Abstract
The research work was performed to investigate the potential of Bacillus thuringiensis strain 199 to induce systemic resistance in tomato against Fusarium wilt. Roots of two-week-old seedlings of tomato plants were primed with bacterial strain. After 10 days of transplantation, some pots of tomato seedlings were provided with inoculum of Fusarium oxysporum lycopersici according to experimental design to induce disease. After 15 days of incubation period, plants challenged with F. oxysporum lycopersici alone were having obvious symptoms of Fusarium wilt. Plants that were treated with B. thuringiensis 199 + F. oxysporum lycopersici were having significant reduction of disease severity. Quantity of total phenolics increased 1.7-fold in bacterial-treated plants as compared to nontreated. Likewise, in case of defense-related enzymes, a significant increase of 1.3-, 1.8-, and 1.4-fold in polyphenol oxidase (PPO), phenyl ammonia lyase (PAL), and peroxidase (PO) was observed in comparison with untreated control. These results, hence, prove the potential of this bacterial strain for use as plant protection agent.
Keywords: Fusarium wilt, induce systemic resistance ISR, peroxidase PO, phenyl ammonia lyase PAL, poly phenol oxidase PPO, tomato, total phenolics
Introduction
Plants are sessile organisms unable to move to save them from stresses and pathogens. To compete with these harmful conditions or diseases, plants, therefore, have evolved different types of mechanisms to minimize harms of these stresses by rapid, dynamic, and complex alterations in their physiology. Resistance to plant disease is supposed to be a dynamic and multifactorial process. It is assumed that plant defense response can be activated by specific recognition of some microorganisms by the plant. There may be whole organisms or products secreted by microorganisms under the influence of which plants initiate defense response [1, 2]. Diverse plant response involves synthesis and accumulation of antimicrobial phytoalexins [3, 4], induction of hypersensitive response [5, 6], production of defense-related proteins [7], production of activated oxygen species [8, 9], and modification of plant cell wall by deposition of callose [10]. When plants are primed with any bioagent, these respond faster and/or more strongly with the activation of defense responses.
Colonization of plant roots by selected strains of nonpathogenic bacteria, such as various species of the general Pseudomonas [11–13], Bacillus [14], or Bradyrhizobium [15], can induce a distinct broad-spectrum resistance response in both below- and above-ground parts of the plant. This type of resistance to diseases is named as induced systemic resistance (ISR) [12, 13, 16]. Fusarium oxysporum is one of soil-borne plant pathogens and is widely distributed in various soil types worldwide [17]. Recently, there has been a growing interest in nonpathogenic bacteria due to their efficacy as biocontrol agents in many crops [18, 19]. Application of some Bacillus strains to seeds or seedlings has been found effective for suppressing soil borne diseases and has successfully induced systemic resistance in the treated plants [14, 20]. Various species of bacillus have been shown to exhibit ISR activity. These are including specific strains of the species, viz., Bacillus amyloliquefaciens, Bacillus subtilis, Bacillus pasteurii, Bacillus cereus, Bacillus pumilus, Bacillus mycoides, and Bacillus sphaericus, that are capable of eliciting significant reductions in the incidence or severity of various diseases on a diversity of hosts [21]. Elicitation of ISR by these strains has been demonstrated in greenhouse or field trials on tomato, bell pepper, muskmelon, watermelon, sugar beet, tobacco, Arabidopsis sp., cucumber, loblolly pine, and two tropical crops and pepper [21]. Protection resulting from ISR elicited by Bacillus spp. has been reported against leaf-spotting fungal and bacterial pathogens, systemic viruses, a crown-rotting fungal pathogen, root-knot nematodes, and a stem-blight fungal pathogen as well as damping-off, blue mold, and late blight diseases.
Methodology
Microbial isolate and plant source
Bacillus thuringiensis strain 199 was procured from bacterial conservatory of Institute of Agriculture Sciences, University of the Punjab, Lahore, Pakistan. This strain was isolated from wheat rhizospheric soil. Seeds of tomato were bought from market.
