Abstract
BACKGROUND AND OBJECTIVES:
There is growing evidence of pubertal maturation occurring at earlier ages, with many studies based on cross-sectional observations. This study examined age at onset of breast development (thelarche), and the impact of BMI and race/ethnicity, in the 3 puberty study sites of the Breast Cancer and the Environment Research Program, a prospective cohort of >1200 girls.
METHODS:
Girls, 6 to 8 years at enrollment, were followed longitudinally at regular intervals from 2004 to 2011 in 3 geographic areas: the San Francisco Bay Area, Greater Cincinnati, and New York City. Sexual maturity assessment using Tanner staging was conducted by using standardized observation and palpation methods by trained and certified staff. Kaplan-Meier analyses were used to describe age at onset of breast maturation by covariates.
RESULTS:
The age at onset of breast stage 2 varied by race/ethnicity, BMI at baseline, and site. Median age at onset of breast stage 2 was 8.8, 9.3, 9.7, and 9.7 years for African American, Hispanic, white non-Hispanic, and Asian participants, respectively. Girls with greater BMI reached breast stage 2 at younger ages. Age-specific and standardized prevalence of breast maturation was contrasted to observations in 2 large cross-sectional studies conducted 10 to 20 years earlier (Pediatric Research in Office Settings and National Health and Nutrition Examination Survey III) and found to have occurred earlier among white, non-Hispanic, but not African American girls.
CONCLUSIONS:
We observed the onset of thelarche at younger ages than previously documented, with important differences associated with race/ethnicity and BMI, confirming and extending patterns seen previously. These findings are consistent with temporal changes in BMI.
Keywords: puberty, girls, breast development, obesity
What’s Known on This Subject:
Several studies have documented earlier onset of pubertal maturation in girls, with several potential factors attributed to the earlier onset.
What This Study Adds:
This study demonstrates earlier maturation in white non-Hispanic girls, with greater BMI linked as a major factor. The entire distribution of pubertal timing has shifted to a younger age, suggesting redefinition of ages for both early and late maturation.
Over the past several years, multiple studies have reported an earlier age at onset of breast development. In 2007, a consensus panel reported there were sufficient data to suggest a trend toward earlier breast development in the United States over the second half of the 20th century.1 This trend has been noted internationally as well; for example, girls in the Copenhagen Puberty Study experienced breast development nearly a year earlier than those born 15 to 16 years previously.2 These findings have been linked temporally to the increase in BMI and prevalence of obesity. The relationship between higher BMI and earlier onset of puberty in girls has been noted previously; in 2 large cross-sectional studies, Pediatric Research in Office Settings (PROS) and the National Health and Nutrition Examination Survey (NHANES) III, earlier maturation occurred in those girls with greater BMI3 and in those with BMI ≥85th percentile.4 Here we report on timing of breast development from a longitudinal cohort of girls recruited at ages 6 to 8 years, to examine pubertal timing in association with higher BMI and by race/ethnicity.
Methods
This project was carried out as part of the National Institute of Environmental Health Sciences/National Cancer Institute Breast Cancer and the Environment Research Program (BCERP). The Puberty Study of the BCERP is investigating environmental exposures and onset of puberty in girls, and has been described in detail elsewhere.5 Girls were enrolled between 2004 and 2008 at 6 through 8 years of age. They were recruited through the 3 puberty study sites of the Breast Cancer and the Environment Research Program: the San Francisco Bay Area in California (through members of Kaiser-Permanente of Northern California), the greater Cincinnati metropolitan area in Ohio and Kentucky (through local schools, and Breast Cancer Registry of Greater Cincinnati), and east and central Harlem in New York City, New York (through community centers, clinics, and local schools).6 Recruitment was through a combined convenience sample at each site, with the sampling frame defined as those age-eligible girls in Kaiser Permanente membership at defined sites, selected schools in greater Cincinnati, or with a clinic appointment in Harlem. Each site had a time frame for recruitment, and used printed material for recruitment, describing the study as “a project of girls growing up today.” The current report includes longitudinal data in scheduled semiannual (Cincinnati) or annual visits (Mount Sinai and Kaiser Permanente of Northern California) through March 2012, with mean follow-up of 4.3 years. The local institutional review board approved the study at each center; consent was obtained from the parent/legal guardian, and assent was obtained from participants once they reached 10 years of age.
Sexual maturity was established through a standardized method based on Tanner staging.7 Breast development was assessed through both observation and palpation.5 Professional staff were trained and certified, and periodic cross-site validation was performed by a master trainer visiting all 3 sites. Onset of breast development was defined as attaining breast stage 2 or greater. We had noted previously that examiners had 87% agreement (ie, same breast stage) with a master trainer in blinded field assessments; the remaining assessments were within 1 stage.5
During the examination visits, trained and certified staff members obtained standardized anthropometric measurements, including height and weight, and made 2 measurements of each parameter. If the difference exceeded a preset amount, or the amount was outside the 5th to 95th percentile values, a third measurement was taken and an average of the values was used for analysis. BMI was calculated from the mean values of height and weight measurements, as weight divided by the square of height. BMI percentile and z score were determined by using the 2000 growth charts from the Centers for Disease Control and Prevention (www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm).
