Abstract
Rationale
Stimulation of nicotinic cholinergic systems has been shown to alleviate ADHD symptoms and to improve cognitive performance. AZD1446 is a selective α4β2* nicotinic acetylcholine receptor agonist with potential effect on the symptoms of ADHD.
Objectives
The purpose of this study is to evaluate the efficacy, safety, and pharmacokinetics of AZD1446 in adults with ADHD treated for 2 weeks.
Method
This was a randomized, double-blind, placebo-controlled crossover trial. Participants were 79 adults with ADHD, grouped according to their use of nicotine-containing products. Nicotine non-users received placebo and two of three AZD1446 treatment regimens (80 mg tid, 80 mg qd, 10 mg tid). Nicotine users received placebo, AZD1446 80 mg tid and 80 mg qd. Efficacy measures included the Conners' Adult ADHD Rating Scale and cognitive measures of immediate and delayed verbal episodic memory, learning, attention, working memory, executive functioning, and spatial problem solving (CogState computerized test battery).
Results
There was no significant effect of AZD1446 on any of the clinical scores irrespective of dose, schedule, or concomitant use of nicotine products. A statistically significant improvement was seen on the Groton Maze Learning Task, a measure of executive functioning, in nicotine non-users after treatment with AZD1446 80 mg qd.
Conclusions
AZD1446 was well tolerated, but did not significantly improve ADHD symptoms after 2 weeks of treatment compared to placebo. While the present study does not support the therapeutic utility of AZD1446 in ADHD, its potential pro-cognitive effects remain to be explored in other neuropsychiatric disorders.
Keywords: ADHD, Nicotinic receptors, AZD1446, Cognition
Background
Attention-deficit/hyperactivity disorder/hyperkinetic syndrome (ADHD/HD, Diagnostic and Statistical Manual of Mental Disorders (DSM-IV)/ICD-10) is one of the most common neuropsychiatric disorders with the reported worldwide-pooled prevalence of 5 % (Polanczyk and Rohde 2007a). Although ADHD is a developmental disorder, there is evidence that clinical symptoms as well as cognitive deficits persist into adulthood (Faraone et al. 2001; Polanczyk et al. 2007b). Moreover, ADHD is associated with considerable impact on quality of life for the individual (underachievement, social stigma, consequences of impulsivity), on family functioning, and costs for society (medical care consumption, work productivity loss, and encounters with the criminal justice system) (Klassen et al. 2004; Bernfort et al. 2008). Though often treated effectively with psychostimulants, there is a recognized need for new medications that would provide efficacious and safe long-term treatment of ADHD.
The nicotinic acethylcholine system may be one of the new targets for the development of alternative drugs for ADHD. Improvement in cognition, facilitation of attention has been observed in different animal species, healthy human volunteers, and ADHD when using nicotine (Conners et al. 1996; Levin et al. 1996, 2002; Potter and Newhouse 2006, 2008). It has been demonstrated that nicotine exerts its beneficial effect selectively on behavioral inhibition and delay aversion tasks, which are known to have good discriminant validity in distinguishing subjects with ADHD from controls (Potter et al. 2006, 2012). Stimulation of neuronal nicotinic acetylcholine receptors (nAChRs) by nicotine may be mediated directly via cholinergic neurotransmission and/or by modulating activity of other neurotransmitters including dopamine, which in turn has a recognized role in the neurobiology of ADHD (Brody et al. 2009; Tomasi and Volkow 2012).
nAChRs are pentameric ligand-gated ion channels distributed broadly across the brain. The most common high-affinity nicotine binding sites, α4β2* nAChRs, have been recognized as a novel target for disorders with cognitive impairment as supported by genetic studies and pharmacological studies in animals. An intron mutation in the α4 subunit gene (CHRNA4, encoding the α4 part of α4β2 receptors) has been linked to severe attention impairment and visuo-spatial attention has been associated with a polymorphism in CHRNA4 (Todd et al. 2003; Greenwood et al. 2012). Deletion and re-expression of the nAChR β subunit has been related to attention impairment and its restoration, respectively (Guillem et al. 2011). Selective agonists for α4β2* nAChRs have been shown to enhance sustained attention performance, to improve vigilance in rodents, to prevent distractibility and improve accuracy in non-human primates (Prendergast et al. 1998; McGaughy et al. 1999; Grottick et al. 2001; Howe et al. 2010; Rezvani et al. 2011, 2012; Young et al. 2013), and to ameliorate behavioral deficits in animal models of ADHD (Ueno et al. 2002). Ispronicline (TC-1734/AZD3480) has shown improved attention in healthy young subjects, producing an EEG pattern similar to that seen with drugs which are known to improve attention and vigilance (Dunbar et al. 2007). The proposed mechanisms of pro-cognitive action of α4β2* nAChRs agonists was related to the modulation of the tonic cholinergic activity (Sarter et al. 2006).
Given such characteristics, α4β2* nAChR agonists were considered a novel therapeutic opportunity for ADHD, which is a disorder with multiple cognitive dysfunctions and with prominent impairment of brain networks underlying sustained attention and reward (Castellanos et al. 2006; Cubillo et al. 2012). Several α4β2* nAChR agonists have reached clinical phase of drug development. Initial results supported their clinical efficacy in ADHD, with specific improvement of inattention and inhibitory behaviors (Wilens et al. 1999, 2006; Apostol et al. 2012; Potter et al. 2013).
AZD1446 is a selective agonist to α4β2/α2β2* nAChRs with demonstrated efficacy on long-term and working memory, spatial learning/memory, and attention tasks in rodents (Mazurov et al. 2012; Szeliga et al. 2012, AZ data on file). In phase I studies, AZD1446 was generally safe and well tolerated (AZ data on file). The main aim of the present study was to examine whether 2 weeks of treatment with AZD1446, compared with placebo, improves the core symptoms of ADHD in adults, as measured by the CAARS-INV total ADHD symptom score. The study also aimed to test the potential effect of nicotine co-administration, as nicotine would compete with AZD1446 at the same receptors and is known to induce nicotine receptor upregulation, desensitization and tolerance (Wells 2008; Mukhin et al. 2008; Srinivasan et al. 2011). Therefore, the study included two cohorts of adult patients, those who were free from nicotine-containing products as well as those who were using them. In addition, the study explored the potential effect of AZD1446 on impaired cognitive functions (as measured by a CogState computerized test battery).
Method
Participants
Subjects were enrolled via clinical referrals and advertisements in the local media. Eligible subjects, aged 18 to 65 years, nicotine non-users and nicotine users, met the diagnostic criteria for ADHD (DSM-IV, APA 2000) as determined using Conners' Adult ADHD Diagnostic Interview for DSM-IV administered by a trained clinician. Subjects also had at least moderate ADHD symptom severity, defined as a score of ≥2 on at least six of nine items in at least one of the subscales of the CAARS-INV Total ADHD Symptoms score (18 items) and ≥4 (at least moderately ill) on the Clinical Global Impressions for ADHD Severity (CGI-S) score. Exclusion criteria were: schizophrenia, bipolar disorder, a history of oppositional defiant disorder, acute anxiety disorders, acute depressive episode, or other unstable psychiatric conditions. In addition, serious medical illness, drug or alcohol dependence, pregnancy, nursing, participation in a previous drug trial in the last 30 days, and treatment with any psychoactive drug in addition to study medication were exclusionary. Use of psychostimulants was not allowed starting 2 weeks prior to randomization and during the study.
The study included two cohorts of patients, those who did not use nicotine-containing products and those who used them. Non-users of nicotine-containing products were defined based on a self-report of not having used any product which contains nicotine during the past 6 months prior to enrolment, and having serum cotinine of 14 ng/mL or less at enrolment.
Users of nicotine-containing products were defined as those having daily use of nicotine products during the past 6 months prior to enrolment and having serum cotinine levels >14 ng/mL at enrolment. Nicotine product users could not have used smoking cessation therapy within 4 weeks prior to enrolment or during the study.
Clinicians and research staff from six study centers in the USA experienced in diagnosing and treating adults with ADHD participated in the study. The study was conducted in accordance with the principles of the Declaration of Helsinki and International Conference on Harmonization/Good Clinical Practice guidelines. All study centers received approval from institutional review boards or independent ethics committees. Following complete explanation of study procedures and risks, each subject provided written informed consent.
