Abstract
A 12-year-old hairy root culture of Cichorium intybus L., a callus culture of the plant as well as roots and leaves of a wild plant of chicory, and roots of two C. intybus L. var. sativum cultivars were examined in respect of their hydroxycinnamate and sesquiterpene lactone compositions and contents. Total phenolics and diphenylpicrylhydrazyl radical scavenging activity of the examined plant tissues were also analyzed. The most active in radical scavenging were extracts from the hairy roots and leaves of chicory. 3,5-Dicaffeoylquinic acid was the major antioxidant present in the hairy roots. Its content in the root biomass reached 5.5 %, calculated on a dry weight basis. 8-Deoxylactucin glucoside (crepidiaside A) was the major sesquiterpene lactone in the hairy roots. Its content reached 1.4 %, calculated on a dry weight basis, and was nearly two orders of magnitude higher than that in the roots of wild chicory plant. The glucosidic derivative of 8-deoxylactucin constituted over 85 % of the total sesquiterpene lactone content in the long-term cultured hairy roots of chicory. Aglycone of this compound was reported to possess anti-inflammatory activity. The qualitative and quantitative analyses of hydroxycinnamates in callus and hairy root cultures of C. intybus were undertaken for the first time.
Keywords: Chicory, Hairy roots, Hydroxycinnamates, Antioxidants, 8-Deoxylactucin glucoside
Introduction
Chicory (Cichorium intybus L., Asteraceae, tribe Cichorieae) is a wild plant species native to Europe, Western Asia, and Northern Africa. It is known as a traditional herbal remedy used to promote appetite and digestion. Commercially grown root cultivars of the plant are raw materials for production of polyfructans [1, 2]. The most characteristic secondary metabolites of C. intybus are bitter-tasting sesquiterpene lactones (mainly lactucin-like guaianolides) [3] and phenolics (hydroxycinnamates and coumarins) [4–7]. Lactucin-like guaianolides are potent anti-inflammatory agents. Of these, 8-deoxylactucin was reported to inhibit DNA binding of the transcription factor NF-κB and was identified as an inhibitor of cyclooxygenase-2 [8]. It also showed particularly high nitric oxide inhibitory activity [9]. Analgesic and sedative activities of lactucin-like guaianolides were confirmed by Wesołowska et al. [10]. Plant hydroxycinnamates are either free acids—hydroxyderivatives of cinnamic acid (e.g., caffeic, ferulic, synapic, and p-coumaric acids) or their conjugates. One of the most commonly occurring conjugates is 5-caffeoylquinic acid (5-CQA) also known as chlorogenic acid. Dicaffeoylquinic acids (DCQAs) along with tri- and tetracaffeoylquinic derivatives as well as mono- and dicaffeoyl conjugates with dicarboxylic aliphatic acids, most commonly tartaric and succinic acid, are characteristic constituents of Asteraceae plants. The compounds are well-documented radical scavengers and lipid peroxidation inhibitors [11–13]. They possess skin protecting activity against UV-induced damage [14, 15] and antiviral activity [16–18]. Moreover, caffeoylquinic acids play a role as infection and insect infestation inhibiting factors in plants [19, 20].
Hairy root cultures may provide an alternative source for production of root-derived biologically active compounds at high and stable levels [21]. In our previous study, sesquiterpene lactones were isolated from Agrobacterium rhizogenes LBA 9402 transformed hairy roots of C. intybus [22]. High activity of a hydroalcoholic extract from the hairy roots in scavenging of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical prompted us to investigate phenolic constituents of the extract. This led to the isolation of four known hydroxycinnamates, including caffeic, 5-CQA, 3,5-DCQA, and 4,5-DCQA acids, and a new neolignan glucoside—(7S, 8R)-3′-demethyl-dehydrodiconiferyl alcohol-3′-O-β-glucopyranoside [23]. In addition, the sesquiterpene lactone 8-deoxylactucin glucoside was obtained for the first time from the hairy roots of C. intybus.
The aim of the present study was to evaluate the hairy roots of chicory as a source of hydroxycinnamates and sesquiterpene lactones based on comparisons of compositions and contents of these compounds, total phenolic contents, and antiradical activities, with those of chicory of wild origin, commercial varieties of root chicory, and callus culture of the plant.
