Abstract
Background and Aims
Stomata formed at high relative air humidity (RH) respond less to abscisic acid (ABA), an effect that varies widely between cultivars. This study tested the hypotheses that this genotypic variation in stomatal responsiveness originates from differential impairment in intermediates of the ABA signalling pathway during closure and differences in leaf ABA concentration during growth.
Methods
Stomatal anatomical features and stomatal responsiveness to desiccation, feeding with ABA, three transduction elements of its signalling pathway (H2O2, NO, Ca2+) and elicitors of these elements were determined in four rose cultivars grown at moderate (60 %) and high (90 %) RH. Leaf ABA concentration was assessed throughout the photoperiod and following mild desiccation (10 % leaf weight loss).
Key Results
Stomatal responsiveness to desiccation and ABA feeding was little affected by high RH in two cultivars, whereas it was considerably attenuated in two other cultivars (thus termed sensitive). Leaf ABA concentration was lower in plants grown at high RH, an effect that was more pronounced in the sensitive cultivars. Mild desiccation triggered an increase in leaf ABA concentration and equalized differences between leaves grown at moderate and high RH. High RH impaired stomatal responses to all transduction elements, but cultivar differences were not observed.
Conclusions
High RH resulted in decreased leaf ABA concentration during growth as a result of lack of water deficit, since desiccation induced ABA accumulation. Sensitive cultivars underwent a larger decrease in leaf ABA concentration rather than having a higher ABA concentration threshold for inducing stomatal functioning. However, cultivar differences in stomatal closure following ABA feeding were not apparent in response to H2O2 and downstream elements, indicating that signalling events prior to H2O2 generation are involved in the observed genotypic variation.
Keywords: Abscisic acid signal transduction, calcium, hydrogen peroxide, methyl jasmonate, nitric oxide, Rosa hybrida, salicylic acid, stomatal closure, stomatal malfunction, transpiration
INTRODUCTION
Abscisic acid (ABA) has been defined as a stress hormone because a rapid increase in its synthesis is elicited by various stresses, mediating plant responses to cope with the applied stress (Leung and Giraudat, 1998; Zhang et al., 2006). It is now well accepted that ABA also exerts an important regulatory role in stomatal functioning in the absence of stress (Franks and Farquhar, 2001; Wigger et al., 2002). For instance, plants grown at high relative air humidity (RH ≥85 %) display very low transpirational water loss and are minimally exposed to water deficit, as manifested by the higher leaf water potential, compared with well-watered plants grown at moderate atmospheric humidity (Fanourakis et al., 2011). However, long-term high RH also results in decreased leaf ABA concentration ([ABA]) (Rezaei Nejad and van Meeteren, 2007; Arve et al., 2013), which in turn induces phenotypes with weak stomatal responsiveness (Fanourakis et al., 2012; Rezaei Nejad and van Meeteren, 2008). As a consequence, in environments where evaporative demand exceeds transport capacity, high-RH-grown plants frequently wilt even when soil water is readily available due to their inability to limit transpiration (Arve et al., 2013; Fanourakis et al., 2013b). Recently, a wide variation in the degree to which this effect appears was found among cultivars of the same species (Fanourakis et al., 2013a; Giday et al., 2013). One illustrative result is that high atmospheric humidity decreased the leaflet hydration level at which the maximum stomatal closure occurred by between 12 and 143 % (Giday et al., 2013). Fanourakis et al. (2013a) discuss the idea that cultivar differences are related to variation in [ABA] or the [ABA] threshold required for inducing stomatal functioning, but do not offer experimental evidence. Assessment of the reasons underlying the described genotypic variation is of significant interest for our understanding of the relationship between [ABA] during growth and the control of water loss by plants.
Like plants with long-term decreased [ABA], a number of ABA-insensitive mutants displayed weak stomatal responsiveness and were thus highly prone to lethal wilting (Koornneef et al., 1984). Later studies demonstrated that certain signalling events of the ABA transduction cascade were impaired or disrupted, and when these were experimentally activated the stomata of ABA-insensitive mutants reacted similarly to wild type (Allen et al., 1999; Iwai et al., 2003). These results indicate that lack or attenuation of intermediate elements in the ABA signalling network do not necessarily affect the signalling components downstream from these regions. ABA induction of stomatal closure is mediated by two responses operating on different time scales (Geiger et al., 2011; Brandt et al., 2012). The slower response (minutes to hours) involves gene expression under transcriptional regulation (Fujii et al., 2009; Kline et al., 2010; for potential effects of high RH see Aliniaeifard and van Meeteren, 2013). The fast response (seconds to minutes) encompasses the ABA receptors [PYRabactin Resistance (PYR)/PYR1-like (PYL)/regulatory components of ABA receptor (RCARs)] together with positive [SNF1-related protein kinase (SnRK2)/open stomata 1 (OST1)] and negative [type 2C protein phosphatases (PP2Cs)] pathway modulators (reviewed by Cutler et al. 2010; for potential effects of high RH see Aliniaeifard and van Meeteren, 2013). Key intermediates, acting downstream of the fast response pathway components, on sequence include hydrogen peroxide (H2O2) (Pei et al., 2000), nitric oxide (NO) (Garcia-Mata et al., 2003; Bright et al., 2006) and calcium (Ca2+) (McAinsh, 1990). Growing evidence suggests that these three elements serve similar functions in the stomatal response pathways of methyl jasmonate (MJ) (Suhita et al., 2004; Munemasa et al., 2011a, b) and salicylic acid (SA) (Mori et al., 2001; Khokon et al., 2011), regulating physiological processes such as plant defence against microorganisms (Suhita et al., 2004; Zeng et al., 2011). Despite a well-documented genotypic effect on the degree to which stomatal sensitivity to ABA is attenuated by elevated atmospheric humidity (Fanourakis et al., 2013a), it is less clear whether it originates from variation in fast response pathway components or downstream intermediates. Given that the ABA, MJ and SA networks converge on the regulation of stomatal closure, it may be expected that the latter two pathways are also differentially impaired by high RH during growth.
Four cultivars with contrasting sensitivity to elevated RH were selected to determine the relationship between [ABA] and regulation of water loss in high-RH-grown plants. We also examined whether the ability of the leaf to accumulate ABA contributed to the intraspecific variation in [ABA]. Another objective of this study was to examine whether intermediate elements (H2O2, NO, Ca2+) in the ABA signalling network contribute to the genotypic variation in stomatal response after leaf development in more humid air. We tested the hypotheses that genotypic variation in the control of water loss is primarily driven by differential effects on [ABA] rather than variation in the [ABA] threshold required for inducing stomatal functioning, and that it originates from intermediate elements in the ABA signalling pathway.