We tested for the difference in median values for BMI percentile across sites by using the 2-sample median test, and for the difference proportions in race/ethnicity and BMI percentile groups across sites using the χ2 and Fisher’s exact tests. Survival analyses were performed by using SAS PROC LIFEREG (SAS Institute, Inc, Cary, NC) first to estimate age of breast development and then SAS PROC LIFETEST to determine the effect of race, education of head of household (as a proxy for socioeconomic status), site, and BMI on age of breast development.
The first step in these analyses was to determine an estimated date of breast development (thelarche). For these analyses, a “breast development interval” was defined for each participant. For girls who reached Tanner breast stage 2 or greater during follow-up (interval censored), the interval was defined as the period from the last research study visit at breast stage 1 to the first visit where the girls was noted to be at breast stage 2 or greater. For some girls, progression through breast stages was not consistent, such as apparent regression of breast stage 2 to breast stage 1 at a subsequent visit. For these girls, the breast development interval encompassed the period of inconsistency, until a consistent B2 was observed. For girls who were left-censored (breast stage 2 or greater at the first study visit), the beginning of the interval was defined as the race-specific 1.0 percentile value of age from the PROS study data (data supplied by M. Herman-Giddens, PhD (personal communication, 2012), and the end of the interval was the date of the initial research study visit when breast stage 2 was observed. For both interval-censored and left-censored girls, an estimated date of breast development was calculated within the interval using age at the beginning and end of the interval as covariates. The estimated date of thelarche was calculated as the date associated with the median probability over the breast development interval. These probabilities were calculated for each girl by using an SAS macro developed by us, which incorporated σ (σ, the scale parameter of the Weibull distribution from LIFEREG)8 and the x’B matrix for the girl, and the dates at the beginning and end of her thelarche interval.
Kaplan-Meier analyses were used to determine mean and median age at breast development, incorporating interval censored data, with the SAS application LIFETEST. The Wilcoxon test was used to test trend across strata. For hazard function estimations, the validity of the proportionality assumption was assessed by plotting the log-log of the estimated survival function against survival time; these plots were parallel, so the proportionality assumption was valid. Time ratios and hazard ratios, with 95% confidence intervals, were calculated from the accelerated failure time model using a Weibull distribution derived by Carroll.8 This model accounts for varying amounts of time and varying ages of the girls while under observation. For these analyses, girls who were right-censored (never at breast stage 2 or greater while under observation) contributed to observational time, but no breast development event, to the analyses. We used PROC GLM to determine the proportion of the variance attributable to each of the covariates.
We compared results of age at breast development with cross-sectional data sets from PROS published by Kaplowitz et al3 and by Herman-Giddens et al.9 Differences between our data and the mean age of attaining breast stage 2 in the PROS data set were tested with a 2-sample t test, assuming unequal variance. Differences in age-specific prevalence of breast stage 2 or greater were examined by using χ2 and Fisher’s exact test.
Results
The baseline cohort included 1239 girls (Table 1). The percentage of BCERP girls with BMI ≥85th percentile included 39% of black, 44% of Hispanic, 26% of white non-Hispanic, and 12% of Asian girls. Differences in the distribution of BMI percentile groups by race and site were examined; the only significant difference noted was that at the end of follow-up, the distribution of BMI percentile groups for blacks differed significantly between Greater Cincinnati and the San Francisco Bay Area (P = .028), but not New York City and the San Francisco Bay Area (P = .174) or New York City and Greater Cincinnati (P = .150). The proportion of girls who had attained breast stage 2 during the study varied by age, race/ethnicity, BMI percentile, and site (Table 1 and 2). Mean and median ages of breast stage 2 varied by race/ethnicity, and were 8.8 and 8.8 years for black, 9.2 and 9.3 for Hispanic, 9.6 and 9.7 for non-Hispanic white, and 9.9 and 9.7 for Asian participants (Table 2). Hispanic BCERP participants had maturation at significantly older ages than black participants, and younger than non-Hispanic white BCERP participants.
TABLE 1.
