Abstract
Background
Various pharmacologic and non-pharmacologic approaches have been applied to reduce sublesional bone loss after spinal cord injury (SCI), and the results are inconsistent across the studies. The objective of this meta-analysis was to investigate whether the two most-studied interventions, bisphosphonate analogues and functional electrical stimulation (FES), could effectively decrease bone mineral density (BMD) attenuation and/or restore lost BMD in the SCI population.
Methods
Randomized controlled trials, quasi-experimental studies, and prospective follow-up studies employing bisphosphonates or FES to treat post-SCI osteoporosis were identified in PubMed and Scopus. The primary outcome was the percentage of BMD change from baseline measured by dual-energy X-ray absorptiometry (DEXA) or computed tomography (CT). Data were extracted from four points: the 3rd, 6th, 12th, and 18th month after intervention.
Results
A total of 19 studies were included in the analysis and involved 364 patients and 14 healthy individuals. Acute SCI participants treated with bisphosphonate therapy demonstrated a trend toward less bone loss than participants who received placebos or usual care. A significant difference in BMD decline was noted between both groups at the 3rd and 12th month post-medication. The subgroup analysis failed to show the superiority of intravenous bisphosphonate over oral administration. Regarding FES training, chronic SCI patients had 5.96% (95% CI, 2.08% to 9.84%), 7.21% (95%CI, 1.79% to 12.62%), and 9.56% (95% CI, 2.86% to 16.26%) increases in BMD at the 3rd, 6th, and 12th months post-treatment, respectively. The studies employing FES ≥5 days per week were likely to have better effectiveness than studies using FES ≤3 days per week.
Conclusions
Our meta-analysis indicated bisphosphonate administration early following SCI effectively attenuated sublesional bone loss. FES intervention for chronic SCI patients could significantly increase sublesional BMD near the site of maximal mechanical loading.
Introduction
Substantial reductions in bone mineral density (BMD) are inevitable below the level of the lesion in spinal cord injury (SCI) patients. Rapid bone loss occurs in the early months following the injury and continues for more than two years until a steady state has been reached [1]. Marked increases in osteoclastic bone resorption and decreases in osteoblastic bone formation likely occur as a result of immobilization, non-weight bearing, and dysregulation of neuroendocrine systems [2,3]. The disruption of the normal skeletal metabolism places the weight bearing bones, such as the femur and proximal tibia, at a greater risk of fragility fractures [2]. Most fractures in the SCI population result from minor trauma and can easily lead to complications, such as breakdown of skin integrity, infection, and fracture malunion, all of which additionally decrease the quality of life and increase healthcare costs. Therefore, an intervention that can effectively attenuate or reverse the bone loss would be beneficial for all SCI patients.
Various treatments including medication, exercise, and physical modalities have been applied to SCI patients to investigate the efficacy of reducing sublesional BMD attenuation [4-8]. Regarding pharmacologic treatments, bisphosphonates emerge as the most-studied regimen and have been tested in paraplegic patients since 1981 [9]. Although bisphosphonate analogues are recognized as effective antiresorptive drugs to prevent osteoporotic fractures in postmenopausal women [10], their role in maintaining BMD following SCI remains controversial [8]. The inconsistent results across the bisphosphonate studies might be derived from various timings and routes of administration, different potencies of analogues, heterogeneity of enrolled patients, and research methodology. In terms of non-pharmacologic therapies, weight bearing, electrical stimulation with or without functional tasks, vibration, pulsed electromagnetic fields, and ultrasound modalities have been evaluated for their effects on modifying BMD in SCI participants [7]. Functional electrical stimulation (FES) is the most extensively investigated approach since it combines the benefits from electrical stimulation and mechanical loading. Although FES intervention has been demonstrated to improve muscle atrophy, solid evidence from a large scale study is still lacking regarding its influence on sublesional BMD attenuation. To our knowledge, a quantitative analysis of BMD changes following either bisphosphonate administration or FES training has never been performed in a specific patient group. The present meta-analysis would aim to investigate the usefulness of both interventions against osteoporosis after SCI and explore whether the effectiveness might vary based on differences in regimens and timing of administration.
Materials and Methods
Study selection
A systematic review was performed by searching PubMed and Scopus from the earliest records to January of 2013. SCI, paraplegia, quadriplegia, BMD, osteoporosis, and osteopenia were entered as medical subject headings and text words to identify relevant articles. Bisphosphonate, alendronate, zoledronic acid, pamidronate, functional electrical stimulation, bicycle, cycling, and exercise were key terms used to extract studies using bisphosphonate analogues or FES to attenuate bone loss. Cochrane Collaboration Central Register of Controlled Clinical Trials, Cochrane Systematic Reviews, ClinicalTrials.gov and bibliographies of included trials and related systematic reviews or meta-analyses were manually scrutinized for additional references.
Eligibility criteria
Randomized controlled trials, quasi-experimental studies, and prospective follow-up studies were included in the review without language restriction. Case reports or case series without a well-designed intervention scheme or outcome measurement were excluded. Studies were eligible if they enrolled adult participants with traumatic SCI. Trials presenting data on pediatric participants and people with specific causes for SCI such as infection, neoplasm, inflammatory diseases, and vasculopathy were ruled out. The interventions of included articles were limited to administration of bisphosphonate analogues and FES. FES was defined as a technique that uses electrical currents to activate nerves innervating paralytic extremities to perform a functional task such as bicycling, ambulation, or resistance training. Research was eliminated if it merely employed electric currents to stimulate paralytic limbs without concomitant exercise regimens. All the selected trials were required to have BMD measured by dual-energy X-ray absorptiometry (DEXA) or a computed tomography (CT).
Data extraction and quality assessment
Two authors (K.V.C. and C.Y.H.) independently evaluated all potential articles eligible for inclusion. Data extracted from selected trials included study designs, patient characteristics, features of bisphosphonate or FES administration, and details of outcome measurements. The Jadad scale was used to assess the quality of the randomized controlled or quasi-experimental trials. The aggregate scores ranged from 0 to 5 points [11]. Trials with scores <3 were assumed to have lower methodologic quality. Prospective cohort or longitudinal follow-up studies were evaluated using the Newcastle-Ottawa scale to assess the quality of selection, comparability, exposure, and outcome [12]. The maximum scores observed were 9 points, and total scores <4 points were considered low in quality. Discrepancies between the 2 independent evaluations of potential articles were resolved by discussion and consensus.
Data synthesis and statistical analysis
Data were extracted from four points: the 3rd, 6th, 12th, and 18th month after intervention. The primary outcome was the percentage of BMD change from baseline and standard deviation (SD) as measured by DEXA or CT. If it was not directly reported, the percentage of BMD change was calculated from the difference of BMD between baseline and follow-up divided by BMD at baseline. The standard deviation was estimated from the square root [(SD of mean BMD at baseline)2 + (SD of BMD during follow-up)2- (SD of BMD at baseline) * (SD of BMD during follow-up)] divided by BMD at baseline [13,14]. The distal femur was the main site used to calculate the BMD. If this data was not reported, the BMD measured at the proximal tibia followed by the femoral trochanter, femoral neck, and the whole lower extremity were utilized instead. In order to maintain comparability with data recorded by DEXA, total BMD rather than trabecular BMD data from CT measurements was used for analysis.
