Abstract
Glucagon-like peptide-1 (GLP-1) is a potent insulin secretagogue released from L-cells in the intestine. Meat hydrolysate (MH) is a powerful activator of GLP-1 secretion in the human enteroendocrine NCI-H716 cell line, but the mechanisms involved in nutrient-stimulated GLP-1 secretion are poorly understood. The objective of this study was to characterize the intracellular signalling pathways regulating MH- and amino acid-induced GLP-1 secretion. Individually, the pharmacological inhibitors, SB203580 (inhibitor of p38 mitogen-activated protein kinase (MAPK)), wortmannin (inhibitor of phosphatidyl inositol 3-kinase) and U0126 (inhibitor of mitogen activated or extracellular signal-regulated protein kinase (MEK1/2) upstream of extracellular signal-regulated kinase (ERK)1/2) all inhibited MH-induced GLP-1 secretion. Further examination of the MAPK pathway showed that MH increased the phosphorylation of ERK1/2, but not p38 or c-Jun N-terminal kinase over 2–15 min. Incubation with SB203580 resulted in a decrease in phosphorylated p38 MAPK and a concomitant increase in the phosphorylation of ERK1/2. Phosphorylation of ERK1/2 was augmented by co-incubation of MH with SB203580. Inhibitors of protein kinase A and protein kinase C did not inhibit MH-induced GLP-1 secretion. In contrast to non-essential amino acids, essential amino acids (EAAs) increased GLP-1 secretion and similar to MH, activated ERK1/2. However, they also activated p38-suggesting type of protein may affect GLP-1 secretion. In conclusion, there appears to be a crosstalk between p38 and ERK1/2 MAPK in the human enteroendocrine cell with the activation of ERK1/2 common to both MH and EAA. Understanding the cellular pathways involved in nutrient-stimulated GLP-1 secretion has important implications for the design of new treatments aimed at increasing endogenous GLP-1 release in type-2 diabetes and obesity.
Introduction
Glucagon-like peptide-1 (GLP-1) is a gut hormone released from intestinal L-cells in response to food ingestion (Kieffer & Habener 1999). In addition to potentiating glucose-dependent insulin secretion, GLP-1 stimulates proinsulin gene expression and biosynthesis (Drucker et al. 1987) and reduces food intake through interactions with the hypothalamus (Turton et al. 1996, Flint et al. 1998). The combined antidiabetic and anorectic effects of GLP-1 make it a promising new therapy for type-2 diabetes.
Previously, we identified the NCI-H716 enteroendocrine cell line as a unique human model for studying the regulation of GLP-1 secretion (Reimer et al. 2001). Similar to several animal cellular models, the NCI-H716 cells respond to activators of protein kinase A (PKA) and protein kinase C (PKC) with increased GLP-1 secretion (Reimer et al. 2001). The neuromediator, gastrin-releasing peptide, is also able to increase GLP-1 secretion at high doses. In addition, Anini & Brubaker (2003) have described a role for M1 and M2 muscarinic receptors in the control of GLP-1 secretion in NCI-H716 cells. Reimann et al. (2004) have recently shown that glutamine is a potent stimulator of GLP-1 secretion from the murine glucagon gene simian virus-40 large T-antigen (GLUTag) cell line. They suggest a possible involvement of the Na-coupled glutamine transporters and using reverse transcriptase (RT)-PCR have identified several Na-coupled amino acid transporters in the GLUTag cells with the strongest bands observed for ATA-2 (electrogenic neutral amino acid transporter), ASCT-2 (sodium dependent neutral amino acid transporter type 2) and y+LAT2 (electroneutral amino acid transporter; Hyde et al. 2003, Reimann et al. 2004). These transporters may play a role in the release of GLP-1 in response to protein and amino acids.
Nutrient ingestion is a major stimulus for GLP-1 release from the L-cells (Drucker 1998). The direct effect of nutrients on GLP-1 secretion has been examined in several cellular models. Saturated fatty acids, palmitic and the unsaturated oleic acids stimulate GLP-1 secretion in human NCI-H716 cells (Reimer et al. 2001). In contrast, only unsaturated fatty acids were effective in the murine GLUTag enteroendocrine cell line and in fetal rat intestinal cells (Brubaker et al. 1998, Rocca et al. 2001). Meat hydrolysate (MH), previously shown in rodent cells to stimulate GLP-1 secretion (Cordier-Bussat et al. 1998), results in a potent stimulation of GLP-1 release in the human NCI-H716 cell line (Reimer et al. 2001). The cellular mechanisms by which MH induces GLP-1 secretion are not known. Given the potential use of modified diets and functional foods to enhance endogenous GLP-1 secretion and improve glucose control in human subjects, understanding the mechanisms involved in nutrient-stimulated GLP-1 secretion is critical. This in vitro work will help identify important targets that can ultimately be tested in future human trials.
The mitogen-activated protein kinase (MAPK) family of signalling molecules, includes extracellular signal-regulated kinase (ERK)1/2, p38 MAPK, c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK), ERK3 and ERK5. MAPKs are the regulators in pathways controlling embryogenesis, cell differentiation, cell proliferation and cell death (Pearson et al. 2001). Glucose-dependent insulinotrophic polypeptide (GIP) and GLP-1 are two incretins, whose actions are mediated via their respective G-protein-coupled receptors (Brubaker & Drucker 2002). The p38 MAPK signal has been shown to mediate GLP-1 induced β-cell proliferation (Buteau et al. 2001) and GIP-stimulated β-(insulinoma cell line (INS)-1) cell survival (Ehses et al. 2003). Many other members of the vasoactive intestinal polypeptide/secretin/glucagon superfamily also exert their downstream effects via activation of MAPKs (Barrie et al. 1997, Montrose-Rafizadeh et al. 1999, Jiang et al. 2001). These studies demonstrate the important role played by the MAPK family of signal molecules in the physiological actions exerted by GLP-1, particularly in the pancreas. However, the role of MAPKs in the stimulation of endogenous GLP-1 secretion by nutrients has never been described. We, hereby, provide evidence for the role of MAPKs in MH-induced GLP-1 secretion in the NCI-H716 cell line. Given the lack of consensus on the role of amino acids in stimulating GLP-1 release, we also demonstrate the ability of essential amino acids (EAAs), but not non-essential amino acids (NEAAs) to trigger secretion.
Materials and Methods
Roswell Park Memorial Institute (RPMI) 1640 medium, Dulbecco’s modified Eagle’s medium (DMEM), L-glutamine, penicillin, streptomycin and fetal bovine serum (FBS) were from Invitrogen. BSA and MH were purchased from Sigma Chemicals, and MH was prepared as an 8% (w/v) stock solution in Krebs-Ringer bicarbonate buffer (KRB; Invitrogen) containing 0·2% BSA (w/v). The pH was adjusted to 7·2 with NaOH, filtered through 0·2 μm filters and aliquots stored at −20 °C. The amino acid solutions (Cat. nos M5550 and M7145) were purchased from Sigma Chemicals. All phospho and total antibodies for p42/44 MAPK (ERK), p38 and JNK were purchased from New England Biolabs (Pickering, Ontario, Canada).
