Summary
The G-protein-activated inwardly rectifying potassium channel Kir3.4 is expressed in the zona glomerulosa cell membrane and transports potassium out of the cell.
Angiotensin II stimulation of aldosterone secretion is mediated in part by suppression of the transcription of KCNJ5, the gene coding for Kir3.4, and blocking channel activity. This results in membrane depolarization, mobilization of intracellular calcium, activation of the calcium-calmodulin pathway, and increasing gene transcription of steroidogenic enzymes required for aldosterone secretion.
In 40–60% of aldosterone-producing adenomas there is a somatic mutation in the region of the KCNJ5 gene that codes for the selectivity filter that decreases potassium selectivity, allowing sodium to leak into the cells, thus depolarizing the membrane and initiating events that result in increased aldosterone synthesis.
The mechanism by which mutated KCNJ5 induces cell proliferation and adenoma formation remains unclear.
Introduction
Primary aldosteronism, the most common form of secondary hypertension, is the cause of high blood pressure in approximately 5–10% of unselected hypertensive patients.1 About half of these have an aldosterone-producing adenoma (APA); the others suffer adrenal zona glomerulosa hyperplasia and hyperfunction of unknown origin, also known as idiopathic hyperaldosteronism. Recent significant advances in exome sequencing has added to our understanding of somatic gene mutations in APA and have uncovered several mutations in the selectivity filter of the G protein-activated inward rectifying potassium channel Kir3.4 (also called KCNJ5 or GIRK4) coded by the KCNJ5 gene. Together, these mutations have been found in 40–60% of APA.2–7 The selectivity filter is the region of the channel pore that allows the specific transport of potassium and exclusion of other cations. Mutations within the filter that allow sodium to enter the zona glomerulosa cell depolarize the membrane, resulting in calcium mobilization and activation of the calcium signal cascade, ultimately increasing aldosterone synthesis and cell proliferation.2 The predominant KCNJ5 mutations are G151R and R168L. Germinal mutations of KCNJ5 have also been found in rare families with familial hyperaldosteronism type 3.2, 8–10 More recently, other less common somatic mutations of the sodium/potassium ATPase gene ATP1A1 and the calcium ATPase gene ATP2B3 have been discovered in APA with normal KCNJ5 genes.11
Potassium Channels in the adrenal
Intracellular recordings from adrenocortical tissues from multiple species have shown that adrenal cells maintain negative resting potentials determined primarily by plasma membrane permeability to K+.12–14 Adrenal zona glomerulosa (ZG) cells are normally hyperpolarized by a predominant potassium conductance mediated by the ‘leak’ K+ channels of the 2-pore domain /4 transmembrane family, TASK1 (KCNK3), TASK3 (KCNK9) and TREK1 (KCNK2), the expression of which varies depending