Summary
Fc receptor-like (FCRL) molecules are preferentially expressed by B lymphocytes and possess tyrosine-based immunoregulatory function. Although they generally inhibit B cell receptor (BCR) signaling, their influence on other activation pathways remains largely unexplored. In humans, FCRL3 encodes a type I transmembrane protein harboring both cytoplasmic ITAM and ITIM elements that can repress BCR activation. Despite this inhibitory property, mounting associations for FCRL3 with autoimmune and lymphoproliferative disorders imply a role for it in promoting B cell pathogenesis. Here we explore its influence on B cell responses to innate Toll-like receptor 9 (TLR9) stimulation. A detailed survey of blood B cell populations found that FCRL3 expression increased as a function of differentiation and was higher among memory subsets with innate-like features. FCRL3 ligation augmented CpG oligodeoxynucleotide TLR9-mediated B cell proliferation, activation, and survival, but surprisingly, abrogated plasma cell differentiation and antibody production. Although FCRL3 amplified the NF-κB and MAPK signaling cascades, it halted CpG triggered BLIMP1 induction in an ERK-dependent fashion. These findings indicate that FCRL3 differentially modulates innate signaling in B cells and provide new insight into the potential of this disease-associated receptor to counter-regulate adaptive and innate immunity.
Keywords: B cells, Fc Receptor-like, Toll-like receptor
Introduction
The balanced transmission of activating or inhibitory signals by cellular immunoreceptors is critical for maintaining homeostatic regulation, optimally responding to pathogens, and avoiding self-induced harm to the host [1]. Multigene families such as the Fc receptors (FCR) for immunoglobulin (Ig) and the paired Ig-like receptors (PIR) encode proteins with either cytoplasmic immunoreceptor tyrosine-based inhibitory motifs (ITIM), immunoreceptor tyrosine-based activation motifs (ITAM), or charged transmembrane residues that permit association with ITAM-bearing adaptor proteins [1]. The more recently identified Fc receptor-like (FCRL1-6) molecules share many characteristics with the classical FCR for IgG and IgE, but differ in their extracellular Ig-like domains, possession of multiple cytoplasmic tyrosines that comprise ITAM-like and/or ITIM sequences, and preferential expression by B cells [2–5]. Until recently, FCRL proteins were orphan receptors, but ligands have now been identified for several members. Whereas the human FCRL6 and mouse FCRL5 representatives interact with MHC-like molecules [6, 7], human FCRL4 and FCRL5 appear to bind IgA and IgG [8]. Though the biological outcomes of these newfound relationships currently remain unclear, the emerging associations of various FCRLs with malignancies [9, 10], infections [11, 12], autoimmune (AI) disorders [13], and immunodeficiencies [14], predict fundamental roles for them in normal and perturbed immunity.
The tyrosine-based regulation of human FCRL1-5 in B cell antigen receptor (BCR) signaling has been explored by several groups. FCRL1 has two ITAM-like sequences which promote BCR-induced calcium flux and cellular proliferation [15]. In contrast, FCRL2-5 all possess one or more ITIM sequences. These motifs restrict BCR-mediated calcium mobilization, mitogen activated protein kinase (MAPK) activation, and whole cell protein tyrosine phosphorylation (pTyr) via recruitment of the SHP-1 and/or SHP-2 SH2 domain-containing phosphatases [16–19]. Among these proteins, FCRL3 has six extracellular Ig-like domains, an uncharged transmembrane region, and a cytoplasmic tail with four tyrosines that anchor possible ITAM, ITIM, and hemi-ITAM sequences [3]. Similar to other ITIM-containing FCRLs, its co-ligation with the BCR induces pTyr of FCRL3 itself, which coincides with decreased whole-cell pTyr and calcium mobilization [18]. Importantly, while FCRL1-5 all mark B lymphocytes, FCRL3 is unique in being additionally expressed by subsets of NK and T lineage cells [5, 20]. Among these, FCRL3 segregates a subpopulation of regulatory CD4+ T cells (Tregs) that is less responsive to antigenic stimulation and has impaired suppression capacity [21, 22].
Despite its inhibitory potential, signaling studies with FcγRIIB/FCRL3 chimeric receptor tyrosine mutants revealed that ITIM deficient variants could enhance BCR calcium flux [18]. In fact, these and earlier studies disclosing its ability to dock the SHP-1 and SHP-2 phosphatases as well as the Syk and ZAP-70 tyrosine kinases [23], suggest that FCRL3 can stimulate as well as inhibit downstream responses. Accordingly, recent studies of FCRL3’s closest relative in mice, FCRL5, demonstrate that it is discretely expressed by innate-like marginal zone and B1 B cells [24] and has dual-functionality conferred by SHP-1 as well as the Lyn Src family kinase [25]. However, under what context(s) these receptors exert their suspected activation properties and the broader functional consequences of these features in B cells, remains unknown.
The biological importance of FCRL3 is further underscored by its association with multiple humoral immune disorders. A survey of single nucleotide polymorphisms (SNP) surrounding the FCRL1-5 gene cluster at chromosome 1q21-23 identified a functional variant in the FCRL3 promoter (−169 C→T) that is situated within a NF-κB consensus binding site and is strongly associated with susceptibility to rheumatoid arthritis and AI [13]. The −169 C allele confers a more orthodox NF-κB localization sequence that increases binding affinity for the p50, p65, and cRel transcription factor components, upregulates FCRL3 transcription and translation, and directly correlates with autoantibody production [13, 26]. Since its identification, the growing number of publications corroborating linkage of this SNP to multiple AI diseases as well as disease activity strongly implicates a pathogenic role for FCRL3 in AI [27, 28]. Interestingly, FCRL3 has also been identified as a biomarker of B cell chronic lymphocytic leukemia (CLL). FCRL3 is upregulated in a subgroup of CLL patients possessing clonal expansions with relatively higher frequencies of Ig heavy-chain variable region (IGHV) gene somatic hypermutation and a more favorable disease course [9].
Its intriguing association with immune diseases and dual-signaling potential led us to investigate the distribution and function of FCRL3 in blood B cells. The present studies find that FCRL3 marks subpopulations of effector B lymphocytes and peaks on memory B cells with innate-like properties. In contrast to its inhibition of adaptive BCR signaling, FCRL3 enhanced B cell function in response to T cell-independent (TI) Toll-like receptor 9 (TLR9) stimulation, but repressed plasma cell (PC) differentiation and Ig production. Phospho-flow and biochemical analyses indicated that FCRL3 enhances TLR9-mediated B cell activation via the NF-κB and MAPK pathways, but inhibits BLIMP1 expression in an ERK-dependent manner. These findings reveal counter-regulatory roles for FCRL3 on B cell responses following TLR9 stimulation and indicate differential influence for it in innate versus adaptive signaling.