BCERP Puberty Study Population Characteristics
| Characteristic | New York City | Cincinnati | San Francisco Bay Area | Total Study Population | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Total no. of participants | 416 | 34 | 379 | 31 | 444 | 36 | 1239 | 100 |
| Age group at enrollment | ||||||||
| 6.0–6.9 y | 154 | 37 | 154 | 41 | 88 | 20 | 396 | 32 |
| 7.0–7.9 y | 136 | 33 | 187 | 49 | 334 | 75 | 657 | 53 |
| ≥8.0 y | 126 | 30 | 38 | 10 | 22 | 5 | 186 | 15 |
| Mean age at enrollment, (y) | 7.4 (0.9) | 7.1 (0.6) | 7.4 (0.4) | 7.3 (0.7) | ||||
| Mean age at end of follow-up, (y) | 11.2 (2.2) | 11.5 (1.9) | 12.0 (1.4) | 11.6 (1.9) | ||||
| Mean length of follow-up, (y) | 3.8 (2.1) | 4.4 (1.8) | 4.6 (1.3) | 4.3 (1.8) | ||||
| Race/Ethnicitya | ||||||||
| Black | 168 | 40 | 127 | 34 | 96 | 22 | 391 | 32 |
| Asian | 0 | 0 | 5 | 1 | 52 | 12 | 57 | 5 |
| Hispanic | 248 | 60 | 15 | 4 | 108 | 24 | 371 | 30 |
| White | 0 | 0 | 232 | 61 | 188 | 42 | 420 | 34 |
| Education of family provider | ||||||||
| Grade School (1-8), Some High School (9-11) or High School Diploma/GED | 232 | 58 | 35 | 10 | 83 | 20 | 350 | 31 |
| Some College/technical/trade/vocational school or associate’s degree | 114 | 29 | 119 | 35 | 136 | 34 | 369 | 32 |
| Bachelor’s degree | 39 | 10 | 106 | 31 | 128 | 32 | 273 | 24 |
| Master’s degree or greater | 13 | 3 | 84 | 24 | 55 | 14 | 152 | 13 |
| BMI% group at enrollmentb | ||||||||
| >95th | 97 | 23 | 52 | 14 | 67 | 15 | 216 | 17 |
| 85th–94.9th | 66 | 16 | 62 | 16 | 65 | 15 | 193 | 16 |
| 50th–84.9th | 135 | 32 | 122 | 32 | 163 | 37 | 420 | 34 |
| <50th | 116 | 28 | 143 | 38 | 149 | 34 | 408 | 33 |
| No BMI data | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Median BMI percentile at enrollmentc | 74.4 | 64.0 | 69.1 | 70.1 | ||||
| BMI% group during follow-upd,e | ||||||||
| >95th | 102 | 25 | 58 | 15 | 67 | 15 | 227 | 18.3 |
| 85th–94.9th | 64 | 15 | 69 | 18 | 72 | 16 | 205 | 16.5 |
| 50th–84.9th | 128 | 31 | 112 | 30 | 154 | 35 | 394 | 31.8 |
| <50th | 120 | 29 | 140 | 37 | 151 | 34 | 411 | 33.2 |
| No BMI data | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0.2 |
| Median BMI% during follow-upd | ||||||||
| All girlsf | 76.3 | 65.8 | 66.4 | 70.9 | ||||
| Girls at/above the 85th percentile onlyg | 96.5 | 94.2 | 94.8 | 95.3 | ||||
| Girls below the 85th percentile onlyh | 52.7 | 44.9 | 50.1 | 48.7 | ||||
| Thelarche | ||||||||
| Breast stage 2+ at enrollment | 90 | 22 | 55 | 15 | 33 | 7% | 178 | 14 |
| Breast stage 2+ observed during study | 244 | 59 | 278 | 73 | 359 | 81% | 881 | 71 |
| Not attained breast stage 2+ by end of follow-up | 82 | 20 | 46 | 12 | 52 | 12% | 180 | 15 |
Racial distributions were significantly different across sites (P < .0001).
BMI percentile groups were significantly different across sites (P = .0021); Greater Cincinnati (P = .0013) and the San Francisco Bay Area (P = .0107) were significantly different from New York City, but not each other (P = .4125).
Median BMI at enrollment in Cincinnati was significantly lower than New York City (P = .0095), but not the San Francisco Bay Area (P = .3095); New York City and the San Francisco Bay Area were not significantly different (P = .1013).
For girls who were breast stage 2+ at enrollment, BMI% during follow-up is BMI% at enrollment. For girls in whom breast stage 2+ was first observed during the study, BMI% during follow-up is the BMI% at the last exam with data before thelarche. For girls who had not attained breast stage 2+ by the end of follow-up, BMI% during follow-up is the BMI% at their last exam.
Distribution of BMI percentile groups for all girls at end of follow-up were significantly different across sites (P = .0021); Greater Cincinnati (P = .0039) and the San Francisco Bay Area (P = .0055) were significantly different from New York City, but not each other (P = .4502).
Median BMI percentile for all girls at end of follow-up in New York City was significantly higher than Cincinnati (P = .0062), and the San Francisco Bay Area (P = .0041); Cincinnati and the San Francisco Bay Area were not significantly different (P = .9699).
Median BMI percentile for girls at/above the 85th percentile at end of follow-up in New York City was significantly higher than Cincinnati (P = .0018), but not the San Francisco Bay Area (P = .0576); Cincinnati and the San Francisco Bay Area were not significantly different (P = .7132).
Median BMI percentile for girls below the 85th percentile at end of follow-up were not significantly different across sites (.0669 ≤ P ≤ .5817).
TABLE 2.