The random effect model was employed to provide a relatively conservative estimate with a 95% confidence interval (CI) of the pooled percent changes in BMD [15]. The heterogeneity across studies was tested by I square and Cochran’s Q test. A P value <0.1 for chi-squared testing of the Q statistic or an I square >50% was regarded as the existence of significant heterogeneity [15]. We performed a subgroup analysis according to the post-injury duration, route of bisphosphonate administration, and the frequency of FES training. A post-injury duration less than two years was defined as acute SCI, whereas chronic SCI indicated patients were undergoing treatments beyond two years following injury. Regarding the acute SCI group, a superiority of intervention was determined by a lower BMD decline in the intervention group without an overlap of the 95% CI of the controls. Regarding the chronic SCI group, we assumed that the BMD attenuation had reached a steady state, and an advantage of intervention referred to an increase in BMD from baseline with a 95% CI above the value of zero. All analyses were performed using Stata 10.0 (StataCorp, Texas, USA).
Results
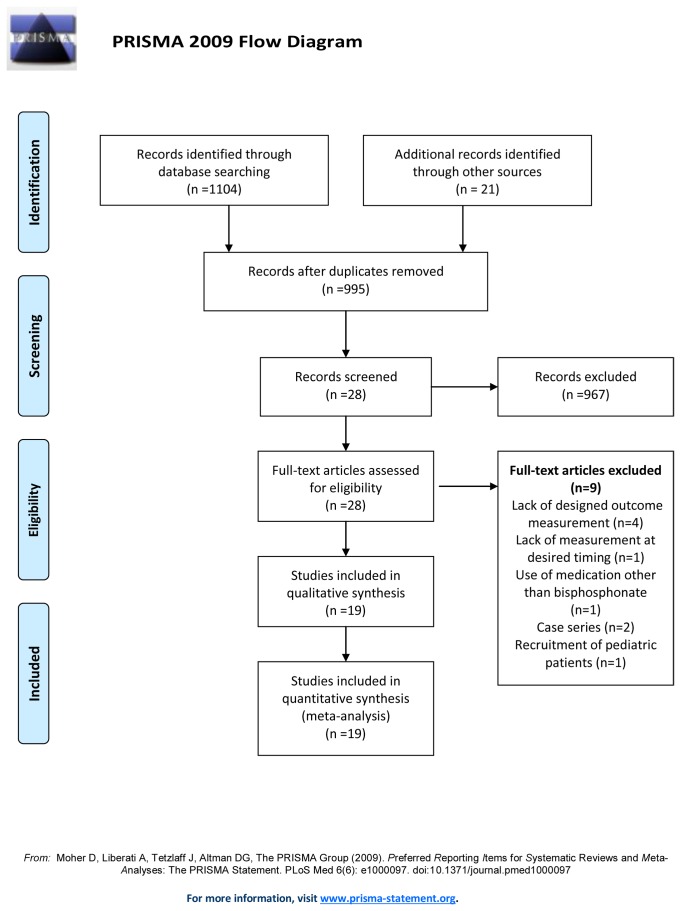
Of the 28 non-duplicate citations identified from the literature, 12 clinical trials associated with bisphosphonate usage and 16 associated with FES administration were screened for eligibility (Figure 1). Four studies were excluded due to medication use other than bisphosphonate analogues [16] or lack of an outcome measured by a DEXA or CT [9,17,18]. An assessment of the remaining 8 articles revealed 7 randomized controlled trials [19-25] and one quasi-experimental study [26]. Among the 7 randomized controlled trials, 3 employed the double-blind placebo controlled scheme [21,23,24]. With regard to bisphosphonate regimens, three studies [20,22,23] used oral alendronate, one study [19] used oral etidronate, two studies [24,25] used intravenous (IV) zoledronic acid, and two studies [21,26] used IV pamidronate. With respect to reference treatments, five studies [19,20,25,26] utilized usual care, two studies [21,24] utilized normal saline injections, and one study [23] utilized placebo tablets.
Figure 1. Flow diagram of the evaluation process for the included and excluded studies.
With respect to FES training, 5 studies were excluded: two studies [27,28] were case series without pre-designed outcome measurements; one study [29] did not report BMD at our desired timing; one study [30] was deficient in BMD assessment; and another study [31] employed pediatric SCI patients. Among the 11 enrolled articles, 9 articles [32-41] were longitudinal follow-up cohort studies whose effectiveness of intervention was determined by the comparison with baseline, and two studies [32,33] used quasi-experimental designs which enrolled SCI participants with usual care. None were randomized controlled trials. In terms of the FES regimens, nine studies [32-34,36,38-42] employed FES cycling ergometry, and 2 studies [35,37] employed FES plus resistance training.
Characteristics of included patients (Table 1)
Table 1. Summary of studies which used bisphosphonate analogues or functional electrical stimulation (FES) to treat bone mineral density (BMD) loss in patients after spinal cord injury (SCI).
| Authors, year | Sample characteristic | Sample number | Study design | Double blind | Intention to treat | Treatment | Frequency | Duration | Device for BMD measurement | Site of BMD measurement | Quality Assessments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies using bisphosphonate analogues to attenuate bone loss in SCI patients | |||||||||||
| Pearson et al, 1997 | Acute SCI. Post-injury duration: within 6 weeks. Injury level: T: C5-T12; C: C6-T12. Age: T: 33.6 ± 4.5 years; C: 35.6 ± 11.6 years | T: 5 (4 males, 1 female); C: 6 (all males) | RCT | No | No | Etidronate, 800 mg, oral | Once daily | 2 weeks for each cycle; total two cycles of treatments separated by 13 weeks | DEXA | Distal femur | 1* |
| Nance et al, 1999 | Acute SCI. Post-injury duration: within 6 weeks. Injury level: T: C4-L1; C: C6-L1. Age: T: 31.8 ± 8.7 years; C: 34.4 ± 12.1 years | T: 12 (11 males, 1 female); C: 7 (all males) | Quasi-experimental study | No | No | Pamidronate, 30 mg, IV | Once per month | 6 months | DEXA | Whole lower extremity | 0* |
| Zehnder et al, 2004 | Mainly chronic SCI. Post-injury duration: T: 10.8 ± 1.4 years; C: 9.9 ± 1.7 years. Injury level: T1-L3. Age: T: 38.8 ± 1.5 years; C: 37.9 ± 2.2 years | T: 29 (all males); C: 26 (all males) | RCT | No | No | Alendronate, 10 mg, oral | Once daily | 24 months | DEXA | Proximal tibia | 2* |
| Moran et al, 2005 | Chronic SCI. Post-injury duration: T: 61.0 ± 77.3 months; C: 38.7 ± 17.1 months. Injury level: not mentioned. Age: T: 30.9 ± 9.5 years; C: 30.8 ± 9.9 years | T: 9 (1 dropout from 8 males, 2 females); C: 8 (1 dropout from 7 males, 2 females) | RCT | No | No | Alendronate,10 mg, oral | Once daily | 6 month | DEXA | Whole lower extremity | 1* |
| Bauman et al, 2005 | Acute SCI. Post-injury duration: 44 ± 18 days. Injury level: T: 3 tetraplegia, 3 paraplegia; C: 2 tetraplegia, 3 paraplegia. Age: T: 39 ± 15 years; C: 30 ± 8 years | T: 6 (4 males, 2 females); C: 5 (4 males, 1 female) | RCT | Yes | No | Pamidronate, 60 mg IV | at baseline and then at 1, 2, 3, 6, 9, and 12 months | 12 month | DEXA | Distal femur | 4* |
