Abstract
Purpose
This study was a 12-month prospective investigation of changes in the medial gastrocnemius (MG) muscle morphology in children aged 2–5 years with spastic cerebral palsy (CP) who had received no previous intramuscular injections of botulinum neurotoxin type-A (BoNT-A) and were randomised to receive either single or multiple (three) BoNT-A injections to the gastrocsoleus. MG morphological changes were compared to age-matched typically developing (TD) peers.
Methods
Thirteen children with spastic CP with a mean age of 45 (15) months and 18 TD children with a mean age of 48 (14) months participated in the study. The principal outcome measures were MG muscle volume, fascicle length, pennation angle and physiological cross-sectional area (PCSA), which were obtained using 2D and 3D ultrasound.
Results
The single and multiple injection frequency groups significantly increased MG muscle volume at 12 months relative to the baseline by 13 and 15 %, respectively. There were no significant differences in the MG muscle volume 28.5 (12.3) versus 30.3 (3.8) ml, fascicle length 48.0 (10.4) versus 44.8 (1.2) mm or PCSA 7.0 (1.2) versus 6.6 (1.7) cm2 between the single and multiple injection groups, respectively, at 12 months follow-up. The change in MG muscle volume in the single and multiple injection groups was significantly lower than the TD peers by 66 and 60 %, respectively.
Interpretation
In young children with spastic CP, naive to BoNT-A treatment, MG muscle growth over 12 months does not appear to be influenced by intramuscular BoNT-A injection frequency. However, MG muscle growth in the spastic CP groups was significantly lower than the age-matched TD peers. It is unclear whether this is an effect of intramuscular BoNT-A injections or reduced growth rates in children with spastic CP in general. Controlled investigations and longitudinal studies with multiple measurement time points are required in order to determine the influence of BoNT-A treatment on muscle physiological and mechanical growth factors in young children with spastic CP.
Keywords: Cerebral palsy, Botulinum neurotoxin type-A, Medial gastrocnemius, Muscle volume, Ultrasound, Physiological cross-sectional area
Introduction
Intramuscular botulinum neurotoxin type-A (BoNT-A) injections result in a partial chemo-denervation of skeletal muscle [1] and are widely used in the management of focal spasticity in children with spastic cerebral palsy (CP) [2, 3]. The use of BoNT-A in children with spastic type CP is not considered to be a stand-alone treatment [4], and when combined with physiotherapy and the use of orthoses, may have a small to moderate effect on improving gait outcomes [5, 6] and functional capacity [7, 8] during the pharmaceutically active period (~2–6 months). Clinically, repeated BoNT-A injections are believed to maintain the time that the BoNT-A is active within the target muscle and, therefore, delay muscle contracture more effectively, with the frequency of injection guided by clinical assessments.
Concerns have been expressed regarding the possible long-term effects of BoNT-A injections on muscle growth in children with CP because of the potential for denervation atrophy, weakness and impaired function [9–11]. Such concerns are, in part, based on animal studies that report reductions in the size and strength of the injected muscle [12–14], as well as adjacent muscles [15], following BoNT-A injection. For example, Fortuna et al. [13] reported a 95 % reduction in rabbit hind limb muscle strength and up to 80 % reductions in muscle contractile material at up to 6 months post-injection. In the only human studies that have assessed muscle growth, BoNT-A injections have been shown to induce significant atrophy in the muscles of two healthy volunteers [16], as well as one child with spastic CP [17]. Given that children with spastic CP already exhibit muscle weakness [18, 19], the short-term functional benefits of BoNT-A may be offset by accelerated long-term structural weakening of muscle.
A recent systematic review [20] concluded, on the basis of cross-sectional studies, that there was consistent evidence for reduced calf muscle size in the paretic limb in spastic CP compared to typically developing (TD) peers [21–25]. Barber et al. [26] subsequently showed that medial gastrocnemius (MG) muscle physiological cross-sectional area (PCSA), but not fascicle length, was reduced by 27 % in children aged 2–5 years with spastic CP who had not commenced BoNT-A treatment compared to TD peers. Such a reduction in PCSA is of functional significance because it results in a proportional decrease in the force-generating capacity of muscle [27] and may contribute to the clinically observed muscle weakness in individuals with CP. However, due to the absence of any prospective studies, it remains unknown to what extent ongoing muscle growth is altered in young children with spastic CP who commence BoNT-A injections, and how muscle growth may be affected by injection frequency.
The purpose of the present study was to prospectively investigate the influence of single versus multiple intramuscular BoNT-A injections on MG muscle growth over 12 months in young children with spastic CP naive to previous BoNT-A treatment and age-matched TD peers. Multiple BoNT-A injections occurred every 4 months to maintain the effects of the BoNT-A in the MG. We hypothesised that children with spastic CP receiving BoNT-A injections would have muscle atrophy and that the children receiving multiple BoNT-A injections would have more muscle atrophy than those receiving a single BoNT-A injection.
