Abstract
Background and Aims
Local adaptation enables plant species to persist under different environmental conditions. Evolutionary change can occur rapidly in invasive annual species and has been shown to lead to local adaptation. However, the patterns and mechanisms of local adaptation in invasive species along colonization sequences are not yet understood. Thus, in this study the alien annual Impatiens glandulifera was used to investigate local adaptation to distinct habitats that have been consecutively invaded in central Europe.
Methods
A reciprocal transplant experiment was performed using 15 populations from alluvial deciduous forests, fallow meadows and coniferous upland forests, and a greenhouse experiment was performed in which plants from these habitats were grown under treatments reflecting the main habitat differentiators (shade, soil acidity, competition).
Key Results
Biomass production, specific leaf area, plant height and relative growth rate differed between habitats in the field experiment and between treatments in the greenhouse, but not between seed origins. Overall, there was no indication of local adaptation in either experiment.
Conclusions
Since I. glandulifera is a successful invader in many habitats without showing local adaptation, it is suggested that the species is coping with environmental variation by means of high phenotypic plasticity. The species seems to follow a ‘jack-and-master’ strategy, i.e. it is able to maintain high fitness under a wide range of environmental conditions, but performs particularly well in favourable habitats. Therefore, the proposed colonization sequence is likely to be based primarily on changes in propagule pressure. It is concluded that invasive alien plants can become dominant in distinct habitats without local adaptation.
Keywords: Biological invasions, colonization history, general-purpose genotype, greenhouse experiment, home site advantage, invasive alien plant, Impatiens glandulifera, jack-and-master strategy, local adaptation, phenotypic plasticity, propagule pressure, reciprocal transplant experiment
INTRODUCTION
Environmental variability causes opposing selection pressures and therefore favours genetic adaptation of plant species. Adaptation, in turn, enables plant species to persist in a set of different environmental conditions (Leimu and Fischer, 2008). If adaptation has taken place, resident genotypes will have a higher relative fitness than foreign ones (‘local vs. foreign’ criterion; Kawecki and Ebert, 2004). While at large scales climatic differences are important for adaptation (Macel et al., 2007), at local scales distinct habitat characteristics might be more relevant (Hereford and Winn, 2008), e.g. soil conditions (Raabová et al., 2011), shade (Godoy et al., 2011) or biotic interactions (Grøndahl and Ehlers, 2008). Many studies have found evidence for adaptation in plant species (e.g. Becker et al., 2008; Hufford et al., 2008; see meta-analyses by Leimu and Fischer, 2008; Hereford, 2009), but others have not (e.g. Leiss and Müller-Schärer, 2001; Hereford and Winn, 2008; Ebeling et al., 2011; Garrido et al., 2012). Recently, progress has been made in explaining the mechanisms influencing local adaptation (e.g. Leimu and Fischer, 2008; Hereford, 2009; Lopez et al., 2009), but it is still a challenge to understand the underlying patterns and drivers of adaptation in plants.
In invasive alien species, evolutionary change can occur rapidly (Maron et al., 2004; Bossdorf et al., 2005). Although there are several mechanisms that are believed to inhibit adaptation, e.g. low genetic variability (Taylor and Keller, 2007), local adaptation of invasive alien plants to distinct habitat types has been demonstrated repeatedly (e.g. Scott et al., 2010; Godoy et al., 2011). Sax et al. (2007) suggested that invasive alien species can be used as model organisms for studying ecological and evolutionary processes in real time. Therefore, invasive alien species are a suitable study system to investigate the evolution of local adaptation.
Local adaptation can broaden species' ecological niches. This is particularly important in secondary invasions (Dietz and Edwards, 2006). According to Dietz and Edwards (2006), plant invasions occur in two stages. During the primary invasion, alien species establish in habitats with the highest propagule pressure, e.g. along transport corridors, while in the secondary invasion additional habitats with distinct environmental conditions are colonized. These two stages do not have to be entered consecutively, but in many plant invasions the most accessible habitats are colonized first, before secondary invasion to new habitat conditions takes place. We expect local adaptation in the secondary invasion to be more pronounced for early-invaded habitats due to longer residence time. When different habitats have been colonized consecutively, a sequence of local adaptation can be studied along this colonization sequence. For example, Erfmeier et al. (2011) found a shift in life history strategy during secondary invasion of a deciduous tree, suggesting on-going adaptation to less favourable habitats. However, there are two possible alternative explanations for secondary invasion in invasive alien plants. First, the species' ecological niche can also be broadened by high phenotypic plasticity (Dietz and Edwards, 2006; Moloney et al., 2009). Invasive plants can profit from high phenotypic plasticity in morphological and physiological traits by two main strategies (Richards et al., 2006): the ‘jack-of-all-trades’ strategy is able to maintain high fitness in a set of distinct habitats (general-purpose genotype; Baker, 1965), while the ‘master-of-some’ strategy can increase fitness in especially favourable habitats (e.g. Sultan, 2001). Second, changing patterns of local propagule pressure may also contribute to secondary invasion. Propagule pressure depends mainly on the distance to (Rouget and Richardson, 2003) and the size of donor populations (Richardson and Pyšek, 2006), i.e. older, larger populations are more likely to donate propagules to other sites. Land use alteration can further change temporal patterns in propagule pressure through alterations of disturbance regimes and transport pathways.
A prominent invasive alien plant species that has colonized distinct habitats in Europe over a long time period is Impatiens glandulifera. In the invaded range, this species frequently occurs in near-natural habitats, primarily in riparian habitats, fenland, mesotrophic grassland and deciduous woodland (Andrews et al., 2005). Impatiens glandulifera is a suitable species to study local adaptation to different habitats because it is an outcrossing annual with potentially fast evolution (Beerling and Perrins, 1993). Previous work showed that I. glandulifera exhibits latitudinal trends in growth which might reflect an adaptation to the length of the growing season (Kollmann and Bañuelos, 2004). In the congeneric I. capensis, potential to develop local adaptation was shown, especially with regard to shade (Dudley and Schmitt, 1995) and density (Donohue et al., 2001). Additionally, Walker et al. (2009) found substantial genetic variation in I. glandulifera in northeast England using microsatellite analysis, and Zybartaite et al. (2011) revealed four major genetic groups of populations in Lithuania using randomly amplified polymorphic DNA.
Historical reconstructions suggest that I. glandulifera colonized different near-natural habitats consecutively in the past 100 years, starting from settlements and riparian habitats (Pyšek and Prach, 1995). In the Czech Republic, for example, the species was first recorded in riparian habitats in 1900, in fallow meadows in 1934 and in forests in 1941 (Pyšek and Prach, 1995). Rivers act as dispersal corridors and it took about 20 years from the first occurrence of the species on main rivers until invasion proceeded upstream along tributaries and laterally away from the rivers (Malíková and Prach, 2010). Invasion in the Czech Republic is still in progress and expected to continue (Malíková and Prach, 2010). Accordingly, first records from southern Germany date to the first two decades of the 20th century (Hegi, 1925–1965) and it can be assumed that habitat colonization in Germany has progressed in a similar way as in the Czech Republic. Deciduous forests along rivers were most probably invaded first, whereas colonization of fallow meadows and coniferous upland forests, which are spatially separated from riverine habitats, started later. These three habitats differ mainly with regard to shade, soil acidity and competition. Forest habitats are characterized by moderate to high shade, while fallow meadows are mainly open. Soils in coniferous forests are usually more acid compared with alluvial deciduous forests and fallow meadows. Competition among herbs is more intense in fallow meadows and alluvial deciduous forests compared with coniferous forests with sparse herb layers. These contrasting habitat conditions should favour local adaptation.
