Abstract
Background
The crucial role of roots in plant nutrition, and consequently in plant productivity, is a strong motivation to study the growth and functioning of various aspects of the root system. Numerous studies on lateral roots, as a major determinant of the root system architecture, mostly focus on the physiological and molecular bases of developmental processes. Unfortunately, little attention is paid either to the morphological changes accompanying the formation of a lateral root or to morphological defects occurring in lateral root primordia. The latter are observed in some mutants and occasionally in wild-type plants, but may also result from application of external factors.
Scope and Conclusions
In this review various morphological aspects of lateral branching in roots are analysed. Morphological events occurring during the formation of a typical lateral root are described. This process involves dramatic changes in the geometry of the developing organ that at early stages are associated with oblique cell divisions, leading to breaking of the symmetry of the cell pattern. Several types of defects in the morphology of primordia are indicated and described. Computer simulations show that some of these defects may result from an unstable field of growth rates. Significant changes in both primary and lateral root morphology may also be a consequence of various mutations, some of which are auxin-related. Examples reported in the literature are considered. Finally, lateral root formation is discussed in terms of mechanics. In this approach the primordium is considered as a physical object undergoing deformation and is characterized by specific mechanical properties.
Keywords: Lateral root formation, plant organ morphology, arabidopsis mutant, growth rates, mechanical stress distribution
INTRODUCTION
The extent of the root system determines the volume of soil available for absorbing water and the nutrients required for the growth of a plant. The root system is also responsible for anchoring a plant in the stratum, thus securing the upright position of the plant and affecting its mechanical resistance to environmental factors such as wind (Kramer and Boyer, 1995). Plant development, productivity (Lynch, 1995) and overall fitness are then dependent on the optimal root system architecture. As lateral branching is the major determinant of this architecture, lateral roots are important to the success of plants.
Investigating lateral root development is obviously of great significance from the point of view of economics. In fact, within the last decades much progress has been made in studies on the formation and functioning of the lateral roots; however, most of these studies concern molecular and cellular processes and the role of genes in their regulation (recently reviewed by Fukaki et al., 2007; Péret et al., 2009a, b; Laskowski, 2013). Unfortunately, very little attention has been paid to the morphology of lateral root development, although knowing this morphology is essential for understanding more about the process of root system branching. From this perspective, research on the natural change of form during lateral root development can be helpful. Studies of morphologically altered lateral root apices in both wild-type plants and mutants defective in lateral root formation might provide insight into the regulation of growth and cell expansion during lateral root morphogenesis and might enable analysis of a possible change in the direction and/or rate of growth in such apices.
My objective in this review is to bring together and to consider studies that have looked at developmental changes in lateral root morphology in order to understand better the process of lateral root development.
THE ROOT SYSTEM AND ITS MOST IMPORTANT REGULATORS
Root systems show significant morphological diversity (reviewed in Hodge et al., 2009), which suggests their high specification and adaptation to various environmental conditions. In dicots and in some monocots the root system consists of a primary root with lateral branchings developing mostly at right angles from pericycle cells. The longitudinal arrangement of the lateral root primordia in various species reveals a high degree of regularity. The site of new primordium initiation is strongly associated with the organization of the vascular system (Barlow, 1984). In diarch roots the primordia are initiated in acropetal order in alternating left and right arrangement, although the developmental sequence of consecutive lateral root formation is not strictly acropetal since, once initiated, some primordia may develop more slowly or even become arrested in development, as was observed in arabidopsis (Dubrovsky et al., 2006) and tomato (Barlow and Adam, 1988).
The development and functioning of lateral roots, and consequently the root system, depend strongly on the culture and environmental conditions. Their effects on root system architecture have been recently described in a number of reviews [e.g. nutrient availability (López-Bucio et al., 2003; Zhang et al., 2007; Niu et al., 2013); abiotic and biotic stresses (Nibau et al., 2008); symbiotic interactions (Gruber et al., 2011)]. Root architecture is also sensitive to external mechanical signals such as a high level of soil compaction (e.g. Iijima and Kono, 1991; Grzesiak, 2009) or contact with a physical barrier to growth (Richter et al., 2009). The effects of mechanical factors on root system architecture have been relatively widely studied but are not considered in detail here; the problem requires a separate review.
ORIGIN AND MORPHOLOGICAL CHANGES OF THE DEVELOPING LATERAL ROOT
Steadily growing mature plant organs such as root and shoot apices usually preserve their form during growth under stable conditions (Hejnowicz and Karczewski, 1993). Unlike the primary root and shoot apices, which originate at the ends of the embryonic plant axis, lateral roots have an endogenous origin and are initiated postembryonically. In general, lateral roots originate from a small number of the founder cells of the internal tissue of the parental root, usually in the pericycle, although in ferns they are derived from endodermis while in some species of water plants they are derived from both pericycle and endodermis (Cutter, 1971; Lin and Raghavan, 1991; Clowes, 1992). In each case, however, their development involves a continuous change of form.
Lateral root formation and functioning are nowadays studied intensively in arabidopsis, which has become a model plant because of its simple anatomy. Initiation takes place a short distance from the root tip (Dubrovsky et al., 2000; Beeckman et al., 2001). The primordia are formed exclusively in the pericycle cells opposite to the xylem poles, so that in diarch arabidopsis roots two files of laterals are produced (Schiefelbein and Benfey, 1994). In lhw mutants, in which the diarch organization is lost, the number of lateral roots is reduced to half as many as in wild-type plants and this is accompanied by the formation of all lateral roots on one side of the primary root (Ohashi-Ito and Bergmann, 2007; Parizot et al., 2008).
Only a limited number of xylem pole pericycle cells gain the identity of founder cells (Péret et al., 2009a), and the mechanisms of their specification are as yet unknown (de Rybel et al., 2010). In this regard, it has been shown recently (Dubrovsky et al., 2011) that pericycle cells are most likely to become founder cells in the auxin-minimum zone of the primary root. The founder cells are specified in the region characterized as a ‘developmental window’ (Dubrovsky et al., 2011) and located between the basal meristem, i.e. the region between the meristem and the elongation zone, and the distal differentiation zone (Benková and Bielach, 2010; de Smet, 2012). Soon after specification they undergo auxin-dependent activation to start dividing. After a series of anticlinal divisions, a periclinal division takes place, leading to the formation of two cell layers (Malamy and Benfey, 1997). Initiation, comprising the events described above, is followed by new meristem formation, accompanied by establishment of the lateral root organization (Celenza et al., 1995; Laskowski et al., 1995). At subsequent stages a coordinated sequence of periclinal and anticlinal divisions occur (Malamy and Benfey, 1997). Some recent observations (Lucas et al., 2013) indicate that the programme of cell divisions may vary among individual primordia. The cellular organization of the developed lateral root apex is similar to that of the main root apex. Such a typical cellular organization, with a pattern of mutually orthogonal periclines and anticlines formed by the cell walls (von Sachs, 1887) and, with the quiescent centre surrounded by initials in the apical part of the forming lateral root, occurs before the primordium emerges over the parent root surface (Malamy and Benfey, 1997).
The continuous development of the cell pattern is accompanied by continuous changes in primordium morphology. During the first divisions of the founder cells the new walls are inserted in anticlinal planes (Casimiro et al., 2001, 2003), which does not change the local geometry as the primordium-to-be now has the form of an arched area comprising one-half the circumference of the pericycle (Sussex et al., 1995). Further periclinal divisions leading to formation of the subsequent cell layers of the primordium are accompanied by the formation of a small protrusion. The cells in the flanks either do not divide or soon stop dividing so the protrusion develops in the central part (developmental stages II and III according to Malamy and Benfey, 1997). With further growth the convexity of the protrusion becomes more pronounced and a small dome-shaped organ develops (stages IV–VI; Péret et al., 2009a). Depending on the stage of development, the dome is more or less flattened, but observed in the axial section its apical part demonstrates mirror symmetry with reference to the axis. The organ elongates to finally reach a form typical of roots: its apical part attains the shape of a dome while the central part is more or less cylindrical (see Fig. 1 in Péret et al., 2009b). Moreover, the apical part usually preserves rotational symmetry in relation to the organ axis, as happens in the case of the main root (Dolan et al., 1993; Hejnowicz and Karczewski, 1993; Scheres et al., 2002; Nakielski, 2008).
Interestingly, the base of the lateral root, in the location where it is attached to the parental root, is elongated along the parental root axis, which results in its oval shape in frontal view (J. Szymanowska-Pułka and M. Lipowczan, unpubl. res.). This is probably a consequence of the origin of the primordium founder cells, i.e. the group of two or three xylem-pole pericycle cells that occupy the area elongated longitudinally (Casimiro et al., 2003). The early primordium base (Dubrovsky et al., 2001; Benková et al., 2003; Kurup et al., 2005) and the base of the developed lateral root form an ellipse-like figure, of which the long diameter/short diameter ratio varies among individual organs (J. Szymanowska-Pułka and M. Lipowczan, unpubl. res.). As the apical part of the lateral root is circular in its cross section, a change of geometry occurs along the lateral root axis. Such a change takes place at the early developmental stages by transition from bilateral to the radial symmetry of the primordium (Lucas et al., 2013).
ANATOMICAL EVENTS ACCOMPANYING THE CHANGE OF GEOMETRY
The above-described changes in primordium morphology are accompanied by specific anatomical events. As mentioned in the previous section, the flanking cells of the newly initiated primordium either do not divide or undergo a few transverse divisions (Malamy and Benfey, 1997), while the central cells of the primordium divide both periclinally and anticlinally, which leads to its expansion. From the early stages on both sides of the primordium axis oblique anticlinal cell divisions occur, which breaks the symmetry of the existing cell pattern and supports the formation of a dome-shaped protrusion. Numerous obliquely oriented division walls were also observed in young radish lateral root primordia, where they resulted in the formation of cuneiform cells (Blakely et al., 1982) and led to the changed geometry of the primordium (Szymanowska-Pułka and Nakielski, 2010). In arabidopsis such divisions first appear at stage II, when the primordium consists of two cell layers (Malamy and Benfey, 1997). At later stages, oblique divisions become more frequent and the cells located near the axis become elongated and sharply pointed towards the apical part due to the elongation of the primordium (Szymanowska-Pułka et al., 2012). These events, leading to the formation of a dome-shaped lateral root apex, can be observed in its axial plane. Also, from the early stages of development the primordium undergoes radialization (Lucas et al., 2013), which means that in the tangential plane its shape changes from elliptical to radial. The events allowing the establishment of radial symmetry take place in daughter cells of the pericycle founder cells. The central group of these cells divide in periclinal and anticlinal planes, adjusting to the existing cell pattern, while the flanking cells undergo oblique divisions that result in the formation of a ring of cells around the central group. At the same time the primordium increases in width (Lucas et al., 2013), which causes a decrease in the long diameter/short diameter ratio towards the apex (J. Szymanowska-Pułka and M. Lipowczan, unpubl. res.) and results in the ellipse-to-round transition.