Cell line and culture conditions
Human NCI-H716 cells were grown in suspension at 37 °C, 5% CO2. The culture medium was RPMI 1640 supplemented with 10% FBS, 2 mM L-glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin. Endocrine differentiation was enhanced by growing cells in dishes coated with Matrigel (Becton Dickinson, Bedford, MA, USA), as described previously (de Bruine et al. 1993).
Secretion studies
Two days before the experiments, 1×106 cells were seeded in 12-well culture plates coated with Matrigel and containing high-glucose DMEM, 10% FBS, 2 mM L-glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin. On the day of the experiment, medium was replaced by KRB containing 0·2% (w/v) BSA with or without test agents and pH adjusted to 7·2. In experiments with pharmacological inhibitors, cells were preincubated with the inhibitor or dimethyl sulphoxide (DMSO) as a control for 30 min prior to the secretion period. Fresh media were added and cells incubated for 2 h at 37 °C with a 2% (w/v) MH solution alone or in combination with the various inhibitors. DMSO was used as a control. Supernatants were collected with the addition of 50 μg/ml phenylmethylsulphonyl fluoride (PMSF) and diprotin-A (34 μg/ml) and frozen at −80 °C for subsequent RIA analysis. Cells were scraped off and sonicated in a homogenization buffer (1 M HCl containing 5% (v/v) HCOOH, 1% (v/v) trifluoroacetic acid and 1% (w/v) NaCl). Peptides were extracted from the cell media and homogenates using an alcohol extraction method as described by the supplier of the GLP-1(7–36) Active RIA Kit (Linco Research, Inc., St Charles, MO, USA). Protein content of the cell homogenate was determined using the Bradford protein assay (Bio-Rad, Inc.).
Western blots
Cells were lysed on ice in buffer (50 mM HEPES, 150 mM NaCl, 10% glycerol, 1·5 mM MgCl2, 1 mM EGTA, 100 mM NaF and 1% NP40) containing 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM sodium othorandanole and 1 mM Na3VO4. Total protein concentrations were determined using the Bradford protein assay (Bio-Rad), and aliquots of each sample representing equal amounts of proteins were subjected to Western immuno-blotting analysis. Cell lysates were suspended in sample buffer (10% glycerol, 62·5 mM Tris–HCl, 2% SDS and 50 mM DTT (pH 6·8)) and subjected to electrophoresis in 10% polyacrylamide SDS gel and transferred to nitrocellulose membranes. The membranes were blocked in buffer (1× Tris-buffered saline (TBS) (50 mM Tris-base, 0·14 M NaCl, 4·8 mM KCl, 0·1% Tween-20)) with skim milk and incubated overnight with a 1:1000 dilution of antibody against phosphorylated p38, 1:5000 dilution of phosphorylated ERK1/2 antibody or 1:500 dilution of JNK antibody. Membranes were washed and incubated for 1 h with a 1:10 000 dilution of peroxidase-conjugated anti-rabbit immunoglobulin (Amersham Pharmacia). Proteins were visualized with electrochemiluminescence (ECL) substrate reagent (Amersham Pharmacia). To control for protein concentration, membranes were stripped and reprobed for total p38 kinase (1:100), total ERK1/2 (1:1000) and total JNK (1:500).
RT-PCR
RT-PCR was performed as described previously (Reimer et al. 2001) using the following primers: PEPT1 (SLC15A1) sense TGTCGCTCTCCATTGTCTAC, antisense TTCCA-CATTGTTGAACTCTGAG; Y+LAT2 (SLC7A6) sense GCACTCATCTACCTCATCG, antisense GATAGCAGC-CAGGACATTC; ASCT2 (SLC1A5) sense GCTTATCCG-CTTCTTCAACTCCTTC, antisense CC-ATTATTCT-CCTCCACGCACTTC; ATA-2 (SLC38A2) sense TGGTAT-CTGAACGGGAACTATTTG, antisense AATTGGCACAG CATAGACAGTC and actin sense GTTGCTATCCAGGC-TGTG, antisense CATAGTCCG-CCTAGAAGC.
Statistical analysis
Data in figures are presented as means±S.E.M. and represent a minimum of three experiments measured in duplicate. Statistical differences were determined using the Kruskal–Wallis’s H-test with post hoc analysis to determine the differences between treatments using the Mann–Whitney’s U-test. Statistical significance was defined as P≤0·05.
Results
The total content of GLP-1 in NCI-H716 cells, as monitored by RIA, was 252·6±40·7 pmol/mg protein. Basal release, during a 2 h incubation period, was 2·6±0·3% (n=27).
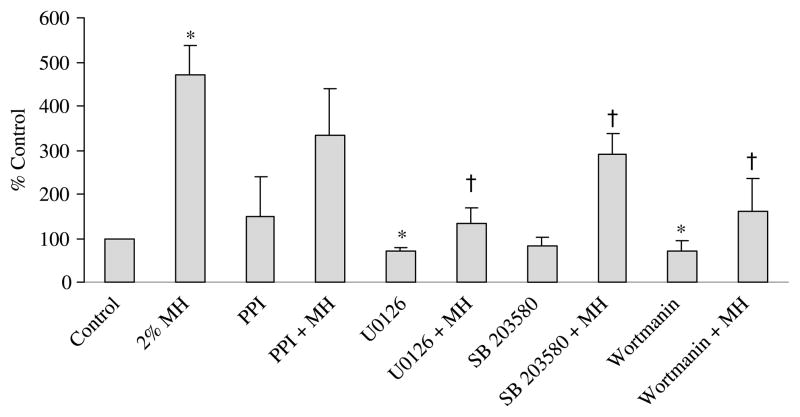
Incubation of NCI-H716 cells with SB203580, a specific inhibitor of p38 MAPK, significantly reduced MH-induced GLP-1 secretion (291·9±46·4% control) compared with MH alone (471·0±65·2% control; P<0·05; Fig. 1). In addition, wortmannin, a phosphatidyl inositol 3 kinase (PI3K) inhibitor, significantly reduced MH-induced GLP-1 secretion (161·1±75·7% control; P<0·05) compared with MH alone (471·0±65·2% control). The U0126 compound, an inhibitor of MEK1/2 upstream from ERK1/2, also significantly reduced MH-induced GLP-1 secretion. Incubation solely with wortmannin or U0126 caused a slight reduction in basal GLP-1 secretion.
Figure 1.
Release of glucagon-like peptide-1 (GLP-1) by NCI-H716 cells in response to treatment with various inhibitors. The treatments were: (1) medium alone (control), (2) 2% meat hydrolysate (MH), (3) 4-amino-5-(4-methylphenyl)-7-(t-butyl)-pyrazolo-3,4-d-pyrimidine (PPI) (10 μM; sarcoma related compliment (Src)-family inhibitor), (4) PPI+2% MH, (5) U0126 (5 μM; MEK1/2 inhibitor), (6) U0126+2% MH, (7) SB203580 (10 μM; p38 MAPK inhibitor), (8) SB203580+2% MH, (9) Wortmannin (100 nM; PI3K inhibitor), (10) Wortmannin+2% MH. Cells were preincubated for 30 min with either medium alone or various inhibitors. Fresh medium was added containing MH with or without the inhibitors for 2 h. Secretion of GLP-1 into the medium is expressed as a percentage of the control value (n=5). Results represent mean±S.E.M. *Significant difference from control (P≤0·0001). Additionally, all treatments involving 2% MH plus an inhibitor were compared with 2% MH alone. †Significant difference between 2% MH and 2% MH in combination with an inhibitor (P<0·05).