MATERIALS AND METHODS
Plant material and growth conditions
Rooted cuttings of four pot rose cultivars (Rosa hybrida ‘Apache Kordana’, ‘Pearl Kordana’, ‘Escimo Kordana’ and ‘Mandarina Kordana’) were planted in 0·55 L pots (four cuttings of the same cultivar per pot) containing a mixture of peat and perlite (9:1, v/v; Meegaa Substrates BV, Rotterdam, the Netherlands) at a commercial nursery (Rosa Danica, Marslev, Denmark). In subsequent parts of this paper, cultivars will be referred to without the (common) second part of their name (e.g. ‘Apache’ in place of ‘Apache Kordana’). Cultivar selection was based on their contrasting water loss rates following growth at high RH: ‘Apache’ and ‘Pearl’ showed transpirational water loss rates similar to plants grown at moderate RH (defined as tolerant to high RH), whereas ‘Escimo’ and ‘Mandarina’ had considerably higher water loss compared with moderate RH-grown plants (defined as sensitive to high RH) (Giday et al., 2013). Four weeks after planting, the plants were pruned, leaving two or three leaves on the shoot. Subsequently, 20 pots per cultivar were obtained from the nursery and placed in two walk-in growth chambers at a density of 25 pots m−2. Each chamber accommodated two tables, established as plots, every plot having five randomly distributed pots of each cultivar. In one chamber the RH was 60 ± 3 % (moderate RH) and in the other it was 90 ± 2 % (high RH). Air temperature was kept constant (20·5 ± 1·4 °C ) in both chambers, resulting in vapour pressure deficits (VPDs) of 0·94 ± 0·07 and 0·23 ± 0·05 kPa for moderate and high RH chambers, respectively. Light was provided by fluorescence lamps (HQI-BT 400W/D Pro, Slovakia) at 400 ± 15 μmol m−2 s−1 photosynthetic photon flux density (PPFD; determined with a LI-250A; LI-COR, Lincoln, NE, USA) for 18 h per day. Air temperature and RH were measured continuously with sensors (Humitter 50U/50Y(X), Vaisala, Helsinki, Finland) placed at the top of fully grown plants (i.e. 60 cm from the root–shoot interface), and data were automatically recorded by loggers (Datataker; Thermo Fisher Scientific Australia Pty Ltd, Scoresby, Australia). Plants were fertigated twice a day (>20 % drainage day−1). Electrical conductivity and pH of the drain water were monitored daily and adjusted to 2 dS m−1 and 5·5, respectively. Border plants (adjacent to chamber walls) were not sampled. All measurements were conducted on fully expanded sunlit leaves, sampled from fully grown plants (defined as bearing at least two flower buds with cylindrical shape and pointed tip).
Stomatal and pore anatomy
To study the effect of high RH on stomatal anatomy, the length, width and density (i.e. number per unit leaf area) of stomata, together with pore length and aperture, were assessed in the four cultivars. The stomatal and pore lengths correspond to the major axes (i.e. longest diameter) of the respective ellipse, while stomatal width and pore aperture were taken as the minor axes (i.e. shortest diameter) (Savvides et al., 2012). Measurements were made by the silicon rubber impression technique (Giday et al., 2013) using a lateral leaflet of the first pentafoliate leaf (counting from the apex). The abaxial (lower) leaflet surface was assessed, since the rose is a hypostomatous species (Fanourakis et al., 2013a). Sampling took place 2 h following the onset of the light period, since this time is required for plants exposed to prolonged darkness (i.e. night-time period) to open stomata and reach a steady-state operating stomatal conductance (Drake et al., 2013). Stomatal (length, width) and pore (length, aperture) anatomical features were determined on 30 randomly selected stomata per leaflet, and stomatal density was counted on five non-overlapping fields of view. Five leaflets (one leaflet per plant, one plant per pot) were assessed.
Stomatal responsiveness to seven closing stimuli
To determine the effect of high RH on stomatal responsiveness to closing stimuli in the four cultivars, terminal leaflets of the first and second five-leaflet leaves (counting from the apex) were sampled, re-cut by submerging their petiole under water (to prevent cavitation of xylem vessels that were opened by cutting) and placed in flasks filled with degassed water. Leaflets were harvested at least 2 h after the beginning of the photoperiod. The environmental conditions in the test room, where all closing stimuli were applied, were as follows: air temperature 21·0 ± 1·2 °C, RH 50 ± 4 % (i.e. VPD 1·24 ± 0·03 kPa), and 50 μmol m−2 s−1 PPFD provided by fluorescent lamps (T5 fluorescent lamp, GE Lighting, Cleveland, OH, USA).
For the desiccation stimulus, leaflets were further incubated at 21 °C, about 100 % RH (i.e. VPD close to 0) and under 15 μmol m−2 s−1 PPFD for 1 h to establish their maximum fresh weight (Fanourakis et al., 2011). The leaflets were then placed on a bench (abaxial surface facing down) in the test room and transpiration rate was recorded for 4 h by gravimetry. At the end of the measurement, leaflet area was determined (LI-250A, LI-COR, Lincoln, NE, USA) and leaflets were dried in a forced-air oven at 80 °C for at least 48 h prior to determining dry weight. Leaflet relative water content (RWC) was calculated using the following equation (Slavik, 1974):
| (1) |
The hormones ABA, MJ and SA, together with three common elements of their signalling pathways (H2O2, NO and Ca2+), were also applied through the transpiration stream, as stomatal closing treatments. For these stimuli, the leaflets were left for 1 h with their petioles in water (based on preliminary experiments) in the test room in order to reach a steady state prior to measurements. Subsequently, the transpiration rate of the leaflets in water was recorded by regular weighings (every 20 min) for 1 h. The leaflets were then transferred to flasks containing water (as a control), 100 μM ABA (Fanourakis et al., 2013a), 200 μM MJ (Herde et al., 1997), 1 mM SA (Manthe et al., 1992), 200 μM H2O2 (Bright et al., 2006), 200 μM sodium nitroprusside [SNP (NO donor)] (He et al., 2004; Rezaei Nejad et al., 2006) or 8 mM Ca2+ (Atkinson et al., 1990). The leaflet transpiration rate was further determined by weighing the flasks every 20 min for 4 h 40 min. Throughout the measurements, leaflets in the vials were maintained inclined at 10 ° to the horizontal, and flasks were sealed with parafilm to prevent evaporation. Following the measurements, leaflet area was determined as mentioned above. Closing stimulus intake was calculated as the product of leaflet transpiration rate and the closing stimulus concentration of the feeding solution (Fanourakis et al., 2013a).
Measurements were carried out on eight (petiole-fed closing stimuli) or nine (desiccation) leaflets (one leaflet per plant, one plant per pot).
Deriving the closing stimulus intake (EC½) inducing 50 % of stomatal response
For the xylem-fed closing stimuli (i.e. ABA, MJ, SA, SNP, H2O2, Ca2+), the change in transpiration rate in response to closing stimulus intake showed the typical features of a dose–response curve (stimulus intake was treated as the dose), and was fitted with a four-parameter logistic model
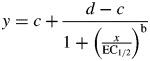 |
(2) |
(Secchi et al., 2013). The coefficients d (maximum value) and c (minimum value) correspond to the transpiration rates prior to (t = 0) and following (t = 4 h 40 min) feeding, respectively. The half-maximal effective concentration (EC½) refers to the stimulus intake required to induce 50 % of the change between the maximum and minimum values (known as the inclination point), while b is the relative slope at EC½ (the so-called Hill slope). The EC½ therefore represents the fed amount of a closing stimulus at which 50 % of its maximal effect is observed. By using eqn (2), values for b, c, d and EC½ were determined in response to the six closing stimuli applied through the petiole for each leaflet (n = 8) in the four studied cultivars. In all cases, an F-test for lack of fit was performed, indicating that data were well described (P > 0·05) by eqn (2).
[ABA] during growth and following desiccation
The effect of ambient humidity on [ABA] was determined in the four cultivars at three different times during the photoperiod. Five-leaflet leaves were collected at 0, 4 and 8 h following the onset of the light period, immediately frozen in liquid nitrogen and stored at –80 °C for further analysis.
In addition, it was assessed whether the [ABA] response to desiccation was affected by the RH of growth in ‘Escimo’ (the most sensitive) and ‘Apache’ (the most tolerant). Terminal leaflets were collected 2 h following the beginning of the photoperiod, and the same procedure as that applied for leaf desiccation was followed, as described above. Leaflets were allowed to dehydrate to 90 % of the initial fresh weight (corresponding to 85 ± 2 % RWC). Immediately afterwards, the leaflets were sealed in polyethylene bags (to prevent further water loss) and stored in darkness (21·0 ± 0·1 °C ) for 6 h. The darkening was applied to prevent ABA degradation, since ABA is light-sensitive (Davies and Jones, 1991). Following the dehydration event, leaflets were sampled at three different times (0, 3 and 6 h), immediately frozen in liquid nitrogen and stored at –80 °C for further analysis. Following 6 h of incubation, further weight loss was less than 1 % of leaflet weight, indicating the effectiveness of the sealing method.