Although formation of the lateral root in arabidopsis has been relatively well described and we know how the anatomical events affect the change of form during development, it is still not known why new directions of cell division are introduced. We may only hypothesize that a local change in growth develops in the region of lateral root formation; this will be discussed in another section.
THE BOUNDARY REGION
Another specific feature of the cell pattern of the developing primordium is a distinct separation from the parental root through the presence of strongly marked external walls of the flanking cells appearing at stage III and visible during further development (Szymanowska-Pułka et al., 2012). It has been shown (de Smet et al., 2008) that the membrane-localized receptor-like kinase ACR4, which is responsible for promoting the first formative divisions in the pericycle, also plays the key role in repressing the divisions in these flanking cells in arabidopsis. At early stages of primordium formation the pericycle cells of acr4 crr3 double mutants undergo increased cell divisions and as a consequence the primordium boundaries are not clearly defined (de Smet et al., 2008). Thus, the non-dividing flanking cells in wild-type plants may assume the status of border cells. Our observations (Szymanowska-Pułka et al., 2012) indicate that in young lateral roots whose apices have emerged over the parent root surface the cells in the flanks in the basal region become abnormally large and very often their walls are curved outward. These cells are situated in the area where the lateral root's outline forms the strongest curvature, which might suggest that they undergo a local compression.
In the course of primordium development the regularity of the cell pattern is lost in the boundary region, i.e. the smooth pattern of periclines and anticlines becomes strongly perturbed (Szymanowska-Pułka et al., 2012). Distinct perturbations in the cell pattern at the basal part of the primordium occur in the puchi-1 mutant (Hirota et al., 2007), in which the number of cells in this region is increased, especially along the apical–basal axis of the parent root. The feature has been attributed to a mutation in the auxin-regulated AP2/EREBP gene PUCHI, encoding a putative APETALA2/ethylene-responsive element binding protein transcription factor. The redundant divisions and pronounced disturbance in the cell division pattern result in swelling of this region in the puchi-1 lateral root. This suggests that PUCHI is required for the coordinated pattern of cell divisions in the primordium and may control the morphology of the forming lateral root in arabidopsis (Hirota et al., 2007).
Shuai et al. (2002) observed β-glucuronidase (GUS) activity at the junction between the primary root and lateral root primordia in a ring of cells localized at the base of the lateral root in both the ET22 enhancer trap line and pLOB5·0:GUS transformants. Expression was maintained in the region in fully developed lateral roots. The LATERAL ORGAN BOUNDARIES (LOB) gene encodes a plant-specific protein of unknown function; however, the authors suggest that a possible function of genes expressed in such a pattern is to define a boundary between the initiating organ primordia and the stem cells from which they are derived. Such a definition of a boundary between the lateral root primordium and the mother root cells is probably important for maintaining the integrity of the stem cells and the initiating organ primordium (Shuai et al., 2002). Similar results were reported by Prasad et al. (2005), who observed GUS activity at the primary and lateral root junctions of pLOJ::GUS transgenic plants. The LOJ (LATERAL ORGAN JUNCTIONS) gene belongs to a class of genes encoding proteins containing a highly conserved pentatricopeptide repeat, designated the PPR domain. Transcript analysis revealed that the expression of the gene is specifically restricted to the lateral organ junctions throughout the life of the plant. Analysing the expression of PIN in lateral roots, Benková et al. (2003) observed expression of PIN6 at the base of lateral root primordia from the earliest stages of development. At later stages, when a protrusion had been formed, expression was detected exclusively in the primordium margins in the form of a ring around its base. In the proPUCHI2·5:GUS line, in which GUS staining was detected at the periphery of the lateral root primordium at the early developmental stage and shortly thereafter, the domain of GUS expression formed a ring that marked the proximal region of the primordium (Hirota et al., 2007).
The examples described above show that a number of genes are involved in ‘separation’ of the lateral branching from the parent root. The border cells may play an essential role in determining the identity of the lateral organ, which is why their identification remains a key, although still open, question.
ATYPICAL MORPHOLOGY IN ARABIDOPSIS LATERAL ROOT PRIMORDIA
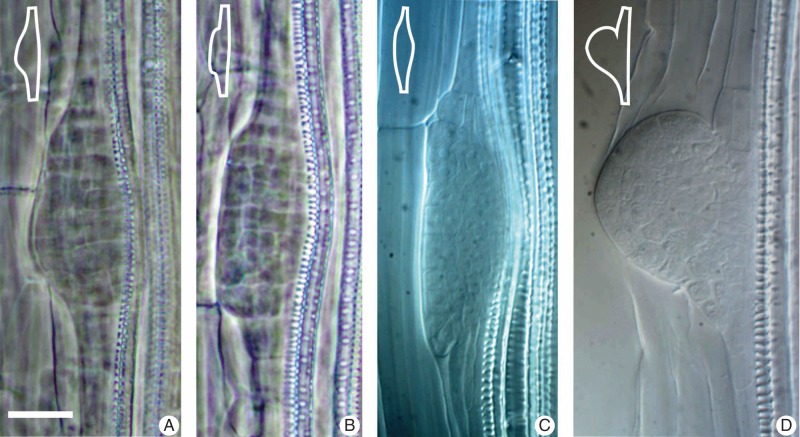
In spite of the fact that the shape of the lateral root primordium is regarded as highly regular (Lucas et al., 2013), some of our recent observations (J. Szymanowska-Pulka et al., unpubl. res.) on the morphological features of lateral roots of arabidopsis have revealed significant deviations from the regular form of a dome in young primordia (18·9 % of the 756 analysed). The most frequent cases of atypical geometry (11·5 %) concern the lack of symmetry in the shape of the apex (Fig. 1A), which is probably a result of unequal growth on the two sides of the organ axis. Some primordia (4·1 %) have a significantly flattened surface (Fig. 1B), which means that although the protrusion has been formed it does not take the shape of a dome, but of a plateau. Other primordia (2·1 %) show a fusiform (spindle-like) form (Fig. 1C), which is usually associated with vascular bundles buckled towards the axis. A possible reason for the buckling is mechanical pressure exerted by the primordium on the bundles. A very specific feature observed in a few (1·2 %) young lateral roots is an unnatural ‘pocket’ formed at the base and visible either on one side (Fig. 1D) or on both sides (not shown) of the primordium in axial view. When the pocket occurs on one side the whole primordium is often deformed in a way suggesting unequal growth, which results in the lack of symmetry and resembles the case shown in Fig. 1A. Irregularly shaped lateral root primordia in wild-type arabidopsis seedlings were also reported by Benková et al. (2003), who observed a few such cases (6 % of the 91 analysed), although the character of the morphological changes was not specified.
Fig. 1.
Examples of the most characteristic types of lateral root primordium deformation in wild-type arabidopsis seedlings. (A) Lack of symmetry in relation to the primordium axis. (B) Flattened surface. (C) Spindle-like form. (D) A pocket formed at the primordium base. Notice that the vascular bundles bend towards the main root axis in (A–C). Insets in the upper left corners are schematic representations of each type of deformation. (A, B) Phase contrast; (C, D) Nomarski contrast. Scale bar = 20 μm.
The abnormal morphological features were usually observed in primordia that had not emerged over the parent root surface, which might suggest that the primordium shape was affected by the overlying tissues of the parent root. This supposition was confirmed by Lucas et al. (2013), who obtained the arabidopsis J0631>>axr3-1 line, which failed to form normal-shaped primordia due to altered (reinforced) mechanical properties of the overlying tissues.
MORPHOGENETIC RESPONSE OF THE ROOT APEX TO EXTERNAL STIMULI
The organization and morphology of the root apical meristem undergo natural changes during the root growth cycle (Rost, 2011). Yet they may also be altered in both primary and lateral roots by various external factors, which may be chemical or mechanical. Roots of Zea mays and Pisum sativum exposed to environmental pollutants exhibit destruction of the rhizodermis and the outer layers of the cortex as well as significant deformation of lateral root primordia (Kummerová et al., 2013). Arabidopsis roots treated with the phytotropin CPD (2-carboxyphenyl-3-phenylpropane-l,2-dione) lose the typical cellular organization of their apices (Ruegger et al., 1997). Various kinds of abiotic stresses may induce morphogenetic responses in roots, such as decreased elongation, increased elongation or abnormal root branching. Some of these morphological changes are correlated with altered auxin distribution (Potters et al., 2007).
Roots of various species subjected to directed mechanical stimulus show reduced elongation associated with radial expansion (Bengough, 1997, 2012), altered cell size and number and enhanced lateral branching (Wilson et al., 1977; Goss and Russell, 1980). A high level of soil compaction changes the general shape of roots from circular to flattened (Lipiec et al., 2012) and causes them to swell radially (Atwell, 1988). Application of mechanical impedance to the root apex may result not only in its deformation but also in a change of meristem organization from closed to open (Potocka et al., 2011), or in enhanced activity of meristematic cells (Iijima et al., 2003). Mechanical impedance was shown to alter the auxin response in arabidopsis roots (Okamoto et al., 2008), which may suggest a possible role of this hormone in mechanical signal transduction. Most of the examples cited above refer to changes in primary roots in response to a mechanical factor. This effect on lateral root morphology is poorly investigated, probably because of technical difficulties connected with the small dimension of this organ and its localization within the tissues of the parental root.
