Abstract
Background and Aims
Several studies have demonstrated trade-offs between depth of seed dormancy and dispersal ability for diaspore-dimorphic species. However, relatively little is known about trade-offs between these two life history traits for a species that produces more than two diaspore morphs. The aim of this study was to investigate the relationship between seed dormancy and dispersal in Ceratocarpus arenarius, an amphi-basicarpic cold desert annual that produces a continuum of dispersal unit morphs.
Methods
A comparison was made of dispersal and dormancy breaking/germination responses of dispersal units from ground level (a), the middle of the plant canopy (c) and the top of the plant canopy (f). Various features of the morphology and mass of dispersal units and fruits (utricles) were measured. The role of bracteoles in diaspore dispersal by wind, settlement onto the soil surface and dormancy/germination was determined by comparing responses of intact dispersal units and fruits. Movement of dispersal units by wind and animals, seed after-ripening, germination phenology and the presence of water-soluble germination inhibitors in bracteoles were tested using standard procedures.
Key Results
Dispersal units a, c and f differed in morphology and mass; in the majority of cases, extremes were exhibited by a and f, with c being intermediate. Overall, relative dispersal ability was f > c > a, whereas relative intensity of dormancy was a > c > f. Bracteoles increased dispersal distance by wind, enhanced settlement of diaspores onto the soil surface and mechanically inhibited germination.
Conclusions
The results provide evidence for a model in which there is a continuous inverse-linear relationship between diaspore dispersal ability and depth of dormancy. Thus, dispersal unit heteromorphism of C. arenarius results in a continuum, from no dispersal ability/high dormancy (dispersal unit a) to high dispersal ability/low dormancy (unit f), which may be a bet-hedging strategy in the cold desert environment.
Keywords: Amphi-basicarpic annual, Ceratocarpus arenarius, cold desert, conceptual model, diaspore heteromorphism, physiological dormancy, seed dispersal, seed germination
INTRODUCTION
While most of the >250 000 species of angiosperms (Soltis et al., 2005; Mabberley, 2008) produce a single morphological type of fruit/seed as the dispersal and germination unit, a few hundred are known to produce two or more fruit/seed morphs (Imbert, 2002). In the latter case, the term fruit (seed) heteromorphism is used to describe the morphologically and physiologically distinct types of dispersal units (i.e. seed, fruit or fruit plus accessory parts) on a single individual plant (Mandák, 1997; Imbert, 2002). This phenomenon is more common in Amaranthaceae (now including Chenopodiaceae), Asteraceae and Brassicaceae, and in annuals, weeds and plants of sandy and saline arid and semi-arid regions than in other groups of plants.
In the past several decades, fruit and seed heteromorphism have been studied most extensively in species with dimorphic aerial diaspores (heterodiaspory sensu Mandák, 1997). These studies have shown that the two fruit or seed morphs may differ in mass (Sorensen, 1978; Venable and Levin, 1985a; Ruiz de Clavijo, 2001), shape (Baker and O'Dowd, 1982; Cordazzo, 2006), colour (Li et al., 2005; Wang et al., 2008) and presence/absence, length and density of the pappus (Venable and Levin, 1985a; Imbert et al., 1997; Brändel, 2007). Functionally, the two morphs may differ in dispersal mode and ability (Sorensen, 1978; Baker and O'Dowd, 1982; Ma et al., 2010), ability to persist in a seed bank (Philipupillai and Ungar, 1984; Venable and Levin, 1985b; Joley et al., 2003) and dormancy/germination characteristics (Venable and Levin, 1985a; El-Keblawy, 2003; Brändel, 2004). However, fewer studies have been done on plants that produce three (trimorphic) fruit/seed types (Venable et al., 1987; Mandák and Pyšek, 2005; Wei et al., 2007) and even fewer on those that produce more than three (polymorphic) fruit/seed types (Ruiz de Clavijo, 1995; Ruiz de Clavijo and Jiménez, 1998; Gardocki et al., 2000).
Ceratocarpus arenarius (Amaranthaceae) is an annual species that is widespread and common in a wide range of plant communities in middle and central Asia (including north-west China; Commissione Redactorum Florae Xinjiangensis, 1994). This is the only species in the genus, and in China it is found only in the cold desert region of northern Xinjiang Province. Ceratocarpus arenarius grows in a diversity of habitats, including sandy deserts, saline–alkaline deserts, gravel deserts and desert grassland. This species is one of the dominant and important components in desert plant communities of this area, and it is ecologically significant in maintaining plant diversity and stability of the cold desert ecosystem [Xinjiang General Exploration Team of Chinese Academy of Sciences (CAS) and Institute of Botany of CAS, 1978; Wang et al., 2006]. The species recently was reported as a problem weed in dryland farming systems (wheat, barley and lentils) in northern Iran (Ebrahimi and Eslami, 2012).
Ceratocarpus arenarius is one of the very common species that germinates from mid-March to early April in the Junggar Desert of Xinjiang. Flowering occurs from late April to late September (Commissione Redactorum Florae Xinjiangensis, 1994), and fruits mature in late summer–early autumn [Xinjiang General Exploration Team of Chinese Academy of Sciences (CAS) and Institute of Botany of CAS, 1978]. The mature fruit with two permanently attached bracteoles is the dispersal and germination unit. Plants of C. arenarius reach 5–30 cm in height, depending on the conditions in which they are growing. An abscission zone develops at the second node above the soil surface (‘node’ in Fig. 1A), and the stem is broken at this zone by wind after the plant is mature (Gao and Wei, 2007). The spherical shape of the plant makes it easy for the individual to be dispersed (rolled or tumbled) by wind far away from the site at which it matured. Thus, C. arenarius is a typical ‘tumbleweed’ (Gao and Wei, 2007; Gao et al., 2008; Zhou, 2009; Ebrahimi and Eslami, 2012).
Fig. 1.
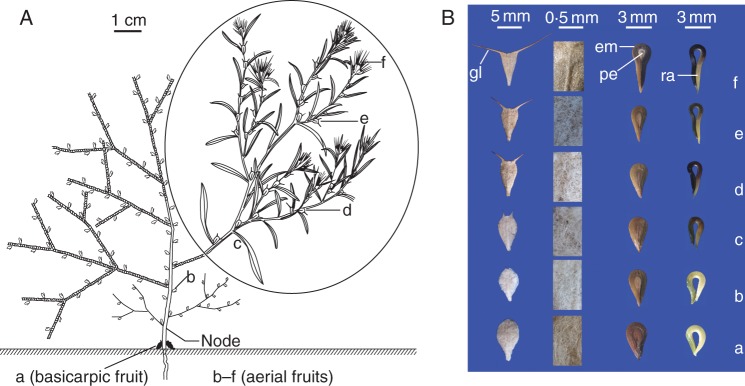
(A) Branching mode and spatial position of basicarpic (a) and aerial (b–f) dispersal units of Ceratocarpus arenarius on an individual plant and (B) morphology of the six types of dispersal units (first column), trichomes on bracts (second column), fruits (third column) and embryos (fourth column) in C. arenarius. a, dispersal units (basicarps) at the soil surface without glochids; b–f, dispersal units above the soil surface with short to long glochids. em, embryo; pe, perisperm; gl, glochid; ra, radicle. The stem of this tumbleweed breaks at the node that is labelled. (Circled material is from www.chinabaike.com)
Gao and Wei (2007), Gao et al. (2008) and Zhou (2009) reported that C. arenarius produces two distinct fruits (dispersal units), i.e. aerial and sub-terranean, which differ in morphology, dispersal and germination. However, we have observed on numerous occasions that plants growing in the field in the Xinjiang Province of China produce a continuum of dispersal units, i.e. from the two basicarps (dispersal unit type a) near the soil surface through aerial dispersal types (b–f) in different canopy positions (Fig. 1A). Following the classification scheme of Barker (2005), C. arenarius is an amphi-basicarpic species. The ratio of dispersal units a (basicarps), c (middle of the plant canopy) and f (top of the plant canopy) on a plant such as the one shown in Fig 1A is approx. 0·03:1:11.
Theory predicts that natural selection should produce a negative correlation between dispersal and dormancy (Venable, 1989; Mandák, 1997), and several studies have demonstrated such a correlation for heteromorphic species (Venable, 1985; Ellner, 1986; El-Keblawy, 2003). Moreover, dimorphic fruits/seeds represent two primary combinations of trade-offs in dispersal and dormancy, i.e. high dispersal ability and low or no dormancy in one fruit/seed morph and low dispersal ability and high dormancy in the other morph. In the former case, diaspores may be dispersed away from the site of the mother plant and germinate under less than certain conditions and over a short period of time; thus, the progeny are exposed to high risk conditions for germination, survival and establishment. In the latter case, diaspores with high dormancy and poor dispersal ability germinate in the vicinity of the mother plant and over a long period of time; thus, the progeny are exposed to low risk conditions for germination, survival and establishment (e.g. Flint and Palmblad, 1978; Venable and Levin, 1985a; Kigel, 1992). As such, then, seed/fruit heteromorphism can be viewed as a bet-hedging strategy that may reflect the unpredictable heterogeneity of the environment in space and time (Venable, 1985; Venable and Levin, 1985a).
