Summary
In the MOG35-55 induced EAE model, autoreactive Th17 cells that accumulate in the central nervous system (CNS) acquire Th1 characteristics via a T-bet dependent mechanism. It remains to be determined whether Th17 plasticity and encephalitogenicity are causally related to each other. Here we show that IL-23 polarized Tbet−/− Th17 cells are unimpaired in either activation or proliferation, and induce higher quantities of the chemokines RANTES and CXCL2 than wildtype (WT) Th17 cells. Unlike their WT counterparts, they retain an IL-17hiIFNγneg-lo cytokine profile following adoptive transfer into syngeneic hosts. This population of highly polarized Th17 effectors is capable of mediating EAE, albeit with a milder clinical course. It has previously been reported that the signature Th1 and Th17 effector cytokines, IFNγ and IL-17, are dispensable for the development of autoimmune demyelinating disease. The current study demonstrates that the “master regulator” transcription factor, T-bet, is also not universally required for encephalitogenicity. Our results contribute to a growing body of data showing heterogeneity of myelin-reactive T cells and the independent mechanisms they employ to inflict damage to CNS tissues, complicating the search for therapeutic targets relevant across the spectrum of individuals with multiple sclerosis.
Keywords: Th17 cells, T-bet, experimental autoimmune encephalomyelitis, multiple sclerosis, neuroimmunology
Introduction
Experimental autoimmune encephalomyelitis (EAE) is a CD4+ T cell mediated autoimmune disease of the central nervous system (CNS), widely used as an animal model of multiple sclerosis (MS). Despite substantial progress in elucidating pathogenic pathways that drive EAE, the mechanisms employed by autoreactive T cells to initiate inflammatory demyelination and, hence, the effector functions that are critical for their encephalitogenicity, are largely unknown. We and others have previously shown that IL-12 polarized Th1 and IL-23 polarized Th17 cells specific for the same myelin antigen are independently capable of inducing EAE following adoptive transfer into naïve syngeneic hosts [1, 2]. Surprisingly, full blown disease occurs in the absence of the signature Th1 and Th17 cytokines, IFNγ and IL-17A/F, either alone or in combination [3-5]. More recently, the master regulatory transcription factor, T-bet, was identified as a critical molecule in the programming of encephalitogenic Th17 as well as Th1 cells [6]. T-bet was originally described as a driver of Th1 differentiation via direct activation of the IFNγ gene and upregulation of the IL-12 receptor β2 chain [7, 8]. Gocke and colleagues subsequently reported that T-bet could also promote the differentiation of autoimmune effector Th17 cells by inducing IL-23 receptor expression [9].
Several laboratories have established a role for T-bet in the plasticity of Th17 cells, particularly in their acquisition of Th1-like characteristics to become so called “ex-Th17” cells [10-12]. Fate mapping experiments using IL-17 reporter mice demonstrated that the majority of CD4+ T cells infiltrating the CNS of C57BL/6 mice actively immunized with a peptide of myelin oligodendrocyte glycoprotein (MOG35-55) are ex-Th17 cells [13, 14]. This observation has led some investigators to speculate that the plasticity of myelin-reactive Th17 cells is causally related to their acquisition of encephalitogenic properties. If they are correct then T-bet would be critical for the development of EAE based on its role in facilitating the transition of myelin reactive Th17 cells into ex-Th17 cells. In the current study we directly assess the requirement of T-bet expression in IL-23 polarized, myelin-reactive T cells for the adoptive transfer of EAE. We find that, unlike their WT counterparts, autoreactive T-bet−/− cells resist conversion to an ex-Th17 phenotype upon in vitro or in vivo reactivation. Moreover, these stable Th17 cells trigger the accumulation of myeloid cells in the spleen and CNS, thereby retaining the ability to induce EAE in WT as well as RAG2-deficient hosts.
