoculation (35, 36). Taken together, these analyses suggest that adaptive immune responses modulate persistent CHIKV infection in a tissue-specific manner.
Fig 3.

CHIKV persistence in tissues of Rag1−/− mice. Three-week-old Rag1−/− congenic C57BL/6 mice were mock inoculated (data not shown) or inoculated with 103 PFU of CHIKV by injection in the left rear footpad. At 3 dpi (n = 6) and 1 (n = 6), 2 (n = 9), 4 (n = 6), 6 (n = 9), and 12 wpi (n = 5), mice were sacrificed and perfused by intracardial injection with 1× PBS, and total RNA was isolated from the left ankle (A), right ankle (B), left quadriceps muscle (C), right quadriceps muscle (D), and spleen (E). Levels of CHIKV RNA were quantified by RT-qPCR. For comparison, values were plotted against values in WT mice. (F) Serum was analyzed for infectious virus via direct plaque assays. Dashed lines indicate the limit of detection. *, P < 0.05; **, P < 0.01; ***, P < 0.001, as determined by two-way ANOVA followed by Bonferroni posttest analysis. Data shown are derived from at least two independent experiments.
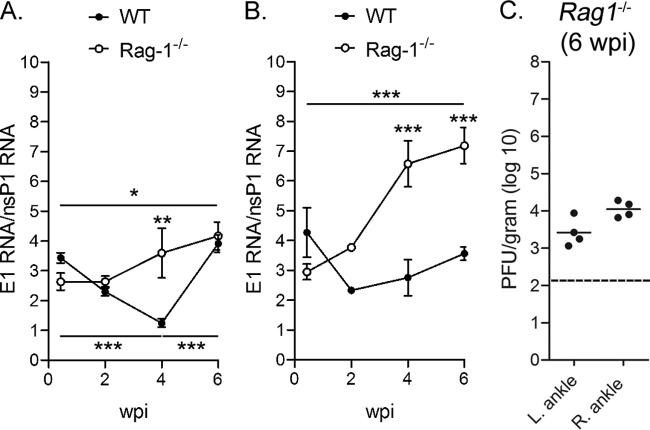
During the alphavirus replication cycle, a 26S positive-sense subgenomic RNA, colinear with the 3′ one-third of the full-length genomic RNA, is synthesized from the 26S subgenomic promoter (37, 38). The 26S subgenomic RNA is synthesized in 3- to 10-fold molar excess of the full-length positive-strand genomic RNA; thus, quantification of this molar excess can be used as an indicator of ongoing alphavirus replication (39–43). We designed an additional sequence-specific RT-qPCR assay with primers and probes complementary to sequences in the E1 coding region which, in contrast to those in the nsP1 gene coding region, are found in both the full-length and subgenomic RNAs produced in infected cells. This assay amplified full-length CHIKV positive-strand genomic RNA with similar efficiency as our nsP1 gene-based assay (data not shown). Utilizing this assay in combination with our nsP1 gene-based assay, we detected a 2.6- to 4.3-fold excess of CHIKV RNA with our E1-based assay in the ankle tissues of WT and Rag1−/− mice at 3 dpi (Fig. 4A and B), suggesting similar levels of replication in these tissues at this time point. The E1 RNA/nsP1 RNA ratios declined from 3 dpi to 4 wpi in the left ankle tissues of WT mice (P < 0.001) to an average ratio of 1.2 at 4 wpi and then increased at 6 wpi (P < 0.001) (Fig. 4A). Similarly, the E1 RNA/nsP1 RNA ratios declined or remained constant over time in the right ankle of WT mice (Fig. 4B). These data suggest that CHIKV replication was restricted in the joint tissues of WT mice during persistence. In contrast, the excess of RNA detected with the E1-based assay increased over time in both the left ankle (P < 0.05) and right ankle (P < 0.001) of Rag1−/− mice to levels that were higher than those of WT mice, suggesting higher levels of CHIKV replication occurred in joint tissues of Rag1−/− mice than in WT mice during persistence. Consistent with these results, infectious CHIKV was detected in ankle joint tissues of Rag1−/− but not WT mice at 42 dpi by direct plaque assay (Fig. 4C and data not shown). These data suggest that CHIKV RNA replication occurs at low, fluctuating levels in joint-associated tissues of WT mice and further support an important role for adaptive immunity in controlling persistent CHIKV infection.
Fig 4.
Increased subgenomic mRNA in tissues of Rag1−/− mice. CHIKV RNA in the left ankle (A) and right ankle (B) of WT and Rag1−/− C57BL/6 mice at the indicated time points was quantified by RT-qPCR assays specific for nucleotide sequences in the nsP1 and the E1 genes in parallel as described in Materials and Methods. The E1/nsP1 ratio was plotted as an indirect measurement of subgenomic mRNA production. *, P < 0.05; **, P < 0.01; ***, P < 0.001, as determined by one-way ANOVA followed by Tukey's multiple comparison test. (C) Infectious virus in the left and right ankles of Rag1−/− C57BL/6 mice at 42 dpi was quantified by direct plaque assay. Dashed line indicates the limit of detection. Data shown are derived from at least two independent experiments.
