Abstract
Background and Aims
The role of flower specialization in plant speciation and evolution remains controversial. In this study the evolution of flower traits restricting access to pollinators was analysed in the bifid toadflaxes (Linaria sect. Versicolores), a monophyletic group of ∼30 species and subspecies with highly specialized corollas.
Methods
A time-calibrated phylogeny based on both nuclear and plastid DNA sequences was obtained using a coalescent-based method, and flower morphology was characterized by means of morphometric analyses. Directional trends in flower shape evolution and trait-dependent diversification rates were jointly analysed using recently developed methods, and morphological shifts were reconstructed along the phylogeny. Pollinator surveys were conducted for a representative sample of species.
Key Results
A restrictive character state (narrow corolla tube) was reconstructed in the most recent common ancestor of Linaria sect. Versicolores. After its early loss in the most species-rich clade, this character state has been convergently reacquired in multiple lineages of this clade in recent times, yet it seems to have exerted a negative influence on diversification rates. Comparative analyses and pollinator surveys suggest that the narrow- and broad-tubed flowers are evolutionary optima representing divergent strategies of pollen placement on nectar-feeding insects.
Conclusions
The results confirm that different forms of floral specialization can lead to dissimilar evolutionary success in terms of diversification. It is additionally suggested that opposing individual-level and species-level selection pressures may have driven the evolution of pollinator-restrictive traits in bifid toadflaxes.
Keywords: Convergence, flower specialization, trait-dependent diversification, species selection, pollination, speciation, reversal, nectar spur, flower tube, toadflax, Linaria sect. Versicolores
INTRODUCTION
Variation of flower morphological traits has long been considered to drive evolution and diversification of angiosperms (Darwin, 1862, 1877; Grant, 1949; Stebbins, 1970; Kay and Sargent, 2009; van der Niet and Johnson, 2012). Adaptation to different pollinator vectors (particularly animal pollinators) has been hypothesized to be a major force shaping flower morphology. This notion gave rise to the concept of pollination syndromes, i.e. sets of flower traits (shape, colour, nectar, scent) that have convergently evolved in distant plant lineages as an adaptation to particular pollinators (bees, birds, moths, etc.) (Faegri and van der Pijl, 1979; Fenster et al., 2004). However, the concept of pollination syndromes, which relies on flower specialization, has been challenged in recent times (Waser et al., 1996; Ollerton et al., 2009). Specialization may still play a relevant role in plant speciation (Kay and Sargent, 2009), but syndrome shifts may not account for the majority of speciation events (e.g. Valente et al., 2012). Therefore, rather than focusing on the evolution of syndromes, the investigation of particular traits, including their evolutionary trends, shifts and correlations, is probably more fruitful for understanding flower evolution in most plant lineages (Smith, 2010).
Traits that restrict the access of pollinators to flower rewards (nectar, pollen) are of exceptional interest because these physical barriers may have evolved as a specialization to particular pollinators. Variations in length and width of flower tubes and nectar spurs have been the subject of several studies (Herrera, 1990; Johnson and Steiner, 1997; Alexandersson and Johnson, 2002; Pérez et al., 2004; Whittall and Hodges, 2007; Tripp and Manos, 2008). An extreme case of restriction of pollinator access is the personate corolla of snapdragons (Antirrhinum) and some relatives of the tribe Antirrhineae and the order Lamiales (Sutton, 1988; Endress, 1994; Kampny, 1995). These species display zygomorphic, gamopetalous, bilabiate corollas in which the lower lip is conspicuously arched upwards, constituting a palate. This structure closes access to pollen and nectar rewards, therefore making the mechanical opening of the corolla necessary for insect pollination. The personate corolla has long been considered as an adaptation to bee pollination (mellitophily), as insects other than bees would not be strong or heavy enough to open it (Hill, 1909; Müller, 1929; Sutton, 1988; Endress, 1992; Vargas et al., 2010).
The relationships between changes in restrictive flower traits and diversification (speciation minus extinction) rates remain poorly understood. Nectar spurs have been hypothesized to represent a key innovation that promotes species diversification by providing a mechanism of pre-zygotic reproductive isolation through differential pollinator visitation (Hodges and Arnold, 1995; Hodges, 1997; but see Hagen and Kadereit, 2003; Cacho et al., 2010). On the other hand, it has been historically argued that ecological specialists usually evolve from generalists, and that specialization constitutes an evolutionary dead end, i.e. a derived state from which both reversal to a generalist state and shift to a different specialized state would be unlikely (Futuyma and Moreno, 1988). There are, however, many examples that contradict this idea (Gómez and Zamora, 2006). In particular, such a view has been challenged by phylogeny-based analyses of flower evolution (Armbruster and Baldwin, 1998; Tripp and Manos, 2008; Fleming et al., 2009). Simultaneous estimations of rates of character change and state-dependent speciation/extinction rates across phylogenetic trees are crucial for a correct understanding of character evolution (Maddison, 2006; Goldberg and Igić, 2008). Recently developed methods (Maddison et al., 2007; FitzJohn et al., 2009; FitzJohn, 2010) enable such estimations and hold great promise for understanding flower evolution (Smith, 2010), yet they have rarely been applied in this context (but see Armbruster et al., 2009; Smith et al., 2010; Valente et al., 2012).
Toadflaxes (Linaria) constitute the most species-rich (∼150 species) genus of the snapdragon lineage (tribe Antirrhineae, Plantaginaceae) (Sutton, 1988). Linaria pollination has historically attracted the interest of botanists and evolutionary biologists (Sprengel, 1793; Darwin, 1876). Toadflaxes constitute a natural group (Vargas et al., 2004; Fernández-Mazuecos et al., 2013) and display a remarkably diverse array of flower traits whose evolution has not, however, been analysed in a phylogenetic framework to date. Several traits of Linaria flowers are potentially linked to pollinator specialization: they have a zygomorphic, bilabiate, usually personate corolla in which a spur of variable length is formed at the base of the lower lip (Fig. 1). The spur contains nectar dripping down from a nectary located at the base of the ovary (Valdés, 1970). The two pairs of anthers are placed at slightly different heights, with the stigma in the space between. While most species have well-developed palates that close access to the corolla throat, in some species belonging to sections Versicolores, Macrocentrum and Lectoplectron the palate is poorly developed, and access to the corolla throat is wide open (e.g. Fig. 1Q). This seems to be usually related to a narrowing of the corolla tube and a broadening of the lower lip (Viano, 1969; Sutton, 1980). Such morphology has been suggested to be related to pollination by long-tongued lepidopterans and dipterans (Hill, 1909; Sutton, 1980, 1988), while the typical personate corolla would be linked to bee pollination (Hill, 1909; Arnold, 1982; Sánchez-Lafuente, 2007; Carrió et al., 2012).
Fig. 1.
Representatives of Linaria sect. Versicolores. Subsect. Versicolores: (A) L. algarviana; (B) L. bipartita; (C) L. clementei; (D) L. gharbensis; (E) L. incarnata; (F) L. maroccana; (G) L. multicaulis subsp. heterophylla; (H) L. onubensis; (I) L. pedunculata; (J) L. salzmannii; (K) L. spartea; (L) L. tenuis; (M) L. viscosa subsp. spicata; (N) L. viscosa subsp. viscosa; (O) L. weilleri. Subsect. Elegantes: (P) L. elegans; (Q) L. nigricans. Floral morphological types (see Fig. 4): Type I (broad tube, variable spur: A, D, F, G, I–O); Type II (broad tube, very short spur: C); Type III (narrow tube, variable spur: B, E, H, P, Q). Photographs by A. Fernández-Mazuecos (A, E), J. Quiles (B, F, K, N, O), J. Ramírez (C, I, J, M), J.L. Blanco-Pastor (D), E. Rico (G), P. Vargas (H, P, Q) and O. Fragman-Sapir (L).
Here we analysed the evolution of flower morphology in a clade of Linaria (sect. Versicolores) that displays remarkable flower diversity. In particular, we used phylogenetic and comparative methods to achieve the following objectives: (1) to get a deeper insight into the phylogenetic relationships within this lineage; (2) to evaluate intra- and inter-specific morphological variation of traits limiting pollinator access to nectar reward; (3) to analyse whether restrictive traits have exerted an effect on diversification rates; and (4) to reconstruct the evolutionary history of flower morphology and to investigate its potential links to pollinators.
MATERIALS AND METHODS
Study group and taxonomic treatment
Linaria sect. Versicolores (bifid toadflaxes) is an assemblage of ∼25 species mainly distributed in the western Mediterranean region (Sutton, 1988) (see examples in Fig. 1). According to phylogenetic analyses based on both nuclear and plastid DNA markers (Fernández-Mazuecos and Vargas, 2011; Fernández-Mazuecos et al., 2013) bifid toadflaxes constitute a monophyletic group within Linaria, formed by two well-supported sister groups: subsect. Elegantes (two species) and subsect. Versicolores (∼23 species). All species are diploid (2n = 12) except for the tetraploid L. hellenica (reviewed by Sutton, 1988). Most species seem to be allogamous (Bruun, 1937; Valdés, 1970; Docherty, 1982; M. Fernández-Mazuecos, unpubl. res.). Section Versicolores is an ideal system for the evolutionary analysis of several flower traits that restrict the access of pollinators to nectar reward: spur length, tube width and palate development. Although morphological affinities among species have not been analysed in detail, some divergent traits have been described. At least the two species of subsect. Elegantes (L. elegans and L. nigricans) display a widely open corolla mouth and a narrow tube (Fig. 1P, Q), while species of subsect. Versicolores usually exhibit typical personate (closed) corollas with a wider tube (e.g. Fig. 1A, K). Some authors, however, have suggested that flowers of certain species of subsect. Versicolores (L. incarnata, L. bipartita; Fig. 1B, E) resemble those of subsect. Elegantes regarding their narrow tubes and broad lower lips (Viano, 1969; Sutton, 1988). In addition, sect. Versicolores exhibits a wide variation in spur length, including some of the shortest (L. clementei; Fig. 1C) and longest (L. elegans; Fig. 1P) spurs in the genus (Sutton, 1988; Sáez and Bernal, 2009). These traits seem to be associated with contrasting species diversities: only a few species display narrow tubes, and spurs as short as those of L. clementei seem to be rare. Nevertheless, inter- and intra-specific morphological variability has not been quantitatively assessed to date.
The potential effects of alpha taxonomy on diversification rate analyses have been pointed out by some authors (Marazzi and Sanderson, 2010; Valente et al., 2010b). Indeed, correct species delimitation is crucial in obtaining accurate estimates of speciation and extinction rates. Therefore, we first conducted a review of the taxonomic literature (Viano, 1978a, b; Sutton, 1988; Dobignard, 1997; Fennane and Ibn Tattou, 1998; De Leonardis et al., 1999; Tan and Iatrou, 2001; De Leonardis et al., 2003; Gómiz, 2004; Hamdi et al., 2009; Sáez and Bernal, 2009) and a survey of herbarium specimens mainly from two herbaria with a broad representation of Linaria sect. Versicolores specimens from Iberia (MA) and northern Africa (RNG) (see Supplementary Data Appendix S1). Although the first modern synthesis of the group is due to Viano (1978a, b), we generally adopted the more inclusive taxonomic treatment of Sutton (1988), except for some modifications detailed in Supplementary Data Appendix S2. In the end, we accepted 30 taxa (including species and subspecies; Table 1) that are morphologically and geographically cohesive.
Table 1.