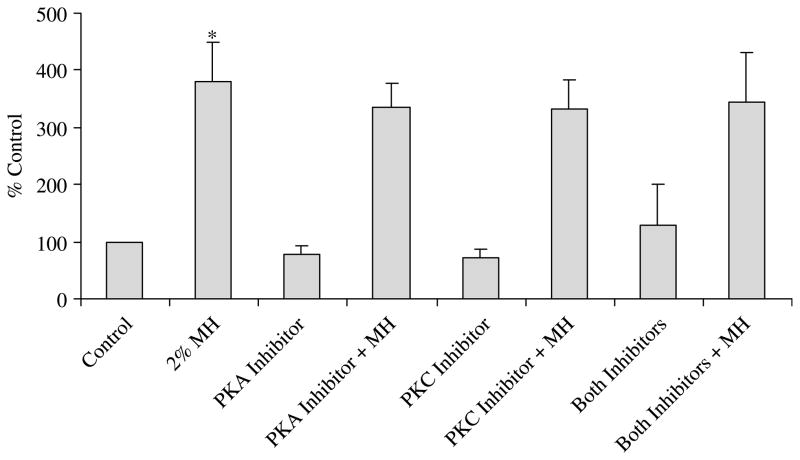
In addition to the aforementioned pathways, we also investigated the role of PKC and PKA in MH-induced GLP-1 secretion. Co-incubation of MH with RpcAMPs (adenosine 3′,5′-cyclic monophosphorothioate, Rp-isomer), a cAMP antagonist that blocks signal transduction of cAMP by inhibiting PKA (Dostmann et al. 1990) did not block MH-induced GLP-1 secretion (Fig. 2). Chelerythrine chloride, a potent, selective and cell permeable inhibitor of PKC (Jarvis et al. 1994) also had no effect on MH-induced GLP-1 secretion (Fig. 2). Incubation of NCI-H716 cells with one of the inhibitors alone did not alter the basal GLP-1 secretion.
Figure 2.
Effect of protein kinase (PKA) and protein kinase C (PKC) inhibitors on meat hydrolysate (MH)-induced glucagon-like peptide-1 (GLP-1) release. Secretion of GLP-1 by NCI-H716 cells is shown in response to treatment with medium alone (control), MH (2%), RpcAMPS (10 μM, PKA inhibitor) and chelerythrine (2 μM, PKC inhibitor) alone or in combination with MH (n=3). Cells were preincubated for 30 min with either medium alone or various inhibitors. Fresh medium was added containing MH with or without the inhibitors for 2 h. Secretion of GLP-1 into the medium is expressed as a percentage of the control value. Results represent mean±S.E.M. *Significant difference between control and 2% MH (P≤0·001). No significant differences were detected with the other treatments.
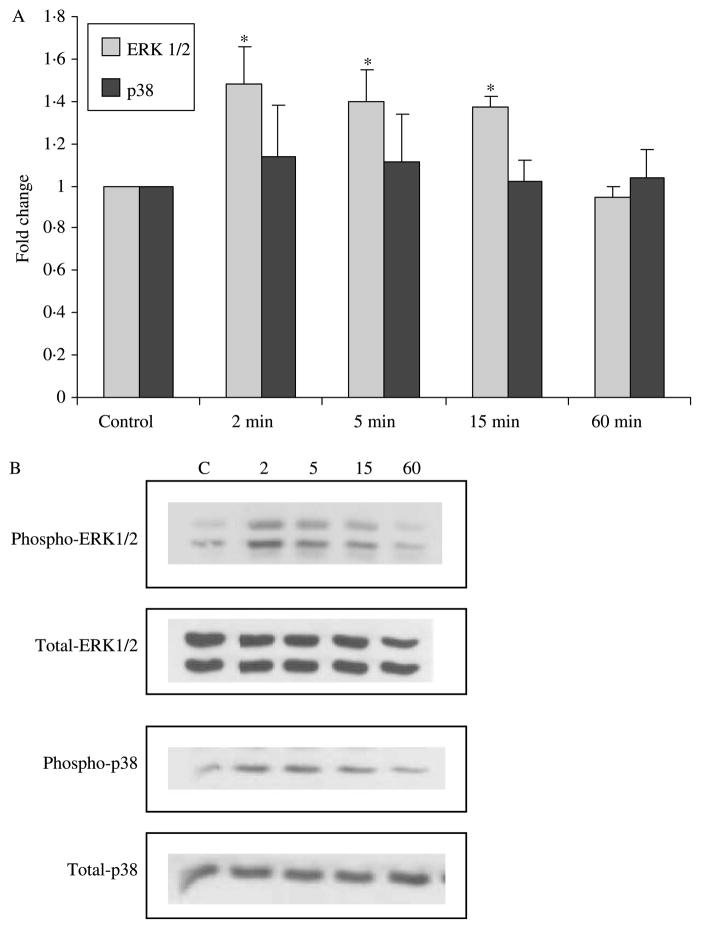
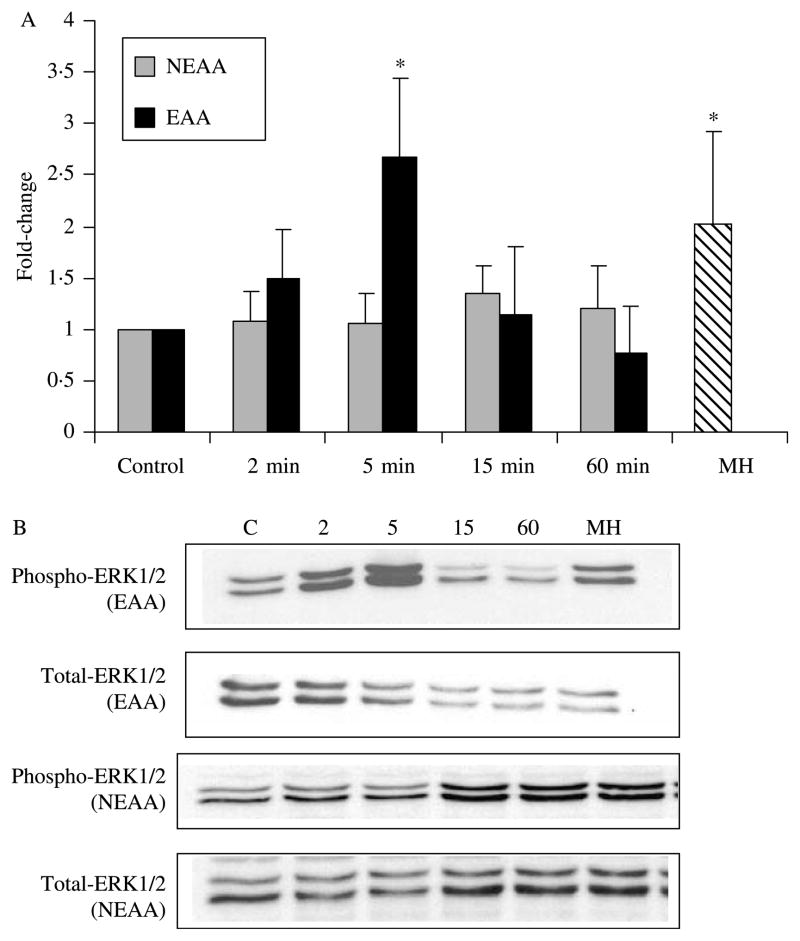
Given the results of the secretory studies implicating MAPK involvement in GLP-1 secretion in response to MH, we went on to first examine the time course of the effect of MH on phosphorylation of three MAPK subfamilies. NCI-H716 cells were incubated with 2% MH followed by Western blot analysis for phosphorylated and total p38 MAPK, ERK1/2 and JNK at timed intervals. Activation of ERK1/2 MAPK was detectable and significant over 2–15 min (P<0·05) and returned to basal levels by 60 min (Fig. 3). In contrast, phosphorylated levels of p38 MAPK remained unchanged. Likewise, no changes were detected in phosphorylation of JNK (data not shown). The amounts of total p38, MAPK and JNK did not change with MH over 2–60 min.
