Abstract
Background
Berberine is an isoquinoline alkaloid widely used to improve the glucidic and lipidic profiles of patients with hypercholesterolemia, metabolic syndrome, and type 2 diabetes. The limitation of berberine seems to be its poor oral bioavailability, which is affected by the presence, in enterocytes, of P-glycoprotein – an active adenosine triphosphate (ATP)-consuming efflux protein that extrudes berberine into the intestinal lumen, thus limiting its absorption. According to some authors, silymarin, derived from Silybum marianum, could be considered a P-glycoprotein antagonist.
Aim
The study aimed to evaluate the role played by a possible P-glycoprotein antagonist (silymarin), when added to a product containing Berberis aristata extract, in terms of benefits to patients with type 2 diabetes.
Methods
The study enrolled 69 patients with type 2 diabetes in suboptimal glycemic control who were treated with diet, hypoglycemic drugs, and in cases of concomitant alterations of the lipid profile, hypolipidemic agents. The patients received an add-on therapy consisting of either a standardized extract of Berberis aristata (titrated in 85% berberine) corresponding to 1,000 mg/day of berberine, or Berberol®, a fixed combination containing the same standardized extract of Berberis aristata plus a standardized extract of Silybum marianum (titrated as >60% in silymarin), for a total intake of 1,000 mg/day of berberine and 210 mg/day of silymarin.
Results
Both treatments similarly improved fasting glucose, total cholesterol, low-density lipoprotein (LDL) cholesterol, triglyceride, and liver enzyme levels, whereas glycosylated hemoglobin (HbA1c) values were reduced to a greater extent by the fixed combination.
Conclusion
The association of berberine and silymarin demonstrated to be more effective than berberine alone in reducing HbA1c, when administered at the same dose and in the form of standardized extracts in type 2 diabetic patients.
Keywords: Berberol®, P-glycoprotein, cholesterol, triglycerides, glycosylated hemoglobin
Introduction
Type 2 diabetes mellitus (T2DM) has continued to increase worldwide, reaching a figure of about 370 million in 2011, with 4.6 million associated deaths per year.1 The standard therapy for this epidemic disease includes diet, exercise, use of oral hypoglycemic drugs, and/or subcutaneous insulin injections.2,3 Several multicenter trials have demonstrated that different pharmacological agents can successfully lower blood glucose and reduce the risk of developing microvascular and macrovascular diabetic complications. However, the large number of limitations and unwanted side effects that still exist limit their use in clinical practice.4,5 Consequently, a small, albeit significant, proportion of diabetic patients are also advised to resort to complementary and alternative medical therapies. Among the effective herbal derivatives, berberine has aroused great interest for its glucose-lowering6–9 and lipid-lowering activity.10,11 Berberine has been demonstrated to be effective in diabetic patients, for whom it has been shown to significantly decrease fasting and postprandial blood glucose and glycosylated hemoglobin (HbA1c) levels. Its effect is similar to that of metformin,12 although it acts with partly different mechanisms, ie, by increasing insulin receptor expression.13 Berberine regulates glucose metabolism through multiple mechanisms of action: 1) stimulation of glucose uptake by glucose transporter type 4 (GLUT-4) upregulation; 2) activation of 5′ adenosine monophosphate (AMP)-activated protein kinase (AMPK), as a consequence of the inhibition of mitochondrial function; 3) suppression of adipogenesis, by inhibiting peroxisome proliferator-activated receptor gamma (PPARγ) and C-enhancer-binding protein alpha (C/EBPα) function; 4) stimulation of glucagon-like peptide-1 (GLP-1) release from ileal cells; 5) suppression of human protein tyrosine phosphatase 1B (h-PTP 1B); 6) stimulation of the pancreatic G protein-coupled receptor 40 (GPR40); and 7) reduction of intestinal glucose absorption, by inhibiting α-glucosidase activity.14 With regard to the lipid profile, berberine upregulates low-density lipoprotein (LDL)-receptor (LDL-r) expression independent of sterol regulatory element-binding proteins but dependent on extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) activation, which results in total cholesterol (C) and LDL-C reduction (by about 30% and 25%, respectively). This upregulation occurs through a posttranscriptional mechanism that stabilizes messenger ribonucleic acid (mRNA), making berberine a cholesterol-lowering drug endowed with a different mechanism of action from that of statins.15 Along with its cholesterol-lowering properties, berberine also reduces triglycerides by about 35%. These actions on the lipid profile have been observed in both animals and humans.15,16 Berberine, an isoquinoline alkaloid of the protoberberine type found in a variety of plants, has been used in Indian and Chinese medicines for many decades. It is found in Hydrastis canadensis (goldenseal), Coptis chinensis (Coptis or goldthread), Berberis aquifolium (Oregon grape), Berberis vulgaris (barberry), and Berberis aristata (tree turmeric).17 In spite of its functions as a glucose-and lipid-lowering agent, berberine remains rather defective in terms of its oral bioavailability.18 In humans, this appears to be due to a P-glycoprotein (P-gp)-mediated gut extrusion process19 and a massive biliary excretion.20 The amount of berberine capable of crossing enterocytes seems to be reduced by about 90% by P-gp, and this suggests that either the use of a potential P-gp inhibitor21 or a chemical modification of berberine that would allow it to overcome P-gp antagonism22 might enhance its poor oral bioavailability, thus increasing its clinical effectiveness. Among the potential P-gp inhibitors, silymarin (derived from Silybum marianum), a herbal drug traditionally used as a liver protectant, could be considered a good candidate, owing to its very poor oral bioavailability and very high safety profile.23 We therefore decided to test the clinical role played by silymarin when added to berberine in the treatment of glycemic and lipid alterations in patients with T2DM.