Results
MOG35-55 immunized T-bet−/− mice generate a Th17 biased response and succumb to EAE
The master transcription factor, T-bet, has been implicated in the pathogenesis of EAE and MS [15-18]. We revisited the role of T-bet in EAE by comparing the clinical courses of C57BL/6 T-bet−/− and WT mice following subcutaneous immunization with an emulsion of MOG35-55 in CFA and intraperitoneal injection of inactivated Bordetella pertussis toxin. Ninety percent of T-bet−/− mice succumbed to moderate to severe EAE, although disease onset was slightly delayed compared to their WT counterparts (Fig. 1A). Examination of cytokine expression by CNS mononuclear cells pooled from representative mice in each group, and by splenocytes harvested from individual mice at peak EAE, revealed skewing towards an IL-17+IFNγ− profile in the T-bet−/− cohort (Fig. 1B and C). Splenocytes from immunized Tbet−/− mice produced significantly higher levels of IL-17 and lower levels of IFNγ than splenocytes from WT mice in response to in vitro challenge with MOG35-55 (Fig. 1D).
Figure 1. MOG35-55 primed T-bet−/− mice mount an enhanced Th17 response and succumb to EAE.
EAE was induced in WT and T-bet−/− C57BL/6 mice via immunization with MOG35-55 in CFA and administration of inactivated pertussis toxin. (A) WT (closed squares) and T-bet−/− (open squares) mice were rated on a daily basis for degree of neurological disability by an examiner blinded to genotype. Clinical scores for each cohort were averaged over two experiments with a total of 8-10 mice/group. (B, C) CNS mononuclear cells (B) and splenocytes (C) were harvested at peak EAE. CNS mononuclear cells, pooled together from mice in each group, or splenocytes harvested from individual mice, were analyzed by intracellular staining and FACS analysis following stimulation with PMA and ionomycin for 6 h. Representative FACS plots are gated on CD4+ T cells. The data shown is representative of three independent experiments. (D) Splenocytes obtained from MOG35-55-immunized WT (closed bars) and T-bet−/− (open bars) mice were cultured with either MOG35-55 at the indicated concentrations, PMA and anti-CD3, or medium only. Supernatants were collected at 96 hours and subjected to sandwich ELISA to measure cytokine levels. The data shown are representative of three experiments. *** P<0.001;** P<0.01; * P<0.05 compared to WT control.
IL-23 polarized T-bet−/− Th17 cells are phenotypically stable in vitro and in vivo
Collectively, these data suggested that in the absence of T-bet, inflammatory demyelination was mediated by myelin-reactive Th17 cells that resist conversion to ex-Th17 cells. In support of this hypothesis, MOG-primed T-bet−/− CD4+ T cells predominantly exhibited an IL-17+IFNγ− profile following a 96 h culture with antigen plus recombinant IL-23 and IL-1β, while a significant percent of WT CD4+ T cells cultured under the same conditions were IL-17−IFNγ+ (Fig. 2A). IL-23 polarized T-bet−/− CD4+ T cells maintained a Th17 phenotype upon secondary challenge with MOG35-55 alone (Fig 2B and C). Multiplex bead immunoassays revealed increased levels of RANTES and CXCL2 in supernatants from primary cultures of Tbet−/− Th17 cells by comparison with WT Th17 cells (Fig 2D). Conversely, the concentration of the IFNγ-induced chemokine CXCL9 was relatively low in supernatants from Tbet−/− Th17 cell cultures. T-bet−/− cells also expressed GM-CSF at a lower frequency than WT cells during primary culture (Fig. 2A). However, T-bet−/− and WT Th17 cells secreted comparable quantities of GM-CSF upon secondary challenge (Supplemental Information Fig. 1). Tbet−/− and WT Th17 cells produced similar quantities of other cytokines and chemokines implicated in EAE pathogenesis, including IL-1α, IL-6, and G-CSF (Fig 2D). The majority of T-bet−/− Th17 cells upregulated activation markers and proliferated in response to antigen to a similar extent as their WT counterparts (Fig. 2E), indicating that their failure to acquire Th1 characteristics was not a consequence of insufficient antigen presentation or TCR engagement. The fact that a relatively high percentage of T-bet−/− cells expressed a CD44+CD69+CD25+CD62Lneg profile could reflect a less differentiated state [19].