Results
FCRL3 expression peaks on memory B cells and discriminates a subset of class-switched B cells with innate-like features
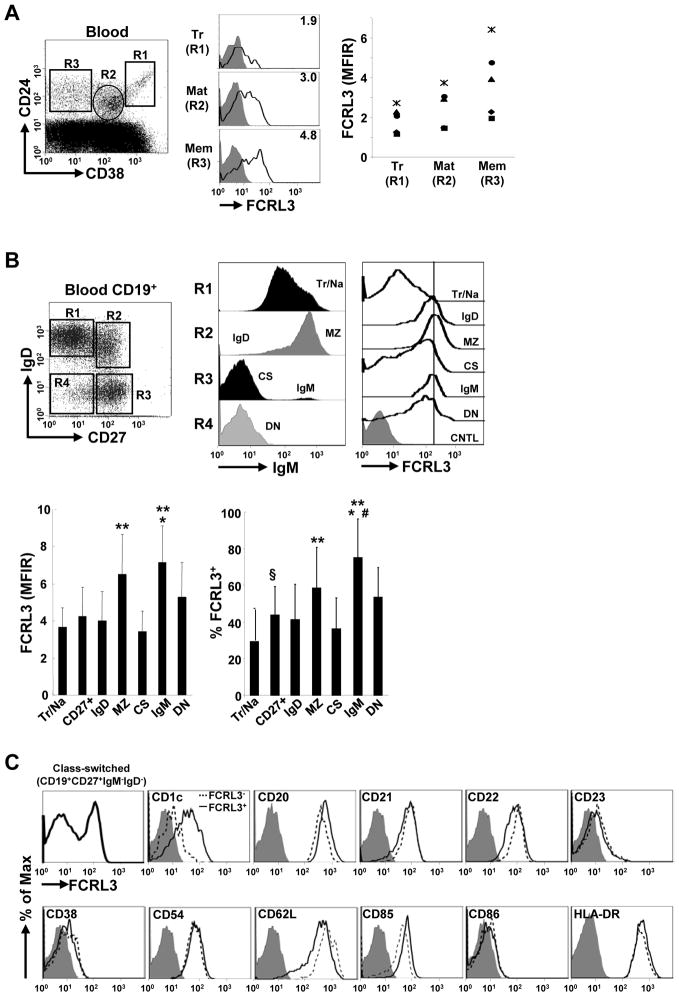
Given a greater appreciation for human B cell developmental heterogeneity over the past several years [29–31], we more extensively investigated the distribution of FCRL3 in the periphery. FCRL3 expression was relatively lower on transitional cells (CD24hiCD38++), but reached progressively higher surface density on mature (CD24intCD38+) and memory (CD24hiCD38−) blood B cells (Fig. 1A). The maturation-dependent increase in FCRL3 by these subsets was consistent among a group of five healthy donors. A comprehensive five color multi-parameter analysis to differentiate six circulating B cell subsets [29, 30] was then performed on a cohort of 20 healthy donors. These studies found higher FCRL3 frequencies on total unfractionated CD27+ memory B cells than CD27−IgM+IgD+ transitional/naïve cells, which make up the majority of B cells in the blood (Fig. 1B). Among memory subpopulations, FCRL3 was more abundant on IgM-only (CD27+IgM+IgD−) and marginal zone/natural effector (MZ) (CD27+IgM+IgD+) cells, but was slightly lower on IgD-only (CD27+IgM−IgD+) and class-switched (CS) (CD27+IgM−IgD−) B cells. Furthermore, the rare double-negative (DN) (CD27−IgD−IgM−) population, that includes CS cells and is expanded in SLE patients [32], also expressed modest to high levels of FCRL3. Intriguingly, it could also segregate CS B cells into two subsets. FCRL3 was co-expressed by CS cells bearing higher levels of the non-classical MHCI molecule CD1c, a characteristic feature of MZ B cells in mice and humans [29, 33], as well as CD20 and CD85, but lower CD22 and CD62L expression (Fig. 1C). These data indicate that FCRL3 expression increases as a function of differentiation and peaks on memory B cells, but exhibits the highest surface density on circulating IgM-only and MZ subsets followed by DN memory B cells. Furthermore, FCRL3 defines a unique subset of CS memory cells distinguished by CD1c expression. Along with the distribution of its mouse FCRL5 ortholog among MZ and B1 B cells [24], these data suggest a potential role for this molecule in memory B cells possessing innate-like characteristics.
Figure 1. FCRL3 expression increases as a function of B cell maturation and is a distinguishing marker of circulating memory B cell subsets.
(A) PBMCs from healthy adults were stained for the indicated markers and the MFI ratios (MFIR) of FCRL3 expression by transitional (Tr), mature (Mat), and memory (Mem) B cell subsets from five donors were compared. The MFIR was determined by dividing the MFI of the antigen-specific fluorochrome-conjugated mAb by the MFI of the irrelevant conjugated isotype-matched negative control mAb. A representative histogram shows FCRL3 (black line) staining versus an isotype-matched control (gray histogram) and the MFIR values for the gated subsets (R1–R3). (B) Negatively selected CD19+ blood B cells were stained with mAbs to CD19, IgD, CD27, IgM, and FCRL3 versus an isotype control. IgM staining by each subpopulation (R1 – R4) is shown and FCRL3 expression by the six different subsets defined by this combination of markers includes: transitional/naïve (Tr/Na), IgD-only (IgD), marginal zone/natural effector (MZ), class-switched (CS), IgM-only (IgM), and double-negative (DN) memory cells. For simplification, IgG1 control (CNTL) staining for total B cells is shown. The two bar graphs indicate the MFIR and mean frequency of FCRL3 expression by these subsets ± SD as determined by staining 20 healthy donors. ** vs Tr/Na, CD27+, IgD and CS P < 0.01; * vs DN P < 0.05; # vs MZ P < 0.05; § vs Tr/Na P < 0.05 by two-tailed t-test. (C) The class-switched (CS) memory B cell gate (CD19+CD27+IgM−IgD−) was analyzed for the indicated markers on FCRL3 positive (solid line) and negative (dashed line) subsets compared to an isotype-matched control (gray histogram).