| Gilchrist et al, 2007 | Acute SCI. Post-injury duration: within 10 days. Injury level: C4-L2. Age: 17-55 years | T: 12 (3 dropouts from 10 males, 5 females); C: 13 (3 dropouts from 12 males, 4 females) | RCT | Yes | No | Alendronate, 70 mg, oral | Once per week | 12 months | DEXA | Femoral shaft | 5* |
| Shapiro et al, 2007 | Acute SCI. Post-injury duration: < 12 weeks. Injury level: C2-T12. Age: T: 30.1 ± 14.2 years; C: 28.4 ± 9.4 years | T: 8; C: 9 (gender not mentioned) | RCT | Yes | No | Zoledronic acid, 4 or 5 mg, IV | At baseline | Once | DEXA | Femoral neck | 5* |
| Bubbear et al, 2011 | Acute SCI. Post-injury duration: within 3 months. Injury level: T: C4-L3; C: C6-T8. Age: 31.6 ± 7.7 years; C: 27.0 ± 14.4 years | T: 6 (1 dropout from 4 males, 3 females); C: 5 (2 dropouts from 5 males, 2 females) | RCT | No | No | Zoledronic acid, 4mg, IV | At baseline | Once | DEXA | Greater trochanter | 3* |
| Studies using FES to attenuate bone loss in SCI patients | |||||||||||
| Leeds et al, 1990 | Chronic SCI. Post-injury duration: 5.17 ± 2.40 years. Injury level: C4-C6. Age: 23.67 ± 3.20 years | 6 (all males) | Longitudinal follow-up study | No | Yes | FES cycling ergometry | 3 sessions per week. Gradually increased training time to 30 mins per session | 6 months | DEXA | Femoral trochanter | 4† |
| BeDell et al, 1996 | Chronic SCI. Post-injury duration: > 2 years. Injury level: C5-T12. Age: 34 ± 6 years | 12 (all males) | Longitudinal follow-up study | No | Yes | FES cycling ergometry | 30 mins, three times per week | 24 sessions | DEXA | Femoral neck | 4† |
| Bloomfield et al, 1996 | Chronic SCI Post-injury duration: T: 6 ± 1.2 years; C: 8.3 ± 2.3 years. Injury level: T: C5-T7; C: C4-T12. Age: T: 28.2 ± 1.8 years; C: 34.4 ± 2.5 years | T: 9 (5 males, 4 females); C: 8 (5 males, 3 females) | Quasi-experimental study | No | Yes | FES cycling ergometry | 3 sessions per week | 9 months | DEXA | Distal femur | 5* |
| Mohr et al, 1997 | Chronic SCI. Post-injury duration: 12.5 ± 2.7 years. Injury level: C6-T4. Age: 35.3 ± 2.3 years | 10 (8 males, 2 females) | Longitudinal follow-up study | No | Yes | FES cycling ergometry | 30 mins per day, 3 days per week | 12 months | DEXA | Proximal tibia | 4† |
| Belanger et al, 2000 | Chronic SCI Post-injury duration: 9.6 ± 6.6 years. Injury level: C5-T5. Age: 32.4 ± 5.9 years | T: 14 (11 males, 3 females); C: 14 age and sex-matched healthy individuals | Longitudinal follow-up study | No | No | FES plus resistive training | 1 hour per day, 5 days per week | 6 months | DEXA | Distal femur | 4† |
| Eser et al, 2003 | Acute SCI. Post-injury duration: T: 4.5 ± 2.9 weeks; C: 4.6 ± 2.9 weeks. Injury level: T: C5-T10; C: C5-T12. Age: T: 32.9 ± 11.5 years; C: 33.8 ± 13.0 years | T: 19 (17 males, 2 females) ; C: 19 (17 males, 2 females) | Quasi-experimental study | No | No | FES cycling ergometry | 30 mins per day, 3 days per week | Average 6 months | CT scanner | Proximal tibia | 5* |
| Chen et al, 2005 | Chronic SCI. Post-injury duration: > 2 years and 7 months. Injury level: C5-T8. Age: 28.67 ± 3.77 years | 15 (all males) | Longitudinal follow-up study | No | Yes | FES cycling ergometry | 30 mins per day, five days per week | 6 months | DEXA | Distal femur | 4† |
| Clark et al, 2007 | Acute SCI. Post-injury duration: within two days. Injury level: T: C4-T10; C: C5-T12. Age: T: 30.0 ± 8.9 years; C: 34.8 ± 11.2 years | T: 23; C: 10 (gender not mentioned) | Longitudinal follow-up study | No | Yes | FES plus resistive training | 15-min to each leg twice daily, 5 days per week | 5 months | DEXA | Whole lower extremity | 5† |
| Frotzler et al, 2008 | Chronic SCI. Post-injury duration: 11.0 ± 7.1 years. Injury level: T3-T9. Age: 41.9 ± 7.5 years | 11 (9 males, 2 females) | Longitudinal follow-up study | No | No | FES cycling ergometry | 60 mins per session. 5 sessions per week | 12 months | Peripheral quantitative CT scanner | Distal femur | 4† |
| Griffin et al, 2009 | Chronic SCI. Post-injury duration: 11.0 ± 3.1 years. Injury level: C4-T7. Age: 40.0 ± 2.4 years | 18 (13 males, 5 females) | Longitudinal follow-up study | No | Yes | FES cycling ergometry | 2-3 times per week | 10 weeks | DEXA | Not mentioned | 4† |
| Lai et al, 2010 | Acute SCI. Post-injury duration: 26-52 days. Injury level: C5-T10. Age: T: 28.9 ± 5.3 years; C: 28.2 ± 5.7 years | T: 12 (10 males, 2 females); C: 12 (10 males, 2 females) | Longitudinal follow-up study | No | Yes | FES cycling ergometry | 3 times per week | 3 months, and then suspend for subsequent 3 months | DEXA | Distal femur | 5† |
Note: * Quality scores derived from the Jadad scale. † Quality scores derived from the Newcastle-Ottawa Scale. Abbreviation: T, treatment group; C, control group; RCT: randomized controlled trial; DEXA: dual-energy X-ray absorptiometry; IV: intravenous.
Our meta-analysis included 364 SCI patients and 14 healthy individuals. The majority were men with ages ranging from 23.7 to 41.9 years. The level of the spinal cord lesion varied from C2 to T12, and most of the studies included patients with both complete and incomplete neurologic injury. Regarding the timing of bisphosphonate or FES administration, nine studies [19,21,23-26,37,40,42] enrolled patients with acute SCI, and ten trials [20,22,32-36,38,39,41] recruited mainly chronic SCI participants.