Methods
Participants
Twenty TD children (11 male, 9 female) and 15 children with spastic CP (11 male, 4 female, 5 hemiplegia, 10 diplegia) aged 2–5 years were assessed at baseline. All participants in the spastic CP group had clinical and video-based gait analysis evidence of either true equinus or jump knee gait. All were independent ambulators and were graded as level I (n = 10) or level II (n = 5) according to age-appropriate Gross Motor Function Classification System (GMFCS) descriptors [28]. Inclusion criteria also included dorsiflexion to neutral, with the knee extended, in order to exclude children with significant fixed contractures of the gastrocsoleus. Participants were excluded if they had previous BoNT-A treatment to the calf muscles, previous calf surgery or if there was a history of previous lower leg injury or other disorders affecting the lower limb. Ethics approval was obtained from the Institutional Human Research Ethics Committee (Royal Children’s Hospital, Melbourne HREC 27062; Southern Health, Victoria HREC 07083C; Griffith University, Queensland PES/29/07/HREC) and written informed consent was obtained from the parents or guardians of participants prior to participation in the study.
Experimental design and procedures
The study was a prospective trial in which participant demographic, anthropometric, ankle range of motion, MG muscle volume, fascicle length and fascicle angle were assessed in the spastic CP and TD groups at baseline and at 12 months follow-up.
The spastic CP group consisted of participants who were randomised to receive either a single intramuscular BoNT-A injection (SCP1) at baseline (total = 6 U/kg BoNT-A; five males, two females, two with hemiplegia, four with diplegia) or multiple intramuscular BoNT-A injections (SCP3) (6 U/kg at baseline and at 4 and 8 months, total = 18 U/kg BoNT-A; six males, two females, three with hemiplegia, four with diplegia). The source of the BoNT-A was Botox® (Allergan Pharmaceuticals). BoNT-A injections were administered to two sites in the MG and to one site in the lateral gastrocnemius and soleus muscle, guided by electrical stimulation. Participants with spastic diplegia had injections in the left and right gastrocnemius muscle and participants with hemiplegia had injections to the gastrocnemius and soleus on the affected side. Following BoNT-A treatment, participants in both spastic CP groups received orthotic and physiotherapy management. Individualised orthotic management [ankle–foot orthoses (AFO) and in-shoe orthoses] was prescribed by a multidisciplinary clinic of paediatric orthopaedic specialists, physiotherapists and orthotists in accordance with the impact of lower limb spasticity and/or contracture and following gait pattern review. Children in the CP groups prescribed an AFO were recommended to wear the AFO for 6–8 h per day. No night splints were worn and no casting was used. Individual children’s AFO prescriptions are outlined in Table 2. Participants in both spastic CP groups received an equivalent time of one-on-one physiotherapy provided by community-based physiotherapists. Physiotherapy treatment included isolated muscle strengthening as part of a functional muscle conditioning programme, muscle stretching, gait re-education, balance and stability training, and functional and play skills. There was no control over additional treatment or therapies pursued personally by the families and children in the study.
Table 2.
Individual participant data for the spastic CP single injection group (SCP1) and multiple injection group (SCP3)
| Sex | Class | GMFCS | AFO | Dose | Muscle volume (ml) | Fascicle length (mm) | PCSA (cm2) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | ||||||
| SCP1 | |||||||||||
| 1 | F | H (R) | I | NO | 96 | 34.1 | 40.4 | 54.0 | 54.0 | 6.5 | 7.4 |
| 2 | M | H (L) | I | SLD (L) | 83 | 16.5 | 18.2 | 22.3 | 29.0 | 7.4 | 7.5 |
| 3 | M | D | I | SLD (B) | 78 | 13.3 | 15.7 | 37.3 | 42.0 | 3.6 | 3.7 |
| 4 | F | D | II | SLD (B) | 79 | 9.0 | 12.9 | 33.0 | 37.3 | 2.7 | 3.4 |
| 5 | M | D | II | HNGD (B) | 96 | 47.6 | 53.3 | 58.6 | 59.3 | 8.1 | 8.8 |
| 6 | M | D | I | SLD (B) | 125 | 34.8 | 38.9 | 57.4 | 58.2 | 6.0 | 6.6 |
| Median | 89.4 | 25.3 | 28.5a | 45.7 | 48.0a | 6.2 | 7.0a | ||||
| MAD | 8.7 | 10.8 | 12.3 | 12.2 | 10.4 | 1.6 | 1.2 | ||||
| r | 0.61 | 0.58 | 0.61 | ||||||||
| SCP3 | |||||||||||
| 1 | M | D | I | HNGD (B) | 297 | 27.5 | 30.3 | 42.8 | 44.8 | 6.4 | 6.6 |
| 2 | M | H (L) | I | NO | 292 | 19.7 | 23.1 | 45.3 | 46.0 | 4.5 | 4.9 |
| 3 | M | D | I | SLD (B) | 346 | 26.6 | 29.9 | 57.0 | 58.5 | 4.7 | 5.0 |
| 4 | F | D | II | SLD | 205 | 11.1 | 15.1 | 51.2 | 52.4 | 2.1 | 2.8 |
| 5 | F | H (R) | I | NO | 320 | 27.4 | 33.1 | 43.1 | 44.8 | 6.4 | 7.2 |
| 6 | M | H (R) | I | NO | 331 | 38.2 | 44.9 | 42.7 | 44.3 | 9.0 | 9.8 |
| 7 | M | D | II | SLD (B) | 329 | 28.7 | 34.1 | 35.1 | 41.8 | 8.1 | 8.2 |
| Median | 320.4 | 27.4 | 30.3a | 43.1 | 44.8a | 6.4 | 6.6a | ||||
| MAD | 23.4 | 1.3 | 3.8 | 2.2 | 1.2 | 1.7 | 1.7 | ||||
| r | 0.61 | 0.63 | 0.61 | ||||||||
There were no significant differences in the baseline or follow-up measures for MG muscle volume, fascicle length or PCSA between the SCP1 and SCP3 groups
D diplegia, H hemiplegia, L left, R right, GMFCS Gross Motor Function Classification System, AFO ankle–foot orthoses, NO no orthosis, HNGD hinged orthosis, SLD solid orthosis, B bilateral, Dose total BoNT-A dose (U) to the involved leg (SCP1 = 1 × 6 U/kg, SCP3 = 3 × 6 U/kg), PCSA physiological cross-sectional area, MAD median absolute deviation, r effect size
aSignificant within-group difference from baseline to 12 months follow-up (p < 0.01)
Height was measured using a stadiometer and weight was measured using a calibrated digital scale. Leg length, fibula length and ankle range of motion were measured in the most involved lower limb in the spastic CP group and the right limb of the TD group. Leg length was the distance between the anterior superior iliac spine to the medial malleolus, and fibula length was the distance from the proximal aspect of the fibula head to the most distal aspect of the lateral malleolus. Ankle maximum dorsiflexion (MDF) and maximum plantarflexion (MPF) range of motion was measured in the sagittal plane using a goniometer.