on the animal species.14–20 In rodents and bovines ‘leak’ channels TASK1, TASK3 and TREK1 appear to set the resting potential;14, 16, 21, 22 in humans it appears to be the TASK1.15 In ZG cells, membrane polarity is also controlled by Kir (K+ inwardly rectifying) channels some of which are members of the G protein-activated inwardly rectifying potassium channel family. Four Kir3 channel subunits coded by the KCNJ genes have been identified in mammals. Kir proteins have two putative membrane-spanning domains (Fig 1)23 and form a tetrameric complex linked by an extracellular pore-forming region and cytoplasmic amino and carboxy terminal domains.24 Kir3.4, coded by KCNJ5, can form homo-tetramers or more commonly, hetero-tetramers with Kir3.1 (KCNJ3), Kir3.2 (KCNJ6) or Kir3.3 (KCNJ9)24 and the combination of Kir3 subunits in each channel varies among tissues and cell types.25 Kir 3.4 is expressed in the zona glomerulosa2 and Kir3.1 is expressed throughout the adrenal including the zona glomerulosa (unpublished). Kir channels hyperpolarize the membrane of excitable cells such as cardiac myocytes and neurons 24. Stimulation of thyrotrophic cells by thyrotropin-releasing hormone causes the vesicles that contain the potassium channel subunit Kir3.4 co-localized with Kir3.1subunit to fuse with the plasma membrane, resulting in an increase in the expression of these subunits in the plasma membrane, thus enhancing potassium currents when the cells are stimulated by dopamine or somatostatin 26 and suppressing TSH release. Dopamine inhibits prolactin release in the pituitary lactotropes by increasing the activity of G-protein activated K+ channels containing Kir3.4 and Kir3.1 by hyperpolarizing the membrane.27 Dopamine also inhibits aldosterone release,28 suggesting that activation of Kir3.4/Kir3.1 heterotetramer in the adrenal zona glomerulosa may inhibit aldosterone production by contributing to the hyperpolarization of the membrane. KCNJ potassium channel selectivity for potassium is conferred by a GYG motif at the narrowest part of the pore29 and mutations of this region decrease the selectivity of the channel for potassium and have been associated with APA.2–5, 7, 8, 30
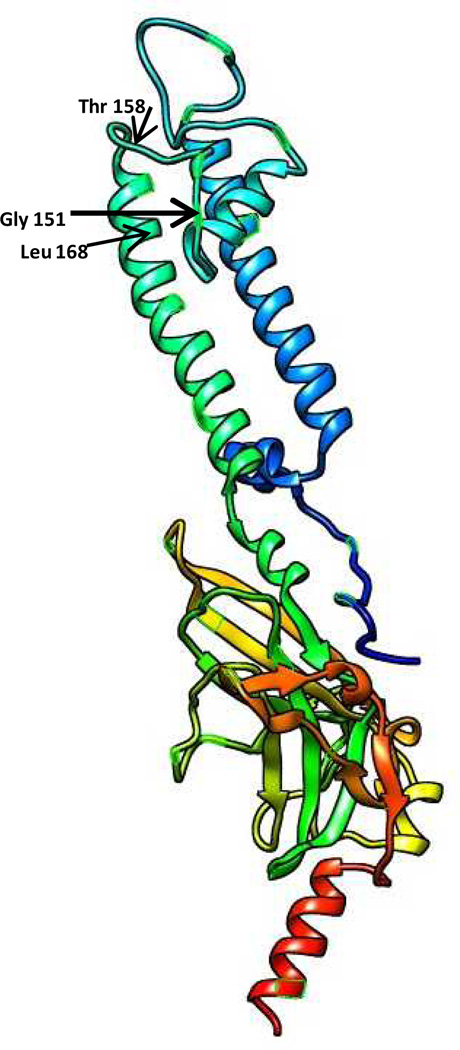
Fig 1.
Model of the crystal structure of the inward-rectifier potassium channel kir2.2 which has very high sequence homology in the selectivity filter with kir3.4. The location of glycine 151, threonine 158 and leucine 168 are shown. The model was based on the Phyre server.23
Role of KCNJ5 in adrenal zona glomerulosa function