Figure 2. IL-23 polarized T-bet−/− Th17 cells are stable in vitro.
WT and T-bet−/− mice were immunized with MOG35-55/CFA. 10-14 d later, single cell suspensions of draining LN cells were cultured with MOG35-55 in the presence of IL-23, IL-1α, anti-IFN-γ and anti-IL-4. (A) At 96 hours cells were harvested, washed and stimulated with PMA and ionomycin for 6 h prior to intracellular staining and FACS analysis. Left panel, percentages of WT (black bars) and T-bet−/− (white bars) CD4+ T cells that expressed cytokines as indicated were averaged over five experiments with 4-6 donors/group. Right panel, representative FACS plots are gated on CD4+ T cells. (B) Following 96 h primary culture, LN cells were washed and re-stimulated with T-depleted syngeneic splenocytes pulsed with MOG35-55. Supernatants collected 24 and 48h later were analyzed by ELISA. (C) LN cells from primed mice were cultured for 96 h in the presence of MOG35-55 and Th17 polarizing factors after which they were rested in the absence of antigen or recombinant cytokines for an additional 96 h. They were then washed and re-stimulated with T-depleted splenocytes and MOG35-55. Cells were harvested 24h later for intracellular staining followed by FACS analysis. Representative FACS plots are gated on CD4+ T cells. (D) Supernatants from cells cultured for 96 h as in (A) were analyzed by the Luminex protein assay. (E) CFSE dilution (top panel) and activation status (bottom panel) of cells cultured as in (C). Cells were stained with CFSE prior to re-challenge with MOG35-55 (solid line) or media alone (shaded) and analyzed by FACS six days later. Activation marker expression was assessed 24 h after re-challenge. Histograms are gated on CD4+ T cells. *** P<0.001;** P<0.01; * P<0.05 compared to WT controls.
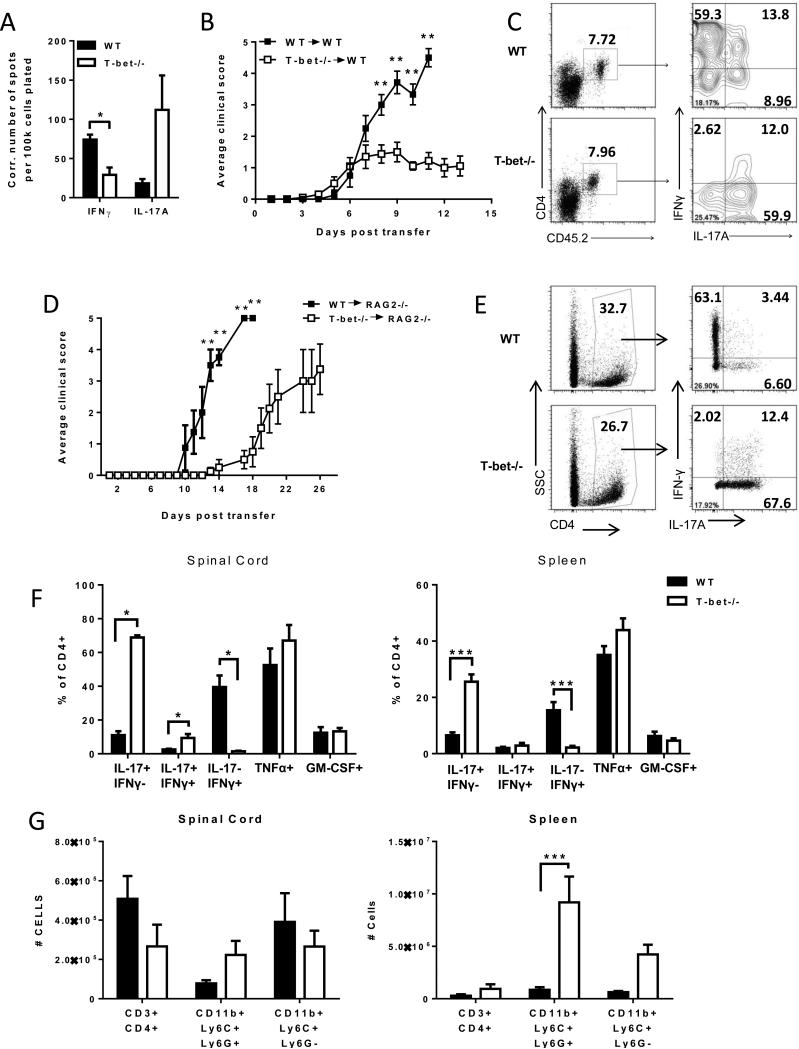
We next compared the stability of MOG-primed, IL-23 polarized T-bet−/− and WT CD4+ CD45.2+ T cells in vivo following transfer into naïve CD45.1 congenic hosts. Spleens harvested from the recipients of Tbet−/− donor cells contained a higher frequency of MOG35-55-specific IL-17 producers and a lower frequency of MOG35-55-specific IFNγ producers than spleens from