Study design and treatments
A multicenter, double-blind, placebo-controlled, randomized, three-way crossover 2-week treatment period study was conducted. Following randomization, patients were administered AZD1446 or placebo for 2 weeks for each of three treatment assignments, with a 21-day washout between each treatment period. A follow-up visit was conducted 7–10 days after the last treatment dose (Fig. 1).
Fig 1.
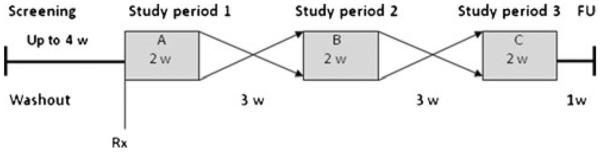
Study design. (A sample randomization sequence is shown using A, B, and C to denote treatment. Treatment randomisation. Follow-up (FU); qd, once daily; tid, three times daily). This three-way crossover study consisted of 2 weeks of treatment with three drug conditions. Nicotine non-users were randomized to one of 18 possible sequences; all sequences had one period of placebo and two periods with AZD1446. Those two periods had two out of three possible dose regimens, resulting in subjects receiving placebo (n=44), AZD1446 10 mg tid (n=28), 80 mg tid (n=31), and 80 mg qd (n=27). Nicotine users were randomized to one of six possible sequences, with three periods, i.e., placebo and AZD1446 80 mg qd and 80 mg tid. Subjects of this cohort received placebo (n=20), AZD1446: 80 mg tid (n=20) and 80 mg qd (n=22)
The study tested two doses of AZD1446 and two dose schedules, a combination of which resulted in 10 mg tid, 80 mg tid, 80 mg qd treatment regimens in the cohort of nicotine non-users and 80 mg tid, 80 mg qd in nicotine users. The choice of dose was based on predictions from preclinical efficacy studies (AZ data on file) and phase I studies showing short half-life of AZD1446 (in the range of 2–3 h) and affinity in vivo of 128 nmol/L (total plasma concentration at 50 % receptor occupancy) (Jucaite et al. 2012). In addition, an observation that efficacy was seen with AZD3480 (TC-1734) at the doses reaching high α4β2* nACh receptor occupancy (AZ data on file) was considered. The choice of different dose schedules was based on the hypothesis that efficacy may be driven by short interaction with α4β2* nACh receptors, as suggested by preclinical studies (AZ data on file).
All doses of AZD1446 and placebo were delivered in identical-appearing capsules and administered orally. Nicotine non-users received placebo and two of three AZD1446 treatment regimens (80 mg tid, 80 mg qd, and 10 mg tid) (detailed randomization description in notes to Fig. 1). Nicotine users received placebo, AZD1446 80 mg tid, and AZD1446 80 mg qd. Blinded study treatment (AZD1446 or placebo) was administered to all patients three times daily during all the three 2-week treatment periods. Treatment periods were separated by a 21-day washout period, during which patients received placebo tid.
Assessments
Each subject underwent a comprehensive clinical evaluation by a study psychiatrist. History of nicotine use was checked and S-cotinine test was performed to control assignment of subjects to the study cohorts.
Clinical rating scales
The primary efficacy measure was a clinical endpoint, the Conner's adult ADHD rating scale investigator administered (CAARS-INV, Conners et al. 2004; see also definition in “Statistics”). The CAARS-INV total ADHD score is the sum of the inattentive score and hyperactive/impulsive score and reflects the 18 symptoms included in the DSM-IV ADHD diagnostic criteria. All other clinical ADHD scores used in the trial were designated secondary endpoints comprising the DSM-IV ADHD symptoms Inattention subscale, Hyperactivity subscale (from the 30-item scoring), the ADHD Index and the CAARS-S/SV, a self-report score. Overall therapeutic effect was assessed with the clinical global impression scale severity score (CGI-S, Guy 1976). The scales were administered at screening, randomization, pre-dose, and end of each treatment period.
Cognitive measurements
A test battery was used to examine effects of AZD1446 on cognitive performance. Tests that measure attention, verbal episodic memory and learning, working memory, response inhibition, and spatial problem solving were chosen and were administered using the computerized CogState Clinical Trials Cognitive Testing System (CogState Ltd., Melbourne, Australia). A practice session was conducted at study screening to familiarize subjects with the tasks. The specific tests in this battery were selected because they are brief, can be given repeatedly without eliciting practice effects, have demonstrated ability to detect cognitive deficits in the domains of interest for individuals having ADHD, and because the tasks have been shown to be sensitive to cognitive change associated with perturbation of the nicotinic cholinergic system (Potter and Newhouse 2004, 2008; Potter et al. 2006, 2012). The following tasks were included:
International Shopping List Task (ISLT) measures immediate and delayed verbal memory and learning and consists of four trials (three learning and one delayed recall trial (as described previously by Lim et al. 2009). Subjects were instructed to remember 12 shopping items that they would typically obtain from their local store. The list was presented three times in the same order, with the list and word order randomized for each patient and assessment time point. During learning trials, participants were asked to recall as many items as possible from the initial list. Then, following a delay of approximately 30 min during which they were administered other cognitive tasks, the subjects completed the delayed recall trial. The primary outcome measure was the number of correctly recalled words.
One Back (OBK) and Two Back (TBK) Tasks are measures of working memory and attention. Participants attended to a playing card presented in the center of the screen and indicated if the card was “exactly the same as the immediately previous card?” (OBK) or “as that shown two cards ago?” (TBK). The primary performance measure was the proportion of correct answers (accuracy), normalized using an arcsine square-root transformation.
Groton Maze Learning Task (GMLT) is a task that measures executive function and spatial problem solving. A 10×10 grid of tiles with a hidden 28-step pathway (maze) was presented on a computer touch screen. The subjects were instructed to move one step–one tile at a time in order to learn the correct pathway through the maze, guided through trial and error feedback. Alternate forms for this task were selected in order to ensure that no subject repeated the same hidden path during the study. Subjects completed each maze five times in each testing session. The primary measure was the total number of errors, with lower scores denoting better performance.
The Stop-Signal Task is a measure of response inhibition, i.e., the ability to withhold a pre-potent response. This is a primary choice reaction time task with an infrequently occurring (25 % of trials) auditory tone (the “stop signal”) indicating that the subject should inhibit their response on that trial (Logan et al. 1984). The task began with a 250-ms delay between the go and stop signals (stop signal delay (SSD)), which was dynamically adjusted by 50 ms after every stop trial to approximate a 0.5 probability of successful inhibition. Subjects completed four blocks of 64 trials. The stop signal reaction time was the main outcome variable, calculated by subtracting the average SSD from the mean Go-RT (Logan et al. 1984).
AZD1446 plasma concentrations and pharmacokinetic data analysis
To determine the concentration of AZD1446 in plasma, venous blood samples (4 mL) were collected by a sparse sampling schedule, with a maximum of 12 PK samples from each individual during the course of the study. Plasma concentrations of AZD1446 were determined using LC-MS/MS after ultrafiltration (Quotient Bioresearch Ltd., England). The PK analysis set included data from all randomized patients who received at least one dose of AZD1446 treatment and had at least one valid PK assessment (48 nicotine non-users and 22 nicotine users). Data collected during the placebo treatment were excluded from the PK analysis set. In total, 314 plasma concentrations were included in the PK analysis. A population PK model was used to obtain individual exposures for the patients using the Empirical Bayes Estimates from the POSTHOC step in NONMEM™, version 7.1.0 (Icon Development Solutions, Ellicott City, MD, USA). The individually predicted parameters were used to obtain secondary PK parameters by employing the analytical solutions to steady-state pharmacokinetic equations valid for one compartment model. The PK parameters of AZD1446 were summarized by cohort.
Safety assessments
Safety and tolerability assessments included records of adverse events; vital signs (pulse, blood pressure); electrocardiogram (ECG); clinical chemistry and hematology assessments; urinalysis. Profile of Mood States (POMS, standard version, 65 items (McNair et al. 1971) was assessed at baseline, and at the start and end of each treatment period. The Columbia Suicide Severity Rating Scale was administered during these same on-site visits and, in addition, via telephone during washout periods. Adverse events and medical/surgical history were classified according to the terminology of the Medical Dictionary for Regulatory Activities, MedDRA, version 13.1 (Nov 1, 2010) (Brown et al. 1999).