Figure 3.
Activation of extracellular signal-related kinase (ERK)1/2 but not p38 MAPK by MH. Western blots were performed with the phosphospecific and non-phosphorylated form of ERK1/2 and p38 MAPK antibodies (n=3). Ratios of phosphorylated:non-phosphorylated signals were calculated and normalized to control. (A) *Significant difference from baseline control (P<0·05). No significant differences were seen in p38 across time. (B) Representative Western blots shown with lane assignments as follows: C, control; 2, 2 min; 5, 5 min; 15, 15 min; 60, 60 min with 2% MH.
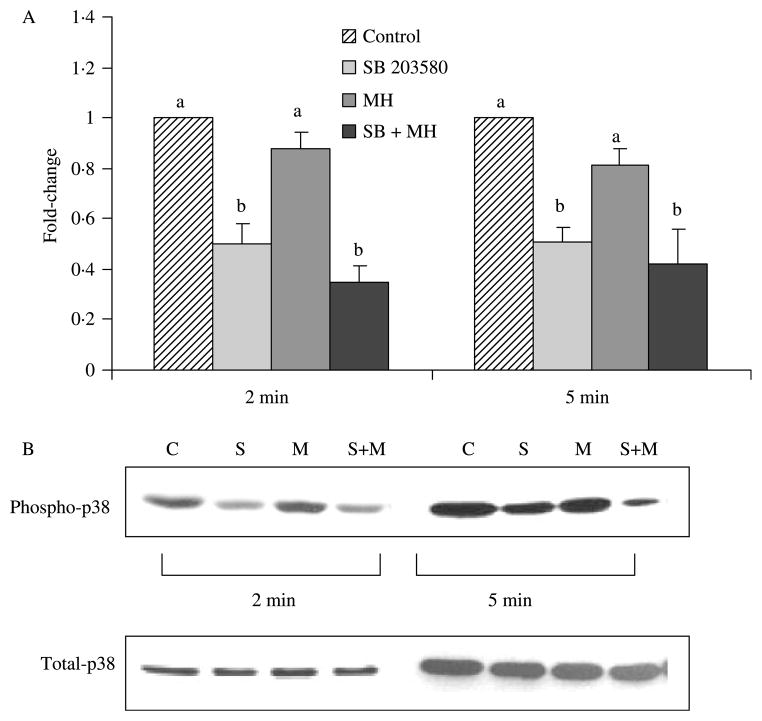
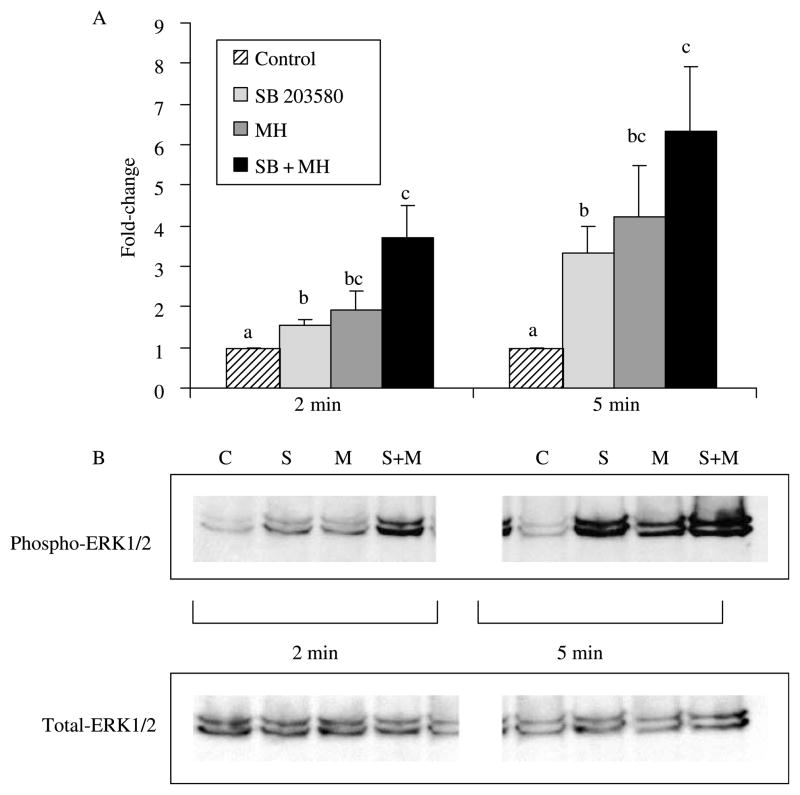
The compound, SB203580, was used to specifically inhibit p38 MAPK in the NCI-H716 cells. As expected, a significant decrease in phosphorylated levels of p38 MAPK occurred at both 2 and 5 min (Fig. 4). Confirming results from the time course, no changes in p38 were seen with MH. The level of phosphorylated p38 that was observed after the co-incubation of SB203580 with MH did not differ from the inhibitor alone. Given the inhibition of p38 with SB203580, there appeared to be a compensatory increase in the phosphorylation of ERK1/2 MAPK, which was significant at 2 and 5 min, but greatly enhanced at the latter time point (P>0·05; Fig. 5). Interestingly, co-incubation of MH with SB203580 resulted in augmented levels of phosphorylated ERK1/2.
Figure 4.
Abundance of phosphorylated p38 MAPK following incubation with MH and/or SB203580 inhibitor. Western blots were performed with the phosphospecific and non-phosphorylated form of the p38 MAPK antibody (n=3). Ratios of phosphorylated:non-phosphorylated p38 MAPK signals were calculated and normalized to control. (A) Treatments with different letters are significantly different from each other within the 2 or 5 min time point (P<0·05). For example, the relevant comparison of MH versus SB+MH at 2 min is significantly different. (B) Representative Western blots of phosphorylated and total p38 MAPK. Lanes 1–4, 2 min; lanes 5–8, 5 min; lanes 1 and 5, control; lanes 2 and 6, SB203580; lanes 3 and 7, 2% MH; lanes 4 and 8, SB203580+2% MH.
Figure 5.
