Abstract
There are two porcine circovirus (PCV) genotypes, PCV-1 and PCV-2. In pigs, PCV-1 infection is asymptomatic but PCV-2 infection can cause severe respiratory disease and other pathology. Although humans ingest PCV-contaminated foods and are exposed to PCV through other sources, the potential of PCV-2 as a zoonotic agent in humans and other species has not been fully explored. Here, four recombinant proteins derived from the PCV-2 capsid gene were examined as antigens using the Luciferase Immunoprecipitation System (LIPS) assay for serological analysis of PCV-2 infection. PCV-2-CAP-Δ1 was the optimum recombinant protein in the LIPS assay with a sensitivity of 93% and specificity of 100% using porcine samples. Testing of healthy human blood donors, equine and bovine serum samples failed to demonstrate the presence of anti-PCV-2 antibodies. Additionally, analysis of two high-risk human groups, cystic fibrosis patients taking porcine derived oral supplements and type I diabetes patients who had undergone porcine islet cell transplantation, showed no evidence of anti-PCV-2 antibodies. These results extend the extensively demonstrated use of LIPS as a robust approach for identifying humoral responses and provide evidence that PCV-2 is not infectious in humans.
Keywords: Antibodies, porcine circovirus, PCV-2, seroepidemiology, porcine-derived supplements
1. Introduction
The porcine circovirus consists of two distinct genotypes known as PCV-1 and PCV-2, which are small, circular, single-stranded DNA viruses of less than 2 kb in size [1]. Both the genomes of PCV-1 and PCV-2 encode only two major open-reading frames, the capsid and the RNA-dependent RNA polymerase. Comparison of the PCV-1 and PCV-2 capsids demonstrates that they have approximately 65% nucleotide identity and 63–68% amino acid identity [2]. PCV-1 was originally discovered as a non-pathogenic contaminant of a porcine kidney cell line [3]. In pigs, PCV-1 infection is asymptomatic, but PCV-2 is pathogenic, causing respiratory disease, nephropathy syndromes, and enteric disease that can lead to death [4]. The exact reason for the differences in pathogenicity between PCV-1 and PCV-2 are not fully understood [1].
Besides pigs, the potential host range of porcine circoviruses is not known. Metagenomic DNA analysis has detected PCV-1 and PCV-2 in a variety of human food products including pig and beef meat [5]. PCV DNA has also been detected in human feces [5] and is found in raw sewage [6]. Using deep sequencing, Delwart and colleagues recently identified PCV-1 contamination in several batches of Rotarix, a childhood vaccine for Rotavirus [7]. PCV-1 and PCV-2 contamination in Rotarix has been confirmed by other research groups [8–10] and in one study the vaccine associated PCV-1 was shown to infect cultured porcine kidney cells [10]. Serologic studies examining potential PCV infection in humans have yielded conflicting results. In one report, low levels of PCV antibody immunoreactivity was detected in humans by immunofluorescence, Western blotting and ELISA [11]. However, two other studies found no serologic evidence of PCV-2 infection in humans [12, 13].
While antibodies against infectious agents are routinely detected using ELISA and Western blotting, these technologies suffer from high backgrounds, low signals, and often miss conformational epitopes. The Luciferase Immunoprecipitation Systems (LIPS), a highly robust technology that employs recombinant light-emitting proteins for detecting antibodies in a liquid phase assay, has been developed to address these deficiencies [14]. LIPS offers several advantages over ELISA, including the production of antigens in mammalian cells with low backgrounds and a large dynamic range of detection that often spans several orders of magnitude [14, 15]. Here we report the development of a LIPS test for detecting antibodies against the PCV-2 capsid protein and its qualification using porcine serum samples. Using this serological assay, detection of antibodies against the PCV-2 capsid was also explored in other animal and human serum samples.
2. Material and methods
2.1 Serum samples
Porcine serum samples were provided by Synbiotics (Exton, PA). These porcine serum samples (n=46) were previously tested by SERELISA® PCV2 Ab Mono Blocking ELISA (Synbiotics). Additional horse (n=20) and bovine samples (n=20) were obtained as residual samples collected for diagnostic or commercial use. No other sample identifiers, except that all animals were living in the New York State area, were provided for these samples. All serum samples were stored at −80°C, thawed, and then left at 4°C for less than a two week prior to processing by LIPS.