Full details of the experimental procedures have been described elsewhere [26]. In brief, MG muscle volume (ml) was measured using a previously validated freehand three-dimensional ultrasound (3DUS) in vivo method [29]. Freehand 3DUS has also been validated in small cadaveric muscles [30] and small dog muscles [31]. The smallest detectible difference [32] for freehand 3DUS measurement of the MG muscle volume was calculated from raw MG muscle volumes (274 ± 75 ml) and small-volume water-filled condom phantom (26–102.5 ml) data from Barber et al. [29], and was found to be 2.34 ml. MG muscle fascicle length (mm) and pennation angle (°) were measured using B-mode ultrasound [33]. All measurements were made with the knee fully extended (0° of flexion), at a neutral ankle angle on the most impaired leg of the participants with spastic CP and the right leg of the TD participants. MG muscle PCSA (cm2), which reflects the force-generating capacity of skeletal muscle [34], was calculated as a function of the MG muscle volume, pennation angle and fascicle length using Eq. 1 [35]:
| 1 |
The average MG growth rate in the period prior to the study was calculated as the ratio of the MG muscle volume at baseline and age (months), and the average MG muscle growth rate during the study period (12 months) was calculated as the ratio of change in the volume to change in age (months). The percentage difference between the MG muscle volume at follow-up relative to that predicted by the average growth rate at baseline (growth rate prior to injection multiplied by age at follow-up) was also calculated.
Statistical analysis
Data were assessed for homogeneity of variance using the non-parametric Levene’s test [36]. A Wilcoxon signed-rank test was used to compare anthropometric, ankle angle, MG volume, fascicle length and PCSA recorded values or measurements at baseline and 12 months follow-up within groups (SPSS Statistics, version 20.0.0; SPSS Inc., Chicago, IL, USA). The Kruskal–Wallis test was used to compare anthropometric values and outcome measures between groups. Mann–Whitney U-tests were run post-hoc for pairwise comparisons as indicated. The selection of covariates for the dependent variables followed the procedure outlined by Mohagheghi et al. [37]. Pearson’s product-moment correlation coefficients were computed for MG fascicle length and leg length, and MG fascicle length and fibula length. If these correlations were significant and the slopes of the regression lines were not significantly different between groups, then the relevant anthropometric variable was used as a covariate in the subsequent group comparisons. For directional hypotheses, one-tailed significance was reported. All data are presented as the median (median absolute deviation, MAD) and, due to multiple statistical comparisons between baseline and 12 months follow-up, the alpha level for assessing statistical significance was set at 0.01 using the Bonferroni correction. The effect size was calculated using Eq. 2, the ratio of the Z-score and the square root of the total number of samples (N) [38]:
| 2 |
Results
Two participants from the TD group (one boy, one girl) and two from the spastic CP groups (both male, with diplegia, one in the single injection group, one in the multiple injection group) were unavailable at follow-up, which resulted in 18 participants in the TD group (10 males, 8 females) and 13 participants in the spastic CP group (9 males, 4 females) were re-assessed at 12 months. Baseline and 12 months follow-up data for the SCP1, SCP3 and TD groups are presented in Table 1. Anthropometric measures (mass, height, leg length, fibula length), MG muscle volume, fascicle length and PCSA were significantly greater at 12 months follow-up relative to the baseline in all three groups. Ankle MDF angle and MPF angle were not significantly different from baseline to 12 months follow-up within groups. MG fascicle length and leg length, and MG fascicle and fibula length were not significantly correlated and the slopes of the regression lines were significantly different between groups. Leg or fibula length were not used as covariates for group comparisons of fascicle length. There were no significant differences in the baseline or follow-up measures for demographic data, anthropometric data or ankle angle data between the SCP1 and SCP3 groups. At baseline, there were no significant differences in the MG muscle volume 25.3 (10.8) versus 27.4 (1.3) ml (Z = 0.00, p = 1.00, r = 0.00), fascicle length 45.7 (12.2) versus 43.1 (2.2) mm (Z = −0.72, p = 0.94, r = 0.20) or PCSA 6.2 (1.6) versus 6.4 (1.7) cm2 (Z = −0.72, p = 0.94, r = 0.20) between the SCP1 and SCP3 groups, respectively (Table 2). There were no significant differences in the MG muscle volume 28.5 (12.3) versus 30.3 (3.8) ml (Z = 0.00, p = 1.00, r = 0.00), fascicle length 48.0 (10.4) versus 44.8 (1.2) mm (Z = −0.29, p = 0.77, r = 0.008) or PCSA 7.0 (1.2) versus 6.6 (1.7) cm2 (Z = −0.30, p = 0.74, r = 0.09) between the SCP1 and SCP3 groups, respectively, at 12 months follow-up (Table 2).
