Abstract
Individual variation in physiological responsiveness to stress mediates risk for mental illness and is influenced by both experiential and genetic factors. Common polymorphisms in the human gene for FK506 binding protein 5 (FKBP5), which is involved in transcriptional regulation of the hypothalamic-pituitary-adrenal (HPA) axis, have been shown to interact with childhood abuse and trauma to predict stress-related psychopathology. In the current study, we examined if such gene-environment interaction effects may be related to variability in the threat-related reactivity of the amygdala, which plays a critical role in mediating physiological and behavioral adaptions to stress including modulation of the HPA axis. To this end 139 healthy, Caucasian youth completed a BOLD fMRI probe of amygdala reactivity and self-report assessments of emotional neglect (EN) and other forms of maltreatment. These individuals were genotyped for six FKBP5 polymorphisms (rs7748266, rs1360780, rs9296158, rs3800373, rs9470080, and rs9394309) previously associated with psychopathology and/or HPA axis function. Interactions between each SNP and EN emerged such that risk alleles predicted relatively increased dorsal amygdala reactivity in the context of higher EN, even after correcting for multiple testing. Two different haplotype analyses confirmed this relationship as haplotypes with risk alleles also exhibited increased amygdala reactivity in the context of higher EN. Our results suggest that increased threat-related amygdala reactivity may represent a mechanism linking psychopathology to interactions between common genetic variants affecting HPA axis function and childhood trauma.
Keywords: FKBP5, amygdala, emotional neglect, stress, PTSD, HPA
Introduction
Childhood adversity predicts over 32% of all psychiatric disorders (Green et al., 2010). Understanding the neurobiological mechanisms that underlie this association and the individual differences that moderate risk, will inform disease etiology and may lead to more effective treatment and prevention strategies. Research across species suggests that early life adversity may precipitate psychopathology by aggravating physiological reactivity, biasing cognitive attention to threatening and stressful stimuli, and disrupting the regulation of emotional responses (Lupien et al., 2009).
The amygdala is critical for recruiting physiological and behavioral resources to adaptively respond to environmental challenges (LeDoux, 2000), particularly threat, with the magnitude of reactivity predicting individual differences in anxiety and depression (Hariri, 2009, Siegle et al., 2002). Consistent with non-human animal research (Tottenham and Sheridan, 2010), emerging human research has linked extreme childhood adversity (e.g., institutional rearing) to heightened amygdala reactivity (Maheu et al., 2010, Tottenham et al., 2011, Gianaros et al., 2008), and volumetric enlargement (Mehta et al., 2009, Tottenham et al., 2010). Because disrupted hypothalamic-pituitary-adrenal (HPA) axis function is associated with dysfunctional stress responsiveness, heightened amygdala reactivity (Maheu et al., 2008), childhood maltreatment (McCrory et al., 2010, Lupien et al., 2009) and psychopathology (Yehuda, 2002), genetic variation affecting HPA axis function may moderate associations between emotional neglect and amygdala reactivity which may, in turn, precipitate stress-related psychopathology.
One intriguing HPA axis candidate gene is FK506 binding protein 5 (FKBP5), located on chromosome 6 (6p21.31). FKBP5 is a co-chaperone that mediates nuclear translocation of the cortisol-glucocorticoid receptor (GR) complex and hence GR-mediated gene transcription. Elevated FKBP5 levels confer reduced GR sensitivity to circulating cortisol, leading to decreased negative feedback regulation of the HPA axis and a slower resolution of the stress response (Binder, 2009). Consistent with these effects, rodent research has linked FKBP5 expression to anxiety-like behavior (Attwood et al., 2011). Of primary importance to the current study, FKBP5 polymorphisms are associated with differential HPA axis function as well as psychopathology in the context of stress (Table 1). However, to our knowledge, neither the effect of FKBP5 nor FKBP5-by-stress interaction effects have been investigated in the context of threat-related brain circuitry. Because heightened threat-related amygdala reactivity has been associated with early adversity, elevated HPA axis function, and stress-related psychopathology, it is a promising candidate mechanism by which FKBP5 genotypes may interact with stress to predict psychopathology.
Table 1.
Associations Between FKBP5 Polymorphisms, Stress Responsiveness and Psychopathology.
| SNP (Location) | Association |
|---|---|
| rs1360780 (Intron) | T allele: Increased FKBP5 protein levels; reduced basal levels of cortisol; impaired HPA negative feedback following dexamethasone (DEX) and psychosocial stress; PTSD symptoms, incidence of depression, depressive symptoms and suicide in the context of childhood maltreatment; increased depression recurrence and more rapid response to antidepressant treatment; increased harm avoidance and reduced cooperativeness (Binder et al., 2004, Binder et al., 2008, Ising et al., 2008, Shibuya et al., 2010, Xie et al., 2010, Brent et al., 2010, Velders et al., 2011, Appel et al., 2011, Zimmermann et al., 2011) |
| rs9296158 (Intron) | A allele: Impaired HPA negative feedback of following DEX; reduced FKBP5 downregulation with increasing PTSD severity; PTSD symptoms and incidence of depression in the context of childhood maltreatment (Binder et al., 2008, Xie et al., 2010, Mehta et al., 2011, Zimmermann et al., 2011) |
| rs9470080 (Intron) | T allele: Depressive symptoms; reduced basal cortisol levels; PTSD in the context of adverse environmental exposure; incidence of depression in the context of childhood maltreatment (Boscarino et al., 2011, Velders et al., 2011, Xie et al., 2010, Zimmermann et al., 2011) |
| rs3800373 (3′ UTR) | G allele: Increased rate of suicide among depressed individuals; more rapid antidepressant response; increased peri-traumatic dissociation following trauma; increased PTSD and incidence of depression in the context of childhood maltreatment; impaired negative feedback of cortisol following psychosocial stress (Binder et al., 2004, Brent et al., 2010, Koenen et al., 2005, Ising et al., 2008, Zimmermann et al., 2011) |
| rs7748266 (Intron) | T allele: Reduced basal cortisol levels (Velders et al., 2011) |
| rs9394309 (Intron) | G allele: Reduced basal cortisol levels (Velders et al., 2011) |
The aim of this study was to assess whether FKBP5 polymorphisms associated with HPA axis function and/or psychopathology (Table 1) predict variation in threat-related amygdala reactivity in the context of childhood adversity. To this end, 139 youth without history of mental illness completed a widely used and well-characterized functional magnetic resonance imaging (fMRI) probe of threat-related amygdala reactivity (Brown et al., 2005, Neumann et al., 2006, Brown et al., 2006, Manuck et al., 2007) and provided self-reports of experienced childhood trauma. This population allowed us to preclude any differences in brain function that may be attributable to psychopathology. Because of the prospective nature of this study, it allows continued assessment of this sample so that the value of our measures in predicting the development of psychopathology may be examined. Because FKBP5 gentoypes only predict psychopathology in the context of adversity and resulting changes in HPA axis regulation are more salient in the context of stress reactivity, we hypothesized that FKBP5 “risk” alleles would be associated with increased threat-related amygdala reactivity, but only in the context of prior childhood emotional neglect.