SOME MUTATIONS ALTER ROOT APEX MORPHOLOGY
Aberrant morphology of root apices may also result from various mutations (overviewed in Table 1). Most studies on arabidopsis mutants defective in root growth and development concentrate on morphological features of the primary root. These mutants often exhibit a shortened primary root (Benfey et al., 1993, Cruz-Ramírez et al., 2004), which happens to be accompanied by radial swelling of the whole root (Holding et al., 1994) or only its apical region (Benfey et al., 1993). In some cases the reduced length results from a reduction in the size of the elongation zone (Ingram et al., 2011) or even from a loss of meristematic and elongation zones (Benfey et al., 1993). Morphological defects occurring in the root cap relate either to its reduced dimensions (Holding et al., 1994) or to its abnormal morphogenesis (Eapen et al., 2003). Some of these mutations cause a significant decrease in the growth rate of roots (Baskin et al., 1992; Ruegger et al., 1997), while others may result in the complete cessation of growth (Benfey et al., 1993; Holding et al., 1994).
Table 1.
Overview of arabidopsis mutants that exhibit altered root morphology
| Mutants | Phenotypes and morphological defects | References |
|---|---|---|
| rsw1, rsw2, rsw3 | Radially swollen PR tip at higher temperature | Baskin et al., 1992 |
| reb1-1, reb1-2 | Radially swollen PR epidermal cell layer | |
| stp1 | Decreased PR growth rate | |
| shr | Short PR, ceased PR growth, large number of secondary roots at PR/hypocotyl junction, lack of meristematic and elongation zones, missing internal root cell layers | Benfey et al., 1993 |
| cob, lit, sab | Expanded apical part of PR, abnormal root cell expansion in LR | |
| 1767 | Shorter PR, reduced cellular elongation | Holding et al., 1994 |
| 4792 | Shorter radially expanded PR, swollen cells, increased number of cells in all tissues | |
| 5905 | Shorter radially expanded PR, swollen cells in all tissues, reduced cellular elongation | |
| 7133 | Swollen epidermal and cortical cells of PR | |
| 7203 | Short PR, ceased PR growth, lack of endodermal cell layer, reduced RC | |
| alf3-1 | Defect in LR maturation, disturbed cellular organization in LR primordia | Celenza et al., 1995 |
| alf4-1 | Defect in LR initiation | |
| rml | Lack of RAM, deformed nodule-like LR primordia | Cheng et al., 1995 |
| tir3 | Reduced LR number, reduced PR elongation | Ruegger et al., 1997 |
| hbt | Disturbed cellular organization of RAM and RC | Willemsen et al., 1998 |
| nhr1 | Enhanced root waving, abnormal RC morphogenesis | Eapen et al., 2003 |
| pin1 | Reduced LR number, irregular shape of LR primordia | Benková et al., 2003 |
| rrd1, rrd2, rrd4 | Aberrant morphology of LR and AR apex at higher temperature | Sugiyama, 2003; Otsuka and Sugiyama 2012 |
| rid1 to 5 | Failure in AR primordium initiation, aberrant LR morphology at higher temperature | Konishi and Sugiyama, 2003; Otsuka and Sugiyama, 2012 |
| rpd1 | Defects in AR primordium development | Konishi and Sugiyama, 2003 |
| rgd1 to 3 | Defects in AR growth after establishment of RAM | |
| srd2, srd2-1 | Reduced LR number, failure in AR primordium initiation, PR development interrupted and aberrant LR morphology at higher temperature | Konishi and Sugiyama, 2003; Ohtani and Sugiyama 2005; Ohtani et al., 2010 |
| xpl | Short PR, increased LR number, abnormal morphology of PR epidermal cells | Cruz-Ramírez et al., 2004 |
| puchi1 | Retarded LR development, swollen LR proximal region | Hirota et al., 2007 |
| pin2,3,7 | Increased LR density, fused LRs | Laskowski et al., 2008 |
| arm | Increased LR number, reduced PR elongation, radial swelling on high nitrate | Hermans et al., 2010 |
| pdr23 | Short PR, reduced elongation zone in the absence of phosphorus, rapid loss of QC identity in the absence of nitrogen | Costa et al., 2011 |
| tir1 afb2 afb3 | LR formed at increased distance from QC, LR primordia of abnormal morphology originated from two-layered pericycle | Dubrovsky et al., 2011 |
| lrd3 | Short PR, increased length and density of LR, meristematic zone of PR reduced in size, decreased length of PR epidermal cells | Ingram et al., 2011 |
| lbd16, lbd18, lbd29 | Reduced LR number, retarded LR formation, knots formed in the LR meristem on auxin-rich medium | Feng et al., 2012 |
PR, primary root; LR, lateral root; AR, adventitious root; RAM, root apical meristem; RC, root cap; QC, quiescent centre.
A number of mutations cause similar morphological defects in lateral roots. In the group of mutants identified by Benfey et al. (1993), lateral roots exhibited atypical expansion. Cheng et al. (1995) isolated a group of rml (root meristemless) mutants in which early arrest of growth and lack of a meristem in either the primary or the lateral root were observed. Growth arrest in the latter resulted in the formation of nodule-like structures consisting of proliferating pericycle cells. Of the large group of pin mutants, in which the distribution of auxin in plant organs is altered (Křeček et al., 2009), lateral root primordia with irregular shape were observed relatively often (29 % of all cases) in pin1 mutants (Benková et al., 2003), while in the triple pin2,3,7 mutant a number of lateral roots that formed adjacent to one another or that fused at the base were present (Laskowski et al., 2008). A similar effect of formation of fused lateral roots was observed in a group of arabidopsis lines defective in response to auxin (de Smet et al., 2010). Other developmental defects were observed in the triple mutant tir1 afb2 afb3, where in some cases divisions in the pericycle failed to result in primordium formation while in other cases a two-layered pericycle occurred and the primordia originating from such a pericycle showed abnormal morphology (Dubrovsky et al., 2011). TIR1 (TRANSPORT INHIBITOR RESPONSE1) is an auxin receptor mediating transcriptional responses to auxin, and the homologous proteins AUXIN-SIGNALING-F-BOX (AFB1, AFB2 and AFB3) are TIR1-like auxin receptors (Mockaitis and Estelle, 2008), which suggests that this mutation is defective in the regulation of developmental responses to auxin. Defects in lateral root initiation and development occur also in the group of alf (aberrant lateral root formation) mutants (Celenza et al., 1995) and in a group of lbd (lateral organ boundaries domain) mutants (Feng et al., 2012).
In the puchi-1 mutant mentioned above (a recessive mutation in PUCHI; Hirota et al., 2007), lateral root primordia appear much shorter than those of wild-type plants. However, those laterals that have emerged through the primary root surface grow normally and the root system in the older plants is indistinguishable from that of wild-type plants. Yet the proximal region of each lateral root in the puchi-1 mutant is significantly swollen and often bent. In the course of primordium development it becomes atypically flat and its overall width is significantly increased. This change of form is connected with cell divisions occurring in a wider area in the mutant primordium. At later stages, when the lateral root meristem of the puchi-1 mutant emerges from the parent root, the lateral forms extra tissue consisting of highly enlarged cells at the periphery of the most proximal region. Abnormal sizes of cells and an altered cell pattern accompany a change in the form of the root apex also in other mutants. Most commonly observed features include defective cellular elongation (Holding et al., 1994; Ingram et al., 2011) and swollen cells in various tissues of the primary root (Baskin et al., 1992; Holding et al., 1994; Eapen et al., 2003; Cruz-Ramírez et al., 2004) or of the lateral root (Benfey et al., 1993). Some mutants show disturbed cellular organization of the primary root apical meristem (Eapen et al., 2003; Ingram et al., 2011) as well as of the secondary root apical meristem and the root cap (Willemsen et al., 1998). In a few cases missing internal root cell layers or increased number of cells in all tissues were reported (Benfey et al., 1993; Holding et al., 1994).
Some arabidopsis mutants show altered morphological features of roots in response to external factors, for example nutrient availability (Hermans et al., 2010; Costa et al., 2011), exogenous auxin (Feng et al., 2012) and higher temperature (Baskin et al., 1992). A large group of temperature-sensitive mutants defective in the development of lateral and adventitious roots and, in some cases, of the primary root apex were isolated by a Japanese team (Konishi and Sugiyama, 2003; Sugiyama, 2003; Ohtani and Sugiyama, 2005; Ohtani et al., 2010; Otsuka and Sugiyama, 2012). Some of these mutants produce lateral roots with aberrant morphology, such as the rrd1 and rrd2 (root redifferentiation defective) mutants, forming lateral roots characterized by a bell-shaped appearance of the root apex (Sugiyama, 2003), and the rid1 (root initiation defective1) mutant with lateral root primordia developing into massive structures (Konishi and Sugiyama, 2003). In some srd (shoot redifferentiation defective) mutants abnormal lateral root primordia lacking functional apical meristems are observed (Ohtani and Sugiyama, 2005). The srd2-1 mutation alters the organization of cells of the primordium and maintains primordial cell division for a long period, resulting in the formation of abnormal hemispherical laterals (Ohtani et al., 2010). Yet the most striking morphological feature occurring in most of these temperature-sensitive mutants is the formation at high frequency of fasciated lateral roots (Konishi and Sugiyama, 2003; Sugiyama, 2003; Otsuka and Sugiyama, 2012). In the fasciated laterals the numbers of internal cell files are increased (Otsuka and Sugiyama, 2012).
Numerous reports on mutants with significantly changed root phenotype often concern some general features of root system architecture, such as altered density of branching (Hermans et al., 2010; Ohtani et al., 2010; Ingram et al., 2011; see also reviews by Casimiro et al., 2003; de Smet et al., 2006; Péret et al., 2009a) or a strongly changed root pattern (Watson et al., 1998; Wu et al., 2007; de Pessemier et al., 2013). Obviously, research on these aspects of root morphology is essential to widen our knowledge of the formation of root system architecture. However, very little is known about the cellular organization of lateral root primordia (Celenza et al., 1995; Hirota et al., 2007) and hardly anything is known about cell pattern formation and the root apical meristem organization of lateral roots in such mutants. Studies on these aspects would provide insight into cell expansion during root morphogenesis.