Materials and methods
Study design
This 4-month, single-blind, randomized, controlled clinical trial was conducted in the setting of routine clinical practice, in accordance with the principles stated in the Declaration of Helsinki and consistent with Good Clinical Practice, as defined by the International Conference on Harmonization and in accordance with the ethical principles underlying European Union Directive 2001/20/EC and the United States Code of Federal Regulations, Title 21, Part 50 (21CFR50).24 The protocol and subject consent and privacy forms were approved by the local review board before the study began. It was carried out in a single center in Italy between October 2012 and May 2013. Suitable patients, identified from the review of case notes and/or computerized clinic registers, were contacted by the investigators while at the center or by telephone. Sixty-nine patients diagnosed with T2DM were enrolled. All patients provided their written informed consent to participate in this study after a full explanation of the study had been given. Sixty-three participants completed the study.
Criteria
The inclusion criteria were: 1) providing informed consent, with a signed and returned privacy agreement; 2) history of continuous (for 3 months) suboptimal glycemic control (HbA1c ranging between 7.0% and 9.0%); 3) age between 25 and 75 years; and 4) a negative pregnancy test, for female patients. The exclusion criteria were: 1) refusal to sign the informed consent or privacy agreement; 2) moderate-to-severe liver disorders, including serum alanine amino-transferase and aspartate amino-transaminase greater than threefold the upper limit of normality and/or abnormal renal function (serum creatinine greater than 115 μmol/L); 3) severe heart dysfunction (New York Heart Association25 class phase III or higher); 4) history of acute diabetic complications, including diabetic ketoacidosis or hyperosmolar hyperglycemic nonketotic coma; 5) psychiatric disease or severe infection; 6) pregnancy or planned pregnancy; 7) fasting plasma glucose with a value of 200 mg/dL or higher; and 8) insulin therapy and use of P-gp antagonists.
Study protocol and treatments
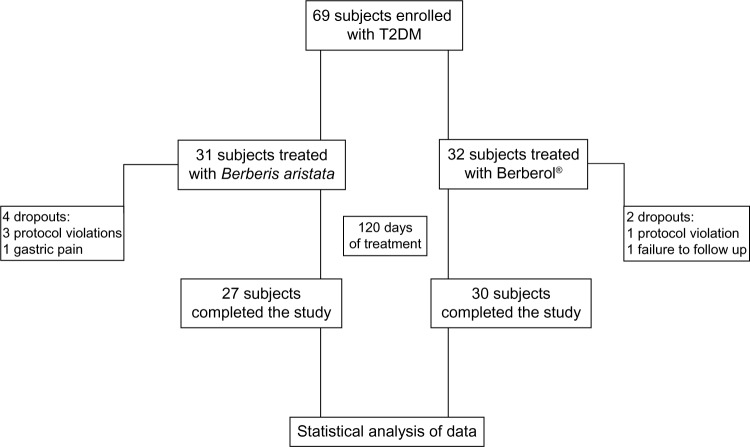
The study scheme is presented in Figure 1. All participants were instructed to follow their usual hypocaloric, low-glycemic index diet throughout the study. The controlled-energy diet (a daily caloric deficit of about 600 kcal) was based on the National Cholesterol Education Program Adult Treatment Panel (NCEP-ATP) III recommendations26 and contained 50% of calories from carbohydrates, 30% from fat (<7% saturated fat, up to 10% polyunsaturated fat, and up to 20% monounsaturated fat), and 20% from proteins, with a maximum cholesterol content of 300 mg/day and 35 g/day of fiber. Standard diet advice was provided by a dietician and/or specialist physician. The participants were also encouraged to maintain their usual standard physical activity (riding a stationary bike for 20 to 30 minutes, three to four times a week or brisk walking for 30-minute sessions, three to four times a week). All the enrolled subjects were randomized by an independent investigator, using a computer-generated random number table, to either of two groups: one receiving Berberis aristata and one receiving Berberis aristata plus Silybum marianum tablets. All the patients of the Berberis aristata group received a galenic preparation containing a standardized extract corresponding to 500 mg of pure berberine per tablet. All the patients of the Berberis aristata group took 1 tablet on an empty