recipients of WT donor cells (Fig. 3A). These stable T-bet−/− Th17 cells induced EAE in 85-90% of hosts, although disease severity was reduced compared to recipients of WT cells (Fig. 3B). IL-23 polarized T-bet−/− Th17 cells did not express FoxP3 and did not mitigate EAE severity when co-transferred with WT Th17 effectors (data not shown).
Figure 3. IL-23 polarized Tbet−/− Th17 cells are encephalitogenic.
(A-C) IL-23 polarized T-bet−/− and WT CD4+ T cells were adoptively transferred into naïve CD45.1 congenic hosts (5 × 106 CD4+ T cells/recipient). (A) Splenocytes harvested from hosts at peak disease were subjected to IL-17 and IFNγ ELISpot assays. Background spots (detected in wells that were not pulsed with antigen) were subtracted to generate the Ag-specific data shown. (B) Adoptive transfer recipients of WT (closed symbols) or Tbet−/− (open symbols) MOG-reactive Th17 cells were rated on a daily basis for degree of neurological disability. The data shown is representative of three independent experiments with 4-6 mice/group. (C) CNS mononuclear cells obtained at peak disease were analyzed for intracellular cytokine expression by FACS following stimulation with PMA and ionomycin for 6 h. Representative FACS plots are gated on CD4+CD45.2+ donor T cells. (D-G) WT (closed symbols) or Tbet−/− (open symbols) Th17 cells were transferred into naïve RAG2−/− hosts (5 × 106 CD4+ T cells/recipient). (D) Recipients were monitored on a daily basis and scored for degree of neurological disability. CNS mononuclear cells (E, F, G) and splenocytes (F, G) were harvested at peak disease and analyzed for intracellular cytokine expression (E, F) and surface marker expression (G) by FACS. Representative FACS plots for intracellular cytokine expression are gated on CD4+ donor T cells. Monocytes were defined as CD11bposLy6Cint-hiLy6Gneg. Granulocytes were defined as CD11bposLy6CintLy6Gpos. *** P<0.001;** P<0.01; * P<0.05 compared to WT controls.
FACS analysis of spinal cord mononuclear cells at peak disease indicated that the majority of infiltrating CD45.2+ T-bet−/− donor cells were IL-17+IFNγ−, while the majority of infiltrating CD45.2+ WT donor cells were IL-17−IFNγ+ (Fig. 3C).
