Abstract
Respiratory signs are common in African children where malaria is highly endemic and, thus, parsing the role of pulmonary pathology in illness is challenging. We examined the lungs of 100 children from an autopsy series in Blantyre, Malawi, in many of whom death was attributed to P falciparum malaria. Our aim was to describe the pathological manifestations of fatal malaria, to understand the role of parasites, pigment, and macrophages, and to catalogue co-morbidities. From available patients which included 55 patients with cerebral malaria and 45 controls, we obtained 4 cores of lung tissue for immunohistochemistry and morphological evaluation. We found that in patients with cerebral malaria, large numbers of malaria parasites were present in pulmonary alveolar capillaries, together with extensive deposits of malaria pigment (hemozoin). The number of pulmonary macrophages in this vascular bed did not differ between patients with cerebral malaria, non-cerebral malaria and non-malarial diagnoses. Co-morbidities found in some cerebral malaria patients included pneumonia, pulmonary edema, hemorrhage, and systemic activation of coagulation. We conclude that the respiratory distress seen in patients with cerebral malaria does not appear to be anatomic in origin but that increasing malaria pigment is strongly associated with cerebral malaria at autopsy.
Introduction
Diseases affecting the respiratory system are a major challenge in many tropical settings where HIV, tuberculosis, pneumonia, and malaria are common. In pediatric malaria, metabolic acidosis causing deep or labored breathing is a frequent manifestation [1–6]. More importantly, acidosis is an independent risk factor for mortality among children with severe pediatric Plasmodium falciparum malaria, and has been associated with case fatality rates of 24.2% to 31% [1, 2, 5, 7–9]. Among the many perturbations to the human host during acute infection with malaria, macrophages are systemically activated in response to the large antigen load and play an important role of in the phagocytosis of parasitized red blood cells. In other disease states characterized by systemic macrophage stimulation, such as transfusion related lung injury, macrophages accumulate in the lungs, causing hypoxia and extensive radiographic opacification [10]. Disseminated intravascular coagulation is a recognized feature of severe malaria, and autopsy studies have demonstrated microthrombi in many tissues [11].
As part of an ongoing clincopathological study of severe malaria, we evaluated the pathology of the lung in fatal malaria. In particular, we aimed to identify whether local pulmonary pathology, with accumulation of activated macrophages and microthrombi might contribute to the pathogenesis of respiratory distress in children with severe malaria infections. Anecdotally, we had subjectively notice variable amounts of macrophage and pulmonary pigment present in this data set and attempted to quantitatively define the subjective histological findings using detailed immunological and morphological tissue counts. An additional aim was to identify pulmonary co-morbidities that might be associated with fatal malaria.
Materials and Methods
Patients
The clinicopathological correlation study of severe and fatal malaria in the Queen Elizabeth Central Teaching Hospital, Blantyre, Malawi commenced in 1996. Clinical management, laboratory investigations, and treatment protocols have been previously described [12]. Blood cultures were sporadically performed at admission but were not available in all patients. In the event of death, a Malawian clinician or nurse met with key family members to consent for an autopsy. Clinical diagnoses were determined prior to each autopsy as previously described [12]. If permission was granted, the post-mortem was performed as quickly as possible in the mortuary at the Queen Elizabeth Central Hospital. Microbiological cultures were not performed at autopsy. In analysis, we compared histological findings between patients without ante mortem respiratory signs and those with a collection of respiratory signs (indicating some form of respiratory distress) including nasal flaring, intercostal recession, deep breathing, grunting, irregular breathing or abnormal chest sounds on auscultation. We also specifically noted the prevalence of anatomic abnormalities in all patients and compared patient groups by the final anatomical diagnosis. The research ethics committees at the University of Malawi College of Medicine, Michigan State University, the University of Liverpool, and the Brigham & Women’s Hospital have approved all or appropriate portions of this study.
Autopsy Procedures
Gross examination, documentation, and histological assessment of the brains and other organs were performed as previously described [12]. Cases were classified after autopsy examination as one of the following final anatomical diagnoses: cerebral malaria with intracerebral histological findings limited to parasite sequestration only (CM1); cerebral malaria with both sequestration and extra-vascular pathology in the brain (CM2); clinically diagnosed cerebral malaria with no sequestration in the brain and with another anatomic cause of death (CM3); other patients enrolled in the study without clinical or pathological evidence of cerebral malaria (Other) – these included parasitemic and aparasitemic patients. Segments of right lung were inflated via the bronchus with formalin and allowed to fix prior to slicing in order to obtain optimal morphological preservation. Segments of the left lung were cut directly and placed in formalin.
Histology
Sections of H&E stained brain tissue were examined and quantified for total parasites as previously described [12]. Standard sections of H&E stained lung tissue were used to make histological assessments except for immunohistochemistry quantification which was performed on tissue microarray (see below). As part of the review of cases for final anatomic diagnosis, increased amounts of apparent macrophages and/or pigment globules were noted in many cases and, thus, a subjective scale for the progressive apparent increase in these elements was used to categorize patients by histological appearance only. Briefly, the low to medium power appearance of the lung histology was graded, blinded to final anatomic diagnosis, by RW as “normal” (graded 0) or as mild, moderate, severe (grades 1–3) by assessing the relative amounts of intravascular accumulation of macrophages and/or pigment AND the absence of other types of pathology (i.e., intra-alveolar inflammatory cell accumulation, interstitial fibrosis or inflammatory cell accumulation and pneumocyte atypia). Sections of representative grades of lung from each case were photographed using polarized light microscopy as previously described [13].
