Abstract
The participation of the mevalonic acid (MVA) and 1-deoxy-D-xylulose 5-phosphate/2-C-methyl-D-erythritol-4-phosphate (DOXP/MEP) pathways in sesquiterpene biosynthesis of grape berries was investigated. There is an increasing interest in this class of terpenoids, since the oxygenated sesquiterpene rotundone was identified as the peppery aroma impact compound in Australian Shiraz wines. To investigate precursor supply pathway utilization, in vivo feeding experiments were performed with the deuterium labeled, pathway specific, precursors [5,5-2H2]-1-deoxy-D-xylulose and [5,5-2H2]-mevalonic acid lactone. Head Space-Solid Phase Micro Extraction-Gas Chromatography-Mass Spectrometry (HS-SPME-GC-MS) analysis of the generated volatile metabolites demonstrated that de novo sesquiterpene biosynthesis is mainly located in the grape berry exocarp (skin), with no detectable activity in the mesocarp (flesh) of the Lemberger variety. Interestingly, precursors from both the (primarily) cytosolic MVA and plastidial DOXP/MEP pathways were incorporated into grape sesquiterpenes in the varieties Lemberger, Gewürztraminer and Syrah. Our labeling data provide evidence for a homogenous, cytosolic pool of precursors for sesquiterpene biosynthesis, indicating that a transport of precursors occurs mostly from plastids to the cytosol. The labeling patterns of the sesquiterpene germacrene D were in agreement with a cyclization mechanism analogous to that of a previously cloned enantioselective (R)-germacrene D synthase from Solidago canadensis. This observation was subsequently confirmed by enantioselective GC-MS analysis demonstrating the exclusive presence of (R)-germacrene D, and not the (S)-enantiomer, in grape berries.
Keywords: Grapevine, Monoterpenes, Diterpenes, 1-Deoxy-D-xylulose, Mevalonic acid, Head space, Solid Phase-Microextration, Gas Chromatography-Mass Spectrometry, Deuterium Labeling
1. Introduction
Terpenoids belong to one of the largest classes of plant metabolites with numerous biological functions, including defense, growth, reproduction, and sensorial attributes (Borg-Karlson, 1990; Köllner et al., 2009; Matich et al., 2003; Price et al., 1990; Schmiderer et al., 2010). Monoterpenes contribute distinctively to the sensorial character of aromatic grape varieties such as Muscat, Gewürztraminer or Riesling (Marais, 1987; Park et al., 1991; Rapp, 1990). Attributes like floral, fruity and citrus can be assigned to the monoterpenes linalool, geraniol, nerol and citronellol, respectively. In addition, C13-norisoprenoids, degradation products of carotenoids, are also considered to be key odorants (Baumes et al., 2002; Mendes-Pinto, 2009). For example, β-damascenone is often described as having a stewed apple, flowery or honey-like aroma, while β-ionone imparts a violet and raspberry-like note. Aroma-active sesquiterpenes are known to occur in various plant species (Haring et al., 1972; Kjeldsen et al., 2003) but, traditionally, relatively little attention has been given to this terpene class in grapevine. However, the sesquiterpene ketone rodundone was recently identified as the key odorant responsible for the peppery attributes of Australian Shiraz wines (Wood et al., 2008), which has led to a growing interest in this class of volatiles. Grapevine flowers produce several sesquiterpenoids with potential roles as attractants for pollinators (Buchbauer et al., 1994, Buchbauer et al., 1995; Lücker et al., 2004; Martin et al., 2009). Furthermore, several sesquiterpene hydrocarbons, most prominently a-ylangene, p-bourbonene, p-caryophyllene, a-humulene and germacrene D, and oxygenated sesquiterpenes, such as farnesol, nerolidol or y-eudesmol, have been described as grape berry and wine constituents (Alves et al., 2005;; Coelho et al., 2006; Robinson et al., 2011; Schreier et al., 1976). An investigation of the profile of grape berry volatiles revealed that their release is developmentally regulated (e.g., increase of sesquiterpenes with a cadinane backbone during ripening) and differed considerably between several varieties (May and Wüst, 2012). The majority of sesquiterpenes was found to be present in grape berry exocarp (skin), whereas only minor amounts were detectable in mesocarp (flesh) at the stage of full ripeness.
While it has been demonstrated that sesquiterpenes are selectively accumulated in the cuticular wax layer of the exocarp (May and Wüst 2011), the site of their biosynthesis and the utilized biochemical pathways are still unknown. In vivo feeding experiments performed with grape berries of the Muscat Ottonel cultivar demonstrated that monoterpenes are almost exclusively synthesized via the DOXP/MEP pathway (Luan and Wüst, 2002), with little if any incorporation of an MVA pathway intermediate. However, the precursor pathway contribution for sesquiterpene synthesis and its localization within the grape berries is still unknown. In some plants, both the primarily cytosolic MVA and the plastidial DOXP/MEP pathway can be involved in the formation of the universal terpenoid pathway intermediates isopentenyl diphosphate (IPP) and dimethylallyl diposphate (DMAPP) (Hemmerlin et al. 2012). This phenomenon, which likely involves an exchange of terpenoid pathway intermediates between cytoplasm and plastids, has been termed “metabolic cross talk”. This exchange and the related compartmentalization in monoterpene and sesquiterpene biosynthesis in plants has been recently reviewed by Gutensohn et al. (2013). To evaluate the origin of precursors for grape berry sesquiterpenes, in vivo feeding experiments were performed using [5,5-2H2]-mevalonic acid lactone (2H2-MVL) and [5,5-2H2]-1-deoxy-D-xylulose (2H2-DOX) with the neutral variety Lemberger and the floral/Muscat-type variety Gewürztraminer. Neutral grape varieties are not dependent upon monoterpenes for their flavor. This classification has been introduced by Strauss et al. (1986). By integrating our previous results on monoterpene biosynthesis (Luan and Wüst, 2002) with those reported here on sesquiterpene precursor pathways, we are providing a more detailed model of precursor utilization in grape berries.