Age at Thelarche and Hazard Ratios by BMI Percentile During Follow-Up, Race/Ethnicity and Site
| Strata | Unadjusted Age (in Years) at Thelarche From Kaplan-Meier Survival Analysisa | Adjusted Likelihood of Thelarche By End of Follow-up From AFT Modelb | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | Hazard Ratio | 95% CI | |||||||||||
| n | Observed | Censored | Median | Mean | SE | Time Ratio | Lower | Upper | Lower | Upper | P value | ||
| All girls | 1239 | 1039 | 200 | 9.40 | 9.26 | 0.04 | — | — | — | — | — | — | — |
| BMI percentile group during follow-upc | |||||||||||||
| >95th | 227 | 195 | 32 | 8.50 | 8.44 | 0.10 | 0.86 | 0.84 | 0.88 | 3.20 | 2.67 | 3.84 | <.0001 |
| 85th–94.9th | 205 | 181 | 24 | 8.70 | 8.69 | 0.11 | 0.89 | 0.87 | 0.91 | 2.60 | 2.18 | 3.09 | <.0001 |
| 50th–84.9th | 394 | 330 | 64 | 9.20 | 9.23 | 0.07 | 0.93 | 0.91 | 0.95 | 1.82 | 1.59 | 2.07 | <.0001 |
| <50th | 411 | 333 | 78 | 10.10 | 9.99 | 0.07 | 1.00 | 1.00 | |||||
| Race/Ethnicity | |||||||||||||
| Non-Hispanic white | 420 | 355 | 65 | 9.70 | 9.62 | 0.06 | 1.06 | 1.04 | 1.08 | 0.62 | 0.54 | 0.72 | <.0001 |
| Asian | 57 | 50 | 7 | 9.70 | 9.92 | 0.15 | 1.04 | 1.00 | 1.08 | 0.72 | 0.56 | 0.94 | .0428 |
| Hispanic | 371 | 310 | 61 | 9.30 | 9.23 | 0.08 | 1.05 | 1.03 | 1.07 | 0.69 | 0.60 | 0.81 | <.0001 |
| Black | 391 | 324 | 67 | 8.80 | 8.76 | 0.08 | 1.00 | 1.00 | |||||
| Site | |||||||||||||
| New York City | 416 | 330 | 86 | 9.10 | 9.03 | 0.08 | 1.00 | 1.00 | |||||
| Cincinnati | 379 | 321 | 58 | 8.90 | 8.88 | 0.08 | 0.94 | 0.92 | 0.97 | 1.58 | 1.37 | 1.81 | <.0001 |
| San Francisco Bay Area | 444 | 388 | 56 | 9.80 | 9.76 | 0.06 | 1.03 | 1.01 | 1.05 | 0.77 | 0.66 | 0.90 | .0028 |
Girls who did not reach thelarche before end of follow-up are censored.
Hazard ratios and 95% confidence intervals calculated as described by Carroll.8
For girls who were breast stage 2+ at enrollment, BMI% during follow-up is BMI% at enrollment. For girls in whom breast stage 2+ was first observed during the study, BMI% during follow-up is the BMI% at the last examination with data before thelarche. For girls who had not attained breast stage 2+ by the end of follow-up, BMI% during follow-up is the BMI% at their last examination.
Girls in all BMI categories >50th percentile were progressively more likely to have reached breast stage 2 than those <50th percentile (P value for trend = .001), adjusting for race/ethnicity and site (Table 2); differences by race/ethnicity remained significant in adjusted models.
We examined the cumulative prevalence of breast stage 2 or greater by age, race/ethnicity, and BMI status (<85th percentile vs ≥85th percentile). As noted in Figs 1 and 2, regardless of race (as well as ethnicity, not shown), participants with BMI ≥85th percentile matured earlier than those <85th percentile.
FIGURE 1.
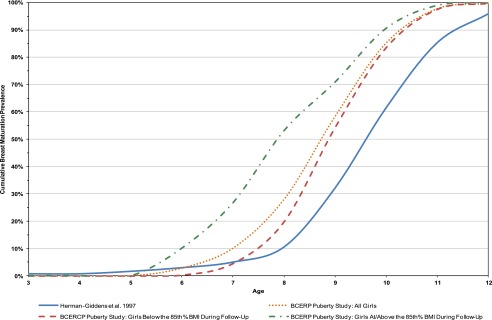
Comparing the cumulative prevalence of Breast Stage 2+ for non-Hispanic white participants between the BCERP Puberty Study and PROS.9
FIGURE 2.