Statistics
The sample size estimates for this study were based on the primary efficacy variable, i.e., clinical endpoint, described as a change from baseline to week 2 in the CAARS-INV Total ADHD Symptoms score. A sample size of 36 nicotine non-users and 24 nicotine users who completed the study was sufficient to ensure >90 % power, assuming an effect size of 0.625 (corresponding to a difference of 5 points on CAARS-INV Total score with a standard deviation of 8 points) in one treatment regimen of AZD1446 versus placebo in either cohort. In addition, to account for dropouts, subjects were randomized until it was predicted that 36 nicotine non-users would complete all three periods (eventually 34 nicotine non-users completed all three periods).
This study had two main objectives, i.e., to show clinical efficacy of AZD1446 separately in each of the two cohorts of patients, nicotine non-users and nicotine users. Identical and parallel analyses were performed for the two cohorts. Statistically significant level of 0.10, with a one-sided test of superiority, was chosen for statistical comparison of AZD1446 efficacy over placebo in CAARS-INV ADHD Symptoms Total after 2 weeks of treatment. Except for the primary analyses, all statistical tests were two-sided with a significance level of 5 % (i.e., α=0.05). Within all analyses, Dunnett's method was used to adjust for comparisons of multiple doses versus placebo. No corrections for multiple comparisons between the tests were made.
Descriptive statistics were used to summarize assessments by visit or treatment period, and either relative to the start of a randomized dose, or relative to the start of study. A mixed effects repeated measures model (MRMM) was used to analyze the primary variable in each cohort with pre-specified fixed factors (period, week, treatment, baseline), fixed interactions (period and week, treatment and week) as fixed effects and random factor (subject), random interaction (subject and period).
The full analysis set was used for all efficacy and safety/tolerability analyses, and included data from all randomized patients who took at least one dose of randomized treatment and had at least one set of post-randomization assessments. There were generally no imbalances in either cohort between treatment groups in any demographic or subject characteristic baseline variables, concomitant medication, or treatment compliance that could have had a potential influence on the results. Most missing data were due to study discontinuation. There were few missing assessments prior to discontinuation, with minimal impact on the analysis. Because the MRMM analyzes observed values and not changes from baseline, a missing baseline score for a patient did not result in all post-baseline observations being dropped from analysis for that patient. All statistical analyses were carried out using SAS (R) software version 9.1.
Results
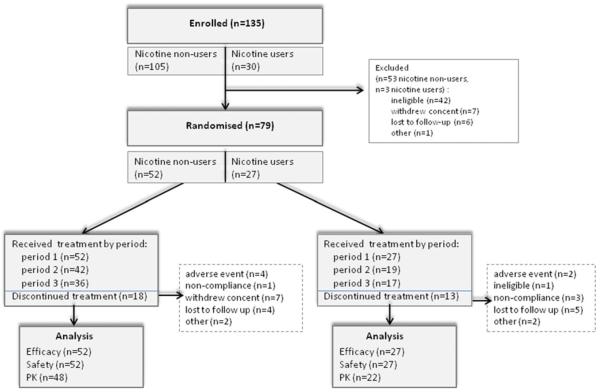
A total of 135 adults with ADHD from six centers in the USA were enrolled in the study, with 79 (69 males and ten females, aged 20 to 62 years of age) randomized to study treatment. The randomized study population included 52 patients who did not use nicotine and 27 patients who used nicotine (Fig. 2). Recruitment occurred between November 2009 and August 2010.
Fig 2.
Study flow diagram
Both cohorts had a greater proportion of patients diagnosed with the combined type of ADHD than the predominantly inattentive type of ADHD (Table 1). Nicotine users had been treated longer for ADHD than nicotine non-users. There were two patients with a history of suicide attempts (one attempt each), both in the nicotine users cohort. Mean duration of exposure was similar across the treatment arms in both study cohorts. Similar number of subjects completed three treatment periods in the two cohorts (65 % (34/52) of nicotine non-users and 52 % (14/27) of nicotine users) (participation by period is described in Fig. 2).
Table 1.
Demographic and clinical characteristics of study sample (intent-to-treat population)
| Characteristics | Nicotine non-users (n=52) | Nicotine users (n=27) |
|---|---|---|
| Age (years) | 34(10.5) | 33 (12.8) |
| Sex (F/M) | 5/47 | 5/22 |
| ADHD type (DSM IV): | ||
| 314.00 (ADHD, predominantly inattentive type) | 14 | 3 |
| 314.01 (ADHD, combined type) | 38 | 24 |
| Years since ADHD diagnosis | 7.2 (9.4) | 12.0 (13.2) |
| Age at first ADHD treatment (years) | 27 (13.0) | 21 (15.6) |
| CAARS-INV DSM-IV total score | 39.4 (7.4) | 43.2 (6.2) |
| CGI-S | 4.7 (0.6) | 4.9 (0.7) |
| Lifetime suicide attempts | 0 | 2 |
Data are presented as mean (SD), except for gender, diagnosis, number of suicide attempts. Lifetime suicide attempts in two subjects, one each. CAARS-INV was investigator-administered
CGI-S Clinical Global Impression Severity Scale
Efficacy
Clinical symptoms
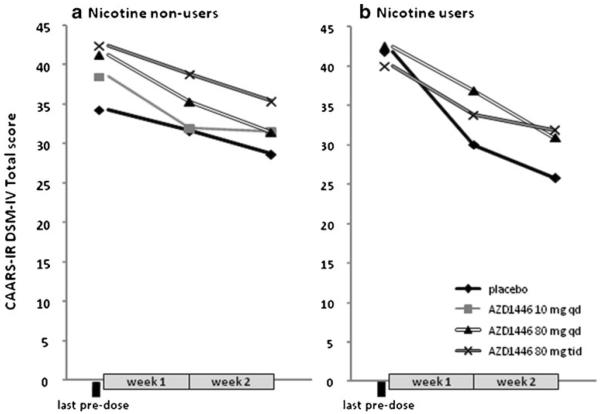
The primary efficacy endpoint analysis showed that AZD1446 did not improve clinical symptoms of ADHD in any of the treatment groups compared to placebo as measured by the CAARS-INV Total ADHD symptoms score (p>0.10) (Table 2). The highest difference in LS mean values, in favour of AZD1446 was observed in nicotine non-users at 80 mg qd dose (difference to placebo −1.28, with a 95 % confidence interval −4.65, 2.10; Table 2). In nicotine users, the difference in effects was in favour of placebo (LS mean 3.37 and 2.19 for 80 mg tid and 80 mg qd, respectively; Table 2). In order assess the efficacy without confounding by period effects, which could potentially be present in crossover studies, data were also presented by first treatment period only. In this subset of data, reduction in CAARS-INV Total ADHD symptoms score was observed in all treatment groups, although, there were no obvious differences between treatments (Fig. 3).
Table 2.