2. Results and Discussion
2.1. Experimental design and analysis of sesquiterpene labeling patterns
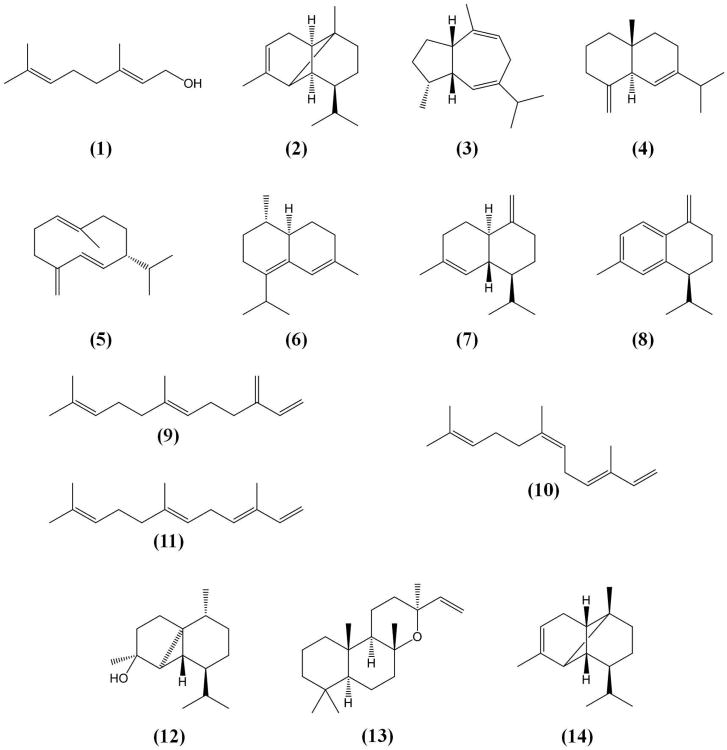
To investigate pathway utilization in sesquiterpene biosynthesis, a Muscat/floral variety with high monoterpene content (Gewürztraminer) and a neutral variety (Lemberger) were chosen. Head Space-Solid Phase Micro Extraction-Gas Chromatography-Mass Spectrometry (HS-SPME-GC-MS) analysis of volatiles released at full berry ripeness indicated that the profile of Lemberger is dominated by bicyclic sesquiterpenes (in order of elution: α-ylangene (2), an unknown volatile (a), guaia-6,9-diene (3), selina-4,6-diene (4), germacrene D (5) as major sesquiterpene, epi-zonarene (6), γ-cadinene (7), an unknown volatile (b), and β-calacorene (8)) (Fig. 1; Fig. 2A). In contrast, acyclic sesquiterpenes predominated in Gewürztraminer (in order of elution: (E,E)-β-farnesene (9), (E,Z)-α-farnesene (10) and (E,E)-α-farnesene (11) as the major sesquiterpene), with small amounts of copaene (14), 5, 7, cubebol (12) and an unknown volatile (c) (Fig. 1; Fig. 2B). In both varieties, the monoterpene geraniol and the volatile diterpene 13-epi-manoyl oxide (13) were detected as well (Fig. 2).
Fig. 1.
Structures of grape volatile terpenoids (absolute stereochemistry not identified with the exception of (R)-germacrene D (5)).
Fig. 2.
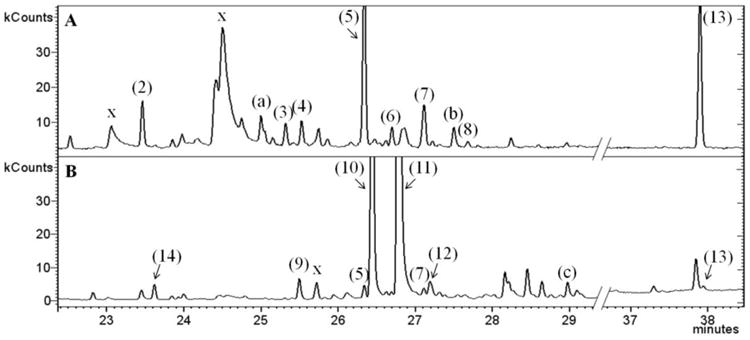
HS-SPME-GC-MS total ion chromatograms of volatiles released from grape berries of the varieties of Lemberger (A) and Gewürztraminer (B). Sesquiterpenes are numbered according to Fig. 1. Peaks labeled with “a, b, or c” belong to volatiles with sesquiterpene like mass spectra. Peaks labeled with “x” were assigned to SPME fiber bleeding.
To evaluate the relative contribution of the MVA and DOXP/MEP pathways for sesquiterpene precursor biosynthesis, [5,5-2H2]-mevalonic acid lactone (2H2-MVL) or [5,5-2H2]-1-deoxy-D-xylulose (2H2-DOX) were injected into the mesocarp (flesh) of intact grape berries or isolated exocarp (skin), and emitted volatiles were subsequently analyzed by HS-SPME-GC-MS. This technique was the method of choice because it can be performed rapidly and at small scale, and previous studies had shown that grape berries emit sesquiterpene hydrocarbons into the head space (May and Wüst, 2012). Even without an absolute quantification of the emitted volatiles we were still able to calculate the percentage of deuterium label incorporation into volatiles (with respect to the unlabeled genuine compound), which provides essential information about pathway utilization.
Since sesquiterpenes are synthesized from one unit of DMAPP and two units of IPP, each of which can harbor up to two deuterium atoms from either 2H2-MVL or 2H2-DOX, the final product may contain up to six deuterium atoms. The degree of deuterium incorporation can be deduced from the corresponding mass spectra, which is facilitated by the fact that genuine und deuterium-labeled sesquiterpenes can be chromatographically separated by GC due to inverse isotope effects (Matucha et al., 1991). Since the signal corresponding to the molecular ion (M•+) of sesquiterpene hydrocarbons is generally low, the ion signal of the characteristic fragment after in-source elimination of an isopropyl group was used for quantification. It is important to note that this fragmentation does not lead to a loss of deuterium.
After administering 2H2-MVL or 2H2-DOX, a deuterium labeling was detected for all major sesquiterpene hydrocarbons in the headspace of all investigated grape cultivars (see Fig. 3 for Lemberger). Feeding with 2H2-DOX and 2H2-MVL leads to an incorporation of label into the same positions in IPP and DMAPP, and the sesquiterpene end products thus have the same deuterium labeling patterns. Most genuine sesquiterpene hydrocarbons with a molecular ion at m/z=204 showed a mass shift by six mass units to m/z=210, which is in agreement with the incorporation of three units of 2H2-IPP/2H2-DMAPP (Fig. 3A, B). Nevertheless, d4- and d2-labeled sesquiterpene hydrocarbons were detectable as well (Supplementary Fig. 1). Interestingly, guaia-6,9-diene incorporated only five deuterium atoms (Fig. 3C), which can be explained by the elimination of deuterium during the terpene synthase-catalyzed cyclization reaction. A reaction pathway to guaia-6,9-diene has been proposed by Steele et al. (1998). However, the obtained labeling pattern that is in agreement with our MS-data can be best rationalized by a cyclization mechanism that combines early cyclization steps from (R)-germacrene D formation (Schmidt et al., 1999), that leads to a germacrene C intermediate, and the last cyclization steps to guaia-6,9-diene according to Steele et al. (1998, Supplementary Fig. 2).
Fig. 3.
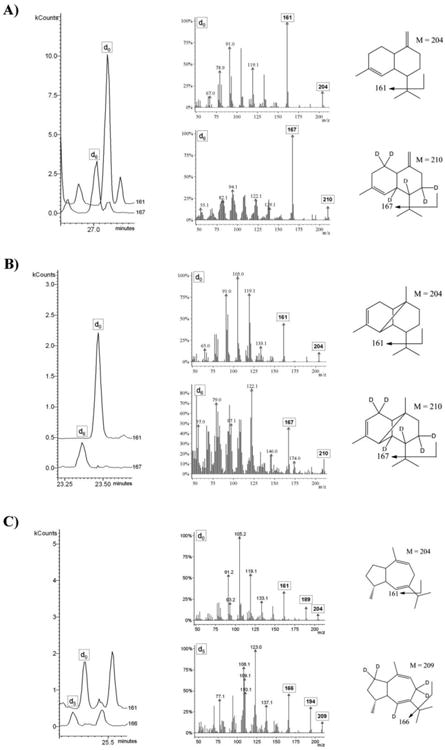
HS-SPME-GC-MS single ion monitoring chromatograms (left panel) and MS spectra of genuine and deuterium-labeled sesquiterpenes (center panel) after administration of [5,5-2H2]-1-deoxy-D-xylulose to isolated exocarp of gape berries (Lemberger cultivar). Expected labeling patterns are depicted for the sesquiterpenes (A) γ-cadinene, (B) α-ylangene and (C) guaia-6,9-diene (right panel). Essentially identical labeling patterns were observed in feeding experiments with [5,5-2H2]-mevalonic acid lactone.