Given that C. arenarius produces dispersal units that exhibit continuous variation in morphology (Fig. 1B), we hypothesized that there are more than two combinations of dispersal ability and degree of dormancy in this species. To test this hypothesis, we chose dispersal units a, c and f (Fig. 1A, B) to compare their (1) morphology and mass; (2) dispersal by wind and animals; and (3) dormancy and germination characteristics. We chose to compare dispersal units a, c and f because they represent basal (a), intermediate (c) and top (f) positions on the plant, respectively, and unit c appeared to be intermediate in morphology and mass between units a and f (see the Results). We further hypothesized that, based on previous studies in Amaranthaceae (e.g. Osman and Ghassali, 1997; Mandák and Pyšek, 2001a; Ungar and Khan, 2001), the persistent bracteoles of the dispersal units of C. arenarius play a significant role in dispersal and in seed dormancy/germination of this species. To test this hypothesis, we compared dispersal and dormancy/germination of dispersal units (with bracteoles) and fruits (without bracteoles).
MATERIALS AND METHODS
Field site description and dispersal unit collection
Freshly matured fruits with khaki-coloured persistent bracteoles were collected from many individuals in three natural populations each of several thousand plants of C. arenarius L. growing on desert sand dunes in the vicinity of Fukang city on the southern edge of the Junggar Basin of Xinjiang, China (44 °09'N, 87 °58'E, 438 m a.s.l.) on 6 October 2010. Dispersal units from individual plants were separated into (1) those at the soil surface and (2) each of five aerial fruit types from the continuum of dispersal units above the soil surface (Fig. 1B). Each of the six dispersal unit types was bulked separately. In the laboratory, dispersal units were separated from other plant material and stored in paper bags under room conditions [18–30 °C, 20–30 % relative humidity (RH)] until used.
This area of the Junggar Basin has a temperate continental climate. Mean annual temperature is 8·3 °C, and the mean temperatures of the coldest (January) and hottest (July) months are –15·6 and 26·0 °C, respectively. Average annual precipitation (including rain and snow) is 222 mm, about two-thirds of which falls in spring and summer, and the snow that falls in winter begins to melt in March or April (data from Fukang weather station, 2001–2010). Annual potential evaporation is >2000 mm (Wei et al., 2003).
Colour, shape, size and mass of dispersal units, fruits and embryos
Colour, shape, size and mass were determined for dispersal units, fruits and embryos of each of the six unit types (Fig. 1B) that had been stored dry in laboratory conditions for 1 month. Length and width of dispersal units, length of glochids (not present on unit a) and length and width of fruits were measured using digital calipers for 30 randomly chosen dispersal units and for 30 fruits (removed from the bracteoles) of each type. The thickness of upper and lower sections of bracteoles of 30 dispersal units of each morph was measured using a calibrated Nikon SMZ1000 light microscope. The angle between glochids was measured for 30 dispersal units of each dispersal unit type (except type a, which does not have glochids) using a protractor. Five replications of 100 dispersal units of each dispersal unit type (5 × 100 × 6) and five replications of 100 fruits of each of the six morphs (5 × 100 × 6) were weighed using a Sartorius BS210S electronic balance (0·0001 g). Then, embryos were dissected out of the fruit using a scalpel, and five replications of 100 embryos of each of the six morphs (5 × 100 × 6) were weighed to the nearest 0·0001 g. For each morph, colour, length, width and mass were determined for dispersal units, and fruits and colour and mass for embryos. The mean and s.e. were calculated for mass and width.
Dispersal
The studies comparing dispersal of intact dispersal units and fruits by wind were done to determine the role of bracteoles in wind dispersal. It was not possible to study dispersal of the fruit (i.e. to remove the bracteoles) in the mammal model study in the field. Also, only intact dispersal units were used in the study on dispersal by ants.
Dispersal by wind: detachment of dispersal units from mother plants
When mature, some aerial dispersal units b–f may be dispersed by wind before the stems break, while others may remain attached to the plant after the stem is broken. Some or all of dispersal units b–f would become detached as the plants tumble across the landscape (Zhou, 2009). If the rolling plant lodges against some inanimate object or other plants, any dispersal units remaining attached to it may be dispersed by wind. The two dispersal units at the base of the plant remain attached to the rooted basal part of the dead plant after the stem breaks, and thus they are not dispersed.
We randomly selected 60 individual whole plants with mature fruits. The stems were broken at the node above the soil surface. The number and percentage of dispersal units c and f on each plant were determined in the laboratory. The ratio of dispersal units c:f was about 1:11. The effect of wind speed on the number and percentage of dispersal units c and f that became detached from and that remained attached to stationary plants was determined in the laboratory in July 2011. Each of 30 individuals was held by hand at a height of 30 cm for 60 s in a constant stream of air (produced by a fan) with velocities of 1 and 4 m s−1; the distance between the plant and the fan was 10 cm. The number of dispersal units c and f that became detached from and that remained attached to mother plants was recorded.
The other 30 individuals were allowed to act as tumbleweeds. Thus, they were exposed to a constant stream of air with a velocity of 4 m s−1 and allowed to roll on the floor of the laboratory. The distance between the plant and fan was 1 cm, and each individual was rolled 1 or 3 m. The number of dispersal units c and f that became detached from and that remained attached to the mother plant as it rolled to 1 or 3 m was recorded.
Wind dispersal of dispersal units
To test the role of the bracteoles in wind dispersal, the dispersal ability of dispersal units a, c and f and of their fruits (i.e. dispersal unit with bracteoles removed) was compared indoors in July 2011 using the following procedures. (1) The fall rate in still air was measured in a tube 120 cm tall and 15 cm in diameter. Each of 50 dispersal units and their fruits was released individually from the top of the tube, and the time required for them to fall from the release point to the bottom of the tube was measured with a digital stopwatch. Fall rate (cm s−1) was calculated for this height (Telenius and Torstensson, 1989; Gravuer et al., 2003). The assumption in this method is that dispersal units and fruits with a slower fall rate would be carried further by the wind than those with a faster fall rate. (2) Dispersal distances were experimentally determined following the method of Telenius and Torstensson (1989). Thus, 50 individual dispersal units and 50 fruits were exposed for 60 s to a constant stream of air (produced by a fan) parallel to the flat seed landing surface. The dispersal units and fruits were released 1 cm from the front of the fan at a height of 30 cm and exposed to wind velocities of 1 and 4 m s−1, and the distances they travelled were measured to 0·001 m.
Dispersal by mammals
Burrowing mammals (mainly Meriones meridianus and Rhombomys opimus) are common in the sand dune habitats of C. arenarius (Ma et al., 1985; Hu and Sheng, 1992). Moreover, glochids on bracteoles of the aerial dispersal units could aid animal transport. Thus, to test the ability of the dispersal units to be moved by mammals, we made a small mammal model by covering a tube 16·5 cm in length and 7·0 cm in diameter with cotton cloth. The ‘mammal’ was tied to a string and dragged along 50 transects of 10 m through a population of several thousand plants with mature fruits in August 2011, following the method of Baker and O'Dowd (1982), Venable et al. (1987) and Mouissie et al. (2005). The number of dispersal units a, c and f that adhered to the mammal was counted for each transect.
Dispersal by ants
Ants collect the dispersal units of C. arenarius in the field, and a large number of them have been found near ant nests (Zhou, 2009; pers. obs.). To quantify collection of the dispersal units by ants, 50 dispersal units of each of the three morphs were placed on a dry sandy soil surface on the north, south, east and west sides of each of three ant nests in the field (50 dispersal units × 3 morphs × 4 directions × 3 nests) in August 2011, following the method of Baker and O'Dowd (1982) and Ma et al. (2010). The distance between each group of dispersal units of each morph and the ant nest was 3 m. Beginning at 7:00 am, the number of dispersal units in each group of each morph remaining at each of the placement sites was counted every hour for 15 h.
Settlement
Both intact dispersal units and fruits were used in this experiment. The reason for using both units was the same as that for using both of them in wind dispersal studies, i.e. to determine the role of the bracteoles.
Water absorption and dehydration of dispersal units and of fruits
Water content of diaspores has implications for adherence to sand/soil particles and also for dormancy break/germination. Thus, an experiment was carried out to determine the role of the bracteoles in water uptake and loss by dispersal units and fruits collected in October 2010 that had been stored dry under laboratory conditions for 2 months. To measure water uptake (imbibition), dispersal units and fruits of morphs a, c and f were placed on Whatman No.1 filter paper moistened with distilled water in 9 cm diameter Petri dishes and kept on a laboratory bench under room conditions (22–25 °C, 30 % RH). Three replicates of 100 dispersal units (3 × 100 × 3) and of 100 fruits for each of the three morphs (3 × 100 × 3) were weighed to the nearest 0·0001 g with an electronic balance. At time 0 and every 10 min until they reached final constant mass, dispersal units and fruits were removed from the dishes, blotted dry with filter paper, weighed and returned to the Petri dishes. Dehydration was monitored under laboratory conditions (22–25 °C, 30 % RH) in the same groups of dispersal units and in fruits that had become fully imbibed. The mass of each group of dispersal units and of fruits was recorded at time 0 (fully imbibed) and every 10 min until final constant mass.