A few cases of mutations causing morphological defects in both primary and lateral root apices of other plant species have been reported. The Pisum sativum crt (curly roots) mutant develops a small, compact curly root system when grown on a high-density substrate (Tsyganov et al., 2000). Yao et al. (2002) isolated the rice srt5 (short root 5) mutant, which has reduced cell size and number and inhibition of elongation of the seminal, crown and lateral roots. The sunn mutation in Medicago truncatula causes shortening of the primary roots (Schnabel et al., 2005). The maize lrt1 (lateral rootless 1) mutant produces impaired lateral roots in the maintenance of the root apical meristem (Husakova et al., 2013).
ROOT BENDING AND LATERAL ROOT POSITIONING
An interesting morphological feature of arabidopsis roots is a wavy growth pattern occurring when seedlings are grown at a 45 ° angle in relation to the gravity vector (Okada and Shimura, 1990) or in response to oxylipin treatment (Vellosillo et al., 2007). de Smet et al. (2007) showed that arabidopsis seedling roots grown vertically (not stimulated gravitropically) may also exhibit a wavy pattern. Root waving may be modified by the ion concentration of the medium and gelling polymers, and by the extent of gas exchange between the inside and the outside of the microenvironment of the growing seedling (Buer et al., 2000).
The left–right alternating pattern of lateral roots along the main root axis correlates with gravity-induced root waving (Lucas et al., 2008) and was shown to depend on AUX1, an auxin influx carrier necessary for gravitropic response (Hobbie and Estelle, 1995; Marchant et al., 1999; Swarup et al., 2005). In gravity-stimulated waving roots the lateral root primordia are initiated on the convex side of the local curvature (Fortin et al., 1989; Lucas et al., 2008). A similar effect is observed in barley when curvature of the root is induced by mechanical pressure on the root tip (Goss and Russell, 1980). Some experiments show that even transient manual mechanical bending of the arabidopsis root induces lateral root formation on the convex side of the bend (Ditengou et al., 2008; Richter et al., 2009). The process of primordium initiation on the convex (locally stretched) side is related to auxin redistribution (Laskowski et al., 2008) resulting from relocalization of the auxin transport protein PIN1 in a protoxylem cell in response to the tropic stimulus (Ditengou et al., 2008). Experiments on auxin-insensitive lateral root-deficient mutants, in which root bending induced new primordium initiation on the convex side of the bend (Ditengou et al., 2008), point to a link between tropically induced auxin redistribution and lateral root positioning. It has been shown that transient bending triggers a transient increase in Ca2+ in the pericycle and in neighbouring tissues on the convex side. Moreover, inhibition of such bend-elicited Ca2+ elevation results in blocking new lateral root formation in the bent region (Richter et al., 2009). These results indicate a possible role of a mechanically induced Ca2+ increase in the signalling pathway triggering lateral root initiation. However, the receptors for external mechanical signals and the signalling mechanisms leading to the reprogramming of development in the pericycle cells remain undefined (Monshausen and Gilroy, 2009).
BREAKING THROUGH TO THE OTHER SIDE
Thus, external mechanical factors not only affect the morphological features of the root apex and root system architecture but also induce the formation of lateral roots. Nevertheless, even an unaffected lateral root primordium is subjected to mechanical factors resulting from its growth and changing geometry (Kwiatkowska and Nakielski, 2011). Moreover, before it emerges through the parent root surface, a primordium needs to push through the overlying tissues, which exert mechanical stress on it. At this time an irreversible separation of the cells of the parent root takes place. The means by which this separation is effected has been the subject of interest since early studies on lateral root development (after Bell and McCully, 1970). One of the first attempt to explain this phenomenon comes from Sutcliffe and Sexton (1968), who observed an increase in activity of glycerophosphatase in the region of lateral root development in P. sativum, and who hypothesized that this enzyme, and probably other hydrolytic enzymes, were induced by mechanical pressure exerted by the growing primordium on the overlying tissues to assist the primordium in penetrating through them.
Contemporary studies have revealed an increase in expression of pectate lyase family genes in arabidopsis roots treated with exogenous auxin (Laskowski et al., 2006). Activity of pectate lyase is restricted to the parent root cells in the vicinity of a developing primordium. The walls of these cells contain substantial amounts of demethylated pectins, serving as a substrate for the enzyme. These results have led the authors to the hypothesis of enzymatic degradation of the middle lamellae of the parental root cells (Laskowski et al., 2006). Other studies have shown that the mechanical properties of the overlying tissues in arabidopsis roots are regulated in an auxin-dependent process. Auxin originating from the lateral root acts as a signal inducing expression of the auxin influx carrier LAX3 (LIKE AUX1) and the cell wall-remodelling enzymes that promote cell separation, finally facilitating lateral root emergence (Swarup et al., 2008). Moreover, local accumulation of primordium-originated auxin represses aquaporin gene expression in the primordium and overlying tissues and is thought to affect water supply and turgor maintenance to facilitate lateral root emergence (Péret et al., 2012).
AUXIN AND ITS ROLE IN SHAPING THE ROOT SYSTEM
The above considerations lead us to the conclusion that auxin and its distribution play a crucial role in many aspects of lateral root morphogenesis. Auxin affects lateral root positioning, initiation and subsequent development, thereby influencing the whole root system architecture (Malamy, 2005; Fukaki et al., 2007; Smith and de Smet, 2012). Local accumulation of this plant hormone in pericycle cells is a signal for the specification of the lateral root founder cells (Dubrovsky et al., 2008). In arabidopsis, auxin reporter maxima occur in the basal meristem at regular intervals of 15 h and correlate with the formation of a consecutive lateral root (de Smet et al., 2007). Auxin is necessary for the first cytological events in the pericycle founder cells and in further development by promoting cell division (Celenza et al., 1995). Treatment with exogenous auxin leads to an increase in new lateral root primordium initiation and growth in different plant species (Torrey, 1950; Blakely et al., 1982, 1988; Laskowski et al., 1995; Sreevidya et al., 2010), while application of the auxin transport inhibitor N-1-naphthylphthalamic acid (NPA) results in the arrest of lateral root development (Reed et al., 1998) by blocking the first transverse divisions (Casimiro et al., 2001). Other hormones regulating root branching are brassinosteroids, which promote lateral root formation through interaction with auxin (Bao et al., 2004), as do cytokinins and ethylene, both in interaction with auxin (Aloni et al., 2006; Ivanchenko et al., 2008). Abscisic acid (de Smet et al., 2003) has an inhibitory effect on lateral root development.
Another argument supporting the possible correlation between auxin and root morphology is the fact that a number of arabidopsis mutants showing aberrant morphology of roots (including lateral roots) are auxin-related. Moreover, many mutations in arabidopsis (Casimiro et al., 2003; de Smet et al., 2006; Fukaki et al., 2007) and other plant species (Dobbelaere et al., 1999; von Behrens et al., 2011; Zhang et al., 2011) exhibit auxin-related defects in lateral root formation, which is considered evidence of the key role of auxin in this process (Casimiro et al., 2003). The experiments with bending roots mentioned above show that an external mechanical factor may alter the auxin distribution in roots, which may suggest that this hormone is somehow sensitive to a mechanical impulse (Laskowski et al., 2008). Thus, in the light of the facts mentioned above we may state that auxin shapes the root system by taking part in various morphogenetic processes.
RATE OF GROWTH AND DEFORMATION
Plant organs are shaped in a way that usually correlates with their functions. The form of an organ develops through a morphogenetic process during which a precise sequence of oriented cell divisions, accompanied by cell expansion and followed by cell differentiation, is realized (Steeves and Sussex, 1972). Growth of a plant organ is highly coordinated because the walls of adjacent cells are glued together by the middle lamellas and usually traversed by plasmodesmata. For this reason the neighbouring cells do not lose contact through their lifetimes. This kind of growth is called symplastic (Erickson, 1986) and may be described in terms of a continuous field of growth rates (Hejnowicz and Romberger, 1984).
The major determinant of the form of an organ, as well as that of the organism as a whole, is its rates of growth in various directions (D'Arcy Thompson, 1942). The growth of most plant organs is anisotropic, which means that growth rates attain different values in various directions, of which three, mutually orthogonal, are the so-called principal directions of growth (Hejnowicz and Romberger, 1984). Two of these directions are directions of extreme growth and the third direction is orthogonal to the plane formed by the first two. The direction of maximal growth usually determines the axis of the organ.
Plant organs are able to regulate their growth rates, for example to adapt to new conditions (Baskin, 2013). A change in growth rate results in altered morphology, so the growth rate is de facto equivalent to the strain rate. This is why the growth of a plant organ may be considered as an irreversible deformation of a body (Green, 1962; Nakielski and Hejnowicz, 2003). Development of the lateral root is a striking example of continuous deformation. In the axial view of the primordium its initial shape may be well represented by a rectangle corresponding to the pericycle cells. With time this simple geometry is replaced by a more complex form similar to that observed in the primary root; thus, an apical part of the primordium attains the shape of a dome. Such a dynamic change of form suggests that growth rates are changeable. Indeed, in contrast to the primary root apex, whose geometry is preserved during growth (Nakielski, 2008), the lateral root has a field of growth rates whose mathematical form is specified in a time-dependent manner (Szymanowska-Pułka, 2007), which means that the rate of growth at a given point in the organ changes with time. Such a field was applied to a uniform rectangular meshwork in computer simulations. When the field was stable, the primordium developed in a steady and typical way. Its shape, represented by the outline of the meshwork (Fig. 2, on the basis of Szymanowska-Pułka, 2007), was regular and preserved a mirror symmetry with reference to its axis (upper shape in Fig. 2). When the field was unstable or even slightly displaced with respect to the meshwork (Szymanowska-Pułka and Nakielski, 2010), the primordium demonstrated disturbed symmetry (middle and lower shapes in Fig. 2) similar to that observed in real cases (Fig. 1D and A, respectively). The analogy between the forms of a real and virtual primordium supports the hypothesis concerning the changeable character of growth rates of the primordium.
Fig. 2.
