Abstract
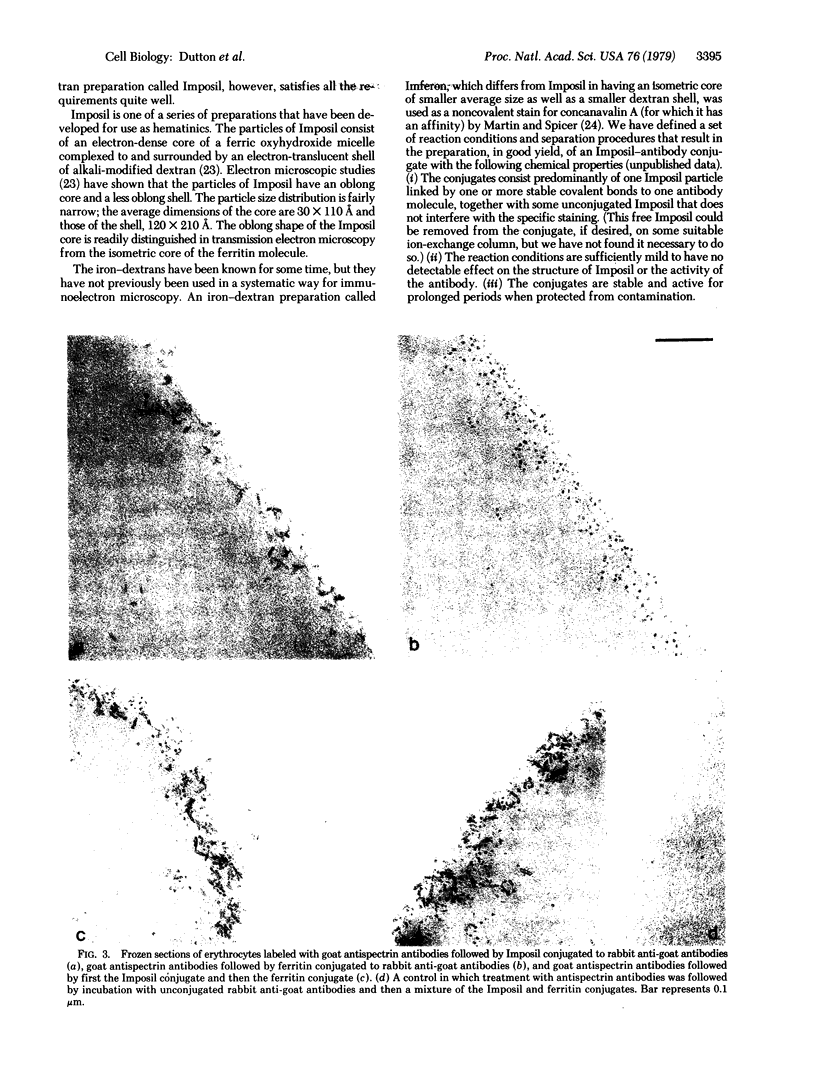
We report the preparation and properties of an electron-dense antibody conjugate, made by the covalent bonding of an iron—dextran (Imposil) particle to an antibody molecule. Transmission electron microscopic experiments with the Imposil—antibody conjugates demonstrate their suitability as specific immunostains at high resolution, particularly for simultaneous double staining experiments in conjunction with ferritin—antibody conjugates.
Keywords: cell ultrastructure, Imposil, ferritin, ultracryotomy
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avol E., Carmichael D., Hegenauer J., Saltman P. Rapid induction of ferritin in laboratory animals prior to its isolation. Prep Biochem. 1973;3(3):279–290. doi: 10.1080/00327487308061512. [DOI] [PubMed] [Google Scholar]
- Faulk W. P., Taylor G. M. An immunocolloid method for the electron microscope. Immunochemistry. 1971 Nov;8(11):1081–1083. doi: 10.1016/0019-2791(71)90496-4. [DOI] [PubMed] [Google Scholar]
- Horisberger M., Rosset J. Colloidal gold, a useful marker for transmission and scanning electron microscopy. J Histochem Cytochem. 1977 Apr;25(4):295–305. doi: 10.1177/25.4.323352. [DOI] [PubMed] [Google Scholar]
- Hämmerling U., Aoki T., Wood H. A., Old L. J., Boyse E. A., de Harvin E. New visual markers of antibody for electron microscopy. Nature. 1969 Sep 13;223(5211):1158–1159. doi: 10.1038/2231158a0. [DOI] [PubMed] [Google Scholar]
- Karnovsky M. J., Unanue E. R., Leventhal M. Ligand-induced movement of lymphocyte membrane macromolecules. II. Mapping of surface moieties. J Exp Med. 1972 Oct 1;136(4):907–930. doi: 10.1084/jem.136.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida Y., Olsen B. R., Berg R. A., Prockop D. J. Two improved methods for preparing ferritin-protein conjugates for electron microscopy. J Cell Biol. 1975 Feb;64(2):331–339. doi: 10.1083/jcb.64.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. J., Spicer S. S. Letter: Concanavalin A-iron dextran technique for staining cell surface mucosubstances. J Histochem Cytochem. 1974 Mar;22(3):206–207. doi: 10.1177/22.3.206. [DOI] [PubMed] [Google Scholar]
- Molday R. S., Dreyer W. J., Rembaum A., Yen S. P. New immunolatex spheres: visual markers of antigens on lymphocytes for scanning electron microscopy. J Cell Biol. 1975 Jan;64(1):75–88. doi: 10.1083/jcb.64.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P K, Pierce G B., Jr Enzyme-labeled antibodies: preparation and application for the localization of antigens. J Histochem Cytochem. 1966 Dec;14(12):929–931. doi: 10.1177/14.12.929. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Marchesi V. T., Singer S. J. The localization of spectrin on the inner surface of human red blood cell membranes by ferritin-conjugated antibodies. J Cell Biol. 1971 Oct;51(1):265–272. doi: 10.1083/jcb.51.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Singer S. J. Ferritin-conjugated plant agglutinins as specific saccharide stains for electron microscopy: application to saccharides bound to cell membranes. Proc Natl Acad Sci U S A. 1971 May;68(5):942–945. doi: 10.1073/pnas.68.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. G., Sheetz M., Singer S. J. Detection and ultrastructural localization of human smooth muscle myosin-like molecules in human non-muscle cells by specific antibodies. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1359–1363. doi: 10.1073/pnas.72.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts C. R., Cox J. S., Fitzmaurice C., Moss G. F. The iron-dextran complex. Nature. 1965 Oct 16;208(5007):237–239. doi: 10.1038/208237a0. [DOI] [PubMed] [Google Scholar]
- SINGER S. J. Preparation of an electron-dense antibody conjugate. Nature. 1959 May 30;183(4674):1523–1524. doi: 10.1038/1831523a0. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J., Wilson D. V. A simple method for coupling proteins to insoluble polysaccharides. Immunology. 1971 Jun;20(6):1061–1065. [PMC free article] [PubMed] [Google Scholar]
- Smith S. B., Revel J. P. Mapping of concanavalin A binding sites on the surface of several cell types. Dev Biol. 1972 Mar;27(3):434–441. doi: 10.1016/0012-1606(72)90183-2. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. A study of positive staining of ultrathin frozen sections. J Ultrastruct Res. 1978 Jun;63(3):287–307. doi: 10.1016/s0022-5320(78)80053-7. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T., Singer S. J. Improved procedures for immunoferritin labeling of ultrathin frozen sections. J Cell Biol. 1976 Dec;71(3):894–906. doi: 10.1083/jcb.71.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]