Abundance of phosphorylated ERK1/2 MAPK following incubation with MH and/or SB203580 inhibitor. Western blots were performed with the phosphospecific and non-phosphorylated form of the ERK1/2 MAPK antibody (n=3). Ratios of phosphorylated:non-phosphorylated ERK1/2 MAPK signals were calculated and normalized to control. (A) Treatments with different letters are significantly different from each other within the 2 or 5 min time point (P<0·05). (B) Representative western blot. Lanes 1–4, 2 min; lanes 5–8, 5 min; lanes 1 and 5, control; lanes 2 and 6, SB203580; lanes 3 and 7, 2% MH; lanes 4 and 8, SB203580+2% MH.
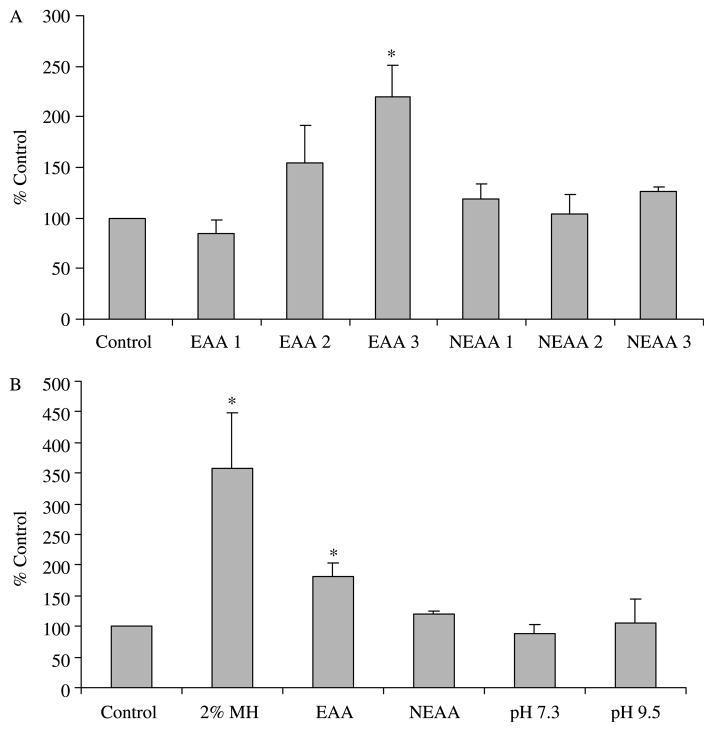
Since MH is a complex, poorly defined protein compound, we also wanted to investigate the effects of well-defined essential and NEAA mixtures on GLP-1 secretion and MAPK activation in the NCI-H716 cell line. The EAAs dose-dependently increased GLP-1 secretion with release reaching significance at the highest concentration tested (6·9 g/l; Fig. 6). NEAAs, however, were not able to trigger GLP-1 release above the baseline control levels. In a separate experiment, we compared GLP-1 secretion under the conditions of equivalent nitrogen content with 2% MH and EAAs and NEAAs as well as two additional treatments, which controlled for differences in the pH of the solutions, namely pH 7·3 and 9·5. Both MH and EAAs significantly increased GLP-1 secretion (P<0·05), but the increase was greater for MH. NEAAs and changes in pH alone did not affect GLP-1 secretion (Fig. 6).
Figure 6.
Effect of essential and non-essential amino acids (NEAAs) on GLP-1 secretion. (A) Cells were incubated for 2 h with increasing concentrations of a mixture of essential amino acids (EAA; MEM, Sigma no. 5550) or a mixture of NEAAs (Sigma no. 7145; n=3). The concentrations displayed for both EAA and NEAA equate to 1=0·69 g/l, 2=3·47 g/l and 3=6·90 g/l. *EAA 3 is significantly different from all other treatments (P<0·05). (B) Cells were incubated for 2 h with equal concentrations (matched for nitrogen content) of MH, EAA or NEAA or two control solutions differing in pH alone (n=3). *Secretion of GLP-1 with MH and EAA was significantly greater than control or NEAA treatments (P<0·05). No effect of pH was seen on GLP-1 secretion.
Similar to the GLUTag cell line (Reimann et al. 2004), we now confirm the presence of the ATA-2, ASCT-2 and y+LAT2 in human NCI-H716 cells (Fig. 7). MH is a complex mixture of smaller peptides and we, therefore, also examined the H+-driven di- and tri-peptide transporter PEPT1, but could not detect expression of this transporter in our cells.
Figure 7.
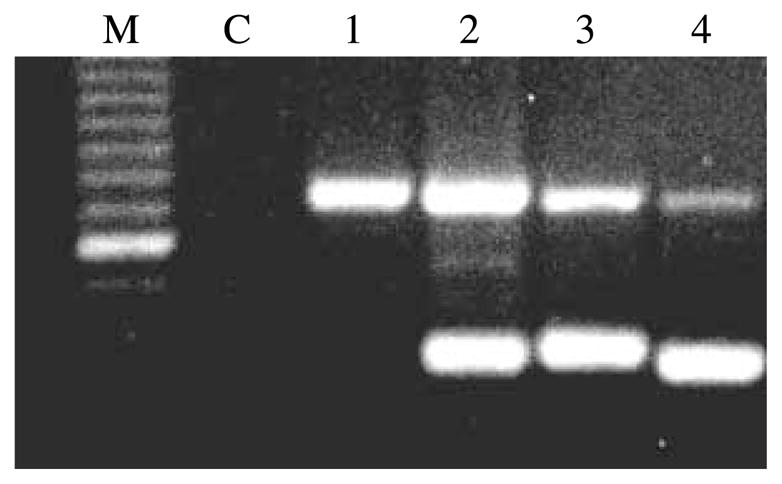
Expression of amino acid and peptide transporters in NCI-H716 cells. Total RNAs were reverse transcribed and amplified by PCR (n=3). The lanes represent M, Marker; C, negative control; 1, oligopeptide transporter PEPT1; 2, Na+-dependent amino acid transporter Y+LAT2; 3, Na+-dependent neutral amino acid transporter ASCT2; 4, Na+-dependent neutral amino acid transporter ATA-2. The intense band on the marker represents a size of 600 bp. Predicted band sizes are 318, 333, 345 and 327 bp respectively and 739 bp for the internal standard of actin.