stomach twice a day (before breakfast and dinner) for the whole length of the study (120 days). All the Berberis aristata tablets were prepared by the same pharmacist and herbal specialist authorized to manufacture galenic preparations according to a medical prescription. All the patients of the Berberis aristata/Silybum marianum group received the add-on oral therapy in a nutraceutical combination, in tablet form (Berberol®; PharmExtracta srl, Pontenure, Italy), containing 588 mg/tablet Berberis aristata extract (titrated in 85% berberine) plus 105 mg/tablet Silybum marianum extract (titrated in >60% flavanolignans). The two active ingredients of the product were provided by SIIT srl (Berberis aristata extract) and Indena (Silybum marianum extract), both of Milan, Italy. The product, in agreement with Italian law, was registered with the Ministry of Health, in 2010 (Registration number: E10 40753Y), as a food supplement, with both active ingredients (Berberis aristata and Silybum marianum standardized extracts) belonging to the list of accepted botanical nutraceuticals, and with all excipients of food grade. Like the patients of the Berberis aristata group, the Berberol patients consumed 1 tablet on an empty stomach twice a day (before breakfast and dinner) for the whole length of the study (120 days). All participants of both groups were instructed to record the onset of any adverse events in a personal daily document, with the specific description of their symptoms (including severity, duration, and possible cause-effect relationship with drug administration), the number of missed tablets, and any changes in diet, physical exercise, or weight.
Figure 1.
Scheme of the study.
Abbreviation: T2DM, type 2 diabetes mellitus.
Concomitant antidiabetic therapies
The glycemic control of the participants of both groups was suboptimal despite a prescribed diet, physical exercise, and/or hypoglycemic drugs. On enrollment, among the patients in the Berberis aristata group, five were only treated with diet and without any antidiabetic drug, nine were on metformin monotherapy, two were on sulphonylurea monotherapy, and 15 were on oral combination therapy (eleven with metformin and sulphonylureas, two with metformin plus dipeptidyl peptidase-4 [DPP-4] inhibitors, one patient with metformin plus pioglitazone, and one patient with metformin plus sulphonylurea and pioglitazone). Sixteen patients in the Berberis aristata group were on statin monotherapy, three were on a combination therapy (two with a statin plus ezetimibe and one with statin plus omega-3 oil), and one patient was taking a fibrate. Eleven participants were not taking any hypolipidemic treatment. On enrollment, among the patients in the fixed combination group (Berberis aristata plus Silybum marianum), four were treated with diet without any antidiabetic drug, four were on metformin monotherapy, one was on sulphonylurea monotherapy, and 23 were on oral combination therapy (of which four were treated with metformin plus sulphonylureas, five with metformin plus DPP-4 inhibitors, one with metformin plus pioglitazone, one with sulphonylurea plus DPP-4 inhibitor, four with metformin plus sulphonylurea and pioglitazone, four with metformin plus sulphonylurea and DPP-4 inhibitors, one with metformin plus pioglitazone and DPP-4 inhibitor, and three with metformin plus sulphonylurea, pioglitazone and acarbose). Twenty-three patients in the Berberis aristata plus Silybum marianum group were on statin monotherapy, and one patient was taking a fibrate. Eight participants were not taking any hypolipidemic treatment.