Table 1.
Demographic, anthropometric and ankle angle data for the single injection CP group (SCP1, n = 6), the multiple injection CP group (SCP3, n = 7) and the typically developing (TD, n = 18) group at baseline and 12 months follow-up
| SCP1 | SCP3 | TD | ||||
|---|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | |
| Age (months) | 49.0 (10.0) | 62.0 (10.0)a | 48.0 (10.0) | 63.0 (10.0)a | 51.0 (8.0) | 63.0 (8.0)a |
| Mass (kg) | 14.9 (1.5) | 17.6 (9.0)a | 17.8 (1.3) | 19.4 (1.4)a | 17.4 (1.8) | 20.6 (3.2)a |
| Height (cm) | 102.6 (7.3) | 106.3 (6.1)a | 105.6 (5.4) | 108.3 (4.5)a | 100.5 (3.7) | 108.5 (5.3)a |
| Leg length (cm) | 43.8 (6.8) | 49.8 (7.1)a | 50.5 (3.5) | 54.0 (5.0)a | 46.5 (4.5) | 51.0 (3.8)a |
| Fibula length (cm) | 18.8 (2.3) | 20.2 (3.0)a | 19.2 (1.2) | 21.0 (1.5)a | 20.0 (1.5) | 22.8 (2.0)a |
| MDF angle (°) | 6.0 (4.0)b | 8.0 (2.5)c | 8.0 (4.0)b | 8.0 (2.0)c | 23.5 (1.5) | 25.5 (2.5) |
| MPF angle (°) | −51.0 (3.5)b | −54.0 (5.5)c | −50.0 (8.0)b | −54.0 (4.0)c | −60.0 (3.0) | −61.0 (1.0) |
Data are shown as median (median absolute deviation, MAD)
MDF maximum dorsiflexion (positive ankle joint angles indicate dorsiflexion), MPF maximum plantarflexion
aSignificant within-group difference from baseline to 12 months follow-up (p < 0.01)
bSignificant between-group difference at baseline compared to TD (p < 0.01)
cSignificant between-group difference at 12 months follow-up compared to TD (p < 0.01)
Ankle MDF angle and ankle MPF angle were significantly smaller in the SCP1 and SCP3 groups compared to the TD group at baseline and at 12 months follow-up (Fig. 1). The MG volume and PCSA were significantly smaller in the SCP1 and SCP3 groups compared to the TD group at 12 months follow-up (Fig. 1). The change in the MG muscle volume from baseline to 12 months follow-up was not significantly different between the SCP1 and SCP3 groups, 3.9 (1.6) and 4.0 (1.2) ml, respectively (Z = −0.36, p = 0.72, r = 0.10), but was significantly lower in the SCP1 (Z = −3.40, p < 0.01, r = 0.69) and SCP3 (Z = −3.40, p < 0.01, r = 0.73) groups compared to the TD peers, 10.9 (3.2) ml.
Fig. 1.
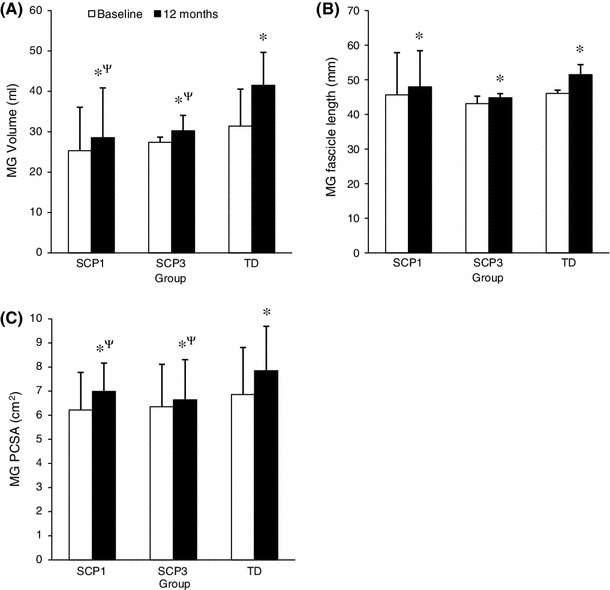
MG volume (a), fascicle length (b) and PCSA (c) at baseline and 12 months follow-up for the spastic CP single injection group (SCP1), the spastic CP multiple BoNT-A injection group (SCP3) and the typically developing group (TD). Data are median (median absolute deviation, MAD). * Indicates significant within-group difference from baseline to 12 months follow-up (p < 0.01). ψ Indicates significant between-group difference at 12 months follow-up compared to TD (p < 0.01)
The average growth rate in the period prior to the study was 0.55 (0.20) ml month−1 for the CP group and 0.68 (0.08) ml month−1 for the TD group. The growth rate during the 12-month study period was 0.36 (0.14), 0.31 (0.14) and 0.90 (0.42) ml month−1 for the SCP1, SCP3 and TD groups, respectively. This corresponds to a reduction in growth rate of 59–64 % after injection, while it increased by 33 % in the TD group over the same time period. The percentage difference between the MG muscle volume at follow-up relative to that predicted by the average growth rate at baseline was −13.9 (6.1), −9.3 (3.8) and 6.2 (3.7) % for the SCP1, SCP3 and TD groups, respectively (negative values represent less growth during the study period than prior to the study period).