KCNJ5 is expressed in the zona glomerulosa of the adrenal.2 The role of KCNJ5 in normal physiology of aldosterone regulation has been studied using the adrenal carcinoma cell line HAC15.31 Angiotensin II stimulation of the human adrenal carcinoma cell line 15 (HAC15) results in down-regulation of KCNJ5 expression at the mRNA (−41.2%) and protein level (−52.7%) and a decrease in membrane potential, leading to the increase in the transcription of enzymes required for adrenal steroid synthesis. The calcium ionophore A23187 produced a similar effect on KCNJ5 expression, suggesting that the effect of angiotensin II on KCNJ5 expression is mediated by intracellular calcium mobilization. Naringin activates the Kir3.1–3.4 heterotetrameric channel by binding the tyrosines 148 and 150 of the M1–M2 loop32 and partially abrogates the effect of angiotensin II on membrane voltage, expression of steroidogenic enzymes, and aldosterone synthesis, further evidence that angiotensin II-induced aldosterone production is mediated in part by Kir3.4.31 Transduction of HAC15 cells with a lentivirus to overexpress KCNJ5 suppressed mRNA expression for StAR, HSD3B2, CYP11B1 and CYP11B2 and blunted the angiotensin II-induced transcriptional activation of the CYP11B2 promoter, as well as basal and angiotensin II-induced aldosterone and cortisol secretion (Fig 2, top panel), membrane voltage, and intracellular Ca2+ concentrations. Downregulation of KCNJ5 using a shRNA-KCNJ5 lentivirus decreased the expression of the Kir3.4 protein, but did not alter membrane voltage, intracellular Ca2+ concentration or aldosterone biosynthesis.31 This suggests that the Kir3.4 channels transfer potassium from inside to outside of the HAC15 cells continuously and that interruption by angiotensin II results in membrane depolarization, mobilization of calcium, and activation of signals resulting in transcription of the CYP11B2, thus increasing aldosterone production (Fig 2,3). Naringin blocks the effect of angiotensin II by activating the Kir3.4 potassium channel, increasing the transfer of potassium ions from the inside to outside and repolarizing the membrane. Overexpression of Kir3.4 maintains or increases the hyperpolarized state (Fig 3) and inhibits aldosterone secretion consistent with reports that activation of the Kir3.4 potassium channel in pituitary lactotropes by dopamine leads to hyperpolarization of the membrane and suppression of prolactin secretion.27
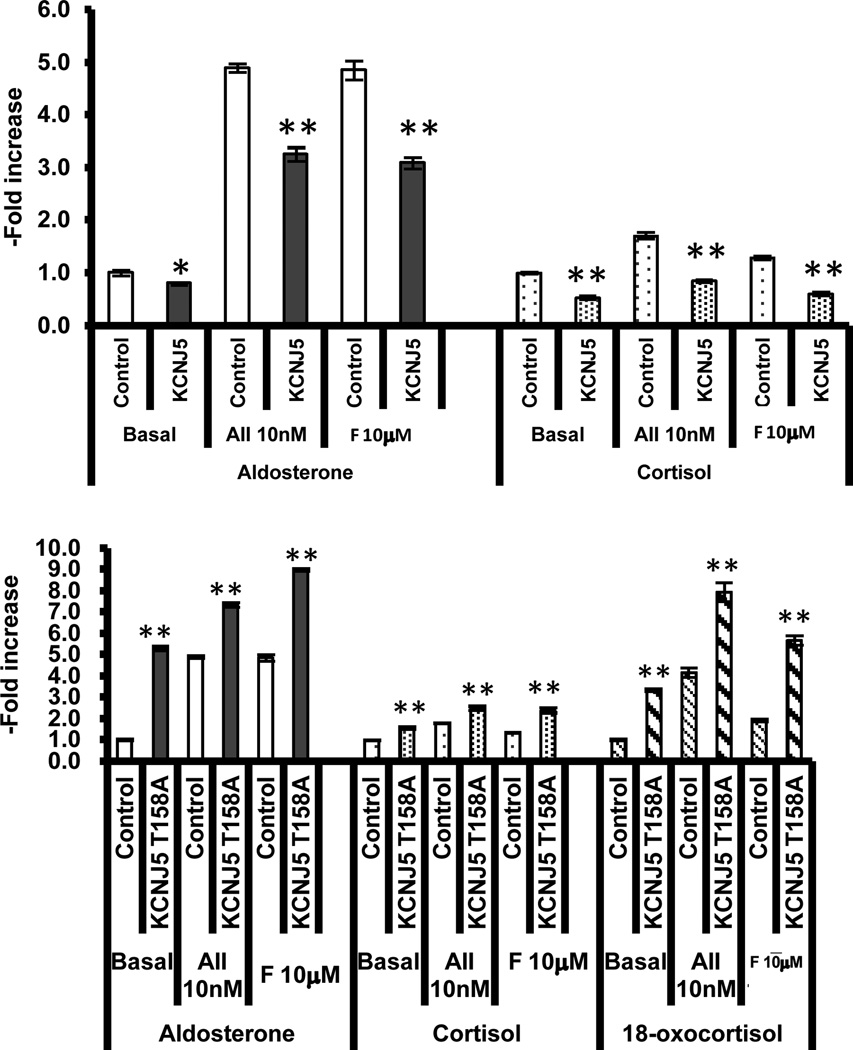
Fig 2.
Effect of KCNJ5 and mutated KCNJ5-T158A lentiviral transduction on steroid synthesis in the HAC15 cell. The upper panel shows the effect of transduction of HAC15 cells with wild type KCNJ5 lentivirus on aldosterone and cortisol secretion in cells under basal conditions and stimulated with angiotensin II (10 nM) and forskolin (10µM) for 24 hrs (*p<0.05, **p<0.01). Transduction of cells resulted in a decrease in basal and stimulated aldosterone and cortisol secretion (data redrawn from Oki et al by permission 31.
The lower panel shows the effect of transduction of HAC15 with a lentivirus with the mutated KCNJ5-T158A on aldosterone, cortisol and 18-oxocortisol secretion under basal and stimulation with angiotensin II (10nM) and forskolin (10 µM). There was a significant enhancement of steroid secretion in the transduced cells with the KCNJ5-T158 lentivirus (data redrawn from Oki et al by permission) 34.