AZD1446 differences to placebo on efficacy measures at week 2 (clinical symptoms, intent-to-treat population)
| Score | Treatment | Nicotine non-users |
Treatment | Nicotine users |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | LS mean | Difference to placebo |
Number | LS mean | Difference to placebo |
|||||||
| LS mean | 95 %CI | P value | LS mean | 95 %CI | P value | |||||||
| CAARS-INVa, DSM-IV total score | Placebo | 40 | 29.0 | Placebo | 17 | 29.4 | ||||||
| 80 mg tid | 26 | 29.1 | 0.09 | −3.26, 3.44 | 0.80 | 80 mg tid | 18 | 32.7 | 3.37 | −2.36, 9.11 | 0.97 | |
| 80 mg qd | 26 | 27.8 | −1.28 | −4.65, 2.10 | 0.39 | 80 mg qd | 20 | 31.6 | 2.19 | −3.49, 7.87 | 0.91 | |
| 10 mg tid | 26 | 28.5 | −0.51 | −3.89, 2.87 | 0.64 | |||||||
| DSM-IV inattention score | Placebo | 40 | 16.7 | Placebo | 17 | 15.6 | ||||||
| 80 mg tid | 26 | 17.0 | 0.25 | −1.95, 2.45 | 0.99 | 80 mg tid | 18 | 18.3 | 2.70 | −0.66, 6.05 | 0.13 | |
| 80 mg qd | 26 | 16.4 | −0.36 | −2.57, 1.84 | 0.97 | 80 mg qd | 20 | 17.4 | 1.80 | −1.51, 5.11 | 0.37 | |
| 10 mg tid | 26 | 16.6 | −0.19 | −2.39, 2.01 | 0.99 | |||||||
| DSM-IV hyperactivity–impulsivity score | Placebo | 40 | 12.4 | Placebo | 17 | 13.9 | ||||||
| 80 mg tid | 26 | 12.5 | 0.13 | −1.67, 1.94 | 1.00 | 80 mg tid | 18 | 14.8 | 0.84 | −2.09, 3.76 | 0.75 | |
| 80 mg qd | 26 | 11.5 | −0.87 | −2.69, 0.95 | 0.55 | 80m qd | 20 | 14.5 | 0.64 | −2.27, 3.54 | 0.84 | |
| 10 mg tid | 26 | 12.4 | −0.04 | −1.86, 1.78 | 1.00 | |||||||
| ADHD Index | Placebo | 40 | 16.2 | Placebo | 17 | 17.4 | ||||||
| 80 mg tid | 26 | 16.2 | −0.02 | −2.14, 2.10 | 1.00 | 80 mg tid | 18 | 19.9 | 2.51 | −0.77, 5.80 | 0.16 | |
| 80 mg qd | 26 | 16.2 | −0.02 | −2.16, 2.11 | 1.00 | 80 mg qd | 20 | 19.9 | 2.54 | −0.72, 5.80 | 0.15 | |
| 10 mg tid | 26 | 16.7 | 0.54 | −1.59, 2.67 | 0.89 | |||||||
| Clinical Global Impression (CGI) Severity of illness score | Placebo | 40 | 4.1 | Placebo | 17 | 4.1 | ||||||
| 80 mg tid | 26 | 4.2 | 0.13 | −0.21, 0.49 | 0.68 | 80 mg tid | 18 | 4.2 | 0.06 | −0.44, 0.56 | 0.95 | |
| 80 mg qd | 26 | 4.1 | 0.05 | −0.30, 0.39 | 0.98 | 80 mg qd | 20 | 4.4 | 0.29 | −0.21, 0.78 | 0.32 | |
| 10 mg tid | 26 | 4.2 | 0.11 | −0.24, 0.46 | 0.82 | |||||||
Note that one-sided test of superiority was used for the primary endpoint CAARS-INV, DSM-IV Total score
CI confidence interval, qd once daily, tid three times daily, LS least squares
Investigator-rated
Fig 3.
CAARS-INV DSM-IV Total score changes by treatment period 1 (observed values, means). a Participants in the nicotine non-users cohort: placebo n=18, AZD1446 10 mg tid n=11, AZD1446 80 mg qd n=11, AZD1446 80 mg tid n=12. b Participants in the nicotine users cohort: placebo n=8, AZD1446 80 mg qd n=10, AZD1446 80 mg tid n=9
The results for the secondary efficacy analyses showed no significant effects of AZD1446 compared to placebo on CAARS-INV subscales, ADHD Index, CGI-S, and CAARS-S/SV, including subscales, in adult patients with ADHD (both nicotine non-users and nicotine users) (Tables 2 and 3).
Table 3.
AZD1446 differences to placebo at week 2 on CAARS self-report scores (intent-to-treat population)
| Score | Treatment | Nicotine non-users |
Treatment | Nicotine users |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | LS mean | Difference to placebo |
Number | LS mean | Difference to placebo |
|||||||
| LS mean | 95 %CI | P value | LS mean | 95 %CI | P value | |||||||
| DSM-IV symptoms, total | Placebo | 41 | 25.9 | Placebo | 17 | 27.1 | ||||||
| 80 mg tid | 26 | 24.9 | −1.04 | −4.13, 2.04 | 0.78 | 80 mg tid | 18 | 29.3 | 2.20 | −3.83, 8.22 | 0.62 | |
| 80 mg qd | 26 | 26.1 | 0.22 | −2.90, 3.34 | 0.99 | 80 mg qd | 19 | 30.5 | 3.41 | −2.57, 9.39 | 0.34 | |
| 10 mg tid | 26 | −0.06 | −3.17, 3.04 | 0.99 | ||||||||
| DSM-IV hyperactive/impulsive symptoms | Placebo | 40 | 10.8 | Placebo | 17 | 12.7 | ||||||
| 80 mg tid | 26 | 9.6 | −1.15 | −2.78, 0.48 | 0.24 | 80 mg tid | 18 | 13.9 | 1.18 | −1.84, 4.20 | 0.59 | |
| 80 mg qd | 26 | 10.3 | −0.43 | −2.08, 1.22 | 0.88 | 80 mg qd | 19 | 13.9 | 1.11 | −1.88, 4.10 | 0.62 | |
| 10 mg tid | 26 | 10.6 | −0.19 | −1.83, 1.45 | 0.99 | |||||||
| DSM-IV inattentive symptoms | ||||||||||||
| Placebo | 41 | 15.2 | Placebo | 17 | 14.4 | |||||||
| 80 mg tid | 26 | 15.2 | 0.01 | −1.85, 1.87 | 1.00 | 80 mg tid | 18 | 15.2 | 0.82 | −2.59, 4.23 | 0.81 | |
| 80 mg qd | 26 | 15.7 | 0.55 | −1.33, 2.43 | 0.85 | 80 mg qd | 19 | 16.6 | 2.22 | −1.17, 5.62 | 0.25 | |
| 10 mg tid | 26 | 15.4 | 0.21 | −1.66, 2.08 | 0.99 | |||||||
| ADHD Index | Placebo | 41 | 14.8 | Placebo | 17 | 17.7 | ||||||
| 80 mg tid | 26 | 13.8 | −1.14 | −3.12, 0.84 | 0.40 | 80 mg tid | 18 | 18.8 | 1.16 | −2.28, 4.60 | 0.67 | |
| 80 mg qd | 26 | 14.215. | −0.62 | −2.61, 1.37 | 0.82 | 80 mg qd | 19 | 19.7 | 2.00 | −2.41, 5.40 | 0.32 | |
| 10 mg tid | 26 | 15.5 | 0.62 | −1.37, 2.62 | 0.82 | |||||||
CAARS-SR scores were self-reported
CI confidence interval, qd once daily, tid three times daily, LS least squares
Cognitive tasks
Statistically significant improvement compared to placebo was seen on the Groton Maze Learning Task (p<0.02) at week 2 in nicotine non-users when treated with AZD1446 80 mg qd (Table 4). Trends for improvement were seen on the International Shopping List Task acquisition trials at 80 mg qd and 80 mg tid (p<0.06=0.08) (Table 4). These data are presented with multiplicity correction for treatment group within each test but were not corrected for multiplicity of cognitive tests. No significant effects on cognitive assessments were observed in nicotine users. There were no statistically significant effects of AZD1446 on any of the other cognitive tasks in any of the cohorts. The Stop Signal Task (SST) data did not pass quality control standards and, therefore, results from the SST are not presented.
Table 4.