Three types of human samples were also analyzed for anti-PCV-2 antibodies. All were anonymized samples obtained under IRB approved protocols at the National Institutes of Health (#99-CC-0168) or the Food and Drug Administration (#08-0868D), Bethesda, Maryland. One group of human serum samples was from adult healthy blood donors (n=40), and another from cystic fibrosis patients (n=37) who chronically ingested porcine derived pancreatic supplements. The third group consisted of nine patients who had been transplanted with porcine β-cell islets as part of a therapeutic clinical trial [16].
2.2. Generation of Renilla luciferase PCV-2 capsid fragments
Based on the PCV-2 capsid sequence, several different constructs were generated by PCR. The primer adapter sequences used to clone each protein coding region are as follows: Full length PCV-2-CAP-FL, 5/-GAGGGATCCACGTATCCAAGGAGGCGT-3/ and 5/-GAGCTCGAGCATTTAGGGTTTAAGTGG-3/; PCV-2-CAP-Δ1 5/-GAGGGATCCAGGAAAAATGGCA TCTTC-3/ and 5/-GAGCTCGAGCATTTAGGGTTTAAGTGG-3/; PCV-2-CAP-Δ2, 5/-GAGGGATCCACCCGCCTCTCCCGCACC-3/ and 5/-GAGCTCGAGCATTTAGGGTTTAAGTGG-3/; and PCV-2-CAP-Δ3, 5/-GAGGGATCCCAGCTTTGGCTGAGGCTA-3/ and 5/-GAGCTCGAGCATTTAGGGTTTAAGTGG-3/. The four PCV capsid fragments were subcloned downstream of Renilla luciferase using the pREN2 vector [15] and the endogenous stop codon was included at the end of the capsid coding sequence. The plasmid DNA was then prepared using a Qiagen Midi preparation kit. DNA sequencing was used to confirm the integrity of the four different fragments.
Cos-1 cells were cultured at 5% CO2, 37°C with DMEM supplemented with 10% FCS. FuGene-6 or XtremeGene was used for transfection of the different Renilla luciferase PCV-2 capsid fusion constructs into Cos-1 cells according to the manufacturer's instructions (Roche, Indianapolis, IN). Cell extracts were obtained 48 h post-transfection in 1.0 ml of lysis buffer (50 mM Tris , pH 7.5, 100 mM NaCl, 5 mM MgCl2, 1% Triton X-100, 50% glycerol and protease inhibitors). The lysates were centrifuged twice at 12,500 g, supernatants collected and used at the time of preparation. The activities of the lysates in light units (LU)/µl were determined using a tube luminometer (20/20 from Turner Scientific) with a coelenterazine substrate mix (Promega, Madison, WI).
2.3 LIPS assay
A standard LIPS assay protocol in a 96-well format at room temperature was used to test all the serum samples [17]. Briefly, serum samples were first diluted 1:10 in assay buffer A (50 mM Tris, pH 7.5, 100 mM NaCl, 5 mM MgCl2, 1% Triton X-100) using a 96-well polypropylene microtiter plate. Antibody levels were measured by adding 40 µl of buffer A, 10 µl of diluted sera (1 µl equivalent), and 1 × 107 LU of each of the Ruc-PCV-2 capsid antigens containing crude Cos-1 cell extract to wells of a polypropylene plate and incubated for 60 minutes at room temperature on a rotary shaker. Next, 5 µl of a 30% suspension of Ultralink protein A/G beads (Pierce Biotechnology, Rockford, IL) in PBS were added to the bottom of each well of a 96-well filter HTS plate (Millipore, Bedford, MA). To this filter plate, the 100 µl antigen-antibody reaction mixture was transferred and incubated for 60 minutes at room temperature on a rotary shaker. The washing steps of the retained protein A/G beads were performed on a Biomek Workstation or Tecan plate washer with a vacuum manifold. After the final wash, LU were measured in a Berthold LB 960 Centro microplate luminometer (Berthold Technologies, Bad Wilbad, Germany) using coelenterazine substrate mix. All LU data were obtained from the average of at least two separate experiments. For the porcine and human samples, the raw LU values were directly used for analysis. For the bovine and equine samples, which were all below the cut-off, the presented values were normalized using the buffer blanks.