FCRL3 ligation enhances TLR9/CpG-induced B cell activation and function
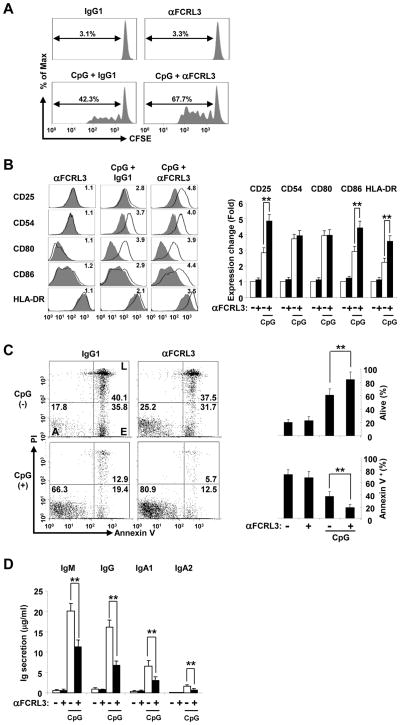
Innate-like and memory B cells constitutively express TLRs and promptly respond to CpG DNA agonists that activate TLR9 in a polyclonal fashion [34, 35]; however, mature-naïve B cells can also be stimulated by this pathway [36]. To explore its role in TI innate responses, we next investigated downstream outcomes of FCRL3 engagement in TLR9 triggered B cells. Given CpG’s broad stimulatory potential and FCRL3’s inducibility by TLR activation ([13] and data below), purified total CD19+ blood B cells were cultured with the CpG 2006 oligodeoxynucleotide TLR9 agonist as well as biotinylated F(ab′)2 digested mouse anti-FCRL3 or control IgG1 monoclonal antibody (mAb) fragments that were cross-linked with streptavidin (SA). Although culture with anti-FCRL3 at various concentrations had no effect on B cell proliferation after SA ligation for 48 hours, the addition of CpG combined with FCRL3 co-ligation enhanced B cell proliferation in a dose-dependent manner according to CFSE dilution and MTT assays (Fig. 2A and Supporting Information Fig. 1). We then examined a panel of activation-sensitive co-stimulatory and adhesion molecules under similar conditions. Cross-linking FCRL3 alone again showed no difference, but culture of CD19+ B cells with CpG up-regulated CD25, CD54, CD80, CD86 and HLA-DR to varying degrees (Fig. 2B). Notably, concomitant FCRL3 stimulation augmented CpG-mediated CD25, CD86, and HLA-DR expression at 48 hours, but did not markedly alter CD54 or CD80 expression. This finding implied that FCRL3 differentially modulates certain activation cascades. Its potential to regulate B cell survival was then addressed. While cross-linking FCRL3 slightly increased the percentage of live (A) cells compared to the control at 48 hours (Annexin-V−PI− 25.2% versus 17.8%) (Fig. 2C), CpG stimulation dramatically decreased early (E) and late (L) apoptosis overall (Annexin-V positive: 32.3% versus 75.9%). Importantly, FCRL3 ligation increased CpG-mediated survival (from 66.3% to 80.9%). These results demonstrate that FCRL3 engagement generally promotes CpG-induced B cell proliferation, activation, and survival.
Figure 2. FCRL3 has differential influence on CpG-mediated B cell activation.
(A) Blood B cells purified by negative selection were labeled with CFSE and cultured with biotinylated F(ab′)2 anti-FCRL3 (3 μg/ml) or an IgG1 control plus SA (20 μg/ml) in the presence or absence of CpG (2.5 μg/ml). Cells were harvested on day 4 and CFSE profiles were analyzed by flow cytometry to assess the frequency among total B cells that had undergone dye dilution. (B) FCRL3 promotes CpG-induced activation marker expression. B cells were cultured for 48 hours as in (A). Cells were stained for the indicated markers following stimulation (black line) versus incubation in medium-only (gray histogram). The fold difference in expression indicated in the histogram was calculated by dividing the post-stimulation MFIR of each antigen by the medium-only control stain. Isotype control stains did not vary and were excluded for simplicity. Mean fold differences in expression quantified from three different donors are detailed in the panel to the right. (C) FCRL3 enhances the pro-survival effects of CpG. B cells were cultured in the indicated conditions and collected at 48 hours to analyze apoptosis by propidium iodide (PI) and annexin-V staining. Numbers in the gates represent percentages of alive (A) cells, as well as early (E), and late (L) apoptotic events. Data representative of three different donors are summarized on the right. (D) FCRL3 inhibits CpG-induced Ig production. B cells were treated as above and after four days culture supernatants were assessed for Ig production by capture ELISA. The data presented are mean ± SD of independent experiments from three different donors. ** P < 0.001 vs CpG plus the IgG1 control by two-tailed t-test.
Given its positive effects on CpG activation, we then examined the influence of FCRL3 on TI B cell differentiation and Ig production. As previously established [34], CpG could induce blood B lymphocytes to differentiate into antibody-secreting cells as indicated by the detection of the IgM, IgG, and IgA isotypes in supernatants by ELISA after 5 days of culture (Fig. 2D). Surprisingly though, coincident FCRL3 cross-linking in these assays uniformly suppressed TLR9-mediated Ig production. These data indicate that although FCRL3 positively regulates several aspects of B cell activation, it inhibits antibody production.
FCRL3 augments CpG-induced B cell division, but inhibits PC and Ig production in a cord blood differentiation model
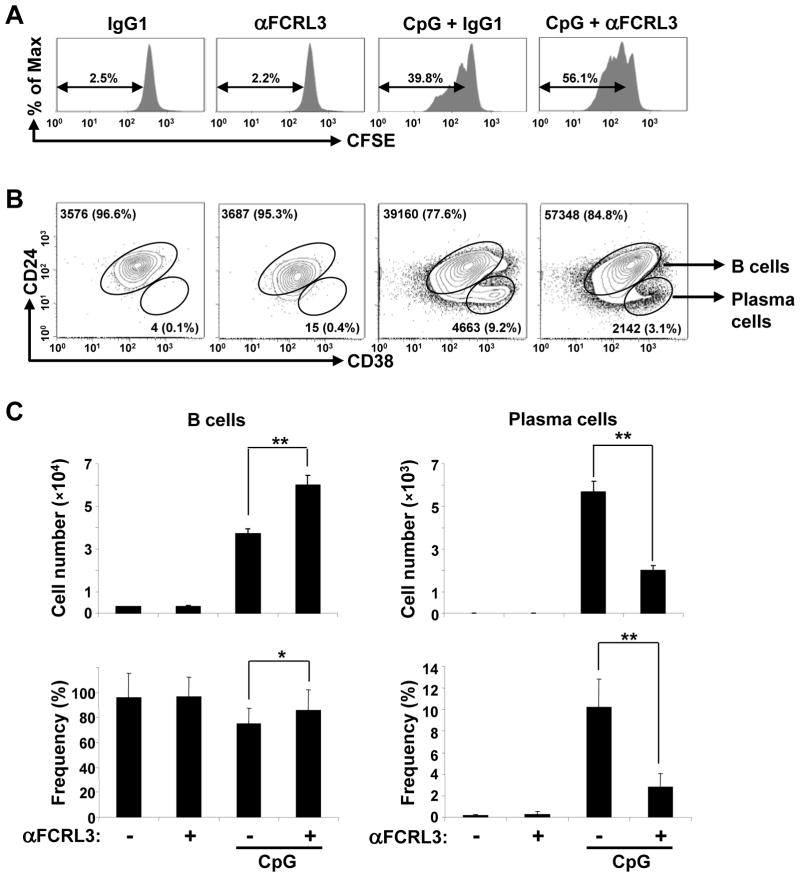
Because of FCRL3’s preferential expression by innate-like B cells and counter-regulatory effects on TLR9-dependent proliferation and Ig production in unfractionated CD19+ blood B cells, we considered work by the Carsetti group who found that transitional B cells, which are chiefly enriched in umbilical cord blood (CB), express TLR9 and can be driven to proliferate, terminally differentiate, and secrete broadly-reactive natural IgM and IgG antibodies in response to TI CpG stimulation [36]. To investigate its role using a defined subpopulation in a similar developmental context, we first determined whether CpG transactivates FCRL3 expression in CB B cells. As in adult blood samples, we could identify the CD24+CD38+ transitional subset, but after negative enrichment of umbilical CB this population comprised >99% of the CD19+ B cells (Supporting Information Fig. 2). Following CpG treatment, FCRL3 became evident on day 1 and peaked by days 2 and 4. As observed by Capolunghi et al. [36], a new subpopulation of CD24lowCD38++ CB-derived PC emerged on day 4 of culture. We then examined the dynamics and immunoregulation of FCRL3 in this system. FCRL3 ligation-alone again had no effect on proliferation as determined by the percentage of CFSE-labeled cells (Fig. 3A). Nor did it impact PC differentiation according to the number and percentage of CD24lowCD38++ cells (Fig. 3B–C). However, as in adult blood, FCRL3 engagement significantly enhanced proliferation in CpG-activated cultures. This was collectively indicated by the increased percentage of CFSE-labeled cells and rise in total cell numbers. While the number and frequency of cells bearing a PC phenotype were expanded in control cultures treated with CpG-only, addition of the anti-FCRL3 mAb significantly inhibited PC differentiation.
