Abstract
People carrying germline mutations in mismatch repair genes are at high risk of colorectal cancer (CRC), yet about half of people from mutation-carrying families decline genetic counselling and/or testing to identify mutation status. We studied the association of quantitative measures of risk perception, risk prediction and self-reported screening colonoscopy in this elusive yet high-risk group. The sample of 26 participants (mean age 43.1 years, 14 women) in the Australasian Colorectal Cancer Family Registry were relatives of mutation carriers; had not been diagnosed with any cancer at the time of recruitment and had declined an invitation to attend genetic counselling and/or testing. A structured elicitation protocol captured perceived CRC risk over the next 10 years. Self-reported colonoscopy screening was elicited during a 45-minute semi-structured interview. Predicted 10-year CRC risk based on age, gender, known mutation status and family history was calculated using “MMRpro.” Mean perceived 10-year risk of CRC was 31% [95% CI 21, 40], compared with mean predicted risk of 4% [2, 7[ (p<0.001); this was independent of age and sex (p = 0.9). Among those reporting any medical advice and any screening colonoscopy (n = 18), those with higher risk perception had less frequent colonoscopy (Pearson’s r = 0.49 [0.02, 0.79]). People who decline genetic testing for CRC susceptibility mutations perceive themselves to be at substantially higher risk than they really are. Those with high perceived risk do not undertake screening colonoscopy more often than those who perceive themselves to be at average risk.
Keywords: colorectal cancer, risk perception, screening colonoscopy, mismatch repair, genetic testing
INTRODUCTION
Germline mutations in one of the mismatch repair genes (MLH1, MSH2, MSH6, PMS2) predispose people to colorectal cancer (CRC), endometrial cancer and some other cancers known as Lynch syndrome (Lynch & Chapelle 2003). Carriers of mutations in these genes have an approximate 30–60% lifetime risk (to age 70 years) of CRC and 30–50% lifetime risk of endometrial cancer depends on age and gender of carriers and the type of gene that is mutated (Baglietto et al. 2010; Bonadona et al. 2011; Senter et al. 2008). The only way to determine if unaffected people in families with a mismatch repair gene mutation have or have not inherited that mutation is genetic testing.
Testing people for a mismatch repair gene mutation is clinically important. Once identified, carriers are recommended annual or biennial colonoscopy screening to reduce CRC risk (de Jong et al. 2006; Jarvinen et al. 2000); and prophylactic hysterectomy and bilateral salpingo-oophrectomy to reduce risk of endometrial and ovarian cancers (Schmeler et al. 2006). Colonoscopy screening reduces risk of CRC by 56% and death by 65% (Jarvinen et al. 2000). Conversely, once identified, non-carriers from mutation-carrying families are at population risk of CRC (Win et al. 2012) and can be relieved of the burden of this intensive screening program. People who learn they are not a carrier have reduced cancer-specific distress lasting at least three years after testing (Collins et al. 2007).
Despite these benefits of genetic testing, about one-half of people from families known to have a mutation carrier do not attend family cancer genetic services to learn about their own genetic results or personal cancer risk (Keogh et al. 2009). But it is not known why people who are at potentially high risk of CRC do not utilise genetic testing and counseling services, and also how this non-attendance relates to screening colonoscopy uptake. Part of the reason for this knowledge gap is that, within standard clinical and research settings, it is difficult to access and enroll people from mutation-carrying families who decline genetic testing.
Personal risk perception is thought to influence precautionary health behaviour such as screening, and therefore it occupies a central role in several health behaviour models. For example, in the “health action process approach,” risk perception is regarded as a crucial step in the decision pathway leading to healthy behaviours (Schwarzer et al. 2008). There are two key aspects to examining the relationship between perceived risk and screening behaviour. Firstly, it is necessary to know whether the people offered genetic testing (usually because of a known family mutation or a strong family history of the disease) consider themselves at increased risk of CRC. Mack et al. (2009) reported a certain degree of uncertainty by people regarding their personal risk level in relation to their family history, although in most similar studies people were aware of their increased risk relative to the general population (Fletcher et al. 2007; Harris & Byles 1997; Harris et al. 1998; Longacre et al. 2006; Palmer et al. 2007). Secondly, it is necessary to understand how perceived risk relates to screening behaviour. Several studies have found a positive correlation between perceived risk and likelihood to screen for CRC (Codori et al. 2001; Gimeno Garcia et al. 2011; Manne et al. 2002; Palmer et al. 2007; Taouqui et al. 2010), while others have found no such correlation (Longacre et al. 2006; Madlensky et al. 2003; Santos et al. 2011).
Although risk perception may influence screening, the reverse may also apply; undergoing screening can actually reduce perceived risk of developing CRC, in concordance with the Risk Reappraisal Hypothesis (Brewer et al. 2004; Glenn et al. 2011). Furthermore, the impact of genetic risk on screening behaviour can be variable, leading to both over- and under-utilisation of services. Shiloh and Ilan (2005) studied the relationship between risk perceptions and women’s interest in predictive genetic testing for breast cancer and screening. They found some conditions under which risk perceptions may enhance screening behaviours and other conditions under which they may not. However, following genetic testing (Collins et al. 2005) or genetic counselling (Armelao et al. 2010) people at high risk for CRC did show an increase in screening appropriate to their risk. In contrast, we know little about risk perception and screening behaviours in people who decline genetic testing. Overall, these studies suggest it is possible that over-estimation of personal CRC risk may be accompanied by under-utilisation of screening.