Experiment
Seeds were surface sterilized with 1.0% solution of sodium hypochlorite for 1–2 min and then thoroughly washed with distilled water. Seeds were sown in sterilized sandy loam soil for seedling development under lab conditions. When seedlings were of 2 weeks old, these were transplanted in plastic pots of 10 inch diameter containing sterilized sandy loamy soil and were subjected to further experiment. There were three replicates with five plants in each replicate. Experimental design was as follows:
Group 1: Supplied with distilled sterilized to serve as control;
Group 2: Challenged with pathogenic strain of F. oxysporum f. sp. lycopersici to serve as disease control;
Group 3: Provided with test bacterial treatment alone to serve as bioagent control;
Group 4: Provided with both bacterial and pathogenic fungi treatment.
Plants were kept under growth room conditions at temperature of 25 °C ± 2 °C under 18 h of light conditions. These were provided with distilled sterilized whenever needed.
Root priming of tomato seedlings with test bacterial genera
Test bacterial strain was cultured onto conical flasks containing 100 ml of Luria–Bertani (LB) broth media. These were kept in incubator at 35 °C for 24 h with agitation. After incubation, material was taken out from flask and centrifuged at 4000 rpm. Supernatant was discarded, and bacterial cells were collected from the pallet. Inoculum of bacterial cells was prepared in normal saline at concentration of 1000 bacterial cell per milliliter. Roots of two-week-old seedlings were primed with bacterial inoculum by keeping then in inoculum for 30 min. After this, these seedlings were transferred in pots prepared as discussed previously.
Inoculation with F. oxysporum f. sp. lycopersici
Culture of F. oxysporum f. sp. lycopersici causing wilt disease in tomato was obtained from First Fungal Culture Bank of Pakistan, Institute of Mycology and Plant Pathology, University of the Punjab, Pakistan. The pathogen inoculum was prepared by culturing the fungus on potato dextrose agar (PDA) medium for 1 week in petri plates. Microconidial suspension was prepared by pouring 30 ml of sterile distilled water in each petri plate. The concentration of micro conidia was adjusted to 1000 conidia per milliliter. This spore suspension was then challenged to allotted pots at rate of 50 ml in each pot.
Plant harvest and analysis
After 20 days of pathogen inoculation, harvest was taken. Beginning after 5 days of inoculation, external symptoms of Fusarium wilt (wilting and yellowing of leaves) were assayed. Disease severity was recorded by the formula given below [22]:
Disease severity = [(Portion of leaves with symptoms)/(The total number of leaves)]×100.
Some biochemical constituents and enzymes related to plant defense were also assayed.
Estimation of total phenolics
One gram shoot sample was extracted with 10 ml of 80% methanol at 70 °C for 15 min. Reaction mixture was containing 1 ml of methanolic extracts, 5 ml of distilled sterilized water, and 250 μl of Folin–Ciocalteau reagent (1 N). This solution was kept at 25 °C. The absorbance of the developed blue color was measured using a spectrophotometer at 725 nm. Gallic acid was used as the standard. The amount of phenolics was expressed as milligram gallic acid per gram plant material [23].
Estimation of defense-related enzymes
Leaf samples were taken at regular intervals from the plants for enzymes assays. One gram of leaf sample was homogenized with 2 ml of 0.1 M sodium phosphate buffer (pH 7.0) in ice bath for enzyme assays. The homogenates were then centrifuged at 10,000 g for 10 min. Supernatants were used to analyze the defense-related enzymes like peroxidase (PO), polyphenol oxidase (PPO), and phenylalanine ammonia lyase (PAL) activities.
Estimation of PPO activity
PPO activity was determined according to the method proposed by Mayer et al. [24]. The reaction mixture was containing 200 μl enzyme extract and 1.5 ml of 0.01 M catechol. Activity was expressed as changes in absorbance at 495 nm·min−1mg−1 protein.
