Abstract
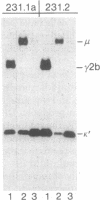
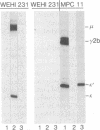
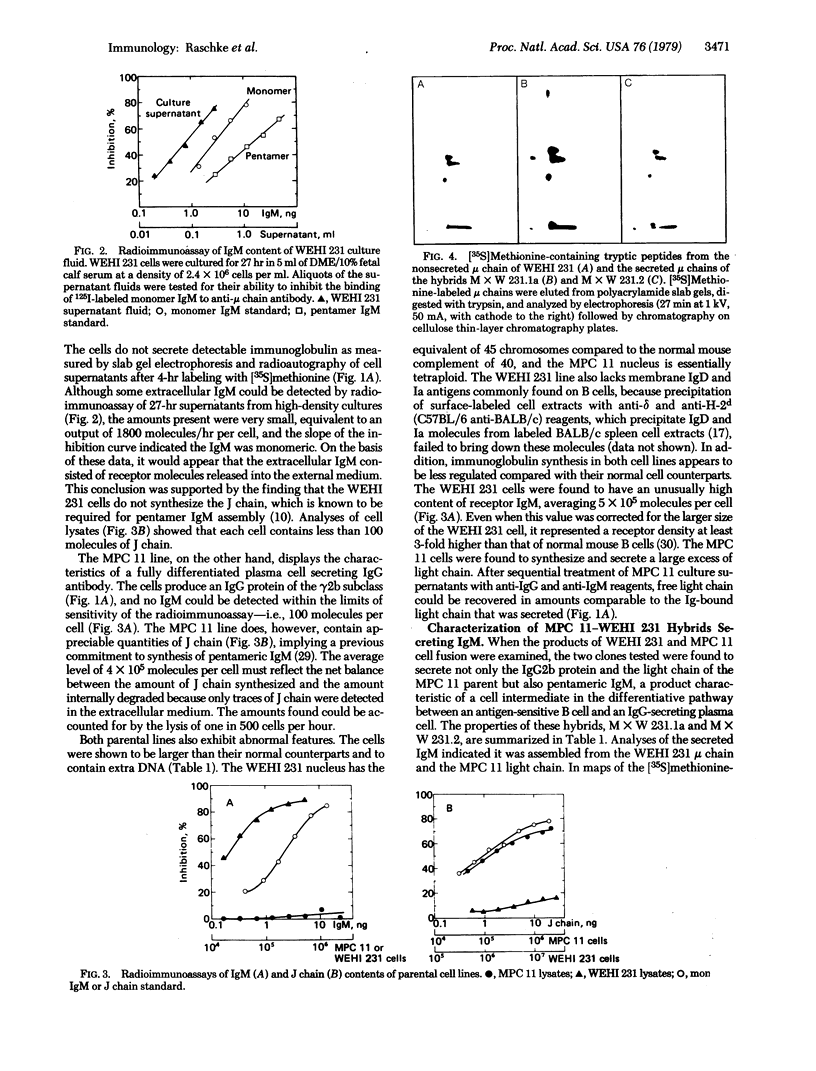
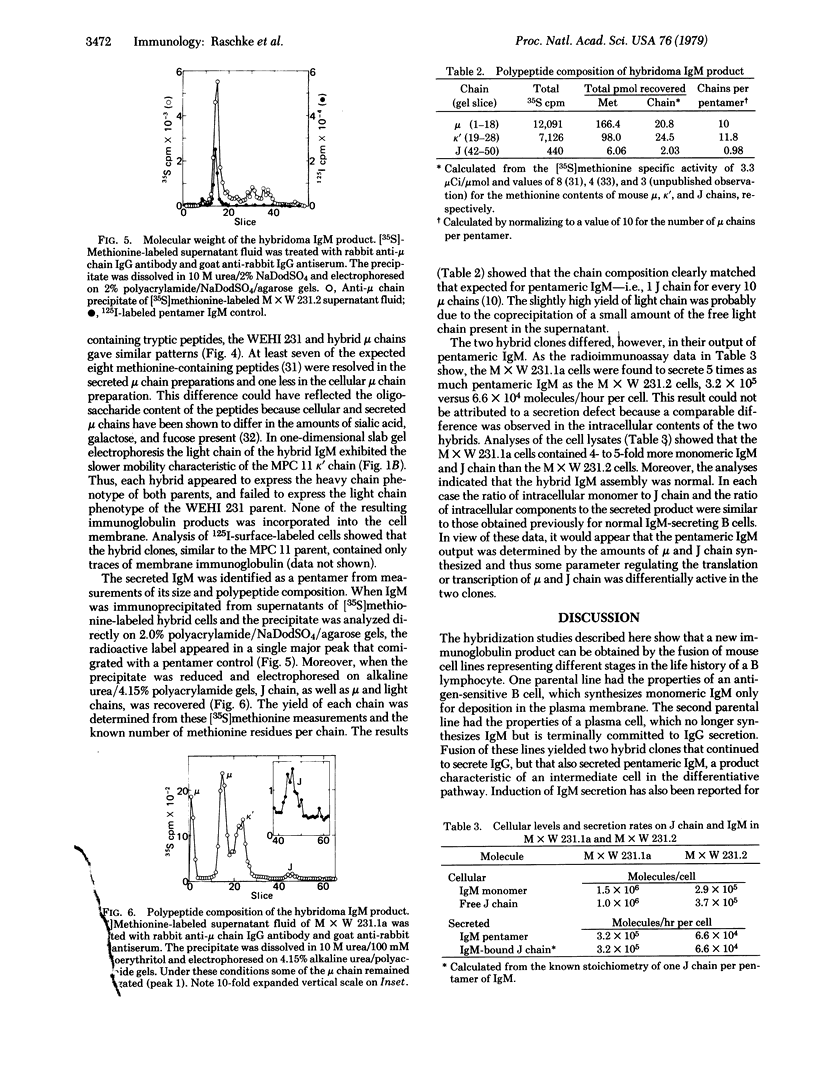
A new immunoglobulin product has been obtained by hybridization of mouse cell lines arrested at different stages in B lymphocyte development. One line was shown to have the characteristics of an undifferentiated B cell that synthesizes monomeric IgM as a membrane receptor but does not express J chain. The second line was represent of a fully differentiated plasma cell synthesizing large amounts of IgG and J chain, but no IgM. Fusion of the two cell types yielded independent hybrid clones that secreted pentameric IgM, normally the first product of antigen-driven B cell differentiation. Analyses of the hybrid cells indicated that the IgM was expressed as a result of complementation between the synthetic capacities of the parental lines. The hybrid cells synthesized both monomeric IgM and J chain and assembled these components into a pentameric molecule with the expected stoichiometry of one J chain per five monomeric units. These findings provided further evidence that the induction of B cell differentiation includes a signal for de novo synthesis of the J chain. Moreover, the complementation achieved by this hybridization provides a system for identifying other intracellular events in B cell differentiation to IgM secretion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Buxbaum J., Citronbaum R., Douglas S., Forni L., Melchers F., Pernis B., Stott D. IgM-producing tumors in the BALB-c mouse: a model for B-cell maturation. J Exp Med. 1974 Sep 1;140(3):742–763. doi: 10.1084/jem.140.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Sjöberg O., Möller G. Mitogens as probes for immunocyte activation and cellular cooperation. Transplant Rev. 1972;11:131–177. doi: 10.1111/j.1600-065x.1972.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Bergman Y., Haimovich J., Melchers F. An IgM-producing tumor with biochemical characteristics of a small B lymphocyte. Eur J Immunol. 1977 Aug;7(8):574–579. doi: 10.1002/eji.1830070815. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Stedman J. D. Efficient fluorography of 3H and 14C on thin layers. Anal Biochem. 1978 Aug 15;89(1):247–256. doi: 10.1016/0003-2697(78)90747-9. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Koshland M. E. Evidence for a long-range conformational change induced by antigen binding to IgM antibody. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5682–5686. doi: 10.1073/pnas.74.12.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crissman H. A., Tobey R. A. Cell-cycle analysis in 20 minutes. Science. 1974 Jun 21;184(4143):1297–1298. doi: 10.1126/science.184.4143.1297. [DOI] [PubMed] [Google Scholar]