Taxonomic treatment followed in this paper, taxon distributions, individuals sampled for phylogenetic and morphometric analyses and flower morphological types
| Taxon | Distribution | No. individuals sampled for phylogenetic analyses | No. individuals sampled for spur and tube measures | No. individuals sampled for geometric morphometric analysis | Morphological type |
|---|---|---|---|---|---|
| Linaria Mill. | |||||
| Linaria sect. Versicolores (Benth.) Wettst. | |||||
| Subsect. Versicolores | |||||
| L. algarviana Chav. | SW Portugal (Algarve) | 1 | 7 | 24 | I |
| L. bipartita (Vent.) Willd. | W Morocco | 2 | 46 | — | III |
| L. bordiana Santa & Simonneau | |||||
| subsp. bordiana | Algeria | 1 | 8 | — | III |
| subsp. kralikiana (Maire) D. A. Sutton | NW Algeria | 2 | 4 | — | I |
| L. clementei Haens. | S Spain (Málaga) | 2 | 22 | 21 | II |
| L. dissita Pomel | NW Africa | — | 6 | — | I |
| L. gattefossei Maire & Weiller | C Morocco | 1 | 6 | — | I |
| L. gharbensis Batt. & Pit. | NW Africa, SW Spain | 2 | 29 | 23 | I |
| L. hellenica Turrill | S Greece | 1 | 2 | — | I |
| L. imzica Gómiz | S Morocco (Anti Atlas) | 1 | 9 | — | I |
| L. incarnata (Vent.) Spreng. | W Iberian Peninsula | 2 | 36 | 48 | III |
| L. mamorensis Mazuecos, Vigalondo & L. Sáez | NW Morocco | 2 | 34 | — | III |
| L. maroccana Hook.f. | Morocco (mainly High Atlas) | 2 | 31 | — | I |
| L. multicaulis (L.) Mill. | |||||
| subsp. multicaulis | Sicily, S Italy (Calabria) | 1 | 4 | — | I |
| subsp. aurasiaca (Pomel) D.A.Sutton | Tunisia, NE Algeria | 1 | 3 | — | I |
| subsp. galioides (Ball) D. A. Sutton | Morocco (High Atlas) | 2 | 31 | — | I |
| subsp. heterophylla (Desf.) D. A. Sutton | NW Africa | 2 | 46 | — | I |
| L. onubensis Pau | SW Spain (Huelva) | 2 | 15 | 45 | III |
| L. pedunculata (L.) Chaz. | S Iberian Peninsula, NW Africa, Balearic Islands | 2 | 39 | 27 | I |
| L. pinifolia (Poir.) Thell. | Tunisia, Algeria | 1 | 5 | — | I |
| L. pseudoviscosa Murb. | Tunisia | 1 | 7 | — | I |
| L. salzmannii Boiss. | S Spain (Málaga) | 1 | 5 | 20 | I |
| L. spartea (L.) Chaz. | Iberian Peninsula, S France | 2 | 87 | 25 | I |
| L. tenuis (Viv.) Spreng. | N Africa, Middle East | 2 | 3 | — | I |
| L. tingitana Boiss. & Reuter | NW Africa | 1 | 12 | — | I |
| L. viscosa (L.) Chaz. | |||||
| subsp. viscosa | S Iberian Peninsula | 2 | 60 | 45 | I |
| subsp. spicata (Kunze) D. A. Sutton | SE Iberian Peninsula | 1 | 45 | 24 | I |
| L. weilleri Emb. & Maire | S Morocco (Anti Atlas) | 1 | 4 | — | I |
| Subsect. Elegantes (Viano) D. A. Sutton | |||||
| L. elegans Cav. | NW Iberian Peninsula | 2 | 58 | 24 | III |
| L. nigricans Lange | SE Spain (Almería) | 2 | 32 | 43 | III |
Phylogenetic relationships and divergence times
Sampling strategy and DNA sequencing. We sampled a total of 45 specimens of Linaria sect. Versicolores, including representatives of 29 of the 30 recognized species and subspecies (one or two specimens per taxon; Table 1; Supplementary Data Table S1). To minimize the impact of recent hybridization, we selected unambiguously identified individuals, and some with intermediate traits or uncertain identification were discarded. We only failed to sample L. dissita, which is a poorly known northern African taxon (Sutton, 1988; Fennane and Ibn Tattou, 1998). We also sampled nine additional species representing six other sections of Linaria, one species of Antirrhinum and one of Chaenorhinum to be used as the outgroup based on previous phylogenetic evidence (Vargas et al., 2004; Fernández-Mazuecos et al., 2013). Plant material was collected in the field and dried in silica gel or obtained from herbarium collections (RNG, MA, ATH, UPOS; Supplementary Data Table S1).
For phylogenetic analyses, we selected one nuclear (ITS) and three plastid (rpl32-trnLUAG, trnK-matK and trnS-trnG) DNA regions employed in our previous phylogenetic and phylogeographic analyses of the genus Linaria (Fernández-Mazuecos and Vargas, 2011; Blanco-Pastor et al., 2012, 2013; Fernández-Mazuecos et al., 2013; Fernández-Mazuecos and Vargas, 2013). One hundred and eighty-one sequences were taken from our previous studies, while the remaining 43 were newly generated. Procedures used for DNA extraction, amplification and sequencing of DNA regions followed Fernández-Mazuecos and Vargas (2011) and Fernández-Mazuecos et al. (2013). Sequences of each DNA region were separately aligned using MAFFT 6 (Katoh et al., 2002) with default parameters, and further adjustments were made by visual inspection. The three ptDNA regions were concatenated in a single matrix after congruence was confirmed in preliminary phylogenetic analyses. All new sequences have been deposited in the GenBank database (see Supplementary Data Table S1 for accession numbers).
Gene tree estimation and dating. Separate phylogenetic analyses were conducted on the ITS and ptDNA matrices using three methods: Bayesian inference (BI; implemented in MrBayes v3·1·2; Ronquist and Huelsenbeck, 2003); maximum likelihood (ML; implemented in RaxML; Stamatakis, 2006); and maximum parsimony (MP; implemented in TNT 1·1; Goloboff et al., 2003) (see Supplementary Data Appendix S2 for details). Based on previous phylogenetic evidence (Vargas et al., 2004), Chaenorhinum was used as the outgroup sequence in all analyses.
In order to obtain time-calibrated gene trees, separate ITS and ptDNA matrices including a single individual per species were analysed through the relaxed molecular clock approach implemented in BEAST 1·6·2 (Drummond et al., 2006; Drummond and Rambaut, 2007). Following previous dating analyses of Linaria sect. Versicolores (Fernández-Mazuecos and Vargas, 2011, 2013), the root node (divergence between Chaenorhinum and Linaria) was calibrated using a normal distribution with mean 23 million years ago (Ma) and standard deviation 4 million years (Myr). This was based on a dating analysis of ndhF sequences of the tribe Antirrhineae (P. Vargas et al., in prep.), which in turn incorporates a calibration of 74 Ma for the divergence time between Oleaceae and Antirrhineae (Bell et al., 2010), and minimum stem-age constraints for Lamiales families and tribes based on five fossils (see Fernández-Mazuecos and Vargas, 2011 for details). Models of nucleotide substitution were selected for each DNA region under the Akaike information criterion (AIC) in jModelTest 0·1 (Posada, 2008). A birth–death process (Gernhard, 2008) was employed as tree prior. The substitution rate variation was modelled using an uncorrelated lognormal distribution. Based on previous estimates for herbaceous plants, uniform prior distributions were set for the substitution rates, with a range of 5 × 10−4 to 5 × 10−2 substitutions per site per Myr for ITS and 1 × 10−4 to 1 × 10−2 substitutions per site per Myr for ptDNA (see Blanco-Pastor et al., 2012 for details). For each dataset, four Markov chain Monte Carlo (MCMC) analyses with 10 million generations each and a sample frequency of 1000 were run through the CIPRES Science Gateway (Miller et al., 2010). Parameter analysis in Tracer 1·5 (Rambaut and Drummond, 2007) showed adequate chain length, with effective sample sizes above 1000. Chains were combined using LogCombiner 1·6·2 after discarding the first 10 % of sampled generations as burn-in. Trees were summarized in a maximum clade credibility (MCC) tree obtained in TreeAnotator 1·6·2 and visualized in FigTree 1·3·1.
Significant incongruence between loci prevented us from concatenating the ITS and ptDNA sequences for total-evidence phylogenetic and dating analyses (Kubatko and Degnan, 2007; Edwards, 2009). Instead, we implemented a species tree estimation analysis (see below).
Species tree estimation. Phylogenetic incongruence between loci is frequently found in plants, particularly in Linaria, due to incomplete lineage sorting and hybridization, among other causes (Blanco-Pastor et al., 2012). While no standard method is currently available for the inference of phylogenetic relationships in the presence of these two processes, a number of coalescent-based methods have been recently proposed for the inference of species trees that account for incongruence between gene trees caused by incomplete lineage sorting (Liu, 2008; Heled and Drummond, 2010). Here we employed the ITS and ptDNA datasets including one or two individuals per taxon to estimate a species phylogeny of Linaria sect. Versicolores under the multi-species coalescent method *BEAST (Heled and Drummond, 2010), implemented in BEAST 1·6·2.
Haplotypic data are needed for coalescent-based analyses, which posed a challenge in the case of the multi-copy ITS region. Cloning of ITS copies was not considered due to the low quality of DNA extracts and PCR products obtained from herbarium material. Instead, in order to reconstruct haplotypes from the unphased ITS sequences, we employed the Bayesian statistical method PHASE 2·1 (Stephens et al., 2001; Stephens and Donnelly, 2003), as implemented in DnaSP v5 (Librado and Rozas, 2009), with default parameters (recombination model MR0, 100 iterations, 100 burn-in iterations, thinning interval 1). A Bayesian phylogenetic analysis of the inferred haplotypes was conducted in MrBayes. Given that close (or unresolved) relationships between haplotypes of the same individual were recovered in all cases (Supplementary Data Fig. S1), the error introduced by potentially incorrect haplotype inference was considered negligible. Therefore, all ITS haplotypes inferred by PHASE were included in subsequent analyses following Blanco-Pastor et al. (2012).
Both datasets (ITS and ptDNA) were included as independent loci in the *BEAST analysis. The tree model and substitution model priors were set as indicated above for dating analyses. Based on results of separate dating analyses of ITS and ptDNA sequences (see above), the crown age of sect. Versicolores was calibrated using a normal prior with mean 6·07 Ma and standard deviation 1·85 Myr. Twenty MCMC analyses were run for 100 million generations each, with a sample frequency of 10 000. Analysis with Tracer 1·5 confirmed convergence and adequate sample sizes, with effective sample sizes above 250. Runs were combined using LogCombiner 1·6·2, after discarding the first 10 % of sampled generations as burn-in. Trees were summarized in an MCC tree obtained in TreeAnotator 1·6·2 and visualized in FigTree 1·3·1. In order to visualize the temporal dynamics of Linaria sect. Versicolores diversification, lineage-through-time (LTT) plots were generated in the R package ape (Paradis et al., 2004) for the MCC species tree and a random sample of 1000 trees from the posterior distribution of the *BEAST analysis.
Analysis of corolla shape
Metric measures. In the personate corolla of Linaria, the main reward for pollinators (nectar) is located at the end of an abaxial spur of variable length (Fig. 1). Three main traits determine nectar accessibility to pollinators: spur length, tube width and palate development. Of these, spur length and tube width can be readily measured in herbarium specimens. In order to characterize the inter- and intra-specific variability of these traits in bifid toadflaxes, the two variables were scored for 696 herbarium specimens representing the 30 recognized species and subspecies of Linaria sect. Versicolores (Supplementary Data Appendix S1). Most specimens were provided by the MA, RNG and ATH herbaria. In addition, specimens from the MPU, RAB, FI, K, BM, LD and S herbaria were electronically surveyed through JSTOR Plant Science (Gallagher, 2010). A single, fully developed flower per specimen was measured. Spur length was measured from the corolla–calyx insertion to the spur tip (Fig. 2A, C). Tube width was measured at the opening level (Fig. 2B, D). In addition, the same variables were measured for 377 living specimens collected from 18 Iberian populations of 12 representative species and subspecies sampled for geometric morphometric analyses (see below). All measurements were obtained from scaled digital photographs using ImageJ 1·44p (Abràmoff et al., 2004).
Fig. 2.