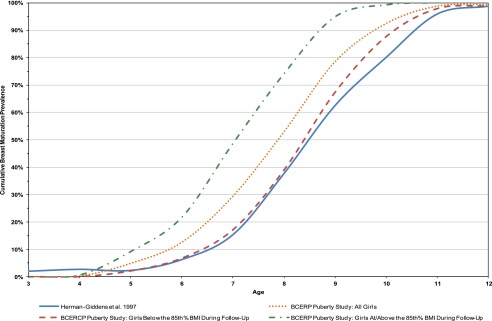
Comparing cumulative prevalence of Breast Stage 2+ for non-Hispanic black participants between the BCERP Puberty Study and PROS.9
Non-Hispanic white BCERP participants matured earlier than white participants in the PROS study (mean age 9.62 vs 9.96 years, P = .0005) (Fig 1). However, non-Hispanic white participants with BMI <85th percentile did not mature earlier than PROS white participants (mean age 9.84 vs 9.96, P = .34). Black BCERP participants matured at similar ages in both studies (P = .36), although those of higher BMI were younger (Fig 2).
Non-Hispanic white BCERP participants matured earlier than white PROS participants at every age between 7 and 12 years (Table 3). Of note, BMI accounted for the greatest amount of variance (14.2%) of all covariates included in the model, contrasted to race, which accounted for 4.4% of the variance. The proxy for socioeconomic status, education level of head of household, did not remain in the model.
TABLE 3.
Comparing the Proportion with Breast Stage 2+ by Age Between BCERP Puberty Study Cross-Sectional Equivalent Data and PROS9
| Age Group | Black | White | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PROS | BCERP Puberty Studya | PROSc | BCERP Puberty Studya | |||||||||
| Total No. of Girls | Percent at Breast Stage 2+ | Total No. of Girls | Percent at Breast Stage 2+ | χ Squareb | P Valueb | Total No. of Girls | Percent at Breast Stage 2+ | Total No. of Girls | Percent at Breast Stage 2+ | χ Squareb | P Valueb | |
| 6.0–6.9 | 126 | 6 | 100 | 18 | 7.43 | .006 | 1506 | 3 | 77 | 4 | 0.24 | .496 |
| 7.0–7.9 | 136 | 15 | 257 | 22 | 2.28 | .131 | 1128 | 5 | 320 | 10 | 11.07 | .001 |
| 8.0–8.9 | 143 | 38 | 294 | 38 | 0.02 | .892 | 1334 | 11 | 363 | 21 | 29.39 | <.0001 |
| 9.0–9.9 | 115 | 63 | 282 | 62 | 0.00 | .971 | 1067 | 32 | 379 | 42 | 12.50 | .000 |
| 10.0–10.9 | 112 | 80 | 269 | 87 | 2.73 | .098 | 1153 | 62 | 340 | 71 | 10.01 | .002 |
| 11.0–11.9 | 126 | 96 | 190 | 96 | 0.02 | .999 | 1104 | 85 | 244 | 92 | 8.15 | .004 |
| 12.0–12.9 | 91 | 99 | 133 | 99 | 0.07 | .999 | 923 | 96 | 156 | 98 | 1.63 | .256 |
Every B visit in the BCERP Puberty Study was treated as if it were a separate girl; missing visits or visits with refused Tanner staging not included.
Fisher’s Exact Test used where cell frequency is ≤5.
PROS did not separate Hispanic and Non-Hispanic white subjects; the BCERP Puberty Study did.
Discussion
This study examined timing of onset of breast development in an ongoing longitudinal study of girls. We observed the onset of breast development in white girls at younger ages than reported in previous publications, suggesting a continued trend to earlier ages of breast development; black girls continue to experience breast development earlier than white girls.
Higher BMI was the strongest predictor of earlier age at breast stage 2 in our study. Similar findings have been reported that noted the association between BMI and body fat with earlier timing of puberty in girls,2–4,10–13 although these data support, but do not establish, causality.
Longitudinal studies have demonstrated this relationship, where BMI, BMI z scores, and adiposity as early as 3 years of age were related to pubertal outcomes, including onset of breast development, onset of the pubertal growth spurt, and age of menarche.2,14–19 The obesity epidemic appears to be a prime driver in the decrease in age at onset of breast development in contemporary girls. In our study, white non-Hispanic BCERP participants with BMI <85th percentile had similar age at breast development as white girls in PROS (9.96 years).3,9 The percentage of girls 6 to 11 years of age who were obese in NHANES III (1988–1994), approximately the same time as PROS, was 9.8% for non-Hispanic white and 17.0% for non-Hispanic black girls. By the time of NHANES 2009–2010 (encompassing the age of girls in our study) it was 14.4% for non-Hispanic white and 24.0% for non-Hispanic black girls.20
Several studies have suggested that earlier onset of breast development may occur independent of activation of the hypothalamic-pituitary-ovarian axis,2 perhaps through endocrine-disrupting chemicals,1,21,22 which we will examine in future manuscripts. In a previous report, Parent et al23 questioned whether the earliest onset had shifted downward, or if the entire distribution shifted downward. Our data suggest that white, non-Hispanic girls throughout the entire distribution of relative timing of puberty (early, on-time, and late-maturing girls) are maturing at younger ages than previously reported.