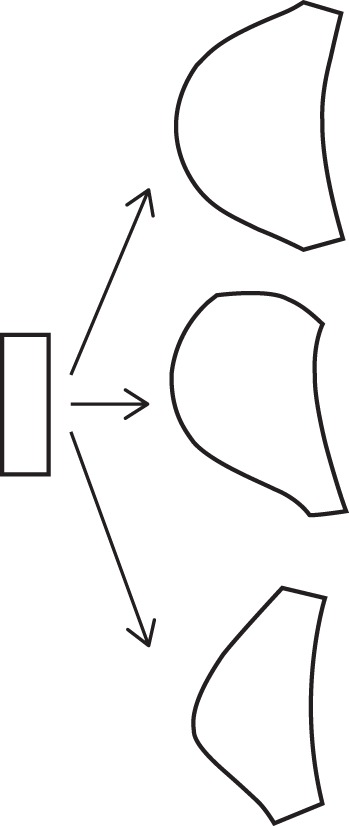
Deformation of the initially rectangular figure under the field of growth rates specified for the lateral root. Application of a stable field of growth rates results in formation of a regular dome-shaped primordium (upper); application of the field displaced constantly in one direction (middle) or randomly (lower) results in formation of an atypically shaped primordium (for details see Szymanowska-Pułka, 2007).
The oblique divisions observed both in the axial and tangential planes of a forming lateral (Szymanowska-Pułka et al., 2012; Lucas et al., 2013) were described above in the section ‘Anatomical events accompanying the change of geometry’. These divisions play a basic role in organ shaping and in this sense they can be considered formative (Smolarkiewicz and Dhonukshe, 2013). Oblique division walls inserted at early stages may suggest the foundation of the new principal directions of growth associated with the field of growth arising in the region of new organ formation, independently of the field of growth of the parent root (Hejnowicz and Hejnowicz, 1991; Szymanowska-Pułka et al., 2012).
MECHANICAL ASPECTS
The shape of a physical body changes under mechanical stress. The character of this change depends on the mechanical properties of the material from which the body is made. For example, elastic material deforms immediately after application of a load and restores its form instantly after removing the load. Viscous material undergoes gradual deformation and plastic material undergoes permanent deformation under mechanical stress. From the point of view of mechanics, plant tissue is considered a viscoelastic material (Fung, 1984), i.e. the relationship between stress and strain depends on time. Viscoelastic materials are characterized by properties of fluids and elastic solids, in that under mechanical stress they exhibit both viscosity and elasticity. Their mechanical parameters may be estimated on the basis of their morphological responses to external mechanical stimuli. The methods of rheology, a branch of mechanics dealing with viscoelastic materials, have been widely applied to the measurement of mechanical parameters of both fresh and fixed plant material (e.g. Alvarez et al., 1998; Balsamo et al., 2003). Experimental application of these methods to roots of sorghum and lateral roots of Pisum have shown that the mechanical properties of root tissues depend on the culture conditions (Tanimoto et al., 2000; Hattori et al., 2003).
In mechanically bent roots in which new primordia are induced on the convex side, even transient bending results in redistribution of stress within the root. This is similar to the process that occurs during the bending of young stems, in which the cells are highly turgid. The cells on the convex side of the bent stem undergo tension while those on the concave side undergo compression. Through osmoregulation the compressed cells lose water while the cells under tension take in water from the outside. This process is reversible; it continues in time and resembles the viscoelastic deformation of any material. However, the compressed and stressed cells adjust their geometry to the current stress distribution through transport of water while deformation of viscoelastic material takes place through displacement of the elements from which the material is formed. The form of viscoelasticity shown by living plant cells has been termed ‘osmoelasticity’, which suitably describes the mechanical stress-dependent phenomena occurring in turgid plant cells (Hejnowicz, 2011).
Observing the continuous change of form of a developing lateral root from the perspective of mechanics, one might assume that a continuous change in the distribution of stress occurs within the emerging organ and in its vicinity. Although there is no direct evidence supporting this assumption, some recent research (Lucas et al., 2013) has shown that there is a relation between mechanical stress distribution and the shape of the lateral root primordium. Another argument derives from studies on phyllotactic pattern formation in shoot apices. The presence and distribution of compressive stresses in the generative region of the sunflower capitulum is considered a critical component of a buckling mechanism whereby the floret primordium is initiated (Dumais and Steele, 2000). The morphogenesis of the arabidopsis shoot apex has also been shown to be indirectly regulated by mechanical stress (Hamant et al., 2008).
The relation between mechanical stress and local change in geometry may probably extend to the level of the single cell, the geometrical features of which may be altered by the changed distribution of the mechanical properties of the cell wall, as hypothesized by Pietruszka et al. (2012). Also, the externally applied load can be perceived by individual plant cells. Embedded in agarose blocks, isolated cells of tobacco subjected to controlled external mechanical stress divide either parallel or perpendicular to the principal direction of stress (Lynch and Lintilhac, 1997). A similar effect was observed in chrysanthemum cells, which under mechanical stress elongated and divided in planes perpendicular to the direction of elongation, corresponding to the principal stress direction (Zhou et al., 2007). Based on the results of these experiments, in which the orientation of the division wall in the cell was forced by a controlled stress distribution, we may hypothesize that the symmetry-breaking oblique cell divisions occurring in lateral root primordia of untreated seedlings of arabidopsis may result from a local change in the distribution of stress in the region of primordium formation.
CONCLUSIONS AND PERSPECTIVES
Both root form and root system architecture are perfectly adjusted to the functioning of the hidden half of the plant. When exposed to physiological or mechanical stresses, roots show extraordinary capacity to adapt to new conditions by regulating growth rates, a process that is eventually manifested as morphogenetic responses. There are opinions (Kennaway et al., 2011) that the oriented growth that is responsible for morphological diversity may be specified through ubiquitous tissue stresses (Hejnowicz, 1997) or through molecular signalling. The above summary suggests that the two may mutually interact. However, we do not know how the mechanical impulse is translated to a molecular signal and vice versa, and what carries the signal. Various reports indicate an important role for auxin in organogenesis and other morphological events and processes, some of which are mechano-dependent. This hormone regulates root system architecture, is related to numerous mutations altering the morphological features of roots, acts as a signal inducing a sequence of events leading to separation of the parent root cells during lateral root emergence, and undergoes redistribution during mechano-induced lateral root initiation. Is it possible that auxin may have another signalling function in plants, namely an involvement in mechano-dependent growth and development? Although contemporary science does not answer this question, it allows us to draw conclusions and to plan further experiments on the basis of the reports discussed above.
In spite of the fact that continuous change in the shape of the lateral root primordium is relatively easy to observe, it seems to be an unexplored aspect of organ development. In recent studies providing direct evidence for the gradual radialization of the primordium, precise molecular methods have been applied to follow the morphological changes that occur (Lucas et al., 2013). Nevertheless, the mechanisms underlying this ‘transformation’ remain unknown. The reasons for atypically shaped primordia, reported both in wild-type plants and mutant lines, are also unclear. The morphology of a plant organ is obviously related to its function, so in order to fully understand the process of lateral root formation and its contribution to the root system, more detailed studies concerning the cellular basis of morphological changes and defects (Casimiro et al., 2003) are needed. Further research on mutants producing lateral roots with aberrant morphology would provide insight into the regulation of growth and would make it possible to follow changes that may occur in both the direction and the rate of growth in the lateral root apical meristem.
Our knowledge of the molecular bases of lateral root initiation and development has increased rapidly within recent decades. Building on these advances, we may try to widen our knowledge of the probable relation between auxin and root system morphology, based in part on the auxin-related mutants whose root growth and development are altered in comparison with wild-type plants. Yet it is important to remember that, as a physical object, the lateral root (as well as other plant organs) also has characteristic physical properties. A change of form of such an object implies either a change in the distribution of mechanical stress or a change in mechanical properties. Direct measurement of both of these remains a challenge, mostly because of technical difficulties. However, the few reports examining the mechanical parameters of tissues of roots show that no challenge in science is so great that it is not taken up.
ACKNOWLEDGEMENTS
I wish to thank my colleagues in the Department of Biophysics and Plant Morphogenesis, University of Silesia, Dr Jerzy Nakielski and Jerzy Karczewski, for their support in my scientific work, and Professor Dorota Kwiatkowska for critical reading of the first version of the manuscript. Special thanks are given to Professor Lewis Feldman of the Department of Plant and Microbial Biology, UC Berkeley, and to Dr Izabela Potocka of the Laboratory of Cell Biology, University of Silesia, for their great patience, encouraging discussion and valuable comments.