Figure 3. FCRL3 augments B cell division, but inhibits PC production in a CpG-inducible CB differentiation model.
(A) B cells purified from umbilical CB by negative selection were labeled with CFSE and treated as in Fig. 2A. Cells were harvested on day 4 and CFSE profiles were analyzed by flow cytometry to assess the dye dilution frequency among total B cells. (B) CB B cells cultured as above were harvested on day 4 and stained for CD19, CD24, and CD38 to detect B cells (CD24+CD38+) and PC (CD24lowCD38++). The values in the contour plots indicate the total number and frequency of each subset determined for one representative sample and results from triplicate cultures shown as the mean ± SD are quantified in (C). The data are representative of experiments from three different donors. ** P < 0.001 vs CpG plus the IgG1 control; * P < 0.05 vs CpG plus the IgG1 control by two-tailed t-test.
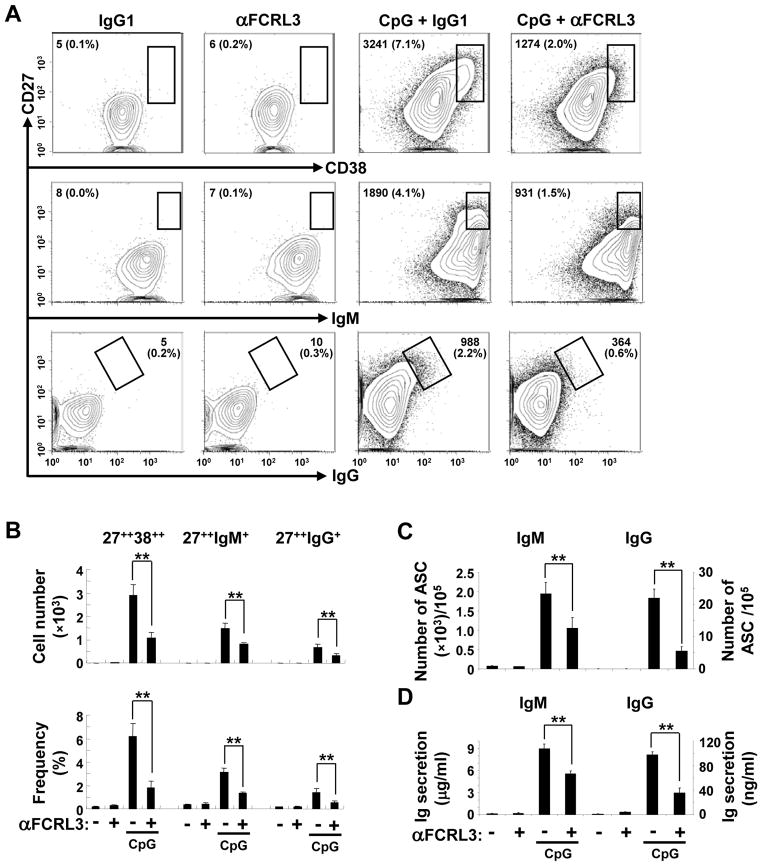
To further examine these CB-derived PCs and their Ig heavy-chain isotypes we also used an alternative phenotyping strategy [36, 37]. On day 4 cells were harvested and characterized for CD19, CD27, CD38, IgM, and IgG surface expression to qualify and quantify the emerging CD27hi PC population. Although very few CD27++ cells were detected in cultures in which FCRL3 or control mAbs were cross-linked alone, the total number and proportion of total CD27++CD38++ as well as CD27++IgM+ and CD27++IgG+ PC was much higher in CpG-stimulated wells (Fig. 4A–B). Importantly, despite an increased number of total B cells, combined stimulation with CpG and FCRL3 yielded a significantly lower proportion of CD27++CD38++ PC (2.4-fold), CD27++IgM+ (2.2-fold), and CD27++IgG+ (2.8-fold) PC compared to control cultures. The number and frequency of antibody-producing cells and their secreted products were then assessed. As shown in Fig. 4C, the absolute number of IgM and IgG-secreting cells following CpG + FCRL3 treatment was significantly lower than the control. The difference in this example, representative of three independent experiments, was 1.9-fold for IgM and 4.0-fold for IgG. Consistent with the ELISPOT data, ELISAs indicated a significant reduction in IgM and IgG production in supernatants derived from CpG + FCRL3 treated wells, with a decreased magnitude of 1.6- to 2.8-fold compared to the control (Fig. 4D). These findings indicate that FCRL3 has variable influence on B cell proliferation, PC differentiation, and Ig secretion.
Figure 4. FCRL3 inhibits CpG-induced PC differentiation and Ig production.
(A) Purified CB B cells were treated and cultured as in Fig. 3. Cells were harvested on day 4 for staining with mAbs specific to CD19, CD27, IgM, IgG, and CD38. Gates within the contour plots encompass CD38++CD27++, IgM+CD27++, and IgG+CD27++ PCs. Values indicate the total number and percentage of cells from all events harvested from single wells of a representative panel and (B) summarizes data enumerated in triplicate. (C) CB B cells treated as in panel A were analyzed by ELISPOT. On day four of culture, cells were plated in light-chain reactive antibody coated wells and incubated overnight prior to PC quantitation. (D) Ig production in supernatants harvested on day 4 was assessed by capture ELISA. The data are mean ± SD of triplicate cultures and are representative of experiments from three different donors. ** P < 0.001 vs CpG plus the IgG1 control by two-tailed t-test.
