Abstract
OBJECTIVE:
To determine whether a limited oxygen strategy (LOX) versus a high oxygen strategy (HOX) during delivery room resuscitation decreases oxidative stress in preterm neonates.
METHODS:
A randomized trial of neonates of 24 to 34 weeks’ gestational age (GA) who received resuscitation was performed. LOX neonates received room air as the initial resuscitation gas, and fraction of inspired oxygen (Fio2) was adjusted by 10% every 30 seconds to achieve target preductal oxygen saturations (Spo2) as described by the 2010 Neonatal Resuscitation Program guidelines. HOX neonates received 100% O2 as initial resuscitation gas, and Fio2 was adjusted by 10% to keep preductal Spo2 at 85% to 94%. Total hydroperoxide (TH), biological antioxidant potential (BAP), and the oxidative balance ratio (BAP/TH) were analyzed in cord blood and the first hour of life. Secondary outcomes included delivery room interventions, respiratory support on NICU admission, and short-term morbidities.
RESULTS:
Forty-four LOX (GA: 30 ± 3 weeks; birth weight: 1678 ± 634 g) and 44 HOX (GA: 30 ± 3 weeks; birth weight: 1463 ± 606 g) neonates were included. LOX decreased integrated excess oxygen (∑Fio2 × time [min]) in the delivery room compared with HOX (401 ± 151 vs 662 ± 249; P < .01). At 1 hour of life, BAP/TH was 60% higher for LOX versus HOX neonates (13 [9–16] vs 8 [6–9]) µM/U.CARR, P < .01). LOX decreased ventilator days (3 [0–64] vs 8 [0–96]; P < .05) and reduced the incidence of bronchopulmonary dysplasia (7% vs 25%; P < .05).
CONCLUSIONS:
LOX is feasible and results in less oxygen exposure, lower oxidative stress, and decreased respiratory morbidities and thus is a reasonable alternative for resuscitation of preterm neonates in the delivery room.
Keywords: newborn, oxidative stress, oxygen, preterm, resuscitation
What’s Known on This Subject:
Preterm infants can be successfully resuscitated with <100% oxygen (O2); however, initiation with room air remains controversial. Current Neonatal Resuscitation Program (NRP) guidelines suggest using air or blended O2 to titrate O2 to meet target preductal saturation goals.
What This Study Adds:
This is the first trial to compare a limited O2 strategy to target NRP–recommended transitional goal saturations versus a high O2 strategy in preterm infants. The limited O2 strategy decreased integrated excess oxygen and oxidative stress and improved respiratory outcomes.
Use of high oxygen (O2) concentrations during delivery room (DR) resuscitation generates bursts of reactive oxygen and nitrogen species that can overwhelm newborn antioxidant capacity and damage cell components such as lipids, proteins, RNA, and DNA.1–8 Even brief 100% O2 exposure during resuscitation increases oxidative stress and reduces antioxidant capacity as measured by using biomarkers such as total hydroperoxide (TH), biological antioxidant potential (BAP), reduced glutathione/oxidized glutathione ratio, and superoxide dismutase activity.7–10 Oxidative stress increases newborn morbidities such as bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), and mortality.4,11–17 Preterm neonates are especially vulnerable to O2 toxicity because they frequently require resuscitation after birth and receive supplemental O2 despite reduced antioxidant defenses that mature later in gestation.18,19
In 2005, the American Academy of Pediatrics/American Heart Association Neonatal Resuscitation Program (NRP) guidelines preferentially recommended the use of 100% O2 but allowed providers to begin resuscitation with less O2 and recommended using pulse oximetry to adjust O2 concentrations as needed.20,21 These guidelines emphasized that no studies justified any particular starting O2 concentration. Subsequently, 4 small trials demonstrated that resuscitation of preterm neonates with an initial fraction of inspired oxygen (Fio2) of 0.21 to 0.30 was feasible when Fio2 was titrated against preductal oxygen saturations (Spo2) and for bradycardia.8,22–24 However, the optimal O2 strategy during resuscitation remains unknown.25,26 Despite no studies comparing outcomes of neonatal resuscitation targeted at various oxyhemoglobin concentrations, the 2010 NRP guidelines recommended that preterm neonates at birth have O2 titrated to achieve O2 saturations in the interquartile range of healthy term newborns27–31 and that excessive O2 should be avoided. To date, no randomized trials of strategies to achieve the NRP-recommended interquartile range of preductal saturations have been conducted in preterm neonates.3
We hypothesized that a limited O2 strategy (LOX) with resuscitation initiated with 0.21 Fio2 and adjusted to meet NRP transitional goal saturations would increase the oxidative balance ratio (BAP/TH) by at least 15% compared with a high O2 strategy (HOX) in which resuscitation was initiated with pure O2 and titrated for a targeted Spo2 of 85% to 94%.
Methods
Patients
This study was a prospective randomized trial conducted from August 2010 to January 2011 at Parkland Hospital, Dallas, Texas. The project was approved by the institutional review board of the University of Texas Southwestern Medical Center. The 2010 NRP guidelines had not been released or implemented at study initiation, and 2005 NRP was in use. Because both LOX and HOX were consistent with the 2005 NRP guidelines and because there was equipoise regarding the 2 treatment arms, the institutional review board permitted the trial to proceed without antenatal consent as long as parental informed consent was subsequently obtained.20,21 All inborn neonates of obstetrical (OB) gestational age (GA) 24 0/7 to 34 6/7 weeks for whom the high-risk resuscitation team was present at birth and who required active resuscitation were included. Active resuscitation was defined as need for blow-by O2 for low Spo2, positive pressure ventilation (PPV), continuous positive airway pressure (CPAP), or ventilation via an endotracheal tube. Neonates were excluded for nonviability, prenatally diagnosed cyanotic congenital heart disease, if no active resuscitation was required, or if preductal Spo2 could not be measured. Multiple gestations were assigned randomly according to the individual neonate.
Procedures
The high-risk neonatal resuscitation team attended delivery of all neonates <35 weeks’ OB GA. Radical oximeters (Masimo Corporation, Irvine, CA) were set to maximal sensitivity with 2-second averaging.8,22,24,32 The probe was placed on the preductal hand within the first 30 seconds of life by using previously described strategies to optimize time to reliable signal.32
A noninvestigator used a permuted design in blocks of 8 to determine randomization sequence and stratified participants into OB-estimated GA groups: 24 0/7 to 28 6/7 weeks and 29 0/7 to 34 6/7 weeks. Allocation was concealed via serially numbered, sealed, opaque envelopes that were opened sequentially by the resuscitation team when the need for resuscitation was recognized. Resuscitation started with room air while the envelope was opened and then immediately changed to LOX or HOX as assigned in a nonblinded fashion.