Tbet−/− Th17 cells transfer EAE to hosts lacking endogenous B or T lymphocytes
Although T-bet−/− donor cells were enriched for the CD4+ T cell subset prior to transfer, we entertained the possibility that immunocompetent host T cells had been activated by contaminating donor APCs bearing MOG35-55/ Class II complexes. Therefore, we repeated the adoptive transfer experiments using RAG2−/− recipients. Consistent with the results obtained in immunocompetent hosts, RAG2−/− mice were susceptible to disease induced by IL-23 polarized T-bet−/− donor cells (Fig 3D). At peak disease, a very high percent of the T-bet−/− cells that had accumulated in the CNS of RAG2−/− recipients were IL-17+IFNγ− (Fig. 3E and F). Similarly, the frequency of IL-17+IFNγ− T-bet−/− cells was significantly higher than that of WT donor Th17 cells in the spleen (Fig. 3F). IFNγ single-producing T-bet−/− cells were virtually undetectable either in the spleen or the CNS. In contrast, IL-17−IFNγ+ cells were more numerous than IL-17+IFNγ− cells among the WT donor population in both the periphery and the CNS. Spleens of RAG2−/− mice that received T-bet−/− donor cells were disproportionately enlarged, primarily due to a local expansion of myeloid cells (Fig 3G, right panel). There was no difference in the absolute numbers of CD4+CD3+ T cells, granulocytes or monocytes infiltrating the spinal cords of T-bet−/− or WT hosts (Fig 3G, left panel).
Discussion
MS is a heterogeneous disease characterized by diversity in both the clinical course and in responsiveness to individual therapeutic agents. At present, no biomarkers have been identified that can guide the selection of an optimal disease modifying regimen. Strategies to manage MS are complicated by the observation that distinct myelin-reactive Th cell subsets can induce inflammatory demyelination via independent cellular and molecular pathways [1]. Therefore it is not surprising that signature Th1 and Th17 cytokines are dispensable for the manifestation of EAE [3-5]. The identification of a molecule that is critical for encephalitogenicity, irrespective of Th effector phenotype, would serve as an ideal therapeutic target. The transcription factor T-bet has been proposed as a candidate therapeutic target in MS, based on its non-redundant roles in Th1 differentiation and in Th17 plasticity. However, in the current study we show that IL-23 polarized myelin-reactive Th17 cells can mediate autoimmune demyelination without expressing T-bet or converting into Th1 (“ex-Th17”) cells. Consistent with our findings, Duhen et. al. recently reported that T-bet deficiency confined to CD4+ T cells does not confer resistance against EAE induced by active immunization with MOG peptide emulsified in CFA [20]. We found that stable Tbet−/− Th17 cells maintain the capacity to produce GM-CSF, and induce augmented production of CXCL2, each of which has been implicated in EAE pathogenesis [21-24]. In ongoing studies we are investigating whether compensatory upregulation of these factors drives the accumulation of myeloid cells (Ly6G+ granulocytes in particular) in the spleens of the recipients of Tbet−/− Th17 donor cells. Engagement of alternative chemokine/ cytokine pathways could underlie the preserved encephalitogenicity of myelin-reactive Tbet−/− Th17 cells.
We consistently found that MOG-specific Tbet−/− Th17 cells induce a milder course of EAE than their WT counterparts. This could be due to reduced production of the pro-inflammatory factor GM-CSF, as we observed in primary cultures of Tbet−/− and WT CD4+ T cells (Fig. 2A). However, we detected similar frequencies of GM-CSF+ cells among Tbet−/− and WT donor cells harvested from the CNS and peripheral lymphoid tissues of adoptive transfer recipients with EAE (Fig. 3F and data not shown). Furthermore, MOG primed, Th17 polarized Tbet−/− and WT cells produce comparable amounts of GM-CSF upon secondary challenge (Supplementary Information Fig. 1). The diminished potency of Tbet−/− donor cells could also be secondary to a failure to express adhesion molecules, such as P-selectin ligand, and chemokine receptors, such as CXCR3, that facilitate efficient CNS trafficking [25]. The delay in clinical onset that we observed following adoptive transfer of Tbet−/− effectors into RAG2−/− hosts (Fig.1 D) is consistent with that hypothesis. Finally, our experiments revealed differences in the composition of myeloid cells that were mobilized and recruited by Tbet−/− versus WT effector cells (Fig. 3G and data not shown) which could be responsible for differences in EAE severity. Each of the above possibilities is currently under investigation in our laboratory.
In conclusion, the current study contributes to a growing body of data that demonstrates that multiple parallel immunopathogenic pathways can potentiate autoimmune neuroinflammation, and it suggests that disease modifying therapies might need to be customized based on immune profiling.