We conducted a reciprocal transplant experiment in southern Germany to test for local adaptation in I. glandulifera to three habitat types along a colonization sequence consisting of alluvial deciduous forests, fallow meadows and coniferous upland forests. Additionally, we manipulated shade, soil acidity and competition as main habitat differentiators in a factorial greenhouse experiment to extract the ecological factors that are likely to lead to local adaptation. Our main aim was to test for local adaptation in I. glandulifera. More specifically we hypothesized (1) higher fitness of local origins when reciprocally sown in the three habitats in the field (‘home habitat advantage’). Based on habitat characteristics, we expected that in the greenhouse (2a) under high shade, low soil acidity and high competition, plants originating from alluvial deciduous forests have higher fitness compared with other origins; (2b) under low shade, low soil acidity and high competition, plants from fallow meadows have highest fitness; and (2c) under high shade, high soil acidity and without competition, plants from coniferous upland forests have the highest fitness. We further predicted local adaptation to be most pronounced in origins from alluvial deciduous forests, followed by those from fallow meadows and those from coniferous upland forests.
MATERIALS AND METHODS
Study species
Impatiens glandulifera (Balsaminaceae) is a herbaceous annual species that was introduced from the Himalaya to Europe as an ornamental plant in the 19th century (Beerling and Perrins, 1993), and has become abundant with considerable impact in 19 European countries within latitudes 30–64 ° N (Kollmann and Bañuelos, 2004). It is common in open and shaded habitats in lowland and lower montane areas (<800 m a.s.l.), but occurs in the Alps up to 1550 m altitude (Kollmann and Bañuelos, 2004). Impatiens glandulifera grows up to 3 m tall, and the basal diameter of the stem can reach 5 cm (Beerling and Perrins, 1993). Germination takes place from February to April. The flowering period is from July to October, and the seeds disperse by dehiscent seed-capsules between August and October (Ammer et al., 2011). They are transported over long distances through human activities and water dispersal (Hartmann et al., 1995). The species has no clonal growth and a short-lived seed bank (Beerling and Perrins, 1993).
Study area and source populations
Seeds of I. glandulifera were collected in the region of Freising, southern Germany (study area: 48·39–48·45 ° N, 11·65–11·88 ° E, ∼140 km2, 366–506 m a.s.l.) in three different habitats, i.e. alluvial deciduous forests, fallow meadows and coniferous forests on nearby hills, each with five replicate populations to cover variability within habitats (hereafter ‘source populations’). The alluvial deciduous forests were located in the floodplain of the River Isar. Impatiens glandulifera populations in this habitat were rather continuous and situated close to the main river channel as well as along forest roads. The tree layer was dominated by Fraxinus excelsior mixed with Acer pseudoplatanus and Salix alba (height 20–30 m), leading to deep shade (canopy cover 70–90 %). The understorey had 60–80 % cover of herbs and shrubs, mainly Aegopodium podagraria and Rubus caesius. The soil was moderately moist with neutral reaction. The fallow meadows occurred at plane to slightly inclined locations on loamy and moist soils with neutral reaction. Impatiens glandulifera populations in this habitat were rather separated by more intense land use around the patches. The vegetation was characterized by tall herbs and grasses, including Arrhenatherum elatius, Galium mollugo and Phalaris arundinacea (cover 80–100 %) with little shade from trees or shrubs. The coniferous upland forests were old-grown spruce plantations on sandy and less moist soils with slightly acidic reaction in the tertiary hills around Freising, with modest slopes under variable orientation. Impatiens glandulifera populations in this habitat were less dense than in the other two habitats, and patches were mostly continuous with small gaps in between. The canopy consisted of 20–30 m tall Picea abies leading to moderate shade (cover 60–80 %). The herb layer was sparser than in the other habitats (cover 30–50 %), including mosses, Oxalis acetosella, Rubus fruticosus agg. and young plants of Quercus robur and Acer pseudoplatanus. The three habitats were all relatively nutrient-rich, while there was a pronounced gradient in soil acidity (see Supplementary Data Table S1 for further information).
The first specimen from the greater study region stored in the two most important herbaria of the federal state is dated to 1909 (Munich) and originates from a riverine site ∼85 km upstream of the study area, situated at River Isar, which runs through the study area. It is assumed that invasion in the study area first covered habitats along River Isar and the tributary River Amper before it proceeded to habitats outside the floodplains. Small tributaries as well as roads are most likely to have served as secondary colonization corridors. From Rivers Isar and Amper, colonization most probably first reached fallow wet meadow habitats, often situated close to tributaries, while colonization of upland coniferous forests began later and is still in progress. The distances between source populations were 5·7 ± 2·9 km (mean ± s.d., accordingly throughout the article) for deciduous forests, 5·3 ± 2·2 km for fallow meadows and 7·5 ± 4·1 km for coniferous forests, and did not differ within habitats (ANOVA, F = 1·19, P = 0·32; see Supplementary Data Table S1 for distances to the closest source population overall and within each habitat).
Annual average temperature in the study region is 7·5 °C and annual precipitation 788 mm (Weihenstephan 1961–1990; Bayerische Landesanstalt für Landwirtschaft, 2012). Monthly mean temperature during the experiment (March–August 2012) was 2 °C higher than the long-term average (1961–1990). Precipitation from March to May 2012 was 54 mm, i.e. 29 % less than normal, while from June to August 2012 it was 125 mm (42 % more than normal in 1961–1990).
Seed material
In each population in the three habitat types we took two to five ripe capsules from 75 randomly chosen plants in September 2011 and again in October 2011 to account for possible temporal differences in seed quality. Seeds were dried at room temperature for 3 weeks and stored at 5 °C for 2 months prior to seed mass determination and stratification. Average seed mass of the plant material used was 13·6 ± 0·9 mg for populations in the deciduous forest, 12·8 ± 0·9 mg in the fallow meadows and 11·8 ± 1·3 mg in coniferous forest (n = 5 × 500 seeds per source population and n = 5 populations per habitat). Seeds were cold-wet stratified on filter paper in Petri dishes and stored at 3 °C. Seed germination rate after 3 weeks (5/15 °C, 12:12 h, without light) was 73 ± 11 % for populations from deciduous forests, 93 ± 4 % for fallow meadows and 79 ± 12 % for coniferous forests (n = 5 × 50 seeds per source population and n = 5 populations per habitat).
Reciprocal seed transplant experiment
In mid-March 2012, we established one experimental plot (0·8 m × 1·6 m) in close proximity to each of the 15 source populations (see Supplementary Data Fig. S1A for the experimental design). The 15 plot sites were chosen to be similar to the source populations, but free of I. glandulifera with a buffer zone of >2 m. The distances between plot and source population ranged from 34 to 962 m, with values of 279 ± 358 m for deciduous forests, 69 ± 39 m for fallow meadows and 379 ± 226 m for coniferous forests. Distances were not different within habitats (Kruskal–Wallis test, P = 0·075; Supplementary Data Table S2).