FCRL3 enhances CpG-induced NF-κB and MAPK signaling in B cells
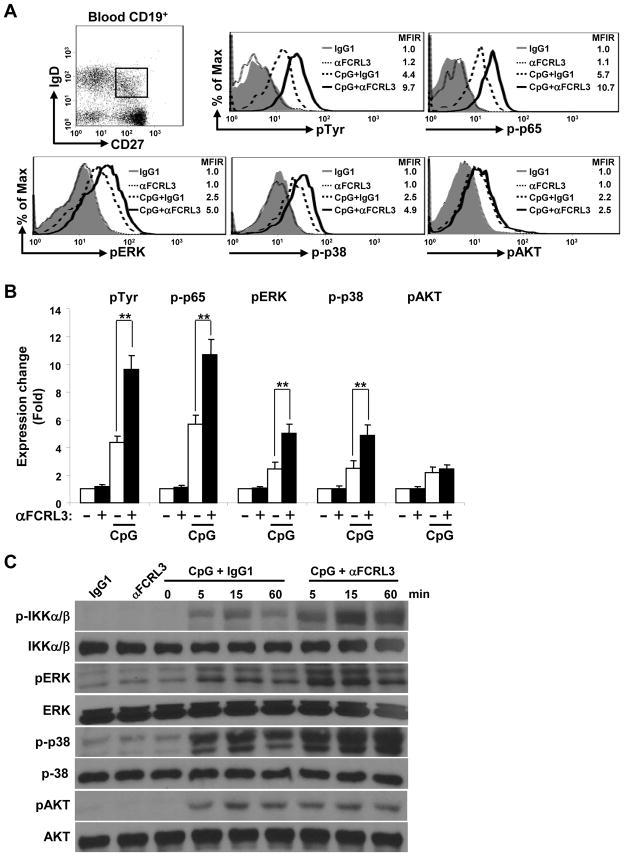
Given FCRL3’s tyrosine-based properties and the consequences of its ligation in TLR9 stimulated B cell responses, we next investigated its modulation of intracellular signaling. The interaction of CpG DNA with TLR9 is known to activate the NF-κB, ERK and p38 MAPK, as well as phosphatidylinositol 3-kinase (PI3K/AKT) pathways [38]. Because FCRL3 might have variable influence in different B cell subsets, we employed CD19+ blood B cells stimulated with CpG and/or FCRL3 for phospho-specific flow cytometry studies. Intracellular whole-cell pTyr as well as phosphorylation of p65 (NF-κB), ERK, p38, and AKT was quantitated in gated subpopulations stained for CD19, IgD, and CD27. Overnight culture with CpG alone promoted pTyr, p-p65, pERK, p-p38, and pAKT activation in all subsets, but the strongest effects were seen within the gated CD19+CD27+IgD+ fraction (Fig. 5A–B and Supporting Information Fig. 3). This population comprises the MZ natural-effector and IgM-only B cell subsets that also express the highest FCRL3 levels (see Fig. 1B). Importantly, cross-linking FCRL3 on CpG activated cells augmented whole-cell Tyr, p65, ERK, and p38 phosphorylation levels. In contrast, pAKT remained relatively unchanged. To validate these observations and further dissect these pathways with sufficient cell quantities, we performed biochemical analyses using the SUDHL5 human B cell line (EBV−; IgMλ). These cells endogenously express FCRL3, but can upregulate it following exposure to CpG (Supporting Information Fig. 4). Immunoblotting of whole-cell lysates confirmed that CpG induces phosphorylation of the IKKα/β NF-κB components, ERK and p38 MAPK elements, as well as AKT within minutes (Fig. 5C). Furthermore, while FCRL3 cross-linking enhanced p-IKKα/β, pERK, and p-p38, it again failed to influence p-AKT by this approach. These results demonstrate that FCRL3 has synergistic effects on NF-κB and MAPK pathway activation in CpG/TLR9 stimulated B cells.
Figure 5. FCRL3 augments CpG-induced NF-κB and MAPK pathway activation.
(A) CD19+ blood B cells purified by negative selection were cultured overnight in the presence or absence of CpG. Cells harvested the next day were stained for CD19, IgD, and CD27 as well as biotinylated F(ab′)2 anti-FCRL3 or an IgG1 control before SA ligation and stimulation for 20 minutes at 37°C. Cells were immediately fixed, permeabilized, and intracellularly labeled with Abs specific for pTyr, p-p65, pERK1/2, p-p38, pAKT or respective conjugated control Abs. The dot plot shows gating of the CD19+CD27+IgD+ population from which staining in the histograms is derived. MFIR values resulting from the indicated stimulation conditions are shown in the histograms. The fold change of the MFIR relative to the unstimulated control is quantified in (B) using the mean MFIR ± SD and is representative of experiments from three different donors. ** P < 0.001 vs CpG plus the IgG1 control by two-tailed t-test. (C) SUDHL5 B cells were treated as in Fig. 2 with the specified stimuli. At the indicated time intervals, cells were lysed and immunoblotted with the listed phospho- or protein-specific Abs to ensure equal loading. One representative of three independent experiments is shown.
FCRL3 impairs CpG-induced antibody production by suppressing BLIMP1 induction in an ERK-dependent manner
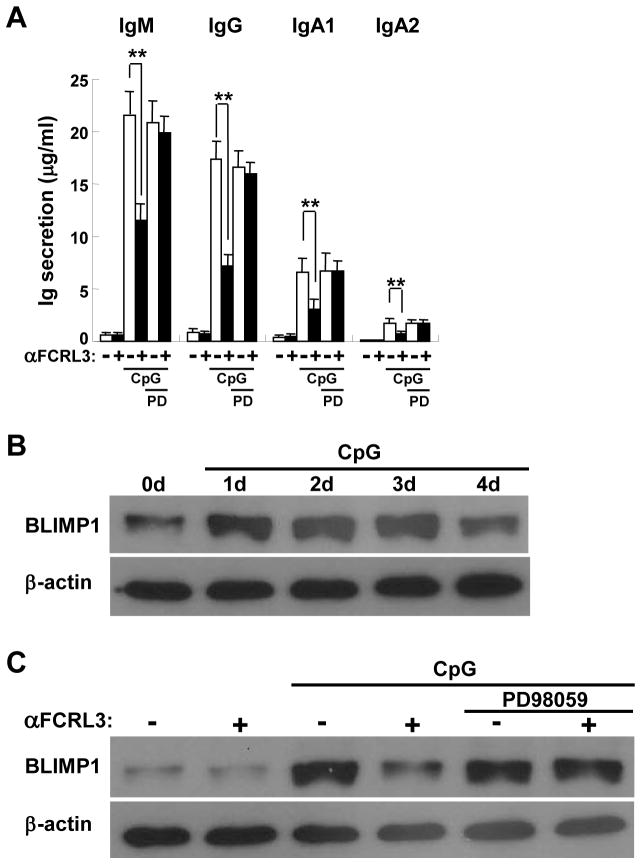
Because of its potent effects on CpG-induced B cell activation coincident with NF-κB and MAPK pathway stimulation, we explored the mechanistic basis for FCRL3’s repression of Ig and PC differentiation. Previous work by the Goodnow and Kurosaki groups highlighted an important role for ERK signaling in BLIMP1 regulation and PC differentiation [39–41]. To specifically assess the impact of ERK, we employed a selective MEK inhibitor, PD98059. Addition of this antagonist at a concentration of 20 μM did not effect Ig secretion by CpG-treated CD19+ purified blood B cells; however, PD98059 treatment blunted FCRL3-mediated suppression and restored Ig production (Fig. 6A). In addition to the findings in Fig. 5, these data indicate that enhanced ERK activation, triggered by FCRL3 co-ligation, inhibits CpG-stimulated antibody generation.
Figure 6. FCRL3 inhibits CpG-mediated Ig production by activating ERK and repressing BLIMP1.
(A) Blood B cells were pretreated with the PD98059 (20 μM) MEK inhibitor or DMSO control for 20 minutes prior to stimulation and analysis as in Fig. 2D. Data are mean ± SD of experiments from three independent donors. ** P < 0.001 vs CpG plus the IgG1 control by two-tailed t-test. (B) SUDHL5 B cells were treated with CpG (2.5 μg/ml) for the indicated times. Cell lysates were immunoblotted for BLIMP1 and β-actin as a loading control. (C) SUDHL5 B cells pretreated with the MEK inhibitor as above were stimulated as in Fig. 2 for 72 hours and lysates were immunoblotted for BLIMP1 and β-actin as a loading control. One representative from three independent experiments is shown.