Materials & Methods
Sample
A total of 139 Caucasian, non-Hispanic, psychiatrically healthy adolescents aged 12 to 15 (Table 2) with genotype information for six FKBP5 polymorphisms (rs9394309, rs7748266, rs1360780, rs9296158, rs3800373, and rs9470080) and functional neuroimaging data were selected from the ongoing Teen Alcohol Outcomes Study (TAOS), which is designed to investigate the contributions of genes, environments, and neural systems to the onset of alcohol use disorders during adolescence. Subjects in the current analyses did not meet threshold or sub-threshold criteria for lifetime/present mood disorder, as assessed by the Kiddies Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present and Lifetime (6–18 Years) Present and Lifetime Episode version (K-SADS-PL) (Ambrosini, 2000, Kaufman et al., 2000).
Table 2.
Demographics, effects of genotype and genotype-by-EN interaction effects on dorsal amygdala ROIs for FKBP5 SNPs.
| SNP/Haplotype | Genotype/Haplotype copies | N | Age, mean (SD) | CTQ, mean (SD) | EN, mean (SD) | Gender, female (%) | R Dorsal | L Dorsal | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Main effect (p) | EN-by-genotype (p) | Main effect (p) | EN-by-genotype (p) | |||||||
| rs3800373 | AA | 74 | 13.5 (1.0) | 32.3 (7.4) | 7.8 (3.5) | 39 (52.7) | 0.091 | 0.003** | 0.082 | 0.212 |
| MAF = 0.26 | AC | 57 | 13.5 (0.9) | 31.9 (7.2) | 7.8 (3.2) | 25 (43.9) | ||||
| CC | 8 | 13.2 (0.9) | 33.3 (4.9) | 8.8 (2.1) | 3 (37.5) | |||||
| rs9296158 | GG | 70 | 13.5 (1.1) | 32.6 (7.5) | 7.9 (3.5) | 37 (52.9) | 0.069 | 0.002** | 0.080 | 0.181 |
| MAF = 0.29 | AG | 58 | 13.5 (0.9) | 31.4 (7.2) | 7.5 (3.1) | 25 (43.1) | ||||
| AA | 11 | 13.3 (0.9) | 33.7 (4.5) | 9.2 (2.3) | 5 (45.5) | |||||
| rs7748266 | CC | 98 | 13.5 (1.0) | 32.6 (7.6) | 7.8 (3.3) | 50 (51.0) | 0.316 | 0.011* | 0.280 | 0.004** |
| MAF = 0.16 | CT/TT | 41 | 13.5 (0.9) | 31.3 (6.0) | 7.8 (3.1) | 17 (41.5) | ||||
| rs1360780 | CC | 72 | 13.5 (1.0) | 32.5 (7.4) | 7.9 (3.5) | 39 (54.2) | 0.062 | 0.002** | 0.082 | 0.207 |
| MAF = 0.28 | CT | 56 | 13.5 (0.9) | 31.5 (7.3) | 7.5 (3.1) | 23 (41.1) | ||||
| TT | 11 | 13.3 (0.9) | 33.7 (4.5) | 9.2 (2.3) | 5 (45.5) | |||||
| rs9394309 | AA | 71 | 13.4 (1.1) | 33.1 (8.3) | 8.0 (3.7) | 37 (52.1) | 0.537 | 0.097 | 0.119 | 0.013* |
| MAF = 0.28 | AG | 57 | 13.6 (0.9) | 30.6 (5.6) | 7.3 (2.7) | 24 (42.1) | ||||
| GG | 11 | 13.3 (0.9) | 34.6 (4.6) | 9.6 (2.3) | 6 (54.5) | |||||
| rs9470080 | CC | 64 | 13.5 (1.1) | 32.7 (7.7) | 8.0 (3.6) | 35 (54.7) | 0.432 | 0.011* | 0.415 | 0.266 |
| MAF = 0.33 | CT | 59 | 13.5 (0.9) | 31.3 (7.1) | 7.4 (3.0) | 25 (42.4) | ||||
| TT | 16 | 13.4 (0.8) | 33.4 (4.8) | 8.8 (2.5) | 7 (43.8) | |||||
| GCCAC | 2 copies | 64 | 13.5 (1.1) | 32.7 (7.7) | 8.0 (3.6) | 35 (54.7) | 0.432 | 0.011* | 0.415 | 0.266 |
| Freq. = 0.67 | 1 copy | 59 | 13.5 (0.9) | 31.3 (7.1) | 7.4 (3.0) | 25 (42.4) | ||||
| 0 copies | 16 | 13.4 (0.8) | 33.4 (4.8) | 8.8 (2.5) | 7 (43.8) | |||||
| TGCC | 2 copies | 72 | 13.5 (1.0) | 32.5 (7.4) | 7.9 (3.5) | 39 (54.2) | 0.062 | 0.002** | 0.082 | 0.207 |
| Freq. = 0.72 | 1 copy | 56 | 13.5 (0.9) | 31.5 (7.3) | 7.5 (3.1) | 23 (41.1) | ||||
| 0 copies | 11 | 13.3 (0.9) | 33.7 (4.5) | 9.2 (2.3) | 5 (45.5) | |||||
| All Groups | 139 | 13.5 (1.0) | 32.2 (7.2) | 7.8 (3.3) | 68 (48.9) | |||||
Significant effect after SNPspD correction
Significant effect after SNPspD correction and correction for number of ROIs
Note: MAF = minor allele frequency. CTQ = childhood trauma questionnaire. EN = Emotional Neglect subscale of the CTQ.