Assessments
Before starting the study, all patients underwent an initial screening assessment that included medical history, physical examination, vital signs (blood pressure and heart rate), a 12-lead electrocardiogram, measurement of height and body weight, calculation of body mass index (BMI), abdominal circumference (waistline, WL), assessment of fasting blood glucose (FG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG). After 120 treatment days the following parameters were evaluated: weight, BMI, WL, HbA1c, FG, TC, LDL-C, HDL-C, TG, aspartate aminotransferase (AST) and alanine aminotransferase (ALT). In all participants, the BMI was calculated, by the investigators, as the weight in kilograms divided by the square of the height in meters. The WL was measured midway between the lateral lower rib margin and the iliac crest, and its reduction was determined with a Gulick anthropometric spring-loaded tape measure (Model 5829; Bell Medical Services, Neptune, NJ, USA).
Plasma analysis
All plasmatic variables were determined after a 12-hour overnight fast. Venous blood samples were drawn from all patients between 8:00 and 9:00 am. We used the plasma obtained by adding 1 mg/mL Na-ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, St Louis, MO, USA) and centrifuged at 3000 rpm for 15 minutes at 4°C. The plasma samples were analyzed immediately after centrifugation. The blood samples were drawn by laboratory technicians, and the assays performed by the biologist in charge of the laboratory. All measurements were performed in a laboratory authorized by the Italian Ministry of Health.
Safety measures
Treatment tolerability was assessed through interviews with the patients by the investigators and the comparison of clinical and laboratory values with baseline levels. Safety monitoring included physical examination, vital sign assessment, weight, electrocardiogram, and adverse event recording.
Statistical analysis
According to the two variables affecting the results – treatment (Berberis aristata versus Berberol) and time (before versus after) – the statistical analysis used was the between-within subject design analysis of variance (ANOVA) and analysis of covariance (ANCOVA). A multiple comparison test (Tukey honestly significant difference [HSD])27 was used to analyze the possible differences between the average values during the observation period. The α level was set at 0.05, and values were considered significant at P<0.05. NCSS 8 Statistical Analysis and Graphics (NCSS, LLC, Kaysville, UT, USA) and JMP 10 (SAS Institute, Inc, Cary, NC, USA) were the two software used for analysis.
Results
A total of 69 patients were enrolled in the trial. Of these, 63 completed the study. A total of 31 were randomized to receive Berberis aristata, and 32 were randomized to receive Berberol. During the study, six patients (four males and two females) did not complete the study, and the reasons for premature withdrawal included protocol violation, failure to follow up, or noncompliance. Specifically, four patients belonged to the Berberis aristata group and two patients to the Berberol group. The characteristics of the sample population completing the study are shown in Table 1. As mentioned in the Materials and methods section, once the enrolment and randomization procedures had been completed, the individuals enrolled in the study started their oral, 120-day treatment with either Berberis aristata or Berberol. The results are shown in Table 2. At time =0 (“Before” in Table 2), the baseline parameters did not show any statistically significant difference between groups. Both treatments did not modify weight, WL, or BMI but significantly reduced, respectively for Berberis aristata and Berberol, fasting glycemia (−19.05% and −18.13%) and HbA1c (−7.18% and −12.35%), with the latter difference being statistically significant for Berberol. With regard to the lipid profile, both Berberis aristata and Berberol significantly reduced TC and TG, whereas only Berberol significantly decreased LDL (−16.92%), with no significant differences between treatments. AST and ALT were equally reduced in both groups. In terms of safety, no patients reported any serious adverse events. In both groups, about 15% reported a mild transient abdominal discomfort. None of the patients experienced any musculoskeletal disorders, such as myopathy, or showed clinical signs of liver toxicity.
Table 1.
Features of participants on enrolment
| Berberis aristata (N=31) | Berberol® (N=32) | |
|---|---|---|
| Sex (males/females) | 16/15 | 15/17 |
| Age (years) | 66.35±9.8 | 67.85±10.81 |
| Height (cm) | 165±11 | 162±10 |
| Diagnosis of T2DM (months) | 142±14 | 149±16 |
Note: All values are expressed as median ± standard deviation.
Abbreviation: T2DM, type 2 diabetes mellitus.
Table 2.