Estimation of PAL activity
PAL activity was determined according to the method of Burrell and Rees [25]. The reaction mixture contained 0.03 M L-phenylalanine and 0.2 ml enzyme extract in a total 2.5 ml of sodium borate buffer (pH 8.8). This reaction mixture was kept in a water bath at 37 °C for 1 h, and 0.5 ml of 1 M (trichloroacetic acid) TCA was added. The amount of trans-cinnamic acid formed from L-phenylalanine was measured spectrophotometrically at 290 nm. Enzyme activity was expressed as microgram of trans-cinnamic acid h−1mg−1 protein.
Estimation of PO activity
The method of Fu and Huang [26] was used to estimate the peroxidase activity. For this purpose, 50 μl of enzyme extract was added to 2.85 ml of 0.1 M phosphate buffer (pH 7.0) and mixed with 0.05 ml of 20 mM guaiacol reagent. The reaction was started by the addition of 0.02 ml of 40 mM hydrogen peroxide to the mixture. Rate of increase in absorbance at 470 nm was measured over 1 min. One unit of enzyme activity was defined by the change in absorbance of 0.01 for 1 g fresh weight per minute.
Statistical analysis
The results represented are mean values of two independent experiments. Data were statistically analyzed by ANOVA and Duncan multiple range test at significance level of p = 0.05 [27].
Results
Disease incidence and disease severity
A microbial strain “Bacillus thuringiensis strain 199” was tested for its capability to manage Fusarium wilt of tomato under pot conditions. For that purpose, tomato plants were cocultivated with pathogen and bacterial strain in different combinations. After incubation, data regarding disease severity were analyzed. A significant reduction of 65.1% was noticed in disease severity in plants treated with bacterial strain as compared to pathogen control (Table 1). Thus, these results provide us evidence that this bacteria strain is helpful for control of Fusarium wilt of tomato.
Table 1.
Efficacy of root priming of B. thuringiensis 199 for control of Fusarium wilts of tomato under growth house conditions
| Treatments | Disease severity |
|---|---|
|
B. thuringiensis + F. oxysporum Lycopersici |
29 ± 2.3A |
| B. thuringiensis | – |
| Pathogen control (F. oxysporum Lycopersici) |
83 ± 5.1B |
| Untreated control | – |
| Values are the mean of three replicates. Capital letters represent significance level at p < 0.05 by DNMRT test. (–) : not determined | |
Change in total phenolic compounds
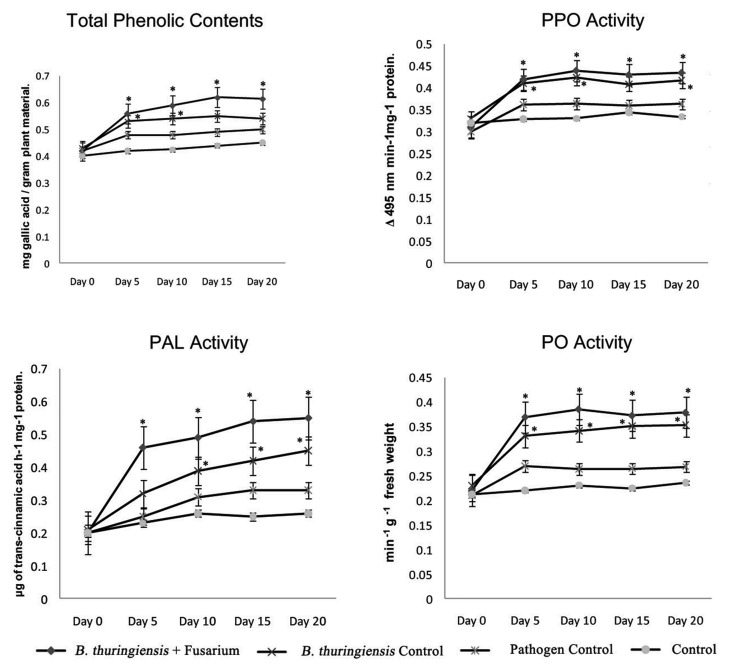
Induction of defense-related biochemicals like total phenolics and some pathogenesis-related enzymes was studied in bacterial- and pathogen-treated plants under different combinations. Presence of our bacterial strains induced significantly higher productions of defense-related biochemicals as governed by ANOVA (p < 0.05). Slight change was observed in pathogenic control whereas no significant change was observed in total phenolic contents of untreated control (Fig. 1). A rapid increase was observed in total phenolics of plants treated with B. thuringiensis up to day 15. A significant increase of 1.7-fold was noted in total phenolic contents of the leaves of tomato plants at day 15 treated and cocultivated with bacterial strain and pathogen.