The impact of earlier maturation in girls has important clinical implications. Clinicians may need to examine additional contemporary studies to decide whether to lower the age for late maturation in girls, and possibly age of precocious puberty. Both the PROS study and our study excluded girls with pathologic conditions known to modify age of pubertal maturation. However, both studies likely included girls with undiagnosed conditions, such as ovarian cysts, which may explain some of the very early ages at breast development and some of the apparent breast development regression that we observed. Previous authors have commented on the impact of timing of pubertal maturation, with several psychosocial and biologic outcomes, perhaps due to a discrepancy between biological and psychological transitions.24 Girls with earlier maturation are at risk for lower self-esteem25 and higher rates of depression.26 They are more likely to be influenced by older peers and more deviant peers,27 and initiate intercourse, substance use, and other norm-breaking behaviors at younger ages.28–30 Although the greatest impact on these psychosocial outcomes appears during the adolescent years, the impact on adult women who matured early includes greater rates of depression,26,29 lower levels of academic achievement,30 and greater number of sexual partners.26 The longer-term impact of early maturation on psychosocial functioning may represent a complex interaction between factors associated with earlier maturation, such as family stress, with developmental outcomes during adolescence, such as depression. Several authors have described that girls maturing earlier are perceived to be aging more rapidly or have an accelerated life course, described as “weathering.”31,32 It is unclear if these adverse psychosocial outcomes associated with early maturation will be sustained when many girls mature at a younger age. The biologic impact of earlier maturation includes greater risk of several cancers, including breast,33 ovarian,34 and endometrial cancer,35 as well as obesity, hyperinsulinemia, and hypertension.36 The increased risk of cancer with earlier maturation might be mediated by the increased risk of obesity (reviewed by Renehan et al),37 or through the association of early maturation with greater peak height velocity,38,39 or longer exposure to endogenous estrogen production.33,40,41
The study does have several potential limitations. The participants in this study are not nationally representative, so this may limit generalizability, but they do include broad racial/ethnic as well as socioeconomic diversity. Additionally, the BMI distribution of our cohort is similar to those published recently regarding the NHANES data.42 There is a potential confounding of early maturation and greater BMI, as several girls were noted to have breast development at time of intake and we did not observe exact age of transition; however, girls in this study with later breast development have lower BMI values, consistent with the impact of BMI on timing of puberty. Additionally, the determination of age of breast development used a somewhat novel application of a well-established approach, an accelerated failure time model (specifically the Weibull model), which both allows determination of event rates as well as extension in survival time.8 The direct comparison of our participants, from a longitudinal study, with participants from 2 cross-sectional studies that used different BMI standards from this study, might appear to be of limited relevance; when we excluded our participants with elevated BMI, the BMI distribution is similar to the results reported by Kaplowitz et al3 and by Rosenfield et al,4 and the remaining participants had similar age of breast maturation. Unlike the NHANES III and most (61%) of PROS participants, we used breast palpation for all of our participants, but this should have increased the age of breast development, rather than decreased it. Although BCERP participants appear to have a younger median age at breast stage 2 than NHANES III participants, because of the differences in study design methods we were unable to conduct statistical analyses to determine the difference in age between the 2 groups. Multiple studies have cautioned against overclassification of breast tissue in girls with greater BMI, particularly after onset of the obesity epidemic. Our study procedure used palpation as well as inspection, which limits misclassification of fat tissue deposited in the chest area. Additionally, as previously reported, we validated our maturation assessment procedures with dual breast-development assessment, which demonstrated substantial agreement between examiners.5 Study site has a significant effect on the age of breast development, with effects for the Cincinnati (hazard ratio 1.58) and the San Francisco Bay Area (hazard ratio 0.77) sites being statistically different than the New York City site, which enrolled only black and Hispanic girls. The earlier age at maturation in Cincinnati may be attributed, in part, to performing maturation assessments every 6 months rather than once each year, which could account for an earlier age by 3 to 4 months (data not shown). The later age at maturation in the San Francisco Bay Area could represent other factors not included, such as country of origin differences not captured in our categorization of race and ethnicity, exposures, or lifestyle factors. In addition, our models may not have adequately accounted for differences in race/ethnicity and BMI at the 3 sites. Last, younger age of breast development may not lead to earlier age of menarche; this relationship will be examined when the sample achieves later pubertal milestones.
Conclusions
We noted earlier onset of breast stage 2 in non-Hispanic white girls, contrasted with 2 previous studies (PROS and NHANES III); this is likely due to greater obesity in the white non-Hispanic girls in our study compared with earlier reports. Girls with BMI >85th percentile matured earlier than those with lower BMI, and BMI explained much of the difference between studies. Black girls experienced breast development at a similar age to blacks from the 2 previous studies, and continued to mature at ages younger than whites.
Acknowledgments
On behalf of the Puberty Studies of the BCERP, we also gratefully acknowledge the study investigators and staff within the Breast Cancer and the Environment Research Centers: Cincinnati Children’s Hospital Medical Center/University of Cincinnati, Fox Chase Cancer Center, Michigan State University, and the University of California San Francisco Comprehensive Cancer Center. We also thank our community collaborators for their insight and support of our research efforts, Marcia Herman-Giddens for providing us with selected maturation data, and the clerical assistance of Lynn Hanrahan.