Limited Oxygen Strategy
Resuscitation was initiated with 21% O2 for LOX neonates. Supplemental O2 was given for the following indications: (1) heart rate (HR) <100 beats per minute after 30 seconds of effective ventilation; or (2) lower limit of goal saturations not achieved. Targeted preductal saturations after birth were derived by approximation of interquartile values for healthy term neonates (Table 1).28,29 Fio2 was adjusted by 10% in 30-second intervals to maintain saturations in the interquartile range. If HR remained <60 beats per minute despite 30 seconds of PPV, Fio2 was increased to 100% until recovery of HR to >100 beats per minute.
TABLE 1.
Targeted Preductal Spo2 After Birth
| Time | Saturation Range |
|---|---|
| 1 min | 60%–65% |
| 2 min | 65%–70% |
| 3 min | 70%–75% |
| 4 min | 75%–80% |
| 5 min | 80%–85% |
| 10 min | 85%–94% |
High Oxygen Strategy
For HOX neonates, resuscitation was initiated with 100% O2 and adjusted every 30 seconds by 10% to meet target Spo2 of 85% to 94%.
Resuscitation Management
Apart from the randomized O2 strategy, resuscitation followed 2005 NRP guidelines.20,21 Treatment failure was defined as HR <60 beats per minute despite 30 seconds of effective PPV. If pulse oximetry did not register stable values, resuscitation continued at the current Fio2 as long as an HR >100 beats per minute was maintained.
Subsequent NICU Management
After NICU admission, all care decisions (including ventilator management) were at the discretion of the attending neonatologist. Target Spo2 was 88% to 94% throughout the NICU stay per unit policy. Infants with an O2 requirement at 36 weeks’ corrected GA underwent an O2 challenge test to define BPD.33
Data Collection
HR and Spo2 were downloaded per pulse oximeter manufacturer instructions. Resuscitation interventions and physiologic responses were recorded at 30-second intervals by the OB circulating nurse per routine practice. Cases were reviewed continuously for adherence to NRP and study protocols.
Sample Collection and Analysis
One mL of umbilical cord blood and 0.25 mL of arterial blood were collected without anticoagulant on NICU admission. Serum was separated immediately by using centrifugation and stored at –80°C. All samples were analyzed within 1 month of collection. Samples were thawed carefully 1 at a time at room temperature, with mixing during and after the thawing process until equilibrium was reached. Thawed samples were analyzed immediately for TH and BAP with a Free Radical Analytical System (FRAS4 analyzer, H&D Srl, Parma, Italy) per manufacturer guidelines. TH represents the total of radical O2 metabolites produced by peroxidation of protein, lipids, and amino acids; it measures oxidative damage and serves as a biomarker of overall free radical attack.10,34–37 BAP measures both endogenous and exogenous antioxidative capacity of serum to reduce oxides by inactivating and eliminating free radicals and reactive O2 species. Although cytoplasmic antioxidants are not measured, BAP, by exploiting the chemical principle of the well-known ferric-reducing ability of plasma, provides a reliable measure of biological antioxidant potential of blood plasma.10,38–41 To estimate global balance between oxidative stress and antioxidant potential, the oxidative balance ratio (BAP/TH) was calculated based on measured values of BAP and TH.
Outcomes
The primary hypothesis was that LOX would improve oxidative balance ratio by 15% compared with HOX. Secondary outcomes were defined a priori as: reduction in admission Pao2 >100 mm Hg, change in Apgar score, total O2 used during resuscitation, integrated excessive O2 (Σ[Fio2 – 0.21] × time [min]), time during resuscitation with saturations >94%, use of rescue high-frequency oscillatory ventilation (HFOV), ventilator and hospital days, in-hospital mortality, and incidence of BPD, ROP, necrotizing enterocolitis, IVH, and PVL.
Statistical Analysis
Statistical analyses were conducted by using SAS version 9.2 (SAS Institute, Cary, NC). Normally distributed variables were compared by using Student’s t test. Nonparametric variables were compared by using Mann-Whitney rank sum tests. HR, Spo2, and Fio2 were compared with a linear mixed model repeated measures analysis, which included factors to assess group, time, and group-by-time interaction. Based on the reported oxidative balance ratio data for preterm neonates, to detect a 15% change, 39 patients were needed for each arm using a 2-sided α level of .05 and a power of 0.8.10 To accommodate a 10% potential patient dropout, 44 neonates were enrolled in each group.
Results
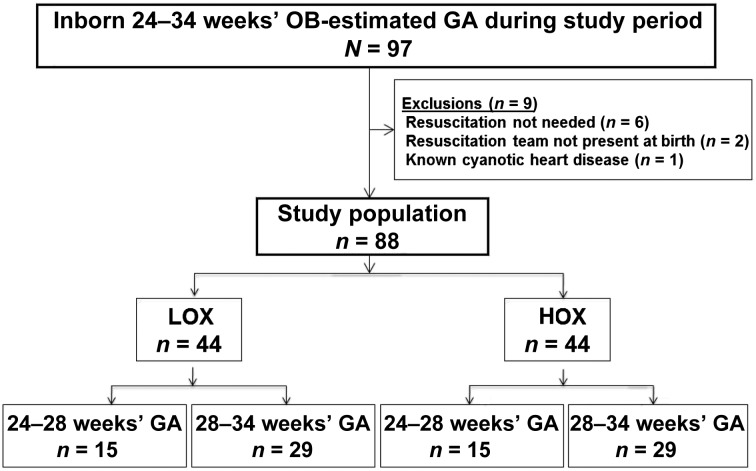
Eligible neonates, comprising those enrolled and randomly assigned to LOX (n = 44) and HOX (n = 44), are shown in Fig 1. Fifteen neonates <28 weeks’ GA and 29 neonates 29 to 34 weeks’ GA were included in each arm. Eleven pairs of twins were included. One pair of monochorionic diamniotic twins were assigned randomly to HOX and 2 pairs of monochorionic diamniotic twins were assigned to LOX. For the remaining 8 pairs, each twin was randomly assigned to different groups. In 1 instance, only 1 twin required resuscitation and was enrolled. LOX and HOX neonates had similar maternal and baseline neonatal characteristics except that breech presentation was higher for HOX neonates (Table 2).