In one half of each plot (0·8 m × 0·8 m) soil remained untreated (‘undisturbed soil’). In the other half all aboveground litter and vegetation were removed and the soil was disturbed with a rake (10 cm deep) 1 week before sowing (‘disturbed soil’). This treatment was included to cover variability within habitats and meant to simulate disturbance by wild animals, e.g. boars. Each half of each plot was divided into 16 subplots. The subplot size (0·2 m × 0·2 m) was chosen based on observed plant densities in the source populations and experiences from a preliminary study in 2011. While one of them remained as a control, 20 seeds of each of the source populations were sown into the other subplots. Subplots were equipped with plastic rings (diameter 10 cm, height 3 cm) that were gently pushed into the soil to prevent seed losses. Nevertheless, we found germination in 7 % of the control subplots, which we consider to have been caused by accidental dispersal from the other subplots. After 7 weeks, seedlings were thinned to a maximum of five per subplot to avoid bias due to intraspecific competition. In deciduous forests, 4 ± 4 seedlings per subplot (across all subplots within the habitat, but excluding controls) were removed, 6 ± 4 in fallow meadows and 1 ± 2 in coniferous forests. The removed seedlings were used to determine aboveground dry biomass per plant (after drying for 3 days at 70 °C).
All plants were harvested after 20 weeks in August 2012. Biomass was used as a proxy for fitness since aboveground biomass and seed production of annual species are often correlated (e.g. Thompson et al., 1991; Shipley and Dion, 1992). In addition, specific leaf area (SLA), plant height and relative growth rate (RGR) were measured to detect plastic plant responses to the main habitat differentiators, i.e. shade, soil acidity and competition. One individual per subplot was chosen randomly to measure height. Three fully developed leaves of the same plant were photographed to determine SLA with the software ImageJ 1·46 (Schneider et al., 2012), and dried afterwards. SLA was calculated as SLA = A WL−1, where A is area and WL is dry mass of the three selected leaves (Cornelissen et al., 2003). We harvested aboveground biomass of all I. glandulifera individuals and determined dry biomass per plant. Mean RGR per subplot was calculated as RGR = [ln(W2)n2−1 – ln(W1)n1−1] (t2 – t1)−1, where W1 and W2 are the aboveground dry biomasses of n individuals harvested at times t1 (week 7) and t2 (week 20), respectively, in each subplot. As suggested by Hoffmann and Poorter (2002), biomass was natural logarithm-transformed before averaging.
Greenhouse experiment
In the same time period, a greenhouse experiment was conducted at the Dürnast Research Centre (located within the study area; www.wzw.tum.de/ghl/) to identify the environmental factors potentially leading to local adaptation. Treatments included shade, soil acidity and competition, giving a full factorial design with a total of eight treatments, including all 15 source populations.
For the shade treatment, plants were grown under a single or a double layer of green fabric, resulting in ∼10 % and 5 %, respectively, photosynthetic active radiation (PAR) in relation to full sunlight, which corresponds to moderate to deep shade, for example in coniferous forests (8·7 ± 4·9 % PAR) and deciduous forests (2·5 ± 1·9 % PAR; Supplementary Data Table S2). As the shade treatment was expected to alter not only light availability but also air humidity and temperature, we performed two shade treatments rather than comparing shade with no shade.
To manipulate soil acidity, commercial peat (Floragard Floratorf; pHCaCl2 3·0–4·0; nitrogen, phosphate and potassium oxide <0·05 kg m−3 each) was mixed with fertilizer (Ferty® 2; nitrogen 0·15 kg m−3, phosphate 0·05 kg m−3, potassium oxide 0·25 kg m−3, magnesium oxide 0·02 kg m−3) and different concentrations of lime. To achieve a moderately low pH treatment, 6 kg m−3 pelleted lime (concentration 50 %) was applied, resulting in pHCaCl2 5·1. For neutral substrate, 14·5 kg m−3 pelleted lime (50 %) and 10 kg m−3 fine lime (95 %) were used. Additionally, Ca(OH)2 (0·3 %) was added with watering to achieve pHCaCl2 6·5; the pHCaCl2 in the two treatments increased during the experiment to 6·4 and 7·2, respectively. The high pH treatment was comparable to the alluvial deciduous forests considered, where pHH2O was around 7·3 (Supplementary Data Table S1) which corresponds to a pHCaCl2 of ∼6·8 (after the Sillanpää equation: pHCaCl2 = 1·044pHH2O – 0·808; Budoi et al., 2003). The low pH treatment was comparable to fallow meadows with pHH2O 6·9, which corresponds to a pHCaCl2 of 6·4. Plot sites had comparable soil reactions (Supplementary Data Table S2).
For the competition treatment, I. glandulifera seedlings were planted alone or together with five individuals of Arrhenatherum elatius. This grass species is known to be a good competitor and has been used in competition experiments for a long time (e.g. Mahmoud and Grime, 1976). Additionally, it grows in one of the habitats considered, i.e. fallow meadows. The grass seedlings were germinated from regional seed material (Rieger-Hofmann GmbH) and introduced 21 days before the target plants to create sufficient competition.
Seeds of I. glandulifera were pre-germinated on a standard growing substrate in multipots. In March 2012, cotyledon length of all seedlings was measured. Forty seedlings of each of the source populations were selected at random and potted individually (pot diameter 19 cm, volume 0·003 m3). Remaining seedlings (minimum of 35 per habitat) were used to determine starting aboveground biomass. Plants were exposed to the eight treatments with five replicates, giving a total of 600 plants. The pots were arranged in five rows (see Supplementary Data Fig. S1B for the experimental design). Half of each row was covered with a double layer of green fabric (high shade), the other half with a single layer (low shade). Each row contained eight blocks. All blocks contained one plant of each source population. Within the two shade levels, the soil acidity and competition treatments were randomized, i.e. each row contained a randomized arrangement of one block per treatment with only the light levels being grouped together. To avoid edge effects, pots were randomized and rotated within the blocks, and blocks of the same shade treatment were rotated within rows once.
After 8 weeks (May 2012), three plants per source population were randomly selected from each treatment; the others were kept for further experiments. Aboveground biomass, plant height and SLA were determined as in the field experiment. To calculate RGR, plant dry mass W1 was estimated based on the correlation between cotyledon length (x) and starting aboveground biomass of remaining seedlings at the beginning of the experiment (for deciduous forest populations, Pearson correlation, W1 = 0·10x + 0·04, r = 0·41, P = 0·004; for fallow meadows, W1 = 0·12x – 0·06, r = 0·70, P < 0·001; and for coniferous forest, W1 = 0·24x – 0·38, r = 0·60, P < 0·001). Additionally, aboveground dry biomass of A. elatius per pot was determined in the competition treatment.