Fig. 1.
A: Tomato plants in growth room. B: Bacterial-treated plants showing less disease severity as compared to pathogen alone. F.O. = F. oxysporum lycopersici, B.T. = B. thuringiensis strain 199
Estimation of defense enzymes
Our study also revealed the significantly higher activity of defense-related proteins against Fusarium wilt pathogen. PO, PPO, and PAL activities were measured in leaves from pathogen-inoculated and bacterial-treated tomato plants. Bacterial strains individually and in a mixture of pathogen stimulate PO, PPO, and PAL in tomato plants. These activities were found to increase rapidly in initial days after inoculation (Fig. 2).
Fig. 2.
Changes in total phenolic contents and Polyphenol oxidase (PPO), Phenylalanine ammonia lyase (PAL) and Peroxidase (PO) activity in tomato plants of different treatments. Bars represent standard error. (*) represents values significantly different at p < 0.05 as governed by ANOVA
There was a significant increase in PPO contents in plants treated with B. thuringiensis. An increase of 1.3-fold was observed at day 5 after inoculum in quantity of PPO as compared to plants that were not treated with bacteria strain (Fig. 2). Plant treated with pathogen alone showed a nonsignificant increase in PPO contents at all. Likewise, phenolics and PPO contents also showed rapid increase in initial days. This trend was also observed in case of PAL and PO activity (Fig. 2). Increase in PAL contents was also significant in bacterial-treated plant that exhibited a maximum increase of 1.8-fold at day 15 (Fig. 2). In the same way, a positive increase was noted in PO activity also. There was an increase of nearly 1.4-fold in PO activity in bacterial-treated plants at day 10.
Discussion
Tomato crop is affected by a number of diseases and disorders under field conditions. Fusarium wilt of tomato caused by F. oxysporum is the most destructive disease in the field [28]. Protection of plants from disease by induction of systemic resistance is a new approach. This is much less harmful to the environment as compared to deadly agrochemicals applied to control plant diseases. Numerous studies have been carried out by various scientists to prove biocontrol effect of bacillus strains on different crop plants. In a study carried out by Yan [29], induced systemic resistance was observed in tomato against late blight, caused by Phytophthora infestans (Mont.) with B. pumilus strain SE34n that was incorporated into the potting medium. In another study, Bacillus megaterium strain 4 was found to be effective to control Fusarium wilt of tomato [30]. B. thuringiensis was used to induce systemic resistance in mung bean plants against root colonizing phytopathogenic fungi [31]. This work was a step further for screening of B. thuringiensis strains capable of inducing systemic response. In our present study, results represented a significant reduction in disease severity of tomato seedlings treated with B. thuringiensis strain 199. Phenolics are the compounds whose quantity is raised when a plant comes under attack by a pathogen [32, 33]. Systemic induction of phenolic compounds under influence of bacterial strains was first reported by Van Peer et al. [34]. However, this alone is not reliable for indication of disease resistance in plant tissues [33]. In this study, a significant increase in total phenolic contents was observed in bacterial-treated plants. Pathogen alone was able to induce phenolic formation in plants but with slightly increased levels.
Pathogenicity-related proteins are usually quantified to assess the activation of defense system of plants. Plants treated with bacterial strain also showed an increase in PPO activity. PPO is a copper containing enzyme, which is responsible for oxidization of phenolics to highly toxic quinines. This enzyme is also involved in terminal oxidation of diseased plant tissue, and this role of this enzyme is attributed in disease resistance [35].
