Abstract
Objectives
No extensive investigation has been performed and thus no representative data are available regarding acute liver failure (ALF) in China. This study aims to investigate the causes and outcomes of ALF in China and establish a prognostic model.
Methods
Patients diagnosed as ALF in seven hospitals in different areas of China from January 2007 to December 2012 were retrospectively selected.
Results
Of the 177 patients included in this study, 112 (63.28%) eventually died. The common causes of ALF were drug toxicity (43.50%), indeterminate etiology (29.38%) and acute viral hepatitis (11.30%). Additionally, traditional Chinese herbs predominated in the causes of drug-induced ALF (30/77). No patients in this study received liver transplantation. In the established model for predicting death in ALF, four variables were finally selected out, including age (P=0.01), the entry hepatic encephalopathy grade (P=0.04), international normalized ratio (P<0.01) and arterial blood ammonia (P=0.02). Using a threshold value of 0.5683, this model had a sensitivity of 95.24% and a specificity of 91.30%.
Conclusions
Traditional Chinese medicine was a major cause of ALF in China. The spontaneous mortality of ALF was high, whereas the rate of liver transplantation was significantly low. The established prognostic model of ALF had superior sensitivity and specificity.
Introduction
Acute liver failure (ALF) is a complex condition characterized by rapid deterioration of liver function in a patient without previously recognized liver disease. In spite of the improvement of artificial liver support system and advancement in liver transplantation, the overall mortality of ALF remains 20%-40% [1], the major reasons of which are the rapid disease progression and the highly unpredictable outcomes depending on various factors. Based on previous reports, patients with ALF had greatly variant outcomes according to the causes that also varied markedly by geographical region [2-4]. So, it is important and necessary to do studies on the etiology and prediction for outcome of ALF in different cohorts [5].
Because of its rarity, studies on ALF are relatively fewer, and moreover, discrepancies in the definitions of ALF hinder in-depth worldwide studies. It is reported that more than 40 definitions exit among different countries or regions and the diversity in ALF definitions becomes the obstacle to comparability and quantitative analysis among studies [6]. In China, though liver disease is a major public health concern and viral hepatitis B is especially prevalent [7], the national diagnostic and treatment guidelines for liver failure were issued by Chinese Society of Hepatology in late 2006 [8]. From then on, the diagnosis of ALF began to arrive at uniformity and studies in this field became standardized in China, but the available data on ALF in Chinese population has been still rare up to date. In this study, we performed an extensive investigation including 177 patients diagnosed as ALF after January 2007, with the intentions of investigating the causes and mortality and establishing a prognostic model based on Chinese patients. To the best of our knowledge, this is the first report on a multi-center study of ALF in Chinese population.
Patients and Methods
Patient Collection
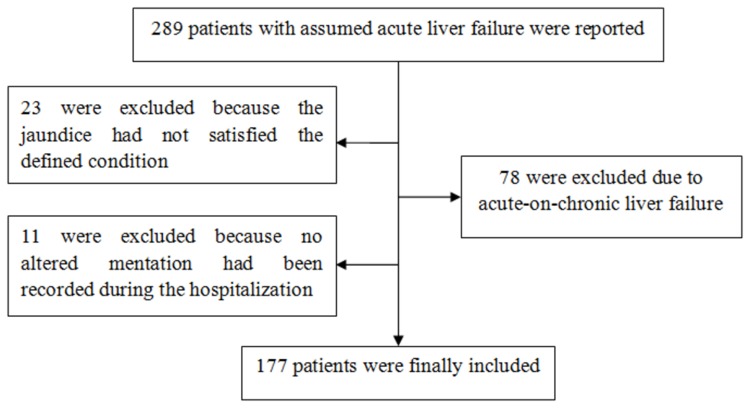
ALF in this study was defined as coagulopathy (prothrombin activity (PTA)≤40% or international normalized ratio (INR)≥1.5), jaundice (serum total bilirubin (TBil) ≥171 μmol/L or a daily increase ≥17.1 μmol/L) and encephalopathy (any degree of altered mentation) within 4 weeks in a patient without pre-existing liver diseases. Patients older than 12 years eligible for the above diagnostic criteria from January 2007 to December 2012 were included in this study. To ensure the representativeness and comparability of the data, we selected seven tertiary military hospitals in different areas of China: Beijing 302 Hospital (located in Beijing, North China), General Hospital of PLA (located in Beijing, North China), Changhai Hospital Affiliated to Second Military Medical University (located in Shanghai, East China), PLA 161 Hospital (located in Wuhan, Hubei Province, South China), PLA 477 Hospital ((located in Wuhan, Hubei Province, South China), General Hospital of Jinan Military Region (located in Jinan, Shandong Province, East China) and General Hospital of Lanzhou Military Region (located in Lanzhou, Gansu Province, West China). Figure 1 shows the enrollment of patients in our study.