LITERATURE CITED
- Aloni R, Aloni E, Langhans M, Ullrich CI. Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Annals of Botany. 2006;97:883–893. doi: 10.1093/aob/mcl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez MD, Canet W, Cuesta F, Lamua M. Viscoelastic characterization of solid foods from creep compliance data: application to potato tissues. Zeitschrift für Lebensmitteluntersuchung und Forschung. 1998;207:356–362. [Google Scholar]
- Atwell BJ. Physiological responses of lupin roots to soil compaction. Plant and Soil. 1988;111:277–281. [Google Scholar]
- Balsamo RA, Bauer AM, Davis SD, Rice BM. Leaf biomechanics, morphology, and anatomy of the deciduous mesophyte Prunus serrulata (Rosaceae) and the evergreen sclerophyllous shrub Heteromeles arbutifolia (Rosaceae) American Journal of Botany. 2003;90:72–77. doi: 10.3732/ajb.90.1.72. [DOI] [PubMed] [Google Scholar]
- Bao F, Shen J, Brady SR, Muday GK, Asami T, Yang Z. Brassinosteroids interact with auxin to promote lateral root development in arabidopsis. Plant Physiology. 2004;134:1624–1631. doi: 10.1104/pp.103.036897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow PW. Positional controls in root development. In: Barlow PW, Carr DJ, editors. Positional controls in plant development. Cambridge: Cambridge University Press; 1984. pp. 281–318. [Google Scholar]
- Barlow PW, Adam JS. The position and growth of lateral roots on cultured root axes of tomato, Lycopersicon esculentum (Solanaceae) Plant Systematics and Evolution. 1988;158:141–154. [Google Scholar]
- Baskin TI. Patterns of root growth acclimation: constant processes, changing boundaries. Wiley Interdisciplinary Reviews. Developmental Biology. 2013;2:65–73. doi: 10.1002/wdev.94. [DOI] [PubMed] [Google Scholar]
- Baskin TI, Betzner AS, Hoggart R, Cork A, Williamson RE. Root morphology mutants in Arabidopsis thaliana. Australian Journal of Plant Physiology. 1992;19:427–437. [Google Scholar]
- Beeckman T, Burssens S, Inzé D. The peri-cell-cycle in Arabidopsis. Journal of Experimental Botany. 2001;52:403–411. doi: 10.1093/jexbot/52.suppl_1.403. [DOI] [PubMed] [Google Scholar]
- von Behrens I, Komatsu M, Zhang Y, et al. Rootless with undetectable meristem 1 encodes a monocotspecific AUX/IAA protein that controls embryonic seminal and post-embryonic lateral root initiation in maize. Plant Journal. 2011;66:341–353. doi: 10.1111/j.1365-313X.2011.04495.x. [DOI] [PubMed] [Google Scholar]
- Bell JK, McCully ME. A histological study of lateral root initiation and development in Zea mays. Protoplasma. 1970;70(179–205) [Google Scholar]
- Benfey PN, Linstead PJ, Roberts K, Schiefelbein JW, Hauser M-T, Aeschbacher RA. Root development in Arabidopsis: four mutants with dramatically altered root morphogenesis. Development. 1993;119:57–70. doi: 10.1242/dev.119.Supplement.57. [DOI] [PubMed] [Google Scholar]
- Bengough AG. A biophysical analysis of root growth under mechanical stress. Plant and Soil. 1997;189:155–164. [Google Scholar]
- Bengough AG. Root elongation is restricted by axial but not by radial pressures: so what happens in field soil? Plant and Soil. 2012;360:15–18. [Google Scholar]
- Benková E, Bielach A. Lateral root organogenesis – from cell to organ. Current Opinion in Plant Biology. 2010;13:677–683. doi: 10.1016/j.pbi.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- Blakely LM, Durham M, Evans TA, Blakely RM. Experimental studies on lateral root formation in radish seedling roots: I. General methods, developmental stages and spontaneous formation of laterals. Botanical Gazette. 1982;143:341–352. [Google Scholar]
- Blakely LM, Blakely RM, Colowit PM, Elliot DS. Experimental studies on lateral root formation in radish seedling roots. II. Analysis of the dose-response to exogenous auxin. Plant Physiology. 1988;87:414–419. doi: 10.1104/pp.87.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Masle J, Wasteneys GO. Growth conditions modulate root-wave phenotypes in Arabidopsis. Plant Cell Physiology. 2000;41:1164–1170. doi: 10.1093/pcp/pcd042. [DOI] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, et al. Dissecting arabidopsis lateral root development. Trends in Plant Science. 2003;8:165–17. doi: 10.1016/S1360-1385(03)00051-7. [DOI] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, et al. Auxin transport promotes arabidopsis lateral root initiation. Plant Cell. 2001;13:843–852. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza JL, Jr, Grisafi PL, Fink GR. A pathway for lateral root formation in Arabidopsis thaliana. Genes & Development. 1995;9:2131–2142. doi: 10.1101/gad.9.17.2131. [DOI] [PubMed] [Google Scholar]
- Cheng J-C, Seeley KA, Sung ZR. RML7 and RML2, Arabidopsis genes required for cell proliferation at the root tip. Plant Physiology. 1995;107:365–376. doi: 10.1104/pp.107.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes FAL. Regeneration of the discrete root epidermis of Pistia stratiotes L. after perturbation of the meristem. New Phytologist. 1992;120:209–213. [Google Scholar]
- Costa CT, Strieder ML, Abel S, Delatorre CA. Phosphorus and nitrogen interaction: loss of QC identity in response to P or N limitation is anticipated in pdr23 mutant. Brazilian Journal of Plant Physiology. 2011;23:219–229. [Google Scholar]
- Cruz-Ramírez A, López-Bucio J, Ramórez-Pimentel G, et al. The xipotl mutant of Arabidopsis reveals a critical role for phospholipid metabolism in root system development and epidermal cell integrity. Plant Cell. 2004;16:2020–2034. doi: 10.1105/tpc.103.018648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutter EG. Plant anatomy. Part 2: Organs. London: E. Arnold; 1971. pp. 5–44. [Google Scholar]
- D'Arcy Thompson W. On growth and form: a new edition. Cambridge: Cambridge University Press; 1942. [Google Scholar]
- Ditengou FA, Teale WD, Kochersperger P, et al. Mechanical induction of lateral root initiation in Arabidopsis thaliana. Proceedings of the National Academy of Science of the USA. 2008;105:18818–18823. doi: 10.1073/pnas.0807814105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere S, Croonenborghs A, Thys A, Vande Broek A, Vanderleyden J. Phytostimulatory effect of Azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant and Soil. 1999;212:155–164. [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, et al. Cellular organisation of the Arabidopsis thaliana root. Development. 1993;119:71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Doerner PW, Colón-Carmona A, Rost TL. Pericycle cell proliferation and lateral root initiation in arabidopsis. Plant Physiology. 2000;124:1648–1657. doi: 10.1104/pp.124.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Rost TL, Colón-Carmona A, Doerner P. Early primordium morphogenesis during lateral root initiation in Arabidopsis thaliana. Planta. 2001;214:30–36. doi: 10.1007/s004250100598. [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Gambetta GA, Hernández-Barrera A, Shishkova S, González I. Lateral root initiation in Arabidopsis: developmental window, spatial patterning, density and predictability. Annals of Botany. 2006;97:903–915. doi: 10.1093/aob/mcj604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, et al. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proceedings of the National Academy of Sciences of the USA. 2008;105:8790–8794. doi: 10.1073/pnas.0712307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Napsucialy-Mendivil S, Duclercq J, et al. Auxin minimum defines a developmental window for lateral root initiation. New Phytologist. 2011;191:970–983. doi: 10.1111/j.1469-8137.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- Dumais J, Steele CR. New evidence for the role of mechanical forces in the shoot apical meristem. Journal of Plant Growth Regulation. 2000;19:7–18. doi: 10.1007/s003440000003. [DOI] [PubMed] [Google Scholar]
- Eapen D, Barroso ML, Campos ME, et al. A no hydrotropic response root mutant that responds positively to gravitropism in Arabidopsis. Plant Physiology. 2003;131:536–546. doi: 10.1104/pp.011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RO. Symplastic growth and symplasmic transport. Plant Physiology. 1986;82:1153. doi: 10.1104/pp.82.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Zhu J, Du X, Cui X. Effects of three auxin-inducible LBD members on lateral root formation in Arabidopsis thaliana. Planta. 2012;236:1227–1237. doi: 10.1007/s00425-012-1673-3. [DOI] [PubMed] [Google Scholar]
- Fortin MC, Pierce FJ, Poff KL. The pattern of secondary root formation in curving roots of Arabidopsis thaliana (L.) Heynh. Plant, Cell and Environment. 1989;12:337–339. doi: 10.1111/j.1365-3040.1989.tb01949.x. [DOI] [PubMed] [Google Scholar]
- Fukaki H, Okushima Y, Tasaka M. Auxin-mediated lateral root formation in higher plants. International Review of Cytology. 2007;256:111–137. doi: 10.1016/S0074-7696(07)56004-3. [DOI] [PubMed] [Google Scholar]
- Fung YC. Biomechanics. Mechanical properties of living tissues. New York: Springer; 1984. [Google Scholar]
- Goss MJ, Russell RS. Effects of mechanical impedance on root growth in barley (Hordeum vulgare L.). III. Observations on the mechanism of response. Journal of Experimental Botany. 1980;31:577–588. [Google Scholar]
- Green PB. Mechanism for plant cellular morphogenesis. Science. 1962;138:1404–1405. doi: 10.1126/science.138.3548.1404. [DOI] [PubMed] [Google Scholar]
- Gruber V, Zahaf O, Diet A, de Zélicourt A, de Lorenzo L, Crespi M. Impact of the environment on root architecture in dicotyledoneous plants. In: Costa de Oliveira A, Varshney RK, editors. Root genomics. Berlin: Springer; 2011. pp. 113–132. [Google Scholar]
- Grzesiak MT. Impact of soil compaction on root architecture, leaf water status, gas exchange and growth of maize and triticale seedlings. Plant Root. 2009;3:10–16. [Google Scholar]
- Hamant O, Heisler MG, Jönsson H, et al. Developmental patterning by mechanical signals in arabidopsis. Science. 2008;322:1650–1655. doi: 10.1126/science.1165594. [DOI] [PubMed] [Google Scholar]
- Hattori T, Inanaga S, Tanimoto E, Lux A, Luxova M, Sugimoto Y. Silicon-induced changes in viscoelastic properties of sorghum root cell walls. Plant and Cell Physiology. 2003;44:743–749. doi: 10.1093/pcp/pcg090. [DOI] [PubMed] [Google Scholar]
- Hejnowicz Z. Graviresponses in herbs and trees: a major role for the redistribution of tissue and growth stresses. Planta. 1997;203:S136–S146. doi: 10.1007/pl00008102. [DOI] [PubMed] [Google Scholar]