Effects of an oral add-on therapy, in patients with T2DM with Berberis aristata or Berberol®, after 120 day of treatment
| Parameter |
Berberis aristata (A)
|
P | Berberol® (B)
|
P | B vs A (after)
|
P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Variation (%) | Before | After | Variation (%) | Variation (%) | ||||
| Weight (kg) | 80.55±19.38 | 80.84±17.65 | 0.36 | ns | 76.81±20.55 | 77.86±16.38 | 1.36 | ns | −3.69 | ns |
| BMI (Kg/m2) | 30.53±6.85 | 30.22±7.01 | −1.07 | ns | 29.90±7.20 | 29.25±5.33 | −2.18 | ns | −3.21 | ns |
| WL (cm) | 100.32±17.19 | 95.43±31.50 | −5.88 | ns | 98.81±14.27 | 96.05±11.18 | −2.80 | ns | 0.64 | ns |
| FG (mg/dL) | 157.34±28.22 | 128.95±3001 | −19.05 | 0.006 | 158.32±34.39 | 131.20±31.70 | −18.13 | 0.007 | 1.74 | ns |
| HbA1c (%) | 7.81±0.88 | 7.25±0.39 | −7.18 | <0.001 | 8.02±0.35 | 7.03±0.27 | −12.35 | <0.001 | −3.04 | <0.05 |
| TC (mg/dL) | 179.81±23.98 | 158.30±35.23 | −11.97 | 0.007 | 177.54±31.65 | 157.93±32.05 | −11.05 | 0.005 | −0.24 | ns |
| HDL–C (mg/dL) | 50.98±13.66 | 49.22±14.19 | −3.46 | ns | 51.88±12.65 | 50.39±14.25 | −2.88 | ns | 2.37 | ns |
| LDL–C (mg/dL) | 96.75±27.69 | 85.02±28.52 | −12.13 | ns | 94.39±29.81 | 78.37±28.19 | −16.92 | 0.004 | −7.83 | ns |
| TG (mg/dL) | 157.32±91.29 | 123.99±78.00 | −21.57 | 0.003 | 155.44±71.22 | 120.33±65.77 | −22.59 | 0.002 | −2.96 | ns |
| AST (U/L) | 28.35±19.33 | 22.88±16.56 | −19.30 | 0.032 | 27.34±12.86 | 21.66±17.34 | −20.88 | 0.018 | −5.34 | ns |
| ALT (U/L) | 32.64±22.31 | 28.39±17.29 | −13.03 | 0.046 | 34.00±23.53 | 29.51±19.83 | −13.21 | 0.040 | 3.94 | ns |
Note: All parameters are expressed as median ± standard deviation.
Abbreviations: ALT, alanine amino-transferase; AST, aspartate amino-transaminase; BMI, body mass index; FG, fasting glucose; HbA1c, glycated hemoglobin; HDL, high density lipoprotein; LDL, low density lipoprotein; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglycerides; WL, waistline.
Discussion
Several studies, mostly performed in the Chinese population, have reported the effects of berberine on the lipidic and glycemic profile,10,11 but very few have reported on the effects of berberine in Caucasians.28–30 At a careful reading, these studies reveal a number of slight differences in the effects of berberine in the Chinese and Caucasians, which probably reflect a genotype/ethnic difference. One of the most important differences is surely efficacy, which seems to be more apparent in the Chinese than in Caucasian subjects. This fact has prompted many formulators to develop compounds of berberine and other herbal/natural ingredients, with the most widely exploited combination being that of berberine with Monascus purpureus. However, these formulations do not consider the low standardization profile of the Monascus raw material or the patient risk arising from the possible presence of mycotoxic contaminants, such as citrinin.31–34 Nevertheless, the association of berberine and Monascus offers real advantages in terms of its capacity to reduce TC, LDL, and TG.35 This is likely due to the berberine downmodulation of proprotein convertase subtilisin/kexin type 9 (PCSK9), a protein reducing the cholesterol-lowering properties of statins.36 This effect of berberine may be of great importance in the context of hypercholesterolemia but is useless when administered to hyperglycemic patients. Berberine is, anyway, rather defective in terms of oral bioavailability, and this appears to be mainly due to the P-gp-mediated gut extrusion process. The results obtained by Shan et al,22 using the berberine analog IMB-Y53, suggest that overcoming the cellular efflux and in particular, the efflux linked to the P-gp function improves berberine bioavailability and its hypoglycemic effect. Chemical modifications of the berberine skeleton and the use of oral bioavailability enhancers coadministered with berberine may represent possible improvements. Among these, silymarin, also known as milk thistle extract, is a herbal drug that has been known for years as a liver protectant. In the standardized form, titrated in flavanolignans, silymarin also suffers from poor oral bioavailability.37,38 In addition, silymarin has been proposed as an enterocyte P-gp antagonist,23 and thus, its use could prove useful to improve berberine efficacy. On this basis, we developed a preparation, Berberol, which contains both Berberis aristata (titrated in 85% berberine) and Silybum marianum (containing silymarin). An early pilot study conducted in statin-intolerant Caucasians39 had demonstrated that the addition of 210 mg silymarin to 1,000 mg berberine, as sole therapy, exerted extremely effective results on the lipid profile (−30% in TC), which had often been described in literature as obtainable only through the administration of 1,500 mg.8–10 We thus decided to verify, in a larger sample enrolled on the base of a T2DM diagnosis, whether the addition of 210 mg silymarin to 1,000 mg berberine could improve upon the antihyperglycemic effect of berberine alone, administered at the same dose of 1,000 mg/day. Although the administration of Berberis