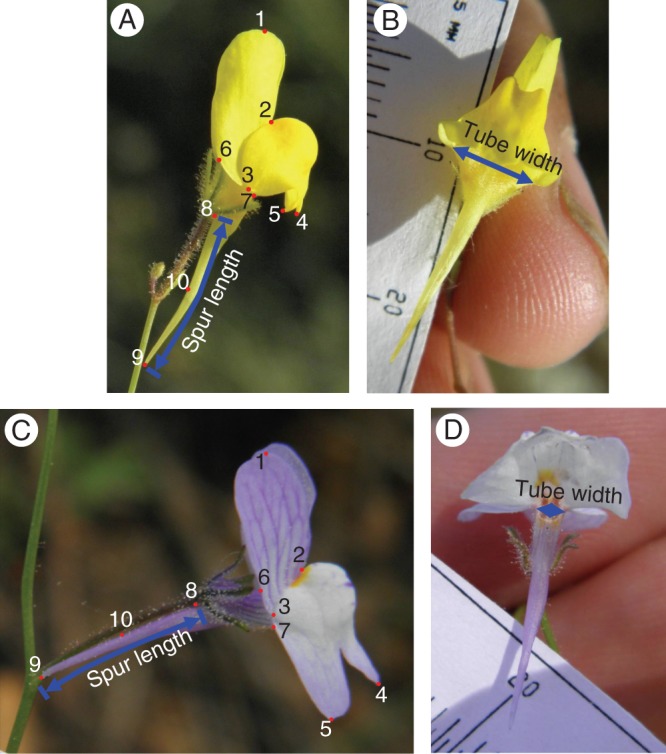
Metric measures (spur length and tube width) and landmarks (1–10) employed in morphometric analyses, shown in two representative taxa of morphological Types I (L. spartea; A, B) and III (L. onubensis; C, D). Photos show lateral (A, C) and ventral (B, D) views. Notice the broad tube in B and the narrow tube in D.
Geometric morphometrics. Geometric morphometrics provides a powerful tool to assess intra- and inter-specific variation in flower morphology (Shipunov and Bateman, 2005; Gómez et al., 2006; Abdelaziz et al., 2011). We used a landmark-based geometric morphometric analysis to describe corolla shape, including palate development, in 18 populations belonging to 12 species and subspecies of Linaria sect. Versicolores from the Iberian Peninsula (Supplementary Data Table S2). These species were considered to represent the full range of corolla shapes of sect. Versicolores based on our metric measures of herbarium specimens of all 30 species and subspecies (see Results). A total of 369 living specimens (7–25 per population; Supplementary Data Table S2) were sampled, and digital photographs were taken of one completely developed flower per individual in lateral view and planar position. Nine landmarks (Fig. 2A, C) were defined at points of evident homology across species (Zelditch et al., 2004). In addition, one pseudolandmark was defined at the mid-point of the spur. Landmarks were captured using tpsDig 2·16 (Rohlf, 2010). The two-dimensional coordinates of the landmarks were determined for each individual, and the generalized orthogonal least-squares Procrustes average configuration of landmarks was calculated using the generalized Procrustes analysis (GPA) superimposition method (Rohlf and Slice, 1990; Slice, 2001). Corolla shape differences among species were assessed using a canonical variate analysis, a multivariate analysis that optimizes between-group differences relative to within-group variation (Albrecht, 1980; Klingenberg and Monteiro, 2005). It generates several canonical variate axes and computes between-group Procrustes distances in the canonical variate space. The analysis was performed with the software MorphoJ (Klingenberg, 2011). Values of canonical variates 1 and 2 for all individuals were plotted. The statistical significance of the between-groups Procrustes distances was determined by randomization tests using 10 000 permutations.
Evolution of flower morphology
Effect of flower morphology on diversification rate. Morphometric analyses allowed the identification of three major floral morphological types (see Results). We estimated the effect of flower morphology on Linaria sect. Versicolores diversification using the binary-state speciation and extinction (BiSSE) model (Maddison et al., 2007; FitzJohn et al., 2009) implemented in the R package diversitree v.0·7–2 (FitzJohn, 2012). We defined two character states: (0) wide-tubed flower (Types I and II, see below); and (1) narrow-tubed flower (Type III) (Table 1). The fact that Type II was found in a single species (L. clementei) prevented us from using the multiple state speciation and extinction (MuSSE) method, which is an extension of BiSSE for more than two character states (FitzJohn, 2012). Instead, Type II was grouped with Type I based on their morphometric similarity (see below). All recognized taxa (species and subspecies) were included as independent entities in this analysis, based on the fact that subspecies belonging to the same species were usually not closely related in phylogenetic analyses (see below). The MCC species tree from the *BEAST analysis [with nodes with posterior probability (PP) <0·5 collapsed] and 10 additional species trees randomly chosen from the Bayesian posterior distribution were analysed. For each tree, we compared a model with state-dependent speciation and extinction and asymmetrical transition rates against nested models with speciation, extinction and transition rate parameters constrained to be equal for both states. We calculated ML parameter values of the unconstrained model (full BiSSE model, six parameters) versus the constrained models (five parameters), and the significance of model differences was assessed by performing likelihood ratio tests. Parameter values of the full BiSSE model were additionally explored for the MCC tree and the ten additional trees in a two-step process using ML values as a prior for an MCMC sampling of parameters, a Bayesian approach (MCMC-BiSSE) that provides a measure of parameter uncertainty (FitzJohn et al., 2009). The trees were analysed with 10 000 steps per tree (chain) and a prior for each parameter exponentially distributed (prioritizing small rates of change, in the absence of evidence to the contrary). After discarding the first 2000 steps of each chain as burn-in, parameter values for each tree were summarized and plotted.
Reconstruction of flower morphology shifts. Ancestral state reconstruction (ASR) of the two morphological types analysed in BiSSE was performed in diversitree using ML under the BiSSE model (ASR-BiSSE), thus accounting for differential speciation, extinction and character transition rates in character optimization (Goldberg and Igić, 2008). To account for phylogenetic uncertainty, analyses were conducted separately for the MCC species tree and the same ten additional trees for which parameter values were estimated using MCMC-BiSSE. A higher number of trees was not analysed due to the high computational demands of this method. Given that the sister group to sect. Versicolores is currently unknown (Fernández-Mazuecos et al., 2013), outgroup taxa, potentially biasing ASRs, were pruned from the analysed trees. Parameter distributions obtained in MCMC-BiSSE analyses were used to account for parameter uncertainty.
For comparison with the ASR-BiSSE approach described above, and to fully account for phylogenetic uncertainty, ASR was also performed using parsimony in Mesquite 2·75 (Maddison and Maddison, 2011). In this case, the three morphological types were included, thus treating Type II as a separate state. Reconstructions were conducted on the full set of species trees of the *BEAST posterior distribution using the ‘trace character over trees’ option. Additionally, the ‘summarize state changes over trees’ option was used to summarize the number of changes between character states across the Bayesian posterior distribution of trees.
Models of spur length and tube width evolution. We tested for the existence of one or more evolutionary optima for spur length and tube width using the ML method implemented in the R package ouch (Butler and King, 2004; King and Butler, 2009). Two models based on an Ornstein–Uhlenbeck process (Hansen, 1997) with one and two optima respectively were tested against a null Brownian motion model. Morphological Types I/II and III were defined as hypothetical selective regimes, and ancestral states were included based on the ASR-BiSSE reconstruction. Model comparisons were performed using AIC values. Analyses were conducted for the MCC species tree and the ten randomly sampled trees.
Pollinator observations
We performed flower visitor surveys in populations of the 12 Iberian species and subspecies of Linaria sect. Versicolores, which represent the full range of corolla shapes of the group. A total of 4618 min of observations (267–941 min per taxon) were performed in 2009, 2010, and 2011 in 14 populations that were also included in geometric morphometric and phylogenetic analyses. Visits were considered legitimate when the visitor touched the anthers and stigma. The placement of pollen on the insect body (thorax or proboscis) was recorded.
RESULTS
Phylogenetic relationships and divergence times
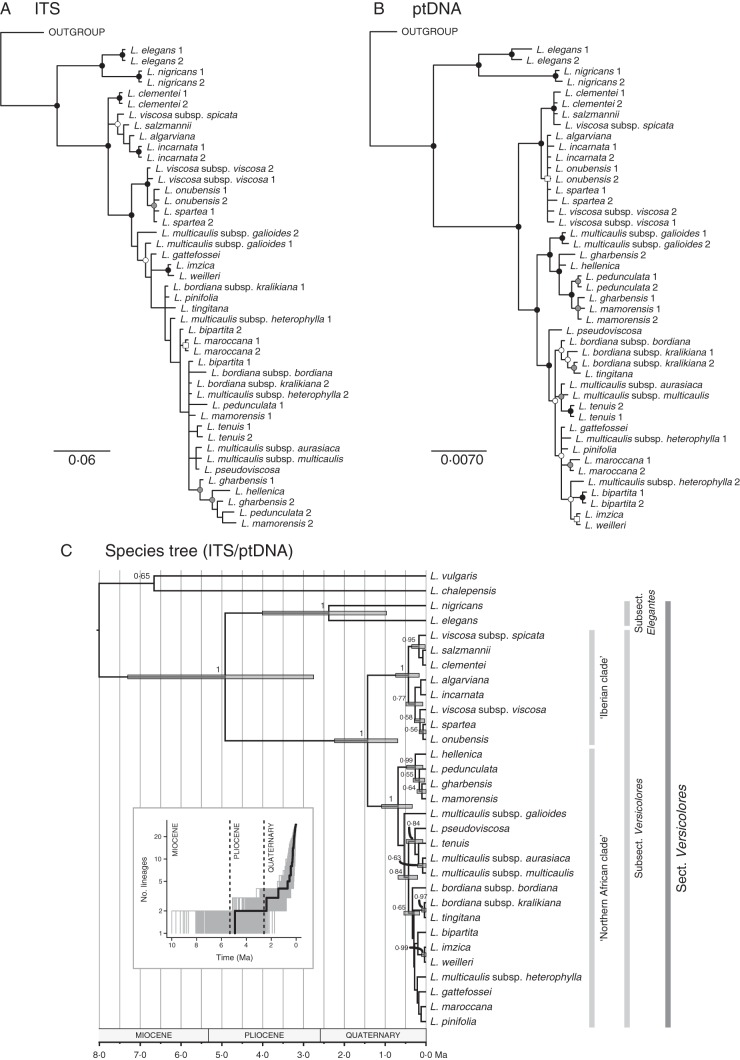
The ITS and ptDNA datasets had total aligned lengths of 610 and 2679 bp respectively (see characteristics of the four DNA regions in Table 2). Overall, the BI, ML and MP analyses yielded congruent topologies, except for some weakly supported clades. The separate phylogenetic analyses of nuclear ITS (Fig. 3A) and ptDNA (Fig. 3B) sequences consistently retrieved sect. Versicolores as a monophyletic group with strong statistical support [PP = 1; bootstrap support (BS) > 90 % in ML and MP analyses] and subsections Elegantes and Versicolores also as monophyletic (PP = 1; BS > 95 %) and sister to each other. However, topological incongruence between both datasets was extensive within subsect. Versicolores. In general, higher clade support values were obtained in ptDNA analyses.
Table 2.
Characteristics of the four DNA regions sequenced for 45 individuals of Linaria sect. Versicolores and 11 outgroup taxa, and employed in phylogenetic analyses
| Nuclear DNA, ITS (ITS1-5·8S-ITS2) | Plastid DNA |
|||
|---|---|---|---|---|
| rpl32-trnLUAG | trnK-matK | trnS-trnG | ||
| Aligned length (bp) | 610 | 830 | 1230 | 619 |
| Ungapped length range (bp) | 577–597 | 582–768 | 1206–1221 | 466–590 |
| Pairwise identity (%) | 90·7 | 94·0 | 98·2 | 92·7 |
| Variable characters | 199 | 211 | 186 | 139 |
| Parsimony-informative characters | 134 | 106 | 82 | 59 |
| Mean G + C content (%) | 56·1 | 23·6 | 32·3 | 27·4 |
| Substitution model | GTR + G | GTR | GTR + G | HKI + I + G |
Fig. 3.
Phylogenetic analyses of Linaria sect. Versicolores. (A, B) Gene trees of ITS (A) and ptDNA (B) sequences. The 50 % majority rule consensus trees obtained in Bayesian analyses are shown. A black dot indicates clade support in BI (PP > 0·95), ML (ML – BS > 70 %) and MP (MP – BS > 70 %) analyses. A grey dot indicates support only in BI and ML analyses. A white dot indicates support only in the BI analysis. A white square indicates support only in the ML analysis. (C) Time-calibrated maximum clade credibility species tree obtained in the Bayesian *BEAST analysis based on ITS and ptDNA sequences. Node bars represent the 95 % highest posterior density intervals for the divergence time estimates. Numbers along the branches are Bayesian posterior probabilities. Major clades are named, including subsections following Sutton (1988). The inset shows a log-lineage-through-time plot for Linaria sect. Versicolores, based on 1000 trees randomly sampled from the posterior distribution of the *BEAST analysis. The thick line corresponds to the MCC species tree.