2.4 Data Analysis
GraphPad Prism software (San Diego, CA) was used for analysis and plotting of the data as well as for statistical analysis. For the calculation of sensitivity and specificity, the results obtained with the anti-PCV-2 ELISA from Synbiotics was used as the gold-standard comparator. The cut-off values for calculating seropositivity for both capsid fragments was calculated using the mean plus 2 standard deviation of the PCV-2 seronegative samples and matched that of a cutoff determined by receiver operator characteristics (ROC) analysis. The Mann-Whitney U test was used to test the statistical significance of the difference in antibody levels between PCV-2 positive and PCV-2 negative porcine samples.
3. Results
3.1 Expression of Renilla luciferase-PCV-2 capsid fusion proteins
Alignment of a representative PCV-1 capsid sequence with the sequence of the PCV-2 capsid template used in this study demonstrates that they show approximately 66% identity and 77% amino acid similarity (Fig. 1). In order to potentially detect antibodies against the capsid of PCV-2 by LIPS, a full length and three progressive N-terminal deletion mutants of the capsid were generated and fused with the C-terminus of Renilla luciferase (Fig 1). Following transfection of each of these constructs into Cos-1 cells, cell extracts were prepared and tested for Renilla luciferase enzymatic activity, which is a surrogate marker for production of the different recombinant proteins, as described in the Material and Methods. Only the two largest capsid constructs, PCV-2-CAP-FL and PCV-2-CAP-Δ1 (missing the first 39 amino acids), demonstrated high levels of luciferase activity with values of 3 million LU/µl and 500,000 LU/µl, respectively. The two smaller fusion proteins, PCV-2-CAP-Δ2 and PCV-2-CAP-Δ3 showed low levels of activity having values of approximately 60,000/µl and were not studied further.
Fig. 1. Comparison of the capsid protein sequences of PCV-2 with PCV-1.
BLASTP analysis shows the homology of the PCV-2 capsid fragment used in this study with the PCV-1 capsid fragment (NP_065679.1). Identical and similar amino acid residues are shown. The four deletion mutants of the PCV-2 capsid used in this study are also shown.
3.2 Identification of anti-PCV-2 capsid-specific antibodies in porcine serum samples
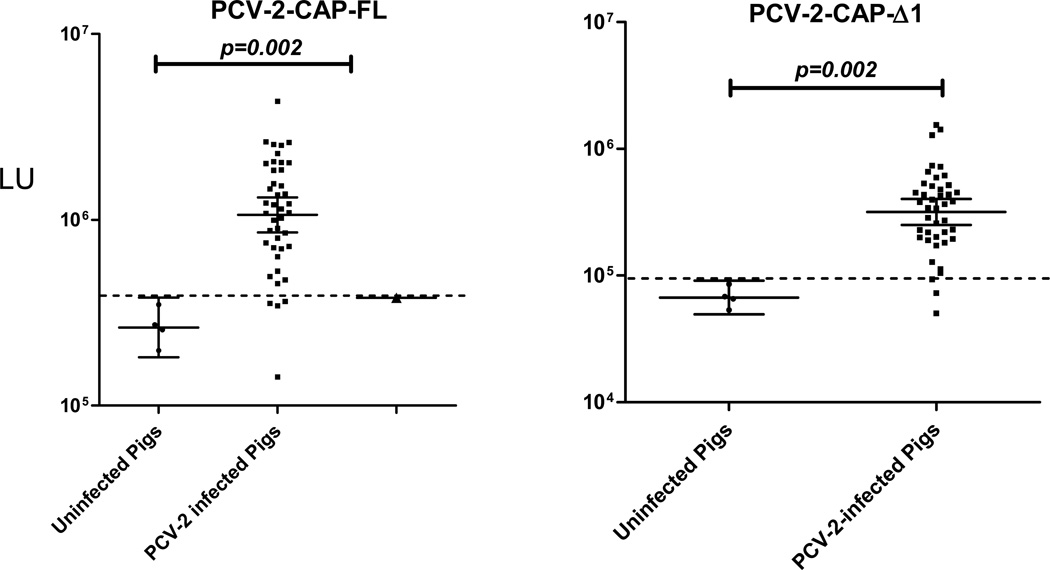
Using extracts of the PCV-2-CAP-FL and PCV-2-CAP-Δ1 Renilla luciferase fusion proteins, porcine serum samples were tested by LIPS and compared to the results obtained using the gold standard. For both the studies, the porcine serum samples (n=46) were tested by LIPS in a status-blinded fashion. Using the PCV-2-CAP-FL antigen, the 42 PCV-2 positive control porcine serum samples had a geometric mean level (GML) of 1,063,000 LU (95% CI; 856,603–1,320,000 LU) compared to 263,434 LU (95% CI; 181,801–381,721) for the 4 PCV-2 negative control porcine serum samples (Fig. 2 A), a statistically significant difference (P=0.002). Using a cut-off value set equal to the mean plus 2 standard deviations of the PCV-2 negative porcine serum samples, the assay had a sensitivity of 90% and a specificity of 100% compared to the competitive ELISA (Fig. 2A). However, comparison of the LIPS values with the antibody titers determined by ELISA showed that they correlated poorly. It should be noted that the background binding of the PCV-2-CAP-FL to the protein A/G beads in the absence of serum was unusually high (~ 300,000 LU) and not typical of other published LIPS tests.