For people at population risk, Cole et al. (2007) found in a community study that risk messages alone were not sufficient to increase uptake of faecal occult blood testing. For people at risk of familial cancers there are limited studies on risk perception and screening behaviour decisions. The majority of studies in this area have been on women with hereditary breast and ovarian cancer. For example, Katapodi et al. (2004) found a positive association between perceived risk and mammography screening, genetic testing and prophylactic mastectomy. However, there are significant differences between this group and people at risk of hereditary CRC. Firstly, the emphasis in counselling of hereditary CRC is on provision of screening, whereas management of risk for hereditary breast and ovarian cancer also focuses on prophylactic mastectomy or oophorectomy. Secondly, both men and women have significantly increased risks of developing CRC and therefore, findings from hereditary breast and ovarian cancer may not be applicable to hereditary CRC settings.
All previous studies of colonoscopy screening following genetic counselling have evaluated people who have agreed to genetic testing. A survey conducted three years after mismatch repair gene mutation testing in an Australian family cancer centre showed screening behaviour in accordance with guidelines for both carriers (n = 19) and non-carriers (n = 54) (Collins et al. 2007). Similar adherence to screening by carriers has been reported elsewhere (Claes et al. 2005; Hadley et al 2004). However there have been, to our knowledge, no studies on screening adherence for the at-risk group who have declined genetic testing. We address this gap by reporting on the risk perception and screening behaviour of people at risk of hereditary colorectal cancer who have not accepted genetic counselling or genetic testing. Specifically, we investigated: 1) personal CRC risk perception in members of families with mismatch repair mutations, and 2) the relationship of this personal risk perception to screening decision-making.
METHODS
Participants
Participants in this study were recruited by the Australasian Colorectal Cancer Family Registry (ACCFR), a large family cohort which is part of the Colon Cancer Family Registry, an international consortium funded by the National Cancer Institute (U.S.A.) (Newcomb et al. 2007). The ACCFR was established in 1997 and currently contains data from approximately 10,500 participants representing approximately 1500 colorectal cancer families. The ACCFR recruited family members via population-based probands (i.e., recently diagnosed CRC cases) from the Victorian Cancer Registry (Australia), and via clinic-based probands from multiple-case families referred to family cancer genetics clinics in Australia (Melbourne, Adelaide, Perth, Brisbane, Sydney) and New Zealand (Auckland).
At the time of recruitment for this study there were 1250 ACCFR participants from 188 mismatch repair gene mutation-carrying families. Deceased participants and those lost to followup were excluded. To be eligible for this study, participants had to be from families in which at least one person had been identified by genetic testing as carrying a deleterious mutation in a mismatch repair gene, had to be between 18 and 69 years old at recruitment into this study, had no previous diagnosis of CRC or any other cancer, and had to have declined an offer within the previous ten years by the ACCFR to attend a genetics service to receive their individual genetic test results (n = 134). Of these, 47 agreed to be contacted about the study; 21 of these were found to be ineligible because of having previous genetic testing or cancer, or they subsequently declined to be interviewed. The 26 participants included in the study represent 22 mismatch repair mutation-carrying families (two participants per family for four families). Screening for germline mutations in MLH1, MSH2, MSH6 and PMS2 was performed for all population-based probands who had a colorectal tumour displaying evidence of impaired mismatch repair function. Impaired mismatch repair was evidenced by either microsatellite instability (MSI), or by lack of mismatch repair protein expression by immunohistochemistry. Screening was performed also for the youngest onset CRC case from each clinic-based family regardless of MSI or mismatch repair protein expression status, and for their family members if they were found to have deleterious mismatch repair gene mutations (described in detail elsewhere) (Win et al. 2012). Participant characteristics including mutation status are summarised in Table 1.
Table I.
MMR mutation, carrier status, perceived risk estimates, predicted risk (MMRpro), gender, age, reported screening history and medical screening advice.