Figure 1. Enrollment of patients in this study.
Ethics Statement
The study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committees of each hospital. Informed written or verbal consent was obtained from the patient or the next of kin.
Data Extraction and Assessment
The following variables were obtained from the electronic medical records and follow-up documents: cause, outcome, sex, age, grade of hepatic encephalopathy (HE) on admission, time from onset of illness to the development of HE, white blood cell count, hemoglobin, platelet count, PTA, INR, serum alanine aminotransferase (ALT), serum aspartate aminotransferase (AST), serum alkaline phosphatase (ALP), serum cholinesterase, serum lactate dehydrogenase (LDH), serum TBil, serum albumin, serum creatinine, serum urea nitrogen, serum glucose, serum Na+, serum K+, serum Cl-, arterial blood ammonia (BLA), arterial blood lactate and arterial blood pH. Grade of HE had been determined in the medical records according to the following criteria: I. loss of sleep rhythm, anxiousness, confusion or flapping tremors; II. loss of sphincter control, drowsiness or behavioral disorder; III. persistent coma, but still responding to shouts; IV. deep coma with no consciousness.
Statistical Analysis
Data analyses were performed using SAS 9.2 software (SAS Institute Inc., Cary, NC, USA). Continuous data were expressed as median (inter-quartile range) or mean±standard deviation. Categorical data were expressed as the number of subjects. Group comparisons were performed using the Wilcoxon rank sum test or t test for continuous variables, and Chi-square test for categorical variables. Logistic regression was used for evaluating prognostic predictors of ALF. Receiver operating characteristic (ROC) curve analysis was used for assessing prognostic accuracy of the outcome prediction model. Tests were two-sided and a probability (P) value of less than 0.05 was considered statistically significant.
Results
Causes, Mortality and Transplantation
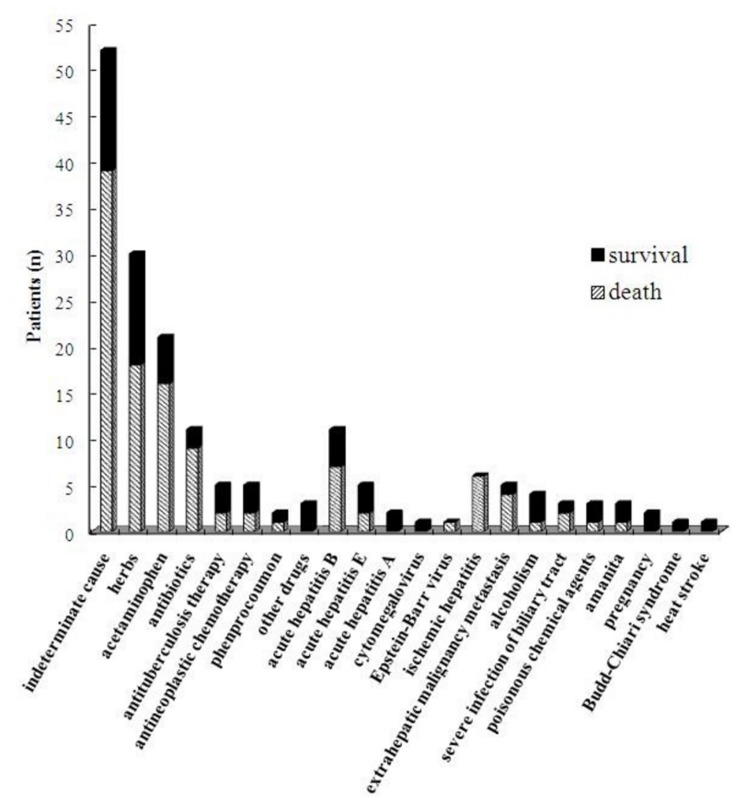
177 patients were totally included in this study. Of these patients, 77 (43.50%) developed ALF caused by drug toxicity, 52 (29.38%) had ALF of indeterminate cause, 20 (11.30%) had acute viral hepatitis and 28 (15.82%) had other causes.