Increase in PAL and PO activity may be due to the hypersensitive reaction that is often observed in many plants when these undercome an attack of insects pests. PAL is the main enzyme in phenyl propanoid pathway and flavonoid pathway [36]. Peroxidase enzyme is related with more than one function in plants. It is responsible for the condensation of phenolics into lignin [37]. This is of immense importance in hypersensitive response in plants. Peroxidase is an integral part of PR-9 family and is responsible for lignin formation in plant defense response. Its increase is also observed in host pathogen interaction [38]. In current research work, reduction in disease severity was attributed to increased levels of PAL and PO enzymes. This detection of increased activity of PAL and PO enzymes in bacterial-treated plants proves that this difference associated with induced resistance is quantitative instead of being qualitative. A similar increase in quantity of both of these two defense-related enzymes was observed in cucumber against colletotrichum fungi when systemic resistance was induced in it by an external agency [39].
F. oxysporum is soil-borne in nature. This can spread through irrigation water. This fungus invades vascular system of a plant internally. It is better to protect the entrance point of this fungus in plant instead of changing the entire soil mycoflora. For this purpose, some microorganisms can be used to induce resistance in plants for combating with this devastating pathogen. Host plant can also provide these microorganisms with food necessary for their nourishment. Many researchers have reported the efficacy of bacterial strains for this purpose. All these studies provide clue that these bacteria have positive influence on quantities of defense-related biochemicals in host plants. From this investigation, it is clear that B. thuringiensis strain 199 can protect plant from Fusarium wilt. The only need is to develop its inoculum for application at field level commercially.
Contributor Information
Waheed Akram, 1Institute of Agricultural Sciences, University of the Punjab, Lahore, Pakistan.
Asrar Mahboob, 2Maize and Millets Research Institute, Yousafwala, Sahiwal, Pakistan.
Asmat Ali Javed, 3Agronomic Research Station, Farooq Abad, Pakistan.
References
- 1.Albersheim P, Valent BS. Host-pathogen interactions in plants. Plants, when exposed to oligosaccharides of fungal origin, defend themselves by accumulating antibiotics. J Cell Biol. 1978 Sep;78(3):627–643. doi: 10.1083/jcb.78.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albersheim P, Anderson-Prouty AJ. Carbohydrates, proteins, cell surfaces, and the biochemistry of pathogenesis. Annu Rev Plant Physiol. 1975;26:31–52. [Google Scholar]
- 3.Ebel J. Phytoalexin synthesis: the biochemical analysis of the induction process. Annu Rev Phytopathol. 1986;24:235–264. [Google Scholar]
- 4.Hammond-Kosack KE, Jones JD. Resistance gene-dependent plant defense responses. Plant Cell. 1996 Oct;8(10):1773–1791. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer DW, Wei ZM, Beer SV, Collmer A. Erwinia chrysanthemi harpinEch: an elicitor of the hypersensitive response that contributes to soft-rot pathogenesis. Mol Plant Microbe Interact. 1995 Jul-Aug;8(4):484–491. doi: 10.1094/mpmi-8-0484. [DOI] [PubMed] [Google Scholar]
- 6.He SY, Huang HC, Collmer A. Pseudomonas syringae pv. syringae harpinPss: a protein that is secreted via the Hrp pathway and elicits the hypersensitive response in plants. Cell. 1993 Jul 2;73(7):1255–1266. doi: 10.1016/0092-8674(93)90354-s. [DOI] [PubMed] [Google Scholar]