FIGURE 1.
Flow diagram showing screening of eligible infants, enrollment, and randomization.
TABLE 2.
Maternal and Neonatal Characteristics
| Characteristic | LOX (n = 44) | HOX (n = 44) |
|---|---|---|
| Maternal | ||
| Age, median (range), y | 26 (16–40) | 26 (16–38) |
| Prenatal clinic attendance | 41 (95) | 40 (91) |
| Antenatal steroids | 24 (55) | 21 (48) |
| Maternal diabetes | 7 (16) | 6 (14) |
| Illicit drug use | 1 (2) | 2 (5) |
| Preeclampsia | 11 (26) | 10 (23) |
| Prolonged rupture of membranes | 11 (25) | 7 (16) |
| Chorioamnionitis | 12 (27) | 10 (23) |
| Abruption | 1 (2) | 1 (2) |
| Previa | 2 (5) | 2 (5) |
| Cesarean delivery | 27 (63) | 32 (73) |
| Neonatal | ||
| Male | 21 (48) | 24 (55) |
| Obstetrical GA, median (range), wks | 30 (24–34) | 30 (24–34) |
| Birth weight, mean ± SD, g | 1678 ± 634 | 1463 ± 606 |
| Intrauterine growth restriction | 5 (11) | 2 (5) |
| Breecha | 10 (23) | 24 (55) |
| Multiple | 12 (27) | 11 (25) |
Unless otherwise noted, data are presented as n (%).
P < .05.
DR Resuscitation Interventions
As determined by the study protocol, the initial FIO2 was different for LOX and HOX neonates (Table 3). Use and maximum level of CPAP, need for PPV, maximum peak inspiratory pressure, and rates of intubation were similar. No neonate required chest compression or epinephrine. There was no difference in arterial cord pH, base deficit, or Apgar scores at 1 and 5 minutes. Two LOX infants and 3 HOX infants had an HR <60 beats per minute after 90 seconds of resuscitation, all of whom responded to ventilation via endotracheal tube. HR did not differ between groups at any time during resuscitation.
TABLE 3.
DR Interventions and Early Parameters on Admission to NICU
| Parameter | LOX (n = 44) | HOX (n = 44) | P |
|---|---|---|---|
| Delivery room interventions | |||
| Initial FiO2a | 0.21 ± 0 | 1.0 ± 0 | .0001 |
| CPAP only, n (%) | 11 (25) | 11 (25) | NS |
| Maximum CPAP, cm H2Ob | 5 (5–7) | 5 (5–8) | NS |
| PPV, n (%) | 29 (66) | 30 (68) | NS |
| Maximum peak inspiratory pressure, cm H2Ob | 25 (20–25) | 25 (20–30) | NS |
| Intubation, n (%) | 9 (20) | 17 (39) | NS |
| Chest compression, n (%) | 0 | 0 | NS |
| Epinephrine, n (%) | 0 | 0 | NS |
| Arterial cord pHa | 7.25 ± 0.08 | 7.23 ± 0.10 | NS |
| Arterial cord base deficita | 6 ± 3 | 7 ± 5 | NS |
| Apgar scoresb | |||
| 1 min | 6 (1–9) | 5 (0–9) | NS |
| 5 min | 8 (2–9) | 7 (3–9) | NS |
| 10 min | 8 (6–9) | 8 (5–9) | NS |
| Final FIO2a | 0.3 ± 0.2 | 0.4 ± 0.2 | NS |
| Inspired oxygen (∑Fio2 × time [min])a | 401 ± 151 | 662 ± 249 | .0001 |
| Integrated excessive O2 (∑[Fio2 – 0.21] × time [min])a | 1.91 ± 1.52 | 4.51 ± 2.49 | .0001 |
| Early parameters on admission to NICU | |||
| No ventilator support, n (%) | 9 (20) | 4 (9) | NS |
| On CPAP, n (%) | 26 (59) | 23 (52) | NS |
| Level of CPAP, cm H2Ob | 5 (4–8) | 5 (4–7) | NS |
| Intubated, n (%) | 9 (20) | 17 (39) | NS |
| Peak inspiratory pressure, cm H2Ob | 20 (18–25) | 20 (16–22) | NS |
| Fio2a | 0.3 ± 0.2 | 0.4 ± 0.3 | NS |
| Breathing 21% O2 on arrival at NICU, n (%) | 18 (41%) | 13 (29%) | NS |
| O2 saturation, %a | 94 ± 4 | 93 ± 4 | NS |
| pHa | 7.28 ± 0.1 | 7.28 ± 0.1 | NS |
| Pao2, mm Hga | 70 ± 32 | 64 ± 25 | NS |
| Paco2, mm Hga | 49 ± 13 | 46 ± 11 | NS |
| Base deficit, mEq/La | 5 ± 2 | 6 ± 5 | NS |
| Pao2 >100 mm Hg | 6 (15) | 3 (7) | NS |
NS, not significant.
Mean ± SD.
Median (range).