Mass of dispersal units and of fruits before and after adherence to soil or sand particles
This experiment was done to determine the role of the bracteoles in adherence of dispersal units to soil and sand particles and thus in the settlement of seeds on the surfaces of these substrates. Four replicates of 100 dry dispersal units of each of the three morphs and of dry fruits were weighed to the nearest 0·0001 g with a Sartorius BS210S electronic balance. Dispersal units and fruits were distributed on water-saturated soil particles and on sand particles in 9 cm diameter Petri dishes under room conditions (22–25 °C, 30 % RH). After they were fully imbibed, the dispersal units and fruits with soil or sand particles attached to them were allowed to re-dry at room temperature. The mass of dry dispersal units and fruits, including attached soil or sand particles, was compared with the mass of dry dispersal units and fruits before adherence to soil or sand particles. During incubation, soil or sand particles adhered to the bracteal trichomes (see Fig. 1B) on the dispersal units (= mass of dispersal units with trichomes plus soil or sand particles) and to pericarps of fruits. The number of times the mass of a dispersal unit and fruit increased (Mt′) after adherence of soil particles or sand particles was calculated as Mt′ = Ms′/Md′, where Ms′ = mass of imbibed dispersal units or of fruit plus soil particles or sand particles and Md′ = mass of dry dispersal units or dry fruits (Lu et al., 2010).
Germination ecophysiology
Effect of dry storage (after-ripening) on germination
Seeds of C. arenarius are physiologically dormant, and one of the ways to break this type dormancy is by dry storage in laboratory conditions (Baskin and Baskin, 1998). The purpose of this study was to compare the depth of dormancy in seeds of dispersal units a, c and f. Dispersal units stored in laboratory conditions for 0 (fresh), 2, 4 and 12 months were tested for germination. For each test, three replicates of 35 dispersal units of each of the three morphs were incubated on two layers of Whatman No.1 filter paper moistened with 2·5 mL of distilled water in 9 cm diameter Petri dishes. Dispersal units were incubated at daily (12/12 h) temperature regimes of 5/2, 15/2, 20/10, 25/15 and 30/15 °C in light (12 h of approx. 100 µmol m−2 s−1, 400–700 nm, cool white fluorescent light each day) or in constant darkness (Petri dishes with dispersal units in them placed in light-proof black bags). Germination (radicle emergence) in light was monitored daily for 28 d; germinated units were removed at each count. Dispersal units incubated in the dark were checked only after 28 d.
After the germination trials were complete, the non-germinated dispersal units were tested for viability. Fruits were dissected out of the dispersal units, cut open and their embryos observed. Fruits with white, firm embryos were counted as viable; all seeds in all trials were viable. The tests of fresh dispersal units (0 months old) were initiated on 3 October 2010, using fruits collected on 1 October, 2010.
Germination phenology
The purpose of this experiment was to determine when dispersal units a, c and f germinate under outdoor conditions and to test the effect of bracteoles on germination under these conditions. Three replications of 200 dispersal units and of 200 fruits of each of the three morphs collected on 6 October 2010 were sown on bare soil in plots (1·5 × 0·8 m) in the experimental garden on the campus of Xinjiang Agricultural University (Urümqi) on 10 October 2010 [(3 replications × 3 dispersal unit morphs) + (3 replications × 3 fruit morphs) = 18 plots]. After the germination trials were complete, three replications of ten non-germinated dispersal units and of ten fruits were retrieved from each of the plots and tested for viability as described above. Germination (seedlings) was monitored at 7 d intervals from sowing to May 2011. Information on temperature, rainfall and snowfall at the study site was obtained from the Xinjiang Agricultural University weather station near the study plots.
Effect of bracteoles and of their trichomes on germination
Bracteoles of the dispersal unit of several species of Amaranthaceae have been reported to inhibit germination (see the Discussion). Thus, we tested this possibility for C. arenarius by the following experiment.
(1) Control: unmanipulated dispersal units a, c and f (intact fruits with bracteoles) were placed in Petri dishes on moist filter paper.
(2) Fruits (i.e. dispersal units a, c and f with bracteoles removed) were placed in Petri dishes. Comparing numbers 1 and 2 would reveal whether the bracteoles inhibited germination.
(3) Dispersal units a, c and f with trichomes removed (but with bracteoles intact) were placed in Petri dishes to determine whether the trichomes inhibited germination.
(4) Dispersal units a, c and f with trichomes removed (but with bracteoles intact) and their removed trichomes were placed together in Petri dishes to test whether water-soluble organic chemicals or salts leached from the trichomes would inhibit germination.
(5) Fruits along with their bracteoles were placed together in Petri dishes to test whether soluble chemicals leached from the bracteoles would inhibit germination.
Experiments with each of the three morphs were conducted at 30/15 °C in light using fresh dispersal units or fruits and those that had been stored dry in the laboratory for 1, 2, 8 and 12 months after they were collected in October 2010. Three replicates of 35 dispersal units or of 35 fruits of each morph were used for each treatment. Germinated dispersal units or fruits were counted daily for 28 d.
Data analysis
All data were analysed for normality and homogeneity of variance prior to analysis to fulfil requirements of t-tests, one-way analysis of varaince (ANOVA), two-way ANOVA and four-way ANOVA. If data were normal and homogeneous, they were subjected to further analysis. If data were not normally distributed or if variances were not homogeneous, they were arcsine (square root %) transformed (percentage data, i.e. percentage of dispersal units c and f remaining on mother plants at 1 and 4 m s−1 wind speeds and after 1 and 3 m dispersal distances, percentage of attached dispersal units on the surface of the mammal model and percentage of dispersal units removed by ants, and germination percentage, germination rate and viability percentage) or log10 transformed (other data, i.e. morphology and mass, fall time, number of dispersal units c and f remaining on mother plants at 1 and 4 m s−1 wind speed and after 1 and 3 m dispersal distances, fall rate and dispersal distance, mass of water absorbed and adherence to soil and sand particles, and rates of hydration and dehydration) before analysis to ensure homogeneity of variance. Non-transformed data appear in all tables and figures.
Paired sample t-tests were used to compare the mass of dispersal units and fruits before and after adherence to soil or sand particles. When variances of non-transformed or transformed data were homogeneous, a one-way ANOVA was used to determine differences in morphology and mass, number and percentage of dispersal units c and f remaining on the mother plants at 1 and 4 m s−1 wind speed and after dispersal for 1 and 3 m, fall time, fall rate and dispersal distances, percentage of attached dispersal units on the surface of the mammal model, percentage removal of dispersal units by ants, mass of water absorbed and adherence to soil or sand particles, rates of hydration and dehydration, germination percentage, germination rate, and viability percentage of dispersal units and of fruits among the three fruit morphs. Four-way ANOVA was used to test for significance of main effects (light, storage time, temperature and dispersal unit type) and their interactions on germination in the ‘Effect of dry storage (after-ripening) on germination’ experiment. When the variance of logarithmically transformed data was still not homogenous, differences among dispersal units and fruit morphs in these characteristics were determined by the non-parametric Kruskal–Wallis test.
Tukey's HSD test was performed for multiple comparisons to determine significant (P < 0·05) differences among the three morphs and treatments. Statistical tests were conducted at P = 0·05. Correlative analyses were used to determine the relationship between dispersal unit and fall time and between dispersal unit and fall rate. All data analyses were performed with the software SPSS 13·0 (SPSS Inc., Chicago, IL, USA). Values are means ± s.e. (Sokal and Rohlf, 1995).
RESULTS
Colour, shape, size and mass of dispersal units, fruits and embryos
The dispersal units (Fig. 1B) are khaki-coloured, their surfaces are covered with trichomes and there is an obvious mid-vein. However, the density of trichomes on the bracteoles of dispersal unit a is obviously greater than that of dispersal unit f. The shapes of the six types of dispersal units vary from inverted ovate to inverted triangular (Fig. 1B), their tops are cupped or straight and there are significant differences among them in length, width and thickness of bracteoles (Supplementary Data Table S1). The length of and angle between glochids increased significantly from dispersal units b to f. Dispersal unit a has the thickest bracteoles and dispersal unit f the thinnest. Moreover, the mass of 100 basicarps (i.e. dispersal unit a) was significantly greater than that of the aerial dispersal units (b–f), which did not differ from each other (Supplementary Data Table S1).
Each dispersal unit contains only one (single-seeded) fruit (utricle), and the colour and shape of fruits from the six types of dispersal units are similar, i.e. dark brown, spatulate and slightly compressed (Fig. 1B). However, the length and width of fruits from the six dispersal unit morphs differed significantly (Supplementary Data Table S1). Fruits from dispersal unit a are shortest and widest. The type of embryo is peripheral (Martin, 1946), and seeds have a small amount of perisperm. The colour of the embryos from the six fruit morphs varied from straw-yellow to dark brown. Also, the mean mass of 100 fruits and of 100 embryos from fruit a is greater than that of fruits and of embryos from the other fruit morphs, and the mass of fruits and of embryos from fruit f is the lightest of all (Supplementary Data Table S1).
The relative relationships for characteristics of these three dispersal units are as follows (Fig. 1B; Supplementary Data Table S1): length of dispersal units, (f > a > c); width of dispersal units, a > (c > f); mass of dispersal units, a > (c > f); length of fruits, f > c > a; width of fruits, f = [a >c]; mass of fruits, a > c > f; mass of embryos, a > c > f; thickness of bracteoles, a > c > f; density of trichomes on bracteoles, a > c > f; length of glochids, f > c (not present on dispersal unit a); and angle between glochids, f > c. Dispersal units in parentheses differ but not significantly, whereas those inside brackets do differ significantly.