| ID | MMR Gene mutate d in the family | Carrier status1 | Risk Estimate | Perce ived Risk: | Gender | Age at | Reported Screening Colonoscopy History (Years) | ||
|---|---|---|---|---|---|---|---|---|---|
| Percei ved | Predic ted (MMR pro) | Predi cted Risk | Inter view | Last Screened | Advised Interval between Screens | ||||
| D18 | MSH2 | Non-carrier | 1 | 1.1 | 0.91 | M | 58 | 2 | 4 |
| D10 | MLH1 | Carrier | 20 | 15.2 | 1.32 | F | 42 | 3 | 1 |
| D6 | MLH1 | Carrier | 40 | 29.4 | 1.36 | M | 44 | 2 | 5 |
| D14 | MSH2 | Carrier | 20 | 10 | 2.00 | M | 32 | Never | Start After 30yo |
| D1 | MSH6 | Non-carrier | 4 | 1.7 | 2.35 | M | 65 | 10 | No Medical Advice |
| D26 | PMS2 | Untested, daughter of carrier | 10 | 3.2 | 3.13 | F | 32 | Never | Not Required Yet |
| D17 | MLH1 | Non-carrier | 3 | 0.6 | 5.00 | M | 50 | 0.52 | 5 |
| D25 | MSH2 | Non-carrier | 4 | 0.8 | 5.00 | F | 30 | 2 | No Medical Advice |
| D5 | MLH1 | Carrier | 50 | 8.9 | 5.62 | F | 55 | Never | No Medical Advice |
| D23 | MSH2 | Untested, brother of carrier | 40 | 6.6 | 6.06 | M | 34 | 4 | 5 |
| D16 | MSH2 | Carrier | 20 | 3 | 6.67 | F | 27 | Never | No Medical Advice |
| D21 | MLH1 | Carrier | 60 | 8.6 | 6.98 | M | 31 | Never | Start After 35yo |
| D12 | MSH2 | Non-carrier | 5 | 0.7 | 7.14 | F | 54 | 0.12 | 3 |
| D22 | MLH1 | Carrier | 40 | 5.1 | 7.84 | M | 28 | 2 | 2 |
| D13 | MSH2 | Untested, daughter of carrier | 40 | 3.5 | 11.43 | F | 33 | Never | No Medical Advice |
| D8 | MLH1 | Non-carrier | 5 | 0.3 | 16.67 | M | 45 | 3 | 5 |
| D9 | MSH2 | Non-carrier | 20 | 1 | 20.00 | F | 51 | 0.12 | 2 |
| D4 | MSH2 | Non-carrier | 30 | 1.4 | 21.43 | F | 68 | 3 | 3 |
| D15 | MLH1 | Non-carrier | 5 | 0.1 | 50.00 | F | 29 | 1 | 5 |
| D7 | MLH1 | Non-carrier | 25 | 0.4 | 62.50 | M | 47 | 1 | 5 |
| D11 | MSH6 | Non-carrier | 76 | 1.1 | 69.09 | M | 58 | 4 | 2 |
| D24 | MSH2 | Non-carrier | 25 | 0.3 | 83.33 | F | 26 | Never | Start After 30yo |
| D2 | MLH1 | Non-carrier | 70 | 0.6 | 116.6 7 | F | 54 | 10 | 1 |
| D19 | MLH1 | Non-carrier | 80 | 0.6 | 133.3 3 | F | 53 | 2 | 2 |
| D3 | MSH2 | Non-carrier | 50 | 0.3 | 166.6 7 | M | 44 | 1 | 2 |
| D20 | MLH1 | Non-carrier | 50 | 0.1 | 500.0 0 | F | 30 | 1.5 | 2 |
| Means | 31 | 4.0 | 50.48 | – | 43 | – | – | ||
As determined by ACCFR protocol 3 0.5 = < 1 year; 0.1 = <1 month
This study was approved by the Human Research Ethics Committee of the University of Melbourne and all participants gave informed consent.
Procedures
All participants in this study were recruited from the established participant pool of the Australasian Colorectal Cancer Family Registry. For the study of risk perception and screening decisions described here, participants agreed to an additional single interview.
Participants’ perceived CRC risk over the next 10 years was captured with a structured elicitation protocol using natural language and visual prompts to elicit risk estimates and subjective confidence in estimates (Figure 1). This method uses a four-step question protocol with estimates elicited in interval format (Spiers-Bridge et al. 2010) for lowest risk, highest risk, best estimate of risk, and estimate of confidence that the interval between the lowest risk and highest risk contains the actual risk (using a 5-step Likert scale to convert the subjective confidence to a numerical value between 50 and 100%). These estimates as well as self-reported colonoscopy screening and self-reported advice about colonoscopy screening from clinicians were elicited during a 45–90 minute face-to-face semi-structured interview covering topics related to genetic testing decision-making, screening knowledge and behaviours, and reported contact with the health care system.
Figure 1.
Elicitation questionnaire for personal estimates of CRC risk over the next 10 years. Female version is illustrated here; both male and female versions were used.
Data Analysis
We used participants’ estimates of the 10-year CRC risk, as this is the relevant clinical measure for comparison to risk predictions using demographic, family and genetic data. It is also the measure used to generate screening recommendations for clinical guidelines. To analyse each participant’s personal perceived 10-year risk of CRC, we used the response to the best estimate question and the confidence in estimate question, “How certain are you that your risk of getting bowel cancer lays between your lowest and highest estimates?” Individual perceived risk estimates were then standardised to 80% confidence intervals for comparison of risk estimates by converting participants’ answers to the confidence in estimate question to numerical values between 50 and 100% as follows:
50% confidence in risk estimate for the response “There is a 50/50 Chance my estimates are correct”
62.5% confidence in risk estimate for the response “There is a Good Chance my estimates are correct”
75% confidence in risk estimate for the response “It is Likely my estimates are correct”
87.5% confidence in risk estimate for the response “I am Almost Certain my estimates”
100% confidence in risk estimate for the response “I am Absolutely Certain my estimates are correct.”
This confidence value was then used to adjust the width of each interval to an 80% certainty level using arcsine transformation that allows comparison of participants’ risk perception estimates (Spiers-Bridge et al. 2010). [Note: the 80% subjective certainty for these elicited intervals is not the same as 95% confidence intervals of mean participant estimates; the latter specifies our confidence that the intervals contain the true population mean and we use these intervals to evaluate the robustness of our sample results. For results reported in this paper, the 80% transformed perceived risk intervals are displayed in the plots to compare participants’ estimates with each other, and the 95% confidence intervals are reported in the plots and the text to evaluate significance of the results.]
Colonoscopy history was summarised from transcribed participant interviews in which all cancer screening practices were elicited. Screening and diagnostic colonoscopies were differentiated by responses to the question “What was the reason for your [first/next] colonoscopy?” Screening colonoscopy events were coded into three categories: within two years of study interview, over two years since study interview, and never. Participants’ reports of general practitioner or specialist medical advice about recommended screening interval were recorded in years between screens, where a recommendation was made.