2.2 Injection of labeled precursors into mesocarp of intact grape berries
2H2-MVL or 2H2-DOX were injected directly into mesocarp of intact grape berries (Lemberger variety) and, after an incubation time of 48 hours, berries were peeled and exocarp and mesocarp analyzed separately by HS-SPME-GC-MS. Label was not incorporated to detectable levels into mesocarp sesquiterpenes from any substrate. In contrast, the injection of precursors into the mesocarp yielded a high degree of labeling in exocarp-derived sesquiterpenes (Table 1), indicating that labeled precursors or end products must have traveled from the mesocarp to the exocarp. It is unknown whether this intriguing translocation is achieved by an active transport mechanism or by simple diffusion. These observations are in agreement with previous metabolite profiling studies, where almost no sesquiterpene hydrocarbons were detectable in the mesocarp, whereas these metabolites were found to be present in exocarp (May and Wüst, 2011). An incorporation of 2H2-DOX, but not 2H2-MVL, into monoterpenes was detectable in both mesocarp (Supplementary Fig. 3) and exocarp (Table 1), which is also consistent with prior studies that demonstrated the presence of monoterpenes in both tissue types (Luan and Wüst, 2002). The diterpene 13-epi-manoyl oxide was found to be labeled following incubation with 2H2-DOX (low incorporation), but not when 2H2-MVL was proffered (Table 1).
Table 1.
Incorporation of [5,5-2H2]-mevalonic acid lactone (2H2-MVL) and [5,5-2H2]-1-deoxy-D-xylulose (2H2-DOX) into terpene volatiles released from intact grape berries (exocarp) of Vitis vinifera cv. Lemberger when labeled precursors were directly injected into mesocarp. The values were obtained from a typical experiment with grapes from the vintage 2010. Incorporation rates were calculated by dividing the peak area of the labeled compound (Al) by the peak area of the genuine compound (Ag) multiplied by 100% i.e. (A1/Ag)*100%. Thus, incorporation rates higher than 100% indicate that the labeled compound is present at a higher concentration than the genuine compound.
| Peak1 | Compound | Identification2 | Diagnostic ions for quantitation (m/z) | Precursor inco 2H2-MVL | rporation [%] 2H2-DOX |
|---|---|---|---|---|---|
| Monoterpenes | |||||
| 1 | Geraniol | a | 69/71 | n.d. | 79 |
| Sesquiterpenes | |||||
| 2 | α-Ylangene | b | 161/167 | 41 | 6 |
| a | Unknown (RI 1432) | e | 189/195 | 139 | 20 |
| 3 | Guaia-6,9-diene | b | 161/166 | 35 | n.q. |
| 4 | Selina-4,6-diene | c | 161/166 | 48 | 5 |
| 5 | Germacrene D | a | 161/167 | 46 | n.q. |
| 6 | epi-Zonarene | b | 161/166 | 16 | 3 |
| 7 | γ-Cadinene | b | 161/167 | 47 | 4 |
| b | Unknown (RI 1534) | e | 122/125 | 33 | 6 |
| 8 | β-Calacorene | b | 142/146 | n.q. | n.q. |
| Diterpenes | |||||
| 13 | 13-epi-Manoyl oxide | d | 257/265 | n.d. | 3 |
n.d., not detectable
n.q., not quantifiable
RI, retention index on a DB5 column
numbers according to Fig. 1
a: identified using standard substances, b: identified by mass spectrum and retention index of the massfinder® library, c: tentatively identified by mass spectrum and retention index of the massfinder® library, d: tentatively identified by mass spectrum and retention index of: R. P. Adams, Identification of essential oil components by gas chromatography/mass spectroscopy, Allured Publishing Corporation, Carol Stream, 1995, e: unknown
2.3 Incubation of isolated grape berry exocarp with labeled precursors
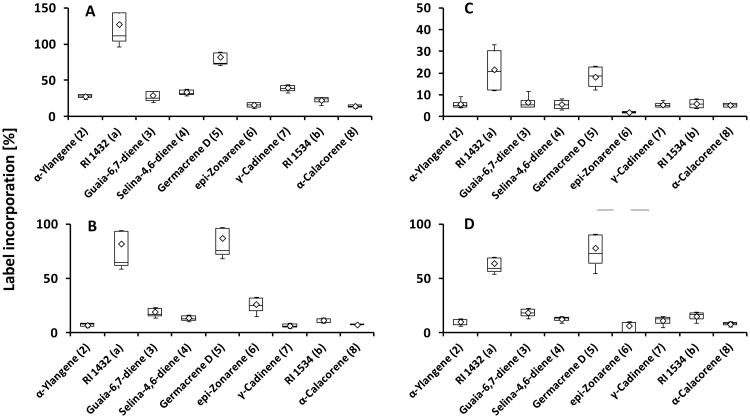
Our preliminary experiments demonstrated that sesquiterpenes in grapevine are synthesized in the fruit exocarp and all follow-up experiments were thus performed with excised exocarp tissue. In experiments performed with grape of the Lemberger cultivar in 2010, the incorporation rates for 2H2-MVL into sesquiterpenes were higher than those obtained with 2H2-DOX, while in a repeated set of experiments (2012) the incorporation rates for both precursors into sesquiterpenes was comparable (Fig. 4). These results indicate that the MVA and DOXP/MEP pathways both contribute to sesquiterpene biosynthesis in the Lemberger cultivar. The highest incorporation rates were detected for germacrene D (5), the major sesquiterpene of the Lemberger variety, and an unidentified sesquiterpene with a retention index of 1432, regardless of which precursor was proffered to fruit skins (Fig. 4). Deuterium label from 2H2-DOX was incorporated at low rates (3 %) into the diterpene 13-epi-manoyl oxide (13), while incorporation of label from 2H2-MVL was very low (< 0.5 %) (Supplementary Fig. 4).
Fig. 4.
Incorporation of deuterium label from terpenoid precursors, administered to isolated fruit exocarp of the Lemberger variety (harvested in 2010 (A and C) and 2012 (B and D)), into sesquiterpene volatiles (n=4). [5,5-2H2]-Mevalonic acid lactone (A and B) or [5,5-2H2]-1-deoxy-D-xylulose (C and D) were used as precursors incorporated via the MVA and DOXP/MEP pathways, respectively. Sesquiterpenes are numbered according to Fig. 1. Box-whisker plots show the 25th (bottom) and 75th (top) percentile, separated by the median. The mean is depicted by a diamond. The lower whisker corresponds to the bottom percentile minus the minimum value, while the upper whisker indicates the maximum value minus the top percentile.