AZD1446 differences to placebo on efficacy measures at week 2 (cognitive measures, intent-to-treat population)
| Score | Treatment | Nicotine non-users |
Treatment | Nicotine users |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | LS mean | Difference to placebo |
Number | LS mean | Difference to placebo |
|||||||
| LS mean | 95 % CI | P value | LS mean | 95 % CI | P value | |||||||
| International | Placebo | 41 | 8.9 | Placebo | 17 | 45.8 | ||||||
| Shopping List | 80 mg tid | 26 | 9.6 | 0.71 | −0.27, 1.68 | 0.22 | 80 mg tid | 17 | 41.4 | −0.08 | −1.15, 0.98 | 0.98 |
| Task-recall | 80 mg qd | 25 | 9.6 | 0.71 | −0.28, 1.70 | 0.22 | 80 mg qid | 19 | 42.8 | 0.36 | −0.67, 1.40 | 0.65 |
| 10 mg tid | 26 | 9.2 | 0.34 | −0.65, 1.33 | 0.77 | |||||||
| International | Placebo | 41 | 26.9 | Placebo | 17 | 9.5 | ||||||
| Shopping List | 80 mg tid | 26 | 28.3 | 1.37 | −0.11, 2.86 | 0.08 | 80 mg tid | 17 | 9.5 | 0.91 | −1.07, 2.89 | 0.48 |
| Task-acquisition | 80 mg qd | 25 | 28.4 | 1.50 | −0.02, 3.02 | 0.06 | 80 mg qid | 19 | 9.9 | 1.29 | −0.66, 3.24 | 0.24 |
| 10 mg tid | 26 | 27.3 | 0.41 | −1.10, 1.93 | 0.87 | |||||||
| One Back | Placebo | 41 | 1.2 | Placebo | 17 | 26.6 | ||||||
| Memory Task | 80 mg tid | 26 | 1.2 | −0.04 | −0.12, 0.04 | 0.61 | 80 mg tid | 17 | 27.5 | −0.04 | −0.18, 0.10 | 0.73 |
| 80 mg qd | 25 | 1.3 | 0.03 | −0.05, 0.11 | 0.71 | 80 mg qid | 19 | 27.9 | −0.01 | −0.15, 0.13 | 0.99 | |
| 10 mg tid | 26 | 1.3 | 0.04 | −0.04, 0.12 | 0.46 | |||||||
| Two Back Memory Task | Placebo | 41 | 1.2 | Placebo | 17 | 1.3 | ||||||
| 80 mg tid | 26 | 1.2 | −0.04 | −0.13, 0.06 | 0.69 | 80 mg tid | 17 | 1.2 | 0.03 | −0.08, 0.13 | 0.80 | |
| 80 mg qd | 25 | 1.2 | −0.04 | −0.13, 0.06 | 0.70 | 80 mg qid | 19 | 1.3 | 0.04 | −0.07, 0.15 | 0.58 | |
| 10 mg tid | 26 | 1.3 | 0.04 | −0.05, 0.14 | 0.59 | |||||||
| Groton Maze Learning Task | Placebo | 41 | 39.8 | Placebo | 17 | 1.2 | ||||||
| 80 mg tid | 26 | 37.3 | −2.56 | −7.87, 2.75 | 0.55 | 80 mg tid | 17 | 1.2 | −4.42 | −11.64, 2.80 | 0.29 | |
| 80 mg qd | 25 | 33.7 | −6.13 | −11.47, −.79 | 0.02a | 80 mg qid | 19 | 1.2 | −3.03 | −9.92, 3.87 | 0.51 | |
| 10 mg tid | 26 | 39.1 | −0.75 | −6.11, 4.61 | 0.98 | |||||||
For the International Shopping List (both), One Back Memory and Two Back Memory tasks, improved performance is indicated by positive numerical change. For the Stop Signal and Groton Maze Learning tasks, improved performance is indicated by negative numerical change. Results are corrected for multiple comparisons between treatment groups
Statistically significant changes
Pharmacokinetics
The predicted half-life of AZD1446 was approximately 2 h and plasma concentrations reached maximum within less than 1 h after administration. There were no differences in AZD1446 exposure between nicotine users and non-users (Table 5).
Table 5.
AZD1446 predicted pharmacokinetic parameters
| Treatment group | Number | tmax, pred (h) | Css, max pred (nmol/L) | AUCss, pred (nmol*h/L) | t1/2pred (h) |
|---|---|---|---|---|---|
| Nicotine non-users | |||||
| 10 mg tid | 28 | 0.63 (0.48–2.18) | 196 (21) | 2,120 (21) | 2.11 (11) |
| 80 mg qd | 27 | 0.76 (0.18–6.24) | 1,240 (44) | 5,710 (19) | 2.12 (7.1) |
| 80 mg tid | 31 | 0.82 (0.52–2.70) | 1,460 (36) | 17,600 (28) | 2.11 (11) |
| Nicotine users | |||||
| 80 mg qd | 19 | 0.76 (0.63–2.58) | 1,360 (29) | 5,730 (19) | 2.09 (8.8) |
| 80 mg tid | 17 | 0.74 (0.64–2.49) | 1,440 (27) | 17,000 (17) | 2.05 (11) |
Results are presented as geometric mean (CV%), except for tmax, pred which is presented as median
Css, max pred predicted maximal plasma concentration at steady-state, tmax, pred predicted time to Css, max, AUCss, pred predicted area under the plasma concentration vs. time curve at steady-state, t1/2pred predicted half-life
Adverse events
In both cohorts, adverse events (AEs) by system organ class and preferred term were similar, thus, pooled data are presented in Table 6. There were no serious adverse events reported in the study. The most common AEs were nausea and headache. Most of the AEs were of mild to moderate intensity and self-resolving. Overall six subjects discontinued the study due to AEs (Fig. 2): in the cohort of nicotine non-users, three subjects treated with AZD1446 80 mg tid (decreased appetite, diarrhea, and dyspepsia) and one subject with placebo (erectile dysfunction); in the cohort of nicotine users, one subject treated with AZD1446 80 mg tid (worsening of insomnia) and one with AZD1446 80 mg qd (anxiety).
Table 6.
Most common adverse events during treatment with AZD1446 and placebo (pooled sample of nicotine non-users and nicotine users)
| Adverse event | Number (%) of patientsa |
|||
|---|---|---|---|---|
| Placebo | AZD1446 10 mg tid | 80 mg qd | 80 mg tid | |
| Number of subjects treated | 64 | 29 | 49 | 51 |
| Number of subjects with at least one adverse events | 25 (39) | 14 (48) | 26 (53) | 32 (63) |
| Most commona adverse events | ||||
| Nausea | 5 (7.8) | 1 (3.4) | 8 (16.3) | 11 (21.6) |
| Headache | 4 (6.2) | 0 | 4 (8.2) | 9 (17.6) |
| Diarrhea | 2 (3.1) | 0 | 2 (4.1) | 5 (9.8) |
| Dyspepsia | 2 (3.1) | 2 (6.9) | 1 (2.0) | 4 (7.8) |
| Nasopharyngitis | 1 (1.6) | 4 (13.8) | 0 | 3 (5.9) |
| Insomnia | 1 (1.6) | 0 | 3 (6.1) | 3 (5.9) |
| Decreased appetite | 0 | 0 | 0 | 3 (5.9) |
Subjects with multiple events in the same category are counted only once in that category, subjects with events in more than one category are counted once in each of those categories. Only AEs occurring in ≥5% of the cases in at least one treatment condition are presented
In both cohorts, assessment of hematology, clinical chemistry, urinalysis, ECG, and vital signs showed no clinically meaningful differences when comparing the AZD1446 treatment groups to placebo. Analysis of the POMS, total, and cluster (vigor, tension, depression, anger, fatigue, confusion) scores had no statistically (MRMM analysis) or clinically meaningful trends in any treatment groups compared to placebo. There were no major differences in suicidal ideation or behavior (C-SSR scale) between the treatment groups.
Discussion
This multicenter, randomized, double-blind, placebo-controlled crossover study evaluated the effects of 2 weeks of treatment with AZD1446 in adults with ADHD. AZD1446, compared to placebo, did not improve core clinical symptoms of ADHD as measured by the CAARS-INV Total ADHD Symptoms score. Effects of AZD1446 on CAARS-INV subscales, ADHD Index and symptom severity, CAARS-S/SV, CGI-S also did not differ significantly from placebo. Improvement compared to placebo was observed for AZD1446 on executive functioning task (GMLT) in nicotine non-users, the 80-mg qd dose. AZD1446 was safe and well tolerated.
There may be several reasons for the negative results of this clinical trial, one being drug pharmacokinetics. Two important questions to address are whether AZD1446 crosses the blood–brain barrier in humans and if adequate central nervous system exposure was achieved with the doses tested. A positron emission tomography study has shown that AZD1446 enters the brain and reaches high (>80 %) occupancy of target receptors at the 80-mg dose (Jucaite et al. 2012). Although receptor occupancy paradigm for agonist dose selection is not established (Grimwood 2009), it could still serve as a framework for the choice of dose range. We also explored the effect of fluctuating exposure of AZD1446. A hypothesis was raised by preclinical studies (AZ data on file) and was in line with studies on atypical antipsychotics, which have suggested that continuous interaction with receptors may not be needed for clinical efficacy (Tauscher-Wisniewski et al. 2002). We also considered use of nicotine among adults with ADHD. Nicotine receptor upregulation or functional changes related to nicotine use (Perry et al. 1996; Mukhin et al. 2008) theoretically may influence effects of nAChR agonists. Therefore, efficacy of AZD1446 in nicotine non-users and users was explored separately. Altogether, the absence of clinical effects of AZD1446 at different doses and schedules in both cohorts suggest the negative results are unlikely to be due to inadequate exposure to AZD1446 or nicotine use.