Discussion
This 12-month prospective study was the first to investigate the influence of intramuscular BoNT-A injection frequency on MG muscle growth in young children with spastic CP naive to previous BoNT-A treatment of the calf muscles. Our hypotheses that children with spastic CP receiving BoNT-A injections would experience muscle atrophy, and to a greater extent with increased injection frequency, were not confirmed. Alternatively, we found a statistically significant longitudinal and cross-sectional muscle growth over 12 months, assessed in terms of MG muscle volume, fascicle length and PCSA, in young children with spastic CP. In addition, we found no significant differences in MG volume, fascicle length and PCSA between the single and multiple injection frequency groups. The study power may be considered a concern of our study; however, the small effect size of the change in muscle volume measured between the treatment groups (r = 0.10) suggests that more than 95 participants in both groups would need to be tested in order to achieve a power >0.5 (two-tailed, α = 0.05). Hence, the differences may not be clinically relevant, although greater numbers would have to be tested to confirm this finding. Our results provide some preliminary support for the view that repeated intramuscular injections may not have compounding effects on muscle growth in young children. These findings are in contrast to previous studies performed in animals reporting large reductions in muscle size and strength in response to BoNT-A injection [13]. The reasons for the disparity between our findings of muscle growth in young children and those of animal studies that indicate substantial muscle atrophy in response to lower relative BoNT-A dose are difficult to reconcile, but would be expected to be multi-factorial. Possible reasons may include altered muscle tissue quality in spastic CP muscle [39, 40], differences in the muscle group treated, follow-up time, exercise loading, extent of muscle denervation and varied species immune responses [41, 15].
This study also found that the increase in MG muscle volume in the spastic CP single and multiple injections groups over 12 months was 60 and 66 % lower than the TD group, respectively. There is evidence for a disparity in growth rates in children with CP compared to TD counterparts [20] but without a control group in this study, it was not possible to reconcile whether this slower growth rate is due to the BoNT-A injection or the underlying mechanisms that lead to reduced growth in CP in general. The mechanisms for the difference in growth rate between the spastic CP and TD groups may include neuronal, endocrinal, nutritional and mechanical growth factors in conjunction with treatments [42]. Genetic alterations have been identified in spastic CP muscle [43], which give rise to competing pathways for muscle hypertrophy and decrease in anabolic growth factors. A deficit in voluntary muscle activation in individuals with CP [44, 45] may limit the contractile stimulus to induce normal muscle growth, and may also reduce muscle growth factor release for protein synthesis [46]. Further, individuals with spastic CP may also have lower physical activity levels [47–49] and, therefore, may experience reduced stimulus for muscle growth than the TD group [50, 51]. Although there was significant growth of the MG muscle from baseline to 12 months follow-up in both CP groups, the proportion of contractile and non-contractile material which composes the muscle was not determined. The impact of growth and BoNT-A injections on muscle tissue quality remains unknown.
The spastic CP participants had not received pharmacological or surgical treatment prior to the study period; therefore, the influence of BoNT-A treatment on the reduced MG muscle growth rate cannot be discounted. In addition, there is some evidence that the growth rate of the muscle slowed after injection. An examination of the growth rate before and after injection (based on an assumption of linear growth) shows that the growth rate slowed to 59–64 % after injection, while it increased by 33 % in the TD group. We also cannot discount the possibility that BoNT-A injections cause muscle atrophy at some stage soon after the injection and that there was a sufficient recovery period to generate growth over the period tested. Further research incorporating a control group and examining the actual time course of muscle growth after BoNT-A injection is certainly required.
It is currently recommended that spasticity should be treated before children with spastic CP reach the age of 5 or 6 years, so that muscle spasticity and the progression of muscle contracture is reduced [52]. The standard of care for spasticity management involves a combination of modalities, including physiotherapy, orthotics, oral pharmacological agents and neuromuscular blocking agents such as BoNT-A [4, 52–54]. The problem of muscle weakness is increasingly being acknowledged as a primary source of functional impairment in spastic CP [55]. However, BoNT-A partial muscle chemo-denervation and the associated short-term muscle weakening of the target spastic muscle may be advantageous for antagonistic and synergistic muscle development in this population. It, therefore, follows that treatment interventions such as BoNT-A need to be considered in terms of their possible short- and long-term effects of muscle spasticity, joint-level stiffness, reduced range of motion and muscle size. Future controlled trials will be required in order to identify the specific effect of BoNT-A treatment, including dosage and frequency, on muscle growth in spastic CP.
Conclusions
This 12-month prospective study confirms that children aged 2–5 years with spastic cerebral palsy (CP) experienced longitudinal and cross-sectional medial gastrocnemius (MG) muscle growth following single and multiple lower limb intramuscular botulinum neurotoxin type-A (BoNT-A) injections, and that the observed growth was not strongly influenced by injection frequency (single versus multiple injections). However, MG growth in young children with spastic CP receiving BoNT-A injections remained 60–66 % lower than in age-matched typically developing (TD) peers. The reduced MG muscle growth rate in the spastic CP group would be expected to contribute to muscle weakness and indicates the need to carefully consider the balance between the short-term benefit of BoNT-A treatment and its possible long-term effects on muscle growth and motor function in spastic CP.