- Davidson R. L. Gene expression in somatic cell hybrids. Annu Rev Genet. 1974;8:195–218. doi: 10.1146/annurev.ge.08.120174.001211. [DOI] [PubMed] [Google Scholar]
- Dennert G., Raschke W. Continuously proliferating allospecific T cells, lifespan and antigen receptors. Eur J Immunol. 1977 Jun;7(6):352–359. doi: 10.1002/eji.1830070606. [DOI] [PubMed] [Google Scholar]
- Dingman C. W., Peacock A. C. Analytical studies on nuclear ribonucleic acid using polyacrylamide gel electrophoresis. Biochemistry. 1968 Feb;7(2):659–668. doi: 10.1021/bi00842a022. [DOI] [PubMed] [Google Scholar]
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Colón S., Campbell P., Rabellino E. Immunoglobulins on the surface of lymphocytes. V. Quantitative studies on the question of whether immunoglobulins are associated with T cells in the mouse. J Immunol. 1972 Oct;109(4):776–783. [PubMed] [Google Scholar]
- Hutchinson M. A., Hunter T., Eckhart W. Characterization of T antigens in polyoma-infected and transformed cells. Cell. 1978 Sep;15(1):65–77. doi: 10.1016/0092-8674(78)90083-1. [DOI] [PubMed] [Google Scholar]
- Hyman R., Stallings V. Complementation patterns of Thy-1 variants and evidence that antigen loss variants "pre-exist" in the parental population. J Natl Cancer Inst. 1974 Feb;52(2):429–436. doi: 10.1093/jnci/52.2.429. [DOI] [PubMed] [Google Scholar]
- Kaji H., Parkhouse R. M. Intracellular J chain in mouse plasmacytomas secreting IgA, IgM and IgG. Nature. 1974 May 3;249(452):45–47. doi: 10.1038/249045a0. [DOI] [PubMed] [Google Scholar]
- Kehry M., Sibley C., Fuhrman J., Schilling J., Hood L. E. Amino acid sequence of a mouse immunoglobulin mu chain. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2932–2936. doi: 10.1073/pnas.76.6.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Koshland M. E. Structure and function of the J chain. Adv Immunol. 1975;20:41–69. doi: 10.1016/s0065-2776(08)60206-0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Levy R., Dilley J. Rescue of immunoglobulin secretion from human neoplastic lymphoid cells by somatic cell hybridization. Proc Natl Acad Sci U S A. 1978 May;75(5):2411–2415. doi: 10.1073/pnas.75.5.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies D. H., Kuehl W. M., Scharff M. D. Somatic cell hybridization of mouse myeloma cells. Cell. 1976 Jul;8(3):405–415. doi: 10.1016/0092-8674(76)90153-7. [DOI] [PubMed] [Google Scholar]