Material and Methods
Standards and Reagents
Caftaric acid (CTA, purity >97 %) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Cichoric acid (DCTA, purity >98 % by HPLC), 5-CQA (purity >97 % by HPLC) and 1,3-dicaffeoylquinic acid (cynarin, purity >99 % by HPLC) were purchased from Roth (Karlsruhe, Germany). 3,5-DCQA and the sesquiterpene lactone 8-deoxylactucin glucoside (crepidiaside A) were isolated in our laboratory from Lactuca virosa and C. intybus hairy root cultures, respectively [23, 24]. Purities of the compounds were ≥90.0 % (by HPLC). The remaining sesquiterpene lactones: 8-deoxylactucin, jacquinelin, jacquinelin glucoside (crepidiaside B), lactucin, 11β,13-dihydrolactucin, and lactucopicrin were isolated in the course of our previous studies from different Cichorium and Lactuca species [3, 25–27]. MeOH, n-hexane, and chloroform of analytical grade were purchased from POCh S.A. (Gliwice, Poland). Water was purified by a Milli-Q system (Millipore Corp., Bedford, MA, USA). MeOH and MeCN of HPLC grade as well as formic acid and glacial acetic acid were purchased from Merck (Darmstadt, Germany).
Plant Material
Leaves and roots of C. intybus L. and roots of C. intybus L. var. sativum (cultivars Dageraad and Sabau 3) were harvested during the first year of their vegetation period from plants grown in the glasshouse of the Garden of Medicinal Plants, Institute of Pharmacology, Polish Academy of Sciences (Kraków, Poland). A callus tissue and hairy roots of C. intybus were harvested from in vitro cultures maintained for over 10 years in our laboratory. The cultures were obtained and cultivated as described elsewhere [22, 28]. The hairy root culture of C. intybus, transformed by A. rhizogenes, was established from aseptic seedlings of the plant obtained from seeds of known wild origin, delivered by the Botanical Garden of Free University in Berlin (Germany). A. rhizogenes strain LBA 9402, containing agropine type Ri plasmid, pRi 1855, was used in the experiment. The hairy roots were induced on leaf explants, excised from the seedlings, and their transformed nature was proven by opine assay and rol genes detection in the plant genomic DNA. The roots were cultivated in a modified liquid MS medium [29], containing ½ strength macronutrients and 3 % sucrose, on a gyrotory shaker (110 rpm.), at 25 °C, under cool white fluorescent tubes (20 μE m−2 s−1) with a 16-h photoperiod, and were subcultured every 4 weeks by inoculating 0.7 g of roots in 250-ml Erlenmeyer flask with 40 ml of the fresh nutrient medium. A time course experiment was performed by harvesting roots 5, 10, 15, 20, and 28 days after the inoculation into the fresh medium. Fresh (FW) and dry (DW) weights of roots, as well as hydroxycinnamate and lactucin-like guaianolide contents were estimated in the harvested plant material. The experiment was done in triplicate.
Quantification of Hydroxycinnamates and Caffeic Acid
Sample Preparation
The dried and pulverized plant material (0.1 g) was extracted with 70 % MeOH (10 ml) at room temperature for 3 h on a rotary shaker (100 rpm). The mixture was filtered and the residue extracted once more with 10 ml of the fresh solvent. The extracts were combined and evaporated to dryness under reduced pressure. The dry residue was redissolved in 70 % MeOH (1 ml) and centrifuged (11,340×g, 5 min) prior to HPLC analysis.
RP-HPLC Analysis
Analytical RP-HPLC separations of the samples were performed using an Agilent 1200 Series HPLC system (Agilent Technologies, USA) equipped with a Rheodyne manual sample injector, quaternary pump, degasser, column oven and a diode array detector. Chromatographic separations of hydroxycinnamates were carried out at 25 °C, on a Zorbax Eclipse XDB-C18 column, 4.6 × 150 mm (Agilent Technologies, USA), with a mobile phase consisting of H2O/HCOOH/CH3COOH 99/0.9/0.1 (solvent A) and MeCN/MeOH/HCOOH/CH3COOH 89/10/0.9/0.1 (solvent B), at a flow rate of 1 ml min−1, using 5 μl injections. The gradient elution conditions described by Spitaler et al. [30] were applied. The analysis allowed identification of the following hydroxycinnamates present in the plant material: CTA, Rt—6.6 min; 5-CQA, Rt—7.3 min; caffeic acid, Rt—8.5 min; DCTA, Rt—20.3 min; 3,5-DCQA, Rt—28.2 min, and 4,5-DCQA, Rt—36.10 min (Fig. 1), based on their retention time values, online UV spectra and by co-chromatography with standard samples. The compounds were quantified using four-point calibration curves based on peak areas measured at 325 nm, prepared for cynarin, caffeic acid, CTA, DCTA, and 5-CQA (concentration range 0.02–1.50 mg ml−1). Moreover, two caffeic acid derivatives of unknown structures being minor constituents of the analyzed extracts could be detected in some of the samples (Rt—22.04 and 24.80 min). Their contents were calculated in reference to the chlorogenic acid calibration curve.