Another possible reason to the lack of efficacy could be related to the study design, e.g., crossover design per se and statistical power. In crossover design studies, there is the risk of confounding period effects which potentially could be the case in this study. However, the results of the first period did not differ from the overall study results, suggesting that such bias was not the main reason for the negative study outcome. The present study was powered to detect a five-point difference between drug treatment and placebo in ADHD CAARSINV Total Score, but the differences observed were much smaller and nonsignificant, even with the less stringent statistical significance level than typically used in pivotal studies (p<0.1 instead of p<0.05). Large reductions in the total score were observed in the placebo groups, similar to the results described in other ADHD trials (Newcorn et al. 2009). This poses a challenge for clinical ADHD studies in general and might have contributed to the lack of efficacy in this trial.
The cognitive test results warrant closer examination in light of the overall negative results of this trial. Executive function impairment is one of the core cognitive deficits in ADHD. Interestingly, treatment with AZD1446 showed numerically better performance on executive function, spatial problem solving, verbal memory, and learning compared to placebo, all for the 80-mg qd dose group in nicotine non-users. It is worth noting that changes observed were selective for higher order functions, with no change in the lower order functions, e.g., reaction time. It may also be that overall effects of AZD1446 on cognitive performance were underestimated, as some tests in the battery were not sufficiently challenging (e.g., potential ceiling effects on ISLT), or were inconclusive (e.g., Stop Signal task, SST). Robust effects of nicotine and other α4β2* nicotinic agonists have been seen on SST performance in both adolescents and adults (Potter et al. 2004, 2008, 2013), which in at least one case correlated with clinical effects. The lack of SST data from this trial unfortunately precluded our ability to validate this intermediate cognitive marker. Presence of effect on SST would have given an additional clue as to whether the drug activated the receptor adequately to produce positive clinical effects. On the other hand, results were at statistical trend levels, not corrected for multiple comparisons between tests and were inconsistent with respect to dose, schedule, and concomitant use of nicotine. Overall, though to be taken with caution, effects on some cognitive tests observed in the present study suggest that AZD1446 may have pro-cognitive pharmacological activity in ADHD, albeit of a magnitude with doubtful clinical significance.
The pro-cognitive effects of AZD1446 in ADHD were predicted based on demonstrated long-lasting, broad dose–response effect on the cognitive performance in rodents. This preclinical efficacy, however, did not translate into the efficacy in adults with ADHD. Importantly, considerable rodent-to-man differences exist in the brain distribution of α4β2* nAChRs, binding specificity and subtype selectivity (Pimlot et al. 2004; Schmaljohann et al. 2004), setting hurdles for translational pharmacology. Another difficult aspect for translational research is the lack of proven in vivo animal model for advancing novel, non-psychostimulant drugs in ADHD (Wickens et al. 2011). The tests used in the preclinical program of AZD1446 included novel object recognition, radial arm maze, water maze, and operant visual signal detection task, which together cover domains of long-term visual episodic/declarative memory, working memory, spatial learning/memory, and sustained and selective attention (Mazurov et al. 2012; Szeliga et al. 2012, AZ data on file). None of the known ADHD animal models were used. Several candidate paradigms, e.g., delayed reinforcement, stop-signal reaction-time tasks, that are assays of distinct types of inhibitory behaviors, have been suggested for use in ADHD (Eagle and Robbins 2003; Humby and Wilkinson 2011; Winstanley 2011), albeit their translatability using nAChR agonists remains to be established. The need of animals with low cholinergic activity to further test cholinergic compounds has been recognized (Sarter and Paolone 2011).
The present study showed no effect of AZD1446 on clinical symptoms of ADHD in adults and by that differs from initial studies of comparable design with other α4β2* nAChRs agonists (ABT-418, ABT-089, AZD3480). There may be several explanations for such differences. Firstly, α4β2* nAChR agonists studied so far in ADHD differ by their molecular and pharmacological characteristics, e.g., their selectivity to β2* subunits (e.g., α4β2*, α2β2*, or α6β2* subtypes) as well as affinity and potency, and different agonism profiles at high/low receptor affinity states, by level of receptor desensitization, modulation of synaptic plasticity (e.g., level of receptor dwell time at synapses) and, not least, potential induction of dopamine release (Arneric et al. 1994; Hahn et al. 2003; Marks et al. 2009; Anderson et al. 2009; von Euler et al. 2011, 2012). Thus, by these intricate difference combinations, compounds may potentially confer different clinical effects. It remains unknown which of the described features in the complex nAChR system would be critical for the efficacy in ADHD.
Secondly, ADHD is a very heterogeneous disorder, making study results highly dependent on the population, bringing to the differences in clinical efficacy between compounds, or even between studies with the same compound. Pilot exploratory crossover design studies with small, rather homogenous ADHD populations, more stable clinical scoring, have demonstrated clinical efficacy of ABT-089 and ABT-418. The strongest effect observed was on inattention scores, further supported by effect on the laboratory measures of attention (e.g., decrease in commission errors during continuous performance task) (Wilens et al. 1999, 2006; Apostol et al. 2012). Encouraging results using ABT-089 were not confirmed in subsequent larger parallel group design studies neither in children (Wilens et al. 2011) nor adults with ADHD (Bain et al. 2012). The lack of efficacy in children could be related to the maturation of the attentional control system (Andrews-Hana et al. 2011). Meanwhile in adults, no significant treatment effect of ABT-089 was judged by the authors (Bain et al. 2012) as true lack of efficacy in ADHD, even though the study was not powered to detect a treatment difference to placebo and had higher proportion of inattentive subtype of ADHD in placebo group potentially enlarging placebo effects. The present study, though not directly comparable due to the use of a different α4β2* nAChRs agonist and a different design, is overall in line with these late observations on ABT-089. Nevertheless, the negative results of the present study do not preclude from further search of nAChR agonists with pharmacological activity in ADHD.
Conclusions
AZD1446 was well tolerated but did not significantly improve ADHD symptoms after 2 weeks of treatment compared to placebo. While these data do not support the therapeutic utility of AZD1446 in ADHD, the potential pro-cognitive effects of AZD1446 remain to be further characterized in other neuropsychiatric disorders.
Acknowledgments
The authors wish to acknowledge the following investigators for their contributions to this study: Jerry C. Steiert, M.D. (Summit Research Network (Seattle), LLC, Seattle, WA); John K. Heussy, M.D. (New York, NY); John F. Prater, M.D. (Nova Southeastern University, Ft. Lauderdale and Gulfcoast Clinical Research Center, Fort Myers, FL); Donald Garcia, M.D. (FutureSearch Trials, LLC, Austin, TX); Nader Oskooilar, M.D., Ph.D. (Pharmacology Research Institute, Los Alamitos, LA). The authors wish to thank members of the AstraZeneca study team: Hans-Göran Hårdemark for the input to the study design, critical reviews; Edwin Johnson for the input to the discussion; Sara Lindholm for the assistance with the study design; Lili Ghavamzadeh for the operational assistance; Dennis Sweitzer for the statistical analysis; and Jacob Brogren for the pharmacokinetic modelling. The authors are grateful to David Hosford (Targacept, Inc.) for the manuscript review and valuable comments.
Declaration of conflicting interests This study was funded by AstraZeneca, R&D, Södertälje, Sweden [ClinicalTrials.gov Identifier: NCT01012375; http://clinicaltrials.gov/ct2/show/NCT01012375?term=AZD1446&rank=4]. AstraZeneca was involved in the original concepts and systematic review of existing trial evidence, the design, the choice of investigators, the control of the allocation schedule, the conduct of the trial, the collection and monitoring of data, data analysis and interpretation, and the writing and approval of this report. The authors have had full control of all primary data and agree to allow the journal to review the data if requested.