In the patients with ALF induced by drugs, 30 (16.95%) had received herbal remedies, 21 (11.86%) had ingested acetaminophen, 11 (6.21%) had used antibiotics, 5 (2.82%) had received antituberculosis therapy, 5 (2.82%) had received antineoplastic chemotherapy, 2 (1.13%) had used phenprocoumon and 3 (1.69%) had ingested other drugs.
In the patients with ALF due to viral hepatitis, 11 (6.21%) was infected with hepatitis B virus (HBV), 5 (2.82%) with hepatitis E virus (HEV), 2 (1.13%) with hepatitis A virus (HAV), 1 (0.57%) with cytomegalovirus and 1 (0.57%) with Epstein-Barr virus.
Other causes of ALF were respectively ischemic hepatitis in 6 (3.39%) patients, extrahepatic malignancy metastasis in 5 (2.82%), alcoholism in 4 (2.26%), severe infection of biliary tract in 3 (1.69%), poisonous chemical agents in 3 (1.69%), amanita in 3 (1.69%), pregnancy in 2 (1.13%), Budd-Chiari syndrome in 1 (0.57%) and heat stroke in 1 (0.57%).
No patients received liver transplantation in this study. In the 177 patients, 65 (36.72%) survived and 112 (63.28%) died. The distribution of causes and outcomes of ALF is demonstrated in Figure 2.
Figure 2. Distribution of causes and outcomes of 177 patients with acute liver failure in China.
Baseline Characteristics on Admission
Table 1 summarizes the baseline characteristics of patients with ALF on admission according to the outcome (death or survival). The median age (P=0.03), INR (P<0.01), arterial BLA (P<0.01) and serum LDH (P<0.01) in patients who died were significantly higher than those in patients who survived, and median platelet count (P=0.01) and PTA (P<0.01) in patients who died were notably lower than those in patients who survived. In addition, the distribution of HE grade (≤II and II) was statistically different (P<0.01). No statistical difference could be detected with respect to other variables.
Table 1. Baseline characteristics of patients with acute liver failure on admission and comparison of variables between patients who survived and died.
| Parameters | Patients who survived (n=65) | Patients who died (n=112) | P value |
|---|---|---|---|
| Sex (male/female) | 33/32 | 49/63 | 0.37 |
| Age (years) | 40.0 (31.0) | 46.0 (31.5) | 0.03 |
| Etiologies | 0.26 | ||
| Drug (non-herb) toxicity | 18 | 29 | |
| Herbs | 12 | 18 | |
| Indeterminate cause | 13 | 39 | |
| Viral causes | 10 | 10 | |
| Other causes | 12 | 16 | |
| Grade of HE | <0.01 | ||
| ≤II | 57 | 31 | |
| II | 8 | 81 | |
| Days from onset of illness to the development of HE | 11 (15) | 10 (15) | 0.30 |
| Serum ALT (U/L) | 796 (1817) | 756 (1162) | 0.90 |
| Serum AST (U/L) | 392.0 (1244.5) | 534.0 (824.0) | 0.43 |
| Serum Alp (U/L) | 162 (83) | 165 (87) | 0.85 |
| Serum TBil (μmol/L) | 326.35±161.32 | 357.57±152.70 | 0.76 |
| Serum albumin (g/L) | 29 (6) | 28 (8) | 0.06 |
| Serum cholinesterase (U/L) | 2964 (2045) | 2895 (1997) | 0.33 |
| Serum LDH (U/L) | 264.0 (164.0) | 366.5 (486.0) | <0.01 |
| Serum creatinine (μmol/L) | 84.5 (38.0) | 88.0 (57.0) | 0.25 |
| Serum urea nitrogen (mmol/L) | 4.00 (4.25) | 3.9 (5.3) | 0.48 |
| Serum glucose (mmol/L) | 5.15 (3.13) | 5.85 (4.00) | 0.06 |
| Serum Na+ (mmol/L) | 137 (6) | 135 (8) | 0.08 |
| Serum K+ (mmol/L) | 3.74±0.59 | 3.86±0.70 | 0.29 |
| Serum Cl- (mmol/L) | 102.9 (6.9) | 100.5 (8.6) | 0.14 |
| White blood cell count (×109) | 8.35 (5.42) | 10.45 (8.61) | 0.11 |
| Platelet count (×109) | 122 (106) | 87 (102) | 0.01 |
| Hemoglobin (g/L) | 116.5 (34.0) | 118.7 (36.5) | 0.37 |
| PTA (%) | 30.00 (17.21) | 16.97 (13.70) | <0.01 |
| INR | 1.78 (0.91) | 3.39 (1.64) | <0.01 |
| Arterial BLA (μmol/L) | 70.50 (28.15) | 153.50 (62.00) | <0.01 |
| Arterial blood lactate (mmol/L) | 2.75(2.25) | 3.40(5.05) | 0.42 |
| Arterial blood pH | 7.47 (0.08) | 7.48 (0.07) | 0.63 |
HE, hepatic encephalopathy; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; TBil, total bilirubin; LDH, lactate dehydrogenase; PTA, prothrombin activity; INR, international normalized ratio; BLA, blood ammonia.
