Abstract
Objective
This study tested cognitive behavior therapy (CBT) in hypnotic-dependent, late middle-age and older adults with insomnia.
Method
Seventy volunteers age 50 and older were randomized to CBT plus drug withdrawal, placebo biofeedback (PL) plus drug withdrawal, or drug withdrawal (MED) only. The CBT and PL groups received eight, 45 minute weekly treatment sessions. The drug withdrawal protocol comprised slow tapering monitored with about six biweekly, 30 minute sessions. Assessment including polysomnography (PSG), sleep diaries, hypnotic consumption, daytime functioning questionnaires, and drug screens collected at baseline, posttreatment, and 1-year follow-up.
Results
Only the CBT group showed significant sleep diary improvement, sleep onset latency significantly decreased at posttreatment. For all sleep diary measures for all groups, including MED, sleep trended to improvement from baseline to follow-up. Most PSG sleep variables did not significantly change. There were no significant between group differences in medication reduction. Compared to baseline, the three groups decreased hypnotic use at posttreatment, down 84%, and follow-up, down 66%. There was no evidence of withdrawal side-effects. Daytime functioning, including anxiety and depression, improved by posttreatment. Rigorous methodological features, including documentation of strong treatment implementation and the presence of a credible placebo, elevated the confidence due these findings.
Conclusions
Gradual drug withdrawal was associated with substantial hypnotic reduction at posttreatment and follow-up, and withdrawal side-effects were absent. When supplemented with CBT, participants accrued incremental self-reported, but not PSG, sleep benefits.
Keywords: hypnotic dependence, drug withdrawal, insomnia, cognitive behavior therapy
Chronic, clinically significant insomnia occurs in about 10% of the population (Ohayon, 2002). National panels have long cautioned against the use of hypnotics in the management of chronic insomnia (Institute of Medicine, 1979; National Institutes of Health, 1984, 1991), but hypnotic use continues to climb (Moloney, Konrad, & Zimmer, 2011).
Depending on dose and type, unwanted effects of hypnotics include physical and psychological dependence, residual sedation, cognitive impairment, and compromised psychomotor performance (Licata & Rowlett, 2008; Vermeeren & Coenen, 2011). In a tradeoff for modest therapeutic gains (Buscemi et al., 2007), hypnotic impairment exposes users to elevated risk for serious accidents (Shuto et al., 2010; Verster, Veldhuijzen, & Volkerts, 2004; Wang, Bohn, Glynn, Mogun, & Avorn, 2001).
The convergent influence of several factors causes the impact of hypnotics to be felt most strongly among older adults. First, insomnia is disproportionately represented among older adults, affecting about one third of people in this age group (Foley, Monjan, Brown, Simonsick, Wallace, & Blazer, 1995). Second, there is greater hypnotic exposure in older adults based on higher prevalence and persistence of hypnotic use in this group (Ashton, 1994; Balkrishnan, Rasu, & Rajagopalan, 2005; Morgan & Clarke, 1997; Stewart et al., 2006). Third, older adults are more vulnerable to the hazards associated with hypnotics than are younger adults. Age-related absorption and metabolic changes in later life can extend drug half-life in the body and promote excess drug accumulation, can heighten the risk of residual daytime cognitive and motor impairment, and can exacerbate sleep-disordered breathing, which is more common in older adults (Guilleminault, 1990; Moran, Thompson, & Nies, 1988; National Institutes of Health, 1984).
Psychological factors interact with the tolerance/dependence pattern often associated with the most common class of hypnotics, benzodiazepine receptor agonists, to prolong hypnotic dependence. Abrupt withdrawal may instigate exacerbation of insomnia and anxiety above baseline levels, termed rebound, after as short a period as 1 week of hypnotic use (Greenblatt, Harmatz, Zinny, & Shader, 1987). Resumption of hypnotics brings rapid relief from these acute symptoms, and psychological dependence on hypnotics is strengthened by this negative reinforcement paradigm. Accordingly, anticipation of rebound effects may discourage attempts to forego reliance on these medications, even when users no longer derive hypnotic benefits (Schneider-Helmert, 1988).
Psychological treatments for people with insomnia were introduced in the late 1960s. They have since been shown to be safe and effective and have gained broad acceptance (Morin et al., 2006). Hypnotic-dependent insomnia refers to current insomnia accompanied by chronic hypnotic use, and there would appear to be a unique and clinically significant role for psychological interventions in the treatment of hypnotic-dependent insomnia.
There is a growing body of literature on psychological management of hypnotic-dependent insomnia, but much of it is poorly controlled and important gaps remain. Thirteen studies investigated this subject and all reported positive results with respect to sleep improvement or reduced hypnotic use. But ten (Baillargeon et al., 2003; Espie, Lindsay, & Brooks, 1988; Kirmil-Gray, Eagleston, Thoresen, & Zarcone, 1985; Lichstein & Johnson, 1993; Lichstein et al., 1999; Morin, Colecchi, Ling, & Sood, 1995; Morin, Stone, McDonald, & Jones, 1994; Riedel et al., 1998; Taylor, Schmidt-Nowara, Jessop, & Ahearn, 2010; Zavesicka, Brunovsky, Matousek, & Sos, 2008) are of diminished interest due to several factors: uncontrolled case study, constricted range of dependent variables, small N, inclusion of over-the-counter hypnotics, or nonrandom assignment to conditions.
There are three methodologically mature studies in this domain (Belleville, Guay, Guay, & Morin, 2007; Morgan, Dixon, Mathers, Thompson, & Tomeny, 2003; Morin et al., 2004). All tested cognitive behavior therapy packages comprised of some combination of sleep hygiene, cognitive therapy, stimulus control, sleep restriction, and relaxation.
We have learned from the above studies that people with insomnia can successfully withdraw from hypnotics, and incremental sleep improvement occurs with supplemental psychological treatment. However, important questions remain. (1) Among the methodologically mature studies, only Morin et al. (2004) obtained polysomnography data to verify sleep status. (2) Only Morin et al. (2004) obtained drug screens to verify medication status. (3) Only Morin et al. (2004) focused on older adults. (4) A placebo-controlled trial has not been conducted.
The present clinical trial addressed these four concerns with hypnotic-dependent insomnia to more clearly understand the role of psychological treatment in the management of this disorder with primarily older adults. Volunteers who had current insomnia combined with hypnotic dependence were randomly assigned to three treatment conditions: multi-component cognitive behavior therapy (CBT) comprising relaxation, stimulus control, and sleep hygiene instructions plus scheduled medication withdrawal, placebo biofeedback (PL) plus scheduled withdrawal, or scheduled withdrawal (MED) only. The CBT and PL groups received eight, 45 minute weekly treatment sessions before commencing drug withdrawal. All three groups were given the same drug withdrawal protocol: slow tapering monitored with about six biweekly, 30 minute sessions, but the length of the drug withdrawal period varied greatly based mainly on the initial dosage level.
Method
Participants
We recruited volunteers from the community through media announcements. Participants were compensated with $300 distributed between posttreatment and 1-year follow-up. This study was approved by the University and Hospital IRBs.
A clinical interview and sleep diaries were used to determine sleep status. To satisfy the diagnosis of hypnotic-dependent insomnia, inclusion criteria were derived from a combination of the Diagnostic and Statistical Manual of Mental Disorders (4th ed.) (DSM-IV) criteria for insomnia (American Psychiatric Association, 1994) and the International Classification of Sleep Disorders criteria for hypnotic-dependent sleep disorder (American Sleep Disorders Association, 1997). The key criteria were: complaint of current difficulty initiating or maintaining sleep lasting at least 6 months, complaint of impaired daytime functioning, and use of prescription sleep medication at least 3 times/week for 6 months. We added the following empirically derived quantitative criteria (Lichstein, Durrence, Taylor, Bush, & Riedel, 2003): sleep onset or awake time during the night must exceed 30 minutes at least three times per week on the baseline sleep diaries.