Fig. 1.
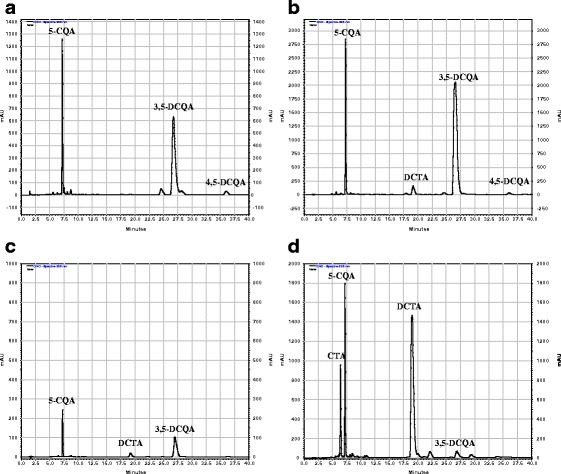
RP-HPLC chromatograms of hydroxycinnamates from hydroalcoholic extracts of Cichorium intybus (detection wavelength = 325 nm): a callus tissue, b hairy roots, c roots of the intact plant, d leaves of the intact plant. CTA caftaric acid, 5-CQA chlorogenic acid, DCTA cichoric acid, 3,5-DCQA 3,5-dicaffeoylquinic acid, 4,5-DCQA 4,5-dicaffeoylquinic acid
Quantification of Lactucin-Like Sesquiterpene Lactones
Sample Preparation
The dried, pulverized plant material (0.1 g) was extracted twice with 10 ml of MeOH at room temperature. The combined extracts were evaporated in vacuo and the residue was dissolved in 70 % MeCN (1 ml), left to stand overnight at 4 °C, centrifuged (11,340×g, 5 min) and analyzed by RP-HPLC.
RP-HPLC Separation and Quantitative Analysis
A sample (5 μl) was injected into a Purospher RP-18e (3 × 125 mm, particle size 5 μm) column (Merck, Darmstadt, Germany) which was eluted with a mobile phase consisting of water and MeCN, at a flow rate of 1 ml min−1, at 40 °C, using the aforementioned Agilent 1200 Series HPLC system. The gradient elution conditions described by Grass et al. [31] were applied. The retention time values of the analyzed compounds were as follows: 11β,13-dihydrolactucin (1), Rt—2.7 min; lactucin (2), Rt—3.5 min; 8-deoxylactucin glucoside (crepidiaside A, 3), Rt—9.0 min; 11β,13-dihydro-8-deoxylactucin glucoside (jacquinelin glucoside, crepidiaside B, 4), Rt—10.2 min; 8-deoxylactucin (5), Rt—13.5 min; 11β,13-dihydro-8-deoxylactucin (jacquinelin) (6), Rt—15.1 min; lactucopicrin/11β,13-dihydrolactucopicrin (7), Rt—26.8 min (Fig. 2). Quantification was done by measurement of peak areas at 260 nm with a reference to the standard curve derived from four concentrations (0.001 to 1.000 mg ml−1) of 8-deoxylactucin glucoside.
Fig. 2.