- 7.Yu LM. Elicitins from Phytophthora and basic resistance in tobacco. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4088–4094. doi: 10.1073/pnas.92.10.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker CJ, Orlandi EW, Mock NM. Harpin, An Elicitor of the Hypersensitive Response in Tobacco Caused by Erwinia amylovora, Elicits Active Oxygen Production in Suspension Cells. Plant Physiol. 1993 Aug;102(4):1341–1344. doi: 10.1104/pp.102.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fellbrich G, Romanski A, Varet A, Blume B, Brunner F, Engelhardt S, Felix G, Kemmerling B, Krzymowska M, Nürnberger T. NPP1, a Phytophthora-associated trigger of plant defense in parsley and Arabidopsis. Plant J. 2002 Nov;32(3):375–390. doi: 10.1046/j.1365-313x.2002.01454.x. [DOI] [PubMed] [Google Scholar]
- 10.Veit S, Wörle JM, Nürnberger T, Koch W, Seitz HU. A novel protein elicitor (PaNie) from Pythium aphanidermatum induces multiple defense responses in carrot, Arabidopsis, and tobacco. Plant Physiol. 2001 Nov;127(3):832–841. [PMC free article] [PubMed] [Google Scholar]
- 11.Ahn IP, Lee SW, Suh SC. Rhizobacteria-induced priming in Arabidopsis is dependent on ethylene, jasmonic acid, and NPR1. Mol Plant Microbe Interact. 2007 Jul;20(7):759–768. doi: 10.1094/MPMI-20-7-0759. [DOI] [PubMed] [Google Scholar]
- 12.van Loon LC, Bakker PA, Pieterse CM. Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol. 1998;36:453–483. doi: 10.1146/annurev.phyto.36.1.453. [DOI] [PubMed] [Google Scholar]
- 13.Van Loon LC. Plant responses to plant growth-promoting rhizobacteria. Eur J Plant Pathol. 2007;119:243–254. [Google Scholar]
- 14.Kloepper JW, Ryu CM, Zhang S. Induced Systemic Resistance and Promotion of Plant Growth by Bacillus spp. Phytopathology. 2004 Nov;94(11):1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259. [DOI] [PubMed] [Google Scholar]
- 15.Cartieaux F, Contesto C, Gallou A, Desbrosses G, Kopka J, Taconnat L, Renou JP, Touraine B. Simultaneous interaction of Arabidopsis thaliana with Bradyrhizobium Sp. strain ORS278 and Pseudomonas syringae pv. tomato DC3000 leads to complex transcriptome changes. Mol Plant Microbe Interact. 2008 Feb;21(2):244–259. doi: 10.1094/MPMI-21-2-0244. [DOI] [PubMed] [Google Scholar]
- 16.De Vleesschauwer D, Hofte M. Rhizobacteria-induced systemic resistance. Adv Bot Res. 2009;51:223–281. [Google Scholar]
- 17.Fravel D, Olivain C, Alabouvette C. Fusarium oxysporum and its biocontrol. New Phytol. 2003;157:493–502. doi: 10.1046/j.1469-8137.2003.00700.x. [DOI] [PubMed] [Google Scholar]
- 18.Kloepper JW, Rodriguez-Kabana R, Zehnder GW, Murphy J, Sikora E, Fernandez, C. Plant root-bacterial interactions in biological control of soilborne diseases and potential extension to systemic and foliar diseases. Australasian Plant Pathol. 1999;28:27–33. [Google Scholar]
- 19.Nagórska K, Bikowski M, Obuchowski M. Multicellular behaviour and production of a wide variety of toxic substances support usage of Bacillus subtilis as a powerful biocontrol agent. Acta Biochim Pol. 2007;54(3):495–508. [PubMed] [Google Scholar]
- 20.Szczech M, Shoda M. The effect of mode of application of Bacillus subtilis RB14-C on its efficacy as a biocontrol agent against Rhizoctonia solani. J Phytopathol. 2006;154:370–377. [Google Scholar]
- 21.Kloepper JW, Ryu CM, Zhang S. Induced Systemic Resistance and Promotion of Plant Growth by Bacillus spp. Phytopathology. 2004 Nov;94(11):1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259. [DOI] [PubMed] [Google Scholar]