Exclusion criteria were age less than 50 years old, history of seizures, consuming more than four alcoholic beverages per week or consuming alcohol at bedtime even once per week; cognitive impairment, a score below 26 on the Mini-Mental State Exam (Folstein, Folstein, & McHugh, 1975) or below 18 (Murden, McRae, Kaner, & Bucknam, 1991) if the participant had less than a 9th grade education; sleep intrusive, unstable medical /psychiatric disorders as per the Structured Clinical Interview for DSM-IV, Axis I (First, Spitzer, Gibbon, & Williams, 1997) and Axis II (First, Gibbon, Spitzer, Williams, & Benjamin, 1997), the Cornell Medical Index (Brodman, Erdmann, Wolff, & Miskovitz, 1986), and follow-up clinical interviews; consuming illicit drugs or sleep active medications, such as sedatives, stimulants, and steroids, besides the designated hypnotics; and presence of other sleep disorders, particularly periodic limb movements (arousal index > 15) or sleep apnea (apnea/hypopnea index > 15).
Research Setting and Apparatus
The study was conducted at two settings, the Psychological Services Center, The University of Memphis, and the Sleep Disorders Center (accredited by the American Academy of Sleep Medicine), Methodist Healthcare of Memphis.
A Nihon Koden #4312 polygraph was used for the all-night sleep studies (PSG). Monitoring consisted of two electroencephalography (EEG) measures, two electrooculography (EOG), and chin electromyography (EMG) according to standard placements (Rechtschaffen & Kales, 1968) to score sleep stages. Supplementary channels included oxygen saturation level, bilateral anterior tibialis EMG, heart rate (EKG), thoracic strain gauge, and a nasal/oral thermistor.
Drug Screens
A urine screen was done for each PSG night and a positive finding for a prohibited substance was a disqualifier. As a preventative, participants were repeatedly reminded of the drug screens.
Dependent Measures
Daytime functioning
We used a group of questionnaires to evaluate sleep, drug, and drug withdrawal effects. The Epworth Sleepiness Scale (ESS, Johns, 1991) measures trait daytime sleepiness. Respondents rate how likely they are to doze in eight commonly encountered restful situations. The Insomnia Impact Scale (IIS, Hoelscher, Ware, & Bond, 1993) questionnaire contains 40 negative statements about the daytime impact of sleep. These statements sample five areas of impairment: physical, cognitive, emotional, social, and occupational. The State-Trait Anxiety Inventory, Trait Form, (STAI) consists of 20 self-descriptive statements that are rated on a 4-point scale indicating how often the statement is true (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). The Geriatric Depression Scale (GDS, Yesavage et al., 1983) consists of 30 questions each asking if a symptom is present. The Fatigue Severity Scale (FSS, Krupp, LaRocca, Muir-Nash, & Steinberg, 1989) is composed of 9 items asserting the intrusion of fatigue in different aspects of living. Each item is rated from 1 = strongly disagree to 7 = strongly agree. The 20-item Benzodiazepine Withdrawal Symptom Questionnaire form 2 (BWSQ2, Tyrer, Murphy, & Riley, 1990) monitored sleep medication withdrawal symptoms, including experiences related to mood change, perceptual-motor disturbance, gastrointestinal distress, and neurological impairment.
Sleep measures
PSG records were manually scored in 30-second epochs by a registered PSG technologist uninformed as to treatment condition, according to the criteria of Rechtschaffen and Kales (1968). A second technologist randomly selected one-third of the records and scored them independently. Discrepancies were resolved by a third technician.
The records yielded sleep stage percent: awake, light sleep (stages 1 and 2), deep sleep (stages 3 and 4), and REM, and absolute values for sleep onset latency (SOL), wake time after sleep onset (WASO), total sleep time (TST), number awakenings during the night (NWAK), and sleep efficiency percent (SE, the ratio of TST to total time in bed × 100).
For self-report sleep data, a sleep diary (given in Lichstein, Durrence, Riedel, Taylor, & Bush, 2004) was used to collect the same sleep pattern information calculated from the PSG: SOL, WASO, TST, NWAK, and SE. The sleep questionnaire also contained a sleep quality rating (SQ, from 1 = very poor to 5 = excellent).
The sleep questionnaire described above also monitored hypnotic consumption. We used a method of quantifying hypnotic consumption we introduced in a prior study (Lichstein et al., 1999). Medication consumed was converted into the number of lowest recommended dosage (LRD) units, as defined by the Physician’s Desk Reference. This method assumes that a minimum dose (1.0 LRD) of different sleep medications delivers equivalent sleep-promoting strength. LRD counts permit between and within-group comparisons.
Therapists
Graduate students in clinical psychology served as therapists, were guided by treatment manuals, and were monitored by audiotape to assess accurate performance. Each treated a similar number of participants within each treatment.
Treatment Implementation Variables
Lichstein, Riedel, and Grieve (1994) proposed a treatment implementation model whereby steps must be taken to ensure the treatment is delivered as intended (delivery), is comprehended by the participant as intended (receipt), and is practiced out of session as intended (enactment). We used induction strategies (such as detailed treatment manuals and mock treatment sessions to induce delivery; tailoring treatments to maximize treatment acceptance by the participant to induce receipt; and reminder sheets to comply with home assignments to induce enactment) to heighten the likelihood that the treatment components were properly implemented. We conducted assessments (such as rating treatment session audiotapes to assess delivery; a medication withdrawal quiz, a stimulus control quiz and relaxation ratings to assess receipt; and compliance logs to assess enactment) to determine the degree of implementation for each treatment component.
Treatment Credibility
Treatment credibility (adapted from Borkovec & Nau, 1972) was evaluated by ratings on four 10-point scales (higher ratings reflected higher credibility) with statements tapping the following dimensions: (1) reasonableness of treatment, (2) opinion of therapist, (3) expectation for improvement, and (4) willingness to recommend treatment to a friend.
Procedures
Volunteers received a screening telephone interview and then were mailed daily sleep questionnaires to cover 14 nights, an IIS, an FSS, an ESS, and a Personality Questionnaire for the SCID. Participants brought this material to a follow-up screening interview at the Psychology Department’s Psychological Services Center, when additional screening questionnaires and informed consent were obtained. Baseline PSG and physical exam followed. Qualifying participants were randomly assigned to the three conditions. Treatment commenced within 2 weeks following baseline. Posttreatment and 1-year follow-up assessment repeated the baseline assessment.
Structure of Treatment Protocols
The CBT and PL conditions consisted of eight weekly individual sessions, each lasting about 45 minutes. Medication withdrawal commenced after the eight treatment sessions were completed. There were typically 4–8 bi-weekly medication withdrawal sessions lasting 15–30 minutes, but this number varied depending on beginning dosage levels and participant ability to tolerate withdrawal. The MED group went directly to the withdrawal protocol following baseline.
The treatment credibility questionnaire was administered at the end of the third treatment session in the two treated groups and at the end of the second medication withdrawal session in the withdrawal phase of all three groups. These points were selected because the participant had sufficient exposure to the procedure and exposure to the therapist to make an informed judgment, but insufficient exposure for treatment response to bias the questionnaire responses.
Treatments
CBT
This treatment combined relaxation training, stimulus control instructions, and sleep hygiene instructions. Disabusing participants of erroneous, exaggerated, and self-defeating cognitions was woven throughout this condition. We used a 10-minute hybrid relaxation procedure (given verbatim in Lichstein, 2000) consisting of promoting a relaxed attitude, slow deep breathing, passive body focusing, and autogenic phrases. Stimulus control consisted of six instructions (Bootzin & Epstein, 2000): (1) Go to bed only when sleepy. (2) Do not use your bed or bedroom for anything but sleep (or sex). (3) If you do not fall asleep within about 15–20 minutes at the beginning of the night or after an awakening, exit the bedroom. Return to bed only when you feel sleepy again. (4) If you do not fall asleep quickly upon returning to bed, repeat instruction 3. (5) Use your alarm to awaken at the same time every morning. (6) Do not nap. Sleep hygiene consisted of five instructions: avoid caffeine after noon and avoid exercise, nicotine, alcohol, and heavy meals within 2 hours of bedtime.