Persistence of CHIKV is associated with pathology.
The data presented thus far suggest that CHIKV RNA persists in mice preferentially in joint-associated tissues. To determine the extent to which the detection of CHIKV RNA is associated with pathology, histological changes in musculoskeletal tissues of uninfected control mice and CHIKV-infected mice at various times postinoculation were evaluated in a blinded manner. Consistent with prior reports (23, 29), arthritis, synovitis, tendonitis, myositis, and myocyte necrosis were most severe during the acute stage (7 dpi) (Fig. 5A to F). Histopathology scores for synovitis (Fig. 5B), arthritis (Fig. 5C), and myositis (Fig. 5D) during the acute stage appeared more severe for WT mice than for Rag1−/− mice, consistent with studies reporting a possible pathogenic role of CD4+ T cells in acute CHIKV-induced disease (36). At this time point, both WT and Rag1−/− mice had an infiltrating inflammatory cell population predominantly composed of macrophages and neutrophils with admixed lymphocytes. At late times postinoculation (4 to 12 wpi), most WT (8 of 11) and Rag1−/− (6 of 9) mice had apparent synovitis (Fig. 5A and B). However, arthritis (Fig. 5C), metatarsal muscle inflammation (Fig. 5D), metatarsal muscle necrosis (Fig. 5E), and tendonitis (Fig. 5F) resolved in WT mice but remained evident in the majority of Rag1−/− mice at 12 wpi. During this chronic stage, the infiltrating inflammatory cell population in tissues of WT mice consisted predominantly of histiocytes and lymphocytes, whereas histiocytes and neutrophils were predominant in the tissues of Rag1−/− mice. Thus, the persistence of CHIKV RNA is associated with pathology in joint-associated tissues, muscle tissue, and tendons. Moreover, T and/or B cell responses appear to prevent the development of more severe chronic disease likely due to their ability to control infection. Nonetheless, synovitis failed to resolve in WT mice for at least 12 wpi, which correlated with the joint tissue-specific persistence of CHIKV RNA (Fig. 1 and Table 1).
Fig 5.
Chronic synovitis in WT and Rag1−/− mice. Three week-old WT and Rag1−/− C57BL/6 mice were mock inoculated or inoculated with 103 PFU of CHIKV by injection in the left rear footpad. (A) At 7 and 42 dpi, 5-μm paraffin-embedded sections were generated from the hind limbs and stained with hematoxylin and eosin. Arrows indicate areas of synovitis, as identified by an anatomic pathologist. Scale bar, 200 μM. Images are representative of three mice per group. (B to F) At 1, 4, 6, and 12 wpi, 5-μm paraffin-embedded sections were generated from the hind limbs, stained with hematoxylin and eosin, and scored in a blinded manner by two anatomic pathologists for the degree of synovitis (B), arthritis (C), metatarsal muscle inflammation (D), metatarsal muscle necrosis (E), and tendonitis (F) based on the following scale for percentage of tissue affected: 0, absent (0%); 1, minimal (<10%); 2, mild (11 to 25%); 3, moderate (26 to 40%); 4, marked (41 to 60%); and 5, severe (>60%).
MAb prophylaxis prevents persistence of CHIKV in tissues of Rag1−/− mice.
The studies described thus far establish a mouse model that can be used for evaluating the efficacy of therapeutic agents against chronic CHIKV infection and joint disease. Recently, a combination of two CHIKV MAbs, CHK-152 and CHK-166 (which recognize discrete epitopes on CHIKV E2 and E1, respectively), was shown to have therapeutic efficacy in murine models of lethal CHIKV infection and acute CHIKV-induced musculoskeletal disease (24). To evaluate whether these neutralizing MAbs could prevent persistent CHIKV infection, 200 μg each of CHK-152 and CHK-166 or 400 μg of a negative control MAb (WNV E16) was administered intraperitoneally to Rag1−/− mice 1 day before and 3 days after inoculation with CHIKV. Persistence of CHIKV was evaluated at 28 days after virus inoculation. Prophylaxis with CHK-152 and CHK-166 reduced levels of infectious CHIKV in the serum to below the limit of detection (Fig. 6A; P < 0.03). In addition, prophylaxis with CHK-152 and CHK-166 reduced CHIKV RNA levels in the left ankle (P < 0.02) and right ankle (P = 0.01) to below the limits of detection in 3/5 and 4/5 mice, respectively (Fig. 6B and C). In the two mice that were positive in these tissues, CHIKV RNA levels were reduced by >99% compared to those in mice treated with the negative-control MAb.