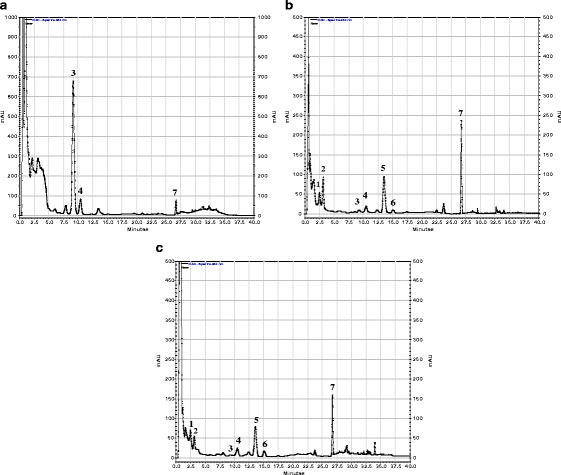
RP-HPLC chromatograms of lactucin-like guaianolides from methanolic extracts of Cichorium intybus L. (detection wavelength = 260 nm): a hairy roots, b roots of the intact plant, c leaves of the intact plant. 1 11β,13-dihydrolactucin, 2 lactucin, 3 8-deoxylactucin glucoside (crepidiaside A), 4 11β,13-dihydro-8-deoxylactucin glucoside (crepidiaside B), 5 8-deoxylactucin, 6 11β,13-dihydro-8-deoxylactucin (jacquinelin), 7 lactucopicrin/11β,13-dihydrolactucopicrin
DPPH Radical Scavenging Assay
The dried and pulverized plant material (100 mg) was extracted twice with 12.5 ml of 50 % MeOH at room temperature. The combined extracts were evaporated in vacuo. The obtained residue was dissolved in 1 ml of 70 % MeOH, left to stand overnight at 4 °C, centrifuged (11,340×g, 5 min) and the supernatant was diluted ten or a hundred times to obtain concentrations corresponding to 10 mg or 1 mg of the dry plant material per 1 ml of the sample, respectively. DPPH (Sigma-Aldrich, USA) was dissolved in methanol to obtain a stable free radical solution (100 μM). A solution (4 mM) of 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox, reference compound) was prepared by dissolving of 100 mg Trolox (Sigma-Aldrich, USA) in 100 ml of methanol. To a spectrophotometric cuvette (1 cm pathlength) containing 480 μl of the methanolic DPPH solution 20 μl of the diluted plant extract (final concentration 10 mg DW ml−1 or 1 mg DW ml−1) were added. A decrease in absorbance at λ = 517 nm was measured by UV/VIS CE 2021 spectrophotometer (Cecil, UK) after ½, 1, 2, 3, 5, 10, 15, 20, and 30 min. DPPH radical scavenging capacity was calculated according to the following equation: Q (quenching, %) = 100 (A o − A c) / A o, where A o is the absorbance of the control (in the absence of any sample) and A c is the value for added sample concentration c [32].
Estimation of Total Phenolic Content
A reducing capacity of the plant material (total phenolic content) was estimated using Folin–Ciocalteu reagent as described by Velioglu et al. [33]. Results are expressed as ferulic and gallic acid equivalents.
Results
Hydroxycinnamate and Caffeic Acid Contents
Results of hydroxycinnamate quantification in the examined plant material are presented in Table 1. Contents of the compounds in hairy roots of C. intybus were at least one order of magnitude higher than those found in roots of the intact plant and reached their maxima at the beginning of the stationary phase of the root growth. The total content of hydroxycinnamates in the biomass from the transformed root culture was also higher (7.0 % DW) than that in the roots (0.3–0.5 % DW) and leaves (3.6 % DW) of chicory. Moreover, substantial quantitative differences in the hydroxycinnamate patterns of the leaf and hairy root extracts were observed (Fig. 1). The transformed roots accumulated mainly 3,5-DCQA (5.5 % DW) whereas DCTA was the major phenolic constituent of the chicory leaves (2.5 % DW). Likewise in the hairy roots, 3,5-DCQA was the most abundant hydroxycinnamate found in the callus tissue. Unbound caffeic acid was present in the analyzed samples in minute amounts. Its content in the hairy roots of chicory reached 0.003±0.0008 % DW and was higher than that found in the roots of the intact plant (0.001 ± 0.0001 % DW). Leaves of C. intybus and the callus tissue of the plant contained up to 0.01 ± 0.0006 % DW of free caffeic acid.
Table 1.
