Abstract
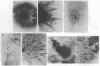
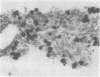
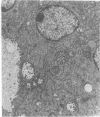
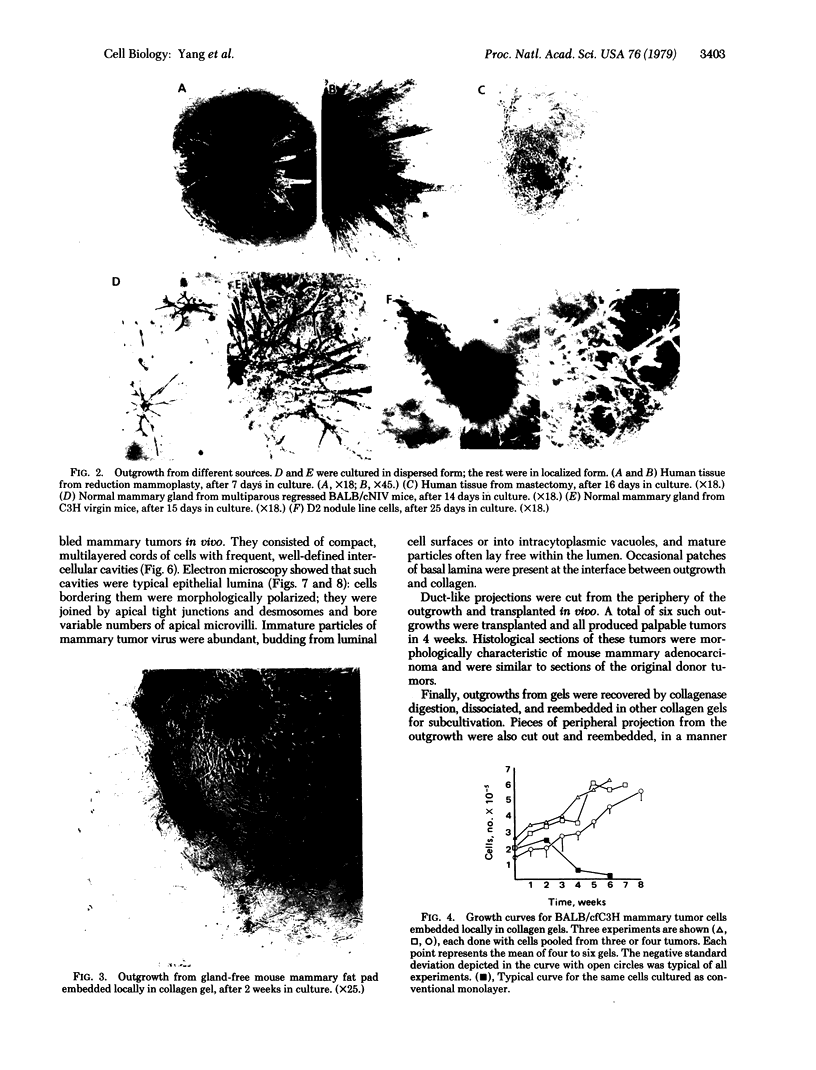
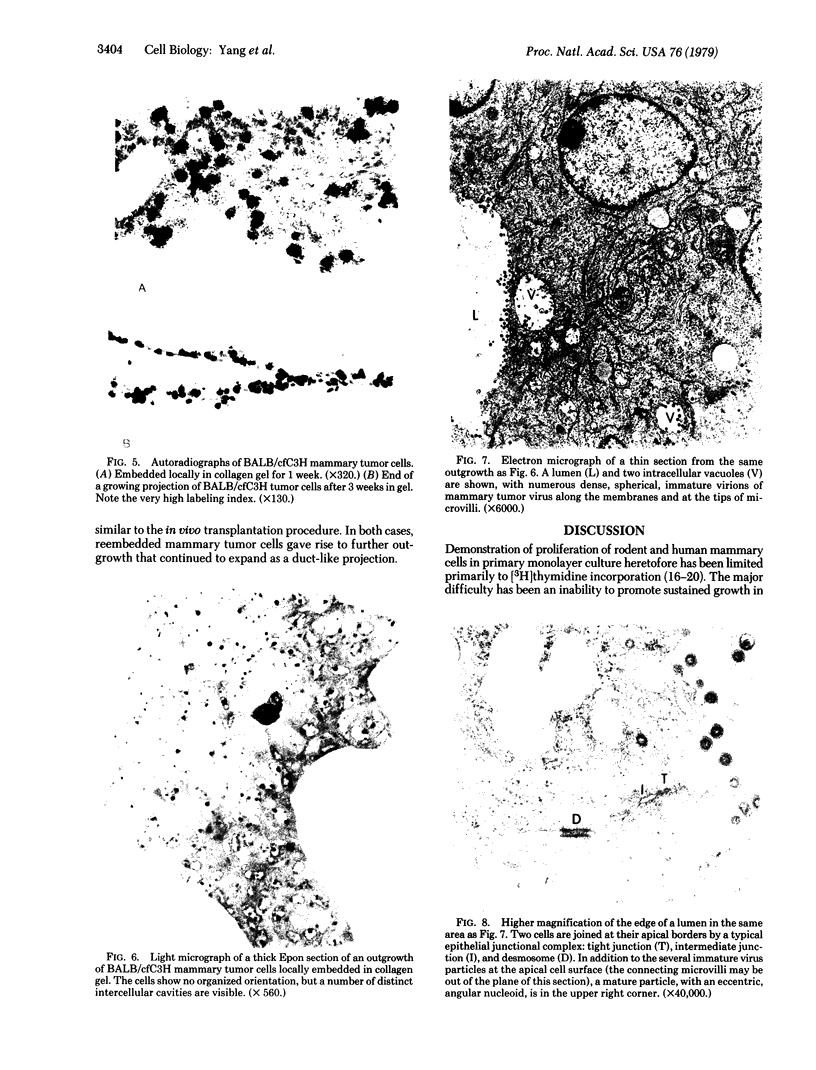
We have developed a method for embedding cells within a collagen matrix which allows sustained growth of mouse mammary tumor epithelial cells in primary culture. A characteristic and reproducible pattern of organization and growth occurs: the cells rearrange themselves and produce duct-like structures extending into the matrix, resulting in a three-dimensional outgrowth. Autoradiography showed continuous [3H]thymidine incorporation during 8 weeks in culture. An increase in DNA content of the cultured cells as a function of time was observed. Mouse mammary tumor cells cultured in the conventional monolayer system failed to show any significant increase in cell number during a culture period of 6 weeks. In addition, in such monolayer systems, cells progressively became detached from the dishes in long-term culture. The mammary epithelial cell origin of the collagen gel cell outgrowths was shown by electron microscopic demonstration of polarized cells containing tight junctions and budding mammary tumor virus particles. In addition, in vivo transplantation of collagen gel outgrowths resulted in the development of mammary adenocarcinoma histologically similar to the donor tumor. Cellular outgrowth patterns resembling those from tumor cells were also seen in similar collagen gel cultures of normal mammary cells from mouse and human and of hyperplastic alveolar nodule cells from mouse. The significance and usefulness of this system in comparison to the conventional monolayer system are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butel J. S., Dudley J. P., Medina D. Comparison of the growth properties in vitro and transplantability of continuous mouse mammary tumor cell lines and clonal derivatives. Cancer Res. 1977 Jun;37(6):1892–1900. [PubMed] [Google Scholar]
- Ceriani R. L., Blank E. W. Response to prolactin and ovarian steroids of normal mammary epithelial cell cultures. Mol Cell Endocrinol. 1977 Aug;8(2):95–103. doi: 10.1016/0303-7207(77)90022-3. [DOI] [PubMed] [Google Scholar]