Fig 6.
Efficacy of MAb prophylaxis and therapy against persistent CHIKV infection in Rag1−/− mice. (A to C) Three-week-old Rag1−/− C57BL/6 mice were injected i.p. with 400 μg of WNV E16 MAb or 200 μg CHK-152 and 200 μg CHK-166 MAbs on days −1 and +3. On day 0, mice were inoculated with 103 PFU of CHIKV in the left rear footpad. At 28 dpi, mice were sacrificed and perfused with PBS via intracardiac injection, and infectious virus in the serum (A) was quantified by plaque assay. Tissues (left ankle/foot [B] and right ankle/foot [C]) were homogenized in TRIzol, and CHIKV RNA was quantified by RT-qPCR. Horizontal lines indicate the means, and dashed lines indicate the limits of detection. P values were determined by Mann-Whitney tests. Data are from two independent experiments. (D to G) Three-week-old Rag1−/− C57BL/6 mice were inoculated with 103 PFU of CHIKV in the left rear footpad. On days 21 and 25, mice were injected i.p. with 400 μg of WNV E16 MAb or 200 μg CHK-152 and 200 μg CHK-166 MAbs. At 28 dpi, mice were sacrificed and perfused with PBS via intracardiac injection. (D) Infectious virus in the serum was quantified by plaque assay. The left ankle (E) and right ankle (F) were homogenized in TRIzol, and CHIKV RNA was quantified by RT-qPCR. (G) CHIKV RNA in the right ankle was quantified by RT-qPCR assays specific for nucleotide sequences in the nsP1 and the E1 genes in parallel as described in Materials and Methods. The E1/nsP1 ratio was plotted as an indirect measurement of subgenomic mRNA production. Horizontal bars indicate the means, and dashed lines indicate the limits of detection. P values were determined by the Mann-Whitney test (D) or two-tailed unpaired t tests (F and G). Data are from two independent experiments.
MAb therapy has tissue-specific effects on persistent CHIKV infection.
To evaluate whether the neutralizing anti-CHIKV MAbs could reduce or eliminate an established persistent infection, 200 μg each of CHK-152 and CHK-166 or 400 μg of a negative-control MAb (WNV E16) was administered intraperitoneally to Rag1−/− mice on days 21 and 25 post-CHIKV inoculation, and viral burdens were evaluated at 28 dpi. As shown in Fig. 6D, therapeutic administration of CHK-152 and CHK-166 eliminated infectious virus from the sera (P < 0.01). In addition, while we detected infectious CHIKV in the quadriceps of 2/3 mice treated with the control MAb, 0/3 mice treated with CHK-152 and CHK-166 had detectable infectious virus (data not shown), suggesting that the CHIKV-specific MAbs could eliminate infectious virus in musculoskeletal tissues. This treatment regimen, however, had no effect on viral RNA levels in the left ankle (Fig. 6E) or the quadriceps muscles (data not shown), although a significant, albeit small (3.2-fold, P < 0.01), reduction of CHIKV RNA was observed in the right ankle (Fig. 6F). In addition, the E1 RNA/nsP1 RNA ratio in the right ankle was reduced in mice treated with CHK-152 and CHK-166 compared to mice treated with the control antibody (P = 0.001) (Fig. 6G), suggesting that the CHIKV-specific MAbs reduced virus replication in this tissue. Thus, this two-dose, 1-week combination MAb therapy was sufficient to reduce burdens of infectious virus, although a more extended regimen may be required to clear CHIKV from some tissues.
DISCUSSION
A defining feature of alphavirus-induced musculoskeletal disease is the development of chronic polyarthralgia and/or polyarthritis, which can be debilitating (1, 17). The underlying processes that result in chronic disease associated with these infections are not well understood. Here, we utilized a recently developed mouse model of acute CHIKV-induced musculoskeletal disease (23) to investigate the sites and duration of CHIKV infection and musculoskeletal tissue pathology, the role of adaptive immunity in control of persistent infection, and a possible strategy to prevent or cure persistent infection. Our findings suggest that CHIKV establishes persistent infections in joint-associated tissues, that persistence of CHIKV RNA is associated with ongoing synovitis, and that the sites of CHIKV persistence and tissue burdens of CHIKV are controlled by adaptive immunity. We also found that an antibody-based treatment prevents persistent CHIKV infection when administered as prophylaxis and has tissue-specific effects when administered therapeutically. Together, these studies support the hypothesis that chronic CHIKV arthritic disease is associated with persistent infection and establishes a small-animal model that can be utilized to investigate molecular mechanisms of chronic disease and test therapies that mitigate persistent CHIKV infection and joint pathology.
Persistence of CHIKV RNA is joint tissue specific and controlled by adaptive immunity.