“Predicted” 10-year CRC risk for each individual was calculated based on age, gender, known mismatch repair gene mutation status and family history, using MMRpro software that calculates risk based on empirical data from observational studies (Chen et al. 2006). The mismatch repair gene mutation status of participants is known to the ACCFR but these data were released to the participants only if requested by participants who then attend a specialist familial cancer service to learn of their mutation status, in accordance with human research subject ethical constraints. For the study of risk perception and screening decisions described in this paper, we interviewed members of ACCFR families who had declined genetic testing, and/or declined the offer to receive genetic testing results. Therefore, although ACCFR researchers know the mutation status of the participants, the participants in the current study chose not to learn their mutation status. We compared the risk perception at interview with an objective measure of risk using a software program (MMRPro) that incorporates family history and mutation status. Although MMRPro may be of limited use in clinical practice, in this instance we use it as an objective benchmark to assess each participant’s over- or underestimation of personal risk.
Pearson’s correlation coefficient was used to compare perceived 10-year CRC risk with predicted risk calculated using MMRPro. Group comparisons of perceived risk estimates by gender and by screening history were conducted using the Student’s t-test using R Statistical Computing software (R Development Core Team 2008).
RESULTS
Of a total of 134 potential participants who met all study criteria from ACCFR population- and clinic-based families in which at least one member had been identified by genetic testing as carrying a deleterious mutation in a mismatch repair gene, 26 were interviewed and entered into the study. Of these 26, 14 were women (54%) and the mean age at interview was 43.1 years (SD = 12.73, range 26–68 years). There were 23 participants who had a mutation or who had a first- and/or second-degree relative with a MMR mutation (88.5%); the remaining three participants had a mutation-carrying cousin. Seven participants were mutation-positive (carriers).
The mean perceived 10-year risk of CRC was 30.5% [95% CI 21.3, 39.7], compared with a mean predicted risk of 4.0% [1.5, 6.5] (Table I). The mean predicted 10-year risk of CRC for potential participants (n = 101) who did not agree to the study was 3.3% [2.1, 4.5]). That is, on average, participants overestimated their risk of CRC over the next 10 years by 26.5 percentage points [17.2, 35.7] (p< 0.001). The ratio of the perceived risk to the predicted risk was on average 8-fold and ranged from 0.9-fold to 500-fold.
There was no evidence of difference in the perceived 10-year risk of CRC between women (mean = 30.6%) and men (mean = 30.3%), p = 0.9 (Figure 2). There was no correlation between perceived 10-year risk and participant age (r = 0.08 [−0.33, 0.47]) or between perceived 10-year risk and predicted risk (r = 0.11 [−0.31, 0.49]). There was no statistical evidence for difference between perceived risk for participants who reported colonoscopy screening within the last two years (mean = 22.1%, n = 9) and perceived risk for participants who reported not screening within the last two years or never (mean = 34.9%, n = 17), p = 0.20. Perceived risk estimates ranked by age, gender and screening category are shown in Figure 2. There were 18 participants reporting at least one colonoscopy (69%); of these 17 (94%) reported screening colonoscopy, and one reported a diagnostic colonoscopy. For participants who reported any screening colonoscopy and any medical screening colonoscopy advice (n = 17), there was a slight positive correlation between risk perception and years since last screening colonoscopy (r = 0.49 [0.02, 0.79]).
Figure 2.
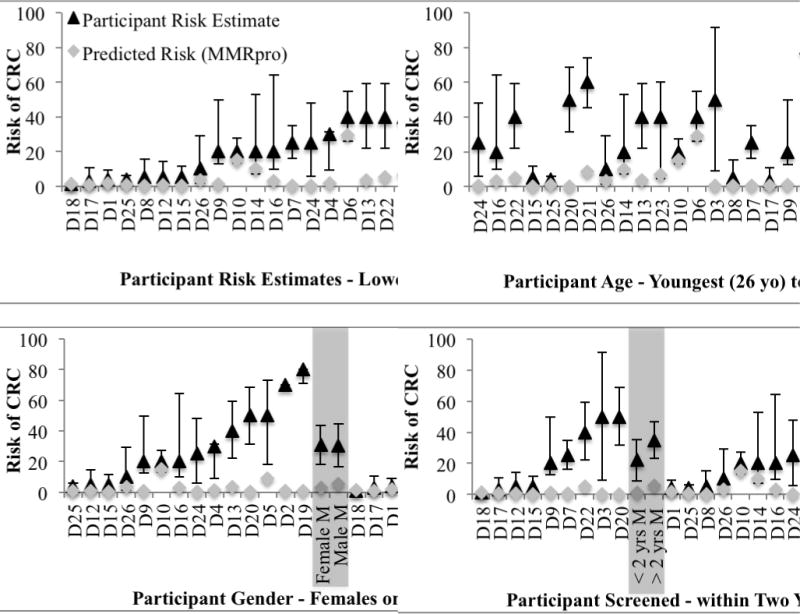
Participant risk perceptions, predicted risks (MMRpro) and age, gender and colonoscopy screening category, with 80% subjective intervals. Mean risk perceptions are marked in grey with 95% Confidence Intervals.
All participants were aged over 25 years at interview, i.e. the recommended age to start screening in this “potentially high risk” group (National Health and Medical Research Council 2005). For the 21 participants who reported receiving medical advice for screening colonoscopy, the recommendations they received varied from annually to every five years (Table I). Nine participants had screened within two years of interview (35%); ten had screened over two years before the interview (38%); and seven reported no screening colonoscopy (27%), including three who had been advised to screen at a later age.