- Hejnowicz Z. Plants as mechano-osmotic transducers. In: Wojtaszek P, editor. Mechanical integration of plant cells and plants, signaling and communication in plants. Berlin: Springer; 2011. pp. 241–267. [Google Scholar]
- Hejnowicz Z, Hejnowicz K. Modeling the formation of root apices. Planta. 1991;184:1–7. doi: 10.1007/BF00208228. [DOI] [PubMed] [Google Scholar]
- Hejnowicz Z, Karczewski J. Modeling of meristematic growth of root apices in natural coordinate system. American Journal of Botany. 1993;80:309–315. [Google Scholar]
- Hejnowicz Z, Rombereger JA. Growth tensor of plant organs. Journal of Theoretical Biology. 1984;110:93–114. [Google Scholar]
- Hermans C, Porco S, Verbruggen N, Bush DR. Chitinase-like protein CTL1 plays a role in altering root system architecture in response to multiple environmental conditions. Plant Physiology. 2010;152:904–917. doi: 10.1104/pp.109.149849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota A, Kato T, Fukaki H, Aida M, Tasaka M. The auxin-regulated AP2/EREBP gene PUCHI is required for morphogenesis in the early lateral root primordium of Arabidopsis. Plant Cell. 2007;19:2156–2168. doi: 10.1105/tpc.107.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie L, Estelle M. The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant Journal. 1995;7:211–220. doi: 10.1046/j.1365-313x.1995.7020211.x. [DOI] [PubMed] [Google Scholar]
- Hodge A, Berta G, Doussan C, Merchan F, Crespi M. Plant root growth, architecture and function. Plant and Soil. 2009;321:153–187. [Google Scholar]
- Holding DR, McKenzie R, Coomber SA. Genetic and structural analysis of five Arabidopsis mutants with abnormal root morphology generated by the seed transformation method. Annals of Botany. 1994;74:193–204. [Google Scholar]
- Husakova E, Hochholdinger F, Soukup A. Lateral root development in the maize (Zea mays) lateral rootless1 mutant. Annals of Botany. 2013;112:417–428. doi: 10.1093/aob/mct043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Kono Y. Interspecific differences of the root system structures of four cereal species as affected by soil compaction. Japanese Journal of Crop Sciences. 1991;60:130–138. [Google Scholar]
- Iijima M, Barlow PW, Bengough AG. Root cap structure and cell production rates of maize (Zea mays) roots in compacted sand. New Phytologist. 2003;160:127–134. doi: 10.1046/j.1469-8137.2003.00860.x. [DOI] [PubMed] [Google Scholar]
- Ingram P, Dettmer J, Helariutta Y, Malamy JE. Arabidopsis Lateral Root Development 3 is essential for early phloem development and function, and hence for normal root system development. Plant Journal. 2011;38:455–467. doi: 10.1111/j.1365-313X.2011.04700.x. [DOI] [PubMed] [Google Scholar]
- Ivanchenko MG, Muday GK, Dubrovsky JG. Ethylene–auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant Journal. 2008;55:335–347. doi: 10.1111/j.1365-313X.2008.03528.x. [DOI] [PubMed] [Google Scholar]
- Kennaway R, Coen E, Green A, Bangham A. Generation of diverse biological forms through combinatorial interactions between tissue polarity and growth. PLoS Computational Biology. 2011;7(6) doi: 10.1371/journal.pcbi.1002071. e1002071. doi:10.1371/journal.pcbi.1002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Sugiyama M. Genetic analysis of adventitious root formation with a novel series of temperature-sensitive mutants of Arabidopsis thaliana. Development. 2003;130:5637–5647. doi: 10.1242/dev.00794. [DOI] [PubMed] [Google Scholar]
- Kramer PJ, Boyer JS. Water relations of plants and soils. New York: Academic Press; 1995. [Google Scholar]
- Křeček P, Skůpa P, Libus J, et al. The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biology. 2009;10(249):1–11. doi: 10.1186/gb-2009-10-12-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummerová M, Zezulka Š, Babula P, Váňová L. Root response in Pisum sativum and Zea mays under fluoranthene stress: morphological and anatomical traits. Chemosphere. 2013;90:665–673. doi: 10.1016/j.chemosphere.2012.09.047. [DOI] [PubMed] [Google Scholar]
- Kurup S, Runions J, Köhler U, Laplaze L, Hodge S, Haseloff J. Marking cell lineages in living tissues. Plant Journal. 2005;42:444–453. doi: 10.1111/j.1365-313X.2005.02386.x. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska D, Nakielski J. Mechanics of meristems. In: Wojtaszek P, editor. Mechanical integration of plant cells and plants, signaling and communication in plants. Berlin: Springer; 2011. pp. 133–172. [Google Scholar]
- Laskowski M. Lateral root initiation is a probabilistic event whose frequency is set by fluctuating levels of auxin response. Journal of Experimental Botany. 2013;64:2609–2617. doi: 10.1093/jxb/ert155. [DOI] [PubMed] [Google Scholar]
- Laskowski M, Biller S, Stanley K, Kajstura T, Prusty R. Expression profiling of auxin-treated Arabidopsis roots: toward a molecular analysis of lateral root emergence. Plant and Cell Physiology. 2006;47:788–792. doi: 10.1093/pcp/pcj043. [DOI] [PubMed] [Google Scholar]
- Laskowski M, Grieneisen VA, Hofhuis H, et al. Root system architecture from coupling cell shape to auxin transport. PLoS Biology. 2008;6(12) doi: 10.1371/journal.pbio.0060307. e307. doi:10.1371/journal.pbio.0060307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski MJ, Williams ME, Nusbaum HC, Sussex IM. Formation of lateral root meristems is a two-stage process. Development. 1995;121:3303–3310. doi: 10.1242/dev.121.10.3303. [DOI] [PubMed] [Google Scholar]
- Lin BL, Raghavan V. Lateral root initiation in Marsilea quadrifolia. I. Origin and histogenesis of lateral roots. Canadian Journal of Botany. 1991;69:123–135. doi: 10.1139/b91-018. [DOI] [PubMed] [Google Scholar]
- Lipiec J, Horn R, Pietrusiewicz J, Siczek A. Effects of soil compaction on root elongation and anatomy of different cereal plant species. Soil & Tillage Research. 2012;121:74–81. [Google Scholar]
- López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L. The role of nutrient availability in regulating root architecture. Current Opinion in Plant Biology. 2003;6:280–287. doi: 10.1016/s1369-5266(03)00035-9. [DOI] [PubMed] [Google Scholar]
- Lucas M, Godin C, Jay-Allemand C, Laplaze L. Auxin fluxes in the root apex co-regulate gravitropism and lateral root initiation. Journal of Experimental Botany. 2008;59:55–66. doi: 10.1093/jxb/erm171. [DOI] [PubMed] [Google Scholar]
- Lucas M, Kenobi K, von Wangenheim D, et al. Lateral root morphogenesis is dependent on the mechanical properties of the overlaying tissues. Proceedings of the National Academy of Sciences of the USA. 2013;110:5229–5234. doi: 10.1073/pnas.1210807110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. Root architecture and plant productivity. Plant Physiology. 1995;109:7–13. doi: 10.1104/pp.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch TM, Lintilhac PM. Mechanical signals in plant development: a new method for single cell studies. Developmental Biology. 1997;181:246–256. doi: 10.1006/dbio.1996.8462. [DOI] [PubMed] [Google Scholar]
- Malamy JE. Intrinsic and environmental response pathways that regulate root system architecture. Plant, Cell and Environment. 2005;28:67–77. doi: 10.1111/j.1365-3040.2005.01306.x. [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- Marchant A, Kargul J, May ST, et al. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO Journal. 1999;18:2066–2073. doi: 10.1093/emboj/18.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockaitis K, Estelle M. Auxin receptors and plant development: a new signaling paradigm. Annual Review of Cell and Developmental Biology. 2008;24:55–80. doi: 10.1146/annurev.cellbio.23.090506.123214. [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Gilroy S. Feeling green: mechanosensing in plants. Trends in Cell Biology. 2009;19:228–235. doi: 10.1016/j.tcb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Nakielski J. The tensor-based model for growth and cell divisions of the root apex. I. The significance of principal directions. Planta. 2008;228:179–189. doi: 10.1007/s00425-008-0728-y. [DOI] [PubMed] [Google Scholar]
- Nakielski J, Hejnowicz Z. The description of growth of plant organs: a continuous approach based on the growth tensor. In: Nation J, Trofimova I, Rand JD, Sulis W, editors. Formal description of developing systems. Netherlands: Kluwer Academic Publishers; 2003. pp. 119–136. [Google Scholar]
- Nibau C, Gibbs DJ, Coates JC. Branching out in new directions: the control of root architecture by lateral root formation. New Phytologist. 2008;179:595–614. doi: 10.1111/j.1469-8137.2008.02472.x. [DOI] [PubMed] [Google Scholar]
- Niu YF, Chai RS, Jin G L, Wang H, Tang CX, Zhang YS. Responses of root architecture development to low phosphorus availability: a review. Annals of Botany. 2013;112:391–408. doi: 10.1093/aob/mcs285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi-Ito K, Bergmann DC. Regulation of the Arabidopsis root vascular initial population by LONESOME HIGHWAY. Development. 2007;134:2959–2968. doi: 10.1242/dev.006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani M, Sugiyama M. Involvement of SRD2-mediated activation of snRNA transcription in the control of cell proliferation competence in Arabidopsis. Plant Journal. 2005;43:479–490. doi: 10.1111/j.1365-313X.2005.02469.x. [DOI] [PubMed] [Google Scholar]
- Ohtani M, Demura T, Sugiyama M. Particular significance of SRD2-dependent snRNA accumulation in polarized pattern generation during lateral root development of arabidopsis. Plant and Cell Physiology. 2010;51:2002–2012. doi: 10.1093/pcp/pcq159. [DOI] [PubMed] [Google Scholar]
- Okada K, Shimura Y. Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science. 1990;250:274–276. doi: 10.1126/science.250.4978.274. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Tsurumi S, Shibasaki K, et al. Genetic dissection of hormonal responses in the roots of arabidopsis grown under continuous mechanical impedance. Plant Physiology. 2008;146:1651–1662. doi: 10.1104/pp.107.115519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka K, Sugiyama M. Tissue organization of fasciated lateral roots of Arabidopsis mutants suggestive of the robust nature of outer layer patterning. Journal of Plant Research. 2012;125:547–554. doi: 10.1007/s10265-011-0471-5. [DOI] [PubMed] [Google Scholar]