Dispersal
Dispersal by wind: detachment of dispersal units from mother plants
A higher proportion of dispersal unit f morph than of unit c morph became detached from both stationary (P < 0·05) (Supplementary Data Fig. S1A) and tumbling (Supplementary Data Fig. S1B) plants (P < 0·05). However, significantly more unit f than unit c morph remained attached to the mother plants in both experiments (P < 0·05 in both cases; Supplementary Data Fig. S1C, D). Moreover, there were significant differences between wind speeds and between tumbling distances (P < 0·05 in all cases; Supplementary Data Fig. S1A–D) in both proportion and number of dispersal units c and f remaining on the mother plants. Thus, both the proportion and number of dispersal units c and f remaining on the mother plants decreased with an increase in wind speed and in distance rolled.
Dispersal ability of dispersal units
In still air, the three types of dispersal units and of their fruits differed significantly in landing time and in fall rate (P < 0·05; Supplementary Data Table S2), with dispersal unit f and fruits of dispersal unit f taking longer to fall. The type of dispersal unit (i.e. a, c and f) was significantly positively correlated with fall time (r = 0·34, P < 0·05) and significantly negatively correlated with fall rate (r = –0·37, P < 0·05). The dispersal distance of dispersal unit f and of fruit f in a parallel stream of air was usually considerably greater than that of those of dispersal units a and c and of their fruits at wind speeds of both 1 and 4 m s−1 (Supplementary Data Table S2).
Dispersal by mammals
Zero, 151 and 661 dispersal units a, c and f, respectively, became attached to the mammal model. The mean number of dispersal units that became attached to the surface of the mammal model for the 50 transects of 10 m was 16·2 ± 1·0 (range 3–37). The percentage of attached dispersal units differed significantly among the three morphs: a (0·0 ± 0·0 %), c (18·5 ± 1·8 %) and f (81·5 ± 1·8 %) (P < 0·05). For each metre of the transect, 1·62 fruits (812 fruits/500 m) become attached to the mammal.
Dispersal by ants
Dispersal unit f was most easily transported by ants. When a dispersal unit f was discovered on the soil surface, ants immediately transported it to the nest. However, the ants did not easily or successfully attempt to carry dispersal unit a to the nest. Some dispersal unit a morphs, even when picked up, usually were dropped and abandoned on the way to the nest. Thus, the average rate of dispersal unit removal by ants differed significantly among the three morphs (Fig. 2; P < 0·05). After 5 h, only about 5 % of the individuals of dispersal unit a had been removed, whereas >45 % of the individuals of dispersal unit c and >70 % of dispersal unit f had been removed. At the end of the experiment, <10 % of the individuals of dispersal unit a had been removed by ants, while about 75 and 95 % of those of dispersal units c and f, respectively, had been removed. The mass of dispersal units and number of dispersal units removed by ants was significantly negatively correlated (r = –0·99; P < 0·05).
Fig. 2.
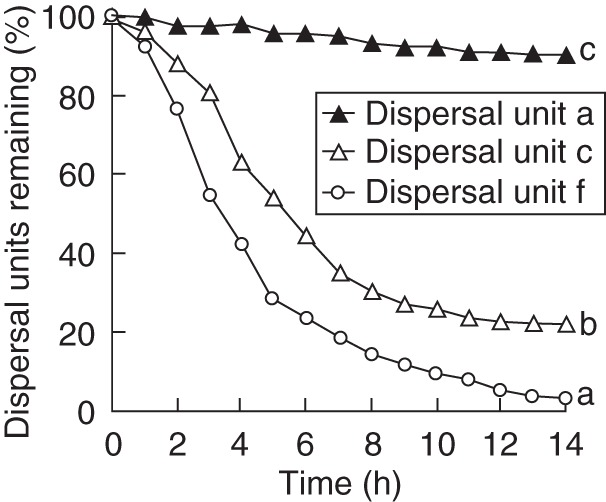
Depletion curves for dispersal units a, c and f removed by ants (mean ± s.e.). Significant differences in percentages of dispersal units remaining after 14 h are indicated by different lower case letters (Tukey's HSD, P < 0·05). Only standard errors ≥1·92 % are shown.
Settlement
Water absorption and dehydration of dispersal units and of fruits
Patterns of water absorption by the three dispersal unit morphs and by their fruits were similar and can be divided into three stages: (1) the rate of water absorption was rapid; (2) the rate of water absorption decreased; and (3) water absorption stopped. Dispersal units and fruits were fully imbibed after 200–300 and 160–200 min, respectively (Supplementary Data Fig. S2). Dispersal unit a had a high capacity to take up water, and mass increased 2000 % (from 0·17 to 3·40 g) within only 60 min from the start of imbibition, which differed significantly from that of dispersal units c and f (P < 0·05). The mass of water absorbed by the three fruit morphs was much less than that of the dispersal units. However, there were significant differences in mass of water imbibed by fruits of dispersal unit a and those of the other two dispersal unit types (P < 0·05). One hundred fruits of dispersal unit a absorbed 1·54 ± 0·16 g of water in 10 min, compared with only 0·51 ± 0·35 and 0·37 ± 0·26 g for 100 fruits of dispersal units c and f, respectively, which did not differ from each other (P = 0·93). Moreover, when they reached their capacity for imbibition, 100 fruits a, c and f had absorbed 2·22, 1·85 and 1·67 g of water, respectively, which was significantly lower than the mass absorbed by 100 dispersal units a, c and f (P < 0·05).
Patterns of dehydration were almost the reverse of those for water absorption and can also be divided into three stages: (1) loss of water was rapid; (2) the rate of dehydration decreased; and (3) fruits had reached their original mass (Supplementary Data Fig. S2). Dispersal units lost water more slowly than fruits. Dispersal units a, c and f retained >50 % of their absorbed water for 40, 20 and 20 min, respectively, and they became fully dehydrated (i.e. had reached the same moisture level as that of dispersal units at the beginning of absorption) after 180–210 min. However, fruits from all three dispersal unit morphs had lost almost 50 % of their water after only 20 min, and they had reached their original mass in 90–120 min.
Mass of dispersal units and of fruits before and after adherence to soil or to sand particles
The mass of dispersal units with adhered soil or sand particles was significantly greater than that of the original mass for the three types of dispersal units (P < 0·05). However, the mass of fruits with adhered soil or sand particles increased very little and did not differ significantly from the original mass (P > 0·05; Supplementary Data Table S3). There were significant differences in the original mass of dispersal units and of fruits (Md′) and in the mass of dispersal units and of fruits after adherence to soil or sand particles (Ms′) among the three dispersal units and among the three fruit types (Supplementary Data Table S3). Moreover, the number of times the dispersal unit mass increased after adherence of soil or of sand particles (Mt′) differed significantly among the three dispersal unit morphs (P < 0·05), but it did not differ among the three fruit types (P = 0·66 and 0·16 after adherence to soil and sand particles, respectively).
Germination ecophysiology
Effect of dry storage (afterripening) on germination
A four-way ANOVA showed that germination was significantly affected by light condition (P < 0·05), storage time (P < 0·05), temperature (P < 0·05) and dispersal unit type (P < 0·05; Supplementary Data Table S4). Freshly harvested dispersal units were dormant at all five temperature regimes in light and in constant darkness, and the highest germination percentages were 0, 13·4 and 16·9 % for dispersal units a, c and f, respectively (Supplementary Data Fig. S3). Dormant dispersal units c and f after-ripened gradually during storage. After 4 months of dry storage, germination in darkness was about 40 % for dispersal unit c at 15/2 °C and 70 % for dispersal unit f at 20/10 °C (Supplementary Data Fig. S3). Dispersal units c and f after-ripened further between 4 and 12 months of dry storage, and 12-month-old morphs germinated to ≥55 % and ≥75 %, respectively, at all temperature regimes in both light and darkness. However, dormancy was not broken in dispersal unit a during storage.
Germination phenology
Even though rainfall during October 2010 was about 50 mm, no dispersal units or fruits in the plots germinated in autumn (October–November) 2010; maximum and minimum daily temperatures were 11·7 and 1·6 °C for October and 4·9 and –4·8 °C for November (Fig. 3A, B). In spring 2011, seedlings from the three fruit morphs emerged 4–6 d earlier than those from dispersal units (Fig. 3B). The greatest number of dispersal units germinated between 26 March and 13 April (mean daily maximum and minimum air temperature 19·0 and 6·5 °C, respectively), and the greatest number of fruits germinated between 20 March and 5 April (mean daily maximum and minimum air temperatures 13·1 and 1·8 °C, respectively). Final germination percentages of dispersal units of the three morphs were significantly lower than those of their fruits (P < 0·05). Thus, of 200 dispersal units and 200 fruits sown in the plots, 13 and 57 of them from dispersal unit a and fruit a, respectively; 32 and 76 from dispersal unit c and fruit c, respectively; and 78 and 118 from dispersal unit f and fruit f, respectively, germinated. Furthermore, the viability of dispersal units of the three morphs was also significantly higher than that of their fruits (P < 0·05) (Fig. 3C). Also, the viability of dispersal unit a was higher than those of dispersal units c and f, which did not differ significantly from each other. Similarly, the viability of fruit a was higher than that of fruits c and f, which did not differ significantly from each other. More than 50 % of fruits c and f lost viability.