Fig. 2.
LIPS detection of antibodies against two different Renilla luciferase fusion proteins of PCV-2. Antibodies to the PCV-2-CAP-FL and PCV-2-CAP-Δ1 were analyzed by LIPS in 46 porcine serum samples. Each symbol represents individual serum samples tested with each protein fragment and the raw LU values are shown on the Y-axis. The geometric mean and 95% confidence interval of the antibody level are also denoted. The Mann Whitney U test was used to test the statistical significance of the differences between the antibody levels in the PCV-2 positive and PCV-2 negative samples. Raw LU values are shown without subtracting background binding to protein A/G beads. The dashed line represents the diagnostic cut-off, derived from the mean plus 2 standard deviations of the PCV-2 seronegative porcine samples.
LIPS analysis of porcine antibody reactivity against the PCV-2-CAP-Δ1 fusion protein revealed that it had a profile similar to that of PCV-2-CAP-FL antigen, but the PCV-2-CAP-Δ1 antigen showed proportionally lower signals in the corresponding samples (Fig. 2). In the absence of serum, PCV-2-CAP-Δ1 fusion protein also showed much lower background binding to the protein A/G beads (~45,000 LU). As shown in Fig. 2B, using the PCV-2-CAP-Δ1 antigen, the GML of the 42 PCV positive porcine samples was 317,510 LU (95% CI; 317,500–401,800), which was significantly (p=0.002) higher than the GML of the PCV-2 negative sample value of 67,151 LU (95% CI; 49,450–91,188 LU). Based on a cut-off value of 50,000 LU, the LIPS assay demonstrated 93% sensitivity and 100% specificity using the PCV-2-CAP-Δ1 antigen. Overall, the PCV-2-CAP-Δ1 had a slightly better performance and lower background than PCV-2-CAP-FL and was used for further studies.
3.3 No serological evidence of PCV-2 infection in humans and other animals
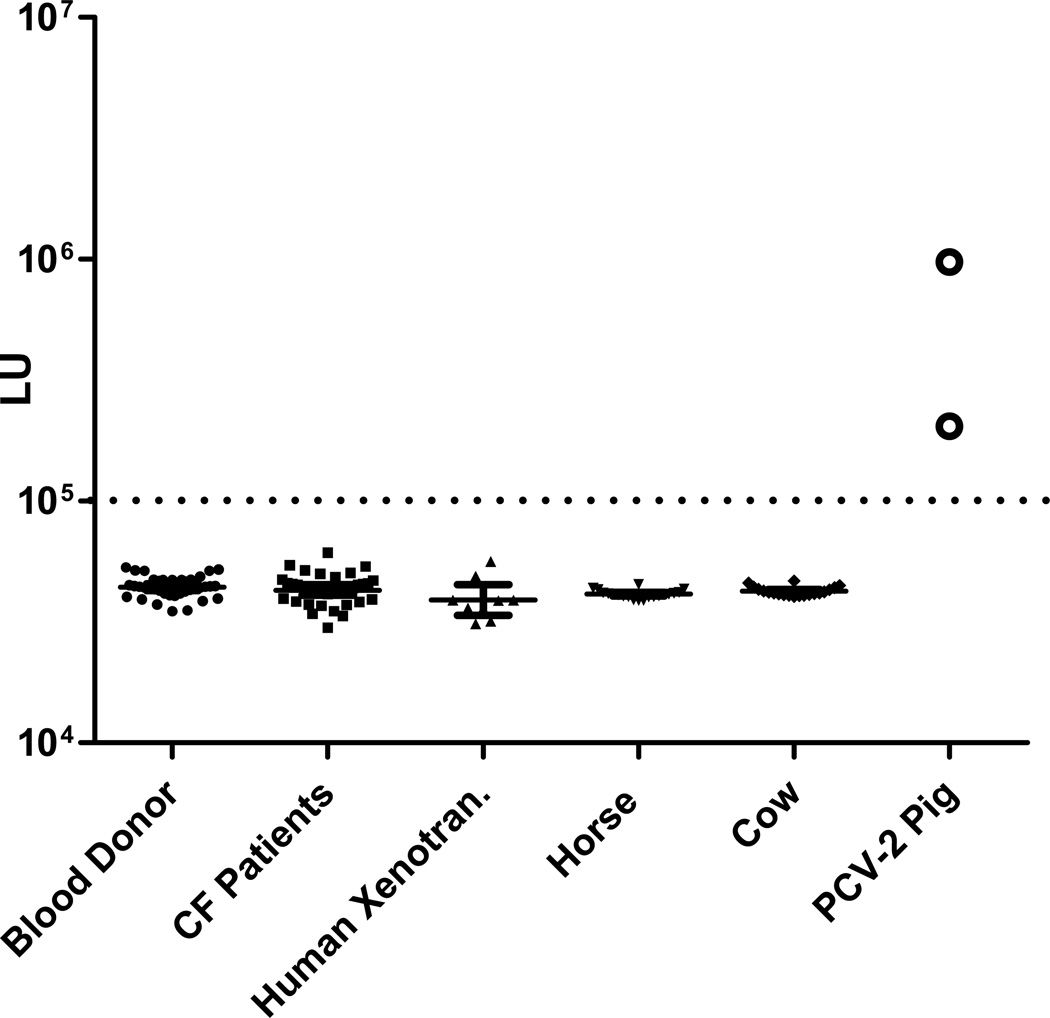