Materials and Methods
Mice
8-12-wk-old C57BL/6 wild-type, CD45.1 congenic, T-bet−/− and RAG2−/− mice were obtained from the Jackson Laboratory and housed in microisolator cages under specific pathogen-free conditions. T-bet−/− and RAG2−/− mice were subsequently bred in our facility. All animal protocols were approved by the University Committee on Use and Care of Animals.
Induction of EAE
Mice were injected subcutaneously with 100 μg MOG35-55 MEVGWYRSPFSRVVHLYRNGK (Biosynthesis) in complete Freund's adjuvant (Difco). For induction of EAE by active immunization, inactivated Bordetella pertussis toxin was administered intraperitoneally on days 0 and 2.
For induction of EAE by adoptive transfer, draining lymph nodes were harvested 10-14 days post-immunization, homogenized, and passed through a 70-μm cell strainer (BD Falcon). LNCs were cultured in vitro with MOG35-55 (50 μg/ml) under conditions favorable to the generation of Th17 cells (rmIL-23, 8 ng/mL; rm IL-1α, 10 ng/ml; anti-IFNγ(clone XMG1.2), 10 μg/mL; anti-IL-4 (clone 11B11), 10 μg/mL). 2 × 106 CD4+ T cells were injected intraperitoneally, and mice were observed daily for signs of EAE as described previously [24].
Flow cytometry
Spinal cords were harvested at peak disease, homogenized in DNase (1 mg/mL) and collagenase A (2 mg/mL) and incubated for 30 min at 37 °C. Mononuclear cells were isolated over a 30/70% Percoll gradient (GE Healthcare). Splenocytes were passed through a 70-μm cell strainer, ACK lysed and washed twice prior to analysis. For intracellular staining, cells were stimulated with PMA (50 ng/mL) and ionomycin (2 μg/mL) in the presence of brefeldin A (10μg/mL) for 6 h or with MOG35-55 for 24 h. Cells were fixed with 4% paraformaldehyde and permeabilized with 0.5% saponin prior to incubation with flourochrome-conjugated antibodies. Flow cytometry was performed using a BD FacsCanto II.
ELISA
Splenocytes were cultured with or without MOG35-55 (50 μg/ml) in a 96 well plate (2 × 106 cells/ well). Supernatants were collected at serial time points and analyzed by sandwich ELISA according to the manufacturer's protocol (eBioscience). In some experiments, cell culture supernatants were analyzed using luminex protein array according to the manufacturer's instructions (Millipore).
ELISPOT assay
The frequency of antigen-specific cytokine producers was determined following culture for 24 h in 96-well filtration plates (Millipore), with or without 50 μg/ml MOG35-55. Antibodies from eBioscience were: anti-IL-17 (TC11-18H10), biotinylated anti-IL-17 (TC11-8H4), IFN-γ (AN18) and biotinylated anti-IFN-γ (R4-6A2). Streptavidin–alkaline phosphatase (Southern Biotech) and an alkaline phosphatase substrate kit (Vector Laboratories) were used to identify trapped cytokine. Spots were counted using the CTL ImmunoSpot Analyzer (Cellular Technology) with ImmunoSpot software, and the number of spots in the medium-only wells subtracted to generate the data shown.
Statistical analysis
Statistical analyses were performed using GraphPad Prism statistical analysis software. Group differences were analyzed by unpaired, two-tailed Students t test. P-values of 0.05 or less were considered significant.
Supplementary Material
Supplemental Information Figure 1. MOG-primed, Th17 polarized CD4+ T cells were rested for 4 days and rechallenged with MOG35-55 pulsed APC as described in Fig. 2C. Supernatants collected 48 hours later were subjected to the Luminex protein assay.
Acknowledgements
This research was supported by a grant from the NINDS, NIH to B.M.S. (R01 NS057670) and by the National Multiple Sclerosis Society Grant FG 1985-A-1 (S. J. L.).