Fig. 3.
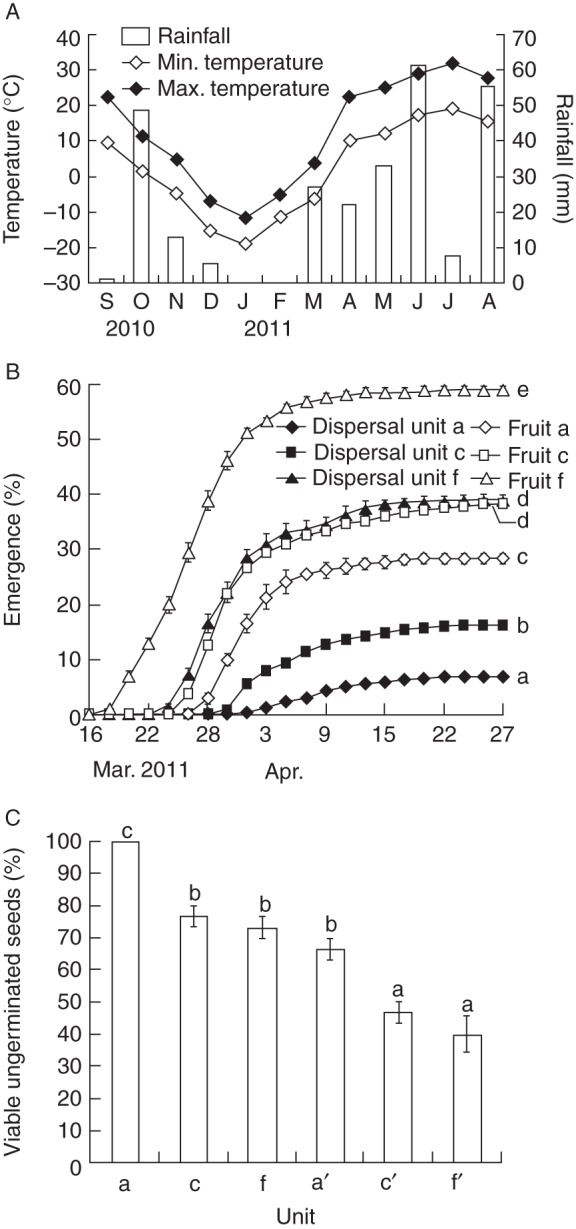
(A) Total monthly rainfall and mean minimum and mean maximum monthly temperatures recorded at the Xinjiang Agricultural University weather station from September 2010 to August 2011. (B) Germination percentages (mean ± s.e.) in spring 2011 of dispersal units a, c and f and of fruits a, c and f of Ceratocarpus arenarius sown in plots in autumn 2010. (C) Percentages (mean ± s.e.) of dispersal units a, c and f and of fruits a (a′), c (c′) and f (f′) remaining viable in plots on 8 May. Only standard errors ≥1·0 % are shown in (B) and (C). Different lower case letters indicate significant differences (Tukey's HSD, P < 0·05). In (B), statistical comparisons are for 27 April 2011. Note: for December 2010, there was both rainfall (shown in the figure) and snowfall, and for January and February 2011 there was no rainfall. Snowfall was 24·8, 14·9 and 9·1 cm in December 2010, January 2011 and February 2011, respectively.
Effect of bracteoles and of their trichomes on germination
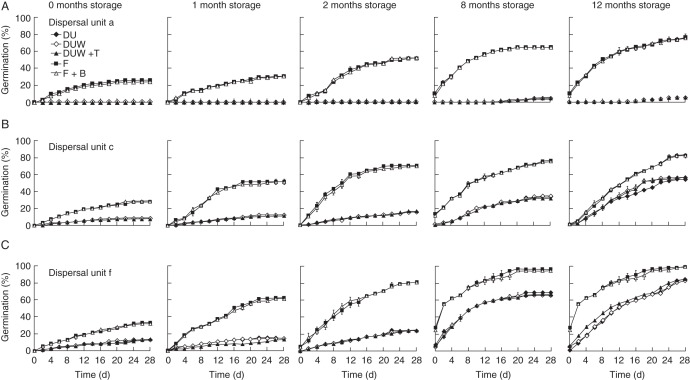
Germination percentages of non-treated freshly matured (0-month-old) dispersal units a, c and f were 0, 8 and 13, respectively (Fig. 4). Furthermore, removal of trichomes from bracteoles did not increase the germination percentage in any of the three dispersal unit types, and neither did the treatment of dispersal units with trichomes removed plus trichomes. However, removing the bracteoles increased germination of fruits from dispersal units a, c and f (bracteoles removed) to 25·7, 27·6 and 33·3 %, respectively (P < 0·05). There were no significant differences in germination percentages between fruits from dispersal units a, c and f and fruits plus their bracteoles for any of the three morphs (all P > 0·05).
Fig. 4.
Effects of bracteoles, trichomes and after-ripening (storage) time on germination percentages (mean ± s.e.) of dispersal units a (upper row), c (middle row) and f (lower row) of Ceratocarpus arenarius in light (12 h photoperiod) at 30/15 °C. Only standard errors ≥2·52 % are shown. B, bracts; DU, intact dispersal unit; DUW, dispersal unit without trichomes; F, fruit; T, trichomes.
Storage (after-ripening) did not increase the germination percentage of dispersal unit a, dispersal unit a with trichomes removed or dispersal unit a with trichomes removed plus trichomes (Fig. 4). After storage for 12 months, germination of dispersal unit a was still <5 %. Furthermore, dispersal unit a with trichomes removed and those with trichomes removed plus trichomes germinated to <6 %. However, storage time influenced germination of fruit a and of fruit a plus bracteoles. After storage for 12 months, germination percentages of fruit a and fruit a plus bracteoles were 76·2 and 75·2 %, respectively.
On the other hand, storage promoted germination of dispersal units c and f in all treatments (Fig. 4). After storage for 12 months, dispersal units c and f with trichomes removed germinated to 56·2 and 82·9 %, respectively. Germination of dispersal units c and f with trichomes removed plus trichomes was not inhibited (Fig. 4). Moreover, germination percentages of fruits c and f and of fruits c and f plus bracteoles were significantly higher than those of dispersal units c and f and of those with trichomes removed (P < 0·05). However, there were no differences in germination percentages between fruit c and fruit c plus bracteoles (P = 0·99) or between fruit f and fruit f plus bracteoles (P = 1·00). Also, there were no significant differences among intact dispersal unit c, dispersal unit c with trichomes removed and dispersal unit c with trichomes removed plus trichomes (P = 0·62); and the same was the case for fruit f (P = 0·54).
DISCUSSION
Polymorphic diaspores represent more than two dispersal–dormancy strategies
Our hypothesis that the dispersal units in C. arenarius represent more than two distinct diaspore dispersal–germination strategies was confirmed via a detailed comparison of dispersal and dormancy/germination along the continuum of dispersal unit morphs (Table 1). Overall, dispersal unit a had low dispersal/high dormancy, dispersal unit c intermediate dispersal/intermediate dormancy and dispersal unit f high dispersal/low dormancy.
Table 1.
Comparison of relative production, dispersal and germination of, and role of bracts in, dispersal units a, c and f of Ceratocarpus arenarius
| Life history trait | Dispersal units | Effect of bracts |
|---|---|---|
| (A) Number of dispersal units produced | f > c > a | –* |
| (B) Dispersal | ||
| Wind | ||
| Number of dispersal units c and f detached from plant | ||
| Stationary plant at 1 and at 4 m s−1 wind speed | f > c | –* |
| Rolling of whole plant for 1m and 3m | f > c | –* |
| Ratio of the numbers of dispersal units c and f remaining on the plant | ||
| Stationary plant at 1 m s−1 wind speed | 1:8 | –* |
| Stationary plant at 4 m s−1 wind speed | 1:5·7 | –* |
| Rolling of whole plant for 1m | 1:7 | –* |
| Rolling of whole plant for 3 m | 1:5·4 | –* |
| Fall rate | a > c > f | Without > with in all units |
| Dispersal distance | (f > c) > a | With > without in all units |
| Settlement onto soil surface | ||
| Mass of water uptake | a > (c > f) | With > without in all units |
| Dehydration time | a > (c > f) | With > without in all units |
| Number of times mass increased after adherence to soil or sand particles | a > (c > f) | With > without in all units |
| Animals | ||
| Mammal | f > c > a | –* |
| Ant | f > c > a | –* |
| (C) Germination/dormancy† | ||
| Germination | ||
| Dispersal units after-ripened for 0–12 months | f > c > a | –* |
| Fruits after-ripened for 0–12 months | f > c > a | Without > with in all units |
| Experimental garden | f > c > a | Without > with in all units |
| Retention of viability in experimental garden | a > c > f | With > without in all units |
All differences between dispersal units are significant except where between parentheses.
*Not tested.
†Dormancy inverse order of germination, i.e. a > c > f.