- Melchers F., Andersson J. IgM in bone marrow-derived lymphocytes. Changes in synthesis, turnover and secretion, and in numbers of molecules on the surface of B cells after mitogenic stimulation. Eur J Immunol. 1974 Mar;4(3):181–188. doi: 10.1002/eji.1830040306. [DOI] [PubMed] [Google Scholar]
- Melchers F. Difference in carbohydrate composition and a possible conformational difference between intracellular and extracellular immunoglobulin M. Biochemistry. 1972 May 23;11(11):2204–2208. doi: 10.1021/bi00761a031. [DOI] [PubMed] [Google Scholar]
- Mosier D. E. A requirement for two cell types for antibody formation in vitro. Science. 1967 Dec 22;158(3808):1573–1575. doi: 10.1126/science.158.3808.1573. [DOI] [PubMed] [Google Scholar]
- Mosmann T., Baumal R. Synthesis but not secretion of J chain by variant mouse myeloma cells which lose alpha-chain-synthesizing ability. J Immunol. 1975 Oct;115(4):955–962. [PubMed] [Google Scholar]
- POTTER M., FAHEY J. L. Studies on eight transplantable plasma-cell neoplasms of mice. J Natl Cancer Inst. 1960 May;24:1153–1165. [PubMed] [Google Scholar]
- Raschke W. C. Expression of murine IgM, IgD and Ia molecules on hybrids of murine LPS blasts with a Syrian hamster B lymphoma. Curr Top Microbiol Immunol. 1978;81:70–76. doi: 10.1007/978-3-642-67448-8_12. [DOI] [PubMed] [Google Scholar]
- Reisfeld R. A., Small P. A., Jr Electrophoretic heterogeneity of polypeptide chains of specific antibodies. Science. 1966 May 27;152(3726):1253–1255. doi: 10.1126/science.152.3726.1253. [DOI] [PubMed] [Google Scholar]
- Sekiguchi T., Sekiguchi F. Interallelic complementation in hybrid cells derived from Chinese hamster diploid clones deficient in hypoxanthine-guanine phosphoribosyl-transferase activity. Exp Cell Res. 1973 Mar 15;77(1):391–403. doi: 10.1016/0014-4827(73)90593-4. [DOI] [PubMed] [Google Scholar]
- Smith G. P. Sequence of the full-length immunoglobulin kappa-chain of mouse myeloma MPC 11. Biochem J. 1978 May 1;171(2):337–347. doi: 10.1042/bj1710337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C. E., 3rd, Koshland M. E. Molecular size and shape of the J chain from polymeric immunoglobulins. Biochemistry. 1973 Aug 14;12(17):3218–3224. doi: 10.1021/bi00741a012. [DOI] [PubMed] [Google Scholar]