Liquid nitrogen-frozen samples were freeze-dried, ground and homogenized (Genogrinder 2000; Metuchen, NJ, USA). Deuterated internal standard (2·4 ng mL−1) was then added to the homogenized samples, before extraction with an accelerated solvent extraction system (ASE 350, Dionex, Hvidovre, Denmark) as described by Pedersen et al. (2011). All extractions were duplicated and extracts were diluted with an equal volume of water before analysis.
Chromatographic separation was performed using an HPLC system (Agilent 1200; Agilent, Horsholm, Denmark) equipped with a 150 × 2·1 mm column (Kinetex 2·6 μm PFP, 100Å; Phenomenex, Macclesfield, UK). Gradient elution was performed with 7 % acetonitrile in 20 mM acetic acid (solvent A) and 78 % acetonitrile in 20 mM acetic acid (solvent B) at a constant flow rate of 200 μL min−1 and injection volume of 20 μL. A gradient profile with the following proportions of solvent B was applied (t (min), %B): (0, 35), (6, 35), (7, 100), (8, 35), (18, 35). Prior to analysis, the system was equilibrated (8–18 min) with 35 % solvent B.
The chromatographic system was interfaced to a liquid chromatography triple quadrupole mass spectrometer (Sciex 3200; Applied Biosystems, Foster City, CA, USA). The analysis was performed using electrospray ionization in negative mode. Multiple reaction monitoring of unlabelled and labelled ABA analogues was based on the 263 > 153 and 267 > 156 mass transitions for cis-ABA (Sigma-Aldrich, Brøndby, Denmark) and its deuterated analogue (d4 trans-ABA; Plant Biotechnology Institute of the National Research Council of Canada, Saskatoon, SK, Canada), respectively. The calibration curve was prepared from seven ABA standard solutions (0·097–6·250 ng mL−1) to which equal amounts (1·2 ng mL−1) of internal standard were added. Subsequently, for each standard solution the analyte area (divided by the internal standard area) was plotted against the known analyte concentration (divided by the internal standard concentration). Data were analysed using the Analyst software v.1.5.1 (Applied Biosystems). The limits of detection (232·8 pmol ABA g−1 dry weight plant tissue) and quantification (620·8 pmol ABA g−1 dry weight plant tissue) were determined based on a recovery experiment consisting of four replicates (0·0195 ng ABA mL−1).
In these measurements, first and second five-leaflet leaves (counting from the apex) were sampled, and eight replicate leaves (one leaf per plant, one plant per pot) were analysed per treatment. The whole leaf (i.e. the five leaflets were pooled) was analysed for the determination of [ABA] at different times in the photoperiod, whereas only the terminal leaflet was sampled when applying desiccation and subsequently measuring [ABA].
Statistical analysis
Data were subjected to analysis of variance using R software (version 2·14·2; www.r-project.org). Because no significant plot effects were revealed by ANOVA (i.e. minimal microsite influence), data for the two plots were allowed to pool (i.e. individual plants were treated as independent observations) for further analysis. Treatment effects were tested at the 5 % probability level and the mean separation was done using least significant differences based on the Bonferroni-adjusted LSD (P = 0·05).
RESULTS
Stomatal and pore anatomy
The effect of high (90 %) RH on stomatal anatomical features was investigated in the four cultivars (Table 1). Leaves expanded at high RH had a higher (5 %) number of stomata per leaf area. Moreover, stomata formed at high RH not only showed enhanced length (3–8 %, though not significant for ‘Apache’) and width (2 %), but also possessed longer (9–16 %) and wider (7 %) pores.
Table 1.
Stomatal and pore anatomical features of four pot rose cultivars grown at moderate (60 %) or high (90 %) relative air humidity (RH)
| Stomatal |
Pore |
|||||
|---|---|---|---|---|---|---|
| Density (mm−2) | Length (μm) | Width (μm) | Length (μm) | Aperture (μm) | ||
| RH | 60 | 49b | 40 | 31b | 22 | 8·1b |
| 90 | 51a | 43 | 32a | 25 | 8·7a | |
| Cultivar | ||||||
| ‘Apache' | 60 | 45 | 39d | 29 | 21c | 6·4 |
| 90 | 45 | 40cd | 29 | 23b | 6·8 | |
| ‘Pearl' | 60 | 55 | 40c | 31 | 20c | 6·7 |
| 90 | 58 | 42b | 32 | 23b | 7·4 | |
| ‘Escimo' | 60 | 37 | 43b | 34 | 24b | 11·0 |
| 90 | 40 | 45a | 34 | 26a | 11·3 | |
| ‘Mandarina' | 60 | 59 | 40cd | 32 | 23b | 8·4 |
| 90 | 62 | 43b | 32 | 27a | 9·3 | |
| F probability | ||||||
| Cultivar | *** | *** | *** | *** | *** | |
| RH | ** | *** | * | *** | *** | |
| Cultivar × RH | NS | *** | NS | ** | NS | |
Measurements were made 2 h following the onset of the light period. Five fields of view (stomatal density) and 30 stomata (stomatal and pore dimensions) were analysed per leaf. Values are the means of five leaves. Means followed by different letters indicate significant differences according to the Bonferroni-adjusted LSD test (comparison in columns).
NS, not significant; *significant at the 0·05 probability level; **significant at the 0·01 probability level; ***significant at the 0·001 probability level.
Stomatal response to desiccation
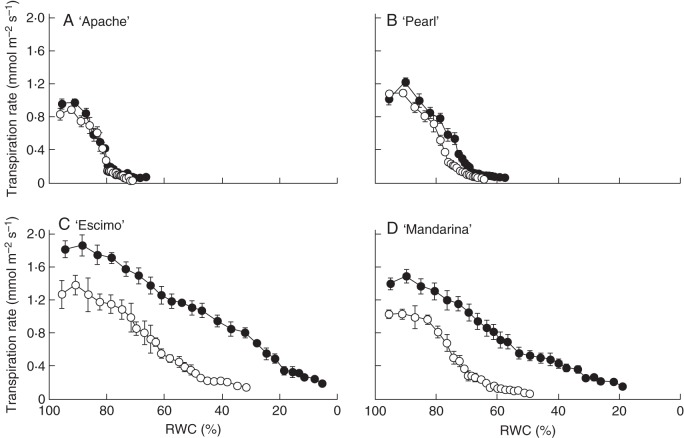
Water loss through the stomatal pores has been identified as the principal determinant of the high-RH-induced increase in leaf transpiration in various rose cultivars, since cuticular water loss makes a minor contribution (Fanourakis et al., 2013a). Against this background, the transpiration rate in response to leaflet desiccation was measured to evaluate the effect of growth RH on stomatal opening in the four cultivars (Fig. 1). Following a transient increase in water loss (known as a wrong-way response), transpiration rate decreased as leaflets dehydrated. In general, stomata on leaves expanded at high RH remained more open, leading to higher transpirational water loss and more dehydrated leaves (i.e. lower RWC) following desiccation. However, the extent to which this effect was observed depended strongly on the cultivar. For instance, the RWC at the end of desiccation was significantly affected by the interaction between RH and cultivar (P < 0·01). RWC at the end of desiccation was slightly decreased (≈7 %; Fig. 1A, B) by high RH in ‘Apache’ and ‘Pearl’ (defined as tolerant to high RH), whereas RWC was considerably lower (52–75 %; Fig. 1C, D) in high-RH-grown leaves of ‘Escimo’ and ‘Mandarina’ (defined as sensitive to high RH).
Fig. 1.