aristata (berberine [1,000 mg/day]) as add-on therapy did not result in statistically significant reductions of the main anthropometric measures (weight, BMI, and WL), it caused statistically significant reductions of a few important glycemic (FG and HbA1c) and lipidic (TC and TG) parameters, while reducing the LDL value to a considerable, albeit not statistically significant, extent. Similarly, the administration of Berberol did not affect the anthropometric parameters but reduced the FG and HbA1c values, as well as TC, LDL, and TG, in a statistically significant manner. The comparison of the results obtained from treatment with Berberol (Berberis aristata and Silybum marianum) against those obtained from the administration of Berberis aristata alone indicates that HbA1c is the parameter that most benefits from the presence of silymarin. Indeed, glycosylated hemoglobin reduced by 7.2% approximately following the administration of 1,000 mg berberine and by about 12.3% following the administration of 1,000 mg berberine and 210 mg silymarin. In the comparison between the two treatments, this result is the only parameter for which the difference was found to be statistically significant. In the light of this evidence, it can be stated that in patients with T2DM, the administration of Berberol (Berberis aristata and Silybum marianum) can improve the glycemic status in a statistically significant manner in comparison with the administration of Berberis aristata alone. This result is not so clear from the point of view of the lipidic profile. Although LDL significantly reduced only in the Berberol arm, the comparison between the groups did not show any statistically significant difference. This may be explained by variations in the study populations, treatments, and enrollment criteria. As a matter of fact, the patients enrolled in the study were either normocholesterolemic (and thus, not receiving any pharmacological therapy) or hypercholesterolemic and thus, treated with statins. In either case, even if the individuals were not really “targeted patients,” they were rather well controlled, with average TC values below 180 mg/dL and average LDL values below 98 mg/dL on enrollment. Perhaps a study designed to enroll individuals with a markedly unbalanced lipidic profile, ie, untreated patients or with residual hyperlipidemia, would have highlighted the additional effect of silymarin when added to berberine. This study obviously has some limitations. First, the study was limited by the small size of the two samples, which makes it impossible to draw final conclusions. Second, the study was not conducted in a double-blind manner, and the individuals could have been influenced by the fact that they knew they were being treated with a galenic preparation (Berberis aristata extract titrated in berberine) or a food supplement registered with the Ministry of Health – the common perception of a galenic is often different from that of a food supplement. Third, we lack clear evidence – such as could be provided with for example, the Caco-2 cell culture method – that the results obtained were due to improved enterocyte crossing caused by the presence of silymarin and were not due to another unexpected type of synergism between berberine and silymarin. Because of this, the next step will be the validation of the fixed mixture Berberis aristata/Silybum marianum in a Caco-2 cell model. The test will also enable a better definition of the most beneficial ratio in terms of dosages. As a matter of fact, the doses of the active ingredients of Berberol have so far, been established on the basis of the limitation arising from the fact that in Italy, Silybum marianum has also been registered as an over-the-counter (OTC) drug. As an OTC medication, the product is used clinically at 420 mg doses. On the other hand, Berberis aristata has never been registered as either a prescription or OTC drug and is marketed in Italy only as food supplement, at 500 mg doses (calculated as pure berberine). Consequently, the combination of 105 mg pure silymarin with 500 mg berberine was chosen to make it impossible to exceed the set dose of 420 mg silymarin even with the (improbable) administration of 2,000 mg pure berberine (4 tablets).
Conclusion
On the basis of the study results, it may be confirmed that use of a fixed (210 mg) amount of silymarin (obtained from Silybum marianum) added to 1,000 mg berberine (obtained from Berberis aristata) and administered as add-on therapy in the form of a nutritional supplement, in tablets (Berberol), is effective in improving lipidic and glycemic profiles in diabetes.40 It may be also concluded that Berberol seems to be equally safe and tolerated and more effective than tablets containing 1,000 mg berberine only in improving HbA1c in patients with a diagnosis of T2DM. Berberine and above all, Berberol could represent a good treatment option before initiating insulin therapy in diabetic patients with suboptimal glycemic control.
Acknowledgment
The authors wish to thank Professor Martino Recchia for the statistical analysis of the results.