Acknowledgments
The authors thank the staff of the Hugh Williamson Gait Analysis Laboratory, The Royal Children’s Hospital, Melbourne, for their assistance with this study. This work was supported by funding from the National Health and Medical Research Council, Australia (Biomedical Postgraduate Scholarship Grant ID: 481953).
Conflict of interest
Professor H. Kerr Graham wishes to disclose a conflict of interest in respect of: (1) unrestricted research grants from Allergan, (2) consultant to Merz.
References
- 1.Antonucci F, Rossi C, Gianfranceschi L, Rossetto O, Caleo M. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28:3689–3696. doi: 10.1523/JNEUROSCI.0375-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brin MF. Botulinum toxin: chemistry, pharmacology, toxicity, and immunology. Muscle Nerve Suppl. 1997;6:S146–S168. doi: 10.1002/(SICI)1097-4598(1997)6+<146::AID-MUS10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Tedroff K, Granath F, Forssberg H, Haglund-Akerlind Y. Long-term effects of botulinum toxin A in children with cerebral palsy. Dev Med Child Neurol. 2009;51:120–127. doi: 10.1111/j.1469-8749.2008.03189.x. [DOI] [PubMed] [Google Scholar]
- 4.Love SC, Novak I, Kentish M, Desloovere K, Heinen F, Molenaers G, O’Flaherty S, Graham HK, Cerebral Palsy Institute Botulinum toxin assessment, intervention and after-care for lower limb spasticity in children with cerebral palsy: international consensus statement. Eur J Neurol. 2010;17:9–37. doi: 10.1111/j.1468-1331.2010.03126.x. [DOI] [PubMed] [Google Scholar]
- 5.Boyd RN, Hays RM. Current evidence for the use of botulinum toxin type A in the management of children with cerebral palsy: a systematic review. Eur J Neurol. 2001;8(Suppl 5):1–20. doi: 10.1046/j.1468-1331.2001.00034.x. [DOI] [PubMed] [Google Scholar]
- 6.Ryll U, Bastiaenen C, De Bie R, Staal B. Effects of leg muscle botulinum toxin A injections on walking in children with spasticity-related cerebral palsy: a systematic review. Dev Med Child Neurol. 2011;53:210–216. doi: 10.1111/j.1469-8749.2010.03890.x. [DOI] [PubMed] [Google Scholar]
- 7.Baker R, Jasinski M, Maciag-Tymecka I, Michalowska-Mrozek J, Bonikowski M, Carr L, MacLean J, Lin JP, Lynch B, Theologis T, Wendorff J, Eunson P, Cosgrove A. Botulinum toxin treatment of spasticity in diplegic cerebral palsy: a randomized, double-blind, placebo-controlled, dose-ranging study. Dev Med Child Neurol. 2002;44:666–675. doi: 10.1111/j.1469-8749.2002.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 8.Molenaers G, Van Campenhout A, Fagard K, De Cat J, Desloovere K. The use of botulinum toxin A in children with cerebral palsy, with a focus on the lower limb. J Child Orthop. 2010;4:183–195. doi: 10.1007/s11832-010-0246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albavera-Hernández C, Rodríguez JM, Idrovo AJ. Safety of botulinum toxin type A among children with spasticity secondary to cerebral palsy: a systematic review of randomized clinical trials. Clin Rehabil. 2009;23:394–407. doi: 10.1177/0269215508099860. [DOI] [PubMed] [Google Scholar]
- 10.Barrett RS. What are the long-term consequences of botulinum toxin injections in spastic cerebral palsy? Dev Med Child Neurol. 2011;53:485. doi: 10.1111/j.1469-8749.2011.03950.x. [DOI] [PubMed] [Google Scholar]
- 11.Gough M, Fairhurst C, Shortland AP. Botulinum toxin and cerebral palsy: time for reflection? Dev Med Child Neurol. 2005;47:709–712. doi: 10.1017/S0012162205001453. [DOI] [PubMed] [Google Scholar]
- 12.Chen CM, Stott NS, Smith HK. Effects of botulinum toxin A injection and exercise on the growth of juvenile rat gastrocnemius muscle. J Appl Physiol. 2002;93:1437–1447. doi: 10.1152/japplphysiol.00189.2002. [DOI] [PubMed] [Google Scholar]
- 13.Fortuna R, Vaz MA, Youssef AR, Longino D, Herzog W. Changes in contractile properties of muscles receiving repeat injections of botulinum toxin (Botox) J Biomech. 2011;44:39–44. doi: 10.1016/j.jbiomech.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Longino D, Frank C, Leonard TR, Vaz MA, Herzog W. Proposed model of botulinum toxin-induced muscle weakness in the rabbit. J Orthop Res. 2005;23:1411–1418. doi: 10.1016/j.orthres.2005.02.016.1100230625. [DOI] [PubMed] [Google Scholar]
- 15.Yaraskavitch M, Leonard T, Herzog W. Botox produces functional weakness in non-injected muscles adjacent to the target muscle. J Biomech. 2008;41:897–902. doi: 10.1016/j.jbiomech.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder AS, Ertl-Wagner B, Britsch S, Schröder JM, Nikolin S, Weis J, Müller-Felber W, Koerte I, Stehr M, Berweck S, Borggraefe I, Heinen F. Muscle biopsy substantiates long-term MRI alterations one year after a single dose of botulinum toxin injected into the lateral gastrocnemius muscle of healthy volunteers. Mov Disord. 2009;24:1494–1503. doi: 10.1002/mds.22661. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder AS, Koerte I, Berweck S, Ertl-Wagner B, Heinen F. How doctors think—and treat with botulinum toxin. Dev Med Child Neurol. 2010;52:875–876. doi: 10.1111/j.1469-8749.2010.03692.x. [DOI] [PubMed] [Google Scholar]