Transpiration rate as a function of relative water content (RWC) during leaflet desiccation of four pot rose cultivars grown at moderate (60 %, open symbols) or high (90 %, closed symbols) relative air humidity. Leaflets were left to desiccate for 4 h. Values are means of nine leaflets ± SEM.
Relationship between stomatal responsiveness and [ABA] in growth conditions
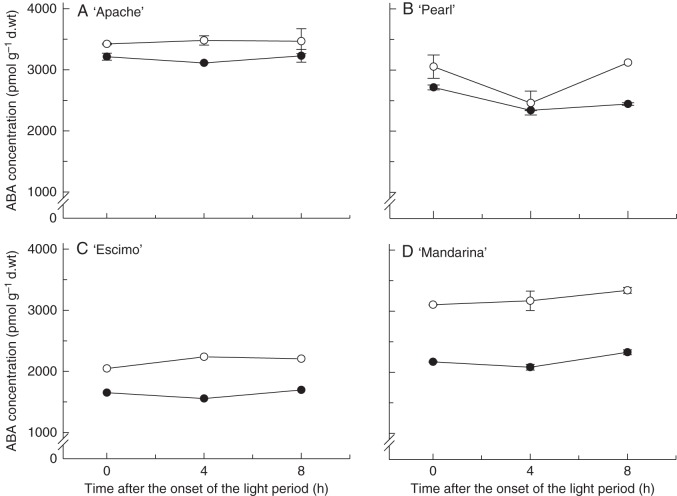
[ABA] was determined at different times during the photoperiod in the studied cultivars grown (and assessed) under both RH conditions. [ABA] was found to be significantly affected by the interaction between RH and cultivar (P < 0·01; Fig. 2). In the tolerant cultivars (‘Apache’, ‘Pearl’), [ABA] was slightly lower (7–11 %; Fig. 2A, B) in leaves developed at high RH, though high-RH-grown leaves of the sensitive cultivars (‘Escimo’, ‘Mandarina’) had a substantially decreased [ABA] (25–32 %; Fig. 2C, D). These differences were similar throughout the light period, since [ABA] did not change significantly during this time (Fig. 2).
Fig. 2.
Endogenous leaflet ABA concentration, expressed on a dry weight (d.wt) basis, at different times after the onset of the light period of four pot rose cultivars grown at moderate (60 %, open symbols) or high (90 %, closed symbols) relative air humidity. Values are the means of either one (C) or two (A, B, D) extractions. Eight leaflets were pooled for extraction.
It was also investigated whether [ABA] at growth conditions underlay variation in stomatal responsiveness, as expressed by dehydration curves. The correlation between [ABA] under growth conditions and RWC following desiccation was found to be highly significant (Fig. 3).
Fig. 3.
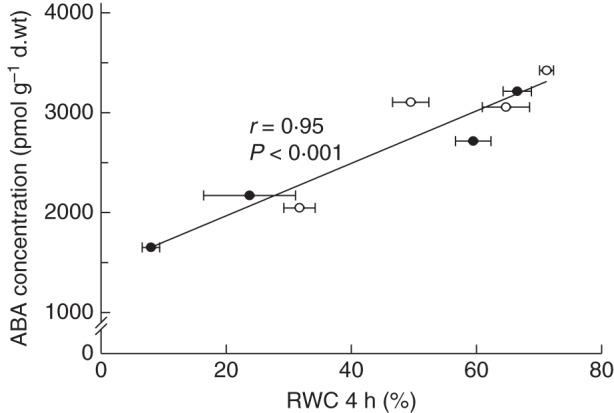
Endogenous leaflet ABA concentration, expressed on a dry weight (d.wt) basis, before the onset of the light period as a function of relative water content (RWC) 4 h after leaflet desiccation of four pot rose cultivars grown at moderate (60 %, open symbols) or high (90 %, closed symbols) relative air humidity. ABA concentrations refer to either one (‘Escimo’) or two (eight leaflets per extraction) extractions, and RWC values are the means of nine leaflets.
[ABA] in response to desiccation
Since [ABA] at growth conditions was lower in high-RH-grown leaves compared to plants developed at moderate RH (Fig. 2), it was tested whether [ABA] response to water deprivation was impaired by high RH. This analysis was performed in the most tolerant (‘Apache’) and most sensitive (‘Escimo’) cultivars. Leaflets were slightly desiccated (to 85 % RWC), and subsequently stored under conditions where further water loss was prevented. In all cases, dehydration induced an increase in [ABA] (Fig. 4). Leaves expanded at high RH had lower [ABA] immediately after desiccation (t = 0), but later on they reached [ABA] similar to that of leaves developed at moderate RH. The sensitive cultivar showed lower [ABA] than the tolerant cultivar following desiccation, but this difference was not noticeable after 6 h.
Fig. 4.
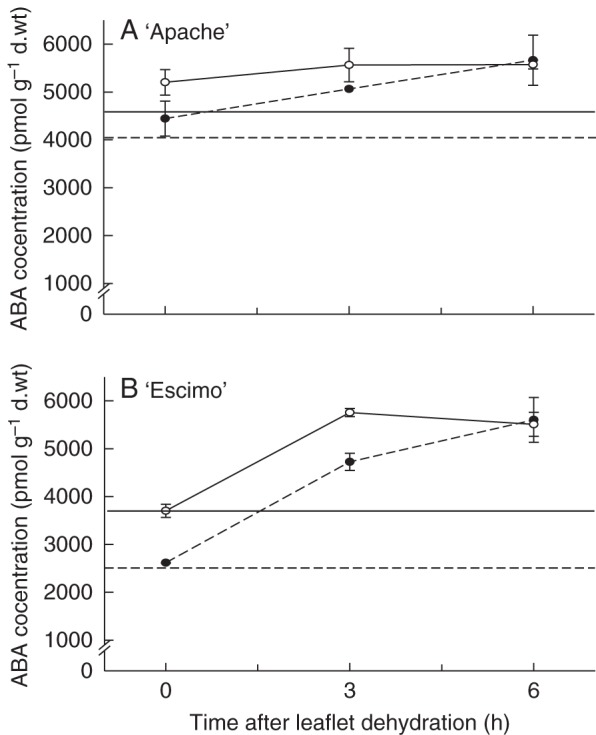
Endogenous leaflet ABA concentration, expressed on a dry weight (d.wt) basis, at different times after leaflet dehydration to 85 % relative water content, of two pot rose cultivars grown at moderate (60 %, open symbols) or high (90 %, closed symbols) relative air humidity (RH). Following dehydration, leaflets were sealed in polyethylene bags and incubated in darkness. Horizontal lines (solid and dashed for 60 and 90 % RH, respectively) denote leaflet ABA concentration before dehydration. Leaflets were sampled 2 h after the onset of the light period, and ABA concentration was calculated as the average of the concentrations at 0 and 4 h (see Fig. 2). Values are the means of two extractions. Eight leaflets were pooled for extraction.
Stomatal responsiveness to closing stimuli applied through the transpiration stream
Stomatal closing ability in response to three hormones (ABA, MJ and SA) and three downstream elements of their signalling pathways [H2O2, NO (induced by SNP) and Ca2+] was investigated in the four cultivars grown at both RH levels. As an example, the temporal dynamics of transpiration rate in response to all six closing stimuli is presented in ‘Apache’ grown at moderate RH (Fig. 5). ABA induced the highest decrease in transpiration rate (P < 0·001), followed by the three assessed signalling elements, which induced similar responses. MJ and SA elicited the least decrease in transpiration (P < 0·001) compared with the rest closing stimuli. Figure 6 shows the changes in transpiration rate in response to closing stimulus intake, the latter being the product of closing stimulus concentration in the feeding solution and the leaflet transpiration rate. It becomes apparent here that ABA induced stomatal closure by the least intake (i.e. the fed amount), while the Ca2+ intake required to elicit the stomatal response was enormously higher (≈80-fold) (P < 0·001). By fitting eqn (2) to these data, the fed amount of a given closing stimulus (EC½) required to generate 50 % of its maximal decrease in the transpiration rate was calculated.