Glossary
- BCERP
Breast Cancer and the Environment Research Program
- NHANES
National Health and Nutrition Examination Survey
- PROS
Pediatric Research in Office Settings
Footnotes
Drs Biro, Greenspan, Galvez, Pinney, Teitelbaum, Windham, Kushi, and Wolff participated in conception of the project, conception of the manuscript, and manuscript preparation; Drs Deardorff and Hiatt participated in conception of the project and manuscript preparation; and Mr Herrick and Dr Succop performed statistical analyses and participated in manuscript preparation.
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Environmental Health Sciences, National Cancer Institute, National Center for Research Resources, National Institutes of Health, Environmental Protection Agency, Agency for Toxic Substances and Disease Registry, or the California Department of Public Health.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Breast Cancer and the Environment Research Program award numbers U01ES012770, U01ES012771, U01ES012800, U01ES012801, U01ES019435, U01ES019453, U01ES019454, and U01ES019457 from the National Institute of Environmental Health Sciences; The National Cancer Institute; P01ES009584 and P30ES006096 from the National Institute of Environmental Health Sciences; UL1RR024131, CSTA-UL1RR029887, and CSTA-UL1RR026314 from the National Center for Research Resources; the Molecular Epidemiology in Children’s Environmental Health training grant (T32-ES10957); and the Avon Foundation. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found on page 1125, and online at www.pediatrics.org/cgi/doi/10.1542/peds.2013-3058.
References
- 1.Euling SY, Herman-Giddens ME, Lee PA, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121(suppl 3):S172–S191 [DOI] [PubMed] [Google Scholar]
- 2.Aksglaede L, Sørensen K, Petersen JH, Skakkebaek NE, Juul A. Recent decline in age at breast development: the Copenhagen Puberty Study. Pediatrics. 2009;123(5). Available at: www.pediatrics.org/cgi/content/full/123/5/e932 [DOI] [PubMed] [Google Scholar]
- 3.Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics. 2001;108(2):347–353 [DOI] [PubMed] [Google Scholar]
- 4.Rosenfield RL, Lipton RB, Drum ML. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics. 2009;123(1):84–88 [DOI] [PubMed] [Google Scholar]
- 5.Biro FM, Galvez MP, Greenspan LC, et al. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics. 2010;126(3). Available at: www.pediatrics.org/cgi/content/full/126/3/e583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiatt RA, Haslam SZ, Osuch J, Breast Cancer and the Environment Research Centers . The breast cancer and the environment research centers: transdisciplinary research on the role of the environment in breast cancer etiology. Environ Health Perspect. 2009;117(12):1814–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll KJ. On the use and utility of the Weibull model in the analysis of survival data. Control Clin Trials. 2003;24(6):682–701 [DOI] [PubMed] [Google Scholar]
- 9.Herman-Giddens ME, Slora EJ, Wasserman RC, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99(4):505–512 [DOI] [PubMed] [Google Scholar]
- 10.Ahmed ML, Ong KK, Dunger DB. Childhood obesity and the timing of puberty. Trends Endocrinol Metab. 2009;20(5):237–242 [DOI] [PubMed] [Google Scholar]
- 11.Jasik CB, Lustig RH. Adolescent obesity and puberty: the “perfect storm”. Ann N Y Acad Sci. 2008;1135:265–279 [DOI] [PubMed] [Google Scholar]
- 12.Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(suppl 3):S208–S217 [DOI] [PubMed] [Google Scholar]
- 13.Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010;140(3):399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buyken AE, Karaolis-Danckert N, Remer T. Association of prepubertal body composition in healthy girls and boys with the timing of early and late pubertal markers. Am J Clin Nutr. 2009;89(1):221–230 [DOI] [PubMed] [Google Scholar]
- 15.Davison KK, Susman EJ, Birch LL. Percent body fat at age 5 predicts earlier pubertal development among girls at age 9. Pediatrics. 2003;111(4 pt 1):815–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heger S, Körner A, Meigen C, et al. Impact of weight status on the onset and parameters of puberty: analysis of three representative cohorts from central Europe. J Pediatr Endocrinol Metab. 2008;21(9):865–877 [DOI] [PubMed] [Google Scholar]
- 17.Lee JM, Appugliese D, Kaciroti N, Corwyn RF, Bradley RH, Lumeng JC. Weight status in young girls and the onset of puberty. Pediatrics. 2007;119(3). Available at: www.pediatrics.org/cgi/content/full/119/3/e624 [DOI] [PubMed] [Google Scholar]