Prediction model for the outcome in ALF
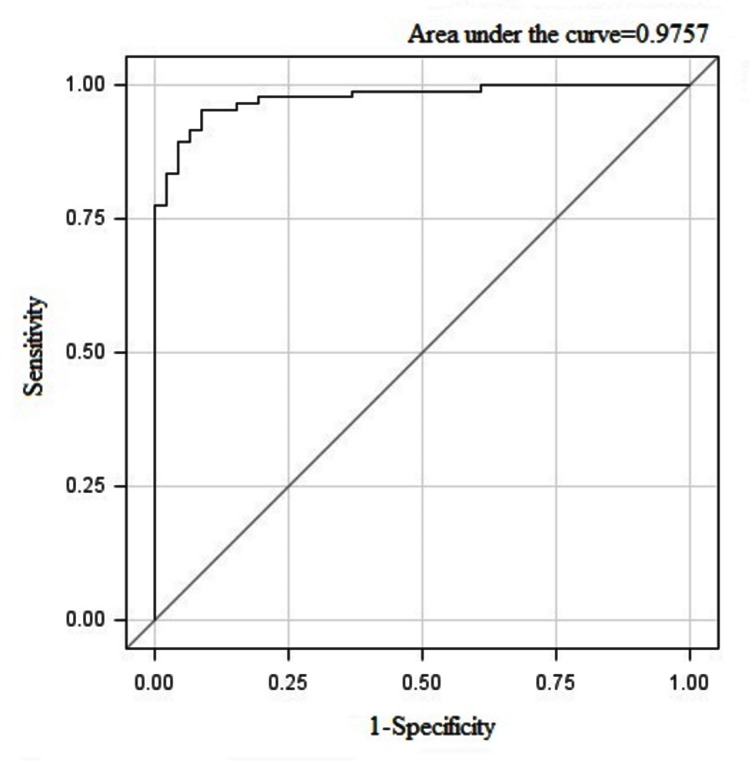
Table 2 represents the final model established using logistic regression. Four variables were eventually selected out to predict the death of ALF, including age (odds ratio (OR) 1.064, 95% confidence interval (CI) 1.015-1.115, P=0.01), the entry HE grade (II vs. ≤II, OR 7.459, 95% CI 1.024-54.327, P=0.04), INR (OR 10.019, 95% CI 2.530-39.677, P<0.01) and arterial BLA (OR 1.035, 95% CI 1.004-1.067, P=0.02). Using a threshold value of 0.5683, this model had a sensitivity of 95.24%, a specificity of 91.30% and an accuracy of 93.85%. Figure 3 shows the ROC curve analysis for predictive power of this model (the area under the ROC curve 0.9757, 95% CI 0.9540-0.9973).
Table 2. A model for prediction of the death in acute liver failure using entry variables.
| Parameters | Odds ratio | 95% CI for Odds ratio | P value |
|---|---|---|---|
| Age (years) | 1.064 | 1.015-1.115 | 0.01 |
| Grade of HE (II vs. ≤II) | 7.459 | 1.024-54.327 | 0.04 |
| INR | 10.019 | 2.530-39.677 | <0.01 |
| Arterial BLA (μmol/L) | 1.035 | 1.004-1.067 | 0.02 |
Somers’D 0.951; Goodman-Kruskal Gamma 0.951; Kendall’s Tau-a 0.438; concordance index 0.976.
HE, hepatic encephalopathy; INR, international normalized ratio; BLA, blood ammonia; CI, confidence interval.
Figure 3. Receiver operating characteristic (ROC) curve analysis for the established prognostic model of acute liver failure.