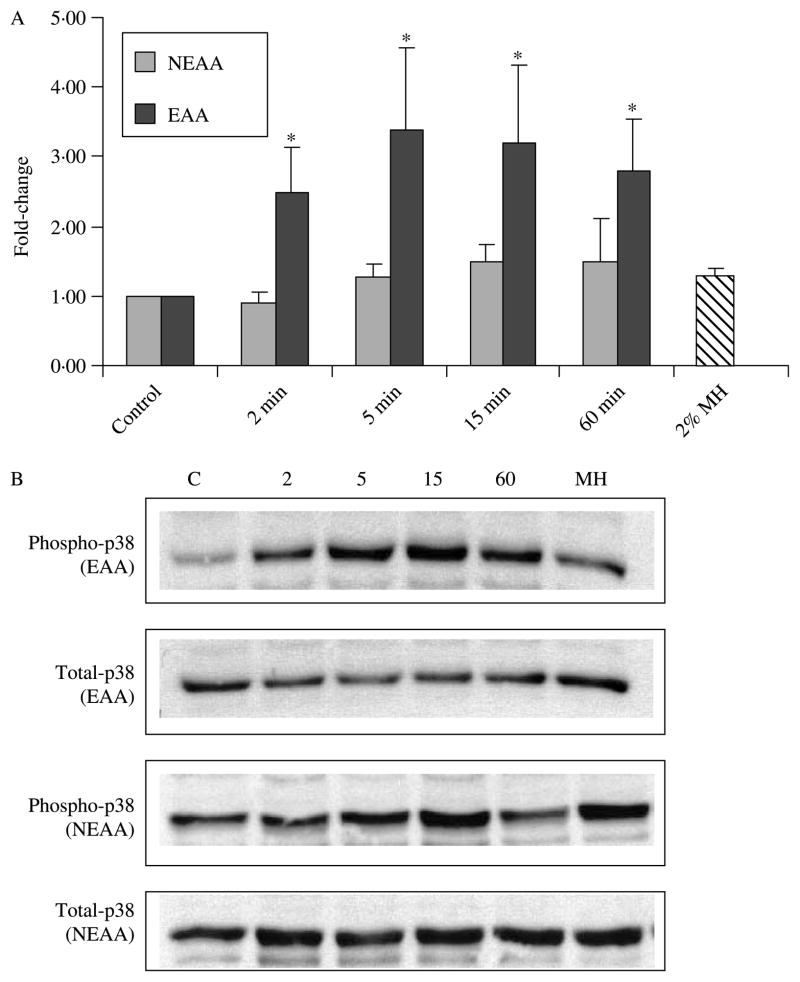
To complete our examination of the effect of amino acids on intracellular pathways, we again performed time-course experiments for EAAs and NEAAs on phosphorylated and total ERK1/2 and p38. In equivalent doses, both EAAs and MH significantly increased phosphorylated ERK1/2 (P<0·05), whereas NEAA had no effect (Fig. 8). In contrast to findings with MH, the EAAs were also able to significantly increase the levels of phosphorylated p38 at 2, 5, 15 and 60 min (Fig. 9). The NEAA and MH had no effect on phosphorylated p38 levels (Fig. 9).
Figure 8.
Activation of ERK1/2 by essential but not NEAAs. Western blots were performed with the phosphospecific and non-phosphorylated form of ERK1/2 antibodies (n=3). Ratios of phosphorylated:non-phosphorylated ERK1/2 signals were calculated and normalized to control. (A) At 5 min, there was a significant (P<0·05) increase in ERK1/2 phosphorylation with EAA compared with control and all other time points for EAA and NEAA. *For comparison, MH also significantly increased ERK1/2 phophorylation at 5 min. (B) Representative Western blots shown with lane assignments as follows: C, control; 2, 2 min; 5, 5 min; 15, 15 min; 60, 60 min; MH, 2% MH at 5 min.
Figure 9.
Abundance of p38 following incubation with essential and NEAAs. Western blots were performed with the phosphospecific and non-phosphorylated form of p38 antibodies (n=3). Ratios of phosphorylated:non-phosphorylated p38 signals were calculated and normalized to control. (A) *Significant activation of p38 following incubation with EAA at 2, 5, 15 and 60 min (P<0·05). (B) Representative Western blots shown with lane assignments as follows: C, control; 2, 2 min; 5, 5 min; 15, 15 min; 60, 60 min; MH, 2% MH at 5 min.
Given the activation of ERK1/2 seen with both MH and EAA, we performed an initial screen for other agonists that potentially stimulate GLP-1 secretion via activation of this MAPK pathway. While epidermal growth factor (100 ng/ml), fibroblast growth factor (100 ng/ml) and insulin (100 ng/ml) all elicit robust activation of ERK1/2 in our NCI-H716 cells, they were unable to trigger GLP-1 secretion at concentrations of either 100 or 300 ng/ml over 2 h (data not shown). As a negative control, lysophosphatidic acid (10 μm), which did not activate ERK1/2 in the NCI-H716 cells did not trigger GLP-1 release. Other compounds tested, including endothelin-1, stromal cell-derived factor-1, platelet-derived growth factor, tumour necrosis factor α, granulocyte–macrophage colony-stimulating factor and lipo-polysaccharide did not elicit an activation of ERK1/2 in the NCI-H716 cells (data not shown). Given that activation of ERK1/2 alone is not sufficient to induce GLP-1 secretion, it may suggest that MH is able to initiate GLP-1 secretion and perhaps then potentiate its release via the ERK1/2 pathway. This will require further examination.
Discussion
MH is a potent stimulator of GLP-1 secretion in human NCI-H716 (Reimer et al. 2001) and murine enteroendocrine GLUTag (Cordier-Bussat et al. 1998) cell lines. Although some controversy remains regarding the effectiveness of protein on triggering GLP-1 secretion from the L-cell (Xiao et al. 1999), Reimann et al. (2004) have recently demonstrated that glutamine, at a concentration similar to normal plasma glutamine levels is able to potently stimulate GLP-1 from murine GLUTag cells. They demonstrate a role for membrane depolarization and increased intracellular calcium in enhancing GLP-1 secretion (Reimann et al. 2004). Results from our present study confirm the ability of a protein hydrolysate derived from meat, as well as a mixture of EAAs to potently stimulate GLP-1 secretion from NCI-H716 cells and demonstrate the involvement of ERK1/2 and p38 MAPK in this secretion.
In addition to the MAPK family, we also determined that the inhibition of PI3K impaired MH-induced GLP-1 secretion. In fact, both p38 MAPK and PI3K have been implicated in the growth promotive effects of GLP-1 (Buteau et al. 1999, 2001). It is, therefore, not unusual that several signalling modules are involved in the cellular response to MH. While glucose is known to cause a rapid and continuous activation of ERK1/2 in β-cells (Arnette et al. 2003), our study is the first to describe the activation of ERK1/2 by MH and furthermore by EAAs in enteroendocrine cells.
Our work is in agreement with recently published evidence that enhanced secretion of GLP-1 by free fatty acids (FFA) in the murine cholecystokinin-secreting murine small intestine endocrine (STC)-1 cell line is accompanied by the activation of ERK1/2 (Hirasawa et al. 2005). GPR120, a G-protein-coupled receptor abundantly expressed in the STC-1 cell line, was identified as a receptor for unsaturated long-chain FFA. Transfection of the STC-1 cells with GPR-120-specific small interfering RNA significantly reduced α-linolenic acid-induced GLP-1 secretion (Hirasawa et al. 2005). Transfection of NCI-H716 cells (which have little expression of GPR120) with GPR120 improved the ability of NCI-H716 cells to secrete GLP-1 in response to α-linolenic acid (Hirasawa et al. 2005). Taken together with our present work, it suggests that ERK1/2 may serve as a common pathway regulating nutrient-induced GLP-1 secretion.