Separate dating analyses of the two loci (Supplementary Data Fig. S2) consistently recovered a crown age for sect. Versicolores around 6 Ma. This age was then used to calibrate the species tree analysis. The time-calibrated species tree obtained in *BEAST (Fig. 3C) strongly supported major clades of Linaria sect. Versicolores, but displayed lower resolution at shallow phylogenetic levels. Divergence between subsections Elegantes (PP = 1) and Versicolores (PP = 1) was dated back to the late Miocene or Pliocene. Two strongly supported sister clades were recognized within subsect. Versicolores (Fig. 3C): the ‘Iberian clade’ (PP = 1) included all species that are endemic or subendemic to the Iberian Peninsula, while the ‘northern African clade’ (PP = 1) included all northern African endemics, plus the Ibero-North African L. pedunculata and L. gharbensis, the southern Italian L. multicaulis subsp. multicaulis and the Greek L. hellenica. A Quaternary diversification of subsect. Versicolores was estimated, with the Iberian and northern African clades diverging 0·69–2·24 Ma and both clades diversifying in the last million years. The LTT plots (Fig. 3C) clearly depicted diversification of Linaria sect. Versicolores since the late Miocene–Pliocene, and primarily during the Quaternary.
Analysis of corolla shape
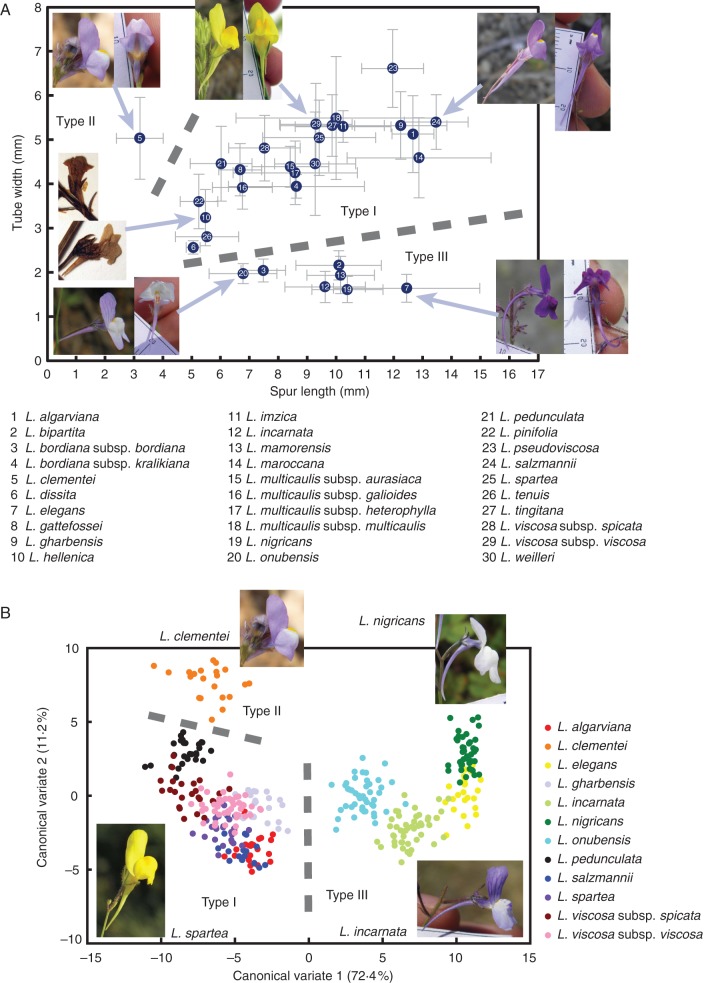
Measures of spur length and tube width from herbarium specimens of the 30 recognized species and subspecies of sect. Versicolores (Fig. 4A; Supplementary Data Table S3) revealed three major morphological types. Type I was the most frequent (22 species and subspecies) and was characterized by a broad tube (>2 mm) and wide variation in spur length (4–18 mm). Type II was only found in L. clementei, and displayed a broad tube (4–7 mm) and a very short spur (1–4·5 mm). Type III, found in seven taxa, had a narrow tube (1–3 mm) and variable spur length (5–19 mm). The same three groups were consistently recovered when measuring living specimens of the 12 taxa present in the Iberian Peninsula (Supplementary Data Fig. S3).
Fig. 4.
Morphological traits of Linaria sect. Versicolores flowers. (A) Scatter plot of tube width versus spur length measured in 696 herbarium specimens representing the 30 species and subspecies of Linaria sect. Versicolores. Means (numbered dots) and standard deviations (bars) for each taxon are plotted. Notice the broad tubes of Types I and II, and the narrow tubes of Type III. (B) Canonical variate analysis of the landmark-based geometric morphometric dataset. Scatter plot of canonical variate 2 versus canonical variate 1 for 369 living specimens sampled in 18 populations belonging to the 12 Iberian species and subspecies (colours), which represent the full range of corolla shapes of Linaria sect. Versicolores. The variance explained by each axis is shown in brackets. In both plots, the three major morphological types discussed in the text are indicated, and representative taxa are shown.
The canonical variate analysis of landmark-based geometric morphometric data of these 12 Iberian taxa (Fig. 4B) recovered the same three morphological types. The first two canonical variates explained 83·6 % of the variance, and all between-taxa Procrustes distances were statistically significant (P < 0·001) based on randomization tests. Variation along canonical variate 1 was related to palate development, since it affected the relative position and shape of the upper and lower lips (Fig. 4B; Supplementary Data Fig. S4A). Variation along canonical variate 2 was related to the shape of the spur and the relative sizes and positions of the upper and lower lips (Fig. 4B; Supplementary Data Fig. S4B). Taxa of Types I and II displayed well-developed palates, while Type III had broader and expanded lower lips. Type II (L. clementei) was clearly related to Type I, from which it was mainly differentiated by its short spur.
Evolution of flower morphology
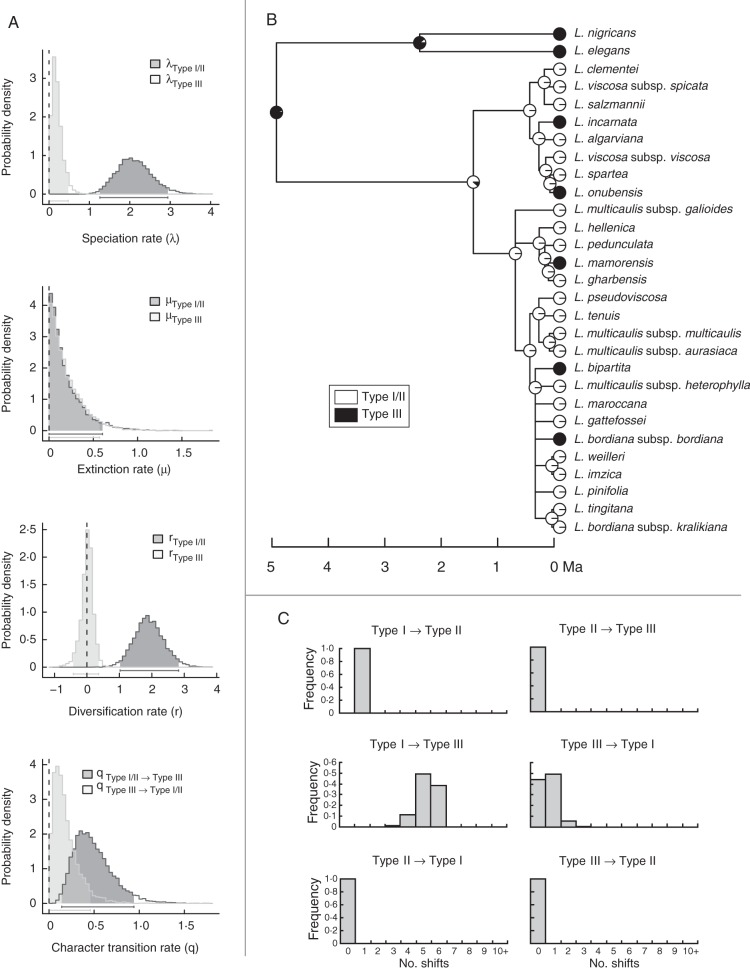
An effect of flower morphology on diversification rates was supported by the likelihood ratio tests of BiSSE models (Table 3). In particular, we detected a significant effect on speciation rates, as the ‘symmetric speciation’ model was rejected for the MCC species tree and nine out of ten randomly chosen trees. Bayesian estimation of BiSSE parameters revealed significantly higher speciation rates for morphological Type I/II than for Type III, as shown by the non-overlapping 95 % credibility intervals obtained when analysing the MCC species tree (Fig. 5A) and the ten randomly chosen trees (Supplementary Data Fig. S5). No effect on extinction rates was detected (see the widely overlapping 95 % credibility intervals in Fig. 5A and Supplementary Data Fig. S5). Diversification (speciation minus extinction) rates were higher for Type I/II, although with certain overlap of the 95 % credibility intervals for three out of ten trees. No significant difference between transition rates was found.
Table 3.
Maximum likelihood estimates of BiSSE parameters and likelihood ratio tests of alternative models based on the maximum clade credibility tree of the *BEAST analysis
| df | λI/II | λIII | μI/II | μIII | qI/II→III | qIII→I/II | lnL | AIC | χ2 | P value | No. trees with P < 0·05 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unconstrained model | 6 | 3·75 | 2·83 × 10−6 | 3·02 | 2·33 | 1·03 | 7·51 × 10−6 | −23·05 | 58·10 | — | — | — |
| Symmetric speciation (λI/II = λIII) | 5 | 2·97 | 2·97 | 1·17 | 6·45 | 2·05 | 5·25 × 10−8 | −25·54 | 61·08 | 4·97 | 0·03* | 9/10 |
| Symmetric extinction (μI/II = μIII) | 5 | 3·53 | 1·93 × 10−9 | 2·61 | 2·61 | 1·19 | 1·55 × 10−7 | −23·09 | 56·19 | 0·09 | 0·77 ns | 0/10 |
| Symmetric transition rate (qI/II→III = qIII→I/II) | 5 | 4·20 | 4·45 × 10−9 | 3·87 | 1·77 | 0·88 | 0·88 | −23·88 | 57·75 | 1·65 | 0·20 ns | 0/10 |
The last column shows the number of trees (out of a random sample of ten trees from the posterior distribution of the *BEAST analysis) where each model was significantly worse (P < 0·05) than the unconstrained model according to a likelihood ratio test. Abbreviations and symbols: df, degrees of freedom; λ, speciation rates; μ, extinction rates; q, character transition rates; lnL, log likelihood; AIC, Akaike information criterion.
*0·01 < P < 0·05; ns, not significant.
Fig. 5.
Analyses of diversification rates and flower type transitions in Linaria sect. Versicolores. (A) Results of the binary-state speciation and extinction (BiSSE) analysis of the MCC species tree (Fig. 3C), considering two character states corresponding to morphological Types I/II (broad tube) and III (narrow tube). Posterior distributions of parameters (speciation rates, extinction rates, diversification rates and character transition rates) obtained in the MCMC-BiSSE analysis are shown. Horizontal bars indicate the 95 % credibility interval for each parameter. (B) Maximum likelihood ancestral state reconstruction of morphological Types I/II and III under state-dependent diversification (ASR-BiSSE), based on parameter estimates shown in A. The tree is the MCC species tree with nodes with PP < 0·5 collapsed. (C) Summary distributions of the number of shifts between flower types inferred when implementing parsimony optimization over the full posterior distribution of trees obtained in the *BEAST analysis.