Discussion
The etiology of ALF showed worldwide variation [9]. Some previous studies reported that synthetic pharmaceuticals (e.g. acetaminophen and phenprocoumon) were the most common cause of ALF in the United States and Europe, while in the Mediterranean region, around 40% of cases with ALF are caused by hepatotropic viruses, most frequently by HBV [2-4]. According to our investigation, the most frequent etiology of ALF in China were drugs, which was in accordance with other reports from western developed countries [2,3,10,11]. However, unlike the overwhelming majority of ALF induced by acetaminophen in western countries, traditional Chinese herbs dominated in the causes in China. So, doctors should pay more attention to the patient’s past medical history on herbal remedy when identifying the etiology of ALF.
Acetaminophen was a well-recognized hepatotoxin [12]. In this study, it was responsible for 11.86% of cases, which was much lower than other reports from the United States and Europe [2,3,10,11]. Unlike the description by Bernal W [13], viral causes of ALF did not predominate in this investigation from China, a developing country, and moreover, acute hepatitis B infection rather than hepatitis E infection, was the main viral cause. In addition to drug-toxicity, indeterminate and viral causes of ALF, 28 (15.82%) cases are classified as other causes, including ischemic hepatitis, extrahepatic malignancy metastasis, alcoholism, etc. Emphatically, ischemic hepatitis was a rare etiology of ALF in China, while it was a common cause of ALF in Sweden [3].
The spontaneous death rates of ALF were reported to range from 10% to 90% in different cohorts [3]. The wide difference was mostly resulted by the discrepancies in the diagnostic criteria of ALF in various studies. In our study, we set coagulopathy, jaundice and encephalopathy as mandatory elements in the definition of ALF. The mortality of ALF without liver transplantation in our study arrived at 63.28%.
Liver transplantation remained the only treatment option in ALF when standard medical therapy failed [14]. In western developed countries, rates of transplant ranged from 25% to 30% in patients with ALF [15], but based on our study, the rate was as low as 0% in China. The reasons for this included the difficulties in obtaining organs in urgent fashion, as well as the economic situation of patients in China. Under such a circumstance, it was critically important to determine the prognosis in ALF as early as possible [16]. With the aim of ensuring the timeliness and practicality of the forecasting model, we used the entry variables rather than the peak ones to predict the outcomes of ALF because it was somewhat late to make further vital decisions when peak value had been reached.
As of now, there have been various prognostic scoring systems applied for predicting outcomes of ALF, including the King’s college hospital criteria, the model for end-stage liver disease score, acute physiology and chronic health evaluation II score and the Clichy criteria [17-19], however, to our knowledge, no prediction model based on Chinese patients with ALF has been established. In this study, the results showed that four common, easily-measured prognostic factors in combination could predict the death in ALF with an accuracy of 93.85%, which were age, the entry HE grade, INR and arterial BLA, and the likelihood of death increased with the levels of the four variables increasing. According to the area under the ROC curve (0.9757), this model also had good discrimination.
The limitation of our research was that we did not set an external validation cohort to verify the established prognostic model.
In conclusion, traditional Chinese medicine was a major cause of ALF in China. The spontaneous mortality of ALF was high, whereas the rate of liver transplantation was significantly low. The established prognostic model of ALF had early applicability and superior sensitivity and specificity.
Funding Statement
The authors have no support or funding to report.