- 18.Rubin C, Maisonet M, Kieszak S, et al. Timing of maturation and predictors of menarche in girls enrolled in a contemporary British cohort. Paediatr Perinat Epidemiol. 2009;23(5):492–504 [DOI] [PubMed] [Google Scholar]
- 19.Windham GC, Zhang L, Longnecker MP, Klebanoff M. Maternal smoking, demographic and lifestyle factors in relation to daughter’s age at menarche. Paediatr Perinat Epidemiol. 2008;22(6):551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307(5):483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biro FM, Wolffe MS. Puberty as a window of susceptibility. In: Russo J, ed. Environment and Breast Cancer. New York, NY: Springer; 2011:29–41 [Google Scholar]
- 22.Buck Louis GM, Gray LEJ, Jr, Marcus M, et al. Environmental factors and puberty timing: expert panel research needs. Pediatrics. 2008;121(suppl 3):S192–S207 [DOI] [PubMed] [Google Scholar]
- 23.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24(5):668–693 [DOI] [PubMed] [Google Scholar]
- 24.Gluckman PD, Hanson MA. Evolution, development and timing of puberty. Trends Endocrinol Metab. 2006;17(1):7–12 [DOI] [PubMed] [Google Scholar]
- 25.Striegel-Moore RH, McMahon RP, Biro FM, Schreiber G, Crawford PB, Voorhees C. Exploring the relationship between timing of menarche and eating disorder symptoms in black and white adolescent girls. Int J Eat Disord. 2001;30(4):421–433 [DOI] [PubMed] [Google Scholar]
- 26.Copeland W, Shanahan L, Miller S, Costello EJ, Angold A, Maughan B. Outcomes of early pubertal timing in young women: a prospective population-based study. Am J Psychiatry. 2010;167(10):1218–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ge X, Conger RD, Elder GH, Jr. Coming of age too early: pubertal influences on girls’ vulnerability to psychological distress. Child Dev. 1996;67(6):3386–3400 [PubMed] [Google Scholar]
- 28.Deardorff J, Gonzales NA, Christopher FS, Roosa MW, Millsap RE. Early puberty and adolescent pregnancy: the influence of alcohol use. Pediatrics. 2005;116(6):1451–1456 [DOI] [PubMed] [Google Scholar]
- 29.Graber JA, Seeley JR, Brooks-Gunn J, Lewinsohn PM. Is pubertal timing associated with psychopathology in young adulthood. J Am Acad Child Adolesc Psychiatry. 2004;43(6):718–726 [DOI] [PubMed] [Google Scholar]
- 30.Johansson T, Ritzén EM. Very long-term follow-up of girls with early and late menarche. Endocr Dev. 2005;8(8):126–136 [DOI] [PubMed] [Google Scholar]
- 31.Foster H, Hagan J, Brooks-Gunn J. Growing up fast: stress exposure and subjective “weathering” in emerging adulthood. J Health Soc Behav. 2008;49(2):162–177 [DOI] [PubMed] [Google Scholar]
- 32.Graber JA, Nichols TR, Brooks-Gunn J. Putting pubertal timing in developmental context: implications for prevention. Dev Psychobiol. 2010;52(3):254–262 [DOI] [PubMed] [Google Scholar]
- 33.Clavel-Chapelon F, E3N Group . Cumulative number of menstrual cycles and breast cancer risk: results from the E3N cohort study of French women. Cancer Causes Control. 2002;13(9):831–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moorman PG, Palmieri RT, Akushevich L, Berchuck A, Schildkraut JM. Ovarian cancer risk factors in African-American and white women. Am J Epidemiol. 2009;170(5):598–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McPherson CP, Sellers TA, Potter JD, Bostick RM, Folsom AR. Reproductive factors and risk of endometrial cancer. The Iowa Women’s Health Study. Am J Epidemiol. 1996;143(12):1195–1202 [DOI] [PubMed] [Google Scholar]
- 36.Frontini MG, Srinivasan SR, Berenson GS. Longitudinal changes in risk variables underlying metabolic Syndrome X from childhood to young adulthood in female subjects with a history of early menarche: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2003;27(11):1398–1404 [DOI] [PubMed] [Google Scholar]
- 37.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578 [DOI] [PubMed] [Google Scholar]
- 38.Ahlgren M, Melbye M, Wohlfahrt J, Sørensen TI. Growth patterns and the risk of breast cancer in women. Int J Gynecol Cancer. 2006;16(suppl 2):569–575 [DOI] [PubMed] [Google Scholar]
- 39.Li CI, Littman AJ, White E. Relationship between age maximum height is attained, age at menarche, and age at first full-term birth and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2144–2149 [DOI] [PubMed] [Google Scholar]
- 40.MacMahon B, Trichopoulos D, Brown J, et al. Age at menarche, urine estrogens and breast cancer risk. Int J Cancer. 1982;30(4):427–431 [DOI] [PubMed] [Google Scholar]
- 41.Henderson BE, Ross RK, Judd HL, Krailo MD, Pike MC. Do regular ovulatory cycles increase breast cancer risk? Cancer. 1985;56(5):1206–1208 [DOI] [PubMed] [Google Scholar]
- 42.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003-2006. JAMA. 2008;299(20):2401–2405 [DOI] [PubMed] [Google Scholar]