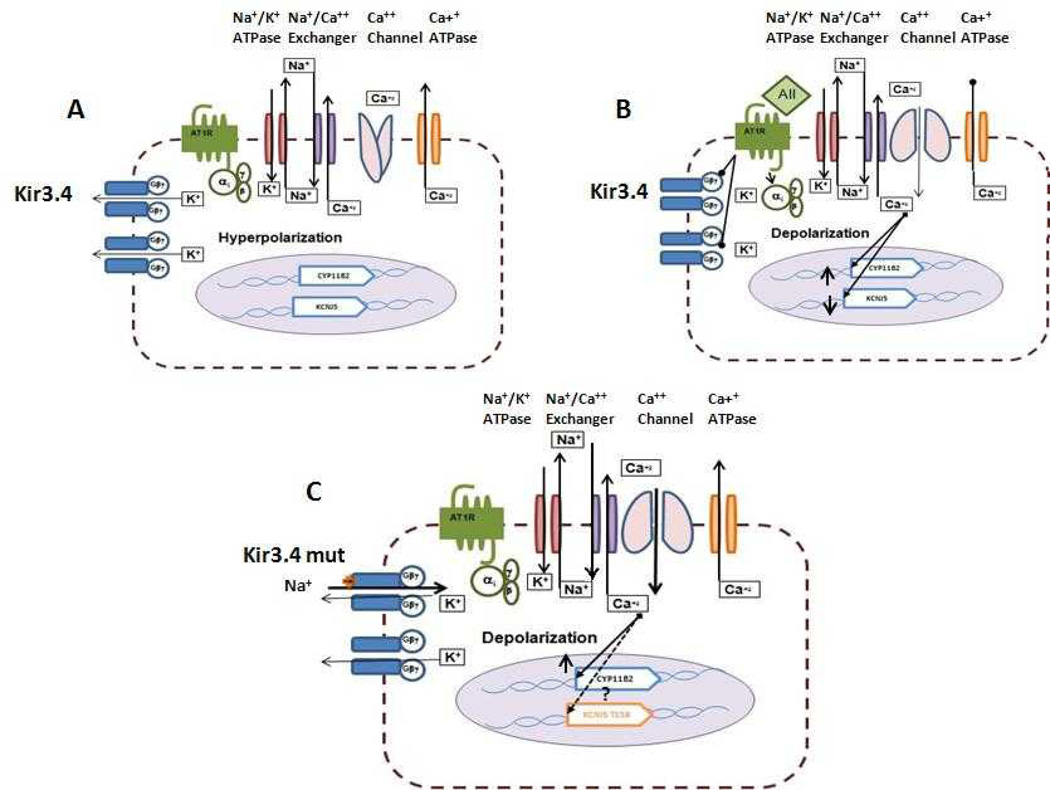
Fig 3.
Proposed mechanisms for the action of Angiotensin II on Kir3.4 and aldosterone secretion and for the effect of the KCNJ5-mutation on aldosterone secretion. A. Under basal conditions there is a continuous outflow of potassium through the Kir3.4 channel and in combination with other potassium channels the cell is hyperpolarized and aldosterone is formed in basal quantities. B. Upon stimulation with Angiotensin II there is a decrease in gene expression of the KCNJ5 (Kir3.4) and blocking of the activity of the channel with membrane depolarization, opening of the calcium channels and increase in intracellular calcium with activation of the calcium-calmodulin cascade resulting in increased expression of the CYP11B2 and aldosterone secretion. C. The KCNJ5 mutation (Kir3.4 mut) results in loss of selectivity of the channel with inflow of sodium, depolarization of the membrane, opening of calcium channels and ultimately increase gene transcription of the CYP11B2 and increased aldosterone secretion.
Aldosterone-producing adenomas and KCNJ5 mutations
Pioneering studies of aldosterone-producing adenomas using exome sequencing by the Lifton group 2 found two recurring mutations around the GYG motif of the KCNJ5 gene in 8 of 22 patients studied. While the channel is normally very selective for potassium, the G151R and L168R mutations result in a decrease in the selectivity, allowing the passage of sodium. An inherited mutation of amino acid T158A was also found in a family presenting with severe hyperaldosteronism and bilateral adrenal hyperplasia which required treatment with bilateral adrenalectomy.2, 33 Expression in HEK293 of the mutated KCNJ5 (G151R, L168R or T158A) with KCNJ3 (required to form the hetero-tetramer channel cells) was shown to result in loss of channel selectivity and membrane depolarization. Expression of the mutated KCNJ5 homo-tetramers also resulted in a similar loss of channel selectivity and membrane depolarization.2 It was postulated that mutations of the selectivity filter that decrease selectivity for potassium and allow sodium to leak into the cells, would depolarize the membrane, increase calcium mobilization, thus stimulate aldosterone secretion and proliferation2 (Fig 3).