DR O2 Use
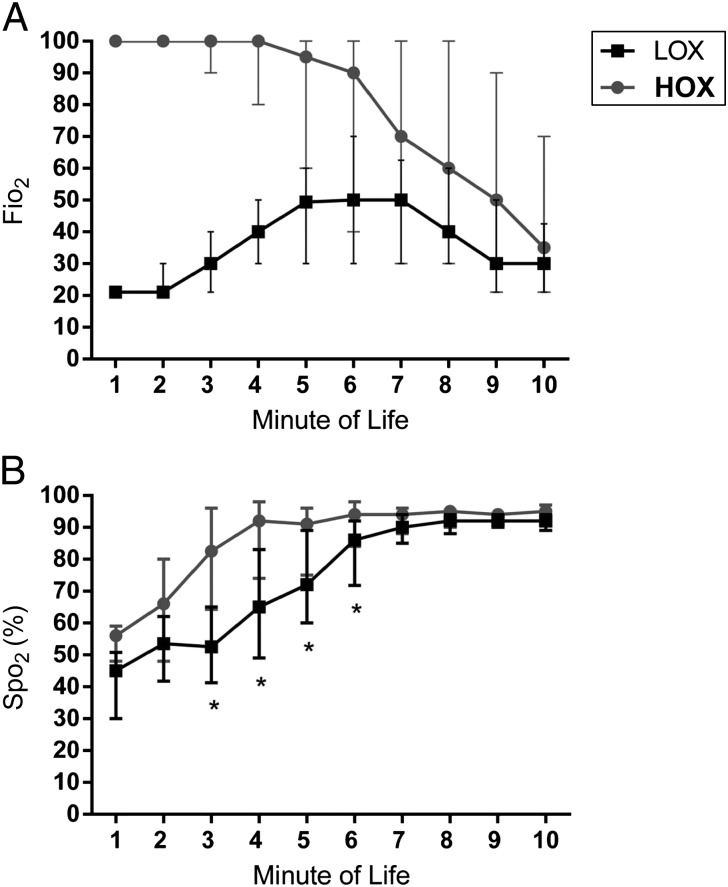
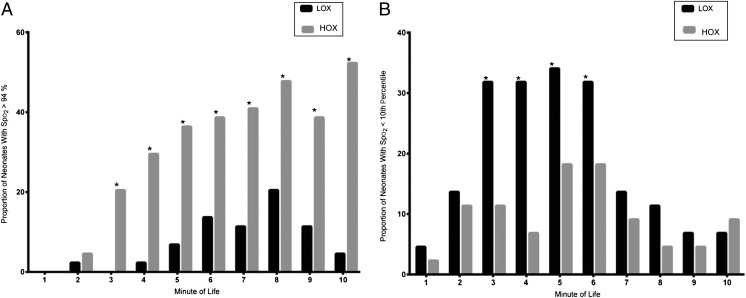
LOX neonates were exposed to less O2 in the DR as noted by lower O2 load and integrated excessive O2 compared with HOX (Table 3). Median Fio2 and interquartile range for the LOX group was increased in a stepwise manner to 0.50 (0.30–0.70) by 6 minutes of life and subsequently weaned to 0.30 (0.21–0.45) by 10 minutes (Fig 2). Conversely, Fio2 for HOX neonates was reduced in a stepwise manner to 0.70 (0.30–1) at 7 minutes of life and then further reduced to 0.35 (0.21–0.70) at 10 minutes. Fio2 differed between LOX and HOX for the first 9 minutes of life. All LOX neonates required some increase in Fio2 above room air to meet target Spo2. Initial Spo2 achieved during the first 2 minutes of life did not differ between groups. HOX neonates had higher Spo2 between 3 and 6 minutes of life, but after 6 minutes, the results were similar between groups. More HOX neonates had Spo2 >94% at each minute of life for the first 10 minutes compared with LOX (Fig 3). More LOX infants had saturations below the 10th centile for the first 10 minutes of life compared with HOX infants.28
FIGURE 2.
A, Median level of O2 administered per minute of life for the first 10 minutes of life in preterm neonates randomly assigned to the LOX group, in which initial Fio2 after birth was 21%, or the HOX group, in which initial Fio2 after birth was 100%. * P < .05. B, Median Spo2 per minute of life for first 10 minutes of life in the DR in LOX and HOX infants. * P < .05.
FIGURE 3.
A, Proportion of neonates with Spo2 >94% per minute for the first 10 minutes of life in the DR in the LOX and HOX groups. * P < .05. B, Proportion of neonates with Spo2 <10th percentile of Dawson curves for the first 10 minutes of life in the DR in the LOX and HOX groups. * P < .05.
Respiratory Support and Other Characteristics on Admission to NICU
Respiratory support, including use and level of CPAP, mechanical ventilation, and peak inspiratory pressure were similar between LOX and HOX infants on NICU admission. Both groups had similar Fio2, PaO2, and Spo2. Similar numbers of neonates had Pao2 >100 mm Hg according to admission arterial blood gas levels.
Oxidative Stress Markers and Antioxidant Capacity
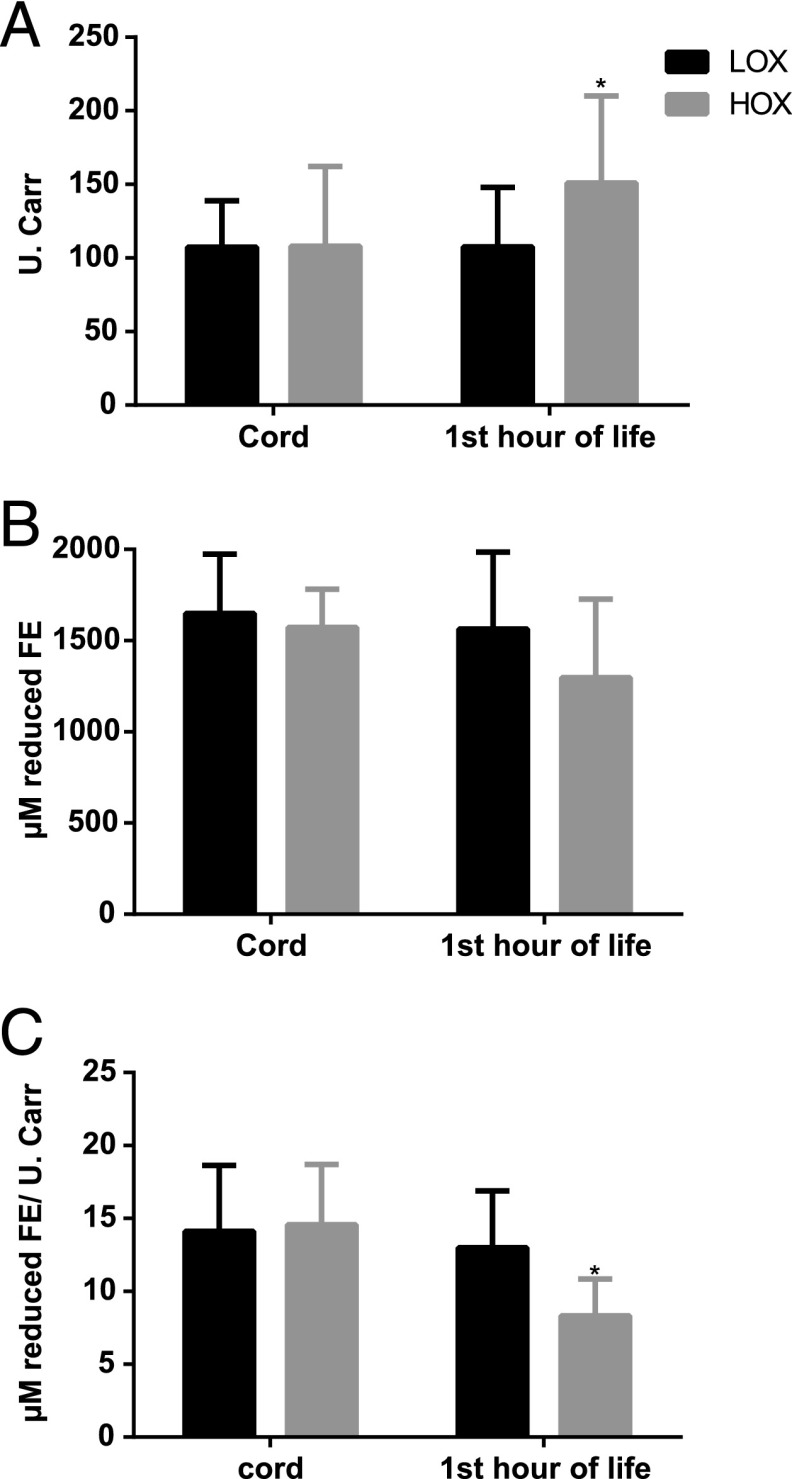
Cord blood TH (107 [29–138] vs 108 [85–161] U.CARR; P > .05), BAP (1649 [1415–1973] vs 1572 [1460–1776] µM; P > .05), and oxidative balance ratio (14 [11–19] vs 15 [9–18] µM/U.CARR; P > .05) were similar for LOX versus HOX. Within 1 hour of admission, TH was lower (107 [91–146] U.CARR vs 151 [124–210] U.CARR; P = .001), BAP was higher (1564 [1216–1963] µM vs 1297 [1029–1705] µM; P = .02), and the oxidative balance ratio (13 [9–16] µM/U.CARR vs 8 [6–9] µM/U.CARR; P < .001) was higher for LOX versus HOX infants. When change from baseline (∆) was analyzed, ∆TH was lower and ∆oxidative balance ratio was higher for LOX infants, with no difference in ∆BAP (Fig 4).