Based on the diagnostic performance of the PCV-2-CAP-Δ1 LIPS test using porcine positive and negative control samples, immunoreactivity was also explored in human samples. Testing of serum samples from 40 healthy human blood donors failed to detect any statistically significant immunoreactivity, with all samples having values below the cut-off and similar to buffer blanks (Fig. 3). In addition to healthy donors, two groups of human subjects at high risk of exposure to PCV-2 were tested for antibodies against PCV-2-CAP-Δ1. One group of samples was from cystic fibrosis patients (n=42), who had ingested long-term, porcine-derived extracts as a daily pancreatic enzymes replacement therapy [18]. The second group was from individuals (n=9) who had undergone xenotransplantation of porcine β-cells of the pancreas in a clinical trial for replacement therapy for type I diabetes [16]. Analysis of the LIPS results (Fig. 3) revealed that none of the cystic fibrosis patients or the xenotransplant recipients had levels of anti-PCV-2 antibody greater than the cut-off and had values similar to that of healthy human blood donors and buffer blanks. These results demonstrate the lack of immunoreactivity against PCV-2 in human samples at high risk for PCV exposure and suggest that PCV-2 is not an infectious agent for humans. Lastly, additional testing of serum samples from 20 cows and 20 horses also failed to detect any statistically significant immunoreactivity to PCV-2, with all samples having values below the cut-off and similar to buffer blanks (Fig. 3). Taken together, these results demonstrate that antibodies to PCV-2 are not commonly found in humans, cows or horses, thus indicating that PCV-2 infection may be restricted to pigs.
Fig. 3.
Serological screening for antibodies against PCV-2 in human serum samples. Immunoreactivity against the PCV-2-CAP-Δ1 fusion protein was determined by LIPS in serum samples from 40 US human blood donors, 40 cystic fibrosis patients (CF Patients) taking porcine enzyme supplements, 9 xenotransplant patients (Human Xenotran.) who had received porcine β-cells, 20 horses, and 20 cows. Two additional PCV-2-infected porcine serum samples were included as positive controls. The geometric mean and 95% confidence interval of the antibody level are also denoted. The dashed line represents the previously described diagnostic cut-off used in Fig. 2B.