DISCUSSION
Of 26 participants at high risk of CRC due to membership in families with MMR mutations and who had chosen not to attend a genetics service to receive genetic test results, only 25% provided interval estimates that captured their CRC risk using risk prediction tools based on their age, gender, known mismatch repair gene mutation status and family history. These results show that most people who have a high risk of CRC, due to being a relative of a mismatch repair mutation carrier and who have declined genetic counselling, testing or receiving genetic test results substantially over-estimate their risk of CRC in the next 10 years. Therefore, the reason for declining genetic testing is not likely because they are underestimating their risk.
We also observed that the participants’ inflated perceived risks were independent of their predicted risk, age, and gender and only slightly associated with reported screening colonoscopy behaviour (years since last colonoscopy). Individual differences in the level of perceived risk in those participants (n = 9) who had screened within two years of interview varied substantially (from 1% to 50%) suggesting that for some people, a perceived risk as low as 1% was sufficient for them to undertake colonoscopy screening, whereas for others a perceived risk greater than 50% was not sufficient for them to have screening. Possible contributing factors for the variation in perceived risk include: difficulty in assigning a numerical value for personal risk, impact of results of previous colonoscopies, and whether guessing mutation status (carrier or not) was included in risk perception. This suggests that risk perception alone is a poor predictor of colonoscopy screening behaviour in people at high risk who decline genetic test information. Further, attempts to mediate health behaviours by methods to increase a high-risk person’s understanding of personal risk may not be effective for increasing uptake of genetic testing. We suggest further epidemiological research into the most influential predictors of colonoscopy screening for this high-risk group, such as ascertaining validated screening practices for people with strong family histories and self-reported clinical recommendations to screen.
Key cognitive heuristics and biases are known to impact subjective judgements, including health risk perception in both lay people and health professionals (Kahneman et al. 1982). Improvements in subjective judgement have been found by using elicitation of intervals (upper and lower bounds) rather than point estimates of risk (Soll & Klayman 2004). Whilst this results in improved accuracy of judgements, interval estimates tended to be too narrow for the degree of expressed uncertainty. This is the overconfidence cognitive bias (Kahneman et al. 1982). A 4-step elicitation protocol for risk estimates has been shown to be effective in de-biasing risk estimates amongst experts including health professionals by increasing the number of points elicited for each estimate (Spiers-Bridge et al. 2010).
Health risk communication techniques were used to design the risk perception measures used in this study. Risk communication improves when natural frequency format data are used, particularly when human figures are used in a linear sequence. Research subjects in a qualitative study of women have been shown to interpret breast cancer risk data in natural frequency format more easily, especially when the data were presented in the form of a human figure (Schapira et al. 2001). Graphical presentation of health risks has been suggested to better inform people of harm compared to the same information presented as numerical data (Visschers et al. 2009). This may be because people find the graphical mode easier for extracting numerical estimates distinguishing between relatively small differences in risk (Ancker et al. 2011). For women, both younger (40 to 49 year olds) and older people prefer estimating risks in 10-year periods, rather than annual risk estimates (Schapira et al. 2001).
Study Strengths and Limitations
Our findings are based on several unique research strengths. Our participants are drawn from the ACCFR, the only family cancer registry in Australia of this size, and one of six major cancer registries forming the international Colon Cancer Family Registry. In the ACCFR, family history of cancer is validated to second-degree relatives using pathology reports, medical records, cancer registry reports and/or death certificates. In addition, it is the largest such registry that distinguishes between screening and diagnostic colonoscopy for evaluating screening decisions. Recall of screening colonoscopy behaviour has been validated for ACCFR participants by comparing recalled colonoscopy with medical records. The positive predictive value for colonoscopy and polyp removal was 81% and the negative predictive value was 86% (Madlensky et al. 2007). There have been, to our knowledge, no studies to date that have been able to capture people from high-risk families who decline an invitation to attend family cancer genetic counselling and testing clinics to receive genetic test results. Finally, our study represents the first adaptation of the risk elicitation protocol for general use, including benefits from the use of a graphical display for health risk estimates (Ancker et al. 2011; Schapira et al. 2001, Visschers et al. 2009) and the more comprehensible denominator of “out of 100 people like you.”
We have used a risk calculation tool to compare subjective participant risk estimates to an objective benchmark, as this is the method for comparing risk judgments among estimators with imperfect or incomplete knowledge of outcomes (e.g., Flander et al. 2012; Speirs-Bridge et al. 2010). These comparisons among participants are not used to judge participants’ performance, as it is likely that participants do not have mutation carrier status information and, in addition, they vary in the extent of what they know of their own family history and other risk factors. Participants are unlikely to have an intuitive risk estimation process akin to that of the risk calculation tool; indeed, we do not know how people estimate personal risk. We do know that risk perception measured using standard methods has not been shown to be a reliable predictor of screening behaviour. This inconsistency in study findings may reflect in part current methods in health risk perception measurement that do not incorporate notions of interval estimation under uncertainty, which has been shown to improve risk estimation under uncertainty (Soll & Klayman 2004; Speirs-Bridge et al. 2010). One caveat in interpreting our results is that the risk perception estimation tool is based on methods developed for expert rather than novice elicitation (Speirs et al 2010). Although experts and novices show similar levels of overconfidence, they differ in the way these biased estimates are expressed (McKensie et al. 2008). Experts produced narrow intervals with the midpoint closer to the truth, whereas novices produced wide intervals with the midpoint further from truth. These differences suggest further study is warranted, to shed light on the nuanced expression of risk perception in individuals who may be at high risk for familial disease.