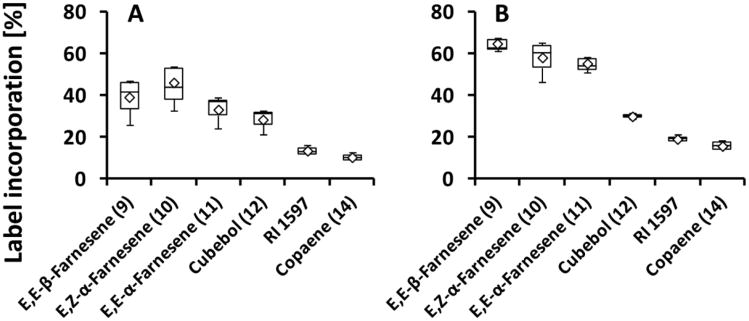
Label from both 2H2-MVL and 2H2-DOX was readily incorporated into sesquiterpenes in the Gewürztraminer variety (Fig. 5). Very similar incorporation rates of deuterium label from 2H2-MVL were observed for (E,E)-β-farnesene (9), (E,Z)-α-farnesene (10), (E,E)-α-farnesene (11) and cubebol (12), while label from 2H2-DOX was preferentially incorporated into 9, 10 and 11, with lower incorporation rates into 12 (Fig. 5). Equal rates of both precursors were incorporated into sesquiterpenes of the variety Syrah, as well. Comparable to the variety Lemberger, highest incorporation rates were achieved for germacrene D and the unidentified sesquiterpene with a retention index of 1432 (Fig. 6).
Fig. 5.
Incorporation of deuterium label from terpenoid precursors, administered to isolated fruit exocarp of the Gewürztraminer variety (harvested in 2012), into sesquiterpene volatiles (n=3). [5,5-2H2]-Mevalonic acid lactone (A) or [5,5-2H2]-1-deoxy-D-xylulose (B) were used as precursors incorporated via the MVA and DOXP/MEP pathways, respectively. Sesquiterpenes are numbered according to Fig. 1. Box-whisker plots show the 25th (bottom) and 75th (top) percentile, separated by the median. The mean is depicted by a diamond. The lower whisker corresponds to the bottom percentile minus the minimum value, while the upper whisker indicates the maximum value minus the top percentile.
Fig. 6.
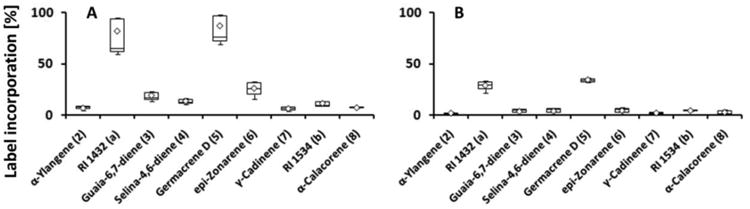
Incorporation of deuterium label from terpenoid precursors, administered to isolated fruit exocarp of the Syrah variety (harvested in 2012), into sesquiterpene volatiles (n=3). [5,5-2H2]-Mevalonic acid lactone (A) or [5,5-2H2]-1-deoxy-D-xylulose (B) were used as precursors incorporated via the MVA and DOXP/MEP pathways, respectively. Sesquiterpenes are numbered according to Fig. 1. Box-whisker plots show the 25th (bottom) and 75th (top) percentile, separated by the median. The mean is depicted by a diamond. The lower whisker corresponds to the bottom percentile minus the minimum value, while the upper whisker indicates the maximum value minus the top percentile.
2.4 Evidence for a transport of farnesyl diphosphate precursors from plastids to the cytosol
Plastidial synthesis of (Z,Z)-farnesyl diphosphate and derived sesquiterpenes has been demonstrated to occur in tomato (Sallaud et al., 2009) but, at this point in time, the available evidence indicates that sesquiterpene synthases encoded in the grape genome localize to the cytosol and use (E,E)-farnesyl diphosphate as substrate (Martin et al., 2012). The labeling data reported here demonstrate that pools of both cytosolic and plastidial IPP/DMAPP (synthesized via the MVA and DOXP/MEP pathways, respectively) can serve as precursors for sesquiterpene biosynthesis in grapevine fruit exocarp. These results are comparable to those obtained with foliage of Vitis vinifera L., were both pathways are used at equal rates in jasmonate-induced sesquiterpene biosynthesis (Hampel et al., 2005). Bick and Lange (2003) characterized a unidirectional proton symport system for the export of isoprenoid intermediates from plastids to cytosol. Isolated chloroplasts from spinach, kale and Indian mustard were demonstrated to be capable of an efficient export of IPP and geranyl diphosphate, while a much less efficient transport was detected for farnesyl diphosphate and DMAPP, and almost none for geranylgeranyl diphosphate and mevalonate (Bick and Lange, 2003). An import of IPP by plastids isolated from cell cultures of Vitis vinifera cv. Muscat de Frontignan was demonstrated by Soler et al. (1993). The genes encoding these transporters have remained elusive.
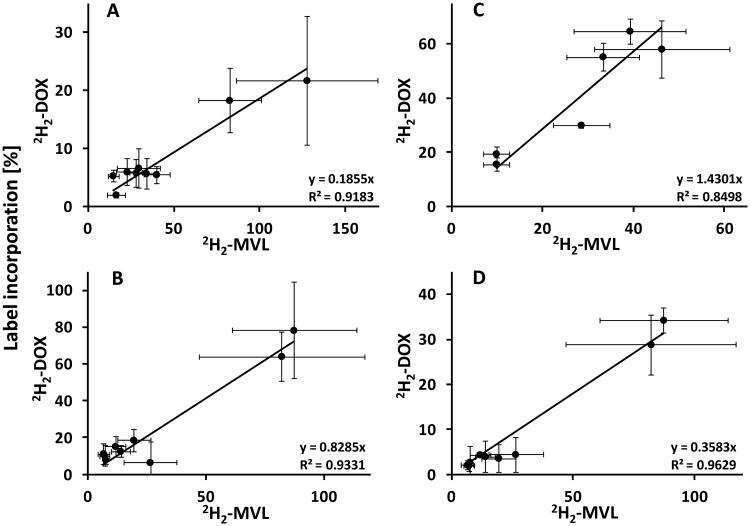
Interestingly, we observed a linear relationship between the incorporation rates of 2H2-MVL and 2H2-DOX into grape sesquiterpenes, which appeared to be independent of the variety (Lemberger, Gewürztraminer or Syrah), the structural class of emitted volatiles (primarily bicyclic sesquiterpenes in Lemberger and Syrah; primarily acyclic sesquiterpenes in Gewürztraminer) and harvest year (feeding experiments with the Lemberger variety in 2010 and 2012) (Fig. 7). Taken together, our results provide evidence that an exchange process generates a homogenous, cytosolic pool of IPP/DMAPP from both cytosolic and plastidial precursors, which is then further metabolized into sesquiterpenes.
Fig. 7.