Statistical analysis
All statistical analyses were performed with R 2·15·1 (R Core Team, 2012), using the packages lme4 (Bates et al., 2012) and multcomp (Hothorn et al., 2009). For the reciprocal transplant experiment we fitted linear mixed effects models using maximum likelihood separately for the two treatments (undisturbed soil, disturbed soil) and the different response variables (biomass, SLA, plant height and RGR of I. glandulifera). Full models contained seed origin, habitat and their interaction. A significant interaction between seed origin and habitat would indicate an adaptation of I. glandulifera to the local environmental conditions (Van Groenendael, 1985; Leiss and Müller-Schärer, 2001). Seed mass and seedling emergence were included as covariates to partially control for maternal effects and varying intraspecific competition before the thinning to five seedlings per subplot after 7 weeks. We added source population nested in habitat and plot nested in habitat as crossed random factors to account for the spatial structure of seed sources and the experimental plot design.
To analyse the effects of seed origin and the eight treatments in the greenhouse on biomass, SLA, plant height and RGR of I. glandulifera, we also used linear mixed effects models fitted with maximum likelihood. We included seed origin, shade, soil acidity and competition with A. elatius and all possible two-way and three-way interactions as fixed factors. Seed mass and grass biomass (competition treatment) were included as covariates to partially control for maternal effects and variation in competition. Source population nested in habitat and block nested in rows within the shade treatment were included as crossed random factors to reflect the spatial component of seed origin and experimental design.
We simplified all models (field and greenhouse experiments) stepwise backwards based on likelihood ratio tests and removed non-significant fixed factors. Model checking plots were inspected to ensure that model assumptions were met. Biomass was natural logarithm-transformed to improve model fitting. No further transformations were necessary. Finally, we calculated post hoc Tukey contrasts for all significant factors with more than two levels in the minimum adequate models.
RESULTS
Plant performance in the field
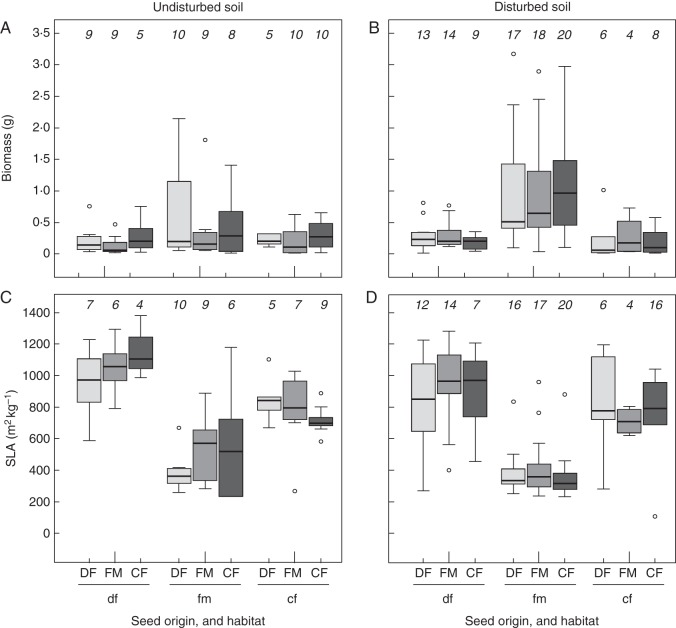
We could not detect any influence of seed origin on aboveground biomass, SLA, plant height and RGR of transplanted I. glandulifera in the field experiment (Table 1). The response of all origins was very similar within each habitat (Fig. 1A–D and Supplementary Data Fig. S2A–D).
Table 1.
Influence of seed origin, habitat and their interaction on aboveground biomass, specific leaf area (SLA), plant height and relative growth rate (RGR) of the invasive alien Impatiens glandulifera in a reciprocal transplant experiment in the invaded range in central Europe
| Biomass |
SLA |
Height |
RGR |
|||||
|---|---|---|---|---|---|---|---|---|
| χ2 | P | χ2 | P | χ2 | P | χ2 | P | |
| Undisturbed soil | ||||||||
| Origin | 3·73 | 0·155 | 2·04 | 0·362 | 1·56 | 0·459 | 0·60 | 0·741 |
| Habitat | 1·41 | 0·493 | 9·58 | 0·008 | 3·34 | 0·188 | 2·82 | 0·245 |
| Origin × habitat | 2·77 | 0·596 | 7·17 | 0·127 | 2·97 | 0·563 | 7·58 | 0·108 |
| Disturbed soil | ||||||||
| Origin | 0·63 | 0·729 | 1·29 | 0·525 | 1·59 | 0·452 | 3·70 | 0·157 |
| Habitat | 9·30 | 0·010 | 8·95 | 0·011 | 8·35 | 0·015 | 4·47 | 0·035 |
| Origin × habitat | 1·95 | 0·745 | 7·36 | 0·118 | 1·14 | 0·887 | 0·97 | 0·617 |
χ2- and P-values are based on maximum likelihood ratio tests for linear mixed effects models. χ2- and P-values of non-significant factors refer to the respective step of the model simplification procedure. Significant terms were tested against the minimum adequate model. See Materials and methods section for information on random factors and covariates. For sample size see Fig. 1 and Supplementary Data Fig. S2.
Significant values (P < 0·05) are shown in bold type.
Fig. 1.
Above-ground biomass (A, B) and specific leaf area (C, D) of the invasive alien Impatiens glandulifera when reciprocally transplanted between alluvial deciduous forests (df/DF), fallow meadows (fm/FM) and coniferous forests (cf/CF) in the invaded range. Seed origins are indicated with capital letters, plot habitats with lower-case letters. Plots remained either untreated (A, C) or were experimentally disturbed before planting (B, D). The number of plant individuals in each group is given in small italic numbers above the boxplots.
In the undisturbed soil treatment, SLA was the only measured trait that was affected significantly by habitat (Table 1): transplants in deciduous forests revealed the highest SLA (Tukey contrasts against both fallow meadows and coniferous forests P < 0·001), followed by those in coniferous forests (against fallow meadows P = 0·002) and fallow meadows (Fig. 1C). In the disturbed soil treatment, plant performance varied considerably across habitats and the influence of habitat was significant for all measured traits (Table 1). Plants transplanted to fallow meadows produced significantly more aboveground biomass than those in the two forest habitats (Tukey contrasts against deciduous forest P = 0·004, against coniferous forests P > 0·001) and did not differ between deciduous and coniferous forests (P = 0·107; Fig. 1B). SLA was similarly high in deciduous and coniferous forests (P = 0·864) and significantly lower in fallow meadows (Tukey contrast against both forest habitats P < 0·001; Fig. 1D). Height of plants in coniferous forests was significantly lower (Tukey contrasts against deciduous forests P = 0·006, against fallow meadows P < 0·001), but comparable between deciduous forests and fallow meadows (P = 0·614; Supplementary Data Fig. S2B). RGR was significantly higher in fallow meadows than in deciduous forests (Tukey contrast P < 0·001; Supplementary Data Fig. S2D).
Seedling emergence in the plots was 44 ± 29 % in deciduous forests (for all subplots together, but excluding the controls), 54 ± 26 % in fallow meadows and 14 ± 21 % in coniferous forests. The response of all five source populations of the same origin was similar for each habitat and treatment, and in all models the population factor within origin explained less than 5 % of the variance of the random factors. The plot site within a habitat explained some of the variance in most models (0–47 %), but most variance of the random factors remained unexplained.