Tissue microarray (TMA) construction
Histological sections of lung tissue were examined and marked for areas of pure alveolar tissue (i.e., avoiding bronchus, lymphoid tissue, large vessels, and tissue of the hilum). Using the corresponding formalin fixed paraffin embedded tissue blocks for 88 sequential autopsies, four cores, each 0.6mm in diameter, were selected from each case from the marked areas of the slide and embedded into a master tissue array block (Supplemental Figure 1). Once constructed, the array included sections of stock normal liver as location controls. Six-micron-thick sections of the tissue microarray were cut and placed on charged slides for histology and immunohistochemistry.
Immunohistochemistry
All immunohistochemistry was performed as per standard protocols of the Hematopathology Immunohistochemistry Laboratory of the Brigham and Women’s Hospital for the following markers: CD31 (endothelium), CD3 (all T-cells), CD4 (T-helper cells), CD8 (cytotoxic T-cells), CD20 (mature B-cells), CD15 (activated B-cells, neutrophils), CD10 (follicular center B-Cells), CD68 (macrophages), CD163 (activated macrophages), iNOS (inducible nitric oxide synthetase) (Figure 1 and Supplemental Figure 2). For each case (i.e., four sections of the cores of tissue per stain), the number of cells per section and the total number of cells per four sections for each marker were recorded by DM and RF after independent examination of the sections. This assessment did not include CD31 which was used as an internal control for the antigen reactivity of the tissue. Inducible nitric oxide synthase (iNOS) was examined to assess its intensity in blood vessels of various sizes, for comparison with the degree of iNOS staining known to occur in brain vessels in cerebral malaria (Supplemental Figure 2)[14]. Each section of core contained 4 high powered fields and all counts were converted to element / 10 hpf for statistical comparison.
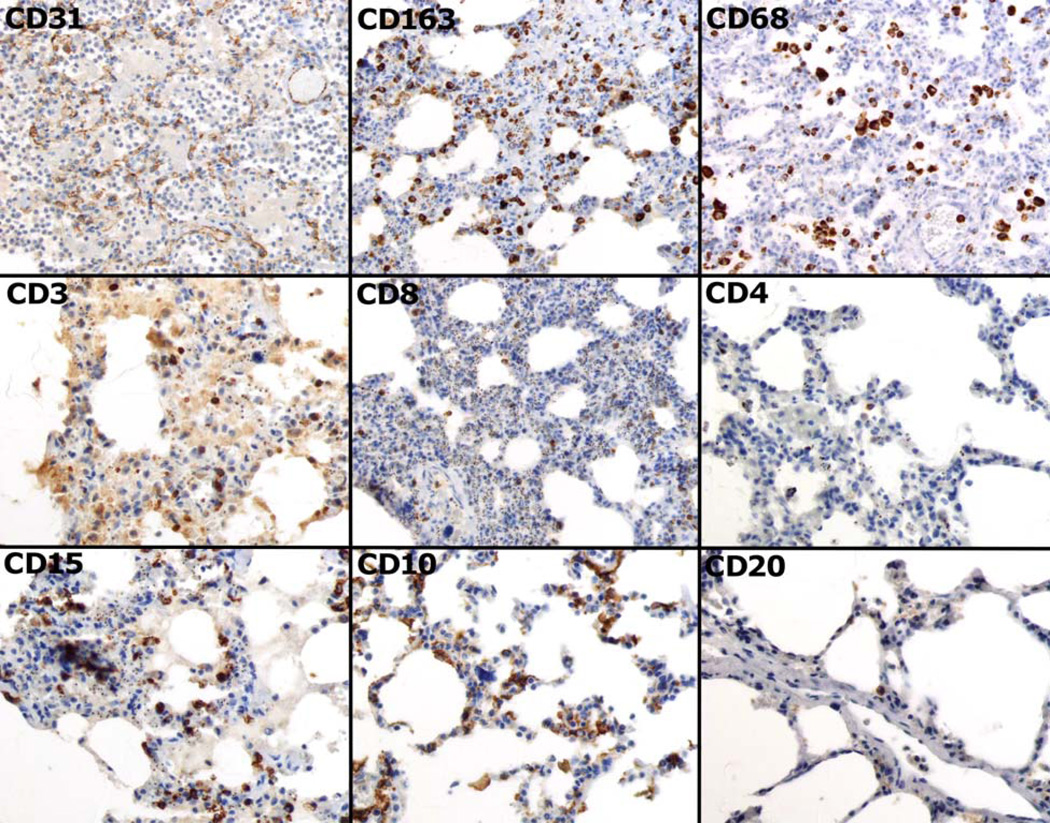
Figure 1.
Images of the immunohistochemistry obtained using the tissue array. CD31 highlights blood vessels throughout the capillary beds of the lung. CD163 and CD68 demonstrate the macrophage population present within the capillary walls and in alveolar spaces. CD3, CD8 and CD4 highlight the total T-cell population, cytotoxic T-cells and helper T-cells respectively. CD15, CD10 (which also highlights endothelium), and C20 were used to characterize B-cells. All images are 400× original magnification.