We and other researchers had previously shown that activation of the PKA and PKC pathways in enteroendocrine cells stimulates GLP-1 secretion (Brubaker & Vranic 1987, Buchan et al. 1987, Drucker et al. 1987, Reimer et al. 2001). In the present study, however, we found that inhibition of the PKA or PKC pathway with RpcAMPS or chelerythrine respectively, did not reduce MH-induced GLP-1 secretion. These findings are in agreement with work by Reimann et al. (2004) in which inhibition of PKA or PKC did not affect glutamine-stimulated GLP-1 secretion. The intracellular amino acid target mammalian target of rapamycin mTOR also did not significantly impair the response to glutamine (Reimann et al. 2004). Inhibition of calmodulin kinase impaired GLP-1 secretion in response to glutamine but also glucose and alanine. In contrast to the lack of effects of PKA and PKC inhibitors, blocking p38 MAPK, PI3K or MEK1/2, all resulted in a reduction in the potential for MH to trigger GLP-1 release. Our specific examination of the MAPK pathways suggests that the ERK1/2 and p38 MAPK subfamilies appear to work in concert to regulate the response to MH by the enteroendocrine cell. This is based on the observations that MH increases ERK1/2 phosphorylation and that inhibition of p38 MAPK further augments the level of phosphorylated ERK1/2 in response to MH. This same crosstalk between ERK1/2 and p38 MAPK has been observed in the rat pineal gland in response to norepinephrine (Mackova et al. 2000) and in a rat hepatoma cell line (Singh et al. 1999).
The work by Reimann & Gribble (2002) also suggests that GLUTag cells are electrically active and respond to increased glucose concentrations with membrane depolarization, triggering enhanced action potential firing and GLP-1 secretion. Interestingly, the non-metabolizable sugar, methyl-α-glucopyranoside, also stimulates electrical activity and GLP-1 secretion by inducing an inward current, largely explained by electrogenic actions of sodium–glucose co-transporters (Gribble et al. 2003). Glutamine has also been shown, by the same group, to both initiate GLP-1 secretion in the GLUTag cell line via triggering membrane depolarization and generating action potentials, as well as potentiating GLP-1 secretion downstream of the calcium signal (Reimann et al. 2004). Although to date, work elucidating the mechanisms by which nutrients stimulate GLP-1 release has been limited, recent studies coupled with our data suggest that the pathways involved are likely complex and in some cases nutrient specific.
MH is a complex compound that is poorly defined, but it is likely to be composed of a mixture of di- and tri-peptides and amino acids. It was, therefore, our intent to provide further insight into the active components of this protein source that stimulate GLP-1 secretion. We effectively demonstrated that a mixture of EAAs, in contrast to a mixture of NEAAs, stimulates GLP-1 secretion in human NCI-H716 cells. While our cells did not express the H+-coupled peptide transporter 1 (PEPT1), which mediates transport of small peptides from the lumen into cells (Terada & Inui 2004), we did confirm the presence of several of the same amino acid transporters (ATA-2, ASCT-2 and y+LAT2) reported by Reimann et al. (2004) in the murine GLUTag cells. ATA-2 is an electrogenic amino acid transporter and evidence from Reimann et al. (2004) demonstrates that the depolarizing action of glutamine contributes to GLP-1 secretion. The role of depolarization by MH or a mixture of EAAs remains to be demonstrated in human NCI-H716 cells.
Since the evidence for the role of proteins and amino acids in GLP-1 secretion has been conflicting; it is important to continue to probe the cellular mechanisms responsible for endogenous secretion. While some show that oral amino acids, but not luminal perfusion induce a rapid plasma GLP-1 response (Herrmann et al. 1995), others show that an oral protein meal (Elliott et al. 1993) but not ileal protein perfusion (Layer et al. 1995) result in elevated plasma GLP-1 levels. While confusing, some explanation may be found in the type of protein utilized based on the findings of Hall et al. (2003), who showed that plasma GLP-1 is higher following a whey preload compared with casein. Whey is considered a ‘fast’ protein, whereas casein is considered a ‘slow’ protein based on their differences in rate of digestion and absorption (Boirie et al. 1997). In addition to the speed with which dietary amino acids are delivered to the plasma (Boirie et al. 1997), whey also contains a high proportion of branched chain amino acids (Ha & Zemel 2003), which are EAAs and may affect GLP-1 secretion. Our results suggest that EAAs can exert a direct stimulatory effect on GLP-1 secretion in the human NCI-H716 cell line. Even though many proteins are absorbed by the time they reach the distal small intestine, it is nevertheless important to understand the mechanisms by which various protein sources, including MH, stimulate GLP-1 secretion. The rapid advances in the area of functional foods have the potential to foster the development of novel foods with specific targeted actions, including GLP-1 release from the distal gut.
In conclusion, we have demonstrated the involvement of ERK1/2 MAPK in the secretion of GLP-1 in response to MH. When a mixture of EAAs was used to give greater definition to the complex hydrolysate, p38 MAPK was activated in addition to ERK1/2. This work provides evidence for the cellular pathways involved in nutrient-stimulated GLP-1 release. While our work suggests that protein hydrolysates and mixtures of EAAs are effective potentiators of GLP-1 secretion, work by other researchers suggests that even single amino acids such as glutamine may be an effective nutrient stimulus (Reimann et al. 2004). Evaluating these compounds in vivo will shed light on the potential for their use in patients perhaps in the form of functional foods. Identification of cellular targets and mechanisms involved in GLP-1 release using this in vitro model will provide important insight for the design of future human trials with the ultimate goal of designing novel nutritional strategies for the treatment of type-2 diabetes and obesity.
Acknowledgments
The author would like to thank Kristine Lee for her extensive technical help and Dr S Robbins for his help with the MAPK inhibitors and Western blots. This work was supported in part by the Canadian Institutes for Health Research, Natural Sciences and Engineering Research Council and the Canadian Foundation for Innovation. The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- Anini Y, Brubaker PL. Muscarinic receptors control glucagon-like peptide 1 secretion by human endocrine L-cells. Endocrinology. 2003;144:3244–3250. doi: 10.1210/en.2003-0143. [DOI] [PubMed] [Google Scholar]
- Arnette D, Beers Gibson T, Lawrence MC, January B, Khoo S, McGlynn K, Vanderbilt CA, Cobb MH. Regulation of ERK1 and ERK2 by glucose and peptide hormones in pancreatic B-cells. Journal of Biological Chemistry. 2003;278:32517–32525. doi: 10.1074/jbc.M301174200. [DOI] [PubMed] [Google Scholar]
- Barrie AP, Clohessy AM, Buensuceso CS, Rogers MV, Allen JM. Pituitary adenylyl cyclase-activating peptide stimulates extracellular signal-regulated kinase 1 or 2 (ERK1/2) activity in a Ras-independent, mitogen-activated protein kinase/ERK kinase 1 or 2-dependent manner in PC-12 cells. Journal of Biological Chemistry. 1997;272:19666–19671. doi: 10.1074/jbc.272.32.19666. [DOI] [PubMed] [Google Scholar]