Fig. 5.
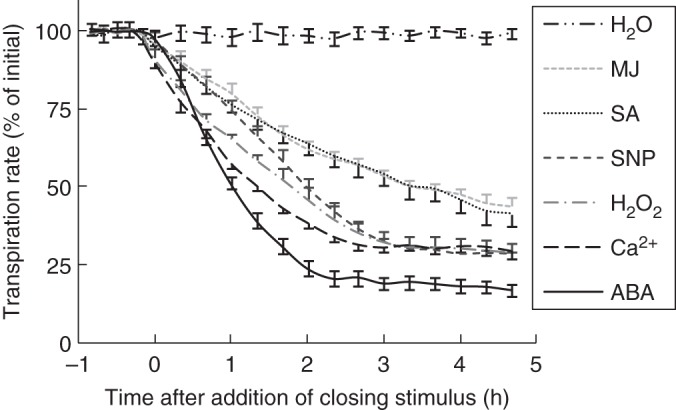
Leaflet transpiration rate as a function of time following feeding through the petiole with a range of closing stimuli (concentrations are given in Table 2) of pot rose ‘Apache’ grown at moderate (60 %) relative air humidity. The transpiration rate of leaflets placed in water was recorded for 1 h prior to the closing stimulus feeding (1·26 ± 0·02 mmol m−2 s−1), and subsequent transpiration rates were expressed relative to that value, i.e. [(transpiration rate in water)–(transpiration rate)]/(transpiration rate in water). Data are averages of eight replications ± SEM.
Fig. 6.
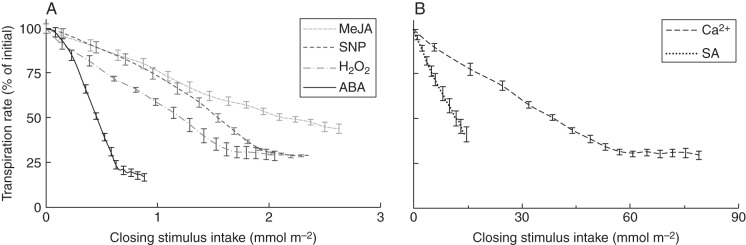
Leaflet transpiration rate as a function of different closing stimuli intake through the petiole (concentrations are given in Table 2) of pot rose ‘Apache’ grown at moderate (60 %) relative air humidity. Closing stimulus intake was calculated as the product of leaflet transpiration rate and closing stimulus concentration. The transpiration rate of leaflets placed in water was recorded for 1 h prior to the closing stimulus feeding (1·26 ± 0·02 mmol m−2 s−1), and subsequent transpiration rates were expressed relative to that value, i.e. [(transpiration rate in water)–(transpiration rate)]/(transpiration rate in water). Data are averages of eight replications ± SEM.
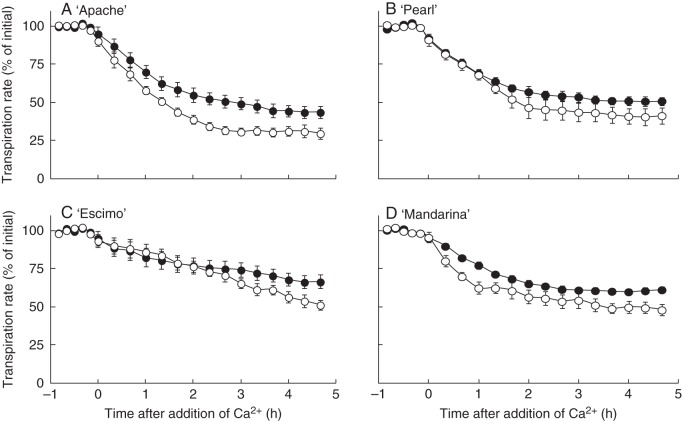
Table 2 shows the effect of growth RH on stomatal closing behaviour in response to the assessed closing stimuli. When examining the decrease in stomatal closure in response to ABA or MJ feeding, a clear separation between tolerant (‘Apache’, ‘Pearl’) and sensitive (‘Escimo’, ‘Mandarina’) cultivars was observed. The decrease in stomatal closure in response to Ca2+ (Fig. 7) or the remaining closing treatments was lower in leaves developed at high RH, but there was no RH by cultivar interaction (i.e. tolerant cultivars reacted like sensitive cultivars; Table 2). Comparable effects were observed when the effect of high RH on EC½ was assessed (Table 2). The EC½ values for ABA and MJ were significantly increased by high RH in the sensitive cultivars, but no significant difference was observed between moderate and high RH in the tolerant cultivars. For the remaining stimuli, there was no effect of high RH on EC½, other than for Ca2+ (26 % higher EC½ in high-RH-grown leaves compared to moderate RH).
Table 2.
Transpiration rate of leaflets placed in water, change in transpiration rate following 4 h 40 min feeding through the petiole (i.e. final value relative to the value prior to feeding) with a range of closing stimuli [100 μM ABA, 200 μM MJ, 1 mM SA, 200 μM H2O2, 200 μM SNP (NO donor), 8 mM Ca2+] and the closing stimulus intake (EC½) required to induce 50 % of this change in four pot rose cultivars grown at moderate (60 %) or high (90 %) relative air humidity (RH)
| Transpiration rate prior to feeding (mmol m−2 s−1) | EC½ (mmol m−2) |
Decrease in transpiration rate ( %) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABA | MJ | SA | H2O2 | SNP | Ca2+ | ABA | MJ | SA | H2O2 | SNP | Ca2+ | |||
| RH | 60 | 1·27 | 0·4 | 1·1 | 6·6 | 1·1 | 1·3 | 39b | 79 | 57 | 54a | 61a | 66a | 59a |
| 90 | 1·32 | 0·6 | 1·6 | 6·7 | 1·0 | 1·5 | 49a | 66 | 40 | 37b | 42b | 45b | 45b | |
| Cultivar | ||||||||||||||
| ‘Apache' | 60 | 1·26 | 0·4b | 1·3abc | 7·5 | 1·1 | 1·5 | 33 | 83ab | 55a | 58 | 70 | 71 | 71 |
| 90 | 1·33 | 0·4b | 1·3abc | 6·5 | 1·1 | 1·7 | 49 | 77ab | 49a | 46 | 54 | 49 | 56 | |
| ‘Pearl' | 60 | 1·17 | 0·4b | 1·1bc | 5·6 | 0·7 | 1·1 | 31 | 81ab | 63a | 66 | 55 | 70 | 60 |
| 90 | 1·19 | 0·5b | 1·0bc | 6·3 | 0·7 | 1·2 | 33 | 82ab | 56a | 42 | 45 | 56 | 50 | |
| ‘Escimo' | 60 | 1·40 | 0·4b | 0·9c | 6·1 | 1·5 | 1·1 | 65 | 67bc | 54a | 51 | 65 | 63 | 49 |
| 90 | 1·44 | 0·9a | 1·9a | 5·7 | 1·4 | 1·8 | 71 | 50d | 25b | 34 | 34 | 38 | 33 | |
| ‘Mandarina' | 60 | 1·23 | 0·4b | 1·0c | 7·1 | 0·9 | 1·4 | 29 | 84a | 58a | 43 | 54 | 61 | 56 |
| 90 | 1·33 | 0·7a | 1·9ab | 8·2 | 0·8 | 1·5 | 43 | 54cd | 31b | 25 | 36 | 36 | 39 | |
| F probability | ||||||||||||||
| Cultivar | ** | *** | NS | NS | * | NS | *** | *** | *** | *** | * | *** | *** | |
| RH | NS | *** | ** | NS | NS | NS | * | *** | *** | *** | *** | *** | *** | |
| Cultivar × RH | NS | *** | ** | NS | NS | NS | NS | *** | ** | NS | NS | NS | NS | |
EC½ was calculated by fitting a four-parameter logistic model [a dose–response relationship, where stimulus intake was treated as the dose; eqn (2)] to the transpiration rate versus closing stimulus intake data. Leaflets were placed in water for 1 h prior to closing stimulus feeding. Data refer to eight replications. Means followed by different letters indicate significant differences according to the Bonferroni-adjusted LSD test (comparison in columns).