Participants were introduced to relaxation, stimulus control, and sleep hygiene during the first session. Remaining sessions were used to practice, refine, and tailor technique, and troubleshoot problems. Participants were strongly encouraged to practice relaxation at home twice a day: once at any time and once at bedtime.
PL
This procedure was modeled after the false biofeedback procedures of Nicassio, Boylan, and McCabe (1982), and was based on EEG theta wave biofeedback (Hauri, Percy, Hellekson, Hartmann, & Russ, 1982). The biofeedback training rationale emphasized the need to increase EEG theta wave activity in order to promote sleep. Treatment was comprised of the attachment of scalp electrodes and simulated auditory EEG biofeedback (BrainMaster, Biofeedback Instrument Corporation). The purported biofeedback tones were from eight recordings of a research team member; and during treatment, all PL participants heard the same set of eight recordings. To prevent active practice with self-relaxation techniques at bedtime, participants were told eight training sessions were optimal and additional home biofeedback practice would not be beneficial.
MED
We implemented gradual hypnotic-withdrawal under the supervision of our collaborating physician and with the approval of the prescribing physician. The MED program consisted of two steps: (1) gradually reducing an individual’s nightly sleep medication to an LRD of 1, and (2) gradually eliminating nightly doses. The first step began by estimating from baseline data the number of nights per week on which hypnotic consumption exceeded 1 LRD. These were designated high dose (HD) nights. In the first week of withdrawal, sleep medication was be cut by 1/4 of the dosage on half of the HD nights (approximately every other night), so long as the reduction did not drop below 1 LRD. This procedure continued until all participants were tapered to 1 LRD. Participants who had no HD nights began withdrawal at Step 2. In week 1 of Step 2, 0.5 LRD was eliminated on 2 nonconsecutive nights. By this pace, it took about 8 weeks to eliminate hypnotic consumption in participants who started at 1 LRD, 7 nights a week.
Participants were given a hand-out explaining the withdrawal program. The instructions included guidelines such as eliminate the most convenient nights first, and if you miss a week, don’t double your withdrawal pace the following week. The entire withdrawal schedule was planned with each participant at the beginning of the withdrawal period, and the participant was given a worksheet laying out the full schedule. This same withdrawal plan was applied to the CBT and PL groups following their treatment sessions.
Results
Participants
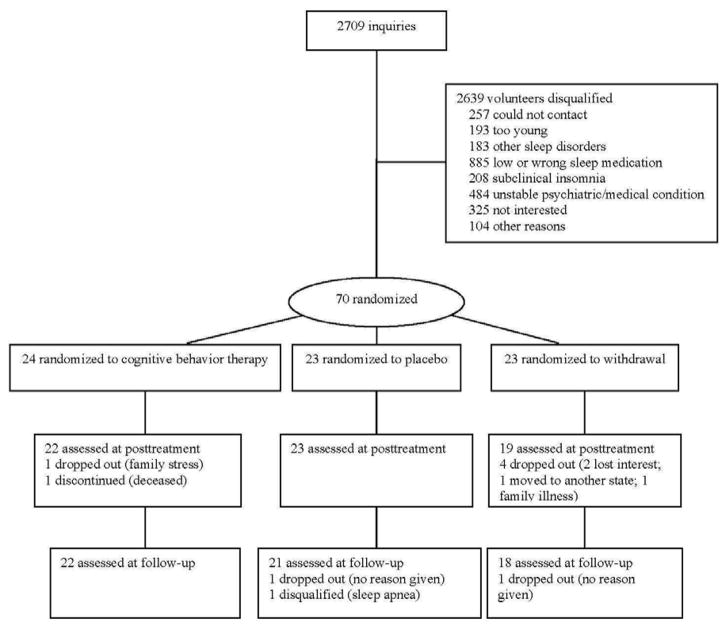
A total of 2709 people inquired about the study, but most did not qualify (see Figure 1). Some of the main reasons for exclusion were not taking sleep medication or taking it too infrequently (885 people), having an unstable, sleep intrusive psychiatric/medical condition (484 people), or having too mild insomnia (208 people).
Figure 1.
CONSORT participant flow diagram.
A total of 70 participants passed the baseline screening process and were randomly assigned to treatment. Subsequently, nine participants were either disqualified or dropped out of the study: two in CBT, two in PL, and five in MED. Table 1 presents subject characteristics by treatment group. Chi square tests comparing gender, χ2(2) = 4.24, race, χ2(2) = 1.18, and insomnia type, χ2(8) = 7.99, counts between groups were all nonsignificant. ANOVAs comparing age, F(2, 67) = 0.15, years of education, F(2, 64) = 2.09, years having insomnia, F(2, 63) = 0.26, and years taking sleep medication, F(2, 66) = 0.91, were all nonsignificant as well.
Table 1.
Subject Characteristics by Treatment Group: Counts and Means (SDs)
| CBT n = 24 | PL n = 23 | MED n = 23 | ||||
|---|---|---|---|---|---|---|
| Gender | ||||||
| Men | 8 | 9 | 3 | |||
| Women | 16 | 14 | 20 | |||
| Race | ||||||
| Black | 1 | 2 | 3 | |||
| White | 23 | 21 | 20 | |||
| Insomnia Typea,b | ||||||
| Onset | 11 | 8 | 5 | |||
| Maintenance | 5 | 7 | 5 | |||
| Terminal | 0 | 0 | 1 | |||
| Mixed | 3 | 3 | 3 | |||
| Combined | 3 | 5 | 9 | |||
|
| ||||||
| M | SD | M | SD | M | SD | |
|
| ||||||
| Age | 64.2 | 8.2 | 63.5 | 7.4 | 62.8 | 10.0 |
| Yrs Education | 14.7 | 2.5 | 15.0 | 2.1 | 13.6 | 2.6 |
| Yrs Insomnia Duration | 10.0 | 9.7 | 8.1 | 9.0 | 8.8 | 8.9 |
| Yrs Medication Duration | 5.3 | 5.0 | 3.9 | 4.7 | 3.6 | 4.1 |
Treatment Groups = CBT, cognitive-behavior therapy; PL, placebo; MED, medication withdrawal only.
Note. All comparisons between groups were ns.
The CBT count for insomnia type does not add to 24 due to missing data.
We defined insomnia types as follows: onset = only satisfied sleep onset criteria; maintenance = only satisfied sleep maintenance criteria; terminal = only satisfied terminal insomnia criteria; mixed = three bad nights a week were satisfied by adding bad onset and maintenance nights; combined = separately satisfied both onset and sleep maintenance criteria.
For all participants, the main medications used were benzodiazepines (45% of participants), nonbenzodiazepine receptor agonists (35%), and sedating antidepressants (16%). Most of our participants (76%) reported previous unsuccessful attempts at hypnotic discontinuation, and 27% of our participants were taking more than one hypnotic at baseline.
Sleep Diary
The following analytic conventions were applied to all sleep diary variables. Data were log-transformed to correct for extreme skewness and within subject phase means on the log-transformed scale were subsequently analyzed. All statistical analyses were carried out with the R system for statistical computing and graphics (R Development Core Team, 2008), version 2.6.2 (2008-02-08), which is available under the GNU General Public License Version 2. Within R, the following packages were used: nlme (Pinheiro & Bates, 2008) and lattice (Sarkar, 2008). The mixed effects model accomplished an intent-to-treat analysis by incorporating all data collected, including that of attriting participants.