No differences between genotype groups were observed for age, CTQ, EN, or gender
TAOS subjects were recruited from the San Antonio, Texas, metro area via commercially available phone lists containing families living within a 30-mile radius of University of Texas Health Science Center at San Antonio (UTHSCA) and likely to have adolescents between the ages of 12 and 15 years. Families with medically healthy adolescents who did not have braces and were in the desired age range completed in-person interviews, which included a comprehensive battery of self-report behavioral assessments. Eligible participants who completed the MRI protocol and blood draw were compensated $140.00, while those only completing the in-person interview received $40.00. All subjects participated after parents provided informed consent per a UTHSCA Institutional Review Board approved protocol.
Childhood Trauma Questionnaire (CTQ)
The CTQ assesses 5 different types of childhood trauma: emotional, physical, and sexual abuse, as well as emotional and physical neglect (Bernstein et al., 2003). Each CTQ subscale has excellent internal consistency and convergent validity (Bernstein et al., 1994). We focused on the emotional neglect subscale because of previous research associating severe forms of emotional neglect with heightened amygdala reactivity (Maheu et al., 2010, Tottenham et al., 2011), and a previous report from our group on a larger TAOS sample documenting associations between emotional neglect, but not other CTQ subscales, and amygdala reactivity (Bogdan et al., in press). Moreover, there was a substantial amount of variability on this scale relative to the other subscales in the present sample.
fMRI Challenge Paradigm
A widely used and validated challenge paradigm was used to elicit robust amygdala reactivity (Hariri et al., 2002, Kleinhans et al., 2010, Carré et al., 2010, Wang et al., 2004). In this task, participants complete 4 blocks of a perceptual face processing task in which they view a trio of faces (expressing either anger or fear) and select 1 of 2 faces (displayed on the bottom) identical to the target stimulus (displayed on top). Each block consists of 6 images derived from a standard set of facial affect pictures (Ekman and Friesen, 1976). Faces are balanced for gender and target affect. Each of the 6 face trios is presented for 4 seconds with a variable inter-stimulus interval of 2-6 seconds, for a total block length of 48 seconds. Interleaved between these blocks, participants complete 5 blocks of a sensorimotor control task during which they view a trio of geometric shapes (circles, horizontal ellipses, vertical ellipses) and select 1 of 2 shapes (displayed on the bottom) identical to the target shape (displayed on top). Each sensorimotor task consists of 6 different shape trios. Each of the 6 different shape trios is presented for 4 seconds with a fixed inter-stimulus interval of 2 seconds, for a total block length of 36 seconds. The total paradigm length is 390 seconds. Reaction times and accuracy were recorded through an MR-compatible button-box. The stimuli used in this task are socially derived threats that are indirect and/or ambiguous (e.g., Should I be afraid of what this person is afraid of? Is this angry person a threat to me?). These types of stimuli engage brain circuitry, particularly the amygdala, responsible for adjusting vigilance to changing contingencies (for review see Davis and Whalen, 2001, Hariri and Whalen, 2011).
Genetic Analyses
Genomic DNA from all participants was isolated from mouthwash samples using the Oragene DNA self-collection kit following the manufacturer's instructions (DNA Genotek, Inc., 2006). FKBP5 SNPs rs1360780, rs9296158, rs3800373 and rs9470080 were genotyped using TaqMan allelic discrimination assay (Livak, 1999). Two additional FKBP5 SNPs (rs7748266 and rs9394309) were derived from an Illumina Human 610-Quad BeadChip assay (Illumina, San Diego, CA, USA). No other polymorphisms from this assay were tested in the current study. All SNPs were in accordance with Hardy-Weinberg Equilibrium (all χ2 < 0.5, all p > 0.4) and genotyping concordance of rs1360780 and rs9470080 across TaqMan genotyping and Illumina BeadChip assays were 100%.
Haploview was used to determine linkage disequilibrium (LD; Figure 1E) and establish haplotype blocks for the candidate FKBP5 SNPs (Barrett et al., 2005). The underlying haplotype phase for each subject was determined using PHASE software (Stephens et al., 2001). Because the genomic region of FKBP5 has a low degree of haplotype complexity (i.e., high LD with common haplotypes) all subjects had posterior pairwise probabilities greater than 95% and were thus included in haplotype analyses. One participant had a posterior probability of 70% and 30% for two different haplotypes within the haplotype block generated from Haploview. However, because this subject would be grouped in the same fashion regardless of block assignment, we included this subject in analyses.
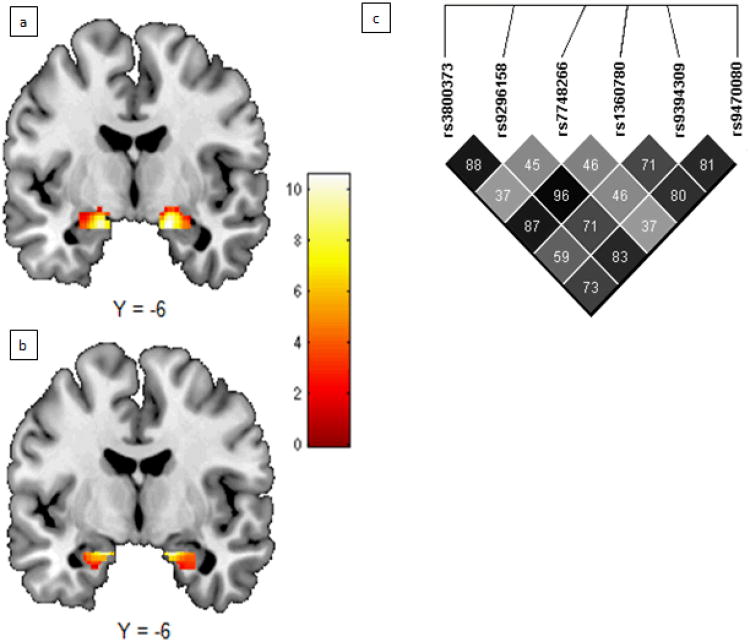
Figure 1. Main and Interaction Effects.
Significant group-level activation in (a) dorsal (left: [-18 -4 -18], k = 152 voxels, t = 10.58; right: [18 -8 -16], k = 164 voxels, t = 10.52) and (b) ventral (left: [-18 -4 -20], k = 67 voxels, t = 9.53; right: [18 -4 -20], k = 89 voxels, t = 9.20) amygdala ROIs (pFWE < 0.05). LD map for FKBP5 SNPs shown in (e). Darker squares denote higher LD and numbers shown are r2 values.