This is the largest such study to date targeting individuals who decline genetic counselling, testing and receiving genetic test results, yet there are constraints due to the small number of participants. We are not able to extrapolate the screening decisions made by these at-risk people with relatives who carry a high-risk mutation to similar at-risk individuals in the population at large.
Practice Implications
The ability to translate developments in genetic testing and effective risk management for the benefit of people at risk of Lynch Syndrome is hampered by limited access to personalised information. Although specialist familial cancer services provide information and counselling for those with high risk family histories, Wong et al. (2007) found that a significant proportion of people with CRC who met the criteria for referral to specialist familial cancer clinics were not referred. Furthermore, not all those who are aware of these services attend and, as this study demonstrates, those that do not attend specialist familial cancer services may over-estimate their risk and under-utilize surveillance. Genetic counselors have limited opportunity to address this directly and there may also be limitations in the effective translation of increased lifetime cancer risks by physicians who manage cancer surveillance. Domanska et al (2009) found that Lynch syndrome family members and their physicians in Sweden had comparable low levels of knowledge about risk and surveillance; the authors call for improved education in genetic medicine for physicians. Their study found that only half of the family members and one-third of the physicians correctly estimated the risk to inherit a predisposing mutation. Family members have also been identified as a means of influencing screening behaviours, particularly amongst younger relatives (Ashida et al. 2011). However, it is difficult to propose and prioritise approaches most likely to be effective without better knowledge of the reasons people at high risk do not proceed with testing. Only qualitative research methods can adequately address this knowledge gap. Reasons for screening and testing decision-making for all people at elevated risk have been difficult to ascertain, as studies of genetic testing for Lynch syndrome cancers recruit from family cancer clinics, so those at high risk of colorectal cancer who have not attended a clinic for genetic testing are excluded (e.g. McCann et al. 2009).
CONCLUSION
This is the first study of risk perception and colonoscopy screening for people who choose not to attend genetic counselling and/or testing services for receipt of genetic test results, despite being at high risk of CRC due to known mismatch repair mutations in their families. We found that both men and women perceived their risk of CRC over the next 10 years as being high and independent of age, self-reported screening, clinical advice and predicted risk. These results suggest that the reason for declining the offer of genetic counselling and/or testing is not likely to be due to a perceived low risk of CRC and that risk perception alone cannot predict appropriate screening colonoscopy decisions.
Acknowledgments
DISCLOSURE OF INTEREST
This work was supported by the Victorian Cancer Agency under #EO109-33, National Cancer Institute, National Institutes of Health under RFA #CA-95-011, and through cooperative agreements with the Australasian Colorectal Cancer Family Registry (U01 CA097735). Mark Jenkins is supported as a Senior Research Fellow by the National Health & Medical Research Council, Australia. Driss Ait Ouakrim was supported by an Australian Commonwealth Scientific and Industrial Research Organisation PhD scholarship (CSIRO, Preventative Heath Flagship). Aung Ko Win is supported by a grant from the Cancer Council Victoria and the Picchi Brothers Foundation, Australia. Authors have full control of all primary data and agree to review if requested.
References
- Ancker JS, Weber EU, Kukafka R. Effects of game-like interactive graphics on risk perceptions and decisions. Med Decis Making. 2011;31:130–42. doi: 10.1177/0272989X10364847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armelao F, Orlandi PG, Tasini E, Franceschini G, Franch R, Paternolli C, et al. High uptake of colonoscopy in first-degree relatives of patients with colorectal cancer in a healthcare region: a population-based, prospective study. Endoscopy. 2010;42:15–21. doi: 10.1055/s-0029-1215324. [DOI] [PubMed] [Google Scholar]
- Ashida S, Hadley DW, Goergen AF, Skapinsky KF, Devlin HC, Koehly LM. The importance of older family members in providing social resources and promoting cancer screening in families with a hereditary cancer syndrome. Gerontologist. 2011;51:833–42. doi: 10.1093/geront/gnr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglietto L, Lindor NM, Dowty JG, White DM, Wagner A, Gomez Garcia EB, et al. Risks of Lynch syndrome cancers for MSH6 mutation carriers. JNCI. 2010;102:193–201. doi: 10.1093/jnci/djp473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer NT, Weinstein ND, Cuite CL, Herrington JE. Risk perceptions and their relation to risk behavior. Ann Behav Med. 2004;27:125–30. doi: 10.1207/s15324796abm2702_7. [DOI] [PubMed] [Google Scholar]
- Bonadona V, Bonaïti B, Olschwang S, Grandjouan S, Huiart L, Longy M, Guimbaud R, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–10. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang W, Lee S, Nafa K, Lee J, Romans K, et al. Prediction of germline mutations and cancer risk in the Lynch syndrome. JAMA. 2006;296:1479–87. doi: 10.1001/jama.296.12.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes E, Denayer L, Evers-Kiebooms G, Boogaerts A, Philippe K, Tejpar S, et al. Predictive testing for hereditary nonpolyposis colorectal cancer: subjective perception regarding colorectal and endometrial cancer, distress, and health-related behavior at one year post-test. Genet Testing. 2005;9:54–65. doi: 10.1089/gte.2005.9.54. [DOI] [PubMed] [Google Scholar]
- Codori AM, Petersen GM, Miglioretti DL, Boyd P. Health beliefs and endoscopic screening for colorectal cancer: potential for cancer prevention. Prev Med. 2001;33:128–36. doi: 10.1006/pmed.2001.0862. [DOI] [PubMed] [Google Scholar]