Hydroxycinnamate contents in roots and leaves of Cichorium intybus and in vitro cultures of the plant, harvested at a stationary phase of growth (means of four measurements ± SD)
| Plant material | Hydroxycinnamate content [% dry weight] | ||||||
|---|---|---|---|---|---|---|---|
| CTA | 5-CQA | DCTA | 3,5-DCQA | 4,5-DCQA | uCA | Total | |
| C. intybus roots | – | 0.062 ± 0.001 | 0.031 ± 0.001 | 0.152 ± 0.001 | 0.005 ± 0.001 | 0.003 ± 0.001 | 0.25 ± 0.01 |
| C. intybus Dageraad roots | 0.010 ± 0.001 | 0.118 ± 0.006 | 0.092 ± 0.005 | 0.285 ± 0.011 | 0.009 ± 0.002 | 0.022 ± 0.003 | 0.54 ± 0.03 |
| C. intybus Sabau 3 roots | 0.007 ± 0.001 | 0.128 ± 0.005 | 0.060 ± 0.004 | 0.229 ± 0.023 | 0.008 ± 0.001 | 0.021 ± 0.002 | 0.45 ± 0.04 |
| C. intybus leaves | 0.388 ± 0.015 | 0.467 ± 0.002 | 2.489 ± 0.125 | 0.108 ± 0.001 | 0.016 ± 0.002 | 0.130 ± 0.010 | 3.60 ± 0.16 |
| C. intybus callus | – | 0.310 ± 0.016 | 0.014 ± 0.001 | 0.990 ± 0.086 | 0.050 ± 0.003 | 0.064 ± 0.004 | 1.43 ± 0.11 |
| C. intybus hairy roots | – | 0.935 ± 0.078 | 0.132 ± 0.016 | 5.567 ± 0.512 | 0.190 ± 0.028 | 0.185 ± 0.018 | 7.00 ± 0.65 |
| C. intybus hairy roots dark grown | 0.006 ± 0.001 | 0.875 ± 0.107 | 0.052 ± 0.008 | 4.240 ± 0.409 | 0.199 ± 0.017 | 0.200 ± 0.009 | 5.57 ± 0.55 |
CTA caftaric acid, 5-CQA chlorogenic acid, DCTA cichoric acid, 3,5-DCQA 3,5-dicaffeoylquinic acid, 4,5-DCQA 4,5-dicaffeoylquinic acid, uCA unidentified caffeic acid derivatives
Lactucin-Like Sesquiterpene Lactone Content
Contents of lactucin-like guaianolides, i.e., 11β,13-dihydrolactucin, lactucin, 8-deoxylactucin glucoside (crepidiaside A), jacquinelin glucoside (crepidiaside B), 8-deoxylactucin, jacquinelin, and lactucopicrin found in the plant material under study are shown in Table 2. The highest content of the analyzed compounds (1.6 % DW) was estimated in the hairy roots of C. intybus. The plant material contained lactucin-like guaianolides almost exclusively in their glucosylated forms (Fig. 2). 8-Deoxylactucin glucoside constituted over 85 % of the sesquiterpene lactones (1.4 % DW). The time course experiment (Table 3) revealed that 10 days after inoculation into the fresh medium, the content of 8-deoxylactucin glucoside in the hairy roots reached its maximum. Lactucopicrin, which was absent from the roots in the initial phase of culture, reached its maximum content at the stationary phase of culture. Trace levels of 8-deoxylactucin (data not shown) could be detected in the cultured roots 5 and 10 days after their transfer to the fresh medium. The compound became undetectable after 15 days of the culture cycle. The leaves and roots of the wild chicory plants contained 0.4 % DW and 0.5 % DW of the investigated sesquiterpene lactones, respectively. The roots of the two commercial varieties of chicory (Dageraad and Sabau 3) accumulated similar amounts of lactucin-like guaianolides as the roots of the wild plant (0.55–0.75 % DW). In accordance with our previous results [28], lactucin-like guaianolides characteristic of the intact plant were not detected in the callus tissue.
Table 2.
Sesquiterpene lactone (1 11β,13-dihydrolactucin; 2 lactucin; 3 8-deoxylactucin glucoside; 4 jacquinelin glucoside; 5 8-deoxylactucin; 6 jacquinelin; 7 lactucopicrin/11β,13-dihydrolactucopicrin) contents in roots and aerial parts of Cichorium intybus and in in vitro cultures of the plant harvested at a stationary phase of growth (means of four measurements ± SD)
| Plant material | Sesquiterpene lactone content [% dry weight] | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| C. intybus roots | 0.023 ± 0.015 | 0.085 ± 0.017 | 0.015 ± 0.007 | 0.028 ± 0.001 | 0.166 ± 0.006 | 0.014 ± 0.001 | 0.173 ± 0.004 |
| C. intybus Dageraad roots | 0.028 ± 0.009 | 0.159 ± 0.009 | 0.134 ± 0.028 | 0.043 ± 0.008 | 0.108 ± 0.006 | 0.012 ± 0.003 | 0.245 ± 0.011 |
| C. intybus Sabau 3 roots | 0.033 ± 0.010 | 0.150 ± 0.029 | 0.047 ± 0.011 | 0.027 ± 0.003 | 0.087 ± 0.014 | 0.012 ± 0.003 | 0.187 ± 0.002 |
| C. intybus leaves | 0.036 ± 0.004 | 0.030 ± 0.005 | 0.004 ± 0.001 | 0.036 ± 0.002 | 0.122 ± 0.010 | 0.026 ± 0.002 | 0.104 ± 0.010 |
| C. intybus callus | – | – | – | – | – | – | – |
| C. intybus hairy roots | – | – | 1.371 ± 0.321 | 0.144 ± 0.027 | – | – | 0.047 ± 0.009 |
| C. intybus hairy roots dark grown | 0.842 ± 0.050 | 0.088 ± 0.002 | 0.038 ± 0.005 | ||||
Table 3.