Correlation analysis of deuterium label incorporation from [5,5-2H2]-mevalonic acid lactone and [5,5-2H2]-1-deoxy-D-xylulose into grape berry sesquiterpenes of the varieties Lemberger (harvested in 2010 (A) or 2012 (B)), Gewürztraminer (C) and Syrah (D). Mean ± SD (n=3-4) is shown for the following sesquiterpenes: α-ylangene (2), an unknown volatile (a), guaia-6,9-diene (3), selina-4,6-diene (4), germacrene D (5) as major sesquiterpene, epi-zonarene (6), γ-cadinene (7), an unknown volatile (b) and β-calacorene (8)) of the varieties Lemberger and Syrah; (E)- β-farnesene (9), (E,Z)-α-farnesene (10) and (E,E)- α-farnesene (11) as the major sesquiterpene), cubebol (12), an unknown volatile (c) and copaene (14) of the Gewürztraminer variety (labeling of sesquiterpenes according to Fig. 1).
The monoterpene geraniol (1) was efficiently labeled when 2H2-DOX was fed to grape berries of the Lemberger variety, but no incorporation of label was detectable in feeding experiments with 2H2-MVL (Table 1). In a previous study based on feeding experiments with identical deuterium labeled precursors, we demonstrated that several grape monoterpenes are almost exclusively synthesized by the DOXP/MEP pathway (Luan and Wüst, 2002). Labeling of the diterpene 13-epi-manoyl oxide (13) was low but clearly detectable with 2H2-DOX (Supplementary Fig. 4), whereas labeling from 2H2-MVL was detectable but below the limit of quantification. Since monoterpene synthases (including the functionally characterized geraniol synthase) and diterpene synthases of grape are predicted to be localized to plastids (Martin et al., 2010), there appears to be only a very limited import of cytosolic IPP/DMAPP into plastids for the biosynthesis of mono- and diterpenes, indicating that a transport of precursors occurs primarily in the direction from plastids to the cytosol.
2.5. Stereochemistry of germacrene D
Germacrene D is a widespread chiral sesquiterpene hydrocarbon. The gape berry moth (Paralobesia viteana (Clemens)) perceives terpenoids, including germacrene D, emitted from Vitis sp. to locate its primary host (Cha et al., 2011). The stereochemistry of germacrene D influences its biological activity, including its effects on herbivors. For example, Stranden et al. (2002) showed that (S)-germacrene D elicits a tenfold stronger effect on a receptor neuron of the cotton bollworm moth Helicoverpa armigera than its enantiomer. The gene encoding germacrene D synthase in grape has been characterized (Martin et al., 2010), but the stereochemistry of germacrene D formed by this enzyme and emitted by grape berries has not yet been investigated. Deuterium-labeled germacrene D, synthesized in grape berries after administration of 2H2-DOX or 2H2-MVA fragmented to a prominent ion at m/z = 167 (Fig. 8A, B), which was identical to the fragmentation pattern observed for the (R)-enantiomer in Solidago canadensis (Steliopoulos et al., 2002). Enantioselective GC-MS confirmed the exclusive presence of the (R)-enantiomer in grape berries (Fig. 8C). This finding is interesting because the (R)-enantiomer is known to occur widely in early vascular plants such as liverworts, but was thought to be less prevalent in angiosperms (König et al., 1996).
Fig. 8.
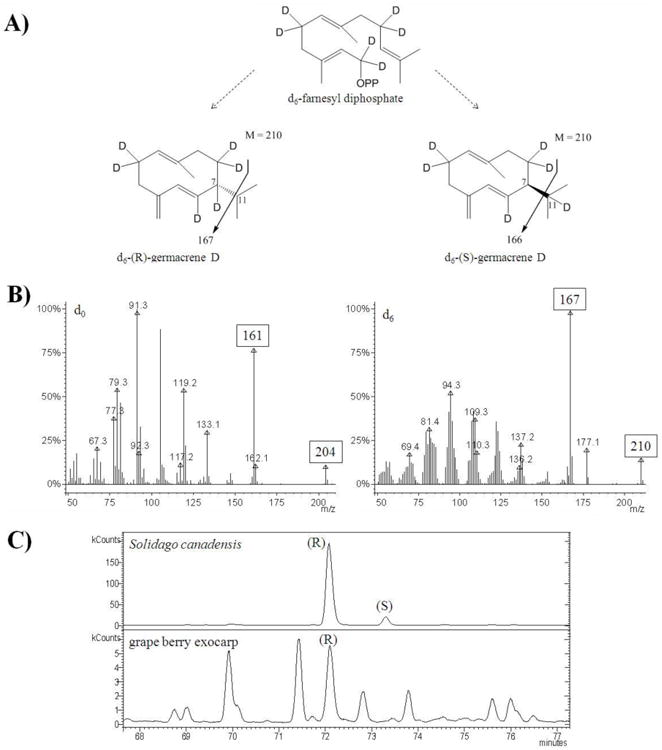
Identification of germacrene D enantiomers in volatiles emitted from grape berries (Lemberger cultivar). (A) Postulated incorporation of labeled precursors according to the enzymatic cyclization mechanism in Solidago canadensis (Schmidt et al., 1999). (B) MS spectra of genuine and deuterium-labeled germacrene. (C) Separation and identification of germacrene D enantiomers by enantio-GC/MS analysis of Solidago canadensis (reference) and Vitis vinifera cv. Lemberger (Syrah contained the same germacrene D enantiomer as Lemberger).
3. Conclusions
The analytical and labeling data obtained so far indicate that sesquiterpene biosynthesis and accumulation in grape berries is restricted to the exocarp and that both routes (MVA and MEP/DOXP) are utilized for sesquiterpene biosynthesis. However Rohmer (1999) has already stated in a remarkable review that “overfeeding of metabolites such as MVA or DX, which are not accumulated by cells, does not represent normal physiological growth conditions. High concentrations of a precursor might activate the corresponding metabolic pathway and consequently lead to an overestimation of its contribution to the production of an isoprenoid.” Hence, pathway utilization in V. vinifera berries might be different under normal physiological conditions. Supplementary experimental techniques like 13C/12C-IRMS at natural abundance level (Jux et al. 2001, Bartram et al. 2006) and feeding more ‘neutral’ precursor such as labeled glucose (for a review see Schuhr et al. 2003) are available and are to be applied to investigate in more detail the metabolic cross talk in sesquiterpene biosynthesis in V. vinifera. Transporters implicated in the excretion of monoterpenes and diterpenes have recently been characterized (Jasinski et al., 2001; Wang et al., 2013), but the intriguing transport system responsible for the exchange of terpenoid pathway intermediates across the plastidial envelope membrane remains to be discovered.
The localization of sesquiterpenes in the exocarp is consistent with their increased release with prolonged skin contact times during fermentation into wine (Caputi et al., 2011). However, it has also been shown that inclusion of stems and leafs in grape must increases the content of the sesquiterpene rotundone (Capone et al., 2012), and further studies into the roles of these organs in the winemaking process are thus warranted.
4. Experimental
4.1. Plant material
Experiments were performed with the varieties Lemberger, obtained from the Institute of Grapevine Breeding, Geisenheim Research Centre/Germany, and Gewürztraminer and Syrah, which were obtained from the Institute of Crop Science and Resource Conservation (INRES) of the University of Bonn. The variety Lemberger was sampled in 2010 and 2012 at full fruit ripeness. The Gewürztraminer and Syrah varieties were sampled at full fruit ripeness in 2012. Golden rod (Soligado canadensis), which was used as a source of germacrene D enantiomers, was field-collected near Bonn (Germany) in autumn 2011.