Plant performance in the greenhouse
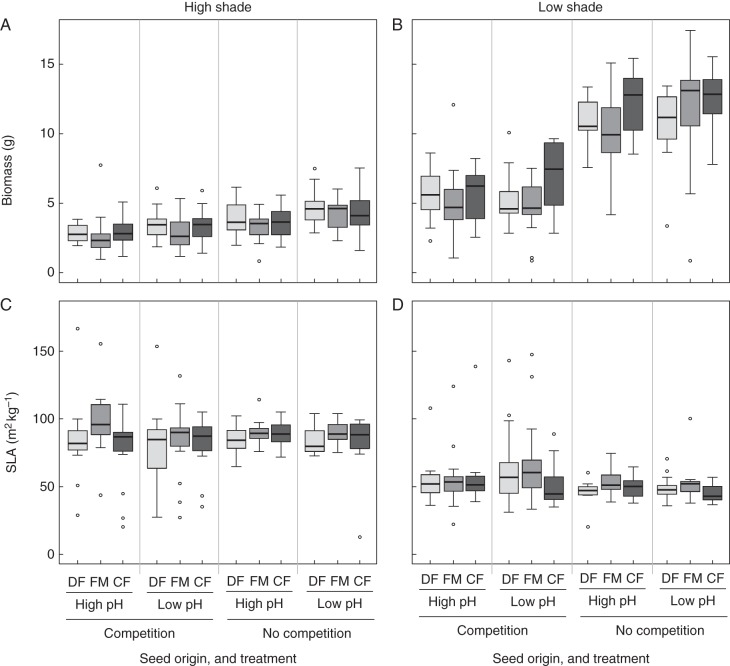
In the greenhouse experiment, seed origin had a significant influence on biomass production (Table 2). Nevertheless, there was no clear pattern and no better performance of each origin in the treatment reflecting its original habitat conditions (Fig. 2A, B). Including all treatments, biomass was highest for plants from coniferous forests (6·5 ± 4·0 g) and lowest for those from fallow meadows (5·6 ± 3·9 g; Tukey contrast P = 0·001). However, the maximum value for biomass was achieved by plants from fallow meadows (17·4 g). Biomass of plants from deciduous forests was in an intermediate range (6·0 ± 3·3 g) and was not significantly different from either fallow meadows (P = 0·086) or coniferous forests (P = 0·385). Biomass was additionally affected by soil acidity and the interaction of shade and competition (Table 2). Plants produced little biomass under high shade also in the absence of competitors, while plants grown under low shade produced remarkably more biomass when released from competition (Fig. 2A, B).
Table 2.
Effects of seed origin, shade, soil acidity, competition and their pairwise interactions on aboveground biomass, specific leaf area (SLA), plant height and relative growth rate (RGR) of the invasive Impatiens glandulifera in a greenhouse experiment
| Biomass |
SLA |
Height |
RGR |
|||||
|---|---|---|---|---|---|---|---|---|
| χ2 | P | χ2 | P | χ2 | P | χ2 | P | |
| Origin | 6·74 | 0·034 | 3·46 | 0·177 | n.a. | n.a. | 7·84 | 0·020 |
| Shade | n.a. | n.a. | 14·11 | < 0·001 | n.a. | n.a. | 37·56 | < 0·001 |
| Soil acidity | 4·04 | 0·045 | 1·24 | 0·265 | n.a. | n.a. | 6·43 | 0·011 |
| Competition | n.a. | n.a. | 0·30 | 0·585 | 9·80 | 0·002 | 1·03 | 0·310 |
| Origin × shade | 4·70 | 0·096 | 4·79 | 0·091 | 6·03 | 0·049 | 4·69 | 0·096 |
| Origin × soil acidity | 1·17 | 0·558 | 0·86 | 0·650 | 8·42 | 0·015 | 1·60 | 0·450 |
| Origin × competition | 2·19 | 0·335 | 1·15 | 0·562 | 0·38 | 0·829 | 0·58 | 0·750 |
| Shade × soil acidity | 3·07 | 0·080 | 0·07 | 0·796 | 1·30 | 0·255 | 0·17 | 0·300 |
| Shade × competition | 5·73 | 0·017 | 1·09 | 0·296 | 0·34 | 0·560 | 0·93 | 0·334 |
| Soil acidity × competition | 0·01 | 0·912 | 0·20 | 0·652 | 2·25 | 0·134 | 0·02 | 0·891 |
χ2- and P-values are based on maximum likelihood ratio tests for linear mixed effects models. χ2- and P-values of non-significant factors refer to the respective step of the model simplification procedure. Significant terms were tested against the minimum adequate model. See Materials and methods section for information on model simplification, random factors and covariates. N =15, except five cases where only 14 replicates were available. Main factors included in a significant interaction were not further explored (n.a., not assessed). All three-way interactions were not significant (not shown).
Significant values (P < 0·05) are shown in bold type.
Fig. 2.
Above-ground biomass (A, B) and specific leaf area (C, D) of invasive populations of Impatiens glandulifera in a greenhouse experiment. Plants were exposed to eight treatments in a full factorial design, including high and low shade, competition by a common grass species (Arrhenatherum elatius) and no competition, as well as low and high soil acidity. Plant material originated from three habitat types, i.e. alluvial deciduous forests (DF), fallow meadows (FM) und coniferous forests (CF). Most groups represent 15 replicates, except five cases where only 14 replicates were available.
For all origins, SLA was significantly higher in the high-shade treatment irrespective of additional treatments and origin (Table 2; Fig. 2C, D). Plants grown without competitors were generally taller than those grown under competition (Table 2; Supplementary Data Fig. S3A, B). Additionally, height was significantly increased under low shade, especially for plants from coniferous forests (significant origin × shade interaction, Table 2). Plants from coniferous forests and fallow meadows grew taller under low compared with high pH, while plants from alluvial deciduous forests were taller under high pH (Fig. 3; significant origin × soil acidity interaction, Table 2). In all treatments, RGR of plants originating from fallow meadows and coniferous forests did not differ (P = 0·503), but RGR was smaller for deciduous forest origins compared with fallow meadows (P < 0·001) and coniferous forests (P =0·027; Supplementary Data Fig. S3C, D). RGR was significantly higher for plants grown under low shade or high soil acidity (Table 2; Supplementary Data Fig. S3C, D).
Fig. 3.
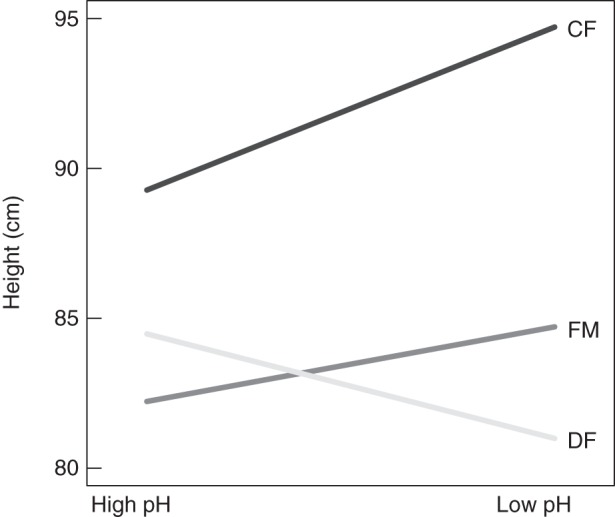
Plant height of invasive populations of Impatiens glandulifera in a greenhouse experiment affected by a significant interaction (χ2 = 8·42, P = 0·015, for methods see text) between pH and seed origin, i.e. alluvial deciduous forests (DF), fallow meadows (FM) und coniferous forests (CF). Graphs were computed pooling all treatments.