To address the downstream mechanism directed by FCRL3 via ERK, we first examined the potential of CpG to induce BLIMP1 expression in SUDHL5 B cells. While constitutive BLIMP1 protein was detectable in this cell line, its expression markedly increased following successive culture in CpG and peaked after 2–3 days (Fig. 6B). Based on this time course we explored the impact of FCRL3 cross-linking on BLIMP1 modulation at 3 days. FCRL3 engagement alone revealed no difference, but BLIMP1 induction was strongly inhibited following CpG treatment (Fig. 6C). Importantly, addition of the PD98059 ERK inhibitor restored BLIMP1 protein levels, reversing the negative regulation driven by FCRL3. These results find that FCRL3 halts CpG-mediated PC differentiation and antibody production by augmenting ERK pathway activation, which in turn suppresses the induction of BLIMP1.
Discussion
This study demonstrates that FCRL3, a potential inhibitor of BCR signaling [18], has counter-regulatory effects on B cells exposed to the TI TLR9 agonist CpG 2006. Examination of its distribution among circulating B lineage cells revealed that FCRL3 reaches highest surface levels on mature and memory subsets, and prominently marks the MZ, IgM-only, and DN populations. FCRL3 could also segregate CD27+ CS cells and was co-expressed by a subpopulation characterized by elevated CD1c expression, a classic feature of innate-like MZ B cells in mice and humans [29, 33]. The discrete expression of its FCRL5 relative by mouse splenic MZ and peritoneal cavity-derived B1 B cells [24], implies evolutionary conservation for the regulation pattern of these two FCRLs in the B lineage. Its distribution is also remarkable given FCRL3’s identification as a biomarker of a more favorable CLL subtype [9]. While the normal cellular counterpart of CLL remains unknown, evidence suggests that it likely derives from a MZ or B1-like B cell equivalent [42]. These relationships between healthy and perturbed B lymphocytes highlight the preferential innate-like qualities of FCRL3+ B cells.
Its induction by TLR9 activation in earlier stage subsets (e.g. transitional B cells) and ability to enhance CpG-driven proliferation, activation marker expression, and survival, all demonstrate that FCRL3 potentiates innate signaling in B cells. A similar dynamic has been observed for the ITAM-coupled FCRL relative FcγRIII (CD16) [43], and is suggested by the contribution of human FCRL4 to TLR9-based CD23 induction [44]. When triggered in tandem, ITAM and TLR-mediated transduction pathways can interface through the CARD11/Bcl10/Malt1 (CBM) adaptor complex to enhance antigen presentation, proliferation, survival, and NF-κB and MAPK activation [45]. Although the ligand for FCRL3 remains unknown, its potential for Syk recruitment [18, 23] and localization akin to FCRL4 in endosomes [44], would provide a substrate for driving activation signals through the CBM complex. Recent data showing that mouse FCRL5 directly recruits Lyn to its ITAM-like sequence and initiates positive signals in B cells suggests it might also operate through this circuit [25].
Although FCRL3 could co-stimulate several TLR9-mediated responses, it inhibited PC differentiation and Ig secretion. An FCRL3-dependent checkpoint at the B cell to PC transition was suggested by studies with adult CD19+ and CB transitional B cells. Consistent with previous work regarding ERK-mediated regulation of PC differentiation [39–41], we found that synergistic ERK activation conducted by FCRL3 could repress TLR-induced BLIMP1 expression. Because ERK antagonism did not halt CpG-triggered Ig production in the absence of FCRL3 cross-linking, these results indicate that perturbation of FCRL3 can impact ERK activity and modulate different downstream effects. Thus, like the BCR [39, 40], FCRL3 is also capable of transducing ERK signals that regulate TLR-driven BLIMP1 transactivation and PC differentiation. Human FCRL5 has also been found to promote CpG-mediated B cell proliferation, but its influence on PC differentiation contrasted with the results shown here for FCRL3 [46]. Notably, this potential disparity could be due to the different technical approaches used. In particular, along with CpG and FCRL5 stimulation, Dement-Brown et al. included BCR triggering with or without T cell help in the form of IL-2. Using this strategy, rather than inhibiting PC differentiation, FCRL5 enhanced the differentiation of antibody secreting cells. Although the mechanistic basis for this was not examined, based our current findings and earlier work by Rui et al [39, 40], the addition of IL-2 could inactivate the ERK-dependent blockade by inducing DUSP5 which can relieve BLIMP1 repression. It will be important to dissect how FCRLs regulate these different stimulatory pathways in future work.
Despite its increasing genetic associations with AI, it is challenging to explain how a receptor that inhibits BCR signaling [18] and is upregulated in disease-susceptible persons with the -169C allele could be pathogenic. Notably, two groups have found that FCRL3 discriminates a Treg subpopulation that exhibits an exhausted phenotype, has defective proliferation following antigen-receptor activation in the presence of IL-2, and is impaired at suppressing effector CD8+ T cell proliferation [21, 22]. While compromised Treg suppression could feasibly hamper tolerance mechanisms, how FCRL3 could foster AI pathogenesis by restraining BCR signaling is harder to rationalize.
Its preferential distribution among innate-like B cells, which parallels the expression of its FCRL5 ortholog in mice, transactivation by TLR stimuli, and NF-κB dependent regulation [13], collectively indicate that FCRL3 influences TI responses. Our recent findings that mouse FCRL5 has SHP-1/Lyn-dependent dual-functionality also uncovered subset-specific differences in its function that varied according the activity of these signaling proteins in MZ versus B1 B cells [24]. The differential responsivity of human B cell subpopulations to TLR9/FCRL3 stimulation also emerged in the phospho-flow analysis reported here. Based on these findings it seems possible that constitutive FCRL3 expression by innate-like B cells may be important for catalyzing prompt responses critical for host protection to innate stimuli. However, FCRL3 can also be induced by innate agonists in other subsets that don’t basally express it. Innate activation fueled by endogenous or foreign antigens may initiate or amplify AI effector responses by B lymphocytes [43, 47]. ERK also plays a key role in tolerogenic maintenance of B cells in response to TLR signaling [39, 40]. Thus, elevated FCRL3 levels associated with the −169C allele could predispose disease-susceptible individuals to heightened TI innate-sensing functions in B cells and possibly other lymphocytes that express it. Importantly, TLR9-dependent Ig production by FCRL3+ B cells was higher in the absence of its exogenous engagement. This finding coupled with FCRL3’s: (i) expression by innate-like B cells that feature broadly-reactive pre-immune Ig repertoires [29, 48], (ii) ability to modulate ERK and BLIMP1, and (iii) genetic SNP-based linkage with autoantibody production and AI disease progression [13, 28], are especially intriguing associations for future study.
In summary, we demonstrate that FCRL3 has potent co-stimulatory influence on TLR9-mediated B cell activation, but inhibits PC differentiation and antibody production by enhancing ERK-dependent suppression of BLIMP1. Elucidating its ligand(s), determining how it counter-regulates responses in the diverse types of effector lymphocytes that express it, and defining whether its regulatory polymorphism perturbs its biology contributing to disease pathogenesis, all remain areas for further exploration that will hopefully improve the understanding and treatment of immune-mediated disorders.