Electron microscopy
For a subset of samples which showed evidence of intense pigment within macrophages, tissue fixed in 2% glutaraldehyde was processed for electron microscopy as per routine protocol, embedded in plastic, and cut as ultra-thin sections for examination under transmission electron miscroscopy (TEM).
Statistics
Clinical variables were compared either by ANOVA (continuous normal data), Kruskal-Wallis (continuous non-normal data), or Fisher’s Exact Test (categorical data). Histological parameters were compared either by Kruskal-Wallis or Fisher’s Exact Test. The comparison of tissue counts with the ordinal categorical variable ‘lung-pigment grades 2–3’ was performed using Kruskal-Wallis and the Cuzick non-parametric trend test. A Fisher’s Exact for a 4 × 4 contingency table was performed for the diagnostic categories vs. pathological categories. All clinical variables were modeled with outcome CM in logistic regression independently (univariate). Clinical variables with a p-value of <0.1 were included in a logistic regression model with lung pigment grades 2–3 and the outcome CM. Post-test estimation for area under the curve was performed. All statistical analysis was performed with Stata v11.0 (College Station, TX).
Results
The complete database of cases at the time of this analysis included 100 autopsies performed on children between the ages of 6 months and 13 years with a mean of 3.6 ± 2.8. The patients included in this study are similar in many respects, but those categorized as CM1 are more likely to be female, have higher heart rates, and a higher HIV seroprevalence. Those with histologically proven CM (CM1 and CM2) are more likely than the others in the study to have had a high-density parasitemia, a low blood hematocrit, a high blood lactate concentration and thrombocytopenia. Those with clinically defined CM but no evidence of sequestration in the brain (CM3) had a shorter mean duration of fever and lower mean blood glucose concentrations on admission than the CM1 and CM2 groups, and those with non-malarial comas (“Other”) have on average less fever than the other groups (Supplemental Table 1). Clinical respiratory signs that had been documented prior to death were distributed equally among all the groups defined by cerebral histology (Supplemental Table 1). There was no association with age and any measured parameter of lung pathology.
By definition, the brains of children dying of cerebral malaria had higher levels of parasite sequestration than those with non-malarial comas, and those with CM2 had higher pigment levels in the brain than those with CM1 (Table 1) [12]. Lung counts of parasites and pigment were similar between CM1 and CM2, and both counts were significantly higher than in the CM3 and Other patients (Table 1).
Table 1.
Summary of histological parameters from the brain and lung
| CM1 | CM2 | CM3 | Other* | P-value | ||
|---|---|---|---|---|---|---|
| Brain Parameters** | (n = 15) | (n = 40) | (n = 19) | (n = 23) | ||
| % of Vessels Parasitized | 78.6 ± 22.4 | 74.2 ± 22.4 | 1.7 ± 1.8 | 3.5 ± 6.3 | <0.0001 | |
| Total Parasites per 100 cap | 301 [234.8, 409.5] | 204.3 [122.3, 324.9] | 0 [0, 1] | 0 [0, 0.9] | 0.0001 | |
| Total Pigment per 100 cap | 15 [7, 26] | 106.2 [41.6, 143.6] | 0.9 [0, 2.7] | 0.9 [0, 2.9] | 0.0001 | |
| Fibrin per 100 cap | 1 [0, 1.7] | 3.2 [0, 9.3] | 0 [0, 0] | 1.8 [0, 6.9] | 0.0034 | |
| Lung Quantitative Parameters | (n = 9) | (n = 35) | (n = 17) | (n = 22) | ||
| Parasites/10 hpf | 42.5 [28.1, 108.8] | 40.6 [18.8, 90] | 9.7 [6.3, 21.9] | 8.1 [1.9, 20] | 0.0001 | |
| Pigment/10 hpf | 193.8 [98.8, 244.4] | 266.3 [180, 417.5] | 50.9 [16.9, 86.3] | 35 [15.6, 121.9] | 0.0001 | |
| T-cells/10 hpf | 76.3 [48.1, 160] | 86.9 [60.6, 156.9] | 111.9 [54.4, 192.5] | 75 [53.1, 92.5] | 0.3907 | |
| Activated Macrophages/10 hpf | 187.5 [129.4, 235] | 246.9 [183.8, 390] | 306.3 [125, 364.4] | 161.9 [139.4, 386.9] | 0.4793 | |
| B-Cells/10 hpf | 13.1 [5.6, 31.3] | 15.6 [10.6, 23.1] | 46.3 [18.1, 66.9] | 13.8 [6.9, 28.1] | 0.0023 | |
| Pigment/macrophage/10hpf | 0.91 [0.67 – 1.2] | 0.98 [0.52 – 2.01] | 0.27 [0.10 – 0.62] | 0.23 [0.10 – 0.41] | 0.0001 | |
| Lung Pathological Parameters | (n = 15) | (n = 40) | (n = 19) | (n = 23) | ||
| Pneumonia (% Positive) | 26.7% | 20.5% | 63.2% | 56.0% | 0.0020 | |
| Pulmonary Edema (% Positive) | 40.0% | 34.0% | 44.0% | 38.0% | 0.8850 | |
| Alveolar Hemorrhage (% Positive) | 20.0% | 2.6% | 26.3% | 30.4% | 0.0005 | |
| Alveolar Fibrin (% Positive) | 20.0% | 12.8% | 21.1% | 30.4% | 0.3910 | |
| Microthrombi (% Positive) | 0.0% | 30.8% | 15.8% | 21.7% | 0.0730 | |
Includes parasitemic and aparasitemic children who did not meet the clinical case definition of cerebral malaria but excludes children with Severe Malarial Anemia
In previous data, we have shown that the pathological findings of cerebral malaria are associated with having > 23% of vessels parasitized and that CM1 and CM2 are distinguishable by the amount of pigment present.