Ancestral state reconstruction under state-dependent diversification (ASR-BiSSE) using the MCC species tree (Fig. 5B) recovered morphological Type III as ancestral to sect. Versicolores and subsect. Elegantes. Type I/II was inferred as ancestral to subsect. Versicolores, which implied an old shift from Type III to Type I/II. Four to five shifts from Type I/II to Type III were inferred within subsect. Versicolores, in both the Iberian and the northern African clade. Similar results were obtained when conducting ASR-BiSSE analysis on ten randomly chosen trees, although with variable ancestral state probabilities (Supplementary Data Fig. S6). Parsimony-based reconstructions yielded congruent results, although with equivocal reconstruction at the root node (Supplementary Data Fig. S7). Accordingly, zero to two shifts from Type III to Type I were estimated when accounting for topological uncertainty (Fig. 5C). Four to six shifts from Type I to Type III were estimated, and one shift from Type I to Type II was unequivocally reconstructed. No shifts were obtained from Type II to Types I and III, and from Type III to Type II (Fig. 5C).
Different models of trait evolution were supported for spur length and tube width in relation to the two main morphological types (Table 4). When analysing the MCC species tree, the Ornstein–Uhlenbeck model with one optimum at 8·98 mm was supported for spur length, while the Ornstein–Uhlenbeck model with two optima at 4·67 (Type I/II) and 1·86 mm (Type III) was preferred for tube width. Similar results were obtained for the ten additionally analysed trees (Table 4).
Table 4.
Testing of evolution models for spur length and tube width
| lnL | AICc | σ2 | α | Optima (mm) | No. trees supporting the model | |
|---|---|---|---|---|---|---|
| Spur length | ||||||
| BM | −83·98 | 172·43 | 230·45 | NA | NA | 0/10 |
| OU1* | −68·36 | 143·68 | 4891·41 | 374·84 | 8·98 (8·94–9·06) | 10/10 |
| OU2 | −68·10 | 145·86 | 2253·02 | 175·54 | 8·77 (8·72–8·87) | 0/10 |
| 9·61 (9·57–9·78) | ||||||
| Tube width | ||||||
| BM | −62·33 | 129·12 | 51·76 | NA | NA | 0/0 |
| OU1 | −51·12 | 109·20 | 6301·93 | 1584·62 | 3·99 (3·81–4·00) | 0/0 |
| OU2* | −32·27 | 74·20 | 726·20 | 669·87 | 4·67 (4·66–4·68) | 10/10 |
| 1·86 (1·83–1·86) |
Three models were tested: Brownian motion (BM), Ornstein–Uhlenbeck process with one optimum (OU1) and Ornstein–Uhlenbeck process with two optima (OU2). Values for the MCC species tree obtained in the *BEAST analysis are shown. Optimum values for the MCC species tree and minimum and maximum values obtained for ten randomly chosen species trees from the Bayesian posterior distribution (in brackets) are indicated. The last column summarizes the number of trees of the same sample where each model was significantly supported based on corrected AICc values. Abbreviations and symbols: *preferred model; lnL, log likelihood; AICc, corrected Akaike information criterion; σ, α, model parameters.
Pollinator observations
Observations of flower visitors in the 12 Iberian species and subspecies of Linaria sect. Versicolores (Table 5; Fig. 6) suggested that flowers of Types I and II are mainly pollinated by bees (Hymenoptera) carrying pollen on the back of the thorax (Fig. 6A), although sporadic visits by nectar-feeding butterflies (Lepidoptera; Fig. 6B) and bee flies (Bombyliidae, Diptera) carrying pollen on the proboscis were also recorded for three taxa: L. spartea, L. viscosa subsp. viscosa (Type I) and L. clementei (Type II). For the four Type III species, a wide variety of flower visitors were observed, most of them displaying a long proboscis: hawk moths (Sphingidae, Lepidoptera), Anthophora-like bees (Anthophorini, Apidae, Hymenoptera) and bee flies (Bombyliidae, Diptera). All of them carried pollen on the proboscis (Fig. 6C).
Table 5.
Potential pollinators of Iberian species of Linaria sect. Versicolores, recorded after 4618 min of observations in 2009, 2010 and 2011
| Taxon | Pollinators |
|---|---|
| Subsect. Versicolores | |
| L. algarviana (I) | Hymenoptera: Ceratina saundersi (S) + |
| Coleoptera: Attagenus sp. (S) | |
| L. clementei (II) | Hymenoptera: Amegilla quadrifasciata (T), Rhodanthidium sticticum (T), Bombus ruderatus (T), Xylocopa sp. (T), Heliophila bimaculata (T) −, Ceratina mocsaryi (S) – |
| Diptera: Dischistus separatus (P) | |
| Lepidoptera (P) | |
| L. gharbensis (I) | Hymenoptera: Anthophora plumipes (T) + |
| L. incarnata (III) | Diptera: Systoechus gradatus (P), Amictus variegatus (P) |
| Lepidoptera: Thymelicus spp. (P) | |
| Hymenoptera: Lasioglossum sp. (S), Ceratina sp. (S) − | |
| L. onubensis (III) | Hymenoptera: Eucera nigrilabris (P) + |
| L. pedunculata (I) | Not seen (autogamous species) |
| L. salzmannii (I) | Hymenoptera: Heliophila bimaculata (T) +, Rhodanthidium sticticum (T), Lasioglossum sp. (S), Hoplitis sp. (S) − |
| L. spartea (I) | Hymenoptera: Apis mellifera (T), Heliophila bimaculata (T), Ceratina cucurbitina (S) −, Lasioglossum sp. (S) − |
| Lepidoptera: Euchloe crameri (P) − | |
| L. viscosa subsp. viscosa (I) | Hymenoptera: Apis mellifera (T) +, Hoplitis sp. (T), Xylocopa uclesiensis (T) −, Heliophila bimaculata (T) −, Lasioglossum sp. (S) − |
| Lepidoptera: Euchloe crameri (P) − | |
| L. viscosa subsp. spicata (I) | Hymenoptera: Rhodanthidium sticticum (T) +, Osmia andrenoides (T) |
| Subsect. Elegantes | |
| L. elegans (III) | Lepidoptera: Macroglossum stellatarum (P) +, Lasiommata megera (P) − |
| Hymenoptera: Anthophora retusa (P) − | |
| Diptera: Bombylius major (P) − | |
| L. nigricans (III) | Hymenoptera: Eucera nigrilabris (P) +, Apis mellifera (P) |
| Lepidoptera: Colias croceus (P) −, Pontia daplidice (P) − |
Observed strategies of pollen placement on insect body are coded as follows: (T) pollen placed on the back of the thorax; (P) pollen placed on the proboscis; (S) small insects with variable pollen placement. +, >50 % of flower visits; −, <5 % of flower visits. Morphological types are indicated in brackets after taxon names.
Fig. 6.
Different behaviours of potential pollinators of morphological Types I (broad tube) and III (narrow tube). (A) Apis mellifera (Hymenoptera) in L. viscosa subsp. viscosa (Type I). (B) Euchloe sp. (Lepidoptera) in L. viscosa subsp. viscosa. (C) Bombyliidae (Diptera) in L. elegans (Type III). White arrows indicate pollen placement on pollinators. Photographs by M. Fernández-Mazuecos (A, B) and P. Vargas (C).
DISCUSSION
This study provides key insights into the evolution and diversification of bifid toadflaxes, with important consequences for understanding the relationship between floral specialization and species diversification. Species with narrow-tubed flowers were found to have evolved recurrently from broad-tubed ancestors, suggesting that similar selective pressures have driven flower evolution in independent lineages. However, the increasing pollinator specialization associated with narrower corolla tubes appears to have prevented narrow-tubed lineages from further diversification. Therefore, our results support a significant effect of floral specialization on the evolutionary success of flowering plant lineages.
Recent diversification of bifid toadflaxes
Our phylogenetic analyses based on nuclear and plastid sequences confirmed the monophyly of Linaria sect. Versicolores (Fernández-Mazuecos and Vargas, 2011; Fernández-Mazuecos et al., 2013). Dating results indicate that diversification of bifid toadflaxes began in the late Miocene or Pliocene. Most inferred cladogenetic events occurred since the onset of the Mediterranean climate (∼3·2 Ma; Suc, 1984) and particularly during the Quaternary (i.e. the last 2·6 Ma), as previously suggested based on plastid markers alone (Fernández-Mazuecos and Vargas, 2011; Fiz-Palacios and Valcárcel, 2013). While our species tree analysis provided strong support for several clades within sect. Versicolores (particularly the two subsections; Fig. 3C), fine-scale relationships between species were mostly unresolved or poorly supported. Low phylogenetic resolution is a common feature of recent radiations (e.g. Hughes and Eastwood, 2006; Scherson et al., 2008; Valente et al., 2010a). It is well known that rapid diversification brings about processes, such as hybridization and incomplete lineage sorting, that cause incongruence between gene trees and therefore may obscure phylogenetic relationships (Degnan and Rosenberg, 2009). This has recently been demonstrated for a different clade of toadflaxes, Linaria sect. Supinae (Blanco-Pastor et al., 2012). Methods based on the multi-species coalescent (such as the one implemented in *BEAST), rather than concatenated analyses, currently constitute the best approach for the inference of species phylogenies in the presence of incongruent gene trees, because they account for incomplete lineage sorting (Edwards, 2009; Leaché and Rannala, 2011). We cannot rule out hybridization as a source of incongruence between gene trees (Fig. 3A, B). However, all phylogenetic comparative methods currently available are tree-based (Nunn, 2011). Incorporation of hybridization will strengthen phylogenetic inference (Yu et al., 2011, 2012), and the development of comparative methods able to deal with reticulate phylogenies will probably lead to the reconstruction of more realistic evolutionary scenarios in the future. At present, however, the assumption of a tree-like phylogeny must be made in order to test evolutionary hypotheses using available tools. To account for uncertainty about phylogenetic relationships at shallow levels (part of which might be due to hybridization), we performed all comparative analyses on the consensus species tree and a random sample of ten species trees from the posterior distribution of the *BEAST analysis. Congruence of results across such sample was interpreted as evidence for a strong evolutionary signal in spite of phylogenetic uncertainty (Huelsenbeck et al., 2000).
Convergence in the evolution of flower shape
Different biotic interactions affecting Linaria flowers have been studied previously, including floral herbivory, nectar robbery and insect pollination (Arnold, 1982; Stout et al., 2000; Newman and Thomson, 2005a, b; Sánchez-Lafuente, 2007). The last is likely the most relevant factor affecting the evolution of morphological traits studied here (Sánchez-Lafuente, 2007). Our phylogenetic comparative analyses suggest that the evolution of flower morphology in bifid toadflaxes has been dominated by shifts between two morphological types mainly differentiated by the width of the tube and the development of the palate (Figs 4 and 5). It is suggested (Table 5; Fig. 6) that these two types constitute divergent strategies of pollen placement on nectar-feeding insects (Armbruster et al., 1994; Grant, 1994; Kay, 2006; Yang et al., 2007). These two strategies of Linaria pollination (Fig. 6) were first identified by Robertson (1888) and Hill (1909). One strategy corresponds to the typically nototribic pollination of broad-tubed species (Type I/II), in which pollen is deposited on the back of the thorax (scutum) of the nectar-feeding insect (Fig. 6A). This is the strategy found in most Linaria species of other sections, and has been demonstrated to result in effective pollination (Macior, 1967; Arnold, 1982; Stout et al., 2000; Newman and Thomson, 2005a; Sánchez-Lafuente, 2007; Sánchez-Lafuente et al., 2011). In sect. Versicolores, the placement of the first optimum inferred by the two-peak Ornstein–Uhlenbeck model (∼4·67 mm; Table 4) suggests an adaptive adjustment to the thorax width of frequent pollinators. Indeed, closely related species pollinated by similar insects should be similarly selected for the floral phenotype that most efficiently uses these pollinators (Kay and Sargent, 2009). In our case, an adjustment of flower tube size to pollinator size probably maximizes pollen transfer in personate, wide-tubed corollas (but see Vargas et al. (2010) for Antirrhinum). The other strategy is displayed by narrow-tubed species (Type III), in which nectar-feeding insects usually carry pollen on the proboscis (Fig. 6C). In this situation, pollen transfer is maximized by narrowing the tube, so that contact of the proboscis with the anthers and the stigma is guaranteed when the insect reaches for nectar contained in the spur (Robertson, 1888; Kampny, 1995). This would lead to the second optimum of tube width (∼1·86 mm; Table 4), which is large enough to fit the anthers (∼0·5 mm) but narrow enough to guarantee pollen transfer by long-proboscis visitors. Similar strategies of pollen placement on pollinators are frequently found in angiosperm lineages with spurred flowers, particularly those pollinated by long-proboscis insects (Herrera, 1993; Johnson and Steiner, 1997; Schiestl and Schlüter, 2009). In Linaria, the narrow-tubed strategy has been previously related to pollination by butterflies (Robertson, 1888; Hill, 1909) and moths (Sutton, 1988; Kampny, 1995). Our observations partially support these predictions (see L. elegans and L. incarnata in Table 5). Additionally, we have found the narrow-tube morphology to be also suited to other long-proboscis pollinators, such as long-tongued bees (mainly from tribe Anthophorini) and bee flies (Bombyliidae) (Table 5; see Supplementary Data Appendix S2 for further discussion on flower visitors).