- Cole SR, Smith A, Wilson C, Turnbull D, Esterman A, Young GP. An advance notification letter increases participation in colorectal cancer screening. J Med Screen. 2007;14:73–5. doi: 10.1258/096914107781261927. [DOI] [PubMed] [Google Scholar]
- Collins V, Meiser B, Gaff C, St John DJ, Halliday J. Screening and preventive behaviors one year after predictive genetic testing for hereditary nonpolyposis colorectal carcinoma. Cancer. 2005;104:273–81. doi: 10.1002/cncr.21183. [DOI] [PubMed] [Google Scholar]
- Collins VR, Meiser B, Ukoumunne OC, Gaff C, St John DJ, Halliday JL. The impact of predictive genetic testing for hereditary nonpolyposis colorectal cancer: three years after testing. Genetics in Medicine. 2007;9:290–7. doi: 10.1097/gim.0b013e31804b45db. [DOI] [PubMed] [Google Scholar]
- de Jong AE, Hendriks YM, Kleibeuker JH, de Boer SY, Cats A, Griffioen G, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology. 2006;130:665–71. doi: 10.1053/j.gastro.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Domanska K, Carlsson C, Bendahl P-O, Nilbert M. Knowledge about hereditary nonpolyposis colorectal cancer; mutation carriers and physicians at equal levels. BMC Med Genet. 2009;10:30. doi: 10.1186/1471-2350-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flander L, Dixon W, McBride M, Burgman M. Facilitated expert judgment of environmental risks: Acquiring and analysing imprecise data. IJRAM. 2012;16:199–212. [Google Scholar]
- Fletcher RH, Lobb R, Bauer MR, Kemp JA, Palmer RC, Kleinman KP, et al. Screening patients with a family history of colorectal cancer. J Gen Intern Med. 2007;22:508–13. doi: 10.1007/s11606-007-0135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno Garcia AZ, Quintero E, Nicolas Perez D, Hernandez M, Jimenez Sosa A. Colorectal cancer screening in first-degree relatives of colorectal cancer: participation, knowledge, and barriers against screening. Eur J Gastroenterol Hepatol. 2011;23:1165–71. doi: 10.1097/MEG.0b013e32834a289e. [DOI] [PubMed] [Google Scholar]
- Glenn BA, Herrmann AK, Crespi CM, Mojica CM, Chang LC, Maxwell AE, et al. Changes in risk perceptions in relation to self-reported colorectal cancer screening among first-degree relatives of colorectal cancer cases enrolled in a randomized trial. Health Psychol. 2011;30:481–91. doi: 10.1037/a0024288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley DW, Jenkins JF, Dimond E, de Carvalho M, Kirsch I, Palmer CG. Colon cancer screening practices after genetic counseling and testing for hereditary nonpolyposis colorectal cancer. J Clin Oncol. 2004;22:39–44. doi: 10.1200/JCO.2004.06.128. [DOI] [PubMed] [Google Scholar]
- Harris MA, Byles JE. A survey of screening compliance among first degree relatives of people with colon cancer in New South Wales. J Med Screen. 1997;4:29–34. doi: 10.1177/096914139700400110. [DOI] [PubMed] [Google Scholar]
- Harris MA, Treloar CJ, Byles JE. Colorectal cancer screening: discussions with first degree relatives. Aust NZ J Pub Health. 1998;22:826–8. doi: 10.1111/j.1467-842x.1998.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Jarvinen HJ, Aarnio M, Mustonen H, Aktan-Collan K, Aaltonen LA, Peltomaki P, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:29–34. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Slovic P, Tversky A. Judgement under Uncertainty: Heuristics and Biases. Cambridge: Cambridge University Press; 1982. [Google Scholar]
- Katapodi MC, Lee KA, Facione NC, Dodd MJ. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analytic review. Prev Med. 2004;38:388–402. doi: 10.1016/j.ypmed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Keogh LA, van Vliet CM, Studdert DM, Maskiell JA, Macrae FA, St John DJ, et al. Is uptake of genetic testing for colorectal cancer influenced by knowledge of insurance implications? Med J Aust. 2009;191:255–8. doi: 10.5694/j.1326-5377.2009.tb02778.x. [DOI] [PubMed] [Google Scholar]
- Longacre AV, Cramer LD, Gross CP. Screening colonoscopy use among individuals at higher colorectal cancer risk. J Clin Gastroenterol. 2006;40:490–6. doi: 10.1097/00004836-200607000-00006. [DOI] [PubMed] [Google Scholar]
- Lynch HT, de la Chapelle A. Hereditary colorectal cancer. NEJM. 2003;348:919–32. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- Mack LA, Cook LS, Temple WJ, Carlson LE, Hilsden RJ, Paolucci EO. Colorectal cancer screening among first-degree relatives of colorectal cancer patients: benefits and barriers. Annals Surg Oncol. 2009;16:2092–2100. doi: 10.1245/s10434-009-0528-z. [DOI] [PubMed] [Google Scholar]
- Madlensky L, Esplen MJ, Gallinger S, McLaughlin JR, Goel V. Relatives of colorectal cancer patients: Factors associated with screening behavior. Am J Prev Med. 2003;25:187–94. doi: 10.1016/s0749-3797(03)00202-2. [DOI] [PubMed] [Google Scholar]
- Madlensky L, Daftary D, Burnett T, Harmon P, Jenkins M, Maskiell J, et al. Accuracy of colorectal polyp self-reports: Findings form the Colon Cancer Family Registry. Cancer Epidemiol Biomarkers Prevent. 2007;16:1898–901. doi: 10.1158/1055-9965.EPI-07-0151. [DOI] [PubMed] [Google Scholar]
- Manne S, Markowitz A, Winawer S, Meropol NJ, Haller D, Rakowski W, et al. Correlates of colorectal cancer screening compliance and stage of adoption among siblings of individuals with early onset colorectal cancer. Health Psychol. 2002;21:3–15. [PubMed] [Google Scholar]
- McCann S, MacAuley D, Barnett Y, Bunting B, Bradley A, Jeffers L, et al. Family communication, genetic testing and colonoscopy screening in hereditary non-polyposis colon cancer: a qualitative study. Psycho-oncol. 2009;18:1208–15. doi: 10.1002/pon.1487. [DOI] [PubMed] [Google Scholar]
- McKenzie C, Liersch M, Yaniv I. Overconfidence in interval estimates: What does expertise buy you? Organ Behav Hum Dec. 2008;107:179–91. [Google Scholar]
- National Health and Medical Research Council: NHMRC. Clinical practice guidelines for the prevention, early detection and management of colorectal cancer. 2005 Accessed December 6, 2011, from http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cp106_0.pdf.