The number of T-cells (as measured by CD3, CD4, or CD8) was similar across all groups despite the higher prevalence of HIV in the CM1 patients (Table 1). Intravascular macrophages (as measured by the activation marker CD163 [15] and the common marker CD68) demonstrated similar ranges across all groups (Table 1). Resting and activated B-cells (as measured by CD20 and CD15) were higher in number in CM3 patients (Table 1). The staining of endothelial cells by CD10 dominated these slides but no precursor B-cells were seen in alveolar capillaries. Inducible nitric oxide synthase staining was prominent in small muscular pulmonary arteries throughout the lungs in all groups while alveolar capillary endothelium, regardless of disease categorization, showed no evidence of staining for iNOS (Supplemental Figure 3).
Pneumonia was the most common co-morbidity, particular among the CM3 and Other patients. A quarter of the CM1 and CM2 patients had histological features of acute pneumonia (Table 1 and Figure 2). Pulmonary edema, though present, was not a specific feature of lung in CM1 or CM2 patients, and its presence bore no relation to respiratory symptoms or signs in life (Supplemental Table 1 & Figure 3A). Microthrombi, indicative of systemic activation of coagulation, were common in the CM2, CM3, and Other group but absent from CM1 (Table 1 & Figure 3B & 3C). In two cases (age 13 and 31 months), the eggs of Schistosoma species trematodes were present in the lungs (S. mansoni and S. haemotobium are both endemic in Malawi) reflecting early exposure and proximity of home to the lake (Figure 3E & 3F). Two other rare findings were scattered alveolar hemorrhages and alveolar fibrin indicative of vascular damage and breakdown in the lungs (Supplemental Figure 3).
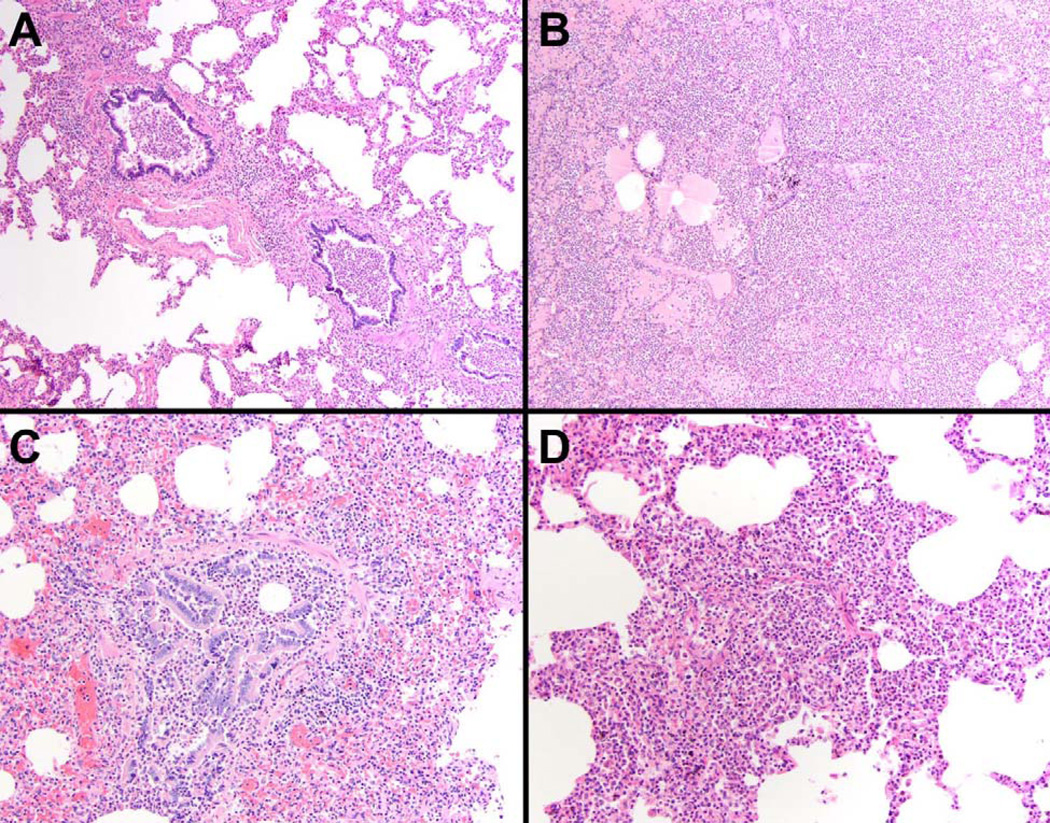
Figure 2.