- 18.Damiano DL, Quinlivan J, Owen BF, Shaffrey M, Abel MF. Spasticity versus strength in cerebral palsy: relationships among involuntary resistance, voluntary torque, and motor function. Eur J Neurol. 2001;8:40–49. doi: 10.1046/j.1468-1331.2001.00037.x. [DOI] [PubMed] [Google Scholar]
- 19.Wiley ME, Damiano DL. Lower-extremity strength profiles in spastic cerebral palsy. Dev Med Child Neurol. 1998;40:100–107. doi: 10.1111/j.1469-8749.1998.tb15369.x. [DOI] [PubMed] [Google Scholar]
- 20.Barrett RS, Lichtwark GA. Gross muscle morphology and structure in spastic cerebral palsy: a systematic review. Dev Med Child Neurol. 2010;52:794–804. doi: 10.1111/j.1469-8749.2010.03686.x. [DOI] [PubMed] [Google Scholar]
- 21.Barber L, Barrett R, Lichtwark G. Passive muscle mechanical properties of the medial gastrocnemius in young adults with spastic cerebral palsy. J Biomech. 2011;44:2496–2500. doi: 10.1016/j.jbiomech.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Elder GC, Kirk J, Stewart G, Cook K, Weir D, Marshall A, Leahey L. Contributing factors to muscle weakness in children with cerebral palsy. Dev Med Child Neurol. 2003;45:542–550. doi: 10.1111/j.1469-8749.2003.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 23.Fry NR, Gough M, McNee AE, Shortland AP. Changes in the volume and length of the medial gastrocnemius after surgical recession in children with spastic diplegic cerebral palsy. J Pediatric Orthop. 2007;27:769–774. doi: 10.1097/BPO.0b013e3181558943. [DOI] [PubMed] [Google Scholar]
- 24.Malaiya R, McNee AE, Fry NR, Eve LC, Gough M, Shortland AP. The morphology of the medial gastrocnemius in typically developing children and children with spastic hemiplegic cerebral palsy. J Electromyogr Kinesiol. 2007;17:657–663. doi: 10.1016/j.jelekin.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Mohagheghi AA, Khan T, Meadows TH, Giannikas K, Baltzopoulos V, Maganaris CN. Differences in gastrocnemius muscle architecture between the paretic and non-paretic legs in children with hemiplegic cerebral palsy. Clin Biomech (Bristol, Avon) 2007;22:718–724. doi: 10.1016/j.clinbiomech.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Barber L, Hastings-Ison T, Baker R, Barrett R, Lichtwark G. Medial gastrocnemius muscle volume and fascicle length in children aged 2 to 5 years with cerebral palsy. Dev Med Child Neurol. 2011;53:543–548. doi: 10.1111/j.1469-8749.2011.03913.x. [DOI] [PubMed] [Google Scholar]
- 27.Barber L, Barrett R, Lichtwark G. Medial gastrocnemius muscle fascicle active torque-length and Achilles tendon properties in young adults with spastic cerebral palsy. J Biomech. 2012;45:2526–2530. doi: 10.1016/j.jbiomech.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Palisano RJ, Rosenbaum PL, Bartlett DJ, Livingstone M. Gross Motor Function Classification System: expanded and revised. Canada: CanChild Centre for Childhood Disability Research, McMaster University; 2007. [Google Scholar]
- 29.Barber L, Barrett R, Lichtwark G. Validation of a freehand 3D ultrasound system for morphological measures of the medial gastrocnemius muscle. J Biomech. 2009;42:1313–1319. doi: 10.1016/j.jbiomech.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Delcker A, Walker F, Caress J, Hunt C, Tegeler C. In vitro measurement of muscle volume with 3-dimensional ultrasound. Eur J Ultrasound. 1999;9:185–190. doi: 10.1016/S0929-8266(99)00023-3. [DOI] [PubMed] [Google Scholar]
- 31.Weller R, Pfau T, Ferrari M, Griffith R, Bradford T, Wilson A. The determination of muscle volume with a freehand 3D ultrasonography system. Ultrasound Med Biol. 2007;33:402–407. doi: 10.1016/j.ultrasmedbio.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Beckerman H, Roebroeck ME, Lankhorst GJ, Becher JG, Bezemer PD, Verbeek AL. Smallest real difference, a link between reproducibility and responsiveness. Qual Life Res. 2001;10:571–578. doi: 10.1023/A:1013138911638. [DOI] [PubMed] [Google Scholar]
- 33.Bénard MR, Becher JG, Harlaar J, Huijing PA, Jaspers RT. Anatomical information is needed in ultrasound imaging of muscle to avoid potentially substantial errors in measurement of muscle geometry. Muscle Nerve. 2009;39:652–665. doi: 10.1002/mus.21287. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien TD, Reeves ND, Baltzopoulos V, Jones DA, Maganaris CN. In vivo measurements of muscle specific tension in adults and children. Exp Physiol. 2010;95:202–210. doi: 10.1113/expphysiol.2009.048967. [DOI] [PubMed] [Google Scholar]