All five source populations of the same origin responded similar to the experimental treatments in the greenhouse, and in all models the population factor explained <5 % of the variance of the random factors. The experimental block within row within shade treatment explained some of the variance in all models (0–14 %), but most variance of the random factors remained unexplained.
DISCUSSION
Explaining the lack of local adaptation
We could not find any indication of local adaptation of the invasive alien I. glandulifera to three distinct habitats, i.e. alluvial deciduous forests, fallow meadows and coniferous forests. Neither an interaction between origin and habitat nor higher fitness of local origins emerged when reciprocally sown to the three habitats in the field (Hypothesis 1), nor did the experimental treatments reflecting the three habitat types in the greenhouse favour the respective provenances (Hypothesis 2a–c). Thus, we could not explain the proposed colonization sequence by different degrees of local adaptation (Hypothesis 3). The lack of local adaptation found in adult plants seems to be consistent for other phases of the study species' life cycle. Our results support the observations by Skálová et al. (2012), who found least local differentiation in seedling traits of I. glandulifera when compared with congeneric I. parviflora, I. capensis and I. noli-tangere under controlled climate chamber conditions.
Performance of I. glandulifera in the reciprocal field experiment was overall rather poor. Aboveground biomass in fallow meadow plots with disturbed soil treatment reached values comparable to those of a field study conducted in the Czech Republic (Skálová and Pyšek, 2009), while most other values were actually lower. SLA was comparable to values observed in a field study in England (∼370–1000 cm2 g−1; Andrews et al., 2009), slightly exceeding them in deciduous forests and slightly falling below them in fallow meadows. Plant height was at the lower margin of values reported from England (Andrews et al., 2005). In the greenhouse experiment, values of biomass and plant height were smaller than in a previous common garden experiment (Kollmann and Bañuelos, 2004).
There are several reasons why an invasive alien species may lack local adaptation. Based on the results of our study, three lines of arguments seem to be relevant. First, residence time in the new range might have been too short (Ross et al., 2009; Haider et al., 2010; Ebeling et al., 2011). Impatiens glandulifera was introduced to England as early as 1839 (Beerling and Perrins, 1993), and the first herbarium specimen from a river ∼85 km upstream from the study area dates back to the beginning of the 20th century. Other studies, however, found adaptation in annual invasive species over comparable time scales, e.g. in Eschscholzia californica, with a residence time in the invaded range of 110–150 years (Leger and Rice, 2007). Still, we cannot exclude that residence time may have been too short until now and local adaptation might evolve in future. Second, it is commonly assumed that high gene flow prevents the evolution of locally adapted genotypes (Haider et al., 2011; 2012). Impatiens glandulifera is self-compatible but protandrous and thus is frequently cross-pollinated by several species of bumblebees, honeybees and wasps (Bartomeus et al., 2010). Pollinators are capable of transferring pollen over several kilometres (Walker et al., 2009), thus enabling long-distance gene flow. Beside pollination, effective seed dispersal can increase gene flow. At the local scale, seeds of I. glandulifera are dispersed up to 6 m by exploding fruits (Chapman and Gray, 2012), but long-distance dispersal via waterways (maximum 20 km; Wadsworth et al., 2000), vehicles and contaminated soil is also common (Hartmann et al., 1995). Long-distance pollen transfer and seed dispersal suggest effective gene flow in I. glandulifera, which probably counteracts local adaptation. Third, strong spatial and temporal fluctuations in populations can act against local adaptation. Although we have no data on the persistence of I. glandulifera populations in our study area, this idea is supported by molecular studies in northeast England that suggest frequent local extinction, re-colonization and repeated anthropogenic dispersal in populations of I. glandulifera (Walker et al., 2009).
Reasons for the success of I. glandulifera in distinct habitats
Despite the observed lack of local adaptation, I. glandulifera was performing well in all studied habitats (see Supplementary Data Table S1 for plant height in the source populations). The most likely reason why the species is able to cope with distinct habitats without showing local adaptation is high phenotypic plasticity (Pigliucci, 2001), which might enable the species to expand its ecological niche (Richards et al., 2006). It has been shown recently that I. glandulifera exhibits higher plasticity in seedling biomass, height and root–shoot ratio than the less invasive congeners I. parviflora and I. capensis (Skálová et al., 2012). We found plasticity in the morphological traits SLA and height, which are particularly plastic (e.g. Flory et al., 2011; Godoy et al., 2011). SLA was larger in shaded habitats (i.e. deciduous and coniferous forests) compared with fallow meadows. Similarly, SLA increased under high shade compared with low shade in the greenhouse. Higher SLA allows plant species to better capture light under shaded conditions and thereby increases fitness (Grotkopp and Rejmánek, 2007). Plant height was comparable in the deciduous forests and fallow meadows, but lower in coniferous forests in the undisturbed soil treatment in the field experiment. In the greenhouse experiment, plants were taller under low shade and in the absence of the competing grass. Plant height is known to be linked to competitive ability, with larger species generally being able to suppress the growth of smaller species (Wang et al., 2010), which in turn is a fitness advantage. As a result, plasticity in SLA and height can generally affect fitness.
From our study we have some indication for both the jack-of-all-trades and master-of-some strategies (Richards et al., 2006). On the one hand, there are no significant fitness differences (measured as biomass) in transplanted I. glandulifera in the undisturbed soil treatment between habitats, suggesting a jack-of-all-trades strategy with high fitness in a set of distinct habitats. On the other hand, some of our results suggest a master-of-some strategy, with increased fitness under favourable conditions. In the disturbed soil treatment, fitness was higher in fallow meadow habitats compared with both forest habitats. In the greenhouse, low shade similarly led to increased biomass, particularly in the absence of the competing grass. Thus, there are indications for both good fitness in all considered habitats and increased fitness under especially favourable conditions. This suggests that I. glandulifera may follow a jack-and-master strategy (Richards et al., 2006).
Alternative factors determining the colonization sequence
As we could not detect local adaptation in I. glandulifera, this mechanism cannot explain the consecutive colonization of different habitats in the invaded range. Therefore, there must be other reasons for the colonization sequence. If I. glandulifera is capable of colonizing distinct habitats due to high phenotypic plasticity, as suggested above, the species will be able to cope with a broad range of environmental conditions. Then, the colonization sequence must be related to landscape and land use characteristics that govern propagule pressure (e.g. Lockwood et al., 2005; Colautti et al., 2006). If propagule pressure instead of adaptation to environmental conditions is the main driver of the proposed colonization sequence, then I. glandulifera will colonize further habitats in the coming decades when propagule pressure continues to increase.