References
- 1. Kantola T, Ilmakunnas M, Koivusalo AM, Isoniemi H (2011) Bridging therapies and liver transplantation in acute liver failure, 10 years of MARS experience from Finland. Scand J Surg 100(1): 8-13. PubMed: 21482500. [DOI] [PubMed] [Google Scholar]
- 2. Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ et al. (2002) Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 137(12): 947-954. doi: 10.7326/0003-4819-137-12-200212170-00007. PubMed: 12484709. [DOI] [PubMed] [Google Scholar]
- 3. Wei G, Bergquist A, Broomé U, Lindgren S, Wallerstedt S et al. (2007) Acute liver failure in Sweden: etiology and outcome. J Intern Med 262(3): 393-401. doi: 10.1111/j.1365-2796.2007.01818.x. PubMed: 17697161. [DOI] [PubMed] [Google Scholar]
- 4. Canbay A, Tacke F, Hadem J, Trautwein C, Gerken G et al. (2011) Acute liver failure--a life-threatening disease. Dtsch Arztebl Int 108(42): 714-720. PubMed: 22114640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wlodzimirow KA, Eslami S, Chamuleau RA, Nieuwoudt M, Abu-Hanna A (2012) Prediction of poor outcome in patients with acute liver failure-systematic review of prediction models: prognostic model research. PLOS ONE 7(12): e50952. doi: 10.1371/journal.pone.0050952. PubMed: 23272081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wlodzimirow KA, Eslami S, Abu-Hanna A, Nieuwoudt M, Chamuleau RA (2012) Systematic review: acute liver failure-one disease, more than 40 definitions. Aliment Pharmacol Ther 35(11): 1245-1256. doi: 10.1111/j.1365-2036.2012.05097.x. PubMed: 22506515. [DOI] [PubMed] [Google Scholar]
- 7. Zhang H, Jiang YF, He SM, Sun J, Gu Q et al. (2011) Etiology and prevalence of abnormal serum alanine aminotransferase levels in a general population in Northeast China. Chin Med. J ( Engl) 124(17): 2661-2668 [PubMed] [Google Scholar]
- 8. Liu Q, Liu Z, Wang T, Wang Q, Shi X et al. (2007) Characteristics of acute and sub-acute liver failure in China: nomination, classification and interval. J Gastroenterol Hepatol 22(12): 2101-2106. doi: 10.1111/j.1440-1746.2006.04362.x. PubMed: 18031366. [DOI] [PubMed] [Google Scholar]
- 9. Ichai P, Samuel D (2011) Epidemiology of liver failure. Clin Res Hepatol. Gastroenterol 35(10): 610-617. [DOI] [PubMed] [Google Scholar]
- 10. Hadem J, Tacke F, Bruns T, Langgartner J, Strnad P et al. (2012) Etiologies and outcomes of acute liver failure in Germany. Clin Gastroenterol Hepatol 10(6): 664-669. doi: 10.1016/j.cgh.2012.02.016. PubMed: 22373724. [DOI] [PubMed] [Google Scholar]
- 11. Bretherick AD, Craig DG, Masterton G, Bates C, Davidson J et al. (2011) Acute liver failure in Scotland between 1992 and 2009; incidence, aetiology and outcome. QJM 104(11): 945-956. doi: 10.1093/qjmed/hcr098. PubMed: 21729878. [DOI] [PubMed] [Google Scholar]
- 12. Claridge LC, Eksteen B, Smith A, Shah T, Holt AP (2010) Acute liver failure after administration of paracetamol at the maximum recommended daily dose in adults. BMJ 341: c6764. doi: 10.1136/bmj.c6764. PubMed: 21127120. [DOI] [PubMed] [Google Scholar]
- 13. Bernal W, Auzinger G, Dhawan A, Wendon J (2010) Acute liver failure. Lancet 376(9736): 190-201. doi: 10.1016/S0140-6736(10)60274-7. PubMed: 20638564. [DOI] [PubMed] [Google Scholar]
- 14. Singhal A, Vadlamudi S, Stokes K, Cassidy FP, Corn A et al. (2012) Liver histology as predictor of outcome in patients with acute liver failure. Transpl Int 25(6): 658-662. doi: 10.1111/j.1432-2277.2012.01470.x. PubMed: 22487361. [DOI] [PubMed] [Google Scholar]
- 15. Lee WM (2012) Recent developments in acute liver failure. Best Pract Res Clin Gastroenterol 26(1): 3-16. doi: 10.1016/j.bpg.2012.01.014. PubMed: 22482521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rutherford A, King LY, Hynan LS, Vedvyas C, Lin W et al. (2012) Development of an accurate index for predicting outcomes of patients with acute liver failure. Gastroenterology 143(5): 1237-1243. doi: 10.1053/j.gastro.2012.07.113. PubMed: 22885329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bechmann LP, Jochum C, Kocabayoglu P, Sowa JP, Kassalik M et al. (2010) Cytokeratin-18 based modification of the MELD score improves prediction of spontaneous survival after acute liver failure. J Hepatol 53(4): 639-647. doi: 10.1016/j.jhep.2010.04.029. PubMed: 20630612. [DOI] [PubMed] [Google Scholar]
- 18. Bhatia V, Singh R, Acharya SK (2006) Predictive value of arterial ammonia for complications and outcome in acute liver failure. Gut 55(1): 98-104. doi: 10.1136/gut.2004.061754. PubMed: 16024550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harbrecht BG (2012) Predicting outcome in patients with acute liver failure: what works best? Crit Care Med 40(5): 1666-1667. doi: 10.1097/CCM.0b013e3182451f9f. PubMed: 22511153. [DOI] [PubMed] [Google Scholar]