Transduction of KCNJ5-T158A into HAC15 cells
The effects of the mutations on channel activity reported by Choi et al2 were studied using transfection of the non-steroidogenic cell HEK293. We recently reported our studies of the T158A mutation of KCNJ5 on the regulation of aldosterone biosynthesis in HAC15.34 Transducing the cell line HAC15 with the lentivirus KCNJ5 T158A resulted in a 5.3 fold increase in basal aldosterone secretion over cells transduced with empty lentivirus (Fig 2, bottom panel) and enhanced the stimulatory effect of angiotensin II and forskolin (an adenyl cyclase activator) on aldosterone synthesis. Forskolin-induced aldosterone secretion in the KCNJ5 T158A cells was greater than that by angiotensin II (Fig 1, bottom panel). Cortisol secretion was also increased, but to a significantly lesser extent than aldosterone. Synthesis of 18-oxocortisol was also increased in transduced HAC15 cells (Fig 1, bottom panel). Patients with APA with and without a KCNJ5 mutation generally have a marked increase in 18-oxocortisol, as well as aldosterone secretion in comparison to normal individuals and patients with idiopathic hyperaldosteronism.35 In one family with familial hyperaldosteronism type 3 with the KCNJ5-T158A mutation the excretion of 18-oxocortisol was found to be very high and not suppressed by dexamethasone.33 Levels of 18-oxocortisol have not yet been reported in other recently described patients with familial hyperaldosteronism type 3.8–10
All mutations of the KCNJ5 gene associated with APA discovered so far are located either in the selectivity filter or adjacent to the channel pore. The G151R or G151E affect the first glycine of the GYG motif, a constant feature of most K+ channels36. Other mutations, T158A, del157 and I157S, probably work by affecting the separation or charge between R155 and E159 that form crucial salt bridges around the pore. The L168R is the most distal mutation discovered to date and may also disrupt the local salt bridge by introducing a positive charge.37, 38 The effect of the KCNJ5 T158A mutation on sodium influx, measured using the cell-impermeant dye CoroNa Green, showed that KCNJ5-T158A cells had a 1.2 fold higher fluorescence for sodium than control cells.34 KCNJ5-T158A cells loaded with an indicator of plasma membrane voltage DiSBAC2 also showed higher plasma membrane voltage than in control cells. KCNJ5-T158A cells loaded with the intracellular calcium indicator Fluo-4 AM showed a 1.6-fold increase in comparison to control cells.34 These results suggest that the KCNJ5-T158A mutation induced an increase in Na+ influx, membrane voltage and intracellular Ca2+ accumulation (Fig 2). Similar results of stimulation of aldosterone biosynthesis were reported with transfections of the HAC15 cell with a mutated cDNA KCNJ5-G151R and KCNJ5-L168R.7 Microarray studies indicated that transfection of the mutated KCNJ5 altered the expression of 36 genes by more than 2.5-fold.7
Transduction of the HAC15 cell with the KCNJ5-T158A mutant lentivirus resulted in a 17.7-fold increase in the mRNA expression of the CYP11B2 and a 5.8-fold for the CYP11B1 enzyme mRNA. The CYP17α mRNA decreased in cells transduced with the KCNJ5-T158A lentivirus, probably explaining why the increase in cortisol secretion by these cells was much less than that of aldosterone (Fig 2, lower panel). Inhibition of L-calcium channels with nifedipine or the calmodulin inhibitor W-7 decreased aldosterone production by 75% and 18%, respectively, indicating the role of intracellular Ca2+ in the regulation of aldosterone biosynthesis.
When mutations of the selectivity filter of the KCNJ5 channel were described, it was postulated that the membrane depolarization and mobilization of intracellular calcium resulted in cellular proliferation responsible for formation of the adenoma, as well as increased aldosterone secretion. However transduction of HAC15 with the KCNJ5-T158A lentivirus decreased proliferation as measured by three different methods without affecting apoptosis.34 Transfection of the HEK293 cell line with a plasmid with the KCNJ5-G151R mutation and an eGFP marker showed that there were significantly less cells at 24 and 36 hrs after transfection expressing the eGFP that those transfected with the wild type KCNJ5. Transfection with KCNJ5-G151E, another mutation found in some families with hyperaldosteronism, produced even fewer marked cells.9 Patients with familial hyperaldosteronism type 3 with the KCNJ5-G151R mutation exhibit adrenal hyperplasia while patients with KCNJ5-G151E mutations do not.9 This suggests that calcium toxicity of adrenal cells in the KCNJ5-G151E is strong and only a small subset of cells proliferate to produce mild hyperaldosteronism. How the KCNJ5-G151R mutation is associated with adenoma formation when transductions of this clone in HAC15 cells decreases proliferation is not clear, however a potential explanation is suggested by the studies by Williams et al,39 who demonstrated that a calcium sensor gene visinin-like 1 (VSNL1) is upregulated in APAs, particularly those harboring a KCNJ5 mutation. Transfection of VSNL1 cDNA increases CYP11B2 expression under basal and angiotensin II stimulation conditions. Silencing of VSNL1 with a siRNA increases apoptosis to ionomycin or when cells are also transfected with a mutated KCNJ5 plasmid.39 Thus VSNL1 appears to exert a protective effect on mutated KCNJ5-induced apoptosis. This suggest that some mutations of the selectivity filter of the KCNJ5 channel result in calcium toxicity and inhibition of cell proliferation, but in adenomas and maybe in familial hyperaldosteronism type 3, the expression of VSNL1 and maybe other genes mitigate against a toxic effect of calcium, resulting in increased proliferation and the development of an adenoma. Proof of this potential mechanism is lacking.