Disclosures Authors AJ, JÖ, JJ, PK, KH, EB, and BP were full-time AstraZeneca employees during the study design, conduct, data analysis and writing period, and received salary and stock from AstraZeneca. Prof. Paul A. Newhouse is supported by the National Institute of Aging (NIMH) Grant, has received speaking fees, consulting fees, and research grants from AstraZeneca, Lilly, and Sanofi-Aventis. PN and AP have no financial involvement or commercial interest in the AstraZeneca products under study and received no financial remuneration.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders (DSM-IV-TR) American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Anderson DJ, Malysz J, Grønlien JH, El Kouhen R, Håkerud M, Wetterstrand C, Briggs CA, Gopalakrishnan M. Stimulation of dopamine release by nicotinic acetylcholine receptor ligands in rat brain slices correlates with the profile of high, but not low, sensitivity α4β2 subunit combination. Biochem Pharmacol. 2009;78:844–851. doi: 10.1016/j.bcp.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Mackiewicz Seghete KL, Claus ED, Burgess GC, Ruzic L, Banich MT. Cognitive control in adolescence: neural underpinnings andrelation to self-report behaviors. PLoS One. 2011;6(6):e21598. doi: 10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol G, Abi-Saab W, Kratochvil CJ, Adler LA, Robieson WZ, Gault LM, Pritchett YL, Feifel D, Collins MA, Saltarelli MD. Efficacy and safety of the novel α4β2 neuronal nicotinic receptor partial agonist ABT-089 in adults with attention-deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled crossover study. Psychopharmacology (Berl) 2012;219:715–725. doi: 10.1007/s00213-011-2393-2. [DOI] [PubMed] [Google Scholar]
- Arneric SP, Sullivan JP, Briggs CA, Donnelly-Roberts D, Anderson DJ, Raszkiewicz JL, et al. (S)-3-methyl-5-(1-methyl-2-pyrrolidinyl) isoxazole (ABT 418): a novel cholinergic ligand with cognition-enhancing and anxiolytic activities: I. In vitro characterization. J Pharmacol Exp Ther. 1994;270:310–318. [PubMed] [Google Scholar]
- Bain EE, Apostol G, Sangal RB, Robieson WZ, McNeill DL, Abi-Saab WM, Saltarelli MD. A randomized pilot study of the efficacy and safety of ABT-089, a novel α4β2 neuronal nicotinic receptor agonist, in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2012;73:783–789. doi: 10.4088/JCP.10m06719. [DOI] [PubMed] [Google Scholar]
- Bernfort L, Nordfeldt S, Persson J. ADHD from a socioeconomic perspective. Acta Paediatrica. 2008;97:239–245. doi: 10.1111/j.1651-2227.2007.00611.x. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Allen-Martinez Z, Scheibal D, Abrams AL, et al. Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacol. 2009;34:282–289. doi: 10.1038/npp.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA) Drug Safety. 1999;20:109–117. doi: 10.2165/00002018-199920020-00002. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci. 2006;10(3):117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Conners CK, Levin ED, Sparrow E, Hinton SC, Erhardt D, Meck WH, et al. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD) Psychopharmacol Bull. 1996;32:67–73. [PubMed] [Google Scholar]
- Conners CK, Erhardt D, Sparrow E. Conners' Adult ADHD Rating Scales. Multi-Health Systems Inc; North Tonawanda, NY: 2004. [Google Scholar]
- Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012;48(2):194–215. doi: 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Dunbar G, Boeijinga PH, Demazières A, Cisterni C, Kuchibhatla R, Wesnes K, Luthringer R. Effects of TC-1734 (AZD3480), a selective neuronal nicotinic receptor agonist, on cognitive performance and the EEG of young healthy male volunteers. Psychopharmacology (Berl) 2007;191:919–929. doi: 10.1007/s00213-006-0675-x. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal Reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci. 2003;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Monuteaux M, Seidman L. Attention deficit hyperactivity disorder and learning disability: a prospective four year follow-up study. J of Atten Disorders. 2001;3:23–25. [Google Scholar]
- Greenwood PM, Parasuraman R, Espeseth T. A cognitive phenotype for a polymorphism in the nicotinic receptor gene CHRNA4. Neuroscience and Biobehav Rev. 2012;36:1331–1341. doi: 10.1016/j.neubiorev.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Grimwood S, Hartig PR. Target site occupancy: emerging generalizations from clinical and preclinical studies. Pharmacol Therapeut. 2009;122:281–301. doi: 10.1016/j.pharmthera.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Wyler R, Higgins GA. A study of the nicotinic agonist SIB-1553A on locomotion and attention as measured by the five-choice serial reaction time task. Pharmacol Biochem Behav. 2001;70:505–513. doi: 10.1016/s0091-3057(01)00639-6. [DOI] [PubMed] [Google Scholar]
- Guillem K, Bloem B, Poorthuis RB, Loos M, Smit AB, Maskos U, Spijker S, Mansvelder HD. Nicotinic acetylcholine receptor β2 subunits in the medial prefrontal cortex control attention. Science. 2011;333(6044):888–891. doi: 10.1126/science.1207079. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment manual for psychopharmacology—revised (DHEW Publ. No. ADM 76–338) U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs; Rockville, MD: 1976. pp. 218–222. [Google Scholar]
- Hahn B, Sharples CGV, Wonnacott S, Shoaib M, Stolerman IP. Attentional effects of nicotinic agonists in rats. Neuropharmacol. 2003;44:1054–1067. doi: 10.1016/s0028-3908(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Howe WM, Ji J, Parikh V, Williams S, Mocaër E, Trocmé-Thibierge C, Sarter M. Enhancement of attentional performance by selective stimulation of alpha4beta2(*) nAChRs: underlying cholinergic mechanisms. Neuropsychopharmacology. 2010;35(6):1391–1401. doi: 10.1038/npp.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humby T, Wilkinson Assaying dissociable elements of behavioral inhibition and impulsivity: translational utility of animal models. Current Opinion in Pharmacol. 2011;11:534–539. doi: 10.1016/j.coph.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Jucaite A, Nyberg S, Takano A, Kågedal M, Johnström P, Halldin C, Jostell KG, Johnson E, Farde L. Relationship between dose, plasma concentration and α4β2 nicotinic receptor occupancy for AZD1446 (TC-6683): a translational approach. Abstract, 28th CINP World Congress of Nueropsychopharmacology; 3–7 June, 2012. [Google Scholar]
- Klassen AF, Miller A, Fine S. Health-related quality of life in children and adolescents who have a diagnosis of attention-deficit/hyperactivity disorder. Pediatrics. 2004;114:e541–547. doi: 10.1542/peds.2004-0844. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, et al. Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacol. 1996;123:55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Lim YY, Prang KH, Cysique L, Pietrzak RH, Snyder PJ, Maruff P. A method for cross-cultural adaptation of a verbal memory assessment. Behav Res Meth. 2009;41:1190–1200. doi: 10.3758/BRM.41.4.1190. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Wageman CR, Grady SR, Gopalakrishnan M, Briggs CA. Selectivity of ABT-089 for alpha4beta2* and alpha6beta2* nicotinic acetylcholine receptors in brain. Biochem Pharmacol. 2009;78:795–802. doi: 10.1016/j.bcp.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurov AA, Miao L, Bhatti BS, Strachan JP, Akireddy S, Murthy S, Kombo D, Xiao YD, Hammond P, Zhang J, Hauser TA, Jordan KG, Miller CH, Speake JD, Gatto GJ, Yohannes D. Discovery of 3-(5-chloro-2-furoyl)-3,7-diazabicyclo[3.3.0]octane (TC-6683, AZD1446), a novel highly selective α4β2 nicotinic acetylcholine receptor agonist for the treatment of cognitive disorders. J Med Chem. 2012;55:9181–994. doi: 10.1021/jm3006542. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Decker MW, Sarter M. Enhancement of sustained attention performance by the nicotinic acetylcholine receptor agonist ABT-418 in intact but not basal forebrain-lesioned rats. Psychopharmacology (Berl) 1999;144:175–182. doi: 10.1007/s002130050991. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppelman LF. Manual for the profile of mood states. Educational and Industrial Testing Service; San Diego: 1971. [Google Scholar]