Freshly matured dispersal units a, c and f of C. arenarius were dormant across a wide range of conditions. Dormancy is probably due to the low growth potential of the fully developed embryo, i.e. physiological dormancy (Baskin and Baskin, 1998, 2004). After dry storage (after-ripening) at room temperature for 12 months, dispersal units c and f germinated to about 60 and 80 %, respectively, in both light and darkness over the range of temperature regimes, whereas unit a germinated to <10 %, i.e. a < c < f. Previous studies have shown that storage (after-ripening), scarification of the pericarp (Gao et al., 2008) and cold stratification (Gao, 2007) increased germination percentages of sub-terranean (our fruit a) and aerial (mixture of our fruits b–f ) fruits of C. arenarius. Cold stratification was more effective in breaking dormancy in aerial fruits than it was in fruit a. However, scarified fruits of morph a and of aerial fruits germinated to >90 % in light at 5/15, 5/25 and 15/25 °C (Gao, 2007).
A study recently has been published on seed germination of C. arenarius growing as an arable weed in dryland crops (wheat, barley and lentils) in northern Iran (Ebrahimi and Eslami, 2012). The authors made a mass collection of seeds (dispersal units), presumably a mixture of our units b–f (Fig. 1). They (Ebrahimi and Eslami, 2012) subsequently removed the pericarp (probably bracteoles) from the fruits [probably fruits (utricles) with enclosing bracteoles] and used the seeds (probably utricles without bracteoles) in the majority of their germination tests. They erroneously concluded that their fruits have physical dormancy, which is not known to occur in Amaranthaceae (including Chenopodiaceae) (Baskin et al., 2000). However, the seeds of this species clearly have physiological dormancy since they have a fully developed embryo (Fig. 1B), are not water impermeable (Supplementary Data Fig. S2) and come out of dormancy during dry storage (after-ripening) and during cold stratification (Gao, 2007; this study).
Germination percentages of dispersal units a, c and f of C. arenarius did not differ much in light/dark and darkness (Supplementary Data Fig. S3). Thus, it seems unlikely that darkness per se would prevent germination of non-dormant dispersal units covered with soil, sand or litter. No dispersal units of C. arenarius germinated in the plots in the experimental garden in autumn 2010, although there was about 50 mm rainfall in October. Thus, lack of germination in autumn is due to innate dormancy. Furthermore, fruits (bracts removed from the dispersal unit) did not germinate in autumn. Dormancy in a portion of the dispersal units and of the fruits was broken during winter and/or early spring. Nevertheless, only about 7, 16 and 39 % of dispersal units a, c and f, respectively, and 29, 38 and 59 % of fruits a, c and f, respectively, emerged in the plots in the experimental garden in spring (late March/early April).
In C. arenarius, the ratio of dispersal units a, c and f produced was about 0·03:1:11. Further, there was an inverse relationship between the number of dispersal units produced (f > c > a) and both the degree of dormancy (a > c > f) and retention of viability (a > c > f). In general, the number of dispersal units produced was also positively correlated with the number that was dispersed furthest by wind. Production of large numbers of dispersal unit f would increase the probability that many individuals of this morph rather than of morph c would remain attached to stationary plants and for a longer time in windy weather to whole plants after they had been rolled to a long distance by wind. Furthermore, compared with dispersal units a and c, more individuals of dispersal unit f became attached to mammals, and more were removed by ants. Few or no individuals of dispersal unit a probably would be moved away from the mother site by either of these means, and a moderate number of individuals of dispersal unit c would be moved an intermediate distance by all of them. Ceratocarpus arenarius is a typical ‘tumbleweed’, and many aerial diaspores become detached from the plant as it rolls. However, significantly more individuals of dispersal unit f than of dispersal unit c remained attached to rolling plants in our laboratory study. Zhou (2009) reported that many aerial fruits became detached from plants of C. arenarius as they rolled for 160 m in the field. She found a significant negative relationship between the number of fruits dispersed from rolling plants and the distance they rolled. A high percentage of the aerial dispersal units of C. arenarius is detached between September and March, with the peak in October (Zhou, 2009).
Dispersal unit a, the heaviest morph, had the fastest fall rate and the shortest dispersal distance at wind speeds of both 1 and 4 m s−1, and dispersal unit f, the lightest morph, the lowest fall rate and greatest dispersal distance. The mass and dispersal distance of dispersal unit c are intermediate between those of dispersal units a and f. Consequently, dispersal unit f has the potential to be dispersed further by wind than dispersal units a and c. Dispersal unit a is not adapted to dispersal by wind even if it becomes detached from the dead mother plant; it has the highest fall rate. In the achene-heteromorphic species Hedypnois rhagadioloides, the mass of dispersal units explained a significant part of the ability to be dispersed by wind. Pappose achenes with the smaller mass were dispersed further than the much heavier epappose achenes (Kigel, 1992). Likewise, in the trimorphic species Garhadiolus papposus (Sun et al., 2008) and Heteracia szovitsii (Cheng and Tan, 2009), the relative dispersal distance at wind speeds of both 1 and 4 m s−1 was central achenes (with the smallest mass and well developed pappus) > intermediate achenes (with mass and pappus between the central and peripheral achene types) > peripheral achenes (with the largest mass and scarcely developed pappus).
Dispersal units c and f of C. arenarius may be dispersed by mammals due to the presence of glochids on the bracteoles. Moreover, many more individuals of dispersal unit f (larger glochids) than of dispersal unit c (shorter glochids) became attached to the model. Likewise, in Lappula duplicicarpa and L. semiglabra, more nutlets with long glochids than of those with short glochids became attached to a mammal model (Ma et al., 2010). In Hypochaeris glabra, non-beaked achenes were more likely to adhere to a mammal model than beaked achenes (Baker and O'Dowd, 1982). Peripheral achenes of Picris echioides (enclosed in involucral bracteoles) with barbs adhered to laboratory mice and were carried for up to 195 s, whereas central achenes with a pappus did not adhere to the mice (Sorensen, 1978). In Heterosperma pinnatum, central achenes with awns were more likely to adhere to an artificial mammal model than were peripheral achenes without awns (Venable et al., 1987). Further, the proportion of achenes that adhered to the model depended on the proportion of central achenes produced in the population.
Ants transported more individuals of dispersal unit f, presumably to the nest to be eaten, than of dispersal unit c. Zhou (2009) reported that ants removed all of the 30 aerial fruits of C. arenarius placed on the soil surface to their nest 8 m away within 10 min. In L. duplicicarpa and L. semiglabra, more nutlets with long than with short glochids were transported by ants (Ma et al., 2010). Very few individuals of dispersal unit a of C. arenarius were picked up by ants, and none was transported to the nests. Thus, ants appear to play a role in dispersal of aerial dispersal units c and f, but not those of unit a, away from the mother habitat. They probably are acting as both a seed predator and a disperser in this system. Ants selectively harvested beaked rather than non-beaked achenes of the dimorphic species H. glabra (Baker and O'Dowd, 1982) and central rather than peripheral achenes of the dimorphic species Leontodon longirrostris (Ruiz de Clavijo, 2001).
Role of bracteoles in dispersal and germination
Our second hypothesis that the persistent bracteoles play an important role in seed dispersal and dormancy of C. arenarius was also supported (Table 1). Thus, bracteoles play an important role in transport of the dispersal units by wind and in seed dormancy and germination. Removal of bracteoles from dispersal units a, c and f decreased their fall time, resulting in lower dispersal distances of fruits than of dispersal units (Supplementary Data Table S2). Mandák and Pyšek (2001b) reported that there was a significant negative relationship between bracteole area and terminal velocity and between bracteole weight and terminal velocity in dispersal units A (without bracteoles) and B and C (with bracteoles) of Atriplex sagittata, and thus the relative potential for dispersal by wind of the three morphs was B > C > A.
Bracteoles of C. arenarius greatly increased the ability of dispersal units a, c and f to adhere to soil and sand surfaces, thereby increasing their mass and decreasing the ease with which they could be moved. The more trichomes on bracteoles, the more soil or sand particles that adhered to the dispersal unit, i.e. a > c > f. The larger mass of unit a and its greater adherence to soil or sand particles may promote settlement of seeds onto the soil surface, thereby preventing them from being dispersed by wind or insects away from the favourable microhabitat of the mother plant (Gutterman, 1994, 2000). When bracteoles were removed from the dispersal units of C. arenarius, the mass of water taken up and rate of absorption and dehydration decreased significantly. Thus, enhancement of water uptake by the bracteoles may be important in settlement of dispersal units of C. arenarius onto a substrate.
Removing the bracteoles from dispersal units a, c and f of C. arenarius significantly promoted germination of all three units. Removal of bracteoles from dispersal units has been shown to improve seed germination of many Amaranthaceae species significantly, e.g. Atriplex centralasiatica (Li et al., 2008) and Salsola vermiculata (Osman and Ghassali, 1997). In A. prostrata, bracteoles were inhibitory to germination at 5/15 °C but not at 5/25 or 25/25 °C (Ungar and Khan, 2001).
Bracteoles slowed or prevented water uptake by dispersal units of Atriplex confertifolia (Garvin and Meyer, 2003) and thus prevented germination in this species. Garvin and Meyer (2003) suggested that the primary role of bracteoles in retarding germination in A. confertifolia was via their influence on cold stratification, perhaps by preventing full water uptake by the utricle. That is, low water potential decreased the effectiveness of the cold stratification process. However, in C. arenarius, water uptake was substantial by both dispersal units and fruits (Supplementary Data Fig. S2), suggesting that the bracteoles did not prevent germination by restricting imbibition. Our results indicate that the bracteoles played a role in inhibiting germination of the three dispersal unit morphs of C. arenarius by mechanical force. Bracteoles mechanically inhibited germination in the chenopods A. prostata (Ungar and Khan, 2001) and Salsola affinis (Wei et al., 2008).