We conclude that invasive alien plants can become dominant in a set of distinct habitat types in the same region without local adaptation. These species may show high degrees of phenotypic plasticity following a jack-and-master strategy. Additionally, in these species the significance of propagule pressure and land use patterns will be high.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We are grateful for valuable comments and suggestions by three anonymous referees on a previous version of the manuscript. We thank Ivonne Jüttner for technical advice in the greenhouse experiment, and Tabea Bartelt, Ingrid Kapps, Caroline von Lavergne-Peguilhen, Charlotte Mason, Juliane Meister, Thomas Wagner and staff at Dürnast Research Centre for practical assistance. Susanne Lachmuth gave useful advice for statistical analyses. This work was supported by a graduate scholarship from Universität Bayern to ATP, the Dr.-Ing. Leonhard-Lorenz-Foundation and the Faculty Graduate Center Weihenstephan of TUM Graduate School at Technische Universität München, Germany.
LITERATURE CITED
- Ammer C, Schall P, Wördehoff R, Lamatsch K, Bachmann M. Does tree seedling growth and survival require weeding of Himalayan balsam (Impatiens glandulifera)? European Journal of Forest Research. 2011;130:107–116. [Google Scholar]
- Andrews M, Maule HG, Raven JA, Mistry A. Extension growth of Impatiens glandulifera at low irradiance: importance of nitrate and potassium accumulation. Annals of Botany. 2005;95:641–648. doi: 10.1093/aob/mci059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews M, Maule HG, Hodge S, Cherrill A, Raven JA. Seed dormancy, nitrogen nutrition and shade acclimation of Impatiens glandulifera: implications for successful invasion of deciduous woodland. Plant Ecology & Diversity. 2009;2:145–153. [Google Scholar]
- Baker HG. Characteristics and modes of origin of weeds. In: Baker HG, Stebbins GL, editors. The genetics of colonizing species. New York: Academic Press; 1965. pp. 147–172. [Google Scholar]
- Bartomeus I, Vilà M, Steffan-Dewenter I. Combined effects of Impatiens glandulifera invasion and landscape structure on native plant pollination. Journal of Ecology. 2010;98:440–450. [Google Scholar]
- Bates D, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. R package version version 0·999999-0. 2012 [Google Scholar]
- Bayerische Landesanstalt für Landwirtschaft. Agrarmeteorologie Bayern. 2012 www.am.rlp.de/Internet/AM/NotesBAM.nsf/bamweb. (22 November 2012) [Google Scholar]
- Becker U, Dostal P, Jorritsma-Wienk LD, Matthies D. The spatial scale of adaptive population differentiation in a wide-spread, well-dispersed plant species. Oikos. 2008;117:1865–1873. [Google Scholar]
- Beerling DJ, Perrins JM. Impatiens glandulifera Royle (Impatiens roylei Walp.) Journal of Ecology. 1993;81:367–382. [Google Scholar]
- Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D. Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia. 2005;144:1–11. doi: 10.1007/s00442-005-0070-z. [DOI] [PubMed] [Google Scholar]
- Budoi GH, Borlan Z, Berca M, et al. Transfer functions in soil science. In: Mitchell N, Zibold G, editors. Proceedings of the 32nd Annual Meeting of ESNA. Ravensburg-Weingarten: Germany; 2003. Fachhochschule Ravensburg-Weingarten University of Applied Sciences, 51–56. [Google Scholar]
- Chapman DS, Gray A. Complex interactions between the wind and ballistic seed dispersal in Impatiens glandulifera (Royle) Journal of Ecology. 2012;100:874–883. [Google Scholar]
- Colautti RI, Grigorovich IA, MacIsaac HJ. Propagule pressure: a null model for biological invasions. Biological Invasions. 2006;8:1023–1037. [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany. 2003;51:335–380. [Google Scholar]
- Dietz H, Edwards PJ. Recognition that causal processes change during plant invasion helps explain conflicts in evidence. Ecology. 2006;87:1359–1367. doi: 10.1890/0012-9658(2006)87[1359:rtcpcd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Donohue K, Pyle EH, Messiqua D, Heschel MS, Schmitt J. Adaptive divergence in plasticity in natural populations of Impatiens capensis and its consequences for performance in novel habitats. Evolution. 2001;55:692–702. doi: 10.1554/0014-3820(2001)055[0692:adipin]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Dudley SA, Schmitt J. Genetic differentiation in morphological responses to simulated foliage shade between populations of Impatiens capensis from open and woodland sites. Functional Ecology. 1995;9:655–666. [Google Scholar]
- Ebeling SK, Stöcklin J, Hensen I, Auge H. Multiple common garden experiments suggest lack of local adaptation in an invasive ornamental plant. Journal of Plant Ecology. 2011;4:209–220. [Google Scholar]
- Erfmeier A, Böhnke M, Bruelheide H. Secondary invasion of Acer negundo: the role of phenotypic responses versus local adaptation. Biological Invasions. 2011;13:1599–1614. [Google Scholar]
- Flory SL, Long F, Clay K. Greater performance of introduced vs native range populations of Microstegium vimineum across different light environments. Basic and Applied Ecology. 2011;12:350–359. [Google Scholar]
- Garrido JL, Rey PJ, Herrera CM, Ramírez JM. Negative evidence of local adaptation to the establishment conditions in a perennial herb. Plant Ecology. 2012;213:1555–1569. [Google Scholar]
- Godoy O, Saldaña A, Fuentes N, Valladares F, Gianoli E. Forests are not immune to plant invasions: phenotypic plasticity and local adaptation allow Prunella vulgaris to colonize a temperate evergreen rainforest. Biological Invasions. 2011;13:1615–1625. [Google Scholar]
- Grøndahl E, Ehlers BK. Local adaptation to biotic factors: reciprocal transplants of four species associated with aromatic Thymus pulegioides and T. serpyllum. Journal of Ecology. 2008;96:981–992. [Google Scholar]
- Grotkopp E, Rejmánek M. High seedling relative growth rate and specific leaf area are traits of invasive species: phylogenetically independent contrasts of woody angiosperms. American Journal of Botany. 2007;94:526–532. doi: 10.3732/ajb.94.4.526. [DOI] [PubMed] [Google Scholar]
- Van Groenendael JM. Differences in life histories between two ecotypes of Plantago lanceolata L. In: White J, editor. Studies on plant demography: a Festschrift for John L. Harper. London: Academic Press; 1985. pp. 51–67. [Google Scholar]
- Haider S, Alexander J, Dietz H, Trepl L, Edwards PJ, Kueffer C. The role of bioclimatic origin, residence time and habitat context in shaping non-native plant distributions along an altitudinal gradient. Biological Invasions. 2010;12:4003–4018. [Google Scholar]
- Haider S, Alexander JM, Kueffer C. Elevational distribution limits of non-native species: combining observational and experimental evidence. Plant Ecology & Diversity. 2011;4:363–371. [Google Scholar]
- Haider S, Kueffer C, Edwards PJ, Alexander JM. Genetically based differentiation in growth of multiple non-native plant species along a steep environmental gradient. Oecologia. 2012;170:89–99. doi: 10.1007/s00442-012-2291-2. [DOI] [PubMed] [Google Scholar]
- Hartmann E, Schuldes H, Kübler R, Konold W. Neophyten: Biologie, Verbreitung und Kontrolle ausgewählter Arten. Landsberg, Germany: Ecomed; 1995. [Google Scholar]