Footnotes
Disclosure
FDP is the main formulator and patent sole inventor of Berberol®. The authors report no other conflicts of interest in this work.
References
- 1.Ginter E, Simko V. Type 2 diabetes mellitus, pandemic in 21st century. Adv Exp Med Biol. 2012;771:42–50. doi: 10.1007/978-1-4614-5441-0_6. [DOI] [PubMed] [Google Scholar]
- 2.Ho J, Leung AK, Rabi D. Hypoglycemic agents in the management of type 2 diabetes mellitus. Recent Pat Endocr Metab Immune Drug Discov. 2011;5(1):66–73. doi: 10.2174/187221411794351879. [DOI] [PubMed] [Google Scholar]
- 3.El-Kaissi S, Sherbeeni S. Pharmacological management of type 2 diabetes mellitus: an update. Curr Diabetes Rev. 2011;7(6):392–405. doi: 10.2174/157339911797579160. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann IS, Roa M, Torrico F, Cubeddu LX. Ondansetron and metformin-induced gastrointestinal side effects. Am J Ther. 2003;10(6):447–451. doi: 10.1097/00045391-200311000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 6.Tang LQ, Wei W, Chen LM, Liu S. Effects of berberine on diabetes induced by alloxan and a high-fat/high-cholesterol diet in rats. J Ethnopharmacol. 2006;108(1):109–115. doi: 10.1016/j.jep.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Li X, Zou D, et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93(7):2559–2565. doi: 10.1210/jc.2007-2404. [DOI] [PubMed] [Google Scholar]
- 8.Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57(5):712–717. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Wei J, Xue R, et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010;59(2):285–292. doi: 10.1016/j.metabol.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 10.Dong H, Zhao Y, Zhao L, Lu F. The effects of berberine on blood lipids: a systemic review and meta-analysis of randomized controlled trials. Planta Med. 2013;79(6):437–446. doi: 10.1055/s-0032-1328321. [DOI] [PubMed] [Google Scholar]
- 11.Dong H, Wang N, Zhao L, Lu F. Berberine in the treatment of type 2 diabetes mellitus: a systemic review and meta-analysis. Evid Based Complement Alternat Med. 2012;2012:591–654. doi: 10.1155/2012/591654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derosa G, Maffioli P, Cicero AF. Berberine on metabolic and cardiovascular risk factors: an analysis from preclinical evidences to clinical trials. Expert Opin Biol Ther. 2012;12(8):1113–1124. doi: 10.1517/14712598.2012.704014. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Xiao X, Feng K, Wang T, Li W, Yuan T, Sun X, Sun Q, Xiang H, Wang H. Berberine Moderates Glucose and Lipid Metabolism through Multipathway Mechanism. Evid Based Complement Alternat Med. 2011;2011 doi: 10.1155/2011/924851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin J, Zhang H, Ye J. Traditional chinese medicine in treatment of metabolic syndrome. Endocr Metab Immune Disord Drug Targets. 2008;8(2):99–111. doi: 10.2174/187153008784534330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong W, Wei J, Abidi P, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10(12):1344–1351. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Davies GE. Berberine inhibits adipogenesis in high-fat diet-induced obesity mice. Fitoterapia. 2010;81(5):358–366. doi: 10.1016/j.fitote.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Singh IP, Mahajan S. Berberine and its derivatives: a patent review (2009–2012) Expert Opin Ther Pat. 2013;23(2):215–231. doi: 10.1517/13543776.2013.746314. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Miao YQ, Fan DJ, et al. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech. 2011;12(2):705–711. doi: 10.1208/s12249-011-9632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan GY, Wang GJ, Liu XD, Fawcett JP, Xie YY. The involvement of P-glycoprotein in berberine absorption. Pharmacol Toxicol. 2002;91(4):193–197. doi: 10.1034/j.1600-0773.2002.t01-1-910403.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsai PL, Tsai TH. Hepatobiliary excretion of berberine. Drug Metab Dispos. 2004;32(4):405–412. doi: 10.1124/dmd.32.4.405. [DOI] [PubMed] [Google Scholar]
- 21.Chae HW, Kim IW, Jin HE, Kim DD, Chung SJ, Shim CK. Effect of ion-pair formation with bile salts on the in vitro cellular transport of berberine. Arch Pharm Res. 2008;31(1):103–110. doi: 10.1007/s12272-008-1127-4. [DOI] [PubMed] [Google Scholar]