Percentage of BMD changes (Table 2)
Table 2. Percent of bone mineral density (BMD) changes compared with the baseline value at the 3rd, 6th, 12th and 18th or more in spinal cord injury (SCI) patients after intervention.
| Authors, year | Participants’ pattern | Intervention | Comparator | Percent of BMD changes at the 3rd month | Percent of BMD changes at the 6th month | Percent of BMD changes at the 12th month | Percent of BMD changes at the 18th month or more |
|---|---|---|---|---|---|---|---|
| Studies using bisphosphonate analogues to attenuate bone loss in SCI patients | |||||||
| Pearson et a al, 1997 | Acute SCI | Etidronate (oral) | SCI patients with usual care | No measurement | T: -8.3 ± 3.6; C: difficulty in data extraction | T: -26.1 ± 7.4; C: difficulty in data extraction | No measurement |
| Nance et al, 1999 | Acute SCI | Pamidronate (IV) | SCI patients with usual care | No measurement | No measurement | T: -4.7 ± 0.4; C: -10.8 ± 0.4 | No measurement |
| Zehnder et al, 2004 | Mainly chronic SCI | Alendronate (oral) | SCI patients with usual care | No measurement | T: 0.4 ± 0.6; C: -1.1 ± 0.4 | T: -0.7 ± 0.8; C: -2.3 ± 0.6 | T: -1.3 ± 1.3; C: -4.0 ± 0.82 |
| Moran et al, 2005 | Chronic SCI | Alendronate (oral) | Calcium 1000mg daily | No measurement | T: -1.0 ± 1.9; C: -0.9 ± 4.6 | No measurement | No measurement |
| Bauman et al, 2005 | Acute SCI | Pamidronate (IV) | Normal saline | T: -1.0 ± 3.0; C: -6.0 ± 7.0 | T: -5.0 ± 4.0; C: -9.0 ± 8.0 | T: -9.0 ± 7.0; C: -12 ± 7.0 | T : -18.0 ± 9.0; C: -19.0 ± 9.0 |
| Gilchrist et al, 2007 | Acute SCI | Alendronate (oral) | Placebo tablet | T: -0.3 ± 5.2; C: -5.6 ± 4.7 | T: -2.3 ± 5.4; C: -13.4 ± 4.9 | T: -3.4 ± 5.2; C: -18.5 ± 4.7 | T: -7.1 ± 4.7; C: -22.6 ± 4.3 |
| Shapiro et al, 2007 | Acute SCI | Zoledronic acid (IV) | Normal saline | No measurement | T: 2.4 ± 4.3; C: -1.7 ± 3.5 | T: -2.1 ± 4.2; C: -12.6 ± 5.2 | No measurement |
| Bubbear et al, 2011 | Acute SCI | Zoledronic acid (IV) | SCI patients with usual care | T: -1.9 ± 2.41; C: -10.84 ± 1.72 | T: -1.5 ± 5.9; C: -18.5 ± 5.9 | T: -4.5 ± 5.7; C: -17.9 ± 9.4 | No measurement |
| Studies using functional electrical stimulation (FES) to attenuate bone loss in SCI patients | |||||||
| Leeds et al, 1990 | Chronic SCI | FES cycling ergometry | Nil | No measurement | T: -5.6 ± 6.5 | No measurement | No measurement |
| Bedell et al, 1996 | Chronic SCI | FES cycling ergometry | Ni; | T: 5.1 ± 17 | No measurement | No measurement | No measurement |
| Bloomfield et al, 1996 | Chronic SCI | FES cycling ergometry | SCI patients with usual care | T: 6.7 ± 2.1; C:-2.1 ± 3.4 | T: 4.8 ± 3.3; C: 2.3 ± 4.4 | No measurement | No measurement |
| Mohr et al, 1997 | Chronic SCI | FES cycling ergometry | Nil | No measurement | No measurement | T: 9.7 ± 3.5 | T: -2 ± 6.9 |
| Belanger et al, 2000 | Chronic SCI | FES plus resistive training | Nil | No measurement | T: 11.1 ± 4.6 | No measurement | No measurement |
| Eser et al, 2003 | Acute SCI | FES cycling ergometry | SCI patients with usual care | T: -0.9 ± 1.8; C: -2.1 ± 2.4 | T: -1.8 ± 3.6; C: -4.2 ± 4.8 | No measurement | No measurement |
| Chen et al, 2005 | Chronic SCI | FES cycling ergometry | Nil | No measuremnt | T: 11.1 ± 0.8 | No measurement | No measurement |
| Clark et al, 2007 | Acute SCI | FES to quadriceps femoris and anterior tibialis | SCI patients with usual care | T: -2.4 ± 3.3; C: -2.3 ± 2.8 | T: -7.1 ± 3.1; C: -4.7 ± 2.7 | No measurement | No measurement |
| Frotzler et al, 2008 | Chronic SCI | FES cycling ergometry | Nil | No measurement | T: 5.2 ± 15.6 | T: 6.6 ± 16 | No measurement |
| Griffin et al, 2009 | Chronic SCI | FES cycling ergometry | Nil | T: -0.6 ± 6.3 | No measurement | No measurement | No measurement |
| Lai et al, 2010 | Acute SCI | FES cycling ergometry | Nil | T: -2.1 ± 0.9; C: -6.6 ± 0.5 | No measurement | No measurement | No measurement |
Note: abbreviation: T: treatment group; C: control group; IV: intravenous
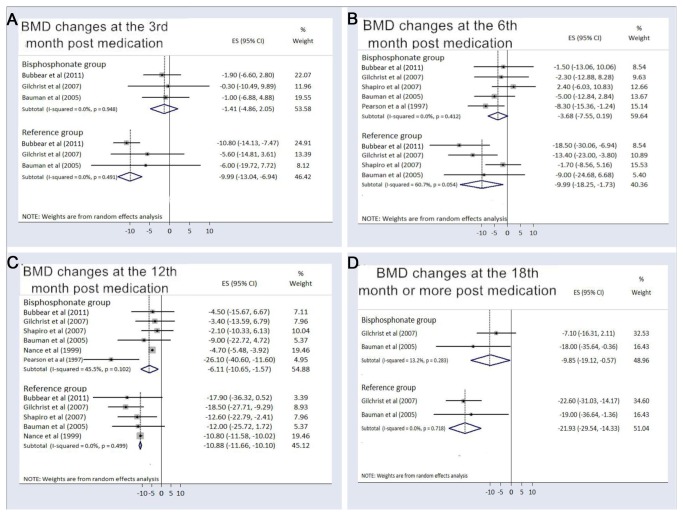
For the acute SCI participants treated with bisphosphonates, the pooled BMD changes compared with baseline were -1.41% (95%CI, -4.86% to 2.05%) at the 3rd month, -3.68% (95% CI, -7.55% to 0.19%) at the 6th month, -6.11% (95% CI, -10.65% to -1.57% ) at the 12th month, and -9.85% (95% CI, -19.12% to -0.57%) at the 18th month or more after medication use. Among the control patients receiving placebo or usual care, the pooled BMD changes were -9.99% (95%CI, -13.04% to -6.94%) at the 3rd month, -9.99 % (95% CI, -18.25% to -1.73%) at the 6th month, -10.88% (95% CI, -11.66% to -10.10%) at the 12th month, and -21.93% (95% CI, -29.54% to -14.33%) at the 18th month or more after recruitment (Figure 2). If we eliminated Pearson’s study [19] that only prescribed bisphosphonates for two weeks at the beginning and end of the research, the pooled BMD changes increased to -1.70 % (95% CI, -6.32% to 2.93%) at the 6th month and -4.68% (95% CI, -5.46% to -3.91% ) at the 12th month. We performed a subgroup analysis for the route of bisphosphonate administration (Figure 3). The pooled BMD changes in trials using IV bisphosphonates were -1.55% (95% CI, -6.70% to 3.59%) at the 6th month and -4.69% (-95% CI, -5.47% to -3.91%) at the 12th month following treatments, compared with -6.45% (95% CI, -12.32% to -0.58%) at the 6th month and -14.14% (95% CI, -36.35% to 8.07%) at the 12th month with oral bisphosphonates (Figure 3). In the present meta-analysis, only two studies administered bisphosphonates in the chronic SCI population, whose pooled BMD change compared with baseline was 0.27% (95% CI, -0.85% to 1.39%) at the 6th month following bisphosphonate treatment.
Figure 2. Percent bone mineral density changes in acute spinal cord injury patients using bisphosphonates.