A haplotype block was formed using the confidence interval (CI) method (Gabriel et al., 2002). This block was composed of 5 of the 6 SNPs included in this study (i.e., rs9296158, rs7748266, rs1360780, rs9394309 and rs9470080) and resulted in the following haplotype frequencies: GCCAC (67.3%), ATTGT (15.5%), ACTGT (9.4%), GCCGT (3.6%), ACTAT (3.2%), ACCAT (0.7%), GCCAT (0.4%). Subjects were categorized based on the presence of the GCCAC haplotype (i.e., 0, 1 or 2 copies), which was comprised of “non-risk” alleles as identified in previous studies (Table 1). A second post-hoc haplotype block was also formed by including the four SNPs (i.e., rs3800373, rs9296158, rs1360780, and rs7748266) with interaction effects surviving both SNPSpD correction and FWE correction for multiple brain regions of interest comparisons (p < 0.0048, see below). This resulted in the following haplotype frequencies: TGCC (71.2%), GATT (13.3%), GACT (12.6%), TATT (2.2%), GACC (0.4%), TACC (0.4%). In a similar fashion to the Haploview generated haplotype, subjects were categorized based on the presence of the TGCC haplotype (i.e., 0, 1 or 2 copies), which was comprised of “non-risk” alleles (Table 1).
fMRI Acquisition Parameters
Blood oxygen level-dependent (BOLD) fMRI data were acquired with a gradient-echo EPI sequence (TR/TE = 2000/25 milliseconds, FOV = 20 cm, matrix = 64 × 64) covering 34 interleaved 3 mm thick axial slices on a Siemens 3T Trio Scanner. Prior to collecting fMRI data for each participant, a reference echoplanar imaging scan was collected; this scan was visually inspected for artifacts and good signal.
Neuroimaging Data Analyses
BOLD fMRI data were processed with SPM8 (Wellcome Department of Imaging Neuroscience, London, England). Images for each participant were realigned to the first volume in the time series to correct for head motion, spatially normalized into a standard stereotactic space (Montreal Neurological Institute template) using a 12- parameter affine model, and smoothed to minimize noise and residual difference in gyral anatomy with a gaussian filter set at 6 mm full-width at half-maximum. Next, the ARtifact detection Tool (ART) (Whitfield-Gabrieli, 2009) was used to generate regressors to account for images with large motion (i.e., >0.6 mm relative to the previous time frame) or spiking artifacts (i.e., global mean intensity 2.5 standard deviations from the entire time series).
After preprocessing, linear contrasts using canonical hemodynamic response functions estimated condition specific (i.e., faces>shapes) BOLD activation for each individual. As we were not interested in neural networks associated with face-specific processing per se, but rather in eliciting a maximal amygdala response across all participants, we chose not to use neutral faces as control stimuli because neutral faces can be subjectively experienced as affectively laden or ambiguous and thus engage the amygdala (Wright et al., 2003, Schwartz et al., 2003). Individual contrast images (i.e., weighted sum of the beta images) were used in a second-level random effects model to determine mean condition-specific responses using a 1-sample t test, corrected for family-wise error (FWE) at a voxel level of p < 0.05 with a voxel extant of 10 contiguous voxels across amygdala regions of interest.
Regions of Interest
Mean BOLD contrast estimates were extracted from functional clusters exhibiting a main effect of task (FWE p < .05; ≥ 10 contiguous voxels) within anatomically defined amygdala regions of interest (ROIs). Extracting parameter estimates from functional clusters activated by our fMRI paradigm, rather than clusters specifically correlated with our independent variables of interest, precludes the possibility of any correlation coefficient inflation that may result when an explanatory covariates is used to select a region of interest (Viviani, 2010). We have used this more conservative and rigorous analytic strategy in recent studies (Hyde et al., 2011, Carré et al., 2010). To account for distinct functional subregions within the amygdala, we constructed separate ventral (i.e., basolateral complex; 1024 mm3/42 voxels) and dorsal (i.e., central nucleus and substantia inominata; 1920 mm3/93.33 voxels) amygdala ROIs as previously described (Manuck et al., 2010, Carré et al., 2010). The ventral and dorsal distinctions allowed for independent examination of regions primarily involved with receiving sensory input and those critical for the expression of responses to threatening stimuli, respectively (Davis and Whalen, 2001, LeDoux, 2007). In humans, fMRI has revealed differences in function between these subregions, such as the conscious versus unconscious processing of fearful faces (Lerner et al., 2011, Etkin et al., 2004), as well as structural and functional connectivity (Lerner et al., 2011). All participants had >98% BOLD signal coverage in these amygdala ROIs.
Statistical Analyses
A general linear model (GLM) was used in Statistica version 7.0 to test emotional neglect-by-FKBP5 SNP genotype interaction (EN × FKBP5) effects for each SNP/haplotype on each of the four amygdala ROIs (left/right dorsal amygdala and left/right ventral amygdala). EN scores, FKBP5 genotype/haplotype (number of minor alleles/haplotypes), EN × FKBP5, age, and gender were used as covariates in the GLM. The interaction term was calculated for each subject by multiplying the EN score by the number of minor alleles. Using a GLM in this manner ensures that significant EN × FKBP5 effects exist even after accounting for independent main effects of EN and FKBP5 (see Hayes and Matthes, 2009). It is important to note that we hypothesized that FKBP5 would not exhibit main effects on amygdala reactivity but would only affect amygdala reactivity in the context of childhood emotional neglect. For one SNP, rs7748266, minor allele homozygotes were combined with heterozygotes for GLM analyses because the minor allele frequency (MAF) was less than 20%.
The program SNPSpD, which uses LD and the number of SNPs to determine a corrected significance threshold, identified α = 0.019 to correct for the six FKBP5 SNPs tested (Nyholt, 2004). An additional bonferroni correction was applied to this threshold to correct for multiple ROI comparisons (α = 0.019/4 = 0.0048). SNPs with individual interaction effects that survived this threshold were used as part of haplotype analyses (see above). One-way ANOVAs and chi-square tests assessed differences in self-report and demographic variables across genotype groups.
Results
Genotype groups did not differ significantly on any self-reported variables including EN (all p's > 0.07), CTQ total (all p's > 0.07), gender ratio (all p's > 0.25), or age (all p's > 0.5; Table 2). Consistent with previous research the task reliably recruited threat-related reactivity of the dorsal and ventral amygdala (Figure 1a,b) (Brown et al., 2005, Neumann et al., 2006, Brown et al., 2006, Manuck et al., 2007). Additionally, consistent with our previous report in a larger sample from TAOS, both right ventral and dorsal ROIs exhibited a positive main effect of EN score (F[1, 137] = 5.87, p = 0.017; F[1, 137] = 4.55, p = 0.035; respectively) (Bogdan et al., in press).