Several independent origins of similarly configured narrow-tubed flowers have been inferred by ancestral state reconstructions (Fig. 5B, C and Supplementary Data Figs S6 and S7). Therefore, this is definitely a case of phenotypic homoplasy, i.e. convergence sensu Scotland (2011). In addition, reversal to an ancestral state (which can be regarded as a special case of convergence; Scotland, 2011) is suggested by the ancestral narrow-tubed flower inferred by the ASR-BiSSE analysis, together with its loss and subsequent repeated reappearance in subsect. Versicolores (Fig. 5B) (Hall, 2003; Porter and Crandall, 2003). The convergent evolution of narrow-tubed phenotypes in several different lineages of bifid toadflaxes may have involved similar genetic and developmental mechanisms (Hall, 2012), in which case it would be interpreted as an instance of parallel evolution sensu Scotland (2011) (note that the definitions of convergence and parallelism are controversial; see also Hall, 2003; Arendt and Reznick, 2008; Wake et al., 2011; Hall, 2012). Homoplasy of morphological traits can result from common adaptive responses to similar selection pressures, coupled with genetic and developmental constraints (Wake, 1991; Brakefield, 2006; Wake et al., 2011). While no information about the latter is yet available for the study group, pollinator observations suggest an adaptive meaning of the two morphological types, as they correspond to different strategies of pollen placement on pollinators. Indeed, the recurrent shifts from broad-tubed flowers to narrow-tubed ones in subsect. Versicolores are not surprising given the fact that broad-tubed species are visited at a low rate by long-proboscis insects, including butterflies and even bee flies, which carry pollen on their proboscis (Table 5; Fig. 6B). Following the ‘pollinator shift’ hypothesis (Grant and Grant, 1965; Stebbins, 1970; Campbell, 2008), when a broad-tubed species is faced with an environment in which such long-proboscis insects are dominant, natural selection would favour a narrowing of the tube that would maximize pollen transfer, a mechanism similar to that previously invoked to explain spur elongation in American columbines (Whittall and Hodges, 2007). Additional research on reproductive and pollination biology at the population level will be needed to shed further light on the microevolutionary mechanisms (including selective pressures) involved in these putatively adaptive morphological shifts.
Trait dependent diversification?
Even though we have inferred a higher number of shifts from broad- to narrow-tubed flowers (4–6) than in the opposite direction (0–2) (Fig. 5B, C), a directional trend in the evolution of morphological types is not supported by BiSSE analyses (Fig. 5A and Supplementary Data Fig. S5). Instead, our analyses of trait-dependent diversification revealed a dissimilar evolutionary success of the broad- and narrow-tube strategies, as shown by the consistently higher speciation rate of broad-tubed species found in BiSSE analyses (Fig. 5A and Supplementary Data Fig. S5). Thus, despite having repeatedly evolved, the narrow-tubed strategy seems to display limited success in terms of speciation. In fact, radiation of subsect. Versicolores (23–28 species) may have been triggered not only by the onset of the Mediterranean climate (see above), but also by a shift from the ancestral narrow tube (as inferred by the ASR-BiSSE analysis; Fig. 5B) to the broad tube in the common ancestor of this clade. This is illustrated by the fact that the sister subsect. Elegantes, which maintained the ancestral state (narrow tube), yielded only two species during the same period of time and under similar climatic conditions (Fig. 3C; see Supplementary Data Appendix S2 for additional analyses and discussion).
BiSSE results suggest that trait-dependent diversification rates, rather than asymmetrical rates of change, are responsible for the contrasting species diversities of the two morphological types. Caution should be exercised in this interpretation, given the small size of our study group (Maddison et al., 2007; Wertheim and Sanderson, 2011; Davis et al., 2013). It is important to note that our analyses were able to reject the null hypothesis of symmetrical speciation rates (Table 3; Fig. 5A) despite the reported low statistical power of BiSSE with small datasets (Davis et al., 2013). However, confounding effects of asymmetrical extinction rates and/or rates of change cannot be ruled out. In any case, the differential diversification success of morphological types is further suggested at the genus level (∼150 species), as shown by the fact that additional Linaria clades displaying narrow tubes are remarkably species-poor (sects Macrocentrum and Lectoplectron, six species) compared with broad-tubed clades (sects Linaria, Speciosae, Supinae, Diffusae and Pelisserianae, >120 species) (Sutton, 1988; Fernández-Mazuecos et al., 2013).
Several flower traits have been found previously to influence diversification rates, including flower symmetry (Sargent, 2004), biotic/abiotic pollination mode (Dodd et al., 1999) and the presence of nectar spurs (Hodges and Arnold, 1995; Hodges, 1997; but see Hagen and Kadereit, 2003; Cacho et al., 2010). In general, it has been proposed that floral specialization promotes diversification (but see Smith et al., 2008), although it has in turn been suggested that high species diversity may promote floral specialization (Armbruster and Muchhala, 2009). Our results suggest that traits restricting pollinator access to rewards and pollen placement on pollinators have significant effects on diversification rates of Linaria sect. Versicolores. Mechanisms of species selection (Stanley, 1975; Jablonski, 2008; FitzJohn, 2010; Rabosky and McCune, 2010) causing such differences in trait-dependent ‘emergent fitness’ (i.e. heritable differences in net diversification rates) are different from those involved in selection at the individual level (Rabosky and McCune, 2010). Indeed, the narrow-tube strategy in bifid toadflaxes has recurrently evolved by means of individual-level selection mechanisms, yet it may have exerted a negative influence on diversification rates, thus leading to the low frequency of this character state (Fig. 5). Specific mechanisms of species selection acting in bifid toadflaxes may include differential opportunities for exploitation of pollinator fauna (e.g. higher diversity of pollinators carrying pollen on the thorax than on the proboscis) and differential extinction risks (e.g. due to the likely higher specialization of narrow-tubed species; Johnson and Steiner, 2000). Even though our analyses have detected significant effects on speciation rather than extinction rates, the latter cannot be ruled out given the reported limitations of phylogeny-based methods to detect variation in extinction rates (FitzJohn, 2010; Rabosky, 2010). Further research will be needed to understand the mechanisms that account for the effects of pollinator-restrictive traits on diversification rates of Linaria and other genera with highly specialized corollas.
Our results support a relationship between resource specialization and evolutionary success in terms of diversification. The intriguing finding that opposing individual-level and species-level selection pressures may drive the evolution of specialized traits is worth further investigation in additional model systems.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Emilio Cano and Fátima Durán for laboratory assistance; Alberto Bañón, Javier Freijanes, Alberto Fernández-Mazuecos, Enrique Sánchez-Gullón, Joaquín Ramírez and Juan Carlos Moreno for field assistance; Concepción Ornosa, Roger Vila, Javier Ortiz and Miguel Carles-Tolrá for assistance in pollinator identification; Juan José Aldasoro, Belén Estébanez, Francisco Gómiz, Santiago Martín-Bravo, Enrique Rico, the ATH, UPOS and SALA herbaria, and particularly the RNG and MA herbaria, their curators Stephen Jury and Mauricio Velayos, and the Flora Iberica project for plant material; the ‘Marismas del Odiel’ natural reserve for collection permissions; José Quiles, Joaquín Ramírez, Enrique Rico and Ori Fragman-Sapir for permission to use their brilliant photographs; and Virginia Valcárcel, Luis Valente and two anonymous reviewers for comments that improved the quality of the manuscript. This work was supported by the Spanish Ministry of Science and Innovation through project CGL2009–10031, by the Spanish Ministry of Education through a FPU fellowship (AP2007–01841) to M. Fernández-Mazuecos, and by the Spanish National Research Council (CSIC) through a JAEpre fellowship to J. L. Blanco-Pastor.
LITERATURE CITED
- Abdelaziz M, Lorite J, Muñoz-Pajares AJ, Herrador MB, Perfectti F, Gómez JM. Using complementary techniques to distinguish cryptic species: a new Erysimum (Brassicaceae) species from North Africa. American Journal of Botany. 2011;98:1049–1060. doi: 10.3732/ajb.1000438. [DOI] [PubMed] [Google Scholar]
- Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Albrecht GH. Multivariate analysis and the study of form, with special reference to canonical variate analysis. American Zoologist. 1980;20:679–693. [Google Scholar]
- Alexandersson R, Johnson SD. Pollinator-mediated selection on flower-tube length in a hawkmoth-pollinated Gladiolus (Iridaceae) Proceedings of the Royal Society Series B, Biological Sciences. 2002;269:631–636. doi: 10.1098/rspb.2001.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt J, Reznick D. Convergence and parallelism reconsidered: what have we learned about the genetics of adaptation? Trends in Ecology & Evolution. 2008;23:26–32. doi: 10.1016/j.tree.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Baldwin BG. Switch from specialized to generalized pollination. Nature. 1998;394:632–632. [Google Scholar]
- Armbruster WS, Muchhala N. Associations between floral specialization and species diversity: cause, effect, or correlation? Evolutionary Ecology. 2009;23:159–179. [Google Scholar]
- Armbruster WS, Edwards ME, Debevec EM. Floral character displacement generates assemblage structure of Western Australian triggerplants (Stylidium) Ecology. 1994;75:315–329. [Google Scholar]
- Armbruster WS, Lee J, Baldwin BG. Macroevolutionary patterns of defense and pollination in Dalechampia vines: adaptation, exaptation, and evolutionary novelty. Proceedings of the National Academy of Sciences of the USA. 2009;106:18085–18090. doi: 10.1073/pnas.0907051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold RM. Pollination, predation and seed set in Linaria vulgaris (Scrophulariaceae) American Midland Naturalist. 1982;107:360–369. [Google Scholar]
- Bell CD, Soltis DE, Soltis PS. The age and diversification of the angiosperms re-revisited. American Journal of Botany. 2010;97:1296–1303. doi: 10.3732/ajb.0900346. [DOI] [PubMed] [Google Scholar]
- Blanco-Pastor JL, Vargas P, Pfeil BE. Coalescent simulations reveal hybridization and incomplete lineage sorting in Mediterranean Linaria. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039089. e39089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Pastor JL, Fernández-Mazuecos M, Vargas P. Past and future demographic dynamics of alpine species: limited genetic consequences despite dramatic range contraction in a plant from the Spanish Sierra Nevada. Molecular Ecology. 2013;22:4177–4195. doi: 10.1111/mec.12383. [DOI] [PubMed] [Google Scholar]
- Brakefield PM. Evo-devo and constraints on selection. Trends in Ecology & Evolution. 2006;21:362–368. doi: 10.1016/j.tree.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Bruun HG. Genetical notes on Linaria, I-II. Hereditas. 1937;22:395–400. [Google Scholar]
- Butler MA, King AA. Phylogenetic comparative analysis: a modeling approach for adaptive evolution. American Naturalist. 2004;164:683–695. doi: 10.1086/426002. [DOI] [PubMed] [Google Scholar]