The figure represents the forest plot of percent bone mineral density (BMD) changes from baseline in the acute spinal cord injury patients using bisphosphonates compared to the reference group at (A) the 3rd, (B) the 6th, (C) the 12th and (D) the 18th month or more following intervention.
Figure 3. Percent bone mineral density changes categorized by the route of administration.
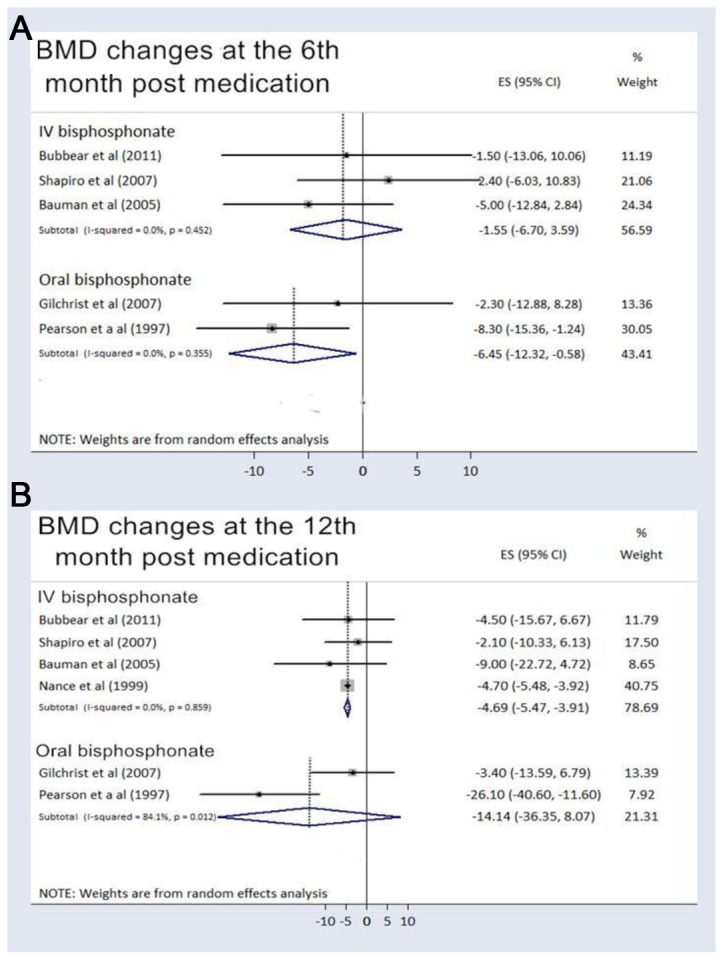
The figure represents the forest plot of percent bone mineral density (BMD) change from baseline in the acute spinal cord injury patients using bisphosphonates categorized by the route of administration at (A) the 6th and (B) the 12th month following intervention.
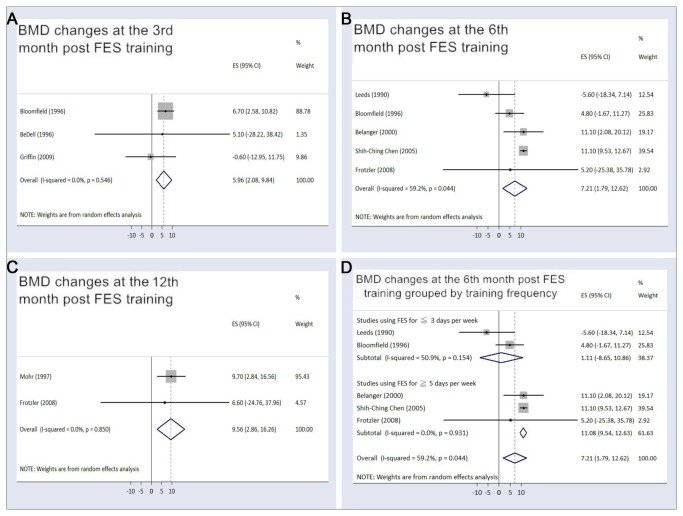
Among the trials that applied FES on acute SCI patients, the pooled BMD changes from baseline were -1.89% (95% CI, -3.42% to -0.36%) at the 3rd month and -1.80% (95% CI, -8.86% to 5.26%) at the 6th month after intervention, whereas the values were -4.41% (95% CI, -7.90% to -0.91%) at the 3rd month and -4.58% (95% CI, -9.19% to 0.03%) at the 6th month in the participants receiving usual care. Among the studies using FES for the chronic SCI population, the pooled BMD changes from baseline were 5.96% (95% CI, 2.08% to 9.84%) at the 3rd month, 7.21% (95%CI, 1.79% to 12.62%) at the 6th month, and 9.56% (95% CI, 2.86% to 16.26%) at the 12th month following FES training. Regarding the influence of FES frequency on the effectiveness of training, a trend of toward higher elevation of BMD (11.08% [95% CI, 9.54% to 12.63%]), was identified in the studies using FES for at least 5 days per week at the 6th month following training. This finding is in stark contrast to the 1.11% (95% CI, -8.65% to 10.86) in the trials conducting FES ≤3 days per week (Figure 4). The temporal relationships of the BMD changes for acute SCI patients undergoing bisphosphonate therapy and chronic SCI patients receiving FES were illustrated in Figure 5.
Figure 4. Percent bone mineral density changes in chronic spinal cord injury patients receiving functional electrical stimulation.
The figure represents the forest plot of percent bone mineral density (BMD) changes from baseline in the chronic spinal cord injury patients receiving functional electrical stimulation (FES) training at (A) the 3rd, (B) the 6th, and (C) the 12th month following intervention; (D) forest plot of the percentage of bone mineral density (BMD) changes from baseline categorized by training frequency at the 6th month post-FES intervention.
Figure 5. Temporal relationships of percent bone mineral density changes in studies employing bisphosphonates or functional electrical stimulation.
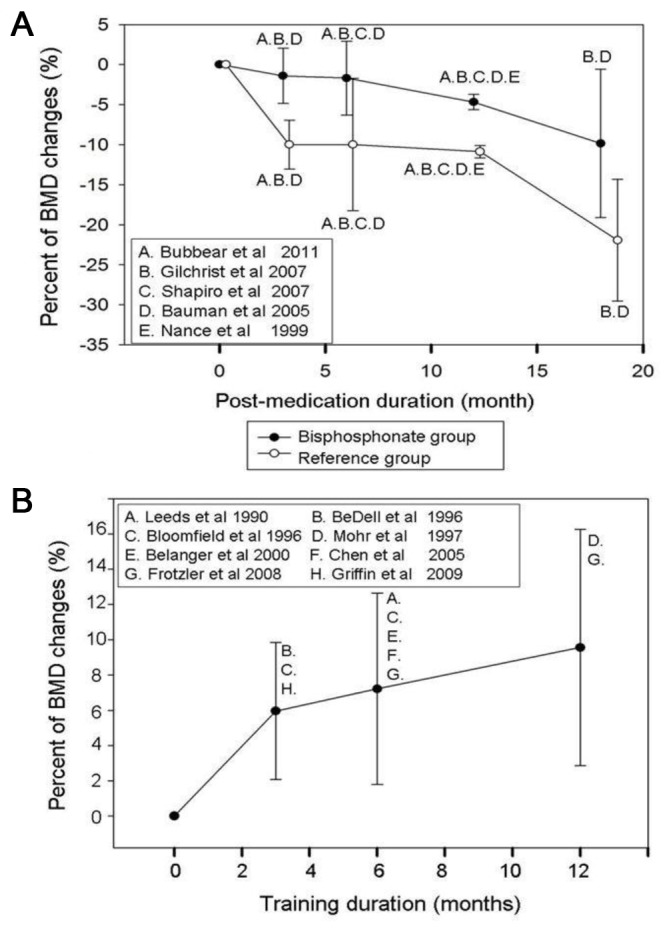
The figure represents the temporal relationships of percent bone mineral density (BMD) changes from baseline in (A) studies prescribing bisphosphonates for acute spinal cord injury (SCI) patients and (B) trials using functional electrical stimulation (FES) for chronic SCI patients. The value was expressed by its pooled point estimate and 95% confidence interval.