GLM revealed significant EN-by-genotype interaction effects on both the right and left dorsal amygdala ROIs (see Table 2), which survived SNPspD correction for multiple testing (p < 0.019). However, no significant interaction effects were observed on ventral amygdala ROIs after accounting for SNPspD correction (All F[5, 133]'s < 4.57, all p's > 0.034). FKBP5 SNPs rs7748266 (F[5, 133] = 6.58, p = 0.011), rs1360780 (F[5, 133] = 10.31, p = 0.002), rs9296158 (F[5, 133] = 9.99, p = 0.002), rs3800373 (F[5, 133] = 9.16, p = 0.003), and rs9470080 (F[5, 133] = 6.59, p = 0.011) had significant interaction effects on right dorsal amygdala reactivity (see Figure 2a-e); while SNPs rs9394309 (F[5, 133] = 6.39, p = 0.013) and rs7748266 (F[5, 133] = 8.51, p = 0.004) had significant interaction effects on left dorsal reactivity (see Figure 3a,b). These effects survived a multiple SNP comparison generated by SNPspD (p < 0.019), and four SNPs (rs7748266, rs1360780, rs9296158, and rs3800373) survived an additional FWE correction for multiple ROI comparisons (p < 0.0048). However, there was no significant main effect of genotype for any SNP on dorsal (see Table 2) or ventral amygdala ROIs (all p's > 0.06). Exploratory haplotype analyses revealed that both the five-SNP haplotype block generated from Haploview (rs9296158, rs7748266, rs1360780, rs9394309 and rs9470080) and the four-SNP haplotype block created post-hoc with SNPs showing the most significant interaction effects (rs3800373, rs9296158, rs1360780 and rs7748266), interacted with EN to predict right dorsal amygdala reactivity, (Haploview: F[5, 133] = 6.59, p = 0.011; Figure 4a; post-hoc composition: F[5, 133] = 9.99, p = 0.002;Figure 4b). Both haplotype analyses are significant after Bonferroni correction for multiple haplotype comparisons (p = 0.025). Results using a whole amygdala ROI (Figure S1) were similar to those seen in the dorsal subregion (Table S2). Additionally, similar results in the dorsal amygdala were obtained when using the total CTQ score (Table S2). We also tested the interactions including subjects (n = 17) with a current or past diagnosis of a mood or anxiety disorder. The results were essentially unchanged; EN × FKBP5 interactions emerged for the same SNPs predicting right (rs7748266, rs9296158, rs1360780, rs3800373, and rs9470080) and left (rs7748266 and rs9394309) dorsal amygdala reactivity (all F[5, 150]'s > 6.4, all p's < 0.012).
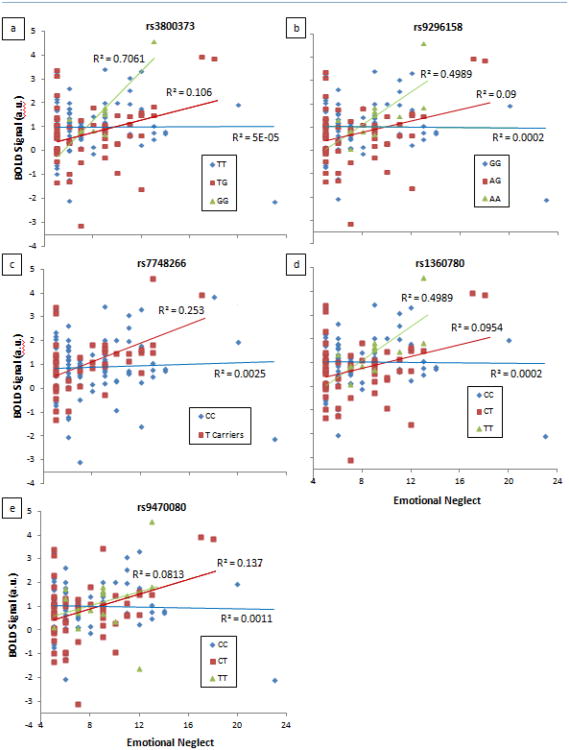
Figure 2. FKBP5 SNPs showing significant interaction effects with emotional neglect on right dorsal amygdala reactivity.
All significance levels survive SNPspD correction for multiple testing of p = 0.019: (a) rs3800373 (p = 0.003), (b) rs9296158 (p = 0.002), (c) rs7748266 (p = 0.011), (d) rs1360780 (p = 0.002), and (e) rs9470080 (p = 0.011). Plots show relationship between EN and amygdala BOLD separated by genotype group.
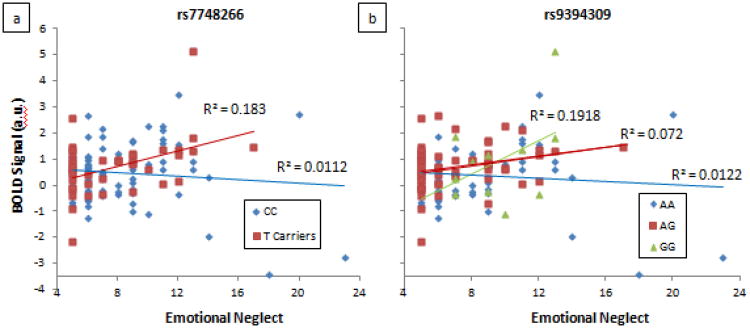
Figure 3. FKBP5 SNPs showing significant interaction effects with emotional neglect on left dorsal amygdala reactivity.
All significance levels survive SNPspD correction for multiple testing of p = 0.019: (a) rs7748266 (p = 0.004) and (b) rs9394309 (p = 0.013). Plots show relationship between EN and amygdala BOLD separated by genotype group.