- Cacho NI, Berry PE, Olson ME, Steinmann VW, Baum DA. Are spurred cyathia a key innovation? Molecular systematics and trait evolution in the slipper spurges (Pedilanthus clade: Euphorbia, Euphorbiaceae) American Journal of Botany. 2010;97:493–510. doi: 10.3732/ajb.0900090. [DOI] [PubMed] [Google Scholar]
- Campbell DR. Pollinator shifts and the origin and loss of plant species. Annals of the Missouri Botanical Garden. 2008;95:264–274. [Google Scholar]
- Carrió E, Güemes J, Herreros R. Pollination biology in an endangered rocky mountain toadflax (Linaria cavanillesii) Plant Biosystems. 2012;147:354–363. [Google Scholar]
- Darwin C. On the various contrivances by which British and foreign orchids are fertilized by insects. London: John Murray; 1862. [PMC free article] [PubMed] [Google Scholar]
- Darwin C. The effects of cross and self fertilisation in the vegetable kingdom. London: John Murray; 1876. [Google Scholar]
- Darwin C. The different forms of flowers on plants of the same species. London: John Murray; 1877. [Google Scholar]
- Davis MP, Midford PE, Maddison W. Exploring power and parameter estimation of the BiSSE method for analyzing species diversification. BMC Evolutionary Biology. 2013;13:38. doi: 10.1186/1471-2148-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan JH, Rosenberg NA. Gene tree discordance, phylogenetic inference and the multispecies coalescent. Trends in Ecology & Evolution. 2009;24:332–340. doi: 10.1016/j.tree.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Dobignard A. Nouvelles observations sur la flore du Maroc. 3. Candollea. 1997;52:119–157. [Google Scholar]
- Docherty Z. Self-incompatibility in Linaria. Heredity. 1982;49:349–352. [Google Scholar]
- Dodd ME, Silvertown J, Chase MW. Phylogenetic analysis of trait evolution and species diversity variation among angiosperm families. Evolution. 1999;53:732–744. doi: 10.1111/j.1558-5646.1999.tb05367.x. [DOI] [PubMed] [Google Scholar]
- Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Ho SYW, Phillips MJ, Rambaut A. Relaxed phylogenetics and dating with confidence. PLoS Biology. 2006;4:699–710. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SV. Is a new and general theory of molecular systematics emerging? Evolution. 2009;63:1–19. doi: 10.1111/j.1558-5646.2008.00549.x. [DOI] [PubMed] [Google Scholar]
- Endress PK. Evolution and floral diversity: the phylogenetic surroundings of Arabidopsis and Antirrhinum. International Journal of Plant Sciences. 1992;153:S106–S122. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Faegri K, van der Pijl L. The principles of pollination ecology. Oxford: Pergamon; 1979. [Google Scholar]
- Fennane M, Ibn Tattou M. Catalogue des plantes vasculaires rares, menacées ou endémiques du Maroc. Bocconea. 1998;8:1–243. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution, and Systematics. 2004;35:375–403. [Google Scholar]
- Fernández-Mazuecos M, Vargas P. Historical isolation versus recent long-distance connections between Europe and Africa in bifid toadflaxes (Linaria sect. Versicolores) PLoS One. 2011;6 doi: 10.1371/journal.pone.0022234. e22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Mazuecos M, Vargas P. Congruence between distribution modelling and phylogeographical analyses reveals Quaternary survival of a toadflax species (Linaria elegans) in oceanic climate areas of a mountain ring range. New Phytologist. 2013;198:1274–1289. doi: 10.1111/nph.12220. [DOI] [PubMed] [Google Scholar]
- Fernández-Mazuecos M, Blanco-Pastor JL, Vargas P. A phylogeny of toadflaxes (Linaria Mill.) based on nuclear internal transcribed spacer sequences: systematic and evolutionary consequences. International Journal of Plant Sciences. 2013;174:234–249. [Google Scholar]
- FitzJohn RG. Quantitative traits and diversification. Systematic Biology. 2010;59:619–633. doi: 10.1093/sysbio/syq053. [DOI] [PubMed] [Google Scholar]
- FitzJohn RG. Diversitree: comparative phylogenetic analyses of diversification in R. Methods in Ecology and Evolution. 2012;3:1084–1092. [Google Scholar]
- FitzJohn RG, Maddison WP, Otto SP. Estimating trait-dependent speciation and extinction rates from incompletely resolved phylogenies. Systematic Biology. 2009;58:595–611. doi: 10.1093/sysbio/syp067. [DOI] [PubMed] [Google Scholar]
- Fiz-Palacios O, Valcárcel V. From Messinian crisis to Mediterranean climate: a temporal gap of diversification recovered from multiple plant phylogenies. Perspectives in Plant Ecology, Evolution and Systematics. 2013;15:130–137. [Google Scholar]
- Fleming TH, Geiselman C, Kress WJ. The evolution of bat pollination: a phylogenetic perspective. Annals of Botany. 2009;104:1017–1043. doi: 10.1093/aob/mcp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futuyma DJ, Moreno G. The evolution of ecological specialization. Annual Review of Ecology and Systematics. 1988;19:207–233. [Google Scholar]
- Gallagher MS. JSTOR Plant Science. In: Nimis PL, Lebbe RV, editors. Tools for identifying biodiversity: progress and problems. Trieste: Edizioni Università di Trieste; 2010. pp. 417–418. [Google Scholar]
- Gernhard T. The conditioned reconstructed process. Journal of Theoretical Biology. 2008;253:769–778. doi: 10.1016/j.jtbi.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Goldberg EE, Igić B. On phylogenetic tests of irreversible evolution. Evolution. 2008;62:2727–2741. doi: 10.1111/j.1558-5646.2008.00505.x. [DOI] [PubMed] [Google Scholar]
- Goloboff P, Farris J, Nixon K. TNT: tree analysis using new technology. 2003 http://www.zmuc.dk/public/phylogeny/tnt/ (last accessed 24 April 2013) [Google Scholar]
- Gómez JM, Zamora R. Ecological factors that promote the evolution of generalization in pollination systems. In: Waser N, Ollerton J, editors. Plant-pollinator interactions: from generalization to specialization. Chicago: University of Chicago Press; 2006. pp. 145–166. [Google Scholar]
- Gómez JM, Perfectti F, Camacho JPM. Natural selection on Erysimum mediohispanicum flower shape: insights into the evolution of zygomorphy. American Naturalist. 2006;168:531–545. doi: 10.1086/507048. [DOI] [PubMed] [Google Scholar]
- Gómiz F. Dos novedades de Marruecos: Linaria imzica Gómiz, sp. nov. (Scrophulariaceae) y Centaurea austromaroccana (Förther & Podlech) Gómiz, comb. & stat. nov. (Compositae) Anales del Jardín Botánico de Madrid. 2004;61:205–208. [Google Scholar]
- Grant V. Pollination systems as isolating mechanisms in angiosperms. Evolution. 1949;3:82–97. doi: 10.1111/j.1558-5646.1949.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Grant V. Modes and origins of mechanical and ethological isolation in angiosperms. Proceedings of the National Academy of Sciences of the USA. 1994;91:3–11. doi: 10.1073/pnas.91.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant V, Grant KA. Flower pollination in the phlox family. New York: Columbia University Press; 1965. [Google Scholar]
- Hagen KB, Kadereit JW. The diversification of Halenia (Gentianaceae): ecological opportunity versus key innovation. Evolution. 2003;57:2507–2518. [PubMed] [Google Scholar]
- Hall BK. Descent with modification: the unity underlying homology and homoplasy as seen through an analysis of development and evolution. Biological Reviews. 2003;78:409–433. doi: 10.1017/s1464793102006097. [DOI] [PubMed] [Google Scholar]
- Hall BK. Parallelism, deep homology, and evo-devo. Evolution & Development. 2012;14:29–33. doi: 10.1111/j.1525-142X.2011.00520.x. [DOI] [PubMed] [Google Scholar]
- Hamdi SMM, Assadi M, Vaezi G, Mirtadzadini M. A new species of Linaria sect. Versicolores (Scrophulariaceae) from Iran. Novon. 2009;18:340–343. [Google Scholar]
- Hansen TF. Stabilizing selection and the comparative analysis of adaptation. Evolution. 1997;51:1341–1351. doi: 10.1111/j.1558-5646.1997.tb01457.x. [DOI] [PubMed] [Google Scholar]
- Heled J, Drummond AJ. Bayesian inference of species trees from multilocus data. Molecular Biology and Evolution. 2010;27:570–580. doi: 10.1093/molbev/msp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera CM. The adaptedness of the floral phenotype in a relict endemic, hawkmoth-pollinated violet. 1. Reproductive correlates of floral variation. Biological Journal of the Linnean Society. 1990;40:263–274. [Google Scholar]
- Herrera CM. Selection on floral morphology and environmental determinants of fecundity in a hawk moth-pollinated violet. Ecological Monographs. 1993;63:251–275. [Google Scholar]
- Hill EJ. Pollination in Linaria with special reference to cleistogamy. Botanical Gazette. 1909;47:454–466. [Google Scholar]
- Hodges SA. Floral nectar spurs and diversification. International Journal of Plant Sciences. 1997;158:81–88. [Google Scholar]
- Hodges SA, Arnold ML. Spurring plant diversification: are floral nectar spurs a key innovation? Proceedings of the Royal Society Series B, Biological Sciences. 1995;262:343–348. [Google Scholar]
- Huelsenbeck JP, Rannala B, Masly JP. Accommodating phylogenetic uncertainty in evolutionary studies. Science. 2000;288:2349–2350. doi: 10.1126/science.288.5475.2349. [DOI] [PubMed] [Google Scholar]
- Hughes C, Eastwood R. Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proceedings of the National Academy of Sciences of the USA. 2006;103:10334–10339. doi: 10.1073/pnas.0601928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski D. Species selection: theory and data. Annual Review of Ecology, Evolution, and Systematics. 2008;39:501–524. [Google Scholar]
- Johnson SD, Steiner KE. Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae) Evolution. 1997;51:45–53. doi: 10.1111/j.1558-5646.1997.tb02387.x. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Steiner KE. Generalization versus specialization in plant pollination systems. Trends in Ecology & Evolution. 2000;15:140–143. doi: 10.1016/s0169-5347(99)01811-x. [DOI] [PubMed] [Google Scholar]
- Kampny CM. Pollination and flower diversity in Scrophulariaceae. Botanical Review. 1995;61:350–366. [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay KM. Reproductive isolation between two closely related hummingbird pollinated neotropical gingers. Evolution. 2006;60:538–552. [PubMed] [Google Scholar]
- Kay KM, Sargent RD. The role of animal pollination in plant speciation: integrating ecology, geography, and genetics. Annual Review of Ecology, Evolution, and Systematics. 2009;40:637–656. [Google Scholar]
- King AA, Butler MA. ouch: Ornstein-Uhlenbeck models for phylogenetic comparative hypotheses (R package) 2009 http://ouch.r-forge.r-project.org. (last accessed 24 April 2013) [Google Scholar]
- Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Molecular Ecology Resources. 2011;11:353–357. doi: 10.1111/j.1755-0998.2010.02924.x. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP, Monteiro LR. Distances and directions in multidimensional shape spaces: implications for morphometric applications. Systematic Biology. 2005;54:678–688. doi: 10.1080/10635150590947258. [DOI] [PubMed] [Google Scholar]
- Kubatko LS, Degnan JH. Inconsistency of phylogenetic estimates from concatenated data under coalescence. Systematic Biology. 2007;56:17–24. doi: 10.1080/10635150601146041. [DOI] [PubMed] [Google Scholar]
- Leaché AD, Rannala B. The accuracy of species tree estimation under simulation: a comparison of methods. Systematic Biology. 2011;60:126–137. doi: 10.1093/sysbio/syq073. [DOI] [PubMed] [Google Scholar]
- De Leonardis W, Giardina G, Zizza A. Linaria multicaulis (L.) Miller subsp. humilis (Guss.) De Leonardis, Giardina and Zizza, comb. et stat. nov., a taxon growing in Sicily. Flora Mediterranea. 1999;9:97–111. [Google Scholar]
- De Leonardis W, De Santis C, Fichera G, Giardina G, Zizza A. Linaria multicaulis (Scrophulariaceae) in Sicily: an investigation within its subspecific and varietal ranks. Bocconea. 2003;16:585–595. [Google Scholar]
- Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Liu L. BEST: Bayesian estimation of species trees under the coalescent model. Bioinformatics. 2008;24:2542–2543. doi: 10.1093/bioinformatics/btn484. [DOI] [PubMed] [Google Scholar]