- Newcomb PA, Baron J, Cotterchio M, Gallinger S, Grove J, Haile R, et al. The Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prevent. 2007;16:2331–43. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- Palmer RC, Emmons KM, Fletcher RH, Lobb R, Miroshnik I, Kemp JA, et al. Familial risk and colorectal cancer screening health beliefs and attitudes in an insured population. Prev Med. 2007;45:336–41. doi: 10.1016/j.ypmed.2007.07.021. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- Santos EM, Lourenco MT, Rossi BM. Risk perception among Brazilian individuals with high risk for colorectal cancer and colonoscopy. Hereditary Cancer in Clinical Practice. 2011;9:4. doi: 10.1186/1897-4287-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira MM, Nattinger AB, McHorney CA. Frequency or probability? A qualitative study of risk communication formats used in health care. Med Decis Making. 2001;21:459–67. doi: 10.1177/0272989X0102100604. [DOI] [PubMed] [Google Scholar]
- Schmeler KM, Lynch HT, Chen LM, Munsell MS, Soliman PT, Clark MB, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. NEJM. 2006;354:261–9. doi: 10.1056/NEJMoa052627. [DOI] [PubMed] [Google Scholar]
- Schwarzer R, Luszczynska A, Ziegelmann JP, Scholz U, Lippke S. Social-cognitive predictors of physical exercise adherence: three longitudinal studies in rehabilitation. Health Psychol. 2008;27:S54–63. doi: 10.1037/0278-6133.27.1(Suppl.).S54. [DOI] [PubMed] [Google Scholar]
- Senter L, Clendenning M, Sotamaa K, Hampel H, Green J, Potter JD, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135:419–28. doi: 10.1053/j.gastro.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh S, Ilan S. To test or not to test? Moderators of the relationship between risk perceptions and interest in predictive genetic testing. J Behav Med. 2005;28:467–79. doi: 10.1007/s10865-005-9017-4. [DOI] [PubMed] [Google Scholar]
- Soll JB, Klayman J, Overconfidence in interval estimates Journal of Experimental Psychology Learning Memory and Cognition. 2004;30:299–314. doi: 10.1037/0278-7393.30.2.299. [DOI] [PubMed] [Google Scholar]
- Speirs-Bridge A, Fidler F, McBride M, Flander L, Cumming G, Burgman M. Reducing overconfidence in the interval judgments of experts. Risk Anal. 2010;30:512–23. doi: 10.1111/j.1539-6924.2009.01337.x. [DOI] [PubMed] [Google Scholar]
- Taouqi M, Ingrand I, Beauchant M, Migeot V, Ingrand P. Determinants of participation in colonoscopic screening by siblings of colorectal cancer patients in France. BMC Cancer. 2010;10:355. doi: 10.1186/1471-2407-10-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visschers VH, Meertens RM, Passchier WW, de Vries NN. Probability information in risk communication: A review of the research literature. Risk Anal. 2009;29:267–87. doi: 10.1111/j.1539-6924.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- Win AK, Dowty JG, English DR, Campbell PT, Young JP, Winship I, et al. Body mass index in early adulthood and colorectal cancer risk for carriers and non-carriers of germline mutations in DNA mismatch repair genes. Brit J Cancer. 2011;105:162–9. doi: 10.1038/bjc.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win AK, Young JP, Lindor N, Tucker KM, Ahnen DJ, Young GP, et al. Colorectal and other cancer risks for carriers and non-carriers of a DNA mismatch repair gene mutation from mutation carrying families: a prospective cohort study. J Clin Oncol. 2012;30:958–64. doi: 10.1200/JCO.2011.39.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Gibbs P, Johns J, Jones I, Faragher I, Lynch E, et al. Value of database linkage: are patients at risk of familial colorectal cancer being referred for genetic counselling and testing? Internal Medicine Journal. 2007;38:328–33. doi: 10.1111/j.1445-5994.2007.01470.x. [DOI] [PubMed] [Google Scholar]