Pneumonia was a common finding in our total patient population although of significantly greater prevalence in non-CM patients than CM patients, with both bronchopneumonia (A & C) and lobar pneumonia (B & D) found. The severity of the pneumonia was variable and, in many cases, was thought to be the primary cause of death (H&E, A & B 100×, C&D 200×).
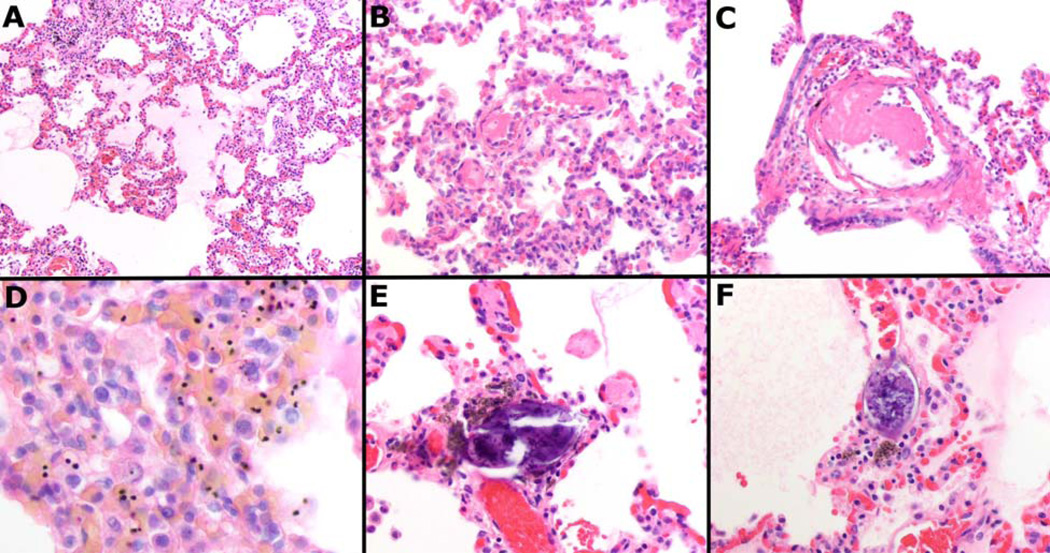
Figure 3.
Representative images of the pathology encountered in the series including pulmonary edema (A), fibrin microthrombi within small (B) and large (C) vessels, sequestration of parasites (D), and Schistosoma species eggs (E & F). All images H&E at 200× to 600× original magnification.
When the grading of lungs (“malaria lung”) was compared with the separately obtained quantitative tissue counts of parasite elements, the pathologic pattern (increasing numbers of parasites and pigment) was consistent with grading (Table 1, Figure 3D, & Supplemental Figure 4). Parasite pigment was easily appreciated on polarizing light microscopy (Supplemental Figure 4). Comparing the histopathologic classification (CM1, CM2, CM3) with the grading of lung pigment-content showed that the majority of CM1 and CM2 patients had high levels of hemozoin in the microvasculature of the lungs (grades 2 and 3) while the CM3 and Other patients had little evidence of parasite and pigment accumulation (grades 0 and 1) (Table 2).
Table 2.
Summary of observations of "Malaria lung"
| Pathological Finding of Malaria Lung* | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | P-value | ||
| Lung Quantitative Parameters | (n = 31) | (n = 30) | (n = 25) | (n = 9) | ||
| Parasites/10 hpf | 6.3 [1.6, 20.3] | 23.8 [11.3, 30.6] | 41.6 [18.8, 90] | 58.8 [24.4, 124.4] | <0.0001** | |
| Pigment/10 hpf | 19.1 [12.8, 85.9] | 98.8 [61.9, 193.8] | 271.3 [126.3, 323.8] | 251.3 [240, 429.4] | <0.0001** | |
| T-cells/10 hpf | 79.4 [51.3, 127.2] | 86.9 [53.4, 159.4] | 74.4 [51.3, 106.9] | 156.9 [128.8, 238.8] | 0.0523*** | |
| Activated Macrophages/10 hpf | 147.5 [125, 364.4] | 292.2 [165.3, 377.2] | 220 [113.8, 280.8] | 299.4 [235, 405.6] | 0.1427*** | |
| B-Cells/10 hpf | 25 [6.9, 50] | 15.9 [11.3, 25] | 13.8 [10, 20.6] | 18.8 [16.3, 26.9] | 0.4320*** | |
| Pigment/macrophage/10hpf | 0.13 [0.07 – 0.56] | 0.51 [0.24 – 0.75] | 1.00 [0.60 – 1.36] | 0.62 [0.50 – 4.49] | <0.0001** | |
| Diagnostic Groups | ||||||
| CM1 (n = 15) | 4 | 7 | 2 | 2 | <0.0001**** | |
| CM2 (n = 39) | 2 | 9 | 22 | 6 | ||
| CM3 (n = 18) | 12 | 5 | 0 | 1 | ||
| Other (n = 23) | 13 | 9 | 1 | 0 | ||
"Malaria Lung" is a histopathological observation of excessive macrophages and/or malaria pigment which was scored blind to the diagnosis by a single pathologist (RW) and graded for severity from 0 (absent) to 3. Quantification was peformed on separate tissue blocks using the tissue microarray by to other observers (RF, DAM)
P-value for Kruskal-Wallis and for non-parametric trend (Cuzick) across groups
Kruskal-Wallis test for non-parametric data across groups
Fischer's Exact test for 4 × 4 contingency table
To determine the association of the lung pigment grading with cerebral malaria, we performed first a univariate analysis with clinically important variables (Supplemental Table 2). This was followed by a multivariate logistic regression for the outcome CM (including CM1 and CM2) vs. Non-CM (CM3 and Other) (Supplemental Table 2). The unadjusted and adjusted ORs of having CM for lung pigment were 5.8 and 3.1 respectively (Supplemental Table 2).