Persistence of alphaviruses in vertebrate hosts was first noted in the central nervous system of both WT and scid mice inoculated intracerebrally (i.c.) with neuroadapted SINV (44, 45). Subsequent studies established that persistent central nervous system (CNS) infection with SINV following an i.c. inoculation was controlled by gamma interferon (IFN-γ) production by T cells and anti-SINV antibodies produced by antibody-secreting B cells residing in the CNS (46–49). In our studies, we found that persistence of CHIKV RNA in WT mice was joint tissue specific, with high levels of viral RNA detected in ankle and wrist tissues, but not in several other tissues, for at least 16 wpi. Our studies also indicated that the establishment of persistent infection in joint-associated tissues of mice is a common property shared by CHIKV strains from all three genotypes. These data are consistent with epidemiological studies which have documented the development of protracted disease symptoms during outbreaks of CHIKV in humans involving any of the three CHIKV genotypes (5). The detection of CHIKV RNA in joint-associated tissues is also consistent with previous studies in humans in which CHIKV antigen was detected in synovial biopsy specimens collected from a patient with chronic disease (12) and in experimentally inoculated rhesus and cynomolgous macaques in which CHIKV RNA was detected in joints at later times postinoculation (22, 50). Analogously, Ross River virus RNA has been detected in knee biopsy specimens collected from patients 5 weeks after the onset of joint symptoms (51).
Similar to WT mice, Rag1−/− mice failed to clear CHIKV RNA from ankle joint-associated tissues. However, the amounts of CHIKV RNA and the E1/nsP1 RNA ratio in ankle joint-associated tissues of Rag1−/− mice were elevated compared to those of WT mice, suggesting that adaptive immunity limits viral burden in these tissues. In contrast, WT but not Rag1−/− mice rapidly cleared CHIKV RNA from quadriceps muscle tissue and infectious virus from the serum. Collectively, these data suggest that T and/or B cell-mediated immunity controls CHIKV burdens in a tissue-specific manner.
Persistence of CHIKV RNA is associated with chronic synovitis.
Similar to acute CHIKV rheumatological disease in WT mice, inoculation of Rag1−/− mice with CHIKV resulted in synovitis, arthritis, myositis, and tendonitis; thus, CHIKV can induce an acute inflammatory response despite the lack of mature T and B cells. These findings are similar to studies in Rag1−/− mice infected with Ross River virus in which affected joints and muscles were infiltrated with macrophages and NK cells (31). However, our detailed assessment of tissue sections at 7 dpi revealed that Rag1−/− mice had less severe tissue pathology than WT mice during the acute stage, suggesting an early pathogenic role for T and/or B cells. These findings agree with studies reporting less severe foot swelling in CHIKV-infected Rag2−/− mice and major histocompatibility complex class II (MHC-II)-deficient mice (36, 52). More specifically, CD4+ T cells were shown to contribute to CHIKV-induced foot swelling and musculoskeletal tissue injury (36).
Assessment of tissue sections from WT and Rag1−/− mice at 4, 6, and 12 wpi revealed that CHIKV infection resulted in a low-level, chronic synovitis. Intraarticular injection of dsRNA directly into murine joint spaces also is arthritogenic (53), suggesting that viral RNA may be sufficient to cause joint inflammation and injury. Thus, persistence of CHIKV in joint-associated tissues may promote chronic inflammation of synovial membranes. In contrast, arthritis, myositis, and tendonitis resolved in the majority of WT mice, but not in Rag1−/− mice, between 4 and 12 wpi. Although Rag1−/− mice had less severe acute disease, in the chronic phase, arthritis, myositis, myocyte necrosis, and tendonitis were present in the majority of Rag1−/− mice. These longitudinal analyses suggest that functional T and/or B cell responses protect against chronic musculoskeletal disease. Our results are consistent with recent findings in rhesus macaques in which persistence of CHIKV in the spleen correlated with defects in adaptive immune responses (54) and with findings in humans in which the rapid appearance of neutralizing IgG3 antibodies correlated with viral clearance and protection from chronic CHIKV disease (55).
Prophylaxis with CHIKV-specific MAbs prevents CHIKV persistence.
Prophylaxis via passive transfer of human immune plasma or CHIKV IgG to highly susceptible Ifnar1−/− mice can protect against CHIKV-induced mortality, suggesting that antibody therapy may be a promising disease prevention option for individuals at high risk of CHIKV infection (56). More recently, prophylaxis with different CHIKV-specific MAbs was shown to protect against lethal CHIKV infection in AGR129 mice (57), which lack receptors for type I and type II interferons as well as Rag2, and also in Ifnar1−/− mice (24). Importantly, prophylaxis with CHIKV-specific MAbs prevented acute joint swelling and inflammatory arthritis in CHIKV-infected WT mice (24). However, none of these previous studies addressed whether antibody-based prophylaxis or treatment could impact persistent CHIKV infection. We found that the combination of two neutralizing MAbs (CHK-152 and CHK-166), which recognize discrete epitopes on CHIKV E2 and E1, respectively, prevented persistent infection in Rag1−/− mice when administered as prophylaxis. Together with previous reports, our findings suggest that antibody-based prophylaxis in targeted at-risk populations may be a promising strategy to prevent both acute and chronic CHIKV disease.