FIGURE 4.
(A) Total hydroperoxides, (B) biological antioxidant potential, and (C) oxidative balance ratio in cord blood and 1 hour of life in preterm neonates resuscitated with LOX or HOX. Data are presented as mean ± SD. Mann-Whitney test was used for comparison of difference over time (cord and admission samples) between the groups. * P < .01.
Respiratory and Other Short-Term NICU Morbidities
Respiratory and other short-term morbidities are shown in Table 4. There were no differences in the incidence of respiratory distress syndrome, need for surfactant, pneumothorax, pulmonary interstitial emphysema, pulmonary arterial hypertension, pneumomediastinum, or pulmonary hemorrhage.
TABLE 4.
Respiratory and Other Short-Term Morbidities
| Parameter | LOX (n = 44) | HOX (n = 44) | P |
|---|---|---|---|
| Respiratory morbidities | |||
| Respiratory distress syndrome | 28 (64) | 25 (57) | NS |
| Received surfactant | 15 (34) | 17 (39) | NS |
| Pneumothorax | 2 (4) | 2 (4) | NS |
| Pulmonary interstitial emphysema | 2 (4) | 5 (11) | NS |
| Pulmonary arterial hypertension | 1 (2) | 3 (7) | NS |
| Pneumomediastinum | 2 (4) | 0 (0) | NS |
| Pulmonary hemorrhage | 2 (4) | 6 (14) | NS |
| BPD | |||
| O2 at 28 d | 8 (19) | 17 (39) | .04 |
| O2 at 36 wk | 3 (7) | 11 (25) | .04 |
| Physiologic definitiona | 2 (4) | 10 (23) | .03 |
| Intravenous steroids for BPD | 1 (2) | 2 (4) | NS |
| HFOV | 2 (4) | 10 (23) | .03 |
| Days on mechanical ventilationb | 3 (0–64) | 8 (0–96) | .045 |
| Days on CPAPb | 4 (0–44) | 6 (0–43) | NS |
| Days on oxygenb | 4 (0–113) | 10 (0–163) | NS |
| Maximum Fio2b | 64 (25–100) | 100 (26–100) | NS |
| Other short-term morbidities | |||
| Sepsis proven | 8 (18) | 10 (23) | NS |
| IVH grade 3 or 4 | 1 (2) | 1 (2) | NS |
| Cystic PVL | 0 (0) | 1 (2) | NS |
| Necrotizing enterocolitis | 1 (2) | 6 (14) | NS |
| Symptomatic patent ductus arteriosus | 6 (14) | 10 (23) | NS |
| Severe ROP/need for laser therapy | 1 (2) | 4 (9) | NS |
| Days of hospitalizationb | 33 (7–126) | 40 (8–166) | NS |
| Death during initial hospitalization | 2 (4) | 3 (7) | NS |
NS, not significant.
The physiologic definition of BPD includes, as a criterion, the receipt of >30% O2 or the need for positive pressure support at 36 weeks or, in the case of infants requiring <30% O2, the need for any O2 at 36 weeks after an attempt at O2 withdrawal.
Median (range).
BPD according to the National Institutes of Health consensus4 and physiologic definition33 was lower for LOX infants, with a number needed to treat of 5 (95% confidence interval: 3–37) to prevent 1 case of BPD. LOX neonates received less rescue HFOV and spent fewer days on mechanical ventilation than HOX neonates. There were no differences in corticosteroid use for BPD, days on O2, maximum Fio2 during NICU stay, culture-proven sepsis, IVH grade 3 or 4, PVL, necrotizing enterocolitis, symptomatic patent ductus arteriosus, ROP stage 3 to 4, need for laser therapy, length of NICU stay, or mortality.
Discussion
We report the first trial comparing LOX using goal saturations now recommended by the 2010 NRP guidelines versus HOX to demonstrate feasibility and decrease O2 exposure in preterm neonates.42 LOX successfully resuscitated preterm neonates without increasing the need for additional respiratory or cardiovascular support in the DR and decreased O2 load by one-half. LOX neonates spent less time with DR Spo2 >94%, developed less oxidative stress by 1 hour of life, and had better preservation of BAP. Importantly, LOX also decreased ventilator days, rescue HFOV, and BPD.
Similar to previous small trials comparing various O2 strategies for DR resuscitation,8,22–24,43 the current study suggests that static delivery of 21% O2 is unlikely to achieve current NRP goal saturations for preterm infants. The majority needed supplemental O2 to meet these goals. Two previous DR O2 studies22,24 concluded that initiation of resuscitation with 21% O2 was inappropriate because the majority of preterm infants needed O2 supplementation to maintain the goal saturations prescribed by their study protocol. Our findings challenge this conclusion. Although it should not be expected that static delivery of room air will be adequate throughout resuscitation and stabilization, starting with 21% O2, provided it is gradually increased by using LOX, results in successful stabilization with no increase in sustained bradycardia, need for PPV, intubation, cardiac compressions, or vasoactive drugs.