- 22.Anitha A, Rabeeth M. Control of Fusarium wilt of tomato by bioformulation of streptomyces griseus in green house condition. African J Basic Appl Sci. 2009;1:9–14. [Google Scholar]
- 23.Zieslin N, Ben-Zaken R. Peroxidase activity and presence of phenolic substances in peduncles of rose flowers. Plant Physiol Biochem. 1993;31:333–339. [Google Scholar]
- 24.Mayer AM, Harel E, Shaul RB. Assay of catechol oxidase a critical comparison of methods. Phytochem. 1965;5:783–789. [Google Scholar]
- 25.Burrell MM, Rees TA. Metabolism of phenylalanine and tyrosine in rice leaves infected by Pyricularia oryzae. Physiol Plant Pathol. 1974;4:497–508. [Google Scholar]
- 26.Fu J, Huang B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ Exp Bot. 2001 Apr;45(2):105–114. doi: 10.1016/s0098-8472(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 27.Steel RGD, Torrie JH. Principles and procedures of statistics. New York: McGraw Publications; 1960. [Google Scholar]
- 28.Jones JB, Jones JP, Stall RE, Zitter TA. Compendium of Tomato Diseases. America: American Phytopathological Society; 1991. [Google Scholar]
- 29.Yan Z, Reddy MS, Kloepper JW. Survival and colonization of rhizobacteria in a tomato transplant system. Can J Microbiol. 2003 Jun;49(6):383–389. doi: 10.1139/w03-051. [DOI] [PubMed] [Google Scholar]
- 30.Terhardt J. Beeinflussung mikrobieller Gemeinschaften der Rhizosphäre nach Blattbehandlung von Pflanzen und biologische Kontrolle von Fusarium oxysporum f. sp. lycopersici und Meloidogyne incognita mit bakteriellen Anatgonisten. Bonn, Germany: Universität Bonn; 1998. PhD theses. [Google Scholar]
- 31.Sheikh LI, Dawar S, Zaki MJ, Ghaffar A. Efficacy of Bacillus thuringiensis and Rhizobium meliloti with nursery fertilizers in the control of root infecting fungi on mung bean and okra plants. Pak J Bot. 2006;38:465–473. [Google Scholar]
- 32.Van Peer R, Niemann GN, Schippers B. Induced resistance and phytoalexin accumulation in biological control of Fusarium wilt in carnation by Pseudomonas sp. strain WCS417r. Phytopathology. 1991;81:728–734. [Google Scholar]
- 33.Waterman PG, Mole S. Analysis of Phenolic Plant Metabolites. London: Blackwell Sci. Publ.; 1994. Method in Ecology. [Google Scholar]
- 34.Van Peer R, Niemann GN, Schippers B. Induced resistance and phytoalexin accumulation in biological control of Fusarium wilt in carnation by Pseudomonas sp. strain WCS417r. Phytopathology. 1991;81:728–734. [Google Scholar]
- 35.Kosuge T. The role of phenolics in host response to infection to infection. Annu Rev Phytopathology. 1969;7:195–222. [Google Scholar]
- 36.Daayf F, Bell-Rhlid R, Belanger RP. Methyl ester of p-coumaric acid; A phytoalexin-like compound from long English cucumber leaves. J Chem Ecol. 1997;23:1517–1526. [Google Scholar]
- 37.Graham MY, Graham TL. Rapid Accumulation of Anionic Peroxidases and Phenolic Polymers in Soybean Cotyledon Tissues following Treatment with Phytophthora megasperma f. sp. Glycinea Wall Glucan. Plant Physiol. 1991 Dec;97(4):1445–1455. doi: 10.1104/pp.97.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott-Carig JS, Kerby KB, Stein BD, Somerville SC. Expression of an extracellular peroxidase that is induced in barley (Hardium vulgar) by powdery mildew pathogen (Erysiphe graminis f. sp. Hordie) Physol Mol Plant Pathol. 1995;47:407–418. [Google Scholar]
- 39.Dalisay RF, Kuc JA. Persistance of reduced penetration by Colletotrichum lagenarium into cucumber leaves with induced systemic re-sistance and its relation ot enhanced peroxidase and chitinase activity. Physiol Mol Plant Pathol. 1995;47:329–338. [Google Scholar]