- Macior LW. Pollen-foraging behavior of Bombus in relation to pollination of nototribic flowers. American Journal of Botany. 1967;54:359–364. [Google Scholar]
- Maddison WP. Confounding asymmetries in evolutionary diversification and character change. Evolution. 2006;60:1743–1746. [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. 2011 http://mesquiteproject.org. (last accessed 24 April 2013) [Google Scholar]
- Maddison WP, Midford PE, Otto SP. Estimating a binary character's effect on speciation and extinction. Systematic Biology. 2007;56:701–710. doi: 10.1080/10635150701607033. [DOI] [PubMed] [Google Scholar]
- Marazzi B, Sanderson MJ. Large-scale patterns of diversification in the widespread legume genus Senna and the evolutionary role of extrafloral nectaries. Evolution. 2010;64:3570–3592. doi: 10.1111/j.1558-5646.2010.01086.x. [DOI] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE) 2010. 2010:1–8. [Google Scholar]
- Müller L. Anatomisch-biomechanische Studien an maskierten Scrophulariaceen-blüten. Österreichische Botanische Zeitschrift. 1929;80:149–161. [Google Scholar]
- Newman DA, Thomson JD. Effects of nectar robbing on nectar dynamics and bumblebee foraging strategies in Linaria vulgaris (Scrophulariaceae) Oikos. 2005a;110:309–320. [Google Scholar]
- Newman DA, Thomson JD. Interactions among nectar robbing, floral herbivory, and ant protection in Linaria vulgaris. Oikos. 2005b;110:497–506. [Google Scholar]
- van der Niet T, Johnson SD. Phylogenetic evidence for pollinator-driven diversification of angiosperms. Trends in Ecology & Evolution. 2012;27:353–361. doi: 10.1016/j.tree.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Nunn CL. The comparative approach in evolutionary anthropology and biology. Chicago: University of Chicago Press; 2011. [Google Scholar]
- Ollerton J, Alarcón R, Waser NM, et al. A global test of the pollination syndrome hypothesis. Annals of Botany. 2009;103:1471–1480. doi: 10.1093/aob/mcp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis E, Claude J, Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- Pérez R, Vargas P, Arroyo J. Convergent evolution of flower polymorphism in Narcissus (Amaryllidaceae) New Phytologist. 2004;161:235–252. [Google Scholar]
- Porter ML, Crandall KA. Lost along the way: the significance of evolution in reverse. Trends in Ecology & Evolution. 2003;18:541–547. [Google Scholar]
- Posada D. ModelTest: phylogenetic model averaging. Molecular Biology and Evolution. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Rabosky DL. Extinction rates should not be estimated from molecular phylogenies. Evolution. 2010;64:1816–1824. doi: 10.1111/j.1558-5646.2009.00926.x. [DOI] [PubMed] [Google Scholar]
- Rabosky DL, McCune AR. Reinventing species selection with molecular phylogenies. Trends in Ecology & Evolution. 2010;25:68–74. doi: 10.1016/j.tree.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Rambaut A, Drummond AJ. Tracer version 1·4. 2007 http://beast.bio.ed.ac.uk/Tracer. (last accessed 24 April 2013) [Google Scholar]
- Robertson C. Zygomorphy and its causes. III. Botanical Gazette. 1888;13:224–230. [Google Scholar]
- Rohlf FJ. tpsDig, version 2·16. 2010 http://life.bio.sunysb.edu/morph/ (last accessed 24 April 2013) [Google Scholar]
- Rohlf FJ, Slice D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Systematic Zoology. 1990;39:40–59. [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Sáez L, Bernal M. Linaria Mill. In: Castroviejo S, Herrero A, Benedí C, Rico E, Güemes J, editors. Flora Iberica. Vol. XIII. Madrid: CSIC; 2009. pp. 232–324. [Google Scholar]
- Sánchez-Lafuente AM. Corolla herbivory, pollination success and fruit predation in complex flowers: an experimental study with Linaria lilacina (Scrophulariaceae) Annals of Botany. 2007;99:355–364. doi: 10.1093/aob/mcl267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Lafuente AM, Rodríguez-Gironés MA, Parra R. Interaction frequency and per-interaction effects as predictors of total effects in plant-pollinator mutualisms: a case study with the self-incompatible herb Linaria lilacina. Oecologia. 2011;168:153–165. doi: 10.1007/s00442-011-2084-z. [DOI] [PubMed] [Google Scholar]
- Sargent RD. Floral symmetry affects speciation rates in angiosperms. Proceedings of the Royal Society Series B, Biological Sciences. 2004;271:603–608. doi: 10.1098/rspb.2003.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherson RA, Vidal R, Sanderson MJ. Phylogeny, biogeography, and rates of diversification of New World Astragalus (Leguminosae) with an emphasis on South American radiations. American Journal of Botany. 2008;95:1030–1039. doi: 10.3732/ajb.0800017. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Schlüter PM. Floral isolation, specialized pollination, and pollinator behavior in orchids. Annual Review of Entomology. 2009;54:425–446. doi: 10.1146/annurev.ento.54.110807.090603. [DOI] [PubMed] [Google Scholar]
- Scotland RW. What is parallelism? Evolution & Development. 2011;13:214–227. doi: 10.1111/j.1525-142X.2011.00471.x. [DOI] [PubMed] [Google Scholar]
- Shipunov AB, Bateman RM. Geometric morphometrics as a tool for understanding Dactylorhiza (Orchidaceae) diversity in European Russia. Biological Journal of the Linnean Society. 2005;85:1–12. [Google Scholar]
- Slice DE. Landmark coordinates aligned by Procrustes analysis do not lie in Kendall's shape space. Systematic Biology. 2001;50:141–149. doi: 10.1080/10635150119110. [DOI] [PubMed] [Google Scholar]
- Smith CI, Pellmyr O, Althoff DM, Balcázar-Lara M, Leebens-Mack J, Segraves KA. Pattern and timing of diversification in Yucca (Agavaceae): specialized pollination does not escalate rates of diversification. Proceedings of the Royal Society Series B, Biological Sciences. 2008;275:249–258. doi: 10.1098/rspb.2007.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SDW. Using phylogenetics to detect pollinator-mediated floral evolution. New Phytologist. 2010;188:354–363. doi: 10.1111/j.1469-8137.2010.03292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SDW, Miller RE, Otto SP, FitzJohn RG, Rausher MD. The effects of flower color transitions on diversification rates in morning glories (Ipomoea subg. Quamoclit, Convolvulaceae) In: Long M, Gu H, Zhou Z, editors. Darwin's heritage today: proceedings of the Darwin 200 Beijing International Conference. Beijing: Higher Education Press; 2010. pp. 202–226. [Google Scholar]
- Sprengel CK. Das entdeckte Geheimniss der Natur im Bau und in der Befruchtung der Blumen. 1793 Berlin: F. Vieweg. [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stanley SM. A theory of evolution above the species level. Proceedings of the National Academy of Sciences. 1975;72:646–650. doi: 10.1073/pnas.72.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GL. Adaptive radiation of reproductive characteristics in angiosperms, I: pollination mechanisms. Annual Review of Ecology and Systematics. 1970;1:307–326. [Google Scholar]
- Stephens M, Donnelly P. A comparison of Bayesian methods for haplotype reconstruction from population genotype data. American Journal of Human Genetics. 2003;73:1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. American Journal of Human Genetics. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Allen JA, Goulson D. Nectar robbing, forager efficiency and seed set: bumblebees foraging on the self incompatible plant Linaria vulgaris (Scrophulariaceae) Acta Oecologica. 2000;21:277–283. [Google Scholar]
- Suc JP. Origin and evolution of the Mediterranean vegetation and climate in Europe. Nature. 1984;307:429–432. [Google Scholar]
- Sutton DA. A new section of Linaria (Scrophulariaceae: Antirrhineae) Botanical Journal of the Linnean Society. 1980;81:169–184. [Google Scholar]
- Sutton DA. A revision of the tribe Antirrhineae. Oxford: Oxford University Press; 1988. [Google Scholar]
- Tan K, Iatrou G. Endemic plants of Greece: the Peloponnese. Copenhagen: Gad; 2001. [Google Scholar]
- Tripp EA, Manos PS. Is floral specialization an evolutionary dead-end? pollination system transitions in Ruellia (Acanthaceae) Evolution. 2008;62:1712–1737. doi: 10.1111/j.1558-5646.2008.00398.x. [DOI] [PubMed] [Google Scholar]
- Valdés B. Taxonomía experimental del género Linaria IV. Reproducción sexual: autogamia y polinización intraespecífica. Boletín de la Real Sociedad Española de Historia Natural. Sección Biológica. 1970;68:79–89. [Google Scholar]
- Valente LM, Savolainen V, Vargas P. Unparalleled rates of species diversification in Europe. Proceedings of the Royal Society Series B, Biological Sciences. 2010a;277:1489–1496. doi: 10.1098/rspb.2009.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente LM, Reeves G, Schnitzler J, et al. Diversification of the African genus Protea (Proteaceae) in the Cape biodiversity hotspot and beyond: equal rates in different biomes. Evolution. 2010b;64:745–760. doi: 10.1111/j.1558-5646.2009.00856.x. [DOI] [PubMed] [Google Scholar]
- Valente LM, Manning JC, Goldblatt P, Vargas P. Did pollination shifts drive diversification in Southern African Gladiolus? Evaluating the model of pollinator-driven speciation. American Naturalist. 2012;180:83–98. doi: 10.1086/666003. [DOI] [PubMed] [Google Scholar]
- Vargas P, Rosselló JA, Oyama R, Güemes J. Molecular evidence for naturalness of genera in the tribe Antirrhineae (Scrophulariaceae) and three independent evolutionary lineages from the New World and the Old. Plant Systematics and Evolution. 2004;249:151–172. [Google Scholar]
- Vargas P, Ornosa C, Ortiz-Sanchez FJ, Arroyo J. Is the occluded corolla of Antirrhinum bee-specialized? Journal of Natural History. 2010;44:1427–1443. [Google Scholar]
- Viano J. Note sur le genre Linaria en Méditerranée occidentale. Le groupe Linaria bipartita (Vent.) Willd. Naturalia Monspeliensia, Série Botanique. 1969;20:219–240. [Google Scholar]
- Viano J. Les linaires à graines aptères du bassin méditerranéen occidental. 1. Linaria sect. Versicolores. Candollea. 1978a;33:33–88. [Google Scholar]
- Viano J. Les linaires à graines aptères du bassin méditerranéen occidental. 2. Linaria sect. Elegantes, Bipunctatae, Diffusae, Speciosae, Repentes. Candollea. 1978b;33:209–267. [Google Scholar]
- Wake DB. Homoplasy: the result of natural selection, or evidence of design limitations? American Naturalist. 1991;138:543–567. [Google Scholar]
- Wake DB, Wake MH, Specht CD. Homoplasy: from detecting pattern to determining process and mechanism of evolution. Science. 2011;331:1032–1035. doi: 10.1126/science.1188545. [DOI] [PubMed] [Google Scholar]
- Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. Generalization in pollination systems, and why it matters. Ecology. 1996;77:1043–1060. [Google Scholar]
- Wertheim JO, Sanderson MJ. Estimating diversification rates: how useful are divergence times? Evolution. 2011;65:309–320. doi: 10.1111/j.1558-5646.2010.01159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittall JB, Hodges SA. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature. 2007;447:706–709. doi: 10.1038/nature05857. [DOI] [PubMed] [Google Scholar]
- Yang CF, Gituru RW, Guo YH. Reproductive isolation of two sympatric louseworts, Pedicularis rhinanthoides and Pedicularis longiflora (Orobanchaceae): how does the same pollinator type avoid interspecific pollen transfer? Biological Journal of the Linnean Society. 2007;90:37–48. [Google Scholar]
- Yu Y, Than C, Degnan JH, Nakhleh L. Coalescent histories on phylogenetic networks and detection of hybridization despite incomplete lineage sorting. Systematic Biology. 2011;60:138–149. doi: 10.1093/sysbio/syq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Degnan JH, Nakhleh L. The probability of a gene tree topology within a phylogenetic network with applications to hybridization detection. PLoS Genetics. 2012;8 doi: 10.1371/journal.pgen.1002660. e1002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelditch ML, Swiderski DL, Sheets HD, Fink WL. Geometric morphometrics for biologists: a primer. San Diego: Elsevier Academic; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.