Materials and methods
Antibodies, reagents, cell lines, and immunoblotting
Details of the Abs, reagents, cell line, and immunoblotting protocol are located in the Supplemental Methods.
B cell isolation and flow cytometry
Venous blood was solicited from healthy donors and remnant umbilical cord blood (CB) samples were obtained from the University of Alabama at Birmingham (UAB) with IRB approval. PBMC were prepared by Ficoll separation and CD19+ B cells were purified from adult or CB by negative selection using the EasySep technique (StemCell Technologies). A second round of selection yielded >99% purity. Stained cells were either analyzed with a FACSCalibur (BD Biosciences) or a CyAn ADP flow cytometer (Beckman Coulter) and data was plotted using FlowJo software (Tree Star).
B cell activation, proliferation, apoptosis, and Ig secretion assays
Purified CD19+ blood B cells or CB B cells were cultured in triplicate (2.5 × 105). For cell proliferation, cells were labeled with CFSE using the CellTrace Cell Proliferation kit (Invitrogen) and incubated with the indicated mAb and/or CpG combinations. Cells were harvested on day 4 and CFSE profiles were analyzed by flow cytometry to assess the dye dilution frequency among total B cells. Apoptosis was determined at 48 hours by flow cytometry after staining with annexin V-FITC and propidium iodide (PI) (Imgenex Corporation). Ig production was quantitated by capture ELISA (Corning). Briefly, plates were coated with Abs overnight and incubated with supernatants for 1 hour at 37°C. After a 1 hour incubation with alkaline phosphatase (AP)-labeled Abs, plates were developed with an AP substrate (Sigma-Aldrich) and quantitated with a spectrophotometer (LabSystem). For ELISPOT assays, cells were added to plates precoated with the polyclonal light-chain Abs. After lysis and debris removal by washing in PBS 0.05% Tween 20, AP-labeled goat anti-human IgM or IgG with 1% gelatin was added and incubated overnight at 4°C. Spots were developed using the BCIP substrate (Sigma-Aldrich) and quantitated using a Nikon Phase Contrast dissecting microscope (Nikon Inc.).
Phospho-specific intracellular staining
For intracellular analyses, 1 × 106 blood B cells were cultured in RPMI 1640 complete medium overnight in the presence or absence of CpG 2006 (2.5 μg/ml). Cells were collected and labeled with mouse anti-human IgD-FITC, CD27-PE-Cy7, and biotinylated F(ab′)2 fragments of anti-FCRL3 or IgG1 control mAbs at different concentrations on ice for 20 minutes. After ligation with SA, cells were incubated for 20 minutes at 37°C, prepped using the Fix and Perm kit (BD), and stained with the indicated phospho-specific Abs before flow cytometry analysis.
Statistics
Data are expressed as means ± SD of at least three independent experiments performed in triplicate. Statistical significance was determined using the unpaired two-tailed Student’s t-test.
Supplementary Material
Acknowledgments
We would like to thank members of the Davis laboratory for their advice and insightful comments, Dr. John F. Kearney for critical evaluation of the manuscript, the donors that participated in this study, and the UAB Tissue Procurement Facility (CA13148). This work was supported in part by NIH grants AI55638, AI067467, and CA161731, the CLL Global Research Foundation, and the Cancer Research Institute (R.S.D.).
Abbreviations
- FCRL
Fc receptor-like
- CLL
chronic lymphocytic leukemia
- TI
T cell-independent
- PC
plasma cell
- SA
streptavidin
- MZ
marginal zone/natural effector
- CS
class-switched
- DN
double-negative
Footnotes
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 2.Hatzivassiliou G, Miller I, Takizawa J, Palanisamy N, Rao PH, Iida S, Tagawa, et al. IRTA1 and IRTA2, novel immunoglobulin superfamily receptors expressed in B cells and involved in chromosome 1q21 abnormalities in B cell malignancy. Immunity. 2001;14:277–289. doi: 10.1016/s1074-7613(01)00109-1. [DOI] [PubMed] [Google Scholar]
- 3.Davis RS, Wang YH, Kubagawa H, Cooper MD. Identification of a family of Fc receptor homologs with preferential B cell expression. Proc Natl Acad Sci USA. 2001;98:9772–9777. doi: 10.1073/pnas.171308498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guselnikov SV, Ershova SA, Mechetina LV, Najakshin AM, Volkova OY, Alabyev BY, Taranin AV. A family of highly diverse human and mouse genes structurally links leukocyte FcR, gp42 and PECAM-1. Immunogenetics. 2002;54:87–95. doi: 10.1007/s00251-002-0436-x. [DOI] [PubMed] [Google Scholar]
- 5.Davis RS. Fc receptor-like molecules. Annu Rev Immunol. 2007;25:525–560. doi: 10.1146/annurev.immunol.25.022106.141541. [DOI] [PubMed] [Google Scholar]
- 6.Schreeder DM, Cannon JP, Wu J, Li R, Shakhmatov MA, Davis RS. Cutting edge: FcR-like 6 is an MHC class II receptor. J Immunol. 2010;185:23–27. doi: 10.4049/jimmunol.1000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell JA, Davis RS, Lilly LM, Fremont DH, French AR, Carayannopoulos LN. Cutting edge: FcR-like 5 on innate B cells is targeted by a poxvirus MHC class I-like immunoevasin. J Immunol. 2010;185:28–32. doi: 10.4049/jimmunol.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson TJ, Fuchs A, Colonna M. Cutting Edge: Human FcRL4 and FcRL5 Are Receptors for IgA and IgG. J Immunol. 2012 doi: 10.4049/jimmunol.1102651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li FJ, Ding S, Pan J, Shakhmatov MA, Kashentseva E, Wu J, Li Y, et al. FCRL2 expression predicts IGHV mutation status and clinical progression in chronic lymphocytic leukemia. Blood. 2008;112:179–187. doi: 10.1182/blood-2008-01-131359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schreeder DM, Pan J, Li FJ, Vivier E, Davis RS. FCRL6 distinguishes mature cytotoxic lymphocytes and is upregulated in patients with B-cell chronic lymphocytic leukemia. Eur J Immunol. 2008;38:3159–3166. doi: 10.1002/eji.200838516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, Kayentao K, et al. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol. 2009;183:2176–2182. doi: 10.4049/jimmunol.0901297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochi Y, Yamada R, Suzuki A, Harley JB, Shirasawa S, Sawada T, Bae SC, et al. A functional variant in FCRL3, encoding Fc receptor-like 3, is associated with rheumatoid arthritis and several autoimmunities. Nat Genet. 2005;37:478–485. doi: 10.1038/ng1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rakhmanov M, Keller B, Gutenberger S, Foerster C, Hoenig M, Driessen G, van der Burg M, et al. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc Natl Acad Sci USA. 2009;106:13451–13456. doi: 10.1073/pnas.0901984106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leu CM, Davis RS, Gartland LA, Fine WD, Cooper MD. FcRH1: an activation coreceptor on human B cells. Blood. 2005;105:1121–1126. doi: 10.1182/blood-2004-06-2344. [DOI] [PubMed] [Google Scholar]