APA with KCNJ5 mutations. The level of Kir3.4 expression in the APA with a KCNJ5 mutation is lower than that of normal human ZG.30 Most patients with APA also have an increase in peri-tumoral nodules and zona glomerulosa hyperplasia, including aldosterone-producing cell clusters.40–44 Patients with APA with KCNJ5 mutations tend to be younger females2–5, 7, 9 except in Japan where there is no sexual preference6 and have more severe hyperaldosteronism and higher lateralization index.4, 45 The finding that the expression of Kir3.4 is lower in adenomas harboring a KCNJ5 somatic mutation might contribute to increased proliferation as the cells would not have as much of a sodium-induced depolarization and calcium toxicity, but just enough intracellular calcium to stimulate proliferation. Some mutations like the KCNJ5-G151E that severely affect channel activity suppress cell proliferation so fewer cells survive, creating a mild hyperaldosteronism phenotype.9, 30 However,other studies have higher expression levels of KCNJ5 in APA patients bearing a mutation.6
Conclusions and perspective
Kir3.4 is a subunit of the G-protein-activated inwardly rectifying potassium channel expressed in the zona glomerulosa of the human adrenal. Part of the mechanism for the stimulation of aldosterone synthesis by angiotensin II is the decrease in transcription of gene KCNJ5 for Kir3.4 and blocking channel activity. All of the somatic mutations in aldosterone-producing adenomas reported so far are in or next to the selectivity filter of the KCNJ5 gene that codes for the Kir3.4 channel. The functional result is the increased mobilization of intracellular calcium and activation of the calcium calmodulin kinase signal cascade (Fig 3). Recently inactivating mutations in the sodium-potassium ATPase alpha subunit (ATP1A1) and the calcium ATPase (ATP2B3)11 have been discovered in some APA. Together, these mutations are present in 50–70% of APAs studied. In the near future other mutations, perhaps of the calcium channels themselves, may be discovered that also cause unregulated aldosterone production in APA or IHA.
Acknowledgements
Some of the studies presented were supported by grants from the NIH HL27255, HL105383 and medical research funds from the Department of Veterans Affairs.
References
- 1.Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 2.Choi M, Scholl UI, Yue P, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akerstrom T, Crona J, Delgado Verdugo A, et al. Comprehensive Re-Sequencing of Adrenal Aldosterone Producing Lesions Reveal Three Somatic Mutations near the KCNJ5 Potassium Channel Selectivity Filter. PLoS One. 2012;7:e41926. doi: 10.1371/journal.pone.0041926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulkroun S, Beuschlein F, Rossi GP, et al. Prevalence, Clinical, and Molecular Correlates of KCNJ5 Mutations in Primary Aldosteronism. Hypertension. 2012;59:592–598. doi: 10.1161/HYPERTENSIONAHA.111.186478. [DOI] [PubMed] [Google Scholar]
- 5.Azizan EA, Murthy M, Stowasser M, et al. Somatic Mutations Affecting the Selectivity Filter of KCNJ5 Are Frequent in 2 Large Unselected Collections of Adrenal Aldosteronomas. Hypertension. 2012;59:587–591. doi: 10.1161/HYPERTENSIONAHA.111.186239. [DOI] [PubMed] [Google Scholar]
- 6.Taguchi R, Yamada M, Nakajima Y, et al. Expression and Mutations of KCNJ5 mRNA in Japanese Patients with Aldosterone-Producing Adenomas. J Clin Endocrinol Metab. 2012;97:1311–1319. doi: 10.1210/jc.2011-2885. [DOI] [PubMed] [Google Scholar]
- 7.Monticone S, Hattangady NG, Nishimoto K, et al. Effect of KCNJ5 mutations on gene expression in aldosterone-producing adenomas and adrenocortical cells. J Clin Endocrinol Metab. 2012;97:E1567–E1572. doi: 10.1210/jc.2011-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulatero P, Tauber P, Zennaro MC, et al. KCNJ5 Mutations in European Families With Nonglucocorticoid Remediable Familial Hyperaldosteronism. Hypertension. 2012;59:235–240. doi: 10.1161/HYPERTENSIONAHA.111.183996. [DOI] [PubMed] [Google Scholar]