NRP’s recommendation for the interquartile range of preductal saturations of healthy term infants as target goal saturations was based on consensus expert opinion as the United States took its first steps to stop the routine use of 100% O2 during resuscitation.30,31,42 The optimal Spo2 percentile for goal saturations during preterm transition is unknown, but ≥10th percentile is considered normal for parameters such as birth weight. Although in the first 10 minutes of life, the majority of LOX neonates had an Spo2 above the 10th percentile on the Dawson curves, more LOX infants had an Spo2 below the 10th percentile compared with HOX.28 Goos et al recently reported retrospectively that preterm neonates initially resuscitated with 30% O2 and adjusted to meet NRP goal saturations also resulted in some with Spo2 below the 10th percentile of the Dawson curves.28,44 The significance of this observation is speculative because the effect of brief DR LOX on long-term neurodevelopmental outcome remains unknown.42 Studies comparing different starting concentrations of O2 to achieve target goal saturations and, more importantly, effects on both short- and long-term clinical outcomes are warranted; however, based on published studies so far, it seems unlikely that 21% vs 30% O2 would result in significant differences.8,22,23,42–45 To optimize balance between excessive O2 and hypoxia, future studies comparing different target Spo2 ranges should be considered.27,46 We speculate that various strategies which improve ventilation and establishes functional residual capacity early may further reduce the need for supplemental oxygen.47,48
The decrease in ventilator days, rescue HFOV, and BPD in LOX neonates is interesting; however, our study was not powered for secondary outcomes. Vento et al8 also reported decreased BPD with the use of a different LOX protocol. Although multiple factors promote lung injury resulting in BPD, oxidant injury is an important contributor.1,2,4,49 High O2 exposure can arrest lung septation in the saccular stage of development.4,12,50,51 Infants with BPD exposed to higher supplemental O2 to achieve higher levels of Spo2 have more persistent lung disease.4,12,52 We speculate that excess O2 exposure in the first few minutes of life may blunt vascular development in preterm infants. Development of strategies to minimize oxygen exposure to prevent tissue injury must be a priority.4
A recent meta-analysis comparing lower (21%–50%) versus higher (>50%) O2 concentrations for DR resuscitation of preterm infants and their effects on morbidity and mortality identified 6 randomized trials.8,22–24,42,53–55 Lower O2 reduced the risk of death, but this effect disappeared when limited to studies with adequate allocation concealment.42 The authors stressed the need for larger trials powered to detect important outcomes, including neurodevelopment and mortality.
The current study has several limitations. Although randomized, the trial was not blinded and is at-risk for bias because the resuscitation team was aware of treatment assignments. However, DR interventions were diligently recorded by the OB circulating nurse who was not involved in the trial (as is our standard practice), and systematic errors that might have unduly influenced randomization or outcome were not found. Video recordings or computerized data acquisition systems other than pulse oximetry for continuous physiologic data were not available. The number of neonates recruited to the trial, which was powered for a difference in oxidative stress markers, did not allow for the evaluation of short-term clinical outcomes such as BPD or long-term neurodevelopmental outcomes with sufficient statistical power.
One of the strengths of the study is that it recapitulates the intended real-world DR use of O2. Adjusting Fio2 in response to Spo2 every 30 seconds is challenging and might interfere with or delay other steps needed to resuscitate a preterm infant. Lack of research personnel assigned to make these changes provided opportunity to study the effect of these complex interactions in a randomized controlled trial setting. We did not have a visual O2 targeting system such as TOTS [Transitional Oxygen Targeting System] to help keep saturations within target range.45 Our study is relevant to most resuscitation situations because the majority of the world’s neonates are resuscitated without a visual targeting system. This is the first randomized trial to use current NRP-recommended O2 saturation targets in preterm infants needing DR resuscitation. Unlike previous trials examining resuscitation of preterm neonates with LOX, our study included all eligible preterm infants due to antenatal waiver of consent, thereby reducing selection bias and increasing the generalizability of the results.8,22–24,42,56 Furthermore, the study population was stratified to 24- to 28-week and 29- to 34-week GA. This approach is important because of limited data on the impact of LOX on more mature preterm neonates.
Conclusions
LOX for DR resuscitation of preterm neonates is feasible, decreases O2 exposure without increasing need for additional resuscitation, and decreases oxidative stress. In addition, LOX seems to decrease ventilator days, HFOV, and risk of BPD. Thus, LOX is a reasonable alternative for DR resuscitation of preterm neonates. Large randomized trials adequately powered for evaluation of the relationship between O2 load during resuscitation and important clinical outcomes such as BPD and long-term neurodevelopment are warranted.53
Acknowledgment
The authors gratefully acknowledge Jie Liao, MD, PhD, for technical support and Beverley Huet, MS, for statistical support.
Glossary
- BAP
biological antioxidant potential
- BPD
bronchopulmonary dysplasia
- CPAP
continuous positive airway pressure
- DR
delivery room
- Fio2
fraction of inspired oxygen
- GA
gestational age
- HFOV
high-frequency oscillatory ventilation
- HOX
high oxygen strategy
- HR
heart rate
- IVH
intraventricular hemorrhage
- LOX
limited oxygen strategy
- NRP
Neonatal Resuscitation Program
- OB
obstetrical
- PPV
positive pressure ventilation
- PVL
periventricular leukomalacia
- ROP
retinopathy of prematurity
Footnotes
Dr Kapadia conceptualized and designed the study, recruited patients, and designed the data collection instruments; participated in collecting the data, analysis of the data, and interpretation of data; and drafted the initial manuscript, wrote the last draft of the manuscript, and approved the final manuscript as submitted. Dr Chalak participated in concept, design, analysis, and interpretation of data; reviewed and revised the manuscript; and approved the final manuscript as submitted. Dr Sparks participated in recruitment, design, analysis, and interpretation of data; and reviewed and approved the final manuscript as submitted. Mr Allen participated in design, data collection, and analysis of data; and reviewed and approved the final manuscript as submitted. Dr Savani participated in concept, design, analysis, and interpretation of data; and critically reviewed and approved the final manuscript as submitted. Dr Wyckoff participated in conceptualization and design of the study; supervised the recruitment, designing of data, and collection instruments; participated in analysis of the data and interpretation of the data; and critically reviewed, revised, and approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifier NCT01697904).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the University of Texas Southwestern Medical Center, Center for Translational Medicine, National Institute of Health/National Center for Advancing Translational Sciences Grant UL1TR000451 (Dr Kapadia), the Children Medical Center Foundation, Dallas (Drs Kapadia and Savani) and the William Buchanan Chair in Pediatrics (Dr Savani). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found on page e1661, online at www.pediatrics.org/cgi/doi/10.1542/peds.2013-1292.