For a subset of cerebral malaria cases, electron microscopy was performed to understand the relationships of pigment, macrophages, parasites, and fibrin to the endothelial lumens of the lung. We confirmed, as seen on H&E at light microscopy resolution, that macrophages were present in the lumens of the capillaries with sequestered parasites (Supplemental Figure 5). Pigment and fibrin were also evident within microvessels.
Discussion
We have studied fatal malaria in children in a country with endemic malaria transmission. In this population, the commonest clinical presentation of life-threatening malaria is coma with convulsions (‘cerebral malaria’, CM), and our study has focused on patients with this presentation. Children with cerebral malaria frequently have multisystem disease, with features that may include acidosis, hypoglycaemia, severe anaemia, hemoglobinuria or jaundice. In this population, parasitemia may be incidental and irrelevant to a patient’s illness, and for the current analysis we have used the presence of intracranial sequestered parasites to indicate the likely role of malaria in causing CM and the patient’s death. We have then compared pulmonary findings between those with and those without cerebral sequestration. We have also attempted to relate pulmonary pathology to ante-mortem clinical respiratory features.
The earliest and now classic descriptions of pathology found in patients who succumb to severe complications of malaria as both children and adults included a wide range of findings in the pulmonary tissues. These early studies suggested that pigment-laden macrophages were present in abundance in the capillary beds of the lungs, occluding most of the vascular space alongside sequestered developing parasites [16]. By contrast, in the patients we have studied, activated macrophages do not appear to be significantly increased in the lungs of children with CM compared to those with other causes of death. Pigment levels (hemozoin both inside and outside of erythrocytes) are, however, significantly higher in the pulmonary vasculature of CM patients, compared to non-CM patients. Similarities in the extent of macrophage accumulation in our patient groups may reflect severe disease afflicting patients in all, whether malarial or other. Comparative studies of lung tissue in non-fatal disease are limited in our site owing to a lack of any pulmonary surgery.
In prior studies, ~75% of blood vessels of the brain in cerebral malaria patients showed some evidence of iNOS staining versus no staining in controls [14]. In the lung, however, we have shown that arterial vessels of any size demonstrated staining across all cases and controls. This suggests that activation of pulmonary vessels is more frequent and from various etiologies (e.g., macrophages, parasites, pneumonia) whereas the effect in brain is most likely resulting for the mass of sequestration of parasites occurring there [17].
Early observations of macrophages with the addition of scattered hemorrhages within the lung tissues are consistent with our findings; however, more overt pathology, as described particularly in adult patients with malaria, including pulmonary edema, hyaline membranes, tissue infarction, and interstitial processes, was not seen in our study [18–22]. Our patients, all of whom were children, lacked these classic features of well developed disease but did have evidence of acute lung injury. The very short duration of illness from admission to death in our patients may preclude the development of classic clinical acute respiratory distress syndrome (ARDS) and associated pathology. Pulmonary edema was present but only in ~40% of patients and across all patient groups at the same level, possibly reflective of the severe illness present in all groups. Another consistent early observation in the literature was a correlation between the intensities of sequestration of parasites in the brain and the lung [18–20, 23]. Our data also demonstrate increased amounts of parasite sequestration in the lungs associated with cerebral malaria.
Post mortem evidence of bronchopneumonia complicating malaria deaths has been often noted, ranging from focal to lobar processes [18–20]. Of the few studies which report findings exclusively in children, congestion, edema, malarial pigment and pneumonia (22.8 to 25% of cases having any feature), as seen in adults[8, 9], are found. In our study, one fourth of CM patients had co-existing histological evidence of pneumonia compared with 50% of the non-CM patients. In many of the CM3 and Other cases, severe pneumonia was present and was considered to be the cause of death. There were increased numbers of B-cells in the non-CM groups which may correspond to the higher rate of pneumonia with coma and the host’s response to bacterial antigen. Thrombi have been observed within the lungs and other organs in previous studies of fatal malaria; we observed similar findings in CM2, CM3, and Other patients; however, thrombi were not seen in the CM1 group [11]. Patients with CM1 have massive sequestration of unruptured parasitized erythrocytes in cerebral vessels, and may constitute a subgroup with a rapid parasite tissue accumulation that is associated with less extensive endothelial stimulation than occurs in CM2. Although fibrin thrombi are seen in the brain in CM1, they are found in only ~10% of vessels, significantly less than in CM2 (Samuel Wassmer, personal communication).