- 9.Scholla UI, Nelson-Williamsa C, Yuec P, et al. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. PNAS. 2012;109:2533–2548. doi: 10.1073/pnas.1121407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charmandari E, Sertedaki A, Kino T, et al. A Novel Point Mutation in the KCNJ5 Gene Causing Primary Hyperaldosteronism and Early-Onset Autosomal Dominant Hypertension. J Clin Endocrinol Metab. 2012;97:E1532–E1539. doi: 10.1210/jc.2012-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beuschlein F, Boulkroun S, Osswald A, et al. Somatic Na+,K+-ATPase and Ca2+-ATPase mutations leading to aldosterone producing adenomas and secondary hypertension. Nature Genetics. 2013;45:440–444. doi: 10.1038/ng.2550. [DOI] [PubMed] [Google Scholar]
- 12.Matthews EK, Saffran M. Effect of ACTH on the electrical properties of adrenocortical cells. Nature. 1968;219:1369–1370. doi: 10.1038/2191369a0. [DOI] [PubMed] [Google Scholar]
- 13.Matthews EK, Saffran M. Ionic dependence of adrenal steroidogenesis and ACTH-induced changes in the membrane potential of adrenocortical cells. J Physiol. 1973;234:43–64. doi: 10.1113/jphysiol.1973.sp010333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enyeart JA, Danthi SJ, Enyeart JJ. TREK-1 K+ channels couple angiotensin II receptors to membrane depolarization and aldosterone secretion in bovine adrenal glomerulosa cells. Am J Physiol Endocrinol Metab. 2004;287:E1154–E1165. doi: 10.1152/ajpendo.00223.2004. [DOI] [PubMed] [Google Scholar]
- 15.Nogueira EF, Gerry D, Mantero F, Mariniello B, Rainey WE. The role of TASK1 in aldosterone production and its expression in normal adrenal and aldosterone-producing adenomas. Clin Endocrinol (Oxf) 2010;73:22–29. doi: 10.1111/j.1365-2265.2009.03738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heitzmann D, Derand R, Jungbauer S, et al. Invalidation of TASK1 potassium channels disrupts adrenal gland zonation and mineralocorticoid homeostasis. Embo J. 2008;27:179–187. doi: 10.1038/sj.emboj.7601934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guagliardo NA, Yao J, Hu C, Barrett PQ. Minireview: aldosterone biosynthesis: electrically gated for our protection. Endocrinology. 2012;153:3579–3586. doi: 10.1210/en.2012-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu C, Rusin CG, Tan Z, Guagliardo NA, Barrett PQ. Zona glomerulosa cells of the mouse adrenal cortex are intrinsic electrical oscillators. J Clin Invest. 2012;122:2046–2053. doi: 10.1172/JCI61996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guagliardo NA, Yao J, Hu C, et al. TASK-3 channel deletion in mice recapitulates low-renin essential hypertension. Hypertension. 2012;59:999–1005. doi: 10.1161/HYPERTENSIONAHA.111.189662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies LA, Hu C, Guagliardo NA, et al. TASK channel deletion in mice causes primary hyperaldosteronism. Proc Natl Acad Sci U S A. 2008;105:2203–2208. doi: 10.1073/pnas.0712000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enyeart JJ, Xu L, Danthi S, Enyeart JA. An ACTH- and ATP-regulated background K+ channel in adrenocortical cells is TREK-1. J Biol Chem. 2002;277:49186–49199. doi: 10.1074/jbc.M207233200. [DOI] [PubMed] [Google Scholar]
- 22.Czirjak G, Enyedi P. TASK-3 dominates the background potassium conductance in rat adrenal glomerulosa cells. Mol Endocrinol. 2002;16:621–629. doi: 10.1210/mend.16.3.0788. [DOI] [PubMed] [Google Scholar]
- 23.Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 24.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 25.Kurachi Y, Ishii M. Cell signal control of the G protein-gated potassium channel and its subcellular localization. J Physiol. 2004;554:285–294. doi: 10.1113/jphysiol.2003.048439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morishige K, Inanobe A, Yoshimoto Y, et al. Secretagogue-induced exocytosis recruits G protein-gated K+ channels to plasma membrane in endocrine cells. J Biol Chem. 1999;274:7969–7974. doi: 10.1074/jbc.274.12.7969. [DOI] [PubMed] [Google Scholar]
- 27.Gregerson KA, Flagg TP, O'Neill TJ, et al. Identification of G protein-coupled, inward rectifier potassium channel gene products from the rat anterior pituitary gland. Endocrinology. 2001;142:2820–2832. doi: 10.1210/endo.142.7.8236. [DOI] [PubMed] [Google Scholar]
- 28.Wu KD, Chen YM, Chu TS, et al. Dopaminergic modulation of aldosterone secretions on changes of sodium intake in aldosterone-producing adenoma. Am J Hypertens. 2002;15:609–614. doi: 10.1016/s0895-7061(02)02929-1. [DOI] [PubMed] [Google Scholar]