MAb therapy after the establishment of persistence enables tissue-specific clearance.
To test whether the neutralizing anti-CHIKV MAbs could reduce or eliminate an established persistent CHIKV infection, we treated persistently infected Rag1−/− mice 3 weeks after CHIKV inoculation with two doses of MAbs and evaluated viral burden 1 week later. Similar to the prophylaxis results, therapeutic administration of CHK-152 and CHK-166 eliminated infectious virus in the sera. MAb treatment also prevented recovery of infectious virus from quadriceps muscle tissue, suggesting that infectious virus in at least some tissues is efficiently eliminated upon treatment. In contrast to prophylaxis, therapeutic administration of CHK-152 and CHK-166 had minimal effects on CHIKV RNA burdens in joint-associated tissues and quadriceps muscle tissue. These findings are similar to those of a study showing that a single dose of an anti-SINV virus MAb administered at 7 days postinfection failed to eliminate virus RNA in the CNS of scid mice (45). Although the latter study is complicated by issues of blood-brain barrier penetration and accumulation of MAb in a restricted compartment, longer MAb treatment regimens may be required to eliminate alphavirus RNA in infected tissues.
A high percentage of CHIKV-infected individuals suffer from chronic arthralgia (13, 14, 16, 17, 58, 59), and chronic CHIKV disease can be debilitating with severe economic consequences (17, 58, 60, 61). Accordingly, an improved understanding of the molecular mechanisms of chronic CHIKV disease and the development of effective therapies to prevent or treat viral persistence are critical. Our studies report a mouse model with long-term CHIKV infection and pathology localized in joint-associated tissues. In this model, adaptive immune responses control rather than contribute to the severity of tissue pathology by restricting CHIKV infection in most but not all tissues. Prophylaxis with a combination of MAbs effectively prevents persistent CHIKV infection, whereas therapeutic administration diminished infectious virus burdens in tissues. The development of this model will facilitate future studies to increase our understanding of the biological basis of chronic CHIKV infection and disease and allow a cost-effective platform for testing new therapies that mitigate infection and pathology.
ACKNOWLEDGMENTS
This research was supported by Public Health Service grant R21-AI096289 (T.E.M.) and R01-AI104545 (M.S.D.) from the National Institute of Allergy and Infectious Diseases. K.A.S. was supported by Public Health Service grant T32 AI052066 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print 16 October 2013
REFERENCES
- 1.Suhrbier A, Jaffar-Bandjee MC, Gasque P. 2012. Arthritogenic alphaviruses—an overview. Nat. Rev. Rheumatol. 8:420–429 [DOI] [PubMed] [Google Scholar]
- 2.Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. 2012. Chikungunya: a re-emerging virus. Lancet 379:662–671 [DOI] [PubMed] [Google Scholar]
- 3.Dupuis-Maguiraga L, Noret M, Brun S, Le Grand R, Gras G, Roques P. 2012. Chikungunya disease: infection-associated markers from the acute to the chronic phase of arbovirus-induced arthralgia. PLoS Negl. Trop. Dis. 6:e1446. 10.1371/journal.pntd.0001446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross RW. 1956. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J. Hyg. 54:177–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powers AM, Logue CH. 2007. Changing patterns of Chikungunya virus: re-emergence of a zoonotic arbovirus. J. Gen. Virol. 88:2363–2377 [DOI] [PubMed] [Google Scholar]
- 6.Volk SM, Chen R, Tsetsarkin KA, Adams AP, Garcia TI, Sall AA, Nasar F, Schuh AJ, Holmes EC, Higgs S, Maharaj PD, Brault AC, Weaver SC. 2010. Genome-scale phylogenetic analyses of Chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J. Virol. 84:6497–6504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsetsarkin KA, Chen R, Sherman MB, Weaver SC. 2011. Chikungunya virus: evolution and genetic determinants of emergence. Curr. Opin. Virol. 1:310–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, Cordioli P, Fortuna C, Boros S, Magurano F, Silvi G, Angelini P, Dottori M, Ciufolini MG, Majori GC, Cassone A. 2007. Infection with Chikungunya virus in Italy: an outbreak in a temperate region. Lancet 370:1840–1846 [DOI] [PubMed] [Google Scholar]