NS, not significant; *significant at the 0·05 probability level; **significant at the 0·01 probability level; ***significant at the 0·001 probability level.
Fig. 7.
Leaflet transpiration rate as a function of time following feeding through the petiole with 8 mM Ca2+ of four pot rose cultivars grown at moderate (60 %, open symbols) or high (90 %, closed symbols) relative air humidity. The transpiration rate of leaflets placed in water was recorded for 1 h prior to the closing stimulus feeding (values are given in Table 2), and subsequent transpiration rates were expressed relative to that value i.e. [(transpiration rate in water)–(transpiration rate)]/(transpiration rate in water). Data are averages of eight replications ± SEM.
DISCUSSION
Plants grown in elevated atmospheric humidity conditions show limited control of water loss and increased lethality upon transfer to environments of increased evaporative demand (reviewed by Aliniaeifard and van Meeteren, 2013; Fanourakis et al., 2013b). The incidence of adverse water relations, however, strongly depends on the cultivar (Giday et al., 2013). This is the first report in which the immediate origin (i.e. during closure) and long-term cause (i.e. in the course of leaf expansion) of genotypic variation in stomatal responsiveness, caused by high growth RH, are revealed.
Genotypic variation is related to [ABA] during growth
Longer stomata with larger pores have been noted following growth at high RH (Table 1); this is an anatomical adaptation typical of several species, in which leaves expanded in more humid air (Fanourakis et al., 2013a). Like stomatal anatomy, stomatal functionality is established during growth (Fanourakis et al., 2011; Pantin et al., 2013b). The effect of high RH on stomatal responsiveness, expressed both by desiccation curves (Fig. 1) and by feeding through the petioles with ABA (Table 2), was clustered into two distinct groups. Stomata of tolerant cultivars (i.e. ‘Apache’ and ‘Pearl’) were little affected by high RH, whereas stomata of sensitive cultivars (i.e. ‘Escimo’ and ‘Mandarina’) were much less responsive after growth at high RH. Observations of reduced stomatal closure in plants grown at high RH and consistent cultivar differences upon desiccation or ABA feeding have also been reported previously (Fanourakis et al., 2013a).
Environmental conditions (e.g. in vitro culture) or genetic factors (e.g. ABA-biosynthetic mutants or transgenic plants expressing an anti-ABA antibody) that cause a long-term decline in [ABA] also result in impaired stomatal closing ability (Artsaenko et al., 1995; Leon-Kloosterziel et al., 1996; Aguilar et al., 2000). By examining a single cultivar, recent reports indicated that plants grown at high RH also have decreased [ABA], which mediates the weak stomatal responsiveness of these plants (Arve et al., 2013; Rezaei Nejad and van Meeteren, 2007). In the present study it was found that the decrease in [ABA], as a result of high RH during growth, primarily occurred in the sensitive cultivars (Fig. 2). More importantly, stomatal responsiveness was positively related to [ABA] during growth among cultivars and RHs (Fig. 3). This indicates that the [ABA] threshold for inducing stomatal functioning was similar among cultivars that differ in their sensitivity to high RH.
Whether the reduced [ABA] of high-RH-grown leaves persisted following water stress was also examined. Following very mild desiccation (i.e. to 85 % RWC), [ABA] increased in high-RH grown leaves and reached the [ABA] of leaves developed at moderate RH (Fig. 4). Consequently, although high RH resulted in lower [ABA] during growth, it did not affect the [ABA] in response to water deficit. Moreover, it was shown that a mild stress during growth at high RH is sufficient to induce [ABA] that is similar to [ABA] of plants developed at moderate RH.
Stomata on desiccating angiosperm leaves respond to both hydraulic (i.e. decrease in water potential) and biochemical (i.e. increase in endogenous ABA) signals, triggering the hydropassive and hydroactive stomatal closure mechanisms, respectively (Brodribb and McAdam, 2011; Franks, 2013). Upon desiccation, ABA redistribution from existing pools and synthesis of new ABA increase endogenous [ABA] (Harris and Outlaw, 1991; Finkelstein and Rock, 2002). We show that although resting [ABA] may be smaller prior to desiccation in high-RH-grown leaves depending on the cultivar (Fig. 2), ABA levels rise substantially following water deprivation (Fig. 4). Consequently, the impaired stomatal closure in response to desiccation observed in leaves developed at high RH (Fig. 1) is the sole result of reduced stomatal sensitivity to hydraulic and biochemical closing signals, rather than a combination of this and reduced ABA accumulation (Fig. 8).
Fig. 8.
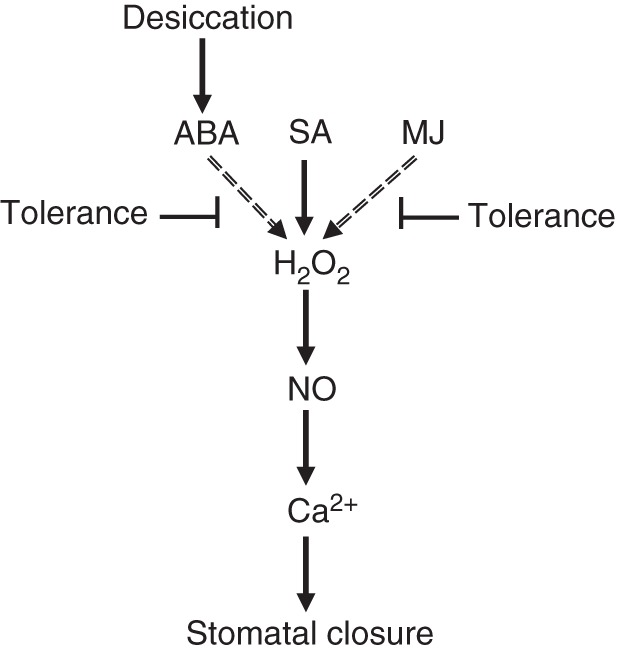
Model summarizing the biochemical signalling elements generating genotypic variation in stomatal closure following growth at high (90 %) relative air humidity. Components connected with dashed arrows reveal cultivar differences. The model does not include the hydraulic signals induced by desiccation and petiole-fed ABA stimuli.
Genotypic variation originates from differential impairment of signalling components between ABA perception and H2O2 generation
ABA elicits stomatal closure via a complex signalling cascade (reviewed by Dodd et al., 2010; Hubbard et al., 2010; Kim et al., 2010). Small signalling molecules (the so-called secondary messengers) are key elements of this pathway. For instance, ABA-induced stomatal closure has been associated with increases in cytosolic free Ca2+ (McAinsh, 1990), preceded by generation of both H2O2 (Pei et al., 2000) and NO (Garcia-Mata et al., 2003). Although Ca2+-independent signalling pathways exist, Ca2+-related mechanisms largely (around 70 %) account for stomatal closure (Siegel et al., 2009). H2O2, NO and Ca2+ have been proposed to also act as secondary messengers in the stomatal response pathways of both MJ (Suhita et al., 2004; Munemasa et al., 2007) and SA (Mori et al., 2001; Khokon et al., 2011).