- 22.Shan YQ, Ren G, Wang YX, et al. Berberine analogue IMB-Y53 improves glucose-lowering efficacy by averting cellular efflux especially P-glycoprotein efflux. Metabolism. 2013;62(3):446–456. doi: 10.1016/j.metabol.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Zhou S, Lim LY, Chowbay B. Herbal modulation of P-glycoprotein. Drug Metab Rev. 2004;36(1):57–104. doi: 10.1081/dmr-120028427. [DOI] [PubMed] [Google Scholar]
- 24.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Postgrad Med. 2002;48(3):206–208. [PubMed] [Google Scholar]
- 25.Atchley AE, Kitzman DW, Whellan DJ, et al. HF-ACTION Investigators Myocardial perfusion, function, and dyssynchrony in patients with heart failure: baseline results from the single-photon emission computed tomography imaging ancillary study of the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) Trial. Am Heart J. 2009;158(4 Suppl):S53–S63. doi: 10.1016/j.ahj.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NCEP Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002. 106(25):3143–4216. [PubMed] [Google Scholar]
- 27.Winer BJ. Statistical Principles in Experimental Design. 2nd edition. McGraw-Hill; New York, NY, USA: 1971. [Google Scholar]
- 28.Di Pierro F, Villanova N, Agostini F, Marzocchi R, Soverini V, Marchesini G. Pilot study on the additive effects of berberine and oral type 2 diabetes agents for patients with suboptimal glycemic control. Diabetes Metab Syndr Obes. 2012;5:213–217. doi: 10.2147/DMSO.S33718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orio F, Muscogiuri G, Palomba S, et al. Berberine improves reproductive features in obese Caucasian women with polycystic ovary syndrome independently of changes of insulin sensitivity. European J Clin Nutr. 2013;8(5):e200–e204. [Google Scholar]
- 30.Hu Y, Ehli EA, Kittelsrud J, et al. Lipid-lowering effect of berberine in human subjects and rats. Phytomedicine. 2012;19(10):861–867. doi: 10.1016/j.phymed.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Affuso F, Ruvolo A, Micillo F, Saccà L, Fazio S. Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr Metab Cardiovasc Dis. 2010;20(9):656–661. doi: 10.1016/j.numecd.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 32.Klimek M, Wang S, Ogunkanmi A. Safety and efficacy of red yeast rice (Monascus purpureus) as an alternative therapy for hyperlipidemia. P T. 2009;34(6):313–327. [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon RY, Cooperman T, Obermeyer W, Becker DJ. Marked variability of monacolin levels in commercial red yeast rice products: buyer beware! Arch Intern Med. 2010;170(19):1722–1727. doi: 10.1001/archinternmed.2010.382. [DOI] [PubMed] [Google Scholar]
- 34.Lin YL, Wang TH, Lee MH, Su NW. Biologically active components and nutraceuticals in the Monascus-fermented rice: a review. Appl Microbiol Biotechnol. 2008;77(5):965–973. doi: 10.1007/s00253-007-1256-6. [DOI] [PubMed] [Google Scholar]
- 35.Kong WJ, Wei J, Zuo ZY, et al. Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism. 2008;57(8):1029–1037. doi: 10.1016/j.metabol.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 36.Cameron J, Ranheim T, Kulseth MA, Leren TP, Berge KE. Berberine decreases PCSK9 expression in HepG2 cells. Atherosclerosis. 2008;201(2):266–273. doi: 10.1016/j.atherosclerosis.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Kidd PM. Bioavailability and activity of phytosome complexes from botanical polyphenols: the silymarin, curcumin, green tea, and grape seed extracts. Altern Med Rev. 2009;14(3):226–246. [PubMed] [Google Scholar]
- 38.Kidd P, Head K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin-phosphatidylcholine complex (Siliphos) Altern Med Rev. 2005;10(3):193–203. [PubMed] [Google Scholar]
- 39.Di Pierro F, Villanova N. L’associazione berberina e silimarina nel trattamento dell’ipercolesterolemia, del diabete e della sindrome metabolica. [The association between berberine and silymarin in hypercholesterolemia, diabetes and metabolic syndrome treatment] Integr Nutr. 2009;12(3):19–24. Italian. [Google Scholar]
- 40.Derosa G, Bonaventura A, Bianchi L, et al. Berberis aristata/Silybum marianum fixed combination on lipid profile and insulin secretion in dyslipidemic patients. Expert Opin Biol Ther. 2013 Aug 24; doi: 10.1517/14712598.2013.832751. Epub. [DOI] [PubMed] [Google Scholar]