- 9.Grandadam M, Caro V, Plumet S, Thiberge JM, Souares Y, Failloux AB, Tolou HJ, Budelot M, Cosserat D, Leparc-Goffart I, Despres P. 2011. Chikungunya virus, southeastern France. Emerg. Infect. Dis. 17:910–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliva O, San Martin JL, Nasci RS. 2011. Preparedness and response for Chikungunya virus: introduction in the Americas, p 1–161 PAHO, Washington, DC [Google Scholar]
- 11.Simon F, Javelle E, Oliver M, Leparc-Goffart I, Marimoutou C. 2011. Chikungunya virus infection. Curr. Infect. Dis. Rep. 13:218–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoarau JJ, Jaffar Bandjee MC, Trotot PK, Das T, Li-Pat-Yuen G, Dassa B, Denizot M, Guichard E, Ribera A, Henni T, Tallet F, Moiton MP, Gauzere BA, Bruniquet S, Jaffar Bandjee Z, Morbidelli P, Martigny G, Jolivet M, Gay F, Grandadam M, Tolou H, Vieillard V, Debre P, Autran B, Gasque P. 2010. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J. Immunol. 184:5914–5927 [DOI] [PubMed] [Google Scholar]
- 13.Borgherini G, Poubeau P, Jossaume A, Gouix A, Cotte L, Michault A, Arvin-Berod C, Paganin F. 2008. Persistent arthralgia associated with Chikungunya virus: a study of 88 adult patients on reunion island. Clin. Infect. Dis. 47:469–475 [DOI] [PubMed] [Google Scholar]
- 14.Simon F, Parola P, Grandadam M, Fourcade S, Oliver M, Brouqui P, Hance P, Kraemer P, Ali Mohamed A, de Lamballerie X, Charrel R, Tolou H. 2007. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean islands. Report of 47 cases. Medicine 86:123–137 [DOI] [PubMed] [Google Scholar]
- 15.Staikowsky F, Le Roux K, Schuffenecker I, Laurent P, Grivard P, Develay A, Michault A. 2008. Retrospective survey of Chikungunya disease in Reunion Island hospital staff. Epidemiol. Infect. 136:196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sissoko D, Malvy D, Ezzedine K, Renault P, Moscetti F, Ledrans M, Pierre V. 2009. Post-epidemic Chikungunya disease on Reunion Island: course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl. Trop. Dis. 3:e389. 10.1371/journal.pntd.0000389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schilte C, Staikovsky F, Couderc T, Madec Y, Carpentier F, Kassab S, Albert ML, Lecuit M, Michault A. 2013. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl. Trop. Dis. 7:e2137. 10.1371/journal.pntd.0002137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calisher CH, el-Kafrawi AO, Al-Deen Mahmud MI, Travassos da Rosa AP, Bartz CR, Brummer-Korvenkontio M, Haksohusodo S, Suharyono W. 1986. Complex-specific immunoglobulin M antibody patterns in humans infected with alphaviruses. J. Clin. Microbiol. 23:155–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chopra A, Anuradha V, Lagoo-Joshi V, Kunjir V, Salvi S, Saluja M. 2008. Chikungunya virus aches and pains: an emerging challenge. Arthritis Rheum. 58:2921–2922 [DOI] [PubMed] [Google Scholar]
- 20.Grivard P, Le Roux K, Laurent P, Fianu A, Perrau J, Gigan J, Hoarau G, Grondin N, Staikowsky F, Favier F, Michault A. 2007. Molecular and serological diagnosis of Chikungunya virus infection. Pathol. Biol. 55:490–494 [DOI] [PubMed] [Google Scholar]
- 21.Ozden S, Huerre M, Riviere JP, Coffey LL, Afonso PV, Mouly V, de Monredon J, Roger JC, El Amrani M, Yvin JL, Jaffar MC, Frenkiel MP, Sourisseau M, Schwartz O, Butler-Browne G, Despres P, Gessain A, Ceccaldi PE. 2007. Human muscle satellite cells as targets of Chikungunya virus infection. PLoS One 2:e527. 10.1371/journal.pone.0000527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, Guigand L, Dubreil L, Lebon P, Verrier B, de Lamballerie X, Suhrbier A, Cherel Y, Le Grand R, Roques P. 2010. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J. Clin. Invest. 120:894–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison TE, Oko L, Montgomery SA, Whitmore AC, Lotstein AR, Gunn BM, Elmore SA, Heise MT. 2011. A mouse model of Chikungunya virus-induced musculoskeletal inflammatory disease: evidence of arthritis, tenosynovitis, myositis, and persistence. Am. J. Pathol. 178:32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pal P, Dowd KA, Brien JD, Edeling MA, Gorlatov S, Johnson S, Lee I, Akahata W, Nabel GJ, Richter MK, Smit JM, Fremont DH, Pierson TC, Heise MT, Diamond MS. 2013. Development of a highly protective combination monoclonal antibody therapy against Chikungunya virus. PLoS Pathog. 9:e1003312. 10.1371/journal.ppat.1003312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoermer KA, Burrack A, Oko L, Montgomery SA, Borst LB, Gill RG, Morrison TE. 2012. Genetic ablation of arginase 1 in macrophages and neutrophils enhances clearance of an arthritogenic alphavirus. J. Immunol. 189:4047–4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanlandingham DL, Hong C, Klingler K, Tsetsarkin K, McElroy KL, Powers AM, Lehane MJ, Higgs S. 2005. Differential infectivities of o'nyong-nyong and Chikungunya virus isolates in Anopheles gambiae and Aedes aegypti mosquitoes. Am. J. Trop. Med. Hyg. 72:616–621 [PubMed] [Google Scholar]