4. Discussion
One major tool for studying the epidemiology of viruses and other infectious agents is to measure the hosts’ adaptive humoral immune response to the agents. In the case of PCV-2, several different solid phase immunoassay formats have been used to detect antibodies against recombinant PCV-2 capsid [19–22]. Here we have established a liquid phase LIPS assay for studying humoral responses to the PCV-2 capsid. Using the PCV-2-CAP fusion protein, the LIPS assay closely approximated the sensitivity (93%) and specificity (100%) of the gold standard competitive ELISA. Despite the modest homology (66%) between the capsids of PCV-2 and PCV-1, a rabbit polyclonal anti-PCV-1 capsid antibody failed to immunoprecipitate the PCV-2 fusion protein in LIPS, suggesting a lack of antibody cross-reactivity between PCV-2 and PCV-1 (data not shown). Thus, future LIPS studies specifically examining antibodies against PCV-1 in porcine, human and other animal sera are needed.
In contrast to the porcine samples, the lack of immunoreactivity in serum samples from horses and cows supports the notion that these animals are not susceptible to PCV-2. Interestingly, there are conflicting reports regarding the possibility of PCV-2 infection in cattle. Two different molecular studies have identified PCV-2 infection in cows with respiratory disease [23] and in calves with fatal hemorrhagic disease [24]. However, two other studies found no evidence of PCV-2 infection in cattle [25, 26]. In the study by Ellis et al., no evidence of PCV-2 infection was detected by serology and PCR testing in horses and cows, even after experimental PCV-2 infection of calves [25]. Possible explanations for the discrepant findings of PCV-2 infection of cows between the studies include contamination of the bovine samples with PCV-2 DNA or that only certain strains of PCV-2, which are geographically isolated, might infect cattle. Based on our limited study, there is no serological evidence of PCV-2 infection in cows or horses from the New York area.
Previous serological studies using immunofluorescence of virus-infected cells, and a competitive ELISA, failed to detect serological evidence of PCV-2 infection in humans [12, 13]. Since liquid phase immunoassays are both highly quantitative and generally able to detect more conformational epitopes than solid phase assays such as ELISA [27], a major goal of study using the liquid phase LIPS assay was to explore potential PCV-2 infection in high risk individuals. Despite the wide dynamic range of antibody levels detected in the PCV-2 infected porcine samples, no serological evidence of PCV-2 infection was detected in three types of human samples, in which the human samples resembled that of the buffer blanks. Our negative serological results with the cystic fibrosis patients suggests that the long-term ingestion of potentially contaminated porcine enzyme supplements are not a concern for these patients, at least in regard to PCV-2 infection. As far as the xenotransplant patients who had received a porcine pancreatic β-cell transplant, no evidence was also found for anti-PCV-2 antibodies in these patients. Overall, no serological evidence was found supporting PCV-2 as a human infectious agent. Our is consistent with a molecular study using a consensus-PCR approach, which did not detect porcine circovirus-like viruses in 1,101 human samples, including samples from immunosuppressed individuals [28]. While circoviruses have been detected in birds, bats and more recently in dogs [29, 30], the absence of humoral responses to PCV-2 in humans suggests that if there is circovirus in humans, it is not closely related to PCV-2. Our simple system of expressing cloned capsid fragments in LIPS makes this a likely useful system for studying immune responses to other viruses.