4.2. Chemicals
[5,5-2H2]-1-deoxy-D-xylulose was synthesized according to Meyer et al. (2004), while [5,5-2H2]-mevalonic acid lactone was prepared according to Simpson et al. (1997). The spectral data of the synthesized compounds were in agreement with the data given in the original references.
4.3. Methods
Incubation of intact grapes
An aqueous solution of each precursor (10 μL, 10 mg/L) was injected into the pulp of intact grape berries (Lemberger variety). The microliter syringe was pierced through the receptacle into the pulp. After an incubation time of 48 hours, grapes were peeled, and mesocarp and exocarp were separated and analyzed separately. Sodium chloride was added to the mesocarp tissue until saturation to increase the volatility of the analytes.
Incubation of grape berry exocarp
Grapes were peeled and remaining mesocarp was removed manually from the exocarp tissue. An aqueous solution of each precursor (100 μL, 1 mg/L) was pipetted into a 10 mL headspace vial. Subsequently, approximately 1 cm² of the isolated skin was placed with the inner side on the solution to allow a direct uptake of the labeled compounds. The outer wax layer of the skin was not wetted, so that the emission of volatiles was not impaired. The skins were incubated for 48 hours at room temperature. The following HS-SPME-GC-MS measurement was performed directly out of the headspace vial without any further preparation.
SPME-Sampling
A 85 μm polyacrylate fiber, from Supelco (Bellefonte, PA, USA) was used for solid phase microextraction (SPME) of volatiles emitted from grape samples. After an equilibration period at 60 °C for 30 min, the SPME fiber was exposed to the sample headspace for 10 minutes. The mesocarp samples were agitated throughout the incubation at 400 rpm. Subsequently, the analytes were thermally desorbed in the GC-injection port.
GC-MS-analysis
GC-MS-analysis was performed using a Varian 450 GC coupled to a Varian 240 MS ion trap mass spectrometer (Palo Alto, CA, United States). The injection port was set to 220 °C. Splitless injection was used and the split valve was opened after 3 min. Separation was achieved using a DB5 column (Varian, length 30 m, 0.25 mm i.d., film thickness = 0.25 μm). The carrier gas (helium, 5.0) was set to 1 mL/min (constant flow). The column temperature program started at 35°C for 3 min, and was increased to 250°C at a rate of 5°C/min. The transfer line temperature was set to 260°C, the ion source to 150°C. An internal EI-ionization (70 eV) was performed and mass spectra were recorded in the range of m/z 35-350 (full scan), at a scan rate of 0.64 sec/scan. Sesquiterpenes were identified by comparison of the retention times and mass spectra with standard substances or by comparing their mass spectra and Kovats retention indices using the Massfinder® library (Hochmuth Scientific Consulting, Hamburg, Germany). Incorporation rates were calculated by dividing the peak area of the labeled compound (A1) by the peak area of the genuine compound (Ag) multiplied by 100% i.e. (A1/Ag)*100%.
Enantioselective GC-MS
Enantioselective GC-MS analysis was performed on the GC-MS system as described above with a fused silica capillary (30 m × 0.25 mm i.d.) coated with 20 % heptakis-(2,3-di-Omethyl-6-O-tert-butyldimethylsilyl)-b-cyclodextrin in BGB 15 (film thickness 0.25 μm) as stationary phase. Separation was achieved with the following temperature program: starting temperature was set at 60 °C for 3 min, and was then increased to 160°C with a rate of 1°C/min. The final temperature was held for further 10 min. All other GC/MS parameters were equal to those listed above.
Supplementary Material
Supplementary Fig. 1. HS-SPME-GC-MS selected ion monitoring chromatogram of genuine (d0), d2, d4 and d6-labeled (E,E)-a-farnesene after administration of [5,5-2H2]-mevalonic acid lactone (A) or [5,5-2H2]-1-deoxy-D-xylulose (B) to Vitis vinifera cv. Gewürztraminer. The characteristic ion traces are m/z 161 (genuine, d0), 163 (d2), 165 (d4) and 167 (d6).
Supplementary Fig. 2. Proposed reaction pathway to guaia-6,9-diene and (R)-germacrene D.
Supplementary Fig. 3. Incorporation of [5,5-2H2]-1-deoxy-D-xylulose (2H2-DOX) into monoterpenes of grape berry mesocarp (Vitis vinifera cv. Lemberger) compared to the feeding experiments with [5,5-2H2]-mevalonic acid lactone (2H2-MVL). A) HS-SPME-GC-MS total ion chromatogram of grape berry mesocarp after 2H2-DOX- and 2H2-MVL-feeding experiments and the corresponding mass spectra of genuine and labeled α-terpineol. B) Proposed fragmentation mechanism of genuine and labeled α-terpineol.
Supplementary Fig. 4. HS-SPME-GC-MS selected ion monitoring chromatogram for characteristic ion traces m/z 257 (genuine 13-epi-manoyl oxide), and 265 (d8-13-epi-manoyl oxide 13) after administration of [5,5-2H2]-1-deoxy-D-xylulose to Vitis vinifera cv. Lemberger (left panel). Mass spectra of genuine and d8-labeled 13-epi-manoyl oxide (center panel). The characteristic ions are M-CH3 (m/z 275 for genuine and m/z 283 for d8-labeled 13) and M-CH3-H2O (m/z 257 for genuine and m/z 265 for d8-labeled 13).
Results demonstrate the de novo sesquiterpenes biosynthesis in grape berry exocarp.
Precursors from both pathways were used for sesquiterpene biosynthesis.
Cross talk occurred primarily in the direction from plastids to the cytosol.
Linear relationship between incorporation rates achieved for both pathways.