For each outcome, we developed a mixed effects model for analysis of data conforming to one between-subjects (3 treatment groups) and one within-subjects (3 points in time) factors. Our model adopted a first order autoregressive covariance pattern with heterogeneous variance to model dependent measure mean and covariance patterns over time. Phase means (SDs) are given in Table 2. Marital status and age consistently correlated with self-reported sleep outcome, and these same analyses were repeated with these covariables. Results were not altered, and the covariance analyses will not be reported.
Table 2.
Sleep Diary Means (SDs) by Treatment Phase
| Baseline
|
Posttreatment
|
1-yr Follow-up
|
||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| SOL (min) | ||||||
| CBT | 50.1 | 36.7 | 23.7 | 21.8 | 24.1 | 16.7 |
| PL | 46.0 | 29.0 | 30.5 | 14.6 | 27.3 | 6.7 |
| MED | 53.0 | 23.2 | 48.0 | 24.4 | 36.9 | 18.0 |
| WASO (min) | ||||||
| CBT | 61.8 | 62.7 | 30.1 | 18.0 | 30.1 | 19.5 |
| PL | 47.9 | 28.5 | 42.0 | 31.5 | 32.7 | 12.8 |
| MED | 69.8 | 64.0 | 62.7 | 82.0 | 48.8 | 80.8 |
| TST (min) | ||||||
| CBT | 347.7 | 73.9 | 398.1 | 63.1 | 421.7 | 61.0 |
| PL | 365.3 | 64.9 | 407.3 | 63.9 | 400.0 | 35.5 |
| MED | 344.9 | 99.2 | 358.8 | 104.7 | 389.9 | 101.1 |
| SE (%) | ||||||
| CBT | 71.2 | 15.5 | 85.0 | 7.5 | 85.4 | 8.3 |
| PL | 74.1 | 8.5 | 80.8 | 12.0 | 81.1 | 5.5 |
| MED | 69.0 | 15.5 | 72.9 | 16.2 | 77.4 | 18.7 |
| SQ (rating) | ||||||
| CBT | 2.73 | 0.63 | 3.37 | 0.70 | 3.50 | 0.55 |
| PL | 2.89 | 0.69 | 3.33 | 0.68 | 3.36 | 0.51 |
| MED | 2.61 | 0.89 | 2.81 | 0.95 | 3.19 | 0.83 |
Treatment Groups = CBT, cognitive-behavior therapy; PL, placebo; MED, medication withdrawal only.
Variables = SOL, sleep onset latency; WASO, wake time after sleep onset; TST, total sleep time; SE, sleep efficiency percent; SQ, sleep quality rating; L-Sleep, light sleep; D-Sleep, deep sleep; REM, rapid eye movement sleep.
Follow-up testing focused on within group change over time which was of primary interest. This minimized the number of t-tests performed and limited the severity of the Bonferroni adjustment. Significant interactions were followed by correlated t-tests comparing pre-post and post follow-up points in three groups producing .05 ÷ 6 = Bonferroni adjusted α of .008.
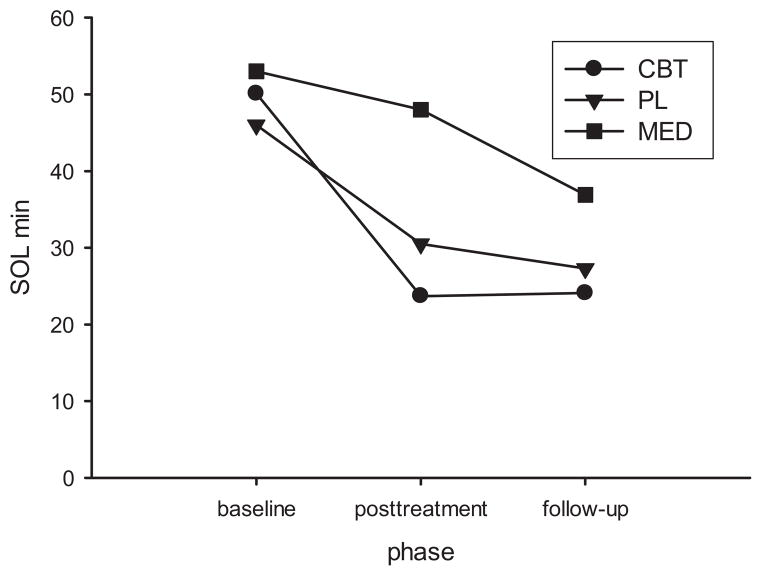
SOL
We obtained a significant treatment × time interaction, F = 2.73, p < .05. Baseline to posttreatment improvement in CBT was the only significant within group SOL change, see Figure 2.
Figure 2.
Sleep diary sleep onset latency changes by phase and treatment condition.
WASO, TST, SE, SQ
The results for these four variables were identical. There was a time main effect, but no group or group × time interaction. Collapsing across groups, there was significant improvement from baseline to posttreatment.
Summary Observation
It is apparent in Figure 2, that although only changes in the CBT group were significant, sleep trended toward improvement over time in all three groups. Indeed, this pattern was consistent without exception. Table 2 presents data on 15 variables- 5 sleep measures × three groups. In every case, average sleep was better at follow-up than baseline.
PSG
The same analytic conventions used with the sleep diary data were applied to the PSG variables with one exception. PSG variables exhibited much less skewness and did not require log transformation.
Table 3 presents PSG results clustered in sleep pattern variables, mirroring the sleep diary measures, and sleep architecture variables that are distinct to PSG. In the latter cluster, we report light sleep (L-Sleep joining stages 1 and 2), deep sleep (D-Sleep joining stages 3 and 4), and REM percent.
Table 3.
Polysomnography Means (SDs) by Treatment Phase
| Baseline
|
Posttreatment
|
1-yr Follow-up
|
||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Sleep Pattern Variables | ||||||
|
| ||||||
| SOL (min) | ||||||
| CBT | 18.6 | 12.3 | 12.0 | 6.7 | 18.1 | 15.0 |
| PL | 24.2 | 14.3 | 21.1 | 16.7 | 14.6 | 9.3 |
| MED | 16.1 | 9.3 | 25.2 | 22.1 | 12.7 | 9.9 |
| WASO (min) | ||||||
| CBT | 68.2 | 40.9 | 70.0 | 34.2 | 58.8 | 34.7 |
| PL | 60.1 | 36.2 | 67.4 | 31.1 | 68.0 | 51.3 |
| MED | 61.1 | 33.4 | 79.0 | 34.9 | 66.7 | 19.3 |
| TST (min) | ||||||
| CBT | 375.4 | 63.3 | 342.7 | 88.5 | 362.2 | 84.5 |
| PL | 399.1 | 64.3 | 391.7 | 48.8 | 379.5 | 67.4 |
| MED | 402.8 | 41.6 | 394.1 | 48.8 | 449.1 | 41.7 |
| SE (%) | ||||||
| CBT | 79.2 | 9.4 | 75.5 | 13.8 | 80.0 | 11.2 |
| PL | 80.5 | 11.0 | 77.4 | 9.3 | 80.1 | 12.2 |
| MED | 81.8 | 7.1 | 76.8 | 8.3 | 83.8 | 4.3 |
|
| ||||||
| Sleep Architecture Variables | ||||||
|
| ||||||
| L-Sleep (%) | ||||||
| CBT | 80.6 | 10.3 | 79.3 | 14.9 | 80.1 | 7.2 |
| PL | 79.9 | 7.5 | 78.0 | 7.8 | 78.4 | 10.5 |
| MED | 78.5 | 7.1 | 76.1 | 5.2 | 76.3 | 8.4 |
| D-Sleep (%) | ||||||
| CBT | 4.6 | 8.4 | 4.1 | 4.0 | 3.7 | 4.0 |
| PL | 2.6 | 3.4 | 4.5 | 5.1 | 5.6 | 3.9 |
| MED | 3.9 | 4.4 | 5.2 | 4.7 | 5.1 | 4.9 |
| REM (%) | ||||||
| CBT | 14.9 | 7.4 | 17.3 | 8.4 | 14.4 | 6.6 |
| PL | 19.8 | 6.3 | 18.4 | 6.9 | 15.3 | 8.3 |
| MED | 18.5 | 5.1 | 20.5 | 5.9 | 17.0 | 5.4 |
Treatment Groups = CBT, cognitive-behavior therapy; PL, placebo; MED, medication withdrawal only.