The pulmonary pathology of pediatric cerebral malaria in our series appears to consist of high parasite and pigment burdens in the lungs without other obvious anatomic lesions. Our data do not support the concept of excessive accumulation of white blood cells in the lung as a feature unique to cerebral malaria. Abundant macrophages were present across all diagnostic groups in great number and their presence may be contributing to inhibition of lung function. The clinical respiratory signs did not correlate with a specific anatomic pathological finding. This is probably because most signs are indicative of a non-anatomic mechanism, usually acidosis or central respiratory depression.
In summary, cerebral malaria is strongly associated with excessive malaria pigment and the sequestration of parasites in the lungs supporting the concept of total body parasite density as a feature of cerebral malaria. A lack of anatomic pathology of the lungs specific to cerebral malaria supports the theory of central respiratory depression possibly secondary to brain swelling as a source of respiratory distress. Co-morbidities, especially pneumonia, should be considered for possible empiric therapy in comatose parasitemic children in Africa.
Supplementary Material
A low power view of a histological section of the tissue array showing 35 of the 358 lung cores used in this experiment. The areas of more solid tissue represent patients with diffuse pneumonia in the lobe sectioned. Variations in fixation and preservation led to variation in the appearance of each core (H&E 20×).
Inducible nitric oxide synthase has been shown to be increased in the cerebral vessels of children with cerebral malaria. Larger vessels in the lung demonstrate increased expression of iNOS (A, B, and C in decreasing order of size) but small capillaries of the alveoli (with and without parasites) were not strongly highlighted by the antibody (D). All images are 400× original magnification).
Damage to the alveolar capillary endothelium manifested as either alveolar hemorrhage (A) or fibrin collection in alveolar spaces (B) was observed in a small number of cases. All images H&E, 200×.
The pattern of lung pathology which was most related to the amount of pigment (either as pigment globules or within pigmented parasites) is best appreciated on both H&E section with standard trans-illumination as well as polarization of the sections. The increasing amounts of pigment from stage 0 through 3 are shown as paired images with “p” denoting the polarized image. All images 200× original magnification; polarized images were maximum bright field using above and below polarized lenses as previously described (see text for reference).
White blood cells outnumbering red blood cells in the capillary lumens as seen by transmission electron microscopy. EC= endothelial cells, M=monocytes, L=lymphocyte, P=neutrophil polymorph, PG=malarial pigment globules in activated monocytes. Electron dense intravascular fibrillar material typical of fibrin is also present. (A & C, Magnification 5,000×). Higher magnification is shown of another degenerate endothelial cell nucleus with large activated intravascular monocytes present (B, Magnification 12,500×). Intravascular white blood cells are numerous and some malarial pigment globules are seen within activated monocytes (D, Magnification 5,000×).
Acknowledgements
We are indebted to the children and their families of Malawi for participation in our clinicopathological studies. We would like to thank the following individuals for their tireless support both in the field and in the laboratory: Alyson Smeedy Campbell, Scott Rodig, Jon Aster, Christine Unitt, Dyann Wirth, and Wales Namanya.
Funding Sources: This work was funded by the National Institutes of Health, USA, and The Wellcome Trust, UK. The following individuals were supported by funds from National Institutes of Health: (DAM (5K23AI072033-05), TET (5R01AI034969-14), KBS (K22). MEM was supported by The Wellcome Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no conflicts of interest to report with regard to the data, its presentation, or this manuscript.
References
- 1.Marsh K, et al. Indicators of Life-Threatening Malaria in in African Children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 2.Taylor WR, C V, White NJ. Pulmonary manifestations of malaria: recognition and management. Treat Respir Med. 2006;5(6):419–428. doi: 10.2165/00151829-200605060-00007. [DOI] [PubMed] [Google Scholar]
- 3.Oduro AR, K K, Rogers W, Atuguba F, Ansah P, Anyorigiya T, Ansah A, Anto F, HA Mensah N, Nkrumah F. Severe falciparum malaria in young children of the Kassena-Nankana district of northern Ghana. Malar J. 2007;27(6):96. doi: 10.1186/1475-2875-6-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dzeing-Ella A, N OP, Tchoua R, Planche T, Mboza B, Mbounja M, JJ Muller-Roemer U, Kendjo E, Ngou-Milama E, Kremsner PG, Krishna S, K M. Severe falciparum malaria in Gabonese children: clinical and laboratory features. Malar J. 2005;9(4):1. doi: 10.1186/1475-2875-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goljan J, N W, Wroczyńska A, Felczak-Korzybska I, Pietkiewicz H. Severe malaria--analysis of prognostic symptoms and signs in 169 patients treated in Gdynia in 1991–2005. Int Marit Health. 2006;57(1–4):149–162. [PubMed] [Google Scholar]