However, even when bracteoles were removed from fresh dispersal units, the presence of the pericarp along with some embryo dormancy prevented germination, i.e. fruits germinated to <40 %. Thus, in fresh seeds and in those after-ripened for only a few months, bracteoles alone were not inhibiting germination. Scarification of the pericarp greatly increased germination of fruits a and of a mixture of aerial fruits (Gao et al., 2008), presumably by lowering its mechanical restriction to embryo growth (Baskin and Baskin, 2004). Thus, the bracteoles act as another layer of mechanical inhibition. The thicker bracteoles of dispersal unit a were much more effective in mechanically restraining germination than were the thinner bracteoles of dispersal units c and f. Even following 12 months of after-ripening, the bracteoles on dispersal unit a still exerted enough mechanical restraint to prevent embryo growth, i.e. radicle elongation (germination).
A conceptual model for dispersal–germination strategies of a polymorphic species
Concepts about trade-offs between dispersal and germination in heteromorphic species mostly have been formulated with those that produce dimorphic above-ground diaspores of various kinds, in particular central vs. peripheral achenes in Asteraceae. The two-stage model includes only ‘stay at home’ and ‘leave home’ strategies for dispersal and germination (Ellner and Shmida, 1981; Venable, 1985; Ellner, 1986; Venable et al., 1987; Mandák, 1997). Our study provides evidence on various aspects of diaspore dispersal and dormancy/germination in C. arenarius to support a model for continuous variation in which there is an inverse-linear relationship between depth of diaspore dormancy and its ability to disperse for this polymorphic species (Table 1; Fig. 5). At one end of the continuum, dispersal unit f, with the greatest ability to disperse the most diaspores the furthest distance from the mother plant and with the least dormancy, is the best ‘colonizer’ type. Potentially, it expands the range of the species but has a low chance to land in a microhabitat in which it can germinate, survive and become established (high-risk strategy). At the other end of the continuum, dispersal unit a, with little or no ability to be dispersed and the highest dormancy, is the ‘maintainer’ type, which remains in the parental environment of proven suitability for germination, growth and reproduction of members of the species (low-risk strategy). Dispersal unit c, representing the part of the continuum between the extremes, has dispersal and dormancy characteristics intermediate between colonizer and maintainer types, i.e. exposure of progeny to a habitat that is between high and low risk.
Fig. 5.
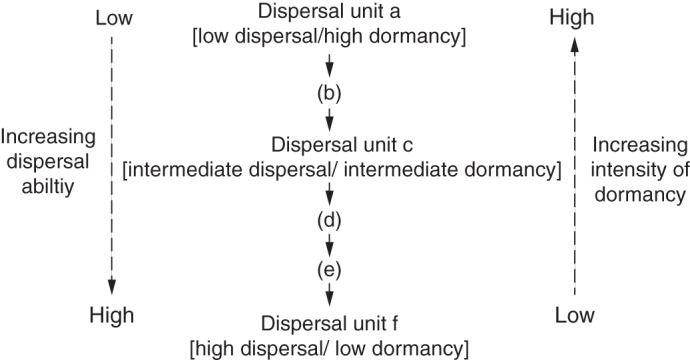
A conceptual model for continuous variation in dispersal–dormancy strategies in Ceratocarpus arenarius. Although we did not collect data for dispersal or dormancy of units b, d or e, we speculate that their positions in the dispersal–dormancy gradient might be as shown in the model.
Although we did not include dispersal units b, d and e in our study, it seems probable that they also would fit into the scheme in our model (Fig. 5). In which case, dispersal units of C. arenarius would exhibit continuous variation in its combinations of dispersal ability and degree of dormancy. Thus, theoretically, the species would have the capability of fine-tuning the dispersal and dormancy aspects of their colonizing ability to a continuous series of habitats. In short, then, in terms of dispersal and dormancy, C. arenarius would be adapted to an environment that varies continuously in space away from the mother plant.
Concluding remarks
The polymorphic dispersal units of the amphi-basicarpic species C. arenarius exhibit continuous variation in morphology and diaspore ecology. The depth of diaspore dormancy and dispersal ability show a continuous inverse-linear relationship. Thus, the basicarps at ground level (dispersal unit a) have the highest degree of dormancy and the least dispersal ability, and aerial unit f, located on the periphery of the plant canopy, the lowest degree of dormancy and the greatest dispersal ability. Theoretically, this ‘cryptic polymorphism’ is an adaptation to an environment that varies continuously away from the mother plant. However, ecological studies are needed to confirm (or not) that this theory applies to the diaspore-polymorphic species C. arenarius in its cold desert environment. Bracteoles enclosing the fruits (utricles) of C. arenarius increase dispersal distance by wind, anchor diaspores to the soil/sand surface and mechanically inhibit germination. Thus, they must play an important role in the diaspore dispersal–dormancy strategy of this species.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was supported in part by the Scientific Research Program of the Higher Education Institution of Xinjiang, China (FSRPHEXJ 2011S12), the National Natural Science Foundation of China (31160093, U1130301), the Key Program for International S & T Cooperation Projects of China (2010ZR002) and the Early Scientific Research Foundation of Xinjiang Agricultural University of China (XJAU201002).
LITERATURE CITED
- Baker GA, O'Dowd DJ. Effects of parent plant density on the production of achene types in the annual Hypochoeris glabra. Journal of Ecology. 1982;70:201–215. [Google Scholar]
- Barker NP. A review and survey of basicarpy, geocarpy, and amphicarpy in the African and Madagascan flora. Annals of the Missouri Botanical Garden. 2005;92:445–462. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press; 1998. [Google Scholar]
- Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Science Research. 2004;14:1–16. [Google Scholar]
- Baskin JM, Baskin CC, Li X. Taxonomy, ecology, and evolution of physical dormancy in seeds. Plant Species Biology. 2000;15:139–152. [Google Scholar]
- Brändel M. Dormancy and germination of heteromorphic achenes of Bidens frondosa. Flora. 2004;199:228–233. [Google Scholar]
- Brändel M. Ecology of achene dimorphism in Leontodon saxatilis. Annals of Botany. 2007;100:1189–1197. doi: 10.1093/aob/mcm214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng XJ, Tan DY. Bet hedging in heteromorphic achenes of Heteracia szovitsii (Asteraceae), a desert ephemeral. Chinese Journal of Plant Ecology. 2009;33:901–910. (in Chinese with English abstract) [Google Scholar]
- Commissione Redactorum Florae Xinjiangensis. Flora Xinjiangensis. 1994 Urumqi: Xinjiang Science & Technology & Hygiene Publishing House. (in Chinese) [Google Scholar]
- Cordazzo CV. Seed characteristics and dispersal of dimorphic fruit segments of Cakile maritima Scopoli (Brassicaceae) population of southern Brazilian coastal dunes. Revista Brasileira de Botanica. 2006;29:259–265. [Google Scholar]
- Ebrahimi E, Eslami V. Effect of environmental factors on seed germination and seedling emergence of invasive Ceratocarpus arenarius. Weed Research. 2012;52:50–59. [Google Scholar]
- El-Keblawy A. Effects of achene dimorphism on dormancy and progeny traits in the two ephemerals Hedypnois cretica and Crepis aspera (Asteraceae) Canadian Journal of Botany. 2003;81:550–559. [Google Scholar]
- Ellner S. Germination dimorphisms and parent–offspring conflict in seed germination. Journal of Theoretical Biology. 1986;123:173–185. [Google Scholar]
- Ellner S, Shmida A. Why are adaptations for long-range seed dispersal rare in desert plants? Oecologia. 1981;51:133–144. doi: 10.1007/BF00344663. [DOI] [PubMed] [Google Scholar]
- Flint SD, Palmblad IG. Germination dimorphism and developmental flexibility in the ruderal weed Heterotheca grandiflora. Oecologia. 1978;36:33–43. doi: 10.1007/BF00344569. [DOI] [PubMed] [Google Scholar]
- Gao R. Amphicarpy and ecological adaptation of Ceratocarpus arenarius L. in Xinjiang. 2007 MS thesis, Xinjiang Agricultural University, Urumqi, China. (in Chinese with English abstract) [Google Scholar]
- Gao R, Wei Y. Amphicarpy of Ceratocarpus arenarius (Chenopodiaceae) in Junggar desert. Acta Botanica Yunnanica. 2007;29:300–302. (in Chinese with English abstract) [Google Scholar]
- Gao R, Wei Y, Yan C. Amphicarpy and seed germination behavior of Ceratocarpus arenarius L. (Chenopodiaceae) Chinese Journal of Ecology. 2008;27:23–27. (in Chinese with English abstract) [Google Scholar]
- Gardocki ME, Zablocki H, El-Keblawy A, Freeman DC. Heterocarpy in Calendula micrantha (Asteraceae): the effects of competition and availability of water on the performance of offspring from different fruit morphs. Evolutionary Ecology Research. 2000;2:701–718. [Google Scholar]