Safety
Among the 87 patients undergoing bisphosphonate therapy, 15 (17.2 %) patients experienced adverse effects. Eight patients receiving zoledronic acid reported flu-like symptoms such as myalgias and fever [24,25]. Other adverse events included gastrointestinal upset (n=4), dizziness (n=1), headache (n=1) after the usage of alendronate, and pruritic rash (n=1) following IV pamidronate. Among 149 patients who received FES, only one patient developed a foot fracture, which was unrelated to FES cycling training [38].
Discussion
This meta-analysis identified 19 clinical trials prescribing bisphosphonate analogues or FES training for SCI patients and evaluated whether or not they could effectively ameliorate or reverse bone loss below the level of the SCI. The bisphosphonate group had less BMD decline than the control patients when the medication was initiated immediately after SCI. Moreover, FES training demonstrated an osteogenic effect on BMD near the distal femur in the chronic SCI group. Our review failed to prove an increase in sublesional BMD in chronic SCI patients using bisphosphonate or a decrease of BMD attenuation in acute SCI patients following FES training.
Two systematic reviews have investigated the utility of bisphosphonate analogues or rehabilitation-oriented methods for sublesional BMD in the SCI population. Bryson et al [8] identified 7 of the 8 bisphosphonate trials included in our meta-analysis and focused on criticisms of their methodology and evidence level. Prophylactic administration of bisphosphonates is not recommended by the authors based on the lower scores of research quality in retrieved studies and the absence of reporting fracture incidence. Biering-Sorensen et al [7] systematically reviewed the literature on non-pharmacologic approaches to treating osteoporosis following SCI and identified 9 of the 11 FES trials in our meta-analysis. A potential advantage of FES on BMD adjacent to the knee joint under a high-frequency and long-period training program was suggested. Both reviews bypassed quantitative analysis due to heterogeneity of the patient populations and outcome measures and were incapable of delineating the temporal relationship of BMD in groups receiving interventions compared with their pre-treatment values or controls. Therefore, we computed the percentage of change of BMD at different intervals, pooled their effect sizes for comparisons, and attempted to provide solid evidence regarding the influence of bisphosphonates or FES on sublesional BMD in SCI patients.
The present study indicated that the acute SCI population undergoing bisphosphonate therapy had less BMD reduction compared with the control patients. A significant superiority of intervention to reference treatments appeared at the 3rd month, and the trend was likely to be statistically significant at the 12th month based on a minimal overlap of the 95% CI of BMD changes between the intervention and control groups (Figure 2 and 5). Consequently, we performed a sensitivity analysis by eliminating Pearson’s study [19], which prescribed oral bisphosphonates for two weeks at the beginning and end of the research. The pooled percentage changes in BMD at the 12th month increased to -4.68% in the treatment population with a 95% CI (-5.46% to -3.91%) which separated from the corresponding value (-11.66% to -10.10%) in the reference group. The result confirmed the effectiveness of bisphosphonates and disclosed the importance of sustained bisphosphonate use in maintaining BMD for acute SCI participants. However, for chronic SCI participants, the pooled BMD change compared with baseline was 0.27% (95% CI, -0.85% to 1.39%) at the 6th month based on two studies using bisphosphonates [20,22]. Only one of the aforementioned studies followed the outcome for two years and found no significant increase in post-treatment BMD in chronic SCI individuals [20]. Therefore, current evidence did not prove an osteogenic effect of bisphosphonate administration in chronic SCI patients.
Some trials advocated the advantage of IV bisphosphonate over oral intake [21,24-26], but the comparison of the effectiveness for improving sublesional BMD has never been studied. IV bisphosphonate use eliminates the need to maintain an upright posture for the 30 minutes that is required with oral dosing, assures higher compliance with therapy, and provides better absorption without the interference of neurogenic bowel disorders in the SCI population [25]. Therefore, we performed a subgroup analysis comparing BMD changes between the use of IV and oral bisphosphonates in the acute SCI population. The crude data showed that bone loss in those patients receiving the IV regimen seemed to be less than in those patients taking oral bisphosphonates (Figure 3). However, if we excluded Pearson’s study [19] for its short use of medication, the point estimate of BMD changes from baseline in Gilchrist’s trial [23] was similar to the value in studies using IV administration (Figure 3). Since current oral bisphosphonates have proven to possess similar efficacy against osteoporotic hip fractures compared with the analogues administered via the IV route [10], our finding supported that the duration and not the route of bisphosphonate administration affected the BMD in acute SCI patients.
Regarding the effectiveness of FES, we found a significant increase in BMD in chronic SCI patients at the 3rd, 6th, and 12th months after intervention. Our results indicated that more than 3 months of FES training was capable of restoring BMD adjacent to the knee joint. Furthermore, a longer period of exercise could achieve better effectiveness (Figure 4 and 5). Since most FES studies used cycling ergometry or knee resistance exercises as their major functional task, the findings were compatible with the augmentation of BMD through FES exclusively occurring near the site directly exposed to mechanical loading, such as the distal femur and proximal tibia. Another concern was that the benefit of osteogenesis could not be maintained after FES was discontinued. According to Chen’s report [36], the BMD returned to the baseline value after 6 months without training, which stressed the importance of a continuous program for FES intervention.
We also conducted a subgroup analysis looking at the training frequency and BMD changes at the 6th month, which was the time interval with the most available BMD data (Figure 4). Our investigation demonstrated that studies using FES ≤3 days per week demonstrated only a pooled 1.11% increase in BMD (95% CI [-8.65% to 10.86]). In contrast, the trials employing FES ≥5 days per week had a pooled 11.08% elevation in BMD, which was statistically significant. This finding suggested 5 days per week should be the minimum training frequency in order to achieve significant improvement in BMD. Finally, compared with control patients, the present meta-analysis failed to show a benefit of FES in decreasing osteoporosis in the acute SCI population.
Our results suggested that the effectiveness of bisphosphonate administration and FES training were in accordance with the unique pathophysiology of osteoporosis in SCI patients. Osteoclastic activity overwhelms osteoblastic activity, which leads to rapid demineralization in the acute SCI following injury [2,43]. Therefore, it was reasonable that bisphosphonate, a potent inhibitor of osteoclast-mediated bone resorption, should help attenuate bone loss in acute SCI patients [8]. With chronic SCI, a steady state between osteogenic and osteolytic activity has been established and the benefit of bisphosphonates to inactivate osteoclasts became less significant [1]. FES training, which uses strenuous muscle contraction and mechanical loading to elicit osteogenesis, was shown to effectively restore BMD at the bony structures adjacent to the stimuli. Our meta-analysis implies that bisphosphonates should be administered from the beginning of the SCI and continued until a steady state has been reached (≈2 yr) in order to prevent loss of BMD. FES training can be used as an adjuvant tool to reverse muscle atrophy and activate bone formation in chronic, stable SCI patients.