Acknowledgements
This work was supported by the Division of Intramural Research, National Institute of Dental and Craniofacial Research, NIH and the CDER, Food and Drug Administration and from NIH grants AI090196, AI081132 and funding from the University of Maryland. We thank Haicheng Song and Chinta Lamichhane at Synbiotics for supplying the porcine serum samples and ELISA results for the samples. We are indebted to Michael Konstan (Case Western Reserve University) for making available the cystic fibrosis serum samples and to Annika Tibell (Karolinska Univ), Bill Switzer and Walid Heneine (CDC) for the xenotransplant serum samples. We also thank Shasta McClenahan and Phil Krause (FDA) for the PCV-1 anti-serum.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mankertz A. Molecular interactions of porcine circoviruses type 1 and type 2 with its host. Virus Res. 2012;164:54–60. doi: 10.1016/j.virusres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Fenaux M, Halbur PG, Gill M, Toth TE, Meng XJ. Genetic characterization of type 2 porcine circovirus (PCV-2) from pigs with postweaning multisystemic wasting syndrome in different geographic regions of North America and development of a differential PCR-restriction fragment length polymorphism assay to detect and differentiate between infections with PCV-1 and PCV-2. J Clin Microbiol. 2000;38:2494–2503. doi: 10.1128/jcm.38.7.2494-2503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tischer I, Mields W, Wolff D, Vagt M, Griem W. Studies on epidemiology and pathogenicity of porcine circovirus. Arch Virol. 1986;91:271–276. doi: 10.1007/BF01314286. [DOI] [PubMed] [Google Scholar]
- 4.Ramamoorthy S, Meng XJ. Porcine circoviruses: a minuscule yet mammoth paradox. Anim Health Res Rev. 2009;10:1–20. doi: 10.1017/S1466252308001461. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Kapoor A, Slikas B, Bamidele OS, Wang C, Shaukat S, et al. Multiple diverse circoviruses infect farm animals and are commonly found in human and chimpanzee feces. J Virol. 2010;84:1674–1682. doi: 10.1128/JVI.02109-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blinkova O, Rosario K, Li L, Kapoor A, Slikas B, Bernardin F, et al. Frequent detection of highly diverse variants of cardiovirus, cosavirus, bocavirus, and circovirus in sewage samples collected in the United States. J Clin Microbiol. 2009;47:3507–3513. doi: 10.1128/JCM.01062-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Victoria JG, Wang C, Jones MS, Jaing C, McLoughlin K, Gardner S, et al. Viral nucleic acids in live-attenuated vaccines: detection of minority variants and an adventitious virus. J Virol. 2010;84:6033–6040. doi: 10.1128/JVI.02690-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baylis SA, Finsterbusch T, Bannert N, Blumel J, Mankertz A. Analysis of porcine circovirus type 1 detected in Rotarix vaccine. Vaccine. 2011;29:690–697. doi: 10.1016/j.vaccine.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Gilliland SM, Forrest L, Carre H, Jenkins A, Berry N, Martin J, et al. Investigation of porcine circovirus contamination in human vaccines. Biologicals. 2012;40:270–277. doi: 10.1016/j.biologicals.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 10.McClenahan SD, Krause PR, Uhlenhaut C. Molecular and infectivity studies of porcine circovirus in vaccines. Vaccine. 2011;29:4745–4753. doi: 10.1016/j.vaccine.2011.04.087. [DOI] [PubMed] [Google Scholar]
- 11.Tischer I, Bode L, Apodaca J, Timm H, Peters D, Rasch R, et al. Presence of antibodies reacting with porcine circovirus in sera of humans, mice, and cattle. Arch Virol. 1995;140:1427–1439. doi: 10.1007/BF01322669. [DOI] [PubMed] [Google Scholar]
- 12.Ellis JA, Wiseman BM, Allan G, Konoby C, Krakowka S, Meehan BM, et al. Analysis of seroconversion to porcine circovirus 2 among veterinarians from the United States and Canada. J Am Vet Med Assoc. 2000;217:1645–1646. doi: 10.2460/javma.2000.217.1645. [DOI] [PubMed] [Google Scholar]
- 13.Allan GM, McNeilly F, McNair I, Curran MD, Walker I, Ellis J, et al. Absence of evidence for porcine circovirus type 2 in cattle and humans, and lack of seroconversion or lesions in experimentally infected sheep. Arch Virol. 2000;145:853–7. doi: 10.1007/s007050050678. [DOI] [PubMed] [Google Scholar]