References
- 1.Gien J, Kinsella JP. Pathogenesis and treatment of bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23(3):305–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welty SE, Smith CV. Rationale for antioxidant therapy in premature infants to prevent bronchopulmonary dysplasia. Nutr Rev. 2001;59(1 pt 1):10–17 [DOI] [PubMed] [Google Scholar]
- 3.Vento M, Escobar J, Cernada M, Escrig R, Aguar M. The use and misuse of oxygen during the neonatal period. Clin Perinatol. 2012;39(1):165–176 [DOI] [PubMed] [Google Scholar]
- 4.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729 [DOI] [PubMed] [Google Scholar]
- 5.Østerholt HC, Dannevig I, Wyckoff MH, et al. Antioxidant protects against increases in low molecular weight hyaluronan and inflammation in asphyxiated newborn pigs resuscitated with 100% oxygen. PLoS ONE. 2012;7(6):e38839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saugstad OD, Sejersted Y, Solberg R, Wollen EJ, Bjørås M. Oxygenation of the newborn: a molecular approach. Neonatology. 2012;101(4):315–325 [DOI] [PubMed] [Google Scholar]
- 7.Vento M, Asensi M, Sastre J, Lloret A, García-Sala F, Viña J. Oxidative stress in asphyxiated term infants resuscitated with 100% oxygen. J Pediatr. 2003;142(3):240–246 [DOI] [PubMed] [Google Scholar]
- 8.Vento M, Moro M, Escrig R, et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics. 2009;124(3). Available at: www.pediatrics.org/cgi/content/full/124/3/e439 [DOI] [PubMed]
- 9.Pallardo FV, Sastre J, Asensi M, Rodrigo F, Estrela JM, Viña J. Physiological changes in glutathione metabolism in foetal and newborn rat liver. Biochem J. 1991;274(pt 3):891–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ezaki S, Suzuki K, Kurishima C, et al. Resuscitation of preterm infants with reduced oxygen results in less oxidative stress than resuscitation with 100% oxygen. J Clin Biochem Nutr. 2009;44(1):111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn JT, Bancalari E, Snyder ES, et al. A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. N Engl J Med. 1992;326(16):1050–1054 [DOI] [PubMed] [Google Scholar]
- 12.Trindade CE, Rugolo LM. Free radicals and neonatal diseases. Neoreviews. 2007;8(12):e522–e532
- 13.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50(5):553–562 [DOI] [PubMed] [Google Scholar]
- 14.Vento M, Sastre J, Asensi MA, Viña J. Room-air resuscitation causes less damage to heart and kidney than 100% oxygen. Am J Respir Crit Care Med. 2005;172(11):1393–1398 [DOI] [PubMed]
- 15.Davis PG, Tan A, O’Donnell CP, Schulze A. Resuscitation of newborn infants with 100% oxygen or air: a systematic review and meta-analysis. Lancet. 2004;364(9442):1329–1333 [DOI] [PubMed] [Google Scholar]
- 16.Rabi Y, Rabi D, Yee W. Room air resuscitation of the depressed newborn: a systematic review and meta-analysis. Resuscitation. 2007;72(3):353–363 [DOI] [PubMed] [Google Scholar]
- 17.Saugstad OD, Ramji S, Soll RF, Vento M. Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology. 2008;94(3):176–182 [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Whitney PL, Frank L. Comparative responses of premature versus full-term newborn rats to prolonged hyperoxia. Pediatr Res. 1994;35(2):233–237 [DOI] [PubMed] [Google Scholar]
- 19.O'Donovan DJ, Fernandes CJ. Free radicals and diseases in premature infants. Antioxid Redox Signal. 2004;6(1):169–176 [DOI] [PubMed]
- 20.International Liaison Committee on Resuscitation . 2005 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Part 7: neonatal resuscitation. Resuscitation. 2005;67(2–3):293–303 [DOI] [PubMed] [Google Scholar]
- 21.Kattwinkel J, ed. Textbook of Neonatal Resuscitation. 5th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2006 [Google Scholar]
- 22.Escrig R, Arruza L, Izquierdo I, et al. Achievement of targeted saturation values in extremely low gestational age neonates resuscitated with low or high oxygen concentrations: a prospective, randomized trial. Pediatrics. 2008;121(5):875–881 [DOI] [PubMed]
- 23.Rabi Y, Singhal N, Nettel-Aguirre A. Room-air versus oxygen administration for resuscitation of preterm infants: the ROAR Study. Pediatrics 2011;128(2). Available at: www.pediatrics.org/cgi/content/full/128/2/e374 [DOI] [PubMed]
- 24.Wang CL, Anderson C, Leone TA, Rich W, Govindaswami B, Finer NN. Resuscitation of preterm neonates by using room air or 100% oxygen. Pediatrics. 2008;121(6):1083–1089 [DOI] [PubMed]
- 25.Higgins RD, Bancalari E, Willinger M, Raju TN. Executive summary of the workshop on oxygen in neonatal therapies: controversies and opportunities for research. Pediatrics. 2007;119(4):790–796 [DOI] [PubMed] [Google Scholar]
- 26.Perlman J, Kattwinkel J, Wyllie J, Guinsburg R, Velaphi S, Nalini Singhal for the Neonatal ILCOR Task Force Group . Neonatal resuscitation: in pursuit of evidence gaps in knowledge. Resuscitation. 2012;83(5):545–550 [DOI] [PubMed] [Google Scholar]
- 27.Vento MAM, Asensi M, Sastre J, García-Sala F, Viña J. Six years of experience with the use of room air for the resuscitation of asphyxiated newly born term infants. Biol Neonate. 2001;79(3–4):261–267 [DOI] [PubMed] [Google Scholar]
- 28.Dawson JA, Kamlin CO, Vento M, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics. 2010;125(6). Available at: www.pediatrics.org/cgi/content/full/125/6/e1340 [DOI] [PubMed]
- 29.Kamlin CO, O’Donnell CP, Davis PG, Morley CJ. Oxygen saturation in healthy infants immediately after birth. J Pediatr. 2006;148(5):585–589 [DOI] [PubMed] [Google Scholar]