Abbreviations
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- MS
multiple sclerosis
- MOG
myelin oligodendrocyte glycoprotein
- WT
wildtype
Footnotes
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J. Exp. Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, Naves R, Han M, Zhong F, Castellanos JG, Mair R, Christakos A, Kolkowitz I, Katz L, Killestein J, Polman CH, de Waal Malefyt R, Steinman L, Raman C. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 4.Haak S, Croxford AL, Kreymborg K, Heppner FL, Pouly S, Becher B, Waisman A. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest. 2009;119:61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroenke MA, Chensue SW, Segal BM. EAE mediated by a non-IFN-γ/non-IL-17 pathway. Eur J Immunol. 2010;40:2340–2348. doi: 10.1002/eji.201040489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Weiner J, Liu Y, Smith AJ, Huss DJ, Winger R, Peng H, Cravens PD, Racke MK, Lovett-Racke AE. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J Exp Med. 2009;206:1549–1564. doi: 10.1084/jem.20082584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 8.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 9.Gocke AR, Cravens PD, Ben LH, Hussain RZ, Northrop SC, Racke MK, Lovett-Racke AE. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J Immunol. 2007;178:1341–1348. doi: 10.4049/jimmunol.178.3.1341. [DOI] [PubMed] [Google Scholar]
- 10.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lexberg MH, Taubner A, Förster A, Albrecht I, Richter A, Kamradt T, Radbruch A, Chang HD. Th memory for interleukin-17 expression is stable in vivo. Eur J Immunol. 2008;38:2654–2664. doi: 10.1002/eji.200838541. [DOI] [PubMed] [Google Scholar]
- 12.Mukasa R, Balasubramani A, Lee YK, Whitley SK, Weaver BT, Shibata Y, Crawford GE, Hatton RD, Weaver CT. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32:616–627. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurschus FC, Croxford AL, Heinen AP, Wörtge S, Ielo D, Waisman A. Genetic proof for the transient nature of the Th17 phenotype. Eur J Immunol. 2010;40:3336–3346. doi: 10.1002/eji.201040755. [DOI] [PubMed] [Google Scholar]
- 15.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovett-Racke AE, Rocchini AE, Choy J, Northrop SC, Hussain RZ, Ratts RB, Sikder D, Racke MK. Silencing T-bet defines a critical role in the differentiation of autoreactive T lymphocytes. Immunity. 2004;21:719–731. doi: 10.1016/j.immuni.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Frisullo G, Angelucci F, Caggiula M, Nociti V, Iorio R, Patanella AK, Sancricca C, Mirabella M, Tonali PA, Batocchi AP. pSTAT1, pSTAT3, and T-bet expression in peripheral blood mononuclear cells from relapsing-remitting multiple sclerosis patients correlates with disease activity. J Neurosci Res. 2006;84:1027–1036. doi: 10.1002/jnr.20995. [DOI] [PubMed] [Google Scholar]
- 18.Nath N, Prasad R, Giri S, Singh AK, Singh I. T-bet is essential for the progression of experimental autoimmune encephalomyelitis. Immunology. 2006;118:384–391. doi: 10.1111/j.1365-2567.2006.02385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, Roychoudhuri R, Ferreyra GA, Shen W, Durum SK, Feigenbaum L, Palmer DC, Antony PA, Chan CC, Laurence A, Danner RL, Gattinoni L, Restifo NP. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duhen R, Glatigny S, Arbelaez CA, Blair TC, Oukka M, Bettelli E. Cutting Edge: The Pathogenicity of IFN-gamma-Producing Th17 Cells Is Independent of T-bet. J Immunol. 2013;190:4478–4482. doi: 10.4049/jimmunol.1203172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 24.Carlson T, Kroenke M, Rao P, Lane TE, Segal B. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J. Exp. Med. 2008;205:811–823. doi: 10.1084/jem.20072404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lord GM, Rao RM, Choe H, Sullivan BM, Lichtman AH, Luscinskas FW, Glimcher LH. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106:3432–3439. doi: 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Information Figure 1. MOG-primed, Th17 polarized CD4+ T cells were rested for 4 days and rechallenged with MOG35-55 pulsed APC as described in Fig. 2C. Supernatants collected 48 hours later were subjected to the Luminex protein assay.