- 14.Burbelo PD, Ching KH, Bush ER, Han BL, Iadarola MJ. Antibody-profiling technologies for studying humoral responses to infectious agents. Expert Rev Vaccines. 2010;9:567–578. doi: 10.1586/erv.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burbelo PD, Ching KH, Mattson TL, Light JS, Bishop LR, Kovacs JA. Rapid antibody quantification and generation of whole proteome antibody response profiles using LIPS (luciferase immunoprecipitation systems) Biochem Biophys Res Commun. 2007;352:889–895. doi: 10.1016/j.bbrc.2006.11.140. [DOI] [PubMed] [Google Scholar]
- 16.Groth CG, Korsgren O, Tibell A, Tollemar J, Moller E, Bolinder J, et al. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994;344:1402–1404. doi: 10.1016/s0140-6736(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 17.Burbelo PD, Ching KH, Klimavicz CM, Iadarola MJ. Antibody profiling by Luciferase Immunoprecipitation Systems (LIPS) J Vis Exp. 2009:32. doi: 10.3791/1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borowitz D, Durie PR, Clarke LL, Werlin SL, Taylor CJ, Semler J, et al. Gastrointestinal outcomes and confounders in cystic fibrosis. J Pediatr Gastroenterol Nutr. 2005;41:273–285. doi: 10.1097/01.mpg.0000178439.64675.8d. [DOI] [PubMed] [Google Scholar]
- 19.Shibata I, Okuda Y, Yazawa S, Ono M, Sasaki T, Itagaki M, et al. PCR detection of Porcine circovirus type 2 DNA in whole blood, serum, oropharyngeal swab, nasal swab, and feces from experimentally infected pigs and field cases. J Vet Med Sci. 2003;65:405–408. doi: 10.1292/jvms.65.405. [DOI] [PubMed] [Google Scholar]
- 20.Marcekova Z, Psikal I, Kosinova E, Benada O, Sebo P, Bumba L. Heterologous expression of full-length capsid protein of porcine circovirus 2 in Escherichia coli and its potential use for detection of antibodies. J Virol Methods. 2009;162:133–141. doi: 10.1016/j.jviromet.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puvanendiran S, Stone S, Yu W, Johnson CR, Abrahante J, Jimenez LG, et al. Absence of porcine circovirus type 1 (PCV1) and high prevalence of PCV 2 exposure and infection in swine finisher herds. Virus Res. 2011;157:92–98. doi: 10.1016/j.virusres.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Jittimanee S, Nuntawan Na Ayudhya S, Kedkovid R, Teankum K, Suradhat S, Thanawongnuwech R. An indirect enzyme-linked immunosorbent assay using a recombinant truncated capsid protein of Porcine circovirus-2. J Vet Diagn Invest. 2012;24:1129–1132. doi: 10.1177/1040638712461251. [DOI] [PubMed] [Google Scholar]
- 23.Nayar GP, Hamel AL, Lin L, Sachvie C, Grudeski E, Spearman G. Evidence for circovirus in cattle with respiratory disease and from aborted bovine fetuses. Can Vet J. 1999;40:277–278. [PMC free article] [PubMed] [Google Scholar]
- 24.Kappe EC, Halami MY, Schade B, Alex M, Hoffmann D, Gangl A, et al. Bone marrow depletion with haemorrhagic diathesis in calves in Germany: characterization of the disease and preliminary investigations on its aetiology. Berl Munch Tierarztl Wochenschr. 2010;123:31–41. [PubMed] [Google Scholar]
- 25.Ellis JA, Konoby C, West KH, Allan GM, Krakowka S, McNeilly F, et al. Lack of antibodies to porcine circovirus type 2 virus in beef and dairy cattle and horses in western Canada. Can Vet J. 2001;42:461–464. [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Arrioja GM, Segales J, Domingo M, Plana-Duran J. Lack of PCV-2 infection in non-porcine species in Spain. Vet Rec. 2003;153:371–372. [PubMed] [Google Scholar]
- 27.Liu E, Eisenbarth GS. Accepting clocks that tell time poorly: fluid-phase versus standard ELISA autoantibody assays. Clin Immunol. 2007;125:120–126. doi: 10.1016/j.clim.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hattermann K, Maerz A, Slanina H, Schmitt C, Mankertz A. Assessing the risk potential of porcine circoviruses for xenotransplantation: consensus primer-PCR-based search for a human circovirus. Xenotransplantation. 2004;11:547–550. doi: 10.1111/j.1399-3089.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 29.Kapoor A, Dubovi EJ, Henriquez-Rivera JA, Lipkin WI. Complete genome sequence of the first canine circovirus. J Virol. 2012;86:7018. doi: 10.1128/JVI.00791-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Todd D. Avian circovirus diseases: lessons for the study of PMWS. Vet Microbiol. 2004;98:169–174. doi: 10.1016/j.vetmic.2003.10.010. [DOI] [PubMed] [Google Scholar]