Variables = SOL, sleep onset latency; WASO, wake time after sleep onset; TST, total sleep time; SE, sleep efficiency percent; L-Sleep, light sleep; D-Sleep, deep sleep; REM, rapid eye movement sleep.
Two nights of PSG monitoring occurred at baseline, posttreatment, and follow-up. To control for first night effects (Agnew, Webb, & Williams, 1966), t-tests compared each of the two nights of data on the seven variables shown in Table 3 at baseline, posttreatment, and follow-up. Of these 21 tests, only three showed a significant difference between night 1 and night 2: REM at baseline and posttreatment, and stage 1 sleep at follow-up. For these three variables, only night 2 data were used in the following PSG analyses. For the remaining 18 variables, we averaged nights 1 and 2 to achieve more stable estimates.
Sleep pattern variables
SOL
We obtained a significant treatment × time interaction, F = 2.84, p < .05. After Bonferroni correction, none of the post hoc tests were significant. SOL increase from baseline to posttreatment and decrease from posttreatment to follow-up in MED were the only contrasts that satisfied the original .05 criterion. Both probabilities were about .03.
WASO
There were no significant main or interaction effects.
TST
There was a time main effect, F = 3.72, p < .05, but no group or group × time interaction. Collapsing across groups, TST decreased 17 minutes from baseline (M = 391.3, SD = 58.2) to posttreatment (M = 374.3, SD = 69.3). Although TST change from posttreatment to follow-up was not significant, average TST at follow-up (M = 390.0, SD = 77.5) had risen to baseline levels.
SE
There was a time main effect, F = 7.74, p < .01, but no group or group × time interaction. Collapsing across groups, SE significantly decreased 3.9% from baseline (M = 80.5, SD = 9.2) to posttreatment (M = 76.5, SD = 10.8). From posttreatment to follow-up (M = 81.0, SD = 10.1), SE significantly increased 4.5%.
Sleep Architecture Variables
L-Sleep, D-Sleep, REM
The results for these three variables were identical. There were no significant main or interaction effects.
Hypnotic Medication
The same analytic conventions used with PSG data were applied to hypnotic medication.
Treatment Phase Changes
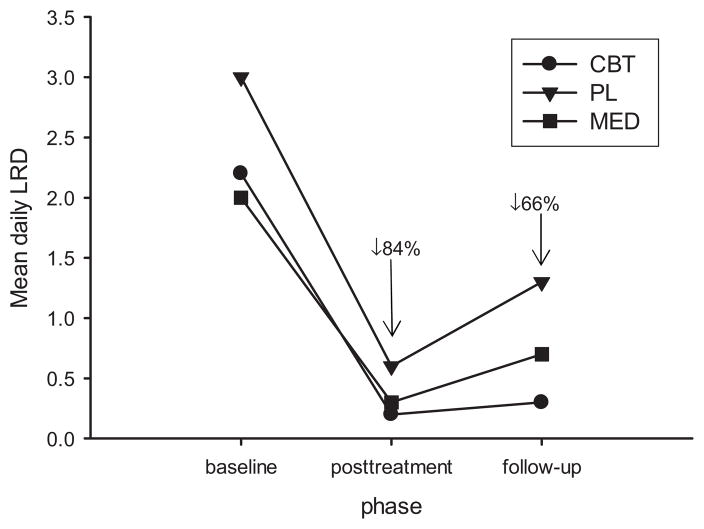
Table 4 presents hypnotic consumption results at baseline, posttreatment, and follow-up in LRD units. These same data are portrayed in Figure 3. There was a time main effect, F = 37.71, p < .01, but no group or group × time interaction. Collapsing across groups, medication significantly decreased from baseline (M = 2.4, SD = 2.4) to posttreatment (M = 0.4, SD = 0.9), an 84.4% decrease. There was also a significant increase from posttreatment to follow-up (M = 0.8, SD = 1.8), a 114.9% increase.
Table 4.
Medication Consumption Means (SDs) by Treatment Phase
| Baseline
|
Posttreatment
|
1-yr Follow-up
|
||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| CBT | 2.2 | 1.8 | 0.2 | 0.6 | 0.3 | 0.6 |
| PL | 3.0 | 3.4 | 0.6 | 1.2 | 1.3 | 2.6 |
| MED | 2.0 | 1.6 | 0.3 | 0.7 | 0.7 | 1.4 |
Treatment Groups = CBT, cognitive-behavior therapy; PL, placebo; MED, medication withdrawal only.
Medication use was measured in units of number of lowest recommended doses, LRDs.
Reported values are mean nightly LRD consumption.
Figure 3.
Self-reported medication consumption by phase and treatment condition.
Comparing baseline to follow-up, medication consumption decreased 66.5%. Considering just the CBT group who reported significant SOL improvement from baseline to posttreatment, these individuals decreased medication consumption 90.0% during this same period. Though nonsignificant due to high variability, Figure 3 shows that the CBT group sustained hypnotic use decrements at follow-up better than the other two groups.
At posttreatment, 16 participants were drug free in the CBT group, 14 in the PL group, and 12 in the MED group, χ2 (2) = 0.02, ns. Jointly, 65.6% of participants were drug free at posttreatment. At follow-up, 12 participants were drug free in the CBT group, 8 in the PL group, and 10 in the MED group, χ2(2) = 0.04, ns. Jointly, 48.4% of participants were drug free at follow-up.
Medication Consumption on PSG Nights
Table 5 presents hypnotic use in the sleep lab on the two PSG assessment nights at baseline, posttreatment, and follow-up in LRD units, and these data parallel those of home use. There was a time main effect, F = 51.88, p < .01, but no group or group × time interaction. Collapsing across groups, medication significantly decreased from baseline (M = 2.8, SD = 2.4) to posttreatment (M = 0.3, SD = 0.7), a 91.0% decrease. Change from posttreatment to follow-up (M = 0.4, SD = 1.5, a 73.1% increase) was nonsignificant. Comparing baseline to follow-up, medication consumption decreased 84.4%.
Table 5.
Medication Consumption Means (SDs) During PSG Nights
| Baseline
|
Posttreatment
|
1-yr Follow-up
|
||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| CBT | 2.6 | 2.0 | 0.04 | 0.2 | 0.2 | 0.5 |
| PL | 3.8 | 3.2 | 0.5 | 1.0 | 0.9 | 2.5 |
| MED | 2.0 | 1.4 | 0.2 | 0.6 | 0.2 | 0.5 |
Treatment Groups = CBT, cognitive-behavior therapy; PL, placebo; MED, medication withdrawal only.
Medication use was measured in units of number of lowest recommended doses, LRDs.
Reported values are mean nightly LRD consumption in the sleep lab.
Daytime Functioning
The same analytic conventions used with PSG data were applied to the daytime functioning variables.
Phase by group results for the six daytime functioning variables are presented in Table 6. There was a significant time main effect, but no group or group × time interaction for GDS (F = 3.33, p < .05), STAI (F = 5.77, p < .01), and BWSQ2 (F = 3.57, p < .05). Collapsing across groups, there was significant improvement from baseline to posttreatment, but no change from posttreatment to follow-up.
Table 6.