Time course of biomass, chlorogenic acid (5-CQA), 3,5-dicaffeoylquinic acid (3,5-DCQA), 8-deoxylactucin glucoside (3), jacquinelin glucoside (4), and lactucopicrin/11β,13-dihydrolactucopicrin (7) accumulation in hairy roots of C. intybus
| Time [days] | FW/flask [g]a | DW/flask [g]a | Secondary metabolite content [% DW]a | ||||
|---|---|---|---|---|---|---|---|
| 5-CQA | 3,5-DCQA | 3 | 4 | 7 | |||
| 5 | 1.71 ± 0.21 | 0.145 ± 0.017 | 0.448 ± 0.005 | 4.201 ± 0.145 | 1.456 ± 0.048 | 0.105 ± 0.006 | – |
| 10 | 4.02 ± 0.72 | 0.250 ± 0.047 | 0.526 ± 0.120 | 4.869 ± 0.760 | 2.502 ± 0.702 | 0.148 ± 0.015 | 0.004 ± 0.001 |
| 15 | 6.77 ± 0.49 | 0.521 ± 0.043 | 0.788 ± 0.118 | 5.454 ± 0.726 | 1.763 ± 0.225 | 0.145 ± 0.006 | 0.029 ± 0.005 |
| 20 | 10.44 ± 0.39 | 0.749 ± 0.074 | 0.945 ± 0.038 | 5.575 ± 0.509 | 1.554 ± 0.238 | 0.123 ± 0.002 | 0.032 ± 0.003 |
| 28 | 11.31 ± 0.80 | 0.731 ± 0.072 | 0.948 ± 0.107 | 5.488 ± 0.697 | 1.476 ± 0.484 | 0.142 ± 0.042 | 0.051 ± 0.001 |
aMeans of three measurements ± SD
DPPH Radical Scavenging
Changes in the DPPH solution absorbance over time, after addition of the tested plant extracts (10 mg DW ml−1) and Trolox solutions: 1.0 and 0.1 mg ml−1 (4.0 and 0.4 mM, respectively) are shown in Fig. 3. The hydroalcoholic extract from the hairy roots of chicory was the most active in this assay, followed by the extract from the leaves of the intact plant. Both extracts exhibited significantly higher activities than those from the normal roots and the callus tissue of the plant.
Fig. 3.
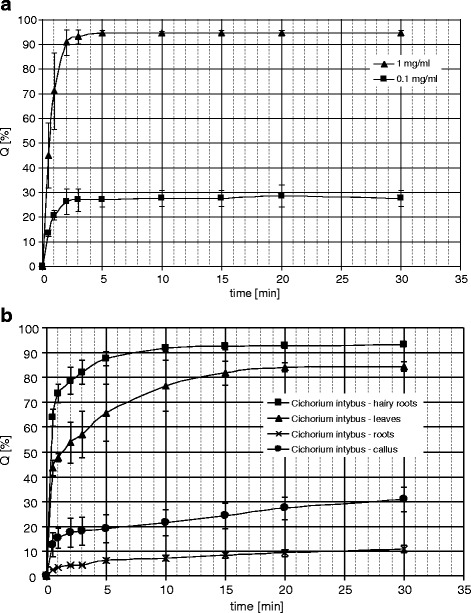
DPPH radical scavenging activity of solutions of the standard compound—Trolox (a), and hydroalcoholic extracts from Cichorium intybus (concentration—10 mg of the dry plant material per 1 ml of the extract) (b). Q [%] percentage of the DPPH reduction
Total Phenolics Content
As expected [34], total phenolics contents in the examined plant material (see Table 3) correlated with DPPH radical scavenging activities of the corresponding extracts.