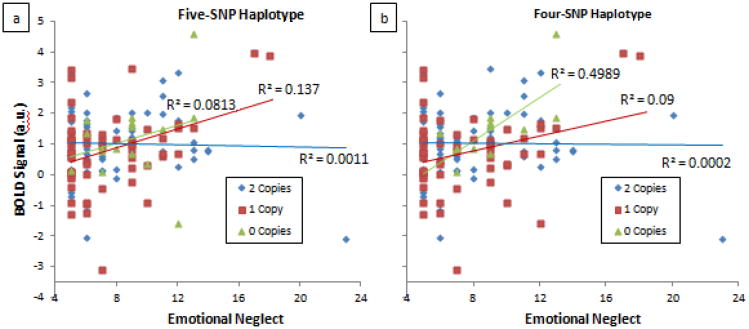
Figure 4. FKBP5 haplotypes showing significant interaction effects with emotional neglect on right dorsal amygdala reactivity.
(a) Five-SNP haplotype (rs9296158, rs7748266, rs1360780, rs9394309 and rs9470080) generated from Haploview (p = 0.011). Subjects were categorized based on the presence of the GCCAC haplotype (i.e. 0, 1 or 2 copies), which is comprised of “non-risk” alleles. (b) Four-SNP haplotype (rs3800373, rs9296158, rs1360780 and rs7748266) constructed from SNPs showing most significant interaction effects (p = 0.002). Subjects were categorized based on the presence of the TGCC haplotype (i.e., 0, 1 or 2 copies), which is comprised of “non-risk” alleles. Plots show relationship between EN and amygdala BOLD separated by haplotype group.
Discussion
This study examined how common FKBP5 polymorphisms previously linked to HPA axis dysfunction and/or stress-related psychopathology impact threat-related amygdala reactivity in the context of childhood adversity. Consistent with predictions, FKBP5 genotypes previously associated with impaired negative feedback of the HPA axis and/or stress-related psychopathology (i.e., rs1360780 T allele, rs9296158 A allele, rs9470080 T allele, rs3800373 G allele, rs7748266 T allele, rs9394309 G allele) interacted with childhood emotional neglect to predict heightened threat-related dorsal amygdala reactivity. While FKBP5 genotype alone may have a modest effect on amygdala reactivity, the further unmasking of this regulatory bias by environmental stressors results in a significant bias in reactivity. This reactivity bias in the context of emotional neglect may represent a mechanism through which individuals carrying these FKBP5 alleles are at increased risk for stress-related psychopathology.
Such heightened threat-related amygdala reactivity is consistent with collective evidence that these FKBP5 risk genotypes are associated with elevated and prolonged cortisol response to stress. First, elevated endogenous cortisol is associated with potentiated amygdala reactivity (Maheu et al., 2008, van Stegeren et al., 2007). Second, extreme forms of childhood emotional neglect (e.g, institutionalization) are associated with relative hyper-responsiveness of the HPA axis (McCrory et al., 2010, Lupien et al., 2009), as well as potentiated amygdala reactivity (Maheu et al., 2010, Tottenham et al., 2011, Gianaros et al., 2008). Third, increased FKBP5 expression (Binder, 2009, Binder et al., 2008, Wochnik et al., 2005) and adverse experiences (Heim et al., 2008, Meaney, 2001) impair negative feedback regulation of the stress response, and FKBP5 is upregulated in the amygdala following stress (Scharf et al., 2011) as well as associated with anxious behavior (Attwood et al., 2011). Thus, HPA axis dysregulation, associated with both FKBP5 risk genotypes and prior emotional neglect, may be further exaggerated by their interaction (i.e., GxE) leading to heightened amygdala reactivity and sensitivity to environmental threat, which may subsequently mediate increased risk for stress-related psychopathology.
Although the amygdala is traditionally analyzed as a unitary structure in neuroimaging studies, this assumption is not consistent with non-human animal research, which suggests there are different roles for the different nuclei within the amygdala (for review see Davis and Whalen, 2001, LeDoux, 2007). Recent human neuroimaging studies (Bzdok et al., 2012, Lerner et al., 2012, Roy et al., 2009, Amunts et al., 2005) support this observed heterogeneity by highlighting differences between the connectivity of the ventral and dorsal subregions, the same a priori ROIs used in this study. Also, differences between ventral and dorsal subregions have been found in the conscious versus unconscious processing of fearful faces (Lerner et al., 2012, Etkin et al., 2004). However, more studies are needed to definitively establish these observed differences. This caveat notwithstanding, our findings were specific to reactivity within the dorsal amygdala, which contains the central nucleus as well as dorsal extended regions including the nucleus basalis of Meynert. Unlike the ventral amygdala, which generally receives sensory, hippocampal and prefrontal afferents, the dorsal amygdala sends efferents to the brainstem and hypothalamus, which drive autonomic and motor functions, as well as prefrontal cortex, which modulates attention and vigilance (LeDoux, 2007). Because all of the EN-by-FKBP5 genotype interactions reported here predicted differences in dorsal, but not ventral amygdala reactivity, the risk associated with these variants may reflect sensitized responses to, as opposed to sensory perceptions, of threat. It is also important to note that we observed nominal, but not corrected, significance levels within whole amygdala ROIs (see supplemental material). This is likely due to differences in the spatial distribution of FKBP5 expression, since animal models show stress-related increases in expression of FKBP5 occurring in the central nucleus, which is part of the dorsal amygdala, and not other regions of the amygdala (Scharf et al. 2011).
These conclusions should be tempered, however, as there were several limitations to this study. First, we did not collect neuroendocrine measures (e.g., cortisol), which would have allowed us to test our speculations regarding HPA axis mechanisms that may drive the observed interaction. Second, we did not collect contemporaneous measures of psychopathology symptomatology making it impossible for us to link amygdala reactivity to individual differences in clinical presentations. Notably, however, TAOS is a prospective study, which will allow us to assess whether genetically-driven variability in brain function interacts with environmental stressors to predict future development of psychopathology. Third, we relied on retrospective self-report of emotional neglect, which could be biased by current mood (Teasdale and Russell, 1983) and personality traits, such as trait anxiety (Reidy and Richards, 1997). It will be important for future studies to use additional measures (e.g., public records, in person interviews) to derive more accurate estimates of adversity. Fourth, while all SNPs showed significant EN-by-genotype interaction effects on right or left dorsal amygdala reactivity, only effects from four SNPs survived both SNPspD correction and correction for multiple ROI comparisons (rs3800373, rs9296158, rs7748266, rs1360780). However, correcting for the number of SNPs tested in this case is quite conservative because of the a priori rationale we had for testing each SNP (Table 1). Additionally, correcting for the number of ROIs tested does not account for any correlation between the regions and is likely overly conservative, as well. Therefore, the SNPspD correction for multiple comparisons is likely more than sufficient to protect against Type 1 error, and for this reason, we include all resulting significant associations. In addition, the observed interaction effects predicting amygdala reactivity were present in a sample of psychiatrically healthy children and adolescents with modest levels of childhood stress (notably, the findings were entirely consistent when including individuals with current or past diagnosis of a mood or anxiety disorder [n =17]). Thus, it will be important for future research to assess whether differential amygdala reactivity conferred by our observed interactions actually predicts meaningful differences in psychopathology and behavior. Indeed, following these samples into peak periods of risk for psychopathology, as TAOS is designed to do, will be particularly valuable (Somerville et al., 2011). Lastly, the SNPs selected do not represent full coverage of the FKBP5 gene as they were carefully selected a priori based on associations with HPA function and/or psychopathology (see Table 1). Thus, there may be FKBP5 variants exhibiting yet to be discovered interaction effects with EN on amygdala function. These limitations notwithstanding, if our results can be replicated, they provide a plausible biological mechanism (i.e., heightened threat-related amygdala reactivity) by which common FKBP5 polymorphisms may confer risk for psychopathology in the context of childhood adversity.