- DEOME K. B., FAULKIN L. J., Jr, BERN H. A., BLAIR P. B. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959 Jun;19(5):515–520. [PubMed] [Google Scholar]
- Das N. K., Hosick H. L., Nandi S. Influence of seeding density on multicellular organization and nuclear events in cultures of normal and neoplastic mouse mammary epithelium. J Natl Cancer Inst. 1974 Mar;52(3):849–861. doi: 10.1093/jnci/52.3.849. [DOI] [PubMed] [Google Scholar]
- Dexter D. L., Kowalski H. M., Blazar B. A., Fligiel Z., Vogel R., Heppner G. H. Heterogeneity of tumor cells from a single mouse mammary tumor. Cancer Res. 1978 Oct;38(10):3174–3181. [PubMed] [Google Scholar]
- Emerman J. T., Enami J., Pitelka D. R., Nandi S. Hormonal effects on intracellular and secreted casein in cultures of mouse mammary epithelial cells on floating collagen membranes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4466–4470. doi: 10.1073/pnas.74.10.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enami J., Yang J., Nandi S. Simultaneous production of casein and mammary tumor virus in mouse mammary epithelial cells grown on floating collagen gels. Cancer Lett. 1979 Feb;6(2):99–105. doi: 10.1016/s0304-3835(79)80007-5. [DOI] [PubMed] [Google Scholar]
- Engel L. W., Young N. A. Human breast carcinoma cells in continuous culture: a review. Cancer Res. 1978 Nov;38(11 Pt 2):4327–4339. [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Gaffney E. V., Polanowski F. P., Blackburn S. E., Lambiase J. T., Burke R. E. Cultures of normal human mammary cells. Cell Differ. 1976 Jul;5(2):69–81. doi: 10.1016/0045-6039(76)90001-4. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Birdwell C. R. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978 Nov;38(11 Pt 2):4155–4171. [PubMed] [Google Scholar]
- Hallowes R. C., Rudland P. S., Hawkins R. A., Lewis D. J., Bennet D., Durbin H. Comparison of the effects of hormones on DNA synthesis in cell cultures of nonneoplastic and neoplastic mammary epithelium from rats. Cancer Res. 1977 Aug;37(8 Pt 1):2492–2504. [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Hosick H. L. A note on growth patterns of epithelial tumor cells in primary culture. Cancer Res. 1974 Jan;34(1):259–261. [PubMed] [Google Scholar]