Discussion
Hydroxycinnamates, flavonoids, phenolic acids, and anthocyanins (in red varieties) are considered to be responsible for high antioxidant and antiradical potential of chicory and lettuce leaves. In the present study, the hydroalcoholic extract from the hairy roots of chicory revealed unexpectedly high activity in the DPPH scavenging assay (Fig. 3). The activity of the hairy root extract was higher than that of leaves and roots of the intact plant and C. intybus callus tissue. A quantitative RP-HPLC analysis of phenolic constituents in the examined samples was performed to explain differences in their antiradical activity. Essentially similar set of signals corresponding to hydroxycinnamates was observed in every of the analyzed samples (Fig. 1). Only chromatograms of the extract from leaves of chicory showed some signals which could be ascribed to flavonoid compounds. The flavonoids (glycosides of quercetin, kaempferol, apigenin, and luteolin), which are partly responsible for antioxidant and radical scavenging activity of chicory leaves [6], were absent from both transformed roots and roots of the intact plant. A reducing capacity of the plant materials was also assessed by means of colorimetric method using Folin–Ciocalteu reagent. The total phenolic contents (Table 4) when compared with the contents of hydroxycinnamates (Table 1) clearly showed that hydroxycinnamates were responsible to high extent for antioxidant activity of the analyzed samples. According to Nicolle et al. [35], contribution of DCTA alone to the total antioxidant power of hydroalcoholic extracts from lettuce (L. sativa) aerial parts exceeds 50 %. In green varieties of chicory, DCTA was also the most efficient phenolic constituent in trapping peroxyl radicals [36]. According to our study, 3,5-DCQA was the most abundant hydroxycinnamate present in the callus and hairy root cultures of chicory. The compound also predominated in callus and hairy roots of L. virosa [24] and a callus culture of L. sativa var. crispa L. [37]. It is noteworthy that the hairy roots of chicory accumulated over two times more of 3,5-DCQA (5.5 % DW) than those of L. virosa (2.6 % DW). However, in the hairy roots of chicory, DCTA content was lower than that found in the hairy roots of lettuce. Similar differences in hydroxycinnamate pattern were observed in callus cultures of the plants. 3,5-DCQA was reported to possess hepatoprotective activity [38, 39], so it could be responsible for anti-hepatotoxic effect of a chicory callus extract described by Zafar and Ali [40]. Total content of hydroxycinnamates in the examined hairy roots of chicory was c. 20 % higher (7.0 % DW) than that found in the chicory hairy roots grown in the dark (5.6 %) and also higher than that found in the transformed roots of L. virosa (4.5 % DW). A hairy root culture of witloof chicory (C. intybus L. cv. Lucknow local) reportedly produced coumarins, esculin and esculetin [41]. The coumarins, however, were absent from the hairy roots cultivated in our laboratory.
Table 4.
Reducing capacities (total phenolic contents) of extracts from roots and leaves of Cichorium intybus and in vitro cultures of the plant harvested at a stationary phase of growth. Results, expressed as ferulic acid equivalents (FA eq) and gallic acid equivalents (GA eq), are means of four measurements (±SD)
| Plant material | Total phenolic content [mg/100 g dry weight] | |
|---|---|---|
| FA eq | GA eq | |
| C. intybus roots | 1,095 ± 85 | 774 ± 39 |
| C. intybus Dageraad roots | 1,463 ± 208 | 1,132 ± 160 |
| C. intybus Sabau 3 roots | 966 ± 20 | 748 ± 15 |
| C. intybus leaves | 5,430 ± 51 | 4,204 ± 46 |
| C. intybus callus | 3,170 ± 71 | 2,454 ± 64 |
| C. intybus hairy roots | 9,832 ± 208 | 7,611 ± 161 |
Lactucin-like guaianolides of chicory are often quantified after hydrolysis of their glycosidic (bounded) forms to corresponding aglycones. The procedure described by Tamaki et al. [42] is frequently adapted [43]. Very recently, another method has been reported by Ferioli and D'Antuono [44] employing a mixture of methanol, water, and formic acid as an extraction solvent. This mixture allows simultaneous extraction of sesquiterpene lactones and phenolic compounds but additional purification steps are necessary. In this study, we have observed that the presence of phenolics in the extract from the hairy roots resulted in difficulty with separation of some sesquiterpene lactones. As could be seen from Fig. 2a, hydroxycinnamates overproduced by the hairy roots are visible as a group of unseparated peaks at the initial part of the chromatogram. If lactucin and 11β,13-dihydrolactucin were present in the extract, their