- 29.Heginbotham L, Abramson T, MacKinnon R. A functional connection between the pores of distantly related ion channels as revealed by mutant K+ channels. Science. 1992;258:1152–1155. doi: 10.1126/science.1279807. [DOI] [PubMed] [Google Scholar]
- 30.Boulkroun S, Golib Dzib JF, Samson-Couterie B, et al. KCNJ5 mutations in aldosterone producing adenoma and relationship with adrenal cortex remodeling. Mol Cell Endocrinol. 2013;371:221–227. doi: 10.1016/j.mce.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 31.Oki K, Plonczynski MW, Lam ML, Gomez-Sanchez EP, Gomez-Sanchez CE. The Potassium Channel, Kir3.4 Participates in Angiotensin II-Stimulated Aldosterone Production by a Human Adrenocortical Cell Line. Endocrinology. 2012;153:4328–4335. doi: 10.1210/en.2012-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yow TT, Pera E, Absalom N, et al. Naringin directly activates inwardly rectifying potassium channels at an overlapping binding site to tertiapin-Q. Br J Pharmacol. 2011;163:1017–1033. doi: 10.1111/j.1476-5381.2011.01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geller DS, Zhang J, Wisgerhof MV, Shackleton C, Kashgarian M, Lifton RP. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 2008;93:3117–3123. doi: 10.1210/jc.2008-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oki K, Plonczynski MW, Luis Lam M, Gomez-Sanchez EP, Gomez-Sanchez CE. Potassium Channel Mutant KCNJ5 T158A Expression in HAC-15 Cells Increases Aldosterone Synthesis. Endocrinology. 2012;153:1774–1782. doi: 10.1210/en.2011-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulatero P, di Cella SM, Monticone S, et al. 18-hydroxycorticosterone, 8-hydroxycortisol, and 18-oxocortisol in the diagnosis of primary aldosteronism and its subtypes. J Clin Endocrinol Metab. 2012;97:881–889. doi: 10.1210/jc.2011-2384. [DOI] [PubMed] [Google Scholar]
- 36.Whorton MR, MacKinnon R. Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell. 2011;147:199–208. doi: 10.1016/j.cell.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murthy M, Azizan EA, Brown MJ, O'Shaughnessy KM. Characterization of a novel somatic KCNJ5 mutation delI157 in an aldosterone-producing adenoma. J Hypertens. 2012;30:1827–1833. doi: 10.1097/HJH.0b013e328356139f. [DOI] [PubMed] [Google Scholar]
- 38.McCoy JG, Nimigean CM. Structural correlates of selectivity and inactivation in potassium channels. Biochim Biophys Acta. 2012;1818:272–285. doi: 10.1016/j.bbamem.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams TA, Monticone S, Crudo V, Warth R, Veglio F, Mulatero P. VSNL1 is upregulated in aldosterone producing adenomas with KCNJ5 mutations and protects from calcium-induced apoptosis. Hypertension. 2012;59:833–839. doi: 10.1161/HYPERTENSIONAHA.111.188532. [DOI] [PubMed] [Google Scholar]
- 40.Boulkroun S, Samson-Couterie B, Golib-Dzib JF, et al. Aldosterone-Producing Adenoma Formation in the Adrenal Cortex Involves Expression of Stem/Progenitor Cell Markers. Endocrinology. 2011;152:4753–4763. doi: 10.1210/en.2011-1205. [DOI] [PubMed] [Google Scholar]
- 41.Boulkroun S, Samson-Couterie B, Dzib JF, et al. Adrenal cortex remodeling and functional zona glomerulosa hyperplasia in primary aldosteronism. Hypertension. 2010;56:885–892. doi: 10.1161/HYPERTENSIONAHA.110.158543. [DOI] [PubMed] [Google Scholar]
- 42.Nishimoto K, Nakagawa K, Li D, et al. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95:2296–2305. doi: 10.1210/jc.2009-2010. [DOI] [PubMed] [Google Scholar]
- 43.Nanba K, Tsuiki M, Sawai K, et al. Histopathological Diagnosis of Primary Aldosteronism Using CYP11B2 Immunohistochemistry. J Clin Endocrinol Metab. 2013;98:1567–1574. doi: 10.1210/jc.2012-3726. [DOI] [PubMed] [Google Scholar]
- 44.Volpe C, Hoog A, Ogishima T, et al. Immunohistochemistry improves histopathologic diagnosis in primary aldosteronism. Journal of clinical pathology. 2013;66:351–354. doi: 10.1136/jclinpath-2012-201287. [DOI] [PubMed] [Google Scholar]
- 45.Seccia TM, Mantero F, Letizia C, et al. Somatic mutations in the KCNJ5 gene raise the lateralization index: implications for the diagnosis of primary aldosteronism by adrenal vein sampling. J Clin Endocrinol Metab. 2012;97:E2307–E2313. doi: 10.1210/jc.2012-2342. [DOI] [PubMed] [Google Scholar]