Daytime Functioning Means (SDs) by Treatment Phase
| Baseline
|
Posttreatment
|
1-yr Follow-up
|
||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| GDS | ||||||
| CBT | 6.8 | 4.0 | 6.0 | 5.0 | 4.6 | 3.2 |
| PL | 6.1 | 4.2 | 5.1 | 6.5 | 5.9 | 5.9 |
| MED | 8.0 | 6.4 | 5.2 | 4.9 | 6.4 | 4.5s |
| STAI | ||||||
| CBT | 37.4 | 6.3 | 34.9 | 9.4 | 31.9 | 7.8 |
| PL | 35.8 | 7.8 | 31.2 | 12.6 | 32.9 | 7.4 |
| MED | 37.6 | 11.9 | 34.9 | 12.4 | 34.7 | 7.7 |
| BWSQ2 | ||||||
| CBT | 4.0 | 3.8 | 2.9 | 2.5 | 2.2 | 2.5 |
| PL | 2.8 | 2.3 | 2.0 | 2.5 | 3.3 | 3.2 |
| MED | 5.2 | 4.3 | 3.4 | 5.0 | 3.1 | 3.4 |
| IIS | ||||||
| CBT | 120.9 | 13.8 | 102.9 | 25.0 | 101.0 | 18.8 |
| PL | 110.1 | 24.3 | 98.3 | 30.6 | 104.1 | 23.1 |
| MED | 129.9 | 24.8 | 113.5 | 27.7 | 112.7 | 40.0 |
| FSS | ||||||
| CBT | 4.3 | 1.2 | 3.8 | 1.1 | 3.7 | 1.6 |
| PL | 4.1 | 1.2 | 3.3 | 1.1 | 3.3 | 1.1 |
| MED | 5.1 | 1.4 | 4.3 | 1.7 | 4.2 | 1.6 |
| ESS | ||||||
| CBT | 7.5 | 5.2 | 6.7 | 5.3 | 5.7 | 3.3 |
| PL | 6.8 | 4.6 | 6.4 | 4.7 | 7.0 | 3.7 |
| MED | 5.4 | 5.0 | 4.2 | 3.1 | 4.5 | 4.2 |
Treatment Groups = CBT, cognitive-behavior therapy; PL, placebo; MED, medication withdrawal only.
Variables = GDS, Geriatric Depression Scale; STAI, State-Trait Anxiety Inventory, Trait-Form Y; BWSQ2, Benzodiazepine Withdrawal Symptom Questionnaire, form 2; IIS, Insomnia Impact Scale; FSS, Fatigue Severity Scale; ESS, Epworth Sleepiness Scale.
There were significant time and group main effects, but no group × time interaction for IIS (F = 12.83, p < .01 and F = 4.90, p < .01, respectively) and FSS (F = 10.33, p < .01 and F = 4.76, p < .01, respectively). In both cases, there was significant improvement from baseline to posttreatment, but no change from posttreatment to follow-up. Also, MED were more impaired than PL. ESS produced no significant results.
Nearly all means in Table 6 are nonpathological. Exceptions are baseline STAI in the CBT (37.4) and MED (37.6) groups and baseline IIS in the MED group (129.9). There are constrained limits to improvement within the subclinical range.
Treatment Implementation
Delivery
Therapy tapes revealed mean accuracy of 97.3% (SD = 2.6) in delivering CBT, 96.1% (SD = 4.3) in PL, and 95.8% (SD = 12.8) in MED. There was no significant difference between groups, F(2, 60) = 0.24.
Receipt
We evaluated two of three components within the CBT condition. Participants averaged 8.8 (SD = 1.2) responses correct on the 10 question stimulus control quiz, 88.0% correct. In the once weekly treatment session inductions, pre-post relaxation ratings significantly increased 2.5 (SD = 0.9) points on the 10 point scale, t(21) = 12.45, p < .01, and significant pre-post heart rate mean decrease was 3.9 (SD = 4.7) beats per minute, t(21) = 3.91, p < .01. Within the MED condition, participants averaged 4.7 (SD = 0.6) responses correct on the 5 question medication withdrawal quiz, 93.3% correct.
Enactment
We evaluated the three components within the CBT condition. Participants averaged 5.7 (SD = 0.3) correct responses out of the 6 point stimulus control home practice prescription, 95.6% correct home enactment. Participants averaged 11.4 relaxation practices a week of the 13 home practices prescribed, 87.6% correct home enactment. Other measures of home relaxation enactment were 119.8 minutes of relaxation practice a week (out of 130 prescribed), significant increase of 2.0 (SD = 0.9) relaxation rating points per practice on the 10 point scale, t(21) = 10.77, p < .01), and significant pre-post heart rate mean decrease of 3.1 (SD = 2.6) beats per minute, t(21) = 5.54, p < .01.
Treatment Credibility
We compared CBT and PL on the four, 10-point credibility questions of logical treatment (CBT M = 8.0, SD = 1.2; PL M = 7.7, SD = 2.0), therapist likability (CBT M = 9.4, SD = 0.9; PL M = 9.7, SD = 0.7), confidence in treatment (CBT M = 7.8, SD = 1.7; PL M = 7.6, SD = 1.8), and recommend to friend (CBT M = 8.4, SD = 1.3; PL M = 8.4, SD = 1.7). All t-tests were nonsignificant.
The same credibility questions were posed for the drug withdrawal part of all three treatments. All four ANOVAs were significant, and only significant post hoc comparisons will be reported. On logical treatment, MED (M = 7.3, SD = 1.9) was significantly lower than either CBT (M = 9.1, SD = 1.5) or PL (M = 9.3, SD = 1.1). On therapist likability, MED (M = 9.4, SD = 1.0) was significantly lower than PL (M = 9.9, SD = 0.3). On confidence in treatment, MED (M = 7.1, SD = 1.8) was significantly lower than either CBT (M = 8.4, SD = 1.5) or PL (M = 8.4, SD = 1.6). On recommend to friend, MED (M = 7.9, SD = 2.4) was significantly lower than either CBT (M = 9.2, SD = 1.3) or PL (M = 9.5, SD = 1.0).
Summary Observation
CBT and PL both earned high credibility evaluations and were not significantly different from each other. When drug withdrawal was presented as a unitary treatment, it had significantly less credibility than when it was joined with a psychological treatment.
Discussion
Scheduled gradual drug withdrawal secured substantial hypnotic reduction at posttreatment and follow-up with our primarily older adult sample. If tapering was supplemented with CBT, incremental SOL self-reported sleep benefits accrued at posttreatment and were maintained to 1-year follow-up. By follow-up, all groups converged for self-reported sleep gains. Only small changes in PSG sleep were observed. There was no evidence of daytime functioning withdrawal side-effects. To the contrary, besides sleep gains, improved daytime functioning was attained on a range of attributes. On a number of sleep and daytime functioning dependent variables, it is noteworthy that the performance of the MED arm approached that of the CBT group.
Methodological controls serve to clarify and strengthen conclusions. Tracking the path of the treatment (treatment implementation of the independent variable) from the integrity of its presentation (delivery), to its mastery by the participant (receipt), and to its home adherence (enactment) confirmed that the treatment we intended to test was tested. Satisfactory assessment of the three aspects of treatment implementation confers elevated confidence that outcome may be attributed to treatment (Lichstein et al., 1994). Similarly, there were no significant differences between the CBT and PL groups on the four credibility dimensions. This established the utility of the PL group in controlling for the influence of such potent factors as expectancy (Greenberg, Constantino, & Bruce, 2006) and therapeutic alliance (Horvath, Del Re, Fluckiger, & Symonds, 2011).
CBT promoted sleep gains but not statistically significant hypnotic withdrawal, except for trends at follow-up seen in Figure 3, and prior research has reported similar results (Belleville et al., 2007; Kirmil-Gray et al., 1985; Lichstein et al., 1999; Morin et al., 2004; Riedel et al., 1998). All conditions attained sizeable self-reported drug reduction by posttreatment that slipped somewhat by 1-year follow-up, the largest regression occurring in the PL and MED groups. Booster sessions placed during the follow-up period may have helped sustain hypnotic gains. Observed hypnotic consumption on PSG nights, verified by drug screens, was even greater than home-based logs. However, these results do not necessarily imply broad generalizability. The people who volunteered for this study were aware of its drug withdrawal aim and embraced this goal. There is a self-selection bias in any treatment study, and undoubtedly, the population of hypnotic-dependent people represents a range of receptivity to the current approach.