- 27.Jupille HJ, Oko L, Stoermer KA, Heise MT, Mahalingam S, Gunn BM, Morrison TE. 2011. Mutations in nsP1 and PE2 are critical determinants of Ross River virus-induced musculoskeletal inflammatory disease in a mouse model. Virology 410:216–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, Huang L, Gorlatov S, Mehlhop E, Marri A, Chung KM, Ebel GD, Kramer LD, Fremont DH, Diamond MS. 2005. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat. Med. 11:522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardner J, Anraku I, Le TT, Larcher T, Major L, Roques P, Schroder WA, Higgs S, Suhrbier A. 2010. Chikungunya virus arthritis in adult wild-type mice. J. Virol. 84:8021–8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Couderc T, Chretien F, Schilte C, Disson O, Brigitte M, Guivel-Benhassine F, Touret Y, Barau G, Cayet N, Schuffenecker I, Despres P, Arenzana-Seisdedos F, Michault A, Albert ML, Lecuit M. 2008. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 4:e29. 10.1371/journal.ppat.0040029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison TE, Whitmore AC, Shabman RS, Lidbury BA, Mahalingam S, Heise MT. 2006. Characterization of Ross River virus tropism and virus-induced inflammation in a mouse model of viral arthritis and myositis. J. Virol. 80:737–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powers AM, Brault AC, Tesh RB, Weaver SC. 2000. Re-emergence of Chikungunya and o'nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J. Gen. Virol. 81:471–479 [DOI] [PubMed] [Google Scholar]
- 33.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68:869–877 [DOI] [PubMed] [Google Scholar]
- 34.Crowley MT, Reilly CR, Lo D. 1999. Influence of lymphocytes on the presence and organization of dendritic cell subsets in the spleen. J. Immunol. 163:4894–4900 [PubMed] [Google Scholar]
- 35.Lum FM, Teo TH, Lee WW, Kam YW, Renia L, Ng LF. 2013. An essential role of antibodies in the control of Chikungunya virus infection. J. Immunol. 190:6295–6302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teo TH, Lum FM, Claser C, Lulla V, Lulla A, Merits A, Renia L, Ng LF. 2013. A pathogenic role for CD4+ T cells during Chikungunya virus infection in mice. J. Immunol. 190:259–269 [DOI] [PubMed] [Google Scholar]
- 37.Jose J, Snyder JE, Kuhn RJ. 2009. A structural and functional perspective of alphavirus replication and assembly. Future Microbiol. 4:837–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strauss JH, Strauss EG. 1994. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 58:491–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raju R, Huang HV. 1991. Analysis of Sindbis virus promoter recognition in vivo, using novel vectors with two subgenomic mRNA promoters. J. Virol. 65:2501–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shirako Y, Strauss JH. 1994. Regulation of Sindbis virus RNA replication: uncleaved P123 and nsP4 function in minus-strand RNA synthesis, whereas cleaved products from P123 are required for efficient plus-strand RNA synthesis. J. Virol. 68:1874–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemm JA, Rice CM. 1993. Roles of nonstructural polyproteins and cleavage products in regulating Sindbis virus RNA replication and transcription. J. Virol. 67:1916–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LaStarza MW, Lemm JA, Rice CM. 1994. Genetic analysis of the nsP3 region of Sindbis virus: evidence for roles in minus-strand and subgenomic RNA synthesis. J. Virol. 68:5781–5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atasheva S, Krendelchtchikova V, Liopo A, Frolova E, Frolov I. 2010. Interplay of acute and persistent infections caused by Venezuelan equine encephalitis virus encoding mutated capsid protein. J. Virol. 84:10004–10015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levine B, Hardwick JM, Trapp BD, Crawford TO, Bollinger RC, Griffin DE. 1991. Antibody-mediated clearance of alphavirus infection from neurons. Science 254:856–860 [DOI] [PubMed] [Google Scholar]