- Boirie Y, Dangin M, Gachon P, Vasson M, Maubois J, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. PNAS. 1997;94:14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker PL, Drucker D. Structure-function of the glucagon receptor family of G protein-coupled receptors: the glucagon, GIP, GLP-1 and GLP-2 receptors. Receptors and Channels. 2002;8:179–188. [PubMed] [Google Scholar]
- Brubaker PL, Vranic M. Fetal rat intestinal cells in monolayer culture: a new in vitro system to study the glucagon-like immunoreactive peptides. Endocrinology. 1987;120:1976–1985. doi: 10.1210/endo-120-5-1976. [DOI] [PubMed] [Google Scholar]
- Brubaker PL, Schloos J, Drucker DJ. Regulation of glucagon-like peptide-1 synthesis and secretion in the GLUTag enteroendocrine cell line. Endocrinology. 1998;139:4108–4114. doi: 10.1210/endo.139.10.6228. [DOI] [PubMed] [Google Scholar]
- de Bruine AP, Dinjens WN, van der Linden EP, Pijls MM, Moerkerk PT, Bosman FT. Extracellular matrix components induce endocrine differentiation in vitro in NCI-H716 cells. American Journal of Pathology. 1993;142:773–782. [PMC free article] [PubMed] [Google Scholar]
- Buchan AM, Barber DL, Gregor M, Soll AH. Morphologic and physiologic studies of canine ileal enteroglucagon-containing cells in short-term culture. Gastroenterology. 1987;93:791–800. doi: 10.1016/0016-5085(87)90442-2. [DOI] [PubMed] [Google Scholar]
- Buteau J, Roduit R, Susini S, Prentki M. Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity. Diabetologia. 1999;42:856–864. doi: 10.1007/s001250051238. [DOI] [PubMed] [Google Scholar]
- Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, Prentki M. Protein kinase C gamma activation mediates glucagon-like peptide-1-induced pancreatic B-cell proliferation. Diabetes. 2001;50:2237–2243. doi: 10.2337/diabetes.50.10.2237. [DOI] [PubMed] [Google Scholar]
- Cordier-Bussat M, Bernard C, Levenez F, Klages N, Laser-Ritz B, Philippe J, Chayvialle JA, Cuber JC. Peptones stimulate both the secretion of the incretin hormone glucagon-like peptide-1 and the transcription of the proglucagon gene. Diabetes. 1998;47:1038–1045. doi: 10.2337/diabetes.47.7.1038. [DOI] [PubMed] [Google Scholar]
- Dostmann WR, Taylor SS, Genieser HG, Jastorff B, Doskeland SO, Ogreid D. Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinases I and II with analogs of adenosine 3′,5′-cyclic phosphorothioates. Journal of Biological Chemistry. 1990;265:10484–10491. [PubMed] [Google Scholar]
- Drucker DJ. Glucagon-like peptides. Diabetes. 1998;47:159–169. doi: 10.2337/diab.47.2.159. [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. PNAS. 1987;84:3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehses JA, Casilla VR, Doty T, Pospisilik JA, Winter KD, Demuth H-U, Pederson RA, McIntosh CH. Glucose-dependent insulinotropic polypeptide promotes B-(INS-1) cell survival via cyclic adenosine monophosphate-mediated caspase-3 inhibition and regulation of p38 mitogen-activated protein kinase. Endocrinology. 2003;144:4433–4445. doi: 10.1210/en.2002-0068. [DOI] [PubMed] [Google Scholar]
- Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7–36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24 h secretion patterns. Journal of Endocrinology. 1993;138:159–166. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. Journal of Clinical Investigation. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003;52:1147–1154. doi: 10.2337/diabetes.52.5.1147. [DOI] [PubMed] [Google Scholar]
- Ha E, Zemel MB. Functional properties of whey, whey components, and essential amino acids: mechanisms underlying health benefits for active people (review) Journal of Nutritional Biochemistry. 2003;14:251–258. doi: 10.1016/s0955-2863(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Hall WL, Millward DJ, Long SJ, Morgan LM. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. British Journal of Nutrition. 2003;89:239–248. doi: 10.1079/BJN2002760. [DOI] [PubMed] [Google Scholar]
- Herrmann C, Goke R, Richter G, Fehmann HC, Arnold R, Goke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56:117–126. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nature Medicine. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- Hyde R, Taylor PM, Hundal HS. Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochemical Journal. 2003;373:1–18. doi: 10.1042/bj20030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis WD, Turner AJ, Povirk LF, Traylor RS, Grant S. Induction of apoptotic DNA fragmentation and cell death in HL-60 human promyelocytic leukemia cells by pharmacological inhibitors of protein kinase C. Cancer Research. 1994;54:1707–1714. [PubMed] [Google Scholar]
- Jiang Y, Cypress AM, Muse ED, Wu C-R, Unson CG, Merrifield RB, Sakmar TP. Glucagon receptor activates extracellular signal-regulated protein kinase 1/2 via cAMP-dependent protein kinase. PNAS. 2001;98:10102–10107. doi: 10.1073/pnas.131200398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer TJ, Habener JF. The glucagon-like peptides. Endocrine Reviews. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- Layer P, Holst JJ, Grandt D, Goebell H. Ileal release of glucagon-like peptide-1 (GLP-1): association with inhibition of gastric acid secretion in humans. Digestive Diseases and Sciences. 1995;40:1074–1082. doi: 10.1007/BF02064202. [DOI] [PubMed] [Google Scholar]
- Mackova M, Man JR, Chik CL, Ho AK. p38MAPK inhibition enhances basal and norepinephrine stimulated p42/44MAPK phosphorylation in rat pinealocytes. Endocrinology. 2000;141:4202–4208. doi: 10.1210/endo.141.11.7797. [DOI] [PubMed] [Google Scholar]
- Montrose-Rafizadeh C, Avdonin P, Garant MJ, Rodgers BD, Kole S, Yang H, Levine MA, Schwindinger W, Bernier M. Pancreatic glucagon-like peptide-1 receptor couples to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology. 1999;140:1132–1140. doi: 10.1210/endo.140.3.6550. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu B-E, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocrine Reviews. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Reimann F, Gribble FM. Glucose-sensing in glucagon-like peptide-1 secreting cells. Diabetes. 2002;51:2757–2763. doi: 10.2337/diabetes.51.9.2757. [DOI] [PubMed] [Google Scholar]
- Reimann F, Williams L, da Silva Xavier G, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia. 2004;47:1592–1601. doi: 10.1007/s00125-004-1498-0. [DOI] [PubMed] [Google Scholar]
- Reimer RA, Darimont C, Nicolas-Metral V, Gremlich S, Rüegg UT, Macé K. A human cellular model for studying the regulation of glucagon-like peptide 1 secretion. Endocrinology. 2001;142:4522–4528. doi: 10.1210/endo.142.10.8415. [DOI] [PubMed] [Google Scholar]
- Rocca AS, LaGreca J, Kalitsky J, Brubaker PL. Monounsaturated fatty acid diets improve glycemic tolerance through increased secretion of glucagon-like peptide-1. Endocrinology. 2001;142:1148–1155. doi: 10.1210/endo.142.3.8034. [DOI] [PubMed] [Google Scholar]
- Singh RP, Dhawan P, Golden C, Kapoor GS, Mehta KD. One-way cross-talk between p38MAPK and p42/44MAPK. Inhibition of p38MAPK induces low density lipoprotein receptor expression through activation of the p42/44MAPK cascade. Journal of Biological Chemistry. 1999;274:19593–19600. doi: 10.1074/jbc.274.28.19593. [DOI] [PubMed] [Google Scholar]
- Terada T, Inui K. Peptide transporters: structure, function, regulation and application for drug delivery. Current Drug Metabolism. 2004;5:85–94. doi: 10.2174/1389200043489153. [DOI] [PubMed] [Google Scholar]
- Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CMB, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Boushey RP, Drucker DJ, Brubaker PL. Secretion of the intestinotropic hormone glucagon-like peptide 2 is differentially regulated by nutrients in humans. Gastroenterology. 1999;117:99–105. doi: 10.1016/s0016-5085(99)70555-x. [DOI] [PubMed] [Google Scholar]