- 6.Waller D, K S, Crawley J, Miller K, Nosten F, Chapman D, ter Kuile FO, BC Craddock C, Holloway PA, et al. Clinical features and outcome of severe malaria in Gambian children. Clin Infect Dis. 1995;21(3):577–587. doi: 10.1093/clinids/21.3.577. [DOI] [PubMed] [Google Scholar]
- 7.Deaton J. Fatal pulmonary edema as a complication of acute falciparum malaria. Am J Trop Med Hyg. 1970;19:196–201. doi: 10.4269/ajtmh.1970.19.196. [DOI] [PubMed] [Google Scholar]
- 8.Attah EDB, Ejeckam GC. Clinico-pathologic correlation in fatal malaria. Trop Geogr Med. 1974;26:359–362. [PubMed] [Google Scholar]
- 9.Edington GM. Cerebral Malaria in the Gold Coast African Four Autopsy Reports. Ann Trop Med Parasitol. 1954;48:300–306. doi: 10.1080/00034983.1954.11685627. [DOI] [PubMed] [Google Scholar]
- 10.Cherry T, et al. Transfusion-related acute lung injury: past, present, and future. Am J Clin Pathol. 2008;129(2):287–297. doi: 10.1309/D3F7BXH466AE3G0P. [DOI] [PubMed] [Google Scholar]
- 11.Jaroonvesama N. Intravascular coagulation in Falciparum malaria. Lancet. 1972;i:221. doi: 10.1016/s0140-6736(72)90621-6. [DOI] [PubMed] [Google Scholar]
- 12.Taylor TE, F W, Carr RA, Whitten RO, Mueller JS, Fosiko NG, Lewallen S, Liomba NG, Molyneux ME. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10(2):143–145. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 13.Whitten R, et al. Liver pathology in Malawian children with fatal encephalopathy. Hum Pathol. 2011;42(9):1230–1239. doi: 10.1016/j.humpath.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maneerat Y, et al. Inducible nitric oxide synthase expression is increased in the brain in fatal cerebral malaria. Histopathology. 2000;37(3):269–277. doi: 10.1046/j.1365-2559.2000.00989.x. [DOI] [PubMed] [Google Scholar]
- 15.Hiraoka A, et al. Expression of CD163 in the liver of patients with viral hepatitis. Pathol Res Pract. 2005;201(5):379–384. doi: 10.1016/j.prp.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Marchiafava F, Bignami Sulle febbri malariche estivo-autunnali. Bull del R Acad Med di Roma. 1892;297(18) [Google Scholar]
- 17.Clark IA, et al. Tissue distribution of migration inhibitory factor and inducible nitric oxide synthase in falciparum malaria and sepsis in African children. Malar J. 2003;2:6. doi: 10.1186/1475-2875-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudgeon LS, Clarke C. An Investigation of Fatal Cases of Pernicious Malaria Caused by Plasmodium falciparum in Macedonia. Quart J Med. 1919:372–390. [Google Scholar]
- 19.Rigdon RH. A consideration of the mechanism of death in acute plasmodium falciparum infection: report of a case. Am J Trop Med Hyg. 1942;36:269–275. [Google Scholar]
- 20.Spitz S. The Pathology of Acute Falciparum Malaria. The Military Surgeon. 1946;99:555–572. [PubMed] [Google Scholar]
- 21.Duarte MIS, et al. Ultrastructure of the lung in falciparum malaria. Am J Trop Med Hyg. 1985;34(1):31–35. doi: 10.4269/ajtmh.1985.34.31. [DOI] [PubMed] [Google Scholar]
- 22.Gopinathan VP, Ganguly SB, Chivukul LK. Cerebral Malaria - A Clinicopathological Study. J Assoc Physcians India. 1986;34(7):473–475. [PubMed] [Google Scholar]
- 23.Dhayagude RG, Purandare NM. Autopsy study of cerebral malaria with reference to malarial granuloma. Arch Pathol. 1943;36:550–558. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A low power view of a histological section of the tissue array showing 35 of the 358 lung cores used in this experiment. The areas of more solid tissue represent patients with diffuse pneumonia in the lobe sectioned. Variations in fixation and preservation led to variation in the appearance of each core (H&E 20×).
Inducible nitric oxide synthase has been shown to be increased in the cerebral vessels of children with cerebral malaria. Larger vessels in the lung demonstrate increased expression of iNOS (A, B, and C in decreasing order of size) but small capillaries of the alveoli (with and without parasites) were not strongly highlighted by the antibody (D). All images are 400× original magnification).
Damage to the alveolar capillary endothelium manifested as either alveolar hemorrhage (A) or fibrin collection in alveolar spaces (B) was observed in a small number of cases. All images H&E, 200×.
The pattern of lung pathology which was most related to the amount of pigment (either as pigment globules or within pigmented parasites) is best appreciated on both H&E section with standard trans-illumination as well as polarization of the sections. The increasing amounts of pigment from stage 0 through 3 are shown as paired images with “p” denoting the polarized image. All images 200× original magnification; polarized images were maximum bright field using above and below polarized lenses as previously described (see text for reference).
White blood cells outnumbering red blood cells in the capillary lumens as seen by transmission electron microscopy. EC= endothelial cells, M=monocytes, L=lymphocyte, P=neutrophil polymorph, PG=malarial pigment globules in activated monocytes. Electron dense intravascular fibrillar material typical of fibrin is also present. (A & C, Magnification 5,000×). Higher magnification is shown of another degenerate endothelial cell nucleus with large activated intravascular monocytes present (B, Magnification 12,500×). Intravascular white blood cells are numerous and some malarial pigment globules are seen within activated monocytes (D, Magnification 5,000×).