- 35.Lieber RL, Fridén J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–1666. doi: 10.1002/1097-4598(200011)23:11<1647::AID-MUS1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 36.Nordstokke DW, Zumbo BD. A new nonparametric Levene test for equal variances. Psicológica. 2010;31:401–430. [Google Scholar]
- 37.Mohagheghi AA, Khan T, Meadows TH, Giannikas K, Baltzopoulos V, Maganaris CN. In vivo gastrocnemius muscle fascicle length in children with and without diplegic cerebral palsy. Dev Med Child Neurol. 2008;50:44–50. doi: 10.1111/j.1469-8749.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- 38.Grissom RJ, Kim JJ. Effect sizes for research: univariate and multivariate applications. New York: Taylor & Francis; 2012. [Google Scholar]
- 39.Foran JR, Steinman S, Barash I, Chambers HG, Lieber RL. Structural and mechanical alterations in spastic skeletal muscle. Dev Med Child Neurol. 2005;47:713–717. doi: 10.1017/S0012162205001465. [DOI] [PubMed] [Google Scholar]
- 40.Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol. 2011;589:2625–2639. doi: 10.1113/jphysiol.2010.203364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eleopra R, Tugnoli V, Quatrale R, Rossetto O, Montecucco C. Different types of botulinum toxin in humans. Mov Disord. 2004;19:S53–S59. doi: 10.1002/mds.20010. [DOI] [PubMed] [Google Scholar]
- 42.Gough M, Shortland AP. Could muscle deformity in children with spastic cerebral palsy be related to an impairment of muscle growth and altered adaptation? Dev Med Child Neurol. 2012;54:495–499. doi: 10.1111/j.1469-8749.2012.04229.x. [DOI] [PubMed] [Google Scholar]
- 43.Smith LR, Pontén E, Hedström Y, Ward SR, Chambers HG, Subramaniam S, Lieber RL. Novel transcriptional profile in wrist muscles from cerebral palsy patients. BMC Med Genomics. 2009;2:44. doi: 10.1186/1755-8794-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rose J, McGill KC. Neuromuscular activation and motor-unit firing characteristics in cerebral palsy. Dev Med Child Neurol. 2005;47:329–336. doi: 10.1017/S0012162205000629. [DOI] [PubMed] [Google Scholar]
- 45.Stackhouse SK, Binder-Macleod SA, Lee SC. Voluntary muscle activation, contractile properties, and fatigability in children with and without cerebral palsy. Muscle Nerve. 2005;31:594–601. doi: 10.1002/mus.20302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 2008;23:160–170. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- 47.Bjornson K, Hays R, Graubert C, Price R, Won F, McLaughlin JF, Cohen M. Botulinum toxin for spasticity in children with cerebral palsy: a comprehensive evaluation. Pediatrics. 2007;120:49–58. doi: 10.1542/peds.2007-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bjornson KF, Belza B, Kartin D, Logsdon R, McLaughlin JF. Ambulatory physical activity performance in youth with cerebral palsy and youth who are developing typically. Phys Ther. 2007;87:248–257. doi: 10.2522/ptj.20060157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orlin MN, Palisano RJ, Chiarello LA, Kang LJ, Polansky M, Almasri N, Maggs J. Participation in home, extracurricular, and community activities among children and young people with cerebral palsy. Dev Med Child Neurol. 2010;52:160–166. doi: 10.1111/j.1469-8749.2009.03363.x. [DOI] [PubMed] [Google Scholar]
- 50.Eliakim A, Scheett T, Allmendinger N, Brasel JA, Cooper DM. Training, muscle volume, and energy expenditure in nonobese American girls. J Appl Physiol. 2001;90:35–44. doi: 10.1152/jappl.2001.90.1.35. [DOI] [PubMed] [Google Scholar]
- 51.McNee AE, Gough M, Morrissey MC, Shortland AP. Increases in muscle volume after plantarflexor strength training in children with spastic cerebral palsy. Dev Med Child Neurol. 2009;51:429–435. doi: 10.1111/j.1469-8749.2008.03230.x. [DOI] [PubMed] [Google Scholar]
- 52.Graham HK, Aoki KR, Autti-Rämö I, Boyd RN, Delgado MR, Gaebler-Spira DJ, Gormley ME, Guyer BM, Heinen F, Holton AF, Matthews D, Molenaers G, Motta F, García Ruiz PJ, Wissel J. Recommendations for the use of botulinum toxin type A in the management of cerebral palsy. Gait Posture. 2000;11:67–79. doi: 10.1016/S0966-6362(99)00054-5. [DOI] [PubMed] [Google Scholar]
- 53.Kerr Graham H, Selber P. Musculoskeletal aspects of cerebral palsy. J Bone Joint Surg Br. 2003;85:157–166. doi: 10.1302/0301-620X.85B2.14066. [DOI] [PubMed] [Google Scholar]
- 54.Koman LA, Smith BP, Shilt JS. Cerebral palsy. Lancet. 2004;363:1619–1631. doi: 10.1016/S0140-6736(04)16207-7. [DOI] [PubMed] [Google Scholar]
- 55.Shortland AP. Muscle volume and motor development in spastic cerebral palsy. Dev Med Child Neurol. 2011;53:486. doi: 10.1111/j.1469-8749.2011.03926.x. [DOI] [PubMed] [Google Scholar]