SOL only in the CBT group showed significant sleep diary improvement from baseline to posttreatment, but all groups on all measures trended to improvement at posttreatment and follow-up. The singular sleep improvement observed in CBT cannot be attributed to placebo, social influence, or time because these factors were controlled for in the two comparison groups.
But what are we to make of significant main effects for sleep diary WASO, TST, SE, and SQ at posttreatment and continuing to follow-up? The case of the MED condition is particularly provocative, keeping in mind that this group received drug tapering unaccompanied by either well established or placebo intervention, and improved subjective sleep was coincident with substantial hypnotic decrement. This outcome merits emphasis: removal of a therapeutic agent was accompanied by either no sleep change or improvement, a finding commonly reported in the hypnotic management literature (Belleville et al., 2007; Lichstein et al., 1999; Morin et al., 2004; Riedel et al., 1998). Further, the MED group had significantly lower rated credibility than drug weaning in the context of CBT or PL, predicting an attenuated MED treatment response. Two default explanations survive the methodological configuration of this study: chronic hypnotic use with concomitant resumptive insomnia is therapeutically inert, or even worse, under these conditions hypnotics are an impediment to good sleep.
CBT studies with or without hypnotic withdrawal often observe dampened PSG effects in the presence of more dynamic sleep diary change (Morin et al., 2004; Morin et al., 2009), as we found herein. There were no significant sleep stage effects, which again speaks to the absence of REM or deep sleep drug withdrawal rebound. Significant, but modest, decreases in SE and TST did occur at posttreatment, but these returned to baseline levels at follow-up. We should not be surprised that PSG and sleep diaries return different results: they are measuring different phenomena by different methods. Sleep diaries sample seven times the number of nights than PSG (in the present study), in the natural environment, relying on perception rather than physiology. Both tap important, but only partly overlapping dimensions of sleep experience.
Though treatment did not directly target daytime functioning, we did observe collateral improvement in this domain, but no negative effects that might have been associated with drug withdrawal. Collapsing across groups, depression (GDS), anxiety (STAI), withdrawal side-effects (BWSQ2), insomnia impact (IIS), and fatigue (FSS) all improved over time. Adopting the same reasoning applied to interpreting sleep effects in the MED condition, we cannot rule out improved daytime functioning derived from drug withdrawal.
Consistent with the current results, there is no evidence for an age effect in withdrawing hypnotics. Studies of mixed age samples (Belleville et al., 2007; Lichstein et al., 1999; Taylor et al., 2010) and older adults (Lichstein & Johnson, 1993; Morgan et al., 2003; Morin et al., 2004) have garnered positive outcomes with respect to sleep and withdrawal success.
This study was probably under powered. A randomized clinical trial with three arms would be enhanced by an N larger than 70. The outcome was populated by many nonsignificant trends favoring the CBT group. Future researchers in this area should heed this caution.
The present study complements a growing body of literature on psychological management of hypnotic-dependent insomnia. The following observations are well-documented based on the current and past studies: the slow pace of drug withdrawal is salient, solitary drug withdrawal is unlikely to have deleterious effects on sleep or daytime functioning and these domains may profit, adding CBT for insomnia to drug withdrawal summons subjective sleep improvement, and sleep gains and hypnotic reduction are well-maintained over time. Future research should consider the following. First, careful research on the sequence of CBT and drug withdrawal has not been conducted. It is not known if therapeutic potency varies when CBT precedes, co-occurs, or follows tapering. Second, many different tapering schedules have been used in our lab across studies and by others. Research has not determined the characteristics of the optimal drug withdrawal schedule. Third, there is little research on the relative difficulty of withdrawing different sedative/hypnotics and how these drugs interact with CBT for insomnia. It may be useful for health care providers to know which drugs are most resistant to weaning.
Hypnotic-dependent insomnia is a common, serious condition.
If the hypnotic is slowly tapered, no sleep change or improved sleep is likely.
Tapering supplemented with cognitive behavior therapy improves sleep.
Acknowledgments
This research was supported by National Institute on Aging grant AG14738.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnew HW, Jr, Webb WB, Williams RL. The first night effect: An EEG study of sleep. Psychophysiology. 1966;2:263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- American Sleep Disorders Association. International classification of sleep disorders: Diagnostic and coding manual, revised. Rochester, MN: Author; 1997. [Google Scholar]
- Ashton H. Guidelines for the rational use of benzodiazepines: When and what to use. Drugs. 1994;48:25–40. doi: 10.2165/00003495-199448010-00004. [DOI] [PubMed] [Google Scholar]
- Baillargeon L, Landreville P, Verreault R, Beauchemin JP, Grégoire JP, Morin CM. Discontinuation of benzodiazepines among older insomniac adults treated with cognitive-behavioural therapy combined with gradual tapering: a randomized trial. Canadian Medical Association Journal. 2003;169:1015–1020. [PMC free article] [PubMed] [Google Scholar]
- Balkrishnan R, Rasu RS, Rajagopalan R. Physician and patient determinants of pharmacologic treatment of sleep difficulties in outpatient settings in the United States. Sleep. 2005;28:715–719. doi: 10.1093/sleep/28.6.715. [DOI] [PubMed] [Google Scholar]
- Belleville G, Guay C, Guay B, Morin CM. Hypnotic taper with or without self-help treatment of insomnia: A randomized clinical trial. Journal of Consulting and Clinical Psychology. 2007;75:325–335. doi: 10.1037/0022-006X.75.2.325. [DOI] [PubMed] [Google Scholar]
- Bootzin RR, Epstein DR. Stimulus control. In: Lichstein KL, Morin CM, editors. Treatment of late-life insomnia. Thousand Oaks, CA: Sage; 2000. pp. 167–184. [Google Scholar]
- Borkovec TD, Nau SD. Credibility of analogue therapy rationales. Journal of Behavior Therapy and Experimental Psychiatry. 1972;3:257–260. [Google Scholar]
- Brodman K, Erdmann AJ, Jr, Wolff HG, Miskovitz PF. Manual: Cornell Medical Index. New York: Cornell University Medical College; 1986. [Google Scholar]
- Buscemi N, Vandermeer B, Friesen C, Bialy L, Tubman M, Ospina M, Klassen TP, Witmans M. The efficacy and safety of drug treatments for chronic insomnia in adults: A meta-analysis of RCTs. Journal of General Internal Medicine. 2007;22:1335–1350. doi: 10.1007/s11606-007-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie CA, Lindsay WR, Brooks DN. Substituting behavioural treatment for drugs in the treatment of insomnia: An exploratory study. Journal of Behavior Therapy and Experimental Psychiatry. 1988;19:51–56. doi: 10.1016/0005-7916(88)90010-9. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: An epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Greenberg RP, Constantino MJ, Bruce N. Are patient expectations still relevant for psychotherapy process and outcome? Clinical Psychology Review. 2006;26:657–678. doi: 10.1016/j.cpr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Greenblatt DJ, Harmatz JS, Zinny MA, Shader RI. Effect of gradual withdrawal on the rebound sleep disorder after discontinuation of triazolam. New England Journal of Medicine. 1987;317:722–728. doi: 10.1056/NEJM198709173171202. [DOI] [PubMed] [Google Scholar]
- Guilleminault C. Benzodiazepines, breathing, and sleep. American Journal of Medicine. 1990;88(Suppl 3A):25S–28S. doi: 10.1016/0002-9343(90)90282-i. [DOI] [PubMed] [Google Scholar]