Supplementary Material
Acknowledgments
This study was supported by an R01AA016274 grant (DEW). MGW is supported by the NIH Postbaccalaureate Intramural Research Training Award. RB is supported by NIH grant P30DA023026. The authors wish to thank members of the TAOS staff for their assistance conducting the study.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- Ambrosini PJ. Historical Development and Present Status of the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) J Am Acad Child Psy. 2000;39:49–58. doi: 10.1097/00004583-200001000-00016. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Appel K, Schwahn C, Mahler J, Schulz A, Spitzer C, Fenske K, Stender J, Barnow S, John U, Teumer A, Biffar R, Nauck M, Volzke H, Freyberger HJ, Grabe HJ. Moderation of Adult Depression by a Polymorphism in the FKBP5 Gene and Childhood Physical Abuse in the General Population. Neuropsychopharm. 2011;36:1982–1991. doi: 10.1038/npp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood BK, Bourgognon JM, Patel S, Mucha M, Schiavon E, Skrzypiec AE, Young KW, Shiosaka S, Korostynski M, Piechota M, Przewlocki R, Pawlak R. Neuropsin cleaves EphB2 in the amygdala to control anxiety. Nature. 2011;473:372–375. doi: 10.1038/nature09938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bernstein D, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiat. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrino. 2009;34:S186–195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Kunzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Kohnlein O, Dabitz H, Bruckl T, Muller N, Pfister H, Lieb R, Mueller JC, Lohmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Muller-Myshok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor iso/val (rs5522) genotype moderates the association between prior childhood emotional neglect and amygdala reactivity. American Journal of Psychiatry. doi: 10.1176/appi.ajp.2011.11060855. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscarino JA, Erlich PM, Hoffman SN, Rukstalis M, Stewart WF. Association of FKBP5, COMT and CHRNA5 polymorphisms with PTSD among outpatients at risk for PTSD. Psychiat Res. 2011;188:173–174. doi: 10.1016/j.psychres.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent D, Melhem N, Ferrell R, Emslie G, Wagner KD, Ryan N, Vitiello B, Birmaher B, Mayes T, Zelazny J, Onorato M, Devlin B, Clarke G, Debar L, Keller M. Association of FKBP5 Polymorphisms With Suicidal Events in the Treatment of Resistant Depression in Adolescents (TORDIA) Study. Am J Psychiat. 2010;167:190–197. doi: 10.1176/appi.ajp.2009.09040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Forbes EE, Hariri AR. Neural basis of individual differences in impulsivity: contributions of corticolimbic circuits for behavioral arousal and control. Emotion. 2006;6:239–245. doi: 10.1037/1528-3542.6.2.239. [DOI] [PubMed] [Google Scholar]
- Brown SM, Peet E, Manuck SB, Williamson DE, Dahl RE, Ferrell RE, Hariri AR. A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Mol Psychiatr. 2005;10:884–888. doi: 10.1038/sj.mp.4001716. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp. doi: 10.1002/hbm.22138. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré JM, Fisher PM, Manuck SB, Hariri AR. Interaction between trait anxiety and trait anger predict amygdala reactivity to angry facial expressions in men but not women. Soc Cogn Affect Neur. 2010;7:213–221. doi: 10.1093/scan/nsq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatr. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J. Individual Differences in Trait Anxiety Predict the Response of the Basolateral Amygdala to Unconsciously Processed Fearful Faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Price JC, Coleman RL, Ziolko SK, Becker C, Moses-Kolko EL, Berga SL, Hariri AR. Medial Prefrontal Cortex 5-HT2A Density Is Correlated with Amygdala Reactivity, Response Habituation, and Functional Coupling. Cereb Cortex. 2009;19:2499–2507. doi: 10.1093/cercor/bhp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, Defelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The Structure of Haplotype Blocks in the Human Genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Hariri AR, Sheu LK, Manuck SB, Matthews KA, Cohen S. Potential neural embedding of parental social standing. Soc Cogn Affect Neur. 2008;3:91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood Adversities and Adult Psychiatric Disorders in the National Comorbidity Survey Replication I: Associations With First Onset of DSM-IV Disorders. Arch Gen Psychiat. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR. The Neurobiology of Individual Differences in Complex Behavioral Traits. Annu Rev Neurosci. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin Transporter Genetic Variation and the Response of the Human Amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Methods. 2009;41:924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The Dexamethasone/Corticotropin-Releasing Factor Test in Men with Major Depression: Role of Childhood Trauma. Biol Psychiat. 2008;63:398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Hyde LW, Gorka A, Manuck SB, Hariri AR. Perceived social support moderates the link between threat-related amygdala reactivity and trait anxiety. Neuropsychologia. 2011;49:651–656. doi: 10.1016/j.neuropsychologia.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising M, Depping AM, Siebertz A, Lucae S, Unschuld PG, Kloiber S, Horstmann S, Uhr M, Muller-Myhsok B, Holsboer F. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur J Neurosci. 2008;28:389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-SADS-PL. J Am Acad Child Psy. 2000;39:1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Weaver K, Johnson LC, Greenson J, Dawson G, Aylward E. Association between amygdala response to emotional faces and social anxiety in autism spectrum disorders. Neuropsychologia. 2010;48:3665–3670. doi: 10.1016/j.neuropsychologia.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Saxe G, Purcell S, Smoller JW, Bartholomew D, Miller A, Hall E, Kaplow J, Bosquet M, Moulton S, Baldwin C. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Mol Psychiatr. 2005;10:1058–1059. doi: 10.1038/sj.mp.4001727. [DOI] [PubMed] [Google Scholar]