- Michalopoulos G., Pitot H. C. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975 Aug;94(1):70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G., Sattler G. L., Pitot H. C. Hormonal regulation and the effects of glucose on tyrosine aminotransferase activity in adult rat hepatocytes cultured on floating collagen membranes. Cancer Res. 1978 Jun;38(6):1550–1555. [PubMed] [Google Scholar]
- Richards J., Nandi S. Neoplastic transformation of rat mammary cells exposed to 7,12-dimethylbenz[alpha]anthracene or N-nitrosomethylurea in cell culture. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3836–3840. doi: 10.1073/pnas.75.8.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J., Nandi S. Primary culture of rat mammary epithelial cells. I. Effect of plating density, hormones, and serum on DNA synthesis. J Natl Cancer Inst. 1978 Sep;61(3):765–771. [PubMed] [Google Scholar]
- Sakai S., Bowman P. D., Yang J., McCormick K., Nandi S. Glucocorticoid regulation of prolactin receptors on mammary cells in culture. Endocrinology. 1979 May;104(5):1447–1449. doi: 10.1210/endo-104-5-1447. [DOI] [PubMed] [Google Scholar]
- Sirbasku D. A. Estrogen induction of growth factors specific for hormone-responsive mammary, pituitary, and kidney tumor cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3786–3790. doi: 10.1073/pnas.75.8.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., King R. J. Effects of steroids on growth of an androgen-dependent mouse mammary carcinoma in cell culture. Exp Cell Res. 1972 Aug;73(2):351–359. doi: 10.1016/0014-4827(72)90058-4. [DOI] [PubMed] [Google Scholar]
- Stoker M. G., Pigott D., Taylor-Papadimitriou J. Response to epidermal growth factors of cultured human mammary epithelial cells from benign tumours. Nature. 1976 Dec 23;264(5588):764–767. doi: 10.1038/264764a0. [DOI] [PubMed] [Google Scholar]
- Yang J., Enami J., Nandi S. Regulation of mammary tumor virus production by prolactin in BALB/cfC3H mouse normal mammary epithelial cells in vitro. Cancer Res. 1977 Oct;37(10):3644–3647. [PubMed] [Google Scholar]