This study shows that the genotypic variation in stomatal response, following growth at high RH, was expressed when ABA or MJ were employed as closing signals, but not in response to SA (Table 2; see also Fig. 8). This suggests that the signalling components that were differentially impaired by high RH likely belong to the common branch of the ABA and MJ pathways and are not required in the SA signalling network. Despite the fact that the SA pathway is the least understood, transduction elements that are independent of the ABA and MJ pathways have already been revealed (Khokon et al., 2011; Zeng et al., 2011). For instance, although H2O2 belongs to the cross-talk among the ABA, MJ and SA pathways, its production is elicited by different oxidase families [NAD(P)H oxidases for ABA and MJ; SHAM-sensitive peroxidases for SA] (Khokon et al., 2011).
When leaves were fed with downstream elements of the ABA, MJ and SA signalling networks [i.e. H2O2, NO (elicited by SNP), and Ca2+], stomata formed at high RH closed less compared to stomata on leaves developed at moderate RH, though this RH effect was consistent among cultivars differing in their sensitivity to high RH (Table 2; see also Fig. 8). Consequently, the high-RH-induced reductions in stomatal closure cannot be reversed by applying signalling elements downstream of the fast response pathway, as described in ABA-insensitive mutants (Allen et al., 1999; Iwai et al., 2003). These results also indicate that high growth RH impairs differentially the signalling components upstream of H2O2 generation, and that these elements contribute to the genotypic variation in stomatal closure. Figure 8 summarizes the proposed model for the high RH-imposed genotypic variation in stomatal responsiveness through defects in ABA signalling. Identification of which specific element(s) is responsible will further advance our understanding of how long-term high RH impairs stomatal functioning in plants.
Our method of assessing ABA-induced stomatal closure could have missed the possibility that xylem-fed ABA also exerts an indirect hydraulic effect on stomata, mediated via decreases in leaf hydraulic conductivity (Pantin et al., 2013a). Similarly to desiccation, it is not possible to distinguish whether hydraulic or biochemical signals elicit cultivar differences in ABA-induced stomatal response. However, the fact that xylem-fed MJ induces genotypic variation in the stomatal response (Table 2) clearly indicates that the biochemical signals were differentially affected by high growth RH.
Conclusions
High (90 %) RH during leaf expansion attenuates stomatal functionality to a cultivar-dependent extent. We have demonstrated here that this genetic variation in stomatal responsiveness is related to leaf ABA concentration during growth rather than the ABA concentration required to stimulate stomatal functioning. Very mild water stress applied to high-RH-grown leaves normalized differences in leaf ABA concentration between plants grown at moderate and high RH. The substantially large cultivar differences in stomatal closure when fed with ABA or MJ did not occur when H2O2 or downstream signalling elements were fed through the petiole. This suggests that genotypic variation in ABA-induced stomatal closure is associated with components lying upstream of H2O2 generation. The differential stomatal responses triggered by both ABA and MJ were not induced by SA, providing further evidence in favour of diverging signalling pathways eliciting H2O2 production by these hormones.
ACKNOWLEDGEMENTS
We thank Ruth Nielsen, Kaj Ole Dideriksen and Helle Kjærsgaard Sørensen for their help in conducting the measurements, as well as Anne Garfield Mortensen and Bente Birgitte Laursen for assisting in ABA analysis. The valuable comments of two anonymous reviewers are greatly acknowledged. This work formed part of a PhD project supported by a grant from Aarhus University and linked to the project GreenGrowing, an Interreg NorthSea project.
LITERATURE CITED
- Aguilar ML, Espadas FL, Coello J, et al. The role of abscisic acid in controlling leaf water loss, survival and growth of micropropagated Tagetes erecta plants when transferred directly to the field. Journal of Experimental Botany. 2000;51:1861–1866. doi: 10.1093/jexbot/51.352.1861. [DOI] [PubMed] [Google Scholar]
- Aliniaeifard S, van Meeteren U. Can prolonged exposure to low VPD disturb the ABA signalling in stomatal guard cells? Journal of Experimental Botany. 2013;64:3551–3566. doi: 10.1093/jxb/ert192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GJ, Kuchitsu K, Chu SP, Murata Y, Schroeder JI. Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell. 1999;11:1785–1798. doi: 10.1105/tpc.11.9.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artsaenko O, Peisker M, zur Nieden U, et al. Expression of a single-chain Fv antibody against abscisic acid creates a wilty phenotype in transgenic tobacco. Plant Journal. 1995;8:745–750. doi: 10.1046/j.1365-313x.1995.08050745.x. [DOI] [PubMed] [Google Scholar]
- Arve LE, Tefra MT, Gislerød HR, Olsen JE, Torre S. High relative air humidity and continuous light reduce stomata functionality by affecting the ABA regulation in rose leaves. Plant, Cell & Environment. 2013;36:382–392. doi: 10.1111/j.1365-3040.2012.02580.x. [DOI] [PubMed] [Google Scholar]
- Atkinson CJ, Mansfield TA, Davies WJ. Does calcium in xylem sap regulate stomatal conductance? New Phytologist. 1990;116:19–27. [Google Scholar]
- Brandt B, Brodsky DE, Xue S, et al. Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proceedings of the National Academy of Sciences of the USA. 2012;109:10593–10598. doi: 10.1073/pnas.1116590109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant Journal. 2006;45:113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM. Passive origins of stomatal control in vascular plants. Science. 2011;331:582–585. doi: 10.1126/science.1197985. [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: emergence of a core signaling network. Annual Review of Plant Biology. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- Davies W, Jones H. Abscisic acid: physiology and biochemistry. Oxford: BIOS Scientific Publishers; 1991. [Google Scholar]
- Dodd AN, Kudla J, Sanders D. The language of calcium signaling. Annual Review of Plant Biology. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- Drake PL, Froend RH, Franks PJ. Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. Journal of Experimental Botany. 2013;64:495–505. doi: 10.1093/jxb/ers347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanourakis D, Carvalho SMP, Almeida DPF, Heuvelink E. Avoiding high relative air humidity during critical stages of leaf ontogeny is decisive for stomatal functioning. Physiologia Plantarum. 2011;142:274–286. doi: 10.1111/j.1399-3054.2011.01475.x. [DOI] [PubMed] [Google Scholar]
- Fanourakis D, Carvalho SMP, Almeida DPF, van Kooten O, van Doorn WG, Heuvelink E. Postharvest water relations in cut rose cultivars with contrasting sensitivity to high relative air humidity during growth. Postharvest Biology and Technology. 2012;64:64–73. [Google Scholar]
- Fanourakis D, Heuvelink E, Carvalho SMP. A comprehensive analysis of the physiological and anatomical components involved in higher water loss rates after leaf development at high humidity. Journal of Plant Physiology. 2013a;170:890–898. doi: 10.1016/j.jplph.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Fanourakis D, Pieruschka R, Savvides A, Macnish AJ, Sarlikioti V, Woltering EJ. Sources of vase life variation in cut roses: a review. Postharvest Biology and Technology. 2013b;78:1–15. [Google Scholar]
- Finkelstein RR, Rock CD. Abscisic acid biosynthesis and response. The Arabidopsis Book. 2002:e0058. doi: 10.1199/tab.0058. doi:10.1199/tab.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ. Passive and active stomatal control: either or both? New Phytologist. 2013;198:325–327. doi: 10.1111/nph.12228. [DOI] [PubMed] [Google Scholar]