- 45.Levine B, Griffin DE. 1992. Persistence of viral RNA in mouse brains after recovery from acute alphavirus encephalitis. J. Virol. 66:6429–6435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burdeinick-Kerr R, Wind J, Griffin DE. 2007. Synergistic roles of antibody and interferon in noncytolytic clearance of Sindbis virus from different regions of the central nervous system. J. Virol. 81:5628–5636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Binder GK, Griffin DE. 2001. Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science 293:303–306 [DOI] [PubMed] [Google Scholar]
- 48.Metcalf TU, Griffin DE. 2011. Alphavirus-induced encephalomyelitis: antibody-secreting cells and viral clearance from the nervous system. J. Virol. 85:11490–11501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tyor WR, Wesselingh S, Levine B, Griffin DE. 1992. Long term intraparenchymal Ig secretion after acute viral encephalitis in mice. J. Immunol. 149:4016–4020 [PubMed] [Google Scholar]
- 50.Chen CI, Clark DC, Pesavento P, Lerche NW, Luciw PA, Reisen WK, Brault AC. 2010. Comparative pathogenesis of epidemic and enzootic Chikungunya viruses in a pregnant rhesus macaque model. Am. J. Trop. Med. Hyg. 83:1249–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soden M, Vasudevan H, Roberts B, Coelen R, Hamlin G, Vasudevan S, La Brooy J. 2000. Detection of viral ribonucleic acid and histologic analysis of inflamed synovium in Ross River virus infection. Arthritis Rheum. 43:365–369 [DOI] [PubMed] [Google Scholar]
- 52.Nakaya HI, Gardner J, Poo YS, Major L, Pulendran B, Suhrbier A. 2012. Gene profiling of Chikungunya virus arthritis reveals significant overlap with rheumatoid arthritis. Arthritis Rheum. 64:3553–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zare F, Bokarewa M, Nenonen N, Bergstrom T, Alexopoulou L, Flavell RA, Tarkowski A. 2004. Arthritogenic properties of double-stranded (viral) RNA. J. Immunol. 172:5656–5663 [DOI] [PubMed] [Google Scholar]
- 54.Messaoudi I, Vomaske J, Totonchy T, Kreklywich CN, Haberthur K, Springgay L, Brien JD, Diamond MS, Defilippis VR, Streblow DN. 2013. Chikungunya virus infection results in higher and persistent viral replication in aged rhesus macaques due to defects in anti-viral immunity. PLoS Negl. Trop. Dis. 7:e2343. 10.1371/journal.pntd.0002343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kam YW, Simarmata D, Chow A, Her Z, Teng TS, Ong EK, Renia L, Leo YS, Ng LF. 2012. Early appearance of neutralizing immunoglobulin G3 antibodies is associated with Chikungunya virus clearance and long-term clinical protection. J. Infect. Dis. 205:1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Couderc T, Khandoudi N, Grandadam M, Visse C, Gangneux N, Bagot S, Prost JF, Lecuit M. 2009. Prophylaxis and therapy for Chikungunya virus infection. J. Infect. Dis. 200:516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fric J, Bertin-Maghit S, Wang CI, Nardin A, Warter L. 2013. Use of human monoclonal antibodies to treat Chikungunya virus infection. J. Infect. Dis. 207:319–322 [DOI] [PubMed] [Google Scholar]
- 58.Moro ML, Grilli E, Corvetta A, Silvi G, Angelini R, Mascella F, Miserocchi F, Sambo P, Finarelli AC, Sambri V, Gagliotti C, Massimiliani E, Mattivi A, Pierro AM, Macini P. 2012. Long-term Chikungunya infection clinical manifestations after an outbreak in Italy: a prognostic cohort study. J. Infect. 65:165–172 [DOI] [PubMed] [Google Scholar]
- 59.Larrieu S, Pouderoux N, Pistone T, Filleul L, Receveur MC, Sissoko D, Ezzedine K, Malvy D. 2010. Factors associated with persistence of arthralgia among Chikungunya virus-infected travellers: report of 42 French cases. J. Clin. Virol. 47:85–88 [DOI] [PubMed] [Google Scholar]
- 60.Couturier E, Guillemin F, Mura M, Leon L, Virion JM, Letort MJ, De Valk H, Simon F, Vaillant V. 2012. Impaired quality of life after Chikungunya virus infection: a 2-year follow-up study. Rheumatology 51:1315–1322 [DOI] [PubMed] [Google Scholar]
- 61.Soumahoro MK, Boelle PY, Gauzere BA, Atsou K, Pelat C, Lambert B, La Ruche G, Gastellu-Etchegorry M, Renault P, Sarazin M, Yazdanpanah Y, Flahault A, Malvy D, Hanslik T. 2011. The Chikungunya epidemic on La Reunion Island in 2005–2006: a cost-of-illness study. PLoS Negl. Trop. Dis. 5:e1197. 10.1371/journal.pntd.0001197 [DOI] [PMC free article] [PubMed] [Google Scholar]