Labeling data and enantio-GC-MS analysis confirmed the presence of (R)-germacrene D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alves RF, Nascimento AMD, Nogueira JMF. Characterization of the aroma profile of Madeira wine by sorptive extraction techniques. Anal Chim Acta. 2005;546:11–21. doi: 10.1016/j.aca.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Bartram S, Jux A, Gleixner G, Boland W. Dynamic pathway allocation in early terpenoids biosynthesis of stress-induced lima bean leaves. Phytochemistry. 2006;67:1661–1672. doi: 10.1016/j.phytochem.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Baumes R, Wirth J, Bureau S, Gunata Y, Razungles A. Biogeneration of C13-norisoprenoid compounds: experiments supportive for an apo-carotenoid pathway in grapevines. Anal Chim Acta. 2002;258:3–14. [Google Scholar]
- Bick JA, Lange BM. Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: unidirectional transport of intermediates across the chloroplast envelope membrane. Arch Biochem Biophys. 2003;415:146–154. doi: 10.1016/s0003-9861(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Borg-Karlson AK. Chemical and ethological studies of pollination in the genus Ophrys (orchidaceae) Phytochemistry. 1990;29:1359–1387. [Google Scholar]
- Buchbauer G, Jirovetz L, Wasicky M, Herlitschka A, Nikiforov A. Aroma von Weiβweinblüten: Korrelation sensorischer Daten mit Headspace-Inhaltsstoffen. Z Lebensm Unters Forsch. 1994;199:1–4. [Google Scholar]
- Buchbauer G, Jirovetz L, Wasicky M, Herlitschka A, Nikiforov A. Aroma von Rotweinblüten: Korrelation sensorischer Daten mit Headspace-Inhaltsstoffen. Z Lebensm Unters Forsch. 1995;200:443–446. [Google Scholar]
- Capone DL, Jeffery DW, Sefton MA. Vineyard and fermentation studies to elucidate the origin of 1,8-cineole in Australian red wine. J Agric Food Chem. 2012;60:2281–2287. doi: 10.1021/jf204499h. [DOI] [PubMed] [Google Scholar]
- Caputi L, Carlin S, Ghiglieno I, Stefanini M, Valenti L, Vrhovsek U, Mattivi F. Relationship of changes in rotundone content during grape ripening and winemaking to manipulation of the ‘peppery’ character of wine. J Agric Food Chem. 2011;59:5565–5571. doi: 10.1021/jf200786u. [DOI] [PubMed] [Google Scholar]
- Cha DH, Linn CE, Teal PEA, Zhang A, Roelofs WL, Loeb GM. Eavesdropping on plant volatiles by a specialist moth: significance of ratio and concentration. PLoS ONE. 2011;6:e17033. doi: 10.1371/journal.pone.0017033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho E, Rocha SM, Delgadillo I, Coimbra MA. Headspace-SPME applied to varietal volatile components evolution during Vitis vinifera L. cv. “Baga” ripening. Anal Chim Acta. 2006;563:204–214. [Google Scholar]
- Gutensohn M, Nagegowda DA, Dudareva N. Involvement of compartimentalization in monoterpene and sesquiterpene biosynthesis in plants. In: Bach TJ, Rohmer M, editors. Isoprenoid Synthesis in Plants and Mcroorganisms – New Concepts and Experimental Approaches. Springer; New York: 2013. [Google Scholar]
- Hampel D, Mosandl A, Wüst M. Induction of de novo volatile terpene biosynthesis via cytosolic and plastidial pathways by methyl jasmonate in foliage of Vitis vinifera L. J Agric Food Chem. 2005;53:2652–2657. doi: 10.1021/jf040421q. [DOI] [PubMed] [Google Scholar]
- Haring HG, Rijkens F, Boelens H, van der Gen A. Olfactory studies on enantiomeric eremophilane sesquiterpenoids. J Agric Food Chem. 1972;20:1018–1021. [Google Scholar]
- Hemmerlin A, Harwood JL, Bach TJ. A raison d'être for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Progr Lipid Res. 2012;51:95–148. doi: 10.1016/j.plipres.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Jasiński M, Stukkens Y, Degand H, Purnelle B, Marchand-Brynaert J, Boutry M. A plant plasma membrane ATP binding cassette-type transporter is involved in antifungal terpenoid secretion. Plant Cell. 2001;13:1095–1107. [PMC free article] [PubMed] [Google Scholar]
- Kjeldsen F, Christensen LP, Edelenbos M. Changes in volatile compounds of carrots (Daucus carota L.) during refrigerated and frozen storage. J Agric Food Chem. 2003;51:5400–5407. doi: 10.1021/jf030212q. [DOI] [PubMed] [Google Scholar]
- Köllner TG, Gershenzon J, Degenhardt J. Molecular and biochemical evolution of maize terpene synthase 10, an enzyme of indirect defense. Phytochemistry. 2009;70:1139–1145. doi: 10.1016/j.phytochem.2009.06.011. [DOI] [PubMed] [Google Scholar]
- König WA, Bülow N, Fricke C, Melching S, Rieck A, Muhle H. The sesquiterpene constituents of the liverwort Preissia quadrata. Phytochemistry. 1996;43:629–633. [Google Scholar]
- Luan F, Wüst M. Differential incorporation of 1-deoxy-D-xylulose into (3S)-linalool and geraniol in grape berry exocarp and mesocarp. Phytochemistry. 2002;60:451–459. doi: 10.1016/s0031-9422(02)00147-4. [DOI] [PubMed] [Google Scholar]
- Lücker J, Bowen P, Bohlmann J. Vitis vinifera terpenoid cyclases: functional identification of two sesquiterpene synthase cDNAs encoding (+)-valencene synthase and (-)-germacrene D synthase and expression of mono- and sesquiterpene synthases in grapevine flowers and berries. Phytochemistry. 2004;65:2649–2659. doi: 10.1016/j.phytochem.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Marais J. Terpene concentrations and wine quality of Vitis vinifera L. cv. Gewürztraminer as affected by grape maturity and cellar practices. Vitis. 1987;26:231–245. [Google Scholar]
- Martin DM, Aubourg S, Schouwey MB, Daviet L, Schalk M, Toub O, Lund ST, Bohlmann J. Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays. BMC Plant Biol. 2010;10:226. doi: 10.1186/1471-2229-10-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DM, Toub O, Chiang A, Lo BC, Ohse S, Lund ST, Bohlmann J. The bouquet of grapevine (Vitis vinifera L. cv. Cabernet Sauvignon) flowers arises from the biosynthesis of sesquiterpene volatiles in pollen grains. Proc Natl Acad Sci USA. 2009;106:7245–7250. doi: 10.1073/pnas.0901387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DM, Chiang A, Lund ST, Bohlmann J. Biosynthesis of wine aroma: transcript profiles of hydroxymethylbutenyl diphosphate reductase, geranyl diphosphate synthase, and linalool/nerolidol synthase parallel monoterpenol glycoside accumulation in Gewürztraminer grapes. Planta. 2012;236:919–929. doi: 10.1007/s00425-012-1704-0. [DOI] [PubMed] [Google Scholar]
- Matich AJ, Young H, Allen JM, Wang MY, Fielder S, McNeilage MA, MacRae EA. Actinidia arguta: volatile compounds in fruit and flowers. Phytochemistry. 2003;63:285–301. doi: 10.1016/s0031-9422(03)00142-0. [DOI] [PubMed] [Google Scholar]
- Matucha M, Jockisch W, Verner P, Anders G. Isotope effect in gas-liquid chromatography of labelled compounds. J Chromatogr A. 1991;588:251–258. [Google Scholar]
- May B, Wüst M. Sesquiterpene profiles of different grape varieties. In: Hofmann T, Meyerhof W, Schieberle P, editors. Advances and Challenges in Flavor Chemistry & Biology. Deutsche Forschungsanstalt für Lebensmittelchemie; Freising: 2011. pp. 323–327. [Google Scholar]