- Franks PJ, Farquhar GD. The effect of exogenous abscisic acid on stomatal development, stomatal mechanics, and leaf gas exchange in Tradescantia virginiana. Plant Physiology. 2001;125:935–942. doi: 10.1104/pp.125.2.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Chinnusamy V, Rodrigues A, et al. In vitro reconstitution of an abscisic acid signalling pathway. Nature. 2009;462:660–664. doi: 10.1038/nature08599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR. Nitric oxide regulates K+ and Cl− channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proceedings of the National Academy of Sciences of the USA. 2003;100:11116–11121. doi: 10.1073/pnas.1434381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Maierhofer T, Al-Rasheid KA, et al. Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Science Signaling. 2011;4:ra32. doi: 10.1126/scisignal.2001346. doi:10.1126/scisignal.2001346. [DOI] [PubMed] [Google Scholar]
- Giday H, Kjær HK, Fanourakis D, Ottosen CO. Smaller stomata require less severe leaf drying to close: a case study in Rosa hydrida. Journal of Plant Physiology. 2013;170:1309–1316. doi: 10.1016/j.jplph.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Outlaw WH. Rapid adjustment of guard cell abscisic acid levels to current leaf water deficit. Plant Physiology. 1991;95:171–173. doi: 10.1104/pp.95.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Tang RH, Hao Y, et al. Nitric oxide represses the Arabidopsis floral transition. Science. 2004;305:1968–1971. doi: 10.1126/science.1098837. [DOI] [PubMed] [Google Scholar]
- Herde O, Pe-a-Cortés H, Willmitzer L, Fisahn J. Stomatal responses to jasmonic acid, linolenic acid and abscisic acid in wild-type and ABA-deficient tomato plants. Plant, Cell & Environment. 1997;20:136–141. [Google Scholar]
- Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI. Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes & Development. 2010;24:1695–1708. doi: 10.1101/gad.1953910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai S, Shimomura N, Nakashima A, Etoh T. New fava bean guard cell signaling mutant impaired in ABA-induced stomatal closure. Plant and Cell Physiology. 2003;44:909–913. doi: 10.1093/pcp/pcg116. [DOI] [PubMed] [Google Scholar]
- Kline KG, Barrett-Wilt GA, Sussman MR. In planta changes in protein phosphorylation induced by the plant hormone abscisic acid. Proceedings of the National Academy of Sciences of the USA. 2010;107:15986–15991. doi: 10.1073/pnas.1007879107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokon MDA, Okuma EIJI, Hossain MA, et al. Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant, Cell & Environment. 2011;34:434–443. doi: 10.1111/j.1365-3040.2010.02253.x. [DOI] [PubMed] [Google Scholar]
- Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annual Review of Plant Biology. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Reuling G, Karssen CM. The isolation and characterization of abscisic-acid insensitive mutants of Arabidopsis thaliana. Physiologia Plantarum. 1984;61:377–383. [Google Scholar]
- Leon-Kloosterziel KM, van de Bunt GA, Zeevaart JAD, Koornneef M. Arabidopsis mutants with a reduced seed dormancy. Plant Physiology. 1996;110:233–240. doi: 10.1104/pp.110.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Giraudat J. Abscisic acid signal transduction. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:199–222. doi: 10.1146/annurev.arplant.49.1.199. [DOI] [PubMed] [Google Scholar]
- Manthe B, Schulz M, Schnabl H. Effects of salicylic acid on growth and stomatal movements of Vicia faba L.: evidence for salicylic acid metabolization. Journal of Chemical Ecology. 1992;18:1525–1539. doi: 10.1007/BF00993226. [DOI] [PubMed] [Google Scholar]
- McAinsh B. Abscisic acid-induced elevation of guard cell cytosolic Ca2+ precedes stomatal closure. Nature. 1990;343:186–188. [Google Scholar]
- Mori IC, Pinontoan R, Kawano T, Muto S. Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant and Cell Physiology. 2001;42:1383–1388. doi: 10.1093/pcp/pce176. [DOI] [PubMed] [Google Scholar]
- Munemasa S, Oda K, Watanabe-Sugimoto M, Nakamura Y, Shimoishi Y, Murata Y. The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiology. 2007;143:1398–1407. doi: 10.1104/pp.106.091298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y. The arabidopsis calcium-dependent protein kinase, CPK6, functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant Physiology. 2011a;155:553–561. doi: 10.1104/pp.110.162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Mori IC, Murata Y. Methyl jasmonate signaling and signal crosstalk between methyl jasmonate and abscisic acid in guard cells. Plant Signaling & Behavior. 2011b;6:939–941. doi: 10.4161/psb.6.7.15439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei Nejad A, van Meeteren U. The role of abscisic acid in disturbed stomatal response characteristics of Tradescantia virginiana during growth at high relative air humidity. Journal of Experimental Botany. 2007;58:627–636. doi: 10.1093/jxb/erl234. [DOI] [PubMed] [Google Scholar]
- Rezaei Nejad A, van Meeteren U. Dynamics of adaptation of stomatal behaviour to moderate or high relative air humidity in Tradescantia virginiana. Journal of Experimental Botany. 2008;59:289–301. doi: 10.1093/jxb/erm308. [DOI] [PubMed] [Google Scholar]
- Rezaei Nejad A, Harbinson J, van Meeteren U. Dynamics of spatial heterogeneity of stomatal closure in Tradescantia virginiana altered by growth at high relative air humidity. Journal of Experimental Botany. 2006;57:3669–3678. doi: 10.1093/jxb/erl114. [DOI] [PubMed] [Google Scholar]
- Pantin F, Monnet F, Jannaud De, et al. The dual effect of abscisic acid on stomata. New Phytologist. 2013a;197:65–72. doi: 10.1111/nph.12013. [DOI] [PubMed] [Google Scholar]
- Pantin F, Renaud J, Barbier F, et al. Developmental priming of stomatal sensitivity to abscisic acid by leaf microclimate. Current Biology. 2013b doi: 10.1016/j.cub.2013.07.050. http://dx.doi.org/10.1016/j.cub.2013.07.050 . [DOI] [PubMed] [Google Scholar]
- Pedersen HA, Laursen B, Mortensen A, Fomsgaard IS. Bread from common cereal cultivars contains an important array of neglected bioactive benzoxazinoids. Food Chemistry. 2011;127:1814–1820. [Google Scholar]
- Pei ZM, Murata Y, Benning G, et al. Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Savvides A, Fanourakis D, van Ieperen W. Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. Journal of Experimental Botany. 2012;63:1135–1143. doi: 10.1093/jxb/err348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secchi F, Irene P, Walter C, Anna KZ, Claudio L, Maciej AZ. The dynamics of embolism refilling in abscisic acid (ABA)-deficient tomato plants. International Journal of Molecular Sciences. 2013;14:359–377. doi: 10.3390/ijms14010359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RS, Xue S, Murata Y, et al. Calcium elevation-dependent and attenuated resting calcium-dependent abscisic acid induction of stomatal closure and abscisic acid-induced enhancement of calcium sensitivities of S-type anion and inward-rectifying K+ channels in Arabidopsis guard cells. The Plant Journal. 2009;59:207–220. doi: 10.1111/j.1365-313X.2009.03872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavik B. Methods of studying plant water relations, London, UK: Chapman and Hall; 1974. [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiology. 2004;134:1536–1545. doi: 10.1104/pp.103.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigger J, Phillips J, Peisker M, et al. Prevention of stomatal closure by immunomodulation of endogenous abscisic acid and its reversion by abscisic acid treatment: physiological behaviour and morphological features of tobacco stomata. Planta. 2002;215:413–423. doi: 10.1007/s00425-002-0771-z. [DOI] [PubMed] [Google Scholar]
- Zeng W, Brutus A, Kremer JM, et al. A genetic screen reveals arabidopsis stomatal and/or apoplastic defenses against Pseudomonas syringae pv. tomato DC3000. PLoS Pathogens. 2011;7:e1002291. doi: 10.1371/journal.ppat.1002291. doi:10.1371/journal.ppat.1002291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jia W, Yang J, Ismail AM. Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Research. 2006;97:111–119. [Google Scholar]