- Hauri PJ, Percy L, Hellekson C, Hartmann E, Russ D. The treatment of psychophysiologic insomnia with biofeedback: A replication study. Biofeedback and Self-Regulation. 1982;7:223–235. doi: 10.1007/BF00998785. [DOI] [PubMed] [Google Scholar]
- Hoelscher TJ, Ware JC, Bond T. Initial validation of the insomnia impact scale. Sleep Research. 1993;22:149. [Google Scholar]
- Horvath AO, Del Re AC, Fluckiger C, Symonds D. Alliance in individual psychotherapy. Psychotherapy. 2011;48:9–16. doi: 10.1037/a0022186. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Sleeping pills, insomnia, and medical practice. Washington, DC: National Academy of Sciences; 1979. [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Kirmil-Gray K, Eagleston JR, Thoresen CE, Zarcone VP., Jr Brief consultation and stress management treatments for drug-dependent insomnia: Effects on sleep quality, self-efficacy, and daytime stress. Journal of Behavioral Medicine. 1985;8:79–99. doi: 10.1007/BF00845513. [DOI] [PubMed] [Google Scholar]
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacology, Biochemistry and Behavior. 2008;90:74–89. doi: 10.1016/j.pbb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichstein KL. Relaxation. In: Lichstein KL, Morin CM, editors. Treatment of late-life insomnia. Thousand Oaks, CA: Sage; 2000. pp. 185–206. [Google Scholar]
- Lichstein KL, Durrence HH, Riedel BW, Taylor DJ, Bush AJ. Epidemiology of sleep: Age, gender, and ethnicity. Mahwah, NJ: Erlbaum; 2004. [Google Scholar]
- Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behaviour Research and Therapy. 2003;41:427–445. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Johnson RS. Relaxation for insomnia and hypnotic medication use in older women. Psychology and Aging. 1993;8:103–111. doi: 10.1037//0882-7974.8.1.103. [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Peterson BA, Riedel BW, Means MK, Epperson MT, Aguillard RN. Relaxation to assist sleep medication withdrawal. Behavior Modification. 1999;23:379–402. doi: 10.1177/0145445599233003. [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Riedel BW, Grieve R. Fair tests of clinical trials: A treatment implementation model. Advances in Behaviour Research and Therapy. 1994;16:1–29. [Google Scholar]
- Moloney ME, Konrad TR, Zimmer CR. The medicalization of sleeplessness: A public health concern. American Journal of Public Health. 2011;101:1429–1433. doi: 10.2105/AJPH.2010.300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MG, Thompson TL, Nies AS. Sleep disorders in the elderly. American Journal of Psychiatry. 1988;145:1369–1378. doi: 10.1176/ajp.145.11.1369. [DOI] [PubMed] [Google Scholar]
- Morgan K, Clarke D. Longitudinal trends in late-life insomnia: Implications for prescribing. Age and Ageing. 1997;26:179–184. doi: 10.1093/ageing/26.3.179. [DOI] [PubMed] [Google Scholar]
- Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M. Psychological treatment for insomnia in the management of long-term hypnotic drug use: a pragmatic randomised controlled trial. British Journal of General Practice. 2003;53:923–928. [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Bastien C, Guay B, Radouco-Thomas M, Leblanc J, Vallieres A. Randomized clinical trial of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. American Journal of Psychiatry. 2004;161:332–342. doi: 10.1176/appi.ajp.161.2.332. [DOI] [PubMed] [Google Scholar]
- Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004) Sleep. 2006;29:1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- Morin CM, Colecchi CA, Ling WD, Sood RK. Cognitive behavior therapy to facilitate benzodiazepine discontinuation among hypnotic-dependent patients with insomnia. Behavior Therapy. 1995;26:733–745. [Google Scholar]
- Morin CM, Stone J, McDonald K, Jones S. Psychological management of insomnia: A clinical replication series with 100 patients. Behavior Therapy. 1994;25:291–309. [Google Scholar]
- Morin CM, Vallieres A, Guay B, Ivers H, Savard J, Merette C, Bastien C, Baillargeon L. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: A randomized controlled trial. Journal of the American Medical Association. 2009;301:2005–2015. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murden RA, McRae TD, Kaner S, Bucknam ME. Mini-Mental State exam scores vary with education in Blacks and Whites. Journal of the American Geriatrics Society. 1991;39:149–155. doi: 10.1111/j.1532-5415.1991.tb01617.x. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health, Consensus Development Conference. Drugs and insomnia: The use of medications to promote sleep. Journal of the American Medical Association. 1984;251:2410–2414. [Google Scholar]
- National Institutes of Health. The treatment of sleep disorders of older people, March 26–28, 1990. Sleep. 1991;14:169–177. doi: 10.1093/sleep/14.2.169. [DOI] [PubMed] [Google Scholar]
- Nicassio PM, Boylan MB, McCabe TG. Progressive relaxation, EMG biofeedback and biofeedback placebo in the treatment of sleep-onset insomnia. British Journal of Medical Psychology. 1982;55:159–166. doi: 10.1111/j.2044-8341.1982.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Medicine Reviews. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D. nlme: linear and nonlinear mixed-effects models (R package version 3.1–88) 2008. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. URL http://www.R-project.org. [Google Scholar]
- Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques and scoring system for sleep stages of human participants. (Public Health Service, NIH Publication No. 204) Washington, DC: U.S. Government Printing Office; 1968. [Google Scholar]
- Riedel BW, Lichstein KL, Peterson BA, Epperson MT, Means MK, Aguillard RN. A comparison of the efficacy of stimulus control for medicated and non-medicated insomniacs. Behavior Modification. 1998;22:3–28. doi: 10.1177/01454455980221001. [DOI] [PubMed] [Google Scholar]
- Sarkar D. Lattice: Lattice graphics. (R package version 0.17–6) 2008. [Google Scholar]
- Schneider-Helmert D. Why low-dose benzodiazepine-dependent insomniacs can’t escape their sleeping pills. Acta Psychiatrica Scandinavica. 1988;78:706–711. doi: 10.1111/j.1600-0447.1988.tb06408.x. [DOI] [PubMed] [Google Scholar]
- Shuto H, Imakyure O, Matsumoto J, Egawa T, Jiang Y, Hirakawa M, Kataoka Y, Yanagawa T. Medication use as a risk factor for inpatient falls in an acute care hospital: A case-crossover study. British Journal of Clinical Pharmacology. 2010;69:535–542. doi: 10.1111/j.1365-2125.2010.03613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stewart R, Besset A, Bebbington P, Brugha T, Lindesay J, Jenkins R, Singleton N, Meltzer H. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2006;29:1391–1397. doi: 10.1093/sleep/29.11.1391. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Schmidt-Nowara W, Jessop CA, Ahearn J. Sleep restriction therapy and hypnotic withdrawal versus sleep hygiene education in hypnotic using patients with insomnia. Journal of Clinical Sleep Medicine. 2010;6:169–175. [PMC free article] [PubMed] [Google Scholar]
- Tyrer P, Murphy S, Riley P. The benzodiazepine withdrawal symptom questionnaire. Journal of Affective Disorders. 1990;19:53–61. doi: 10.1016/0165-0327(90)90009-w. [DOI] [PubMed] [Google Scholar]
- Vermeeren A, Coenen AML. Effects of the use of hypnotics on cognition. Progress in Brain Research. 2011;190:89–103. doi: 10.1016/B978-0-444-53817-8.00005-0. [DOI] [PubMed] [Google Scholar]
- Verster JC, Veldhuijzen DS, Volkerts ER. Residual effects of sleep medication on driving ability. Sleep Medicine Reviews. 2004;8:309–325. doi: 10.1016/j.smrv.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Zolpidem use and hip fractures in older people. Journal of the American Geriatrics Society. 2001;49:1685–1690. doi: 10.1111/j.1532-5415.2001.49280.x. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zavesicka L, Brunovsky M, Matousek M, Sos P. Discontinuation of hypnotics during cognitive behavioural therapy for insomnia. BMC Psychiatry. 2008;8:80. doi: 10.1186/1471-244X-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]