Several limitations should be considered in the interpretation of the present meta-analysis. First, none of the studies had sufficient time to follow up subsequent fracture events. Although our findings demonstrated potential benefits of bisphosphonates or FES for improving sublesional BMD, some uncertainty remains regarding the effectiveness to reduce the rates of osteoporosis-related fractures. Secondly, most of the included FES studies used a pre-post test design without random assignment of enrolled participants or comparison with usual care. These fundamental flaws rendered those FES trials low in research quality and level of evidence. Thirdly, diversity of bisphosphonate dosage and protocols of administration existed across studies. The limitation was partly compensated by subgroup analysis based on the routes of administration and timings of BMD measurement. Besides, heterogeneity between trials usually biases the treatment effect toward a null result. However, our investigation demonstrated some significant results which made the effectiveness of both therapeutic approaches more convincing. Finally, most of the patients were males with diverse injury levels and post-injury durations. The heterogeneity with respect to injury level and duration of injury tended to reduce the precision of the outcome measurements and caused potentially insignificant results. Therefore, we analyzed the BMD changes separately in acute and chronic SCI populations and demonstrated some favorable results of both therapeutic approaches after compensating for bias from different post-injury periods. We should also be cautious about generalizing our study findings to female SCI patients.
In conclusion, the present meta-analysis revealed that bisphosphonate administration immediately after the SCI event was effective at attenuating the rate of bone loss below the SCI level. Sustained use of bisphosphonates until a steady state between osteogenic and osteolytic activities is reached is crucial in reducing osteoporosis in the acute SCI population. FES intervention could significantly increase sublesional BMD adjacent to the site of maximal mechanical loading in patients with chronic SCI, and training a minimum of 5 days per week was associated with higher effectiveness.
Supporting Information
PRISMA Checklist for systematic review and meta-analysis.
(DOC)
Funding Statement
This research was supported by grants from the National Science Council (102-2314-B-002-013, 100-2314-B-002-012-MY3 and NSC 99-2320-B-002-005-MY3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dionyssiotis Y (2011) Spinal cord injury-related bone impairment and fractures: an update on epidemiology and physiopathological mechanisms. J Musculoskelet Neuronal Interact 11: 257-265. PubMed: 21885901. [PubMed] [Google Scholar]
- 2. Maïmoun L, Fattal C, Micallef JP, Peruchon E, Rabischong P (2006) Bone loss in spinal cord-injured patients: from physiopathology to therapy. Spinal Cord 44: 203-210. doi: 10.1038/sj.sc.3101832. PubMed: 16158075. [DOI] [PubMed] [Google Scholar]
- 3. Charmetant C, Phaner V, Condemine A, Calmels P (2010) Diagnosis and treatment of osteoporosis in spinal cord injury patients: A literature review. Ann Phys Rehabil Med 53: 655-668. doi: 10.1016/j.rehab.2010.10.001. PubMed: 21094110. [DOI] [PubMed] [Google Scholar]
- 4. Galea MP (2012) Spinal cord injury and physical activity: preservation of the body. Spinal Cord 50: 344-351. doi: 10.1038/sc.2011.149. PubMed: 22158253. [DOI] [PubMed] [Google Scholar]
- 5. Gater DR Jr, Dolbow D, Tsui B, Gorgey AS (2011) Functional electrical stimulation therapies after spinal cord injury. Neurorehabilitation 28: 231-248. PubMed: 21558629. [DOI] [PubMed] [Google Scholar]
- 6. Dolbow DR, Gorgey AS, Daniels JA, Adler RA, Moore JR et al. (2011) The effects of spinal cord injury and exercise on bone mass: a literature review. Neurorehabilitation 29: 261-269. PubMed: 22142760. [DOI] [PubMed] [Google Scholar]
- 7. Biering-Sørensen F, Hansen B, Lee BS (2009) Non-pharmacological treatment and prevention of bone loss after spinal cord injury: a systematic review. Spinal Cord 47: 508-518. doi: 10.1038/sc.2008.177. PubMed: 19172152. [DOI] [PubMed] [Google Scholar]
- 8. Bryson JE, Gourlay ML (2009) Bisphosphonate use in acute and chronic spinal cord injury: a systematic review. J Spinal Cord Med 32: 215-225. PubMed: 19810623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Minaire P, Berard E, Meunier PJ, Edouard C, Goedert G et al. (1981) Effects of disodium dichloromethylene diphosphonate on bone loss in paraplegic patients. J Clin Invest 68: 1086-1092. doi: 10.1172/JCI110331. PubMed: 6457060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jansen JP, Bergman GJ, Huels J, Olson M (2011) The efficacy of bisphosphonates in the prevention of vertebral, hip, and nonvertebral-nonhip fractures in osteoporosis: a network meta-analysis. Semin Arthritis Rheum 40: 275-284 e271-272 [DOI] [PubMed] [Google Scholar]
- 11. Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T et al. (2008) Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther 88: 156-175. doi: 10.2522/ptj.20070147. PubMed: 18073267. [DOI] [PubMed] [Google Scholar]
- 12. Sheth U, Simunovic N, Klein G, Fu F, Einhorn TA et al. (2012) Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am 94: 298-307. doi: 10.2106/JBJS.K.00154. PubMed: 22241606. [DOI] [PubMed] [Google Scholar]
- 13. Chang KV, Chen SY, Chen WS, Tu YK, Chien KL (2012) Comparative effectiveness of focused shock wave therapy of different intensity levels and radial shock wave therapy for treating plantar fasciitis: a systematic review and network meta-analysis. Arch Phys Med Rehabil 93: 1259-1268. doi: 10.1016/j.apmr.2012.02.023. PubMed: 22421623. [DOI] [PubMed] [Google Scholar]
- 14. Chang KV, Hsiao MY, Chen WS, Wang TG, Chien KL (2013) Effectiveness of Intra-Articular Hyaluronic Acid for Ankle Osteoarthritis Treatment: A Systematic Review and Meta-Analysis. Arch Phys Med Rehabil, 94: 951–60. PubMed: 23149311. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557-560. doi: 10.1136/bmj.327.7414.557. PubMed: 12958120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bauman WA, Spungen AM, Morrison N, Zhang RL, Schwartz E (2005) Effect of a vitamin D analog on leg bone mineral density in patients with chronic spinal cord injury. J Rehabil Res Dev 42: 625-634. doi: 10.1682/JRRD.2004.11.0145. PubMed: 16586188. [DOI] [PubMed] [Google Scholar]
- 17. Minaire P, Depassio J, Berard E, Meunier PJ, Edouard C et al. (1987) Effects of clodronate on immobilization bone loss. Bone 8 Suppl 1: S63-S68. PubMed: 2961357. [PubMed] [Google Scholar]
- 18. Chappard D, Minaire P, Privat C, Berard E, Mendoza-Sarmiento J et al. (1995) Effects of tiludronate on bone loss in paraplegic patients. J Bone Miner Res 10: 112-118. PubMed: 7747617. [DOI] [PubMed] [Google Scholar]