- Parizot B, Laplaze L, Ricaud L, et al. Diarch symmetry of the vascular bundle in Arabidopsis root encompasses the pericycle and is reflected in distich lateral root initiation. Plant Physiology. 2008;146:140–148. doi: 10.1104/pp.107.107870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, De Rybel B, Casimiro I, et al. Arabidopsis lateral root development: an emerging story. Trends in Plant Science. 2009a;14:399–408. doi: 10.1016/j.tplants.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Péret B, Larrieu A, Bennett MJ. Lateral root emergence: a difficult birth. Journal of Experimental Botany. 2009b;60:3637–3643. doi: 10.1093/jxb/erp232. [DOI] [PubMed] [Google Scholar]
- Péret B, Li G, Zhao J, et al. Auxin regulates aquaporin function to facilitate lateral root emergence. Nature Cell Biology. 2012;14:991–998. doi: 10.1038/ncb2573. [DOI] [PubMed] [Google Scholar]
- de Pessemier J, Chardon F, Juraniec M, Delaplace P, Hermans C. Natural variation of the root morphological response to nitrate supply in Arabidopsis thaliana. Mechanisms of Development. 2013;130:45–53. doi: 10.1016/j.mod.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Pietruszka M, Lipowczan M, Geitmann A. Persistent symmetry frustration in pollen tubes. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0048087. e48087. doi:10.1371/journal.pone.0048087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocka I, Szymanowska-Pułka J, Karczewski J, Nakielski J. Effect of mechanical stress on Zea root apex. I. Mechanical stress leads to the switch from closed to open meristem organization. Journal of Experimental Botany. 2011;62:4583–4593. doi: 10.1093/jxb/err169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MAK. Stress-induced morphogenic responses: growing out of trouble? Trends in Plant Science. 2007;12:98–105. doi: 10.1016/j.tplants.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Prasad AM, Sivanandan C, Resminath R, Thakare DR, Bhat SR, Srinivasan Cloning and characterization of a pentatricopeptide protein encoding gene (LOJ) that is specifically expressed in lateral organ junctions in Arabidopsis thaliana. Gene. 2005;353(67– 79) doi: 10.1016/j.gene.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday GK. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in arabidopsis. Plant Physiology. 1998;118:1369–1378. doi: 10.1104/pp.118.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter GL, Monshausen GB, Krol A, Gilroy S. Mechanical stimuli modulate lateral root organogenesis. Plant Physiology. 2009;151:1855–1866. doi: 10.1104/pp.109.142448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost TL. The organization of roots of dicotyledonous plants and the positions of control points. Annals of Botany. 2011;107:1213–1222. doi: 10.1093/aob/mcq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, et al. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell. 1997;9:745–757. doi: 10.1105/tpc.9.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rybel B, Vassileva V, Parizot B, et al. A novel Aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Current Biology. 2010;20:1697–1706. doi: 10.1016/j.cub.2010.09.007. [DOI] [PubMed] [Google Scholar]
- von Sachs J. Lectures in plant physiology. Oxford: Clarendon Press; 1887. Relations between growth and cell-division in the embryonic tissues; pp. 431–459. [Google Scholar]
- Scheres B, Benfey P, Dolan L. Root development. The Arabidopsis Book. 2002;1 doi: 10.1199/tab.0101. e0101. doi:10.1199/tab.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW, Benfey PN. Root development in Arabidopsis. In: Meyerowitz EM, Somerville CR, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 335–353. [Google Scholar]
- Schnabel E, Journet EP, de Carvalho-Niebel F, Duc G, Frugoli J. The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Molecular Biology. 2005;58:809–822. doi: 10.1007/s11103-005-8102-y. [DOI] [PubMed] [Google Scholar]
- Shuai B, Reynaga-Peňa CG, Springer PS. The LATERAL ORGAN BOUNDARIES gene defines a novel, plant-specific gene family. Plant Physiology. 2002;129:747–761. doi: 10.1104/pp.010926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smet I. Lateral root initiation: one step at a time. New Phytologist. 2012;193:867–873. doi: 10.1111/j.1469-8137.2011.03996.x. [DOI] [PubMed] [Google Scholar]
- de Smet I, Signora L, Beeckman T, Inze D, Foyer CH, Zhang H. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant Journal. 2003;33:543–555. doi: 10.1046/j.1365-313x.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- de Smet I, Vanneste S, Inzé D, Beeckman T. Lateral root initiation or the birth of a new meristem. Plant Molecular Biology. 2006;60:871–887. doi: 10.1007/s11103-005-4547-2. [DOI] [PubMed] [Google Scholar]
- de Smet I, Tetsumura T, de Rybel B, et al. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development. 2007;134:681–690. doi: 10.1242/dev.02753. [DOI] [PubMed] [Google Scholar]
- de Smet I, Vassileva V, de Rybel B, et al. Receptor-like kinase ACR4 restricts formative cell divisions in the Arabidopsis root. Science. 2008;322:594–597. doi: 10.1126/science.1160158. [DOI] [PubMed] [Google Scholar]
- de Smet I, Lau S, Voβ U, Vanneste S, et al. Bimodular auxin response controls organogenesis in Arabidopsis. Proceedings of the National Academy of Sciences of the USA. 2010;107:2705–2710. doi: 10.1073/pnas.0915001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, de Smet I. Root system architecture: insights from Arabidopsis and cereal crops. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2012;367:1441–1452. doi: 10.1098/rstb.2011.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolarkiewicz M, Dhonukshe P. Formative cell divisions: principal determinants of plant morphogenesis. Plant and Cell Physiology. 2013;54:333–342. doi: 10.1093/pcp/pcs175. [DOI] [PubMed] [Google Scholar]
- Sreevidya VS, Hernandez-Oane RJ, Gyaneshwar P, Lara-Flores M, Ladha JK, Reddy PM. Changes in auxin distribution patterns during lateral root development in rice. Plant Science. 2010;178:531–538. [Google Scholar]
- Steeves TA, Sussex IM. Patterns in plant development. Englewood Cliffs, NJ: Prentice-Hall; 1972. [Google Scholar]
- Sugiyama M. Isolation and initial characterization of temperature-sensitive mutants of Arabidopsis thaliana that are impaired in root redifferentiation. Plant and Cell Physiology. 2003;44:588–596. doi: 10.1093/pcp/pcg077. [DOI] [PubMed] [Google Scholar]
- Sussex IM, Godoy JA, Kerk NM, et al. Cellular and molecular events in a newly organizing lateral root meristem. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1995;350:39–43. doi: 10.1098/rstb.1995.0135. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JF, Sexton IM. β-Glycerophosphatase and lateral root development. Nature. 1968;217:1285. [Google Scholar]
- Swarup R, Kramer EM, Perry P, et al. Root gravitropism requires lateral root cap and epidermal cells for transport and response to amobile auxin signal. Nature Cell Biology. 2005;7:1057–1065. doi: 10.1038/ncb1316. [DOI] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R, et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nature Cell Biology. 2008;10:946–954. doi: 10.1038/ncb1754. [DOI] [PubMed] [Google Scholar]
- Szymanowska-Pułka J. Application of a changing field of growth rates to a description of root apex formation. Journal of Theoretical Biology. 2007;247:650–656. doi: 10.1016/j.jtbi.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Szymanowska-Pułka J, Nakielski J. The tensor-based model for growth and cell divisions of the root apex. II. Lateral root formation. Planta. 2010;232:1207–1218. doi: 10.1007/s00425-010-1239-1. [DOI] [PubMed] [Google Scholar]
- Szymanowska-Pułka J, Potocka I, Karczewski J, Jiang K, Nakielski J, Feldman LJ. Principal growth directions in development of the lateral root in Arabidopsis thaliana. Annals of Botany. 2012;110:491–501. doi: 10.1093/aob/mcs129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto E, Fujii S, Yamamoto R, Inanaga S. Measurement of viscoelastic properties of root cell walls affected by low pH in lateral roots of Pisum sativum L. Plant and Soil. 2000;226:21–28. [Google Scholar]
- Torrey JG. The induction of lateral roots by indoleacetic acid and root decapitation. American Journal of Botany. 1950;37:257–264. [Google Scholar]
- Tsyganov VE, Pavlova ZB, Kravchenko LV, et al. New gene crt (Curly Roots) controlling pea (Pisum sativum L.) root development. Annals of Botany. 2000;86:975–981. [Google Scholar]
- Vellosillo T, Martinez M, Lopez MA, et al. Oxylipins produced by the 9-lipoxygenase pathway in Arabidopsis regulate lateral root development and defence responses through a specific signaling cascade. Plant Cell. 2007;19:831–846. doi: 10.1105/tpc.106.046052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MB, Emory KK, Piatak RM, Malmberg RL. Arginine decarboxylase (polyamine synthesis) mutants of Arabidopsis thaliana exhibit altered root growth. Plant Journal. 1998;13:231–239. doi: 10.1046/j.1365-313x.1998.00027.x. [DOI] [PubMed] [Google Scholar]
- Willemsen V, Wolkenfelt H, de Vrieze G, Weisbeek P, Scheres B. The HOBBIT gene is required for formation of the root meristem in the Arabidopsis embryo. Development. 1998;125:521–531. doi: 10.1242/dev.125.3.521. [DOI] [PubMed] [Google Scholar]
- Wilson AJ, Robards AW, Goss MJ. Effects of mechanical impedance on root growth in barley, Hordeum vulgare L. Effects on cell development in seminal roots. Journal of Experimental Botany. 1977;28:1216–1227. [Google Scholar]
- Wu G, Taketa S, Lewis DR, Spalding EP. Mutations in Arabidopsis Multidrug Resistance-Like ABC transporters separate the roles of acropetal and basipetal auxin transport in lateral root development. Plant Cell. 2007;19:1826–1837. doi: 10.1105/tpc.106.048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S-G, Taketa S, Ichii M. A novel short-root gene that affects specifically early root development in rice (Oryza sativa L.) Plant Science. 2002;163:207–215. [Google Scholar]
- Zhang H, Rong H, Pilbeam D. Signalling mechanisms underlying the morphological responses of the root system to nitrogen in Arabidopsis thaliana. Journal of Experimental Botany. 2007;58:2329–2338. doi: 10.1093/jxb/erm114. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang D, Hao Z. Review on mutation in lateral root of rice. Journal of Northeast Agricultural University. 2011;18:75–82. [Google Scholar]
- Zhou J, Wang B, Li Y, Wang Y, Zhou L. Responses of chrysanthemum cells to mechanical stimulation require intact microtubules and plasma membrane-cell wall adhesion. Journal of Plant Growth Regulation. 2007;26:55–68. [Google Scholar]