- 16.Ehrhardt GR, Davis RS, Hsu JT, Leu CM, Ehrhardt A, Cooper MD. The inhibitory potential of Fc receptor homolog 4 on memory B cells. Proc Natl Acad Sci USA. 2003;100:13489–13494. doi: 10.1073/pnas.1935944100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haga CL, Ehrhardt GR, Boohaker RJ, Davis RS, Cooper MD. Fc receptor-like 5 inhibits B cell activation via SHP-1 tyrosine phosphatase recruitment. Proc Natl Acad Sci USA. 2007;104:9770–9775. doi: 10.1073/pnas.0703354104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kochi Y, Myouzen K, Yamada R, Suzuki A, Kurosaki T, Nakamura Y, Yamamoto K. FCRL3, an autoimmune susceptibility gene, has inhibitory potential on B-cell receptor-mediated signaling. J Immunol. 2009;183:5502–5510. doi: 10.4049/jimmunol.0901982. [DOI] [PubMed] [Google Scholar]
- 19.Jackson TA, Haga CL, Ehrhardt GR, Davis RS, Cooper MD. FcR-like 2 Inhibition of B cell receptor-mediated activation of B cells. J Immunol. 2010;185:7405–7412. doi: 10.4049/jimmunol.1002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polson AG, Zheng B, Elkins K, Chang W, Du C, Dowd P, Yen L, et al. Expression pattern of the human FcRH/IRTA receptors in normal tissue and in B-chronic lymphocytic leukemia. Int Immunol. 2006;18:1363–1373. doi: 10.1093/intimm/dxl069. [DOI] [PubMed] [Google Scholar]
- 21.Nagata S, Ise T, Pastan I. Fc receptor-like 3 protein expressed on IL-2 nonresponsive subset of human regulatory T cells. J Immunol. 2009;182:7518–7526. doi: 10.4049/jimmunol.0802230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swainson LA, Mold JE, Bajpai UD, McCune JM. Expression of the autoimmune susceptibility gene FcRL3 on human regulatory T cells is associated with dysfunction and high levels of programmed cell death-1. J Immunol. 2010;184:3639–3647. doi: 10.4049/jimmunol.0903943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu MJ, Zhao R, Cao H, Zhao ZJ. SPAP2, an Ig family receptor containing both ITIMs and ITAMs. Biochem Biophys Res Commun. 2002;293:1037–1046. doi: 10.1016/S0006-291X(02)00332-7. [DOI] [PubMed] [Google Scholar]
- 24.Won WJ, Foote JB, Odom MR, Pan J, Kearney JF, Davis RS. Fc receptor homolog 3 is a novel immunoregulatory marker of marginal zone and b1 B cells. J Immunol. 2006;177:6815–6823. doi: 10.4049/jimmunol.177.10.6815. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Z, Li R, Li H, Zhou T, Davis RS. FCRL5 exerts binary and compartment-specific influence on innate-like B-cell receptor signaling. Proc Natl Acad Sci U S A. 2013;110:E1282–1290. doi: 10.1073/pnas.1215156110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibson AW, Li FJ, Wu J, Edberg JC, Su K, Cafardi J, Wiener H, et al. The FCRL3 −169CT promoter single-nucleotide polymorphism, which is associated with systemic lupus erythematosus in a Japanese population, predicts expression of receptor protein on CD19+B cells. Arthritis Rheum. 2009;60:3510–3512. doi: 10.1002/art.24915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chistiakov DA, Chistiakov AP. Is FCRL3 a new general autoimmunity gene? Hum Immunol. 2007;68:375–383. doi: 10.1016/j.humimm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Bajpai UD, Swainson LA, Mold JE, Graf JD, Imboden JB, McCune JM. A functional variant in FCRL3 is associated with higher Fc receptor-like 3 expression on T cell subsets and rheumatoid arthritis disease activity. Arthritis Rheum. 2012;64:2451–2459. doi: 10.1002/art.34457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanz I, Wei C, Lee FE, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol. 2008;20:67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berkowska MA, Driessen GJ, Bikos V, Grosserichter-Wagener C, Stamatopoulos K, Cerutti A, He B, et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood. 2011;118:2150–2158. doi: 10.1182/blood-2011-04-345579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, Lee EH, et al. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. Journal of Immunology. 2007;178:6624–6633. doi: 10.4049/jimmunol.178.10.6624. [DOI] [PubMed] [Google Scholar]
- 33.Amano M, Baumgarth N, Dick MD, Brossay L, Kronenberg M, Herzenberg LA, Strober S. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: beta 2-microglobulin-dependent and independent forms. J Immunol. 1998;161:1710–1717. [PubMed] [Google Scholar]
- 34.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 35.Gururajan M, Jacob J, Pulendran B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS One. 2007;2:e863. doi: 10.1371/journal.pone.0000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capolunghi F, Cascioli S, Giorda E, Rosado MM, Plebani A, Auriti C, Seganti G, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol. 2008;180:800–808. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 37.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeshita F, Gursel I, Ishii KJ, Suzuki K, Gursel M, Klinman DM. Signal transduction pathways mediated by the interaction of CpG DNA with Toll-like receptor 9. Semin Immunol. 2004;16:17–22. doi: 10.1016/j.smim.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Rui LX, Vinuesa CG, Blasioli J, Goodnow CC. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nature Immunology. 2003;4:594–600. doi: 10.1038/ni924. [DOI] [PubMed] [Google Scholar]
- 40.Rui L, Healy JI, Blasioli J, Goodnow CC. ERK signaling is a molecular switch integrating opposing inputs from B cell receptor and T cell cytokines to control TLR4-driven plasma cell differentiation. J Immunol. 2006;177:5337–5346. doi: 10.4049/jimmunol.177.8.5337. [DOI] [PubMed] [Google Scholar]
- 41.Yasuda T, Kometani K, Takahashi N, Imai Y, Aiba Y, Kurosaki T. ERKs induce expression of the transcriptional repressor Blimp-1 and subsequent plasma cell differentiation. Sci Signal. 2011;4:ra25. doi: 10.1126/scisignal.2001592. [DOI] [PubMed] [Google Scholar]
- 42.Chiorazzi N, Ferrarini M. Cellular origin(s) of chronic lymphocytic leukemia: cautionary notes and additional considerations and possibilities. Blood. 2011;117:1781–1791. doi: 10.1182/blood-2010-07-155663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sohn HW, Krueger PD, Davis RS, Pierce SK. FcRL4 acts as an adaptive to innate molecular switch dampening BCR signaling and enhancing TLR signaling. Blood. 2011;118:6332–6341. doi: 10.1182/blood-2011-05-353102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ivashkiv LB. Cross-regulation of signaling by ITAM-associated receptors. Nature Immunology. 2009;10:340–347. doi: 10.1038/ni.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dement-Brown J, Newton CS, Ise T, Damdinsuren B, Nagata S, Tolnay M. Fc receptor-like 5 promotes B cell proliferation and drives the development of cells displaying switched isotypes. J Leukoc Biol. 2012;91:59–67. doi: 10.1189/jlb.0211096. [DOI] [PubMed] [Google Scholar]
- 47.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunol Rev. 2000;175:70–79. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.