- Ledoux J. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Ledoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lerner Y, Singer N, Gonen T, Weintraub Y, Cohen O, Rubin N, Ungerleider LG, Hendler T. Feeling without Seeing? Engagement of Ventral, but Not Dorsal, Amygdala during Unaware Exposure to Emotional Faces. J Cogn Neurosci. 2011;24:531–542. doi: 10.1162/jocn_a_00165. [DOI] [PubMed] [Google Scholar]
- Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal. 1999;14:143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, Jenness J, Lau JY, Ackerman JP, Pine DS, Ernst M. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn Affect Behav Neurosci. 2010;10:34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheu FS, Mazzone L, Merke DP, Keil MF, Stratakis CA, Pine DS, Ernst M. Altered amygdala and hippocampus function in adolescents with hypercortisolemia: A functional magnetic resonance imaging study of Cushing syndrome. Dev Psychopathol. 2008;20:1177–1189. doi: 10.1017/S0954579408000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB, Brown SM, Forbes EE, Hariri AR. Temporal Stability of Individual Differences in Amygdala Reactivity. Am J Psychiat. 2007;164:1613–1614. doi: 10.1176/appi.ajp.2007.07040609. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Marsland AL, Flory JD, Gorka A, Ferrell RE, Hariri AR. Salivary testosterone and a trinucleotide (CAG) length polymorphism in the androgen receptor gene predict amygdala reactivity in men. Psychoneuroendocrino. 2010;35:94–104. doi: 10.1016/j.psyneuen.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E, De Brita SA, Viding E. Research Review: The neurobiology and genetics of maltreatment and adversity. J Child Psychol Psyc. 2010;51:1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Mehta D, Gonik M, Klengel T, Rex-Haffner M, Menke A, Rubel J, Mercer KB, Putz B, Bradley B, Holsboer F, Ressler KJ, Muller-Myhsok B, Binder EB. Using Polymorphisms in FKBP5 to Define Biologically Distinct Subtypes of Posttraumatic Stress Disorder: Evidence From Endocrine and Gene Expression Studies. Arch Gen Psychiat. 2011;68:901–910. doi: 10.1001/archgenpsychiatry.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Golembro NI, Nosarti C, Colvert E, Mota A, Williams SCR, Rutter M, Sonuga-Barke EJS. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees Study Pilot. J Child Psychol Psyc. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- Neumann SA, Brown SM, Ferrell RE, Flory JD, Manuck SB, Hariri AR. Human Choline Transporter Gene Variation Is Associated with Corticolimbic Reactivity and Autonomic-Cholinergic Function. Biol Psychiat. 2006;60:1155–1162. doi: 10.1016/j.biopsych.2006.03.059. [DOI] [PubMed] [Google Scholar]
- Nyholt DR. A Simple Correction for Multiple Testing for Single-Nucleotide Polymorphisms in Linkage Disequilibrium with Each Other. Am J Hum Genet. 2004;74:765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy J, Richards A. A memory bias for threat in high-trait anxiety. Pers Indiv Differ. 1997;23:653–663. [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf SH, Liebl C, Binder EB, Schmidt MV, Müller MB. Expression and Regulation of the Fkbp5 Gene in the Adult Mouse Brain. PLoS ONE. 2011;6:e16883. doi: 10.1371/journal.pone.0016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: Adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Shibuya N, Suzuki A, Sadahiro R, Kamata M, Matsumoto Y, Goto K, Hozumi Y, Otani K. Association study between a functional polymorphism of FK506-binding protein 51 (FKBP5) gene and personality traits in healthy subjects. Neurosci Lett. 2010;485:194–197. doi: 10.1016/j.neulet.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiat. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Fani N, McClure-Tone EB. Behavioral and neural representation of emotional facial expressions across the lifespan. Dev Neuropsychol. 2011;36:408–428. doi: 10.1080/87565641.2010.549865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A New Statistical Method for Haplotype Reconstruction from Population Data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD, Russell ML. Differential effects of induced mood on the recall of positive, negative and neutral words. Br J Clin Psychol. 1983;22:163–171. doi: 10.1111/j.2044-8260.1983.tb00597.x. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, Eigsti IM, Thomas KM, Freed PJ, Booma ES, Gunnar MR, Altemus M, Aronson J, Casey BJ. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2010;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stegeren AH, Wolf OT, Everaerd W, Scheltens P, Barkhof F, Rombouts SARB. Endogenous cortisol level interacts with noradrenergic activation in the human amygdala. Neurobiol Learn Mem. 2007;87:57–66. doi: 10.1016/j.nlm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Velders FP, Kuningas M, Kumari M, Dekker MJ, Uitterlinden AG, Kirschbaum C, Hek K, Hofman A, Verhulst FC, Kivimaki M, van Duijn CM, Walker BR, Tiemeier H. Genetics of cortisol secretion and depressive symptoms: A candidate gene and genome wide association approach. Psychoneuroendocrino. 2011;36:1053–1061. doi: 10.1016/j.psyneuen.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani R. Unbiased ROI selection in neuroimaging studies of individual differences. Neuroimage. 2010;50:184–189. doi: 10.1016/j.neuroimage.2009.10.085. [DOI] [PubMed] [Google Scholar]
- Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY. Neural Correlates of Facial Affect Processing in Children and Adolescents With Autism Spectrum Disorder. J Am Acad Child Psy. 2004;43:481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S. Artifact Detection and QA Manual. MIT; 2009. [Google Scholar]
- Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, Schwartz CE, Shin LM, Fischer H, McMullin K, Rauch SL. Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex. Neuroimage. 2003;18:660–669. doi: 10.1016/s1053-8119(02)00037-x. [DOI] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharm. 2010;35:1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Post-traumatic stress disorder. N Engl J Med. 2002;346:108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Bruckl T, Nocon A, Pfister H, Binder EB, Uhr M, Lieb R, Moffitt TE, Caspi A, Holsboer F, Ising M. Interaction of FKBP5 Gene Variants and Adverse Life Events in Predicting Depression Onset: Results From a 10-Year Prospective Community Study. Am J Psychiat. 2011;168:1107–1116. doi: 10.1176/appi.ajp.2011.10111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.