- 19. Pearson EG, Nance PW, Leslie WD, Ludwig S (1997) Cyclical etidronate: its effect on bone density in patients with acute spinal cord injury. Arch Phys Med Rehabil 78: 269-272. doi: 10.1016/S0003-9993(97)90032-0. PubMed: 9084348. [DOI] [PubMed] [Google Scholar]
- 20. Zehnder Y, Risi S, Michel D, Knecht H, Perrelet R et al. (2004) Prevention of bone loss in paraplegics over 2 years with alendronate. J Bone Miner Res 19: 1067-1074. doi: 10.1359/JBMR.040313. PubMed: 15176988. [DOI] [PubMed] [Google Scholar]
- 21. Bauman WA, Wecht JM, Kirshblum S, Spungen AM, Morrison N et al. (2005) Effect of pamidronate administration on bone in patients with acute spinal cord injury. J Rehabil Res Dev 42: 305-313. doi: 10.1682/JRRD.2004.05.0062. PubMed: 16187243. [DOI] [PubMed] [Google Scholar]
- 22. Moran de Brito CM, Battistella LR, Saito ET, Sakamoto H (2005) Effect of alendronate on bone mineral density in spinal cord injury patients: a pilot study. Spinal Cord 43: 341-348. doi: 10.1038/sj.sc.3101725. PubMed: 15700052. [DOI] [PubMed] [Google Scholar]
- 23. Gilchrist NL, Frampton CM, Acland RH, Nicholls MG, March RL et al. (2007) Alendronate prevents bone loss in patients with acute spinal cord injury: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 92: 1385-1390. doi: 10.1210/jc.2006-2013. PubMed: 17227802. [DOI] [PubMed] [Google Scholar]
- 24. Shapiro J, Smith B, Beck T, Ballard P, Dapthary M et al. (2007) Treatment with zoledronic acid ameliorates negative geometric changes in the proximal femur following acute spinal cord injury. Calcif Tissue Int 80: 316-322. doi: 10.1007/s00223-007-9012-6. PubMed: 17417700. [DOI] [PubMed] [Google Scholar]
- 25. Bubbear JS, Gall A, Middleton FR, Ferguson-Pell M, Swaminathan R et al. (2011) Early treatment with zoledronic acid prevents bone loss at the hip following acute spinal cord injury. Osteoporos Int 22: 271-279. doi: 10.1007/s00198-010-1221-6. PubMed: 20358358. [DOI] [PubMed] [Google Scholar]
- 26. Nance PW, Schryvers O, Leslie W, Ludwig S, Krahn J et al. (1999) Intravenous pamidronate attenuates bone density loss after acute spinal cord injury. Arch Phys Med Rehabil 80: 243-251. doi: 10.1016/S0003-9993(99)90133-8. PubMed: 10084430. [DOI] [PubMed] [Google Scholar]
- 27. Pacy PJ, Hesp R, Halliday DA, Katz D, Cameron G et al. (1988) Muscle and bone in paraplegic patients, and the effect of functional electrical stimulation. Clin Sci (Lond) 75: 481-487. PubMed: 3267113. [DOI] [PubMed] [Google Scholar]
- 28. Sloan KE, Bremner LA, Byrne J, Day RE, Scull ER (1994) Musculoskeletal effects of an electrical stimulation induced cycling programme in the spinal injured. Paraplegia 32: 407-415. doi: 10.1038/sc.1994.67. PubMed: 8090549. [DOI] [PubMed] [Google Scholar]
- 29. Hangartner TN, Rodgers MM, Glaser RM, Barre PS (1994) Tibial bone density loss in spinal cord injured patients: effects of FES exercise. J Rehabil Res Dev 31: 50-61. PubMed: 8035360. [PubMed] [Google Scholar]
- 30. Giangregorio L, Craven C, Richards K, Kapadia N, Hitzig SL et al. (2012) A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: effects on body composition. J Spinal Cord Med 35: 351-360. doi: 10.1179/2045772312Y.0000000041. PubMed: 23031172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lauer RT, Smith BT, Mulcahey MJ, Betz RR, Johnston TE (2011) Effects of cycling and/or electrical stimulation on bone mineral density in children with spinal cord injury. Spinal Cord 49: 917-923. doi: 10.1038/sc.2011.19. PubMed: 21423253. [DOI] [PubMed] [Google Scholar]
- 32. Leeds EM, Klose KJ, Ganz W, Serafini A, Green BA (1990) Bone mineral density after bicycle ergometry training. Arch Phys Med Rehabil 71: 207-209. PubMed: 2317139. [PubMed] [Google Scholar]
- 33. Bedell KK, Scremin AM, Perell KL, Kunkel CF (1996) Effects of functional electrical stimulation-induced lower extremity cycling on bone density of spinal cord-injured patients. Am J Phys Med Rehabil 75: 29-34. doi: 10.1097/00002060-199601000-00008. PubMed: 8645435. [DOI] [PubMed] [Google Scholar]
- 34. Mohr T, Podenphant J, Biering-Sorensen F, Galbo H, Thamsborg G et al. (1997) Increased bone mineral density after prolonged electrically induced cycle training of paralyzed limbs in spinal cord injured man. Calcif Tissue Int 61: 22-25. doi: 10.1007/s002239900286. PubMed: 9192506. [DOI] [PubMed] [Google Scholar]
- 35. Bélanger M, Stein RB, Wheeler GD, Gordon T, Leduc B (2000) Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil 81: 1090-1098. doi: 10.1053/apmr.2000.7170. PubMed: 10943761. [DOI] [PubMed] [Google Scholar]
- 36. Chen SC, Lai CH, Chan WP, Huang MH, Tsai HW et al. (2005) Increases in bone mineral density after functional electrical stimulation cycling exercises in spinal cord injured patients. Disabil Rehabil 27: 1337-1341. doi: 10.1080/09638280500164032. PubMed: 16321917. [DOI] [PubMed] [Google Scholar]
- 37. Clark JM, Jelbart M, Rischbieth H, Strayer J, Chatterton B et al. (2007) Physiological effects of lower extremity functional electrical stimulation in early spinal cord injury: lack of efficacy to prevent bone loss. Spinal Cord 45: 78-85. doi: 10.1038/sj.sc.3101929. PubMed: 16636686. [DOI] [PubMed] [Google Scholar]
- 38. Frotzler A, Coupaud S, Perret C, Kakebeeke TH, Hunt KJ et al. (2008) High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone 43: 169-176. doi: 10.1016/j.bone.2008.03.004. PubMed: 18440891. [DOI] [PubMed] [Google Scholar]
- 39. Griffin L, Decker MJ, Hwang JY, Wang B, Kitchen K et al. (2009) Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol 19: 614-622. doi: 10.1016/j.jelekin.2008.03.002. PubMed: 18440241. [DOI] [PubMed] [Google Scholar]
- 40. Lai CH, Chang WH, Chan WP, Peng CW, Shen LK et al. (2010) Effects of functional electrical stimulation cycling exercise on bone mineral density loss in the early stages of spinal cord injury. J Rehabil Med 42: 150-154. doi: 10.2340/16501977-0499. PubMed: 20140411. [DOI] [PubMed] [Google Scholar]
- 41. Bloomfield SA, Mysiw WJ, Jackson RD (1996) Bone mass and endocrine adaptations to training in spinal cord injured individuals. Bone 19: 61-68. doi: 10.1016/8756-3282(96)00109-3. PubMed: 8830990. [DOI] [PubMed] [Google Scholar]
- 42. Eser P, de Bruin ED, Telley I, Lechner HE, Knecht H et al. (2003) Effect of electrical stimulation-induced cycling on bone mineral density in spinal cord-injured patients. Eur J Clin Invest 33: 412-419. doi: 10.1046/j.1365-2362.2003.01156.x. PubMed: 12713456. [DOI] [PubMed] [Google Scholar]
- 43. Jiang SD, Dai LY, Jiang LS (2006) Osteoporosis after spinal cord injury. Osteoporos Int 17: 180-192. doi: 10.1007/s00198-005-2028-8. PubMed: 16217589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist for systematic review and meta-analysis.
(DOC)