quantification would be impossible. Fortunately, both compounds were absent from the hairy root biomass as shown in our previous studies [22, 23]. Figure 2 also shows that we could directly analyze both free (aglycones) and glycosidically bound sesquiterpene lactones in the methanolic extracts from the examined plant materials by RP-HPLC/DAD. The applied gradient solvent system allowed simultaneous quantification of lactucin-like guaianolides and their 11β,13-dihydroderivatives, except for lactucopicrin and 11β,13-dihydrolactucopicrin which could not be resolved in the HPLC runs. The quantification of 8-deoxylactucin, described in our earlier paper [22], was done following the procedure by Peters and Amerongen [45] based on at least partial hydrolysis of sesquiterpene lactone glycosides before chromatographic analysis. The content of the compound in the transformed roots reached 0.35 % calculating on a dry-weight basis and was a sum of 8-deoxylactucin liberated after partial hydrolysis of its glucoside (crepidiaside A) and unbounded aglycone. In the present study, we have shown that 12-year-old hairy roots of chicory accumulated 8-deoxylactucin exclusively in its glucosylated form. In general, the transformed roots of chicory after 12 years of culture retained their capability to produce and accumulate lactucin-like guaianolides. The diversity of compounds accumulated by the hairy roots, however, remained limited in comparison to the biosynthetic potential of the intact plant [3]. The capability of roots of C. intybus intact plant to accumulate 8-deoxylactucin as the dominant sesquiterpene lactone was retained by the hairy roots of the plant, however, in the culture conditions the compound was glucosylated and stored in the cells, as no sesquiterpene lactones could be found in the culture medium. Łuczkiewicz et al. [46] reported on high content of pulchelin E in hairy roots of Rudbeckia hirta cultivated under constant light (88 ± 8 μE m−2 s−1). The sesquiterpene lactone was absent from the intact plant and from the plant tissue cultures grown in the dark. The presence of light seems to be not a prerequisite for biosynthesis of sesquiterpene lactones in hairy roots of Cichorieae plants. Transformed roots of L. virosa, grown in the dark, produced sesquiterpene lactones characteristic of the intact plant, including lactucin-like guaianolides [24]. The hairy roots of chicory maintained in the dark also accumulated sesquiterpene lactones (Table 2). The contents of the compounds, however, were lower than those found in the roots grown under the photoperiod.
Conclusions
The biomass from A. rhizogenes-transformed root culture of chicory could be an alternative source for the production of hydroxycinnamates with well-documented antioxidative, antiradical, and hepatoprotective activities. The plant material is an exceptionally rich source of 3,5-DCQA and 5-CQA acids. Moreover, the hairy roots accumulate the sesquiterpene lactone 8-deoxylactucin glycoside in high amounts. Its aglycone is known as an anti-inflammatory agent. Studies on regulatory mechanisms affecting the biosynthesis and accumulation of both phenolics and sesquiterpenes, using the investigated hairy roots as a model system, would be of interest.
Abbreviations
- 5-CQA
Chlorogenic acid
- CTA
Caftaric acid
- DCQA
Dicaffeoylquinic acid
- DCTA
Cichoric acid
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- DW
Dry weight
- FW
Fresh weight
- RP-HPLC/DAD
Reversed-phase high-performance liquid chromatography with photodiode array detection
Contributor Information
Janusz Malarz, Phone: +48-12-6623217, FAX: +48-12-6374500, Email: malarzj@if-pan.krakow.pl.
Anna Stojakowska, Email: stoja@if-pan.krakow.pl.
Wanda Kisiel, Email: kisielw@if-pan.krakow.pl.
References
- 1.Baert JRA. Industrial Crops and Products. 1997;6:195–199. doi: 10.1016/S0926-6690(97)00008-3. [DOI] [Google Scholar]
- 2.Amaducci S, Pritoni G. Industrial Crops and Products. 1998;7:345–349. doi: 10.1016/S0926-6690(97)00067-8. [DOI] [Google Scholar]
- 3.Kisiel W, Zielińska K. Phytochemistry. 2001;57:523–527. doi: 10.1016/S0031-9422(01)00072-3. [DOI] [PubMed] [Google Scholar]
- 4.Rees SB, Harborne JB. Phytochemistry. 1985;24:2225–2231. doi: 10.1016/S0031-9422(00)83015-0. [DOI] [Google Scholar]
- 5.Innocenti M, Gallori S, Giaccherini C, Ieri F, Vincieri FF, Mulinacci N. Journal of Agricultural and Food Chemistry. 2005;53:6497–6502. doi: 10.1021/jf050541d. [DOI] [PubMed] [Google Scholar]
- 6.Heimler D, Isolani L, Vignolini P, Romani A. Food Chemistry. 2009;114:765–770. doi: 10.1016/j.foodchem.2008.10.010. [DOI] [Google Scholar]
- 7.Jaiswal R, Kiprotich J, Kuhnert N. Phytochemistry. 2011;72:