- Mukhin AG, Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, Horti AG, Vaupel DB, Pavlova O, Stein EA. Greater nicotinic acetylcholine receptor density in smokers than in non-smokers: a PET study with 2-18F-FA-85380. J Nucl Med. 2008;49:1628–1635. doi: 10.2967/jnumed.108.050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcorn JH, Sutton VK, Zhang S, Wilens T, Kratochvil C, Emslie GJ, D'souza DN, Schuh LM, Allen AJ. Characteristics of placebo responders in pediatric clinical trials of attention-deficit/hyperactivity disorder. J Am Acad Child and Adolescent Psych. 2009;48:1165–1172. doi: 10.1097/CHI.0b013e3181bc730d. [DOI] [PubMed] [Google Scholar]
- Perry DC, Dávila-García MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exper Therapeut. 1996;289:1545–1552. [PubMed] [Google Scholar]
- Pimlott SL, Piggott M, Owens J, et al. Nicotinic acetylcholine receptor distribution in Alzheimer's disease, dementia with Lewy bodies, Parkinson's disease, and vascular dementia: in vitro binding study using 5-[125I]-A-85380. Neuropsychopharmacol. 2004;29:108–116. doi: 10.1038/sj.npp.1300302. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Rohde LA. Epidemiology of attention-deficit/hyperactivity disorder across the lifespan. Cur Opin Psych. 2007;20:386–392. doi: 10.1097/YCO.0b013e3281568d7a. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacol (Berl) 2004;176:182–194. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Bucci DJ (2006) Central nicotinic cholinergic systems: a role in the cognitive dysfunction in attention-deficit/hyperactivity disorder? Behav Brain Res. 2006;175:201–211. doi: 10.1016/j.bbr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol Biochem Behav. 2008;88:407–417. doi: 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Potter AS, Bucci DJ, Newhouse PA. Manipulation of nicotinic acetylcholine receptors differentially affects behavioral inhibition in human subjects with and without disordered baseline impulsivity. Psychopharmacol (Berl) 2012;220:331–340. doi: 10.1007/s00213-011-2476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter AS, Dunbar G, Mazzulla E, Hosford D, Newhouse PA. Nicotinic agonist treatment with AZD3480 improves behavioral inhibition and clinical measures in adult Attention Deficit/Hyperactivity Disorder (manuscript in review) 2013. [Google Scholar]
- Prendergast MA, Jackson WJ, Terry AV, Jr, Decker MW, Arneric SP, Buccafusco JJ. Central nicotinic receptor agonists ABT-418, ABT-089, and (−)-nicotine reduce distractibility in adult monkeys. Psychopharmacology (Berl) 1998;136:50–58. doi: 10.1007/s002130050538. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Cauley M, Sexton H, Xiao Y, Brown ML, Paige MA, McDowell BE, Kellar KJ, Levin ED. Sazetidine-A, a selective α4β2 nicotinic acetylcholine receptor ligand: effects on dizocilpine and scopolamine-induced attentional impairments in female Sprague–Dawley rats. Psychopharmacology (Berl) 2011;215:621–630. doi: 10.1007/s00213-010-2161-8. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Cauley MC, Johnson EC, Gatto GJ, Levin ED. Effects of AZD3480, a neuronal nicotinic acetylcholine receptor agonist, and donepezil on dizocilpine-induced attentional impairment in rats. Psychopharmacology (Berl) 2012;223:251–258. doi: 10.1007/s00213-012-2712-2. [DOI] [PubMed] [Google Scholar]
- Sarter M, Paolone G. Deficits in attentional control: cholinergic mechanismsand circuitry-based treatment approaches. Behav Neurosci. 2011;125:825–835. doi: 10.1037/a0026227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Pantoja R, Moss FJ, Mackey ED, Son CD, Miwa J, Lester HA. Nicotine up-regulates alpha4beta2 nicotinic receptors and ER exit sites via stoichiometry-dependent chaperoning. J Gen Physiol. 2011;137:59–79. doi: 10.1085/jgp.201010532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohann J, Minnerop M, Karwath P, Gündisch D, Falkai P, Guhlke S, Wüllner U. Imaging of central nACh Receptors with 2-[18F]F-A85380: optimized synthesis and in vitro evaluation in Alzheimer's disease. Appl Radiat Isot. 2004;61:1235–1240. doi: 10.1016/j.apradiso.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Szeliga KT, Jordan KG, Hauser TA, Mazurov AA, Yohannes D, Rezvani AH, Levin ED, von Euler G, Johnson EC, Gatto GJ. AZD1446 (TC-6683), a novel alpha4beta2 nAChR agonist with cognitive-enhancing properties. Abstract. Society for Neuroscience Meeting; New Orleans. Oct 13–17, 2012. [Google Scholar]
- Tauscher-Wisniewski S, Kapur S, Tauscher J, Jones C, Daskalakis ZJ, Papatheodorou G, Epstein I, Christensen BK, Zipursky RB. Quetiapine: an effective antipsychotic in first-episode schizophrenia despite only transiently high dopamine-2 receptor blockade. J Clin Psychiatry. 2002;63:992–997. doi: 10.4088/jcp.v63n1106. [DOI] [PubMed] [Google Scholar]
- Todd RD, Lobos EA, Sun LW, Neuman RJ. Mutational analysis of the nicotinic acetylcholine receptor alpha 4 subunit gene in attention deficit/hyperactivity disorder: evidence for association of an intronic polymorphism with attention problems. Mol Psychiatry. 2003;8:103–108. doi: 10.1038/sj.mp.4001257. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Abnormal functional connectivity in children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;71:443–450. doi: 10.1016/j.biopsych.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K, Togashi H, Matsumoto M, Ohashi S, Saito H, Yoshioka M. Alpha4beta2 nicotinic acetylcholine receptor activation ameliorates impairment of spontaneous alternation behavior in stroke-prone spontaneously hypertensive rats, an animal model of attention deficit hyperactivity disorder. J Pharmacol Exp Ther. 2002;302:95–100. doi: 10.1124/jpet.302.1.95. [DOI] [PubMed] [Google Scholar]
- von Euler G, Bertrand D, Johnson EC. Comparison of pharmacologic properties of AZD3480 and AZD1446 on neuronal nicotinic receptor subtypes. Biochem Pharmacol. 2011;82:1026. [Google Scholar]
- von Euler G, Bertrand D, Gatto GJ, Johnson EC. Agonistic and desensitization properties of clinically tolerated compounds on neuronal nicotinic receptor subtypes. Abstract 532.16/B43, Society for Neuroscience Annual Conference; New Orleans, USA. 2012. [Google Scholar]
- Wells GB. Structural answers and persistent questions about how nicotinic receptors work. Front Biosci. 2008;13:5479–510. doi: 10.2741/3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens JR, Hyland BI, Tripp G. Animal models to guide clinical drug development in ADHD: lost in translation? Br J Pharmacol. 2011;164:1107–1128. doi: 10.1111/j.1476-5381.2011.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, Bostic J, Prince J, Monuteaux MC, Soriano J, Fine C, Abrams A, Rater M, Polisner D. A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1931–1937. doi: 10.1176/ajp.156.12.1931. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Verlinden MH, Adler LA, Wozniak PJ, West SA. ABT-089, a neuronal nicotinic receptor partial agonist, for the treatment of attention-deficit/hyperactivity disorder in adults: results of a pilot study. Biol Psychiatry. 2006;59:1065–1070. doi: 10.1016/j.biopsych.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Gault LM, Childress A, Kratochvil CJ, Bensman L, Hall CM, Olson E, Robieson WZ, Garimella TS, Abi-Saab WM, Apostol G, Saltarelli MD. Safety and efficacy of ABT-089 in pediatric attention-deficit/hyperactivity disorder: results from two randomized placebo-controlled clinical trials. J Am Acad Child Adolescent Psych. 2011;50:73–84. doi: 10.1016/j.jaac.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA. The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br J Pharmacol. 2011;164:1301–1321. doi: 10.1111/j.1476-5381.2011.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Meves JM, Geyer MA. Nicotinic agonist-induced improvement of vigilance in mice in the 5-choice continuous performance test. Behav Brain Res. 2013 Mar 1;240:119–33. doi: 10.1016/j.bbr.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]