- Garvin SC, Meyer SE. Multiple mechanisms for seed dormancy regulation in shadscale (Atriplex confertifolia: Chenopodiaceae) Canadian Journal of Botany. 2003;81:601–610. [Google Scholar]
- Gravuer K, Von Wettberg EJ, Schmitt J. Dispersal biology of Liatris scariosa var. novae-angliae (Asteraceae), a rare New England grassland perennial. American Journal of Botany. 2003;90:1159–1167. doi: 10.3732/ajb.90.8.1159. [DOI] [PubMed] [Google Scholar]
- Gutterman Y. Strategies of seed dispersal and germination in plants inhabiting deserts. Botanical Review. 1994;60:373–425. [Google Scholar]
- Gutterman Y. Environmental factors and survival strategies of annual plant species in the Negev Desert, Israel. Plant Species Biology. 2000;15:113–125. [Google Scholar]
- Hu DF, Sheng HL. Space-food resource use of sandy desert rodent community within ephemeral existing period in southern fringe of Dzungaria Basin. Acta Theriologica Sinica. 1992;19:25–36. (in Chinese with English abstract) [Google Scholar]
- Imbert E. Ecological consequences and ontogeny of seed heteromorphism. Perspectives in Plant Ecology, Evolution and Systematics. 2002;5:13–36. [Google Scholar]
- Imbert E, Escarré J, Lepart J. Seed heteromorphism in Crepis sancta (Asteraceae): performance of two morphs in different environments. Oikos. 1997;79:325–332. [Google Scholar]
- Joley DB, Maddox DM, Schoenig SE, Mackey BE. Parameters affecting germinability and seed bank dynamics in dimorphic achenes of Centaurea solstitialis in California. Canadian Journal of Botany. 2003;81:993–1007. [Google Scholar]
- Kigel J. Diaspore heteromorphism and germination in populations of the ephemeral Hedypnois rhagadioloides (L.) F. W. Schmidt (Asteraceae) inhabiting a geographic range of increasing aridity. Acta Oecologica. 1992;13:45–53. [Google Scholar]
- Li WQ, Liu XJ, Khan MA, Yamaguchi S. The effect of plant growth regulators, nitric oxide, nitrate, nitrite and light on the germination of dimorphic seeds of Suaeda salsa under saline conditions. Journal of Plant Research. 2005;118:207–214. doi: 10.1007/s10265-005-0212-8. [DOI] [PubMed] [Google Scholar]
- Li WQ, Liu XJ, Khan MA, Tsuji W, Tanaka K. The effect of light, temperature and bracteoles on germination of polymorphic seeds of Atriplex centralasiatica Iljin under saline conditions. Seed Science and Technology. 2008;36:325–338. [Google Scholar]
- Lu JJ, Tan DY, Baskin JM, Baskin CC. Fruit and seed heteromorphism in the cold desert annual ephemeral Diptychocarpus strictus (Brassicaceae) and possible adaptive significance. Annals of Botany. 2010;105:999–1014. doi: 10.1093/aob/mcq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WB, Zhao XJ, Tan DY, Baskin CC, Baskin JM, Xue JH. Nutlet dimorphism in individual flowers of two cold desert annual Lappula species (Boraginaceae): implications for escape by offspring in time and space. Plant Ecology. 2010;209:361–374. [Google Scholar]
- Ma Y, Wang FG, Jin SK, Li SH. Classification and distribution of rodents in Northern Xinjiang. Sciences and Technology Publishing House Press: Beijing; 1985. (in Chinese) [Google Scholar]
- Mabberley DJ. Mabberlery's plant-book. A portable dictionary of plants, their classification and uses. 3rd edn. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- Mandák B. Seed hetermorphism and the life cycle of plants: a literature review. Preslia. 1997;69:129–159. [Google Scholar]
- Mandák B, Pysĕk P. The effects of light quality, nitrate concentration and presence of bracteoles on germination of different fruit types of the heterocarpous Atriplex sagittata. Journal of Ecology. 2001a;89:149–158. [Google Scholar]
- Mandák B, Pysĕk P. Fruit dispersal and seed banks in Atriplex sagittata: the role of heterocarpy. Journal of Ecology. 2001b;89:159–165. [Google Scholar]
- Mandák B, Pyšek P. How does seed heteromorphism influence the life history stages of Atriplex sagittata (Chenopodiaceae)? Flora. 2005;200:516–526. [Google Scholar]
- Martin AC. The comparative internal morphology of seeds. American Midland Naturalist. 1946;36:513–660. [Google Scholar]
- Mouissie AM, Lengkeek W, Diggelen RV. Estimating adhesive seed-dispersal distances: field experiments and correlated random walks. Functional Ecology. 2005;19:478–486. [Google Scholar]
- Osman AE, Ghassali F. Effects of storage conditions and presence of fruiting bracts on the germination of Atriplex halimus and Salsola vermiculata. Experimental Agriculture. 1997;33:149–156. [Google Scholar]
- Philipupillai J, Ungar IA. The effect of seed dimorphism on the germination and survival of Salicornia europaea L. populations. American Journal of Botany. 1984;71:542–549. [Google Scholar]
- Ruiz de Clavijo E. The ecological significance of fruit heteromorphism in the amphicarpic species Catananche lutea (Asteraceae) International Journal of Plant Sciences. 1995;156:824–833. [Google Scholar]
- Ruiz de Clavijo E. The role of dimorphic achenes in the biology of the annual weed Leontodon longirrostris. Weed Research. 2001;41:275–286. [Google Scholar]
- Ruiz de Clavijo E, Jiménez MJ. The influence of achene type and plant density on growth and biomass allocation in the heterocarpic annual Catananche lutea (Asteraceae) International Journal of Plant Sciences. 1998;159:637–647. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 3rd edn. San Francisco: Freeman; 1995. [Google Scholar]
- Soltis DE, Soltis PS, Endress PK, Chase MW. Phylogeny and evolution of angiosperms. Sunderland, MA: Sinauer Associates; 2005. [Google Scholar]
- Sorensen AE. Somatic polymorphism and seed dispersal. Nature. 1978;276:174–176. [Google Scholar]
- Sun HZ, Tan DY, Qu RM. Characteristics of heteromorphic achenes of Garhadiolus papposus, an ephemeral Asteraceae species, with reference to their adaptations to desert environment. Biodiversity Science. 2008;16:353–361. (in Chinese with English abstract) [Google Scholar]
- Telenius A, Torstensson P. The seed dimorphism of Spergularia marina in relation to dispersal by wind and water. Oecologia. 1989;80:206–210. doi: 10.1007/BF00380152. [DOI] [PubMed] [Google Scholar]
- Ungar IA, Khan MA. Effect of bracteoles on seed germination and dispersal of two species of Atriplex. Annals of Botany. 2001;87:233–239. doi: 10.1006/anbo.2000.1321. [DOI] [PubMed] [Google Scholar]
- Venable DL. The evolutionary ecology of seed heteromorphism. American Naturalist. 1985;126:577–595. [Google Scholar]
- Venable DL. The ecology of seed banks. Parker VT, Leck MA, Simpson RL. Orlando, FL: Academic Press; 1989. Modeling the evolutionary ecology of seed banks; pp. 67–87. [Google Scholar]
- Venable DL, Levin DA. Ecology of achene dimorphism in Heterotheca latifolia. I. Achene structure, germination and dispersal. Journal of Ecology. 1985a;73:133–145. [Google Scholar]
- Venable DL, Levin DA. Ecology of achene dimorphism in Heterotheca latifolia. II. Demographic variation within populations. Journal of Ecology. 1985b;73:743–755. [Google Scholar]
- Venable DL, Búrquez A, Corral G, Morales E, Espinosa F. The ecology of seed heteromorphism in Heterosperma pinnatum in central Mexico. Ecology. 1987;68:65–76. [Google Scholar]
- Wang L, Huang ZY, Baskin CC, Baskin JM. Germination of dimorphic seeds of the desert annual halophyte Suaeda aralocaspica (Chenopodiaceae), a C4 plant without kranz anatomy. Annals of Botany. 2008;102:757–769. doi: 10.1093/aob/mcn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XQ, Jiang J, Wang YC, Luo WL, Song CW, Chen JJ. Responses of ephemeral plant germination and growth to water and heat conditions in the southern part of Gurbantunggut desert. Chinese Science Bulletin. 2006;51(Suppl.):110–116. [Google Scholar]
- Wei WS, He Q, Liu MZ, Gao WD. Climate change and the desert environment in Junggar Basin, Xinjiang, China. Journal of Desert Research. 2003;23:101–105. (in Chinese) [Google Scholar]
- Wei Y, Dong M, Huang ZY. Seed polymorphism, dormancy and germination of Salsola affinis (Chenopodiaceae), a dominant desert annual inhabiting the Junggar Basin of Xinjiang, China. Australian Journal of Botany. 2007;55:464–470. [Google Scholar]
- Wei Y, Dong M, Huang ZY, Tan DY. Factors influencing seed germination of Salsola affinis (Chenopodiaceae), a dominant annual halophyte inhabiting the deserts of Xinjiang, China. Flora. 2008;203:134–140. [Google Scholar]
- Xinjiang General Exploration Team of Chinese Academy of Sciences (CAS), Institute of Botany of CAS. Vegetation of Xinjiang and its utilization. 1978 Beijing: Science Publishing House. (in Chinese) [Google Scholar]
- Zhou XQ. Life history strategy of amphicarpy in Ceratocarpus arenarius L. 2009 MS thesis, Xinjiang Agricultural University, Urumqi, China. (in Chinese with English abstract) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.