- May B, Wüst M. Temporal development of sesquiterpene hydrocarbon profiles of different grape varieties during ripening. Flavour Fragr J. 2012;27:280–285. [Google Scholar]
- Mendes-Pinto MM. Carotenoid breakdown products the-norisoprenoids-in wine aroma. Arch Biochem Biophys. 2009;483:236–245. doi: 10.1016/j.abb.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Meyer O, Hoeffler JF, Grosdemange-Billiard C, Rohmer M. Practical synthesis of 1-deoxy-D-xylulose and 1-deoxy-d-xylulose 5-phosphate allowing deuterium labelling. Tetrahedron. 2004;60:12153–12162. [Google Scholar]
- Park SK, Morrison JC, Adams DO, Noble AC. Distribution of free and glycosidically bound monoterpenes in the skin and mesocarp of Muscat of Alexandria grapes during development. J Agric Food Chem. 1991;39:514–518. [Google Scholar]
- Jux A, Gleixner G, Boland W. Classification of terpenoids to the methylerythritolphosphate or the mevalonate pathway with natural 12C/13C isotope ratios: dynamic allocation of resources in induced plants. Angew Chem, Int Ed. 2001;40:2091–2093. doi: 10.1002/1521-3773(20010601)40:11<2091::AID-ANIE2091>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Price KR, Dupont MS, Shepherd R, Chan HWS, Fenwick GR. Relationship between the chemical and sensory properties of exotic salad crops—coloured lettuce (Lactuca sativa) and chicory (Cichorium intybus) J Sci Food Agric. 1990;53:185–192. [Google Scholar]
- Rapp A. Natural flavours of wine: correlation between instrumental analysis and sensory perception. Fresenius J Anal Chem. 1990;337:777–785. [Google Scholar]
- Robinson AL, Boss PK, Heymann H, Solomon PS, Trengove RD. Development of a sensitive non-targeted method for characterizing the wine volatile profile using headspace solid-phase microextraction comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. J Chromatogr A. 2011;1218:504–517. doi: 10.1016/j.chroma.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Rohmer M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep. 1999;16:565–574. doi: 10.1039/a709175c. [DOI] [PubMed] [Google Scholar]
- Sallaud C, Rontein D, Onillon S, Jabès F, Duffé P, Giacalone C, Thoraval S, Escoffier C, Herbette G, Leonhardt N, Causse M, Tissier A. A novel pathway for sesquiterpene biosynthesis from Z,Z-farnesyl pyrophosphate in the wild tomato Solanum habrochaites. Plant Cell. 2009;21:301–317. doi: 10.1105/tpc.107.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiderer C, Grausgruber-Gröger S, Grassi P, Steinborn R, Novak J. Influence of gibberellin and daminozide on the expression of terpene synthases and on monoterpenes in common sage (Salvia officinalis) J Plant Physiol. 2010;167:779–786. doi: 10.1016/j.jplph.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Schmidt CO, Bouwmeester HJ, Franke S, König WA. Mechanisms of the Biosynthesis of Sesquiterpene Enantiomers (+)- and (-)-Germacrene D in Solidago canadensis. Chirality. 1999;11:353–362. [Google Scholar]
- Schreier P, Drawert F, Junker A. Sesquiterpen-Kohlenwasserstoffe in Trauben. Z Lebensm Unters Forsch. 1976;160:271–274. doi: 10.1007/BF01132291. [DOI] [PubMed] [Google Scholar]
- Schuhr CA, Radykewicz T, Sagner S, Latzel C, Zenk MH, Arigoni D, Bacher A, Rohdich F, Eisenreich W. Quantitative assessment of crosstalk between the two isoprenoid biosynthesis pathways in plants by NMR spectroscopy. Phytochem Rev. 2003;2:3–16. [Google Scholar]
- Simpson TJ, Ahmed SA, Rupert McIntyre C, Scott FE, Sadler IH. Biosynthesis of polyketide-terpenoid (meroterpenoid) metabolites andibenin B and andilesin A in Aspergillus variecolor. Tetrahedron. 1997;53:4013–4034. [Google Scholar]
- Soler E, Clastre M, Bantignies B, Marigo G, Ambid C. Uptake of isopentenyl diphosphate by plastids isolated from Vitis vinifera L. cell suspensions. Planta. 1993;191:324–329. [Google Scholar]
- Steele CL, Crock J, Bohlmann J, Croteau R. Sesquiterpene Synthases from Grand Fir (Abies grandis) J Biol Chem. 1998;273:2078–2089. doi: 10.1074/jbc.273.4.2078. [DOI] [PubMed] [Google Scholar]
- Steliopoulos P, Wüst M, Adam KP, Mosandl A. Biosynthesis of the sesquiterpene germacrene D in Solidago canadensis: 13C and (2)H labeling studies. Phytochemistry. 2002;60:13–20. doi: 10.1016/s0031-9422(02)00068-7. [DOI] [PubMed] [Google Scholar]
- Stranden M, Borg-Karlson A, Mustaparta H. Receptor neuron discrimination of the germacrene D enantiomers in the moth Helicoverpa armigera. Chem Senses. 2002;27:143–152. doi: 10.1093/chemse/27.2.143. [DOI] [PubMed] [Google Scholar]
- Strauss CR, Wilson B, Gooley PR, Williams PJ. Role of Monoterpenes in Grape and Wine Flavor. ACS Symposium Series. 1986;317:222–242. [Google Scholar]
- Wang Y, Lim L, DiGuistini S, Robertson G, Bohlmann J, Breuil C. A specialized ABC efflux transporter GcABC-G1 confers monoterpene resistance to Grosmannia clavigera, a bark beetle-associated fungal pathogen of pine trees. New Phytol. 2013;197:886–898. doi: 10.1111/nph.12063. [DOI] [PubMed] [Google Scholar]
- Wood C, Siebert TE, Parker M, Capone DL, Elsey GM, Pollnitz AP, Eggers M, Meier M, Vössing T, Widder S, Krammer G, Sefton MA, Herderich MJ. From wine to pepper: rotundone, an obscure sesquiterpene, is a potent spicy aroma compound. J Agric Food Chem. 2008;56:3738–3744. doi: 10.1021/jf800183k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. HS-SPME-GC-MS selected ion monitoring chromatogram of genuine (d0), d2, d4 and d6-labeled (E,E)-a-farnesene after administration of [5,5-2H2]-mevalonic acid lactone (A) or [5,5-2H2]-1-deoxy-D-xylulose (B) to Vitis vinifera cv. Gewürztraminer. The characteristic ion traces are m/z 161 (genuine, d0), 163 (d2), 165 (d4) and 167 (d6).
Supplementary Fig. 2. Proposed reaction pathway to guaia-6,9-diene and (R)-germacrene D.
Supplementary Fig. 3. Incorporation of [5,5-2H2]-1-deoxy-D-xylulose (2H2-DOX) into monoterpenes of grape berry mesocarp (Vitis vinifera cv. Lemberger) compared to the feeding experiments with [5,5-2H2]-mevalonic acid lactone (2H2-MVL). A) HS-SPME-GC-MS total ion chromatogram of grape berry mesocarp after 2H2-DOX- and 2H2-MVL-feeding experiments and the corresponding mass spectra of genuine and labeled α-terpineol. B) Proposed fragmentation mechanism of genuine and labeled α-terpineol.
Supplementary Fig. 4. HS-SPME-GC-MS selected ion monitoring chromatogram for characteristic ion traces m/z 257 (genuine 13-epi-manoyl oxide), and 265 (d8-13-epi-manoyl oxide 13) after administration of [5,5-2H2]-1-deoxy-D-xylulose to Vitis vinifera cv. Lemberger (left panel). Mass spectra of genuine and d8-labeled 13-epi-manoyl oxide (center panel). The characteristic ions are M-CH3 (m/z 275 for genuine and m/z 283 for d8-labeled 13) and M-CH3-H2O (m/z 257 for genuine and m/z 265 for d8-labeled 13).