- 30.Kattwinkel J. Neonatal Resuscitation Textbook. 6th ed. Elk Grove Village, IL: American Academy of Pediatrics and American Heart Association; 2011 [Google Scholar]
- 31.Kattwinkel J, Perlman JM, Aziz K, et al. Neonatal resuscitation: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics 2010;126(5). Available at: www.pediatrics.org/cgi/content/full/126/5/e1400 [DOI] [PubMed]
- 32.O’Donnell CP, Kamlin CO, Davis PG, Morley CJ. Obtaining pulse oximetry data in neonates: a randomised crossover study of sensor application techniques. Arch Dis Child Fetal Neonatal Ed. 2005;90(1):F84–F85 [DOI] [PMC free article] [PubMed]
- 33.Walsh MC, Yao Q, Gettner P, et al. National Institute of Child Health and Human Development Neonatal Research Network . Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114(5):1305–1311 [DOI] [PubMed] [Google Scholar]
- 34.Buonocore G, Perrone S, Longini M, Terzuoli L, Bracci R. Total hydroperoxide and advanced oxidation protein products in preterm hypoxic babies. Pediatr Res. 2000;47(2):221–224 [DOI] [PubMed] [Google Scholar]
- 35.Kakita H, Hussein MH, Yamada Y, et al. High postnatal oxidative stress in neonatal cystic periventricular leukomalacia. Brain Dev. 2009;31(9):641–648 [DOI] [PubMed] [Google Scholar]
- 36.Trotti R, Carratelli M, Barbieri M. Performance and clinical application of a new, fast method for the detection of hydroperoxides in serum. Panminerva Med. 2002;44(1):37–40 [PubMed] [Google Scholar]
- 37.Cavalleri AC, Colombo C, Venturelli E, et al. Evaluation of reactive oxygen metabolites in frozen serum samples. Effect of storage and repeated thawing. Int J Biol Markers. 2004;19(3):250–253 [DOI] [PubMed] [Google Scholar]
- 38.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76 [DOI] [PubMed] [Google Scholar]
- 39.Stiller R, von Mering R, König V, Huch A, Huch R. How well does reflectance pulse oximetry reflect intrapartum fetal acidosis? Am J Obstet Gynecol. 2002;186(6):1351–1357 [DOI] [PubMed] [Google Scholar]
- 40.Dohi K, Satoh K, Ohtaki H, et al. Elevated plasma levels of bilirubin in patients with neurotrauma reflect its pathophysiological role in free radical scavenging. In Vivo. 2005;19(5):855–860 [PubMed] [Google Scholar]
- 41.Celi P, Sullivan M, Evans D. The stability of the reactive oxygen metabolites (d-ROMs) and biological antioxidant potential (BAP) tests on stored horse blood. Vet J. 2010;183(2):217–218 [DOI] [PubMed] [Google Scholar]
- 42.Brown JV, Moe-Byrne T, Harden M, McGuire W. Lower versus higher oxygen concentration for delivery room stabilisation of preterm neonates: systematic review. PLoS ONE. 2012;7(12):e52033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dawson JA, Kamlin CO, Wong C, et al. Oxygen saturation and heart rate during delivery room resuscitation of infants <30 weeks’ gestation with air or 100% oxygen. Arch Dis Child Fetal Neonatal Ed. 2009;94(2):F87–F91 [DOI] [PubMed]
- 44.Goos TG, Rook D, van der Eijk AC, et al. Observing the resuscitation of very preterm infants: are we able to follow the oxygen saturation targets? Resuscitation. 2013;84(8):1108–1113 [DOI] [PubMed] [Google Scholar]
- 45.Gandhi B, Rich W, Finer N. Achieving targeted pulse oximetry values in preterm infants in the delivery room. J Pediatr. 2013;163(2):412–415 [DOI] [PubMed]
- 46.Higgins RD, Bancalari E, Willinger M, Raju TN. Executive summary of the workshop on oxygen in neonatal therapies: controversies and opportunities for research. Pediatrics. 2007;119(4):790–796 [DOI] [PubMed] [Google Scholar]
- 47.Lista G, Castoldi F, Cavigioli F, Bianchi S, Fontana P. Alveolar recruitment in the delivery room. J Matern Fetal Neonatal Med. 2012;25(suppl 1):39–40 [DOI] [PubMed]
- 48.Lista G, Fontana P, Castoldi F, Cavigioli F, Dani C. Does sustained lung inflation at birth improve outcome of preterm infants at risk for respiratory distress syndrome? Neonatology. 2011;99(1):45–50 [DOI] [PubMed] [Google Scholar]
- 49.Trembath A, Laughon MM. Predictors of bronchopulmonary dysplasia. Clin Perinatol. 2012;39(3):585–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coalson JJ, Winter V, deLemos RA. Decreased alveolarization in baboon survivors with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 1995;152(2):640–646 [DOI] [PubMed]
- 51.Warner BB, Stuart LA, Papes RA, Wispé JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol. 1998;275(1):L110–L117 [DOI] [PubMed]
- 52.Group TS-RMS. Supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP-ROP): a randomized, controlled trial. I: primary outcomes. Pediatrics. 2000;105(2):295–310 [DOI] [PubMed]
- 53.Saugstad OD, Rootwelt T, Aalen O. Resuscitation of asphyxiated newborn infants with room air or oxygen: an international controlled trial: the Resair 2 study. Pediatrics. 1998;102(1). Available at: www.pediatrics.org/cgi/content/full/102/1/e1 [DOI] [PubMed] [Google Scholar]
- 54.Harling AE, Beresford MW, Vince GS, Bates M, Yoxall CW. Does the use of 50% oxygen at birth in preterm infants reduce lung injury? Arch Dis Child Fetal Neonatal Ed. 2005;90(5):F401–F405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lundstrøm KE, Pryds O, Greisen G. Oxygen at birth and prolonged cerebral vasoconstriction in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1995;73(2):F81–F86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rich WD, Auten KJ, Gantz MG, et al. National Institute of Child Health and Human Development Neonatal Research Network . Antenatal consent in the SUPPORT trial: challenges, costs, and representative enrollment. Pediatrics. 2010;126(1). Available at: www.pediatrics.org/cgi/content/full/126/1/e215 [DOI] [PMC free article] [PubMed] [Google Scholar]