- Hegi G. Illustrierte Flora von Mittel-Europa. München, Germany: Carl Hansen; 1925–1965. [Google Scholar]
- Hereford J. A quantitative survey of local adaptation and fitness trade-offs. American Naturalist. 2009;173:579–588. doi: 10.1086/597611. [DOI] [PubMed] [Google Scholar]
- Hereford J, Winn AA. Limits to local adaptation in six populations of the annual plant Diodia teres. New Phytologist. 2008;178:888–896. doi: 10.1111/j.1469-8137.2008.02405.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann WA, Poorter H. Avoiding bias in calculations of relative growth rate. Annals of Botany. 2002;90:37–42. doi: 10.1093/aob/mcf140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufford KM, Mazer SJ, Camara MD. Local adaptation and effects of grazing among seedlings of two native California bunchgrass species: implications for restoration. Restoration Ecology. 2008;16:59–69. [Google Scholar]
- Hothorn T, Bretz F, Westfall P, Heiberger RM. multcomp: simultaneous inference for general linear hypotheses. 2009 http://CRAN.R-project.org/package=multcomp , R package version, 1-0. [Google Scholar]
- Kawecki TJ, Ebert D. Conceptual issues in local adaptation. Ecology Letters. 2004;7:1225–1241. [Google Scholar]
- Kollmann J, Bañuelos MJ. Latitudinal trends in growth and phenology of the invasive alien plant Impatiens glandulifera (Balsaminaceae) Diversity and Distributions. 2004;10:377–385. [Google Scholar]
- Leger EA, Rice KJ. Assessing the speed and predictability of local adaptation in invasive California poppies (Eschscholzia californica) Journal of Evolutionary Biology. 2007;20:1090–1103. doi: 10.1111/j.1420-9101.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- Leimu R, Fischer M. A meta-analysis of local adaptation in plants. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0004010. e4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiss K, Müller-Schärer H. Performance of reciprocally sown populations of Senecio vulgaris from ruderal and agricultural habitats. Oecologia. 2001;128:210–216. doi: 10.1007/s004420100649. [DOI] [PubMed] [Google Scholar]
- Lockwood JL, Cassey P, Blackburn T. The role of propagule pressure in explaining species invasions. Trends in Ecology & Evolution. 2005;20:223–228. doi: 10.1016/j.tree.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Lopez S, Rousset F, Shaw FH, Shaw RG, Ronce O. Joint effects of inbreeding and local adaptation on the evolution of genetic load after fragmentation. Conservation Biology. 2009;23:1618–1627. doi: 10.1111/j.1523-1739.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- Macel M, Lawson CS, Mortimer SR, et al. Climate vs soil factors in local adaptation of two common plant species. Ecology. 2007;88:424–433. doi: 10.1890/0012-9658(2007)88[424:cvsfil]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mahmoud A, Grime JP. An analysis of competitive ability in three perennial grasses. New Phytologist. 1976;77:431–435. [Google Scholar]
- Malíková L, Prach K. Spread of alien Impatiens glandulifera along rivers invaded at different times. Ecohydrology & Hydrobiology. 2010;10:81–85. [Google Scholar]
- Maron JL, Vilà M, Bommarco R, Elmendorf S, Beardsley P. Rapid evolution of an invasive plant. Ecological Monographs. 2004;74:261–280. [Google Scholar]
- Moloney KA, Knaus F, Dietz H. Evidence for a shift in life-history strategy during the secondary phase of a plant invasion. Biological Invasions. 2009;11:625–634. [Google Scholar]
- Pigliucci M. Phenotypic plasticity: beyond nature and nurture. Baltimore: Johns Hopkins University Press; 2001. [Google Scholar]
- Pyšek P, Prach K. Invasion dynamics of Impatiens glandulifera – a century of spreading reconstructed. Biological Conservation. 1995;74:41–48. [Google Scholar]
- R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2012. [Google Scholar]
- Raabová J, Münzbergová Z, Fischer M. The role of spatial scale and soil for local adaptation in Inula hirta. Basic and Applied Ecology. 2011;12:152–160. [Google Scholar]
- Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecology Letters. 2006;9:981–993. doi: 10.1111/j.1461-0248.2006.00950.x. [DOI] [PubMed] [Google Scholar]
- Richardson DM, Pyšek P. Plant invasions: merging the concepts of species invasiveness and community invasibility. Progress in Physical Geography. 2006;30:409–431. [Google Scholar]
- Ross CA, Faust D, Auge H. Mahonia invasions in different habitats: local adaptation or general-purpose genotypes? Biological Invasions. 2009;11:441–452. [Google Scholar]
- Rouget M, Richardson DM. Inferring process from pattern in plant invasions: a semimechanistic model incorporating propagule pressure and environmental factors. American Naturalist. 2003;162:713–724. doi: 10.1086/379204. [DOI] [PubMed] [Google Scholar]
- Sax DF, Stachowicz JJ, Brown JH, et al. Ecological and evolutionary insights from species invasions. Trends in Ecology & Evolution. 2007;22:465–471. doi: 10.1016/j.tree.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, Meyer SE, Merrill KR, Anderson VJ. Local population differentiation in Bromus tectorum L. in relation to habitat-specific selection regimes. Evolutionary Ecology. 2010;24:1061–1080. [Google Scholar]
- Shipley B, Dion J. The allometry of seed production in herbaceous angiosperms. American Naturalist. 1992;139:467–483. [Google Scholar]
- Skálová H, Havlíčková V, Pyšek P. Seedling traits, plasticity and local differentiation as strategies of invasive species of Impatiens in central Europe. Annals of Botany. 2012;10:1429–1438. doi: 10.1093/aob/mcr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skálová H, Pyšek P. Germination and establishment of invasive and native Impatiens species in species-specific microsites. In: Pyšek P, Pergl J, editors. Biological invasions: towards a synthesis. Neobiota. Vol. 8. 2009. pp. 101–109. [Google Scholar]
- Sultan SE. Phenotypic plasticity for fitness components in Polygonum species of contrasting ecological breadth. Ecology. 2001;82:328–343. [Google Scholar]
- Taylor DR, Keller SR. Historical range expansion determines the phylogenetic diversity introduced during contemporary species invasion. Evolution. 2007;61:334–345. doi: 10.1111/j.1558-5646.2007.00037.x. [DOI] [PubMed] [Google Scholar]
- Thompson BK, Weiner J, Warwick SI. Size-dependent reproductive output in agricultural weeds. Canadian Journal of Botany. 1991;69:442–446. [Google Scholar]
- Wadsworth RA, Collingham YC, Willis SG, Huntley B, Hulme PE. Simulating the spread and management of alien riparian weeds: are they out of control? Journal of Applied Ecology. 2000;37:28–38. [Google Scholar]
- Walker N, Hulme PE, Hoelzel A. Population genetics of an invasive riparian species, Impatiens glandulifera. Plant Ecology. 2009;203:243–252. [Google Scholar]
- Wang P, Stieglitz T, Zhou DW, Cahill JF., Jr. Are competitive effect and response two sides of the same coin, or fundamentally different? Functional Ecology. 2010;24:196–207. [Google Scholar]
- Zybartaite L, Zukauskiene J, Jodinskiene M, Janssens SB, Paulauskas A, Kupcinskiene E. RAPD analysis of genetic diversity among Lithuanian populations of Impatiens glandulifera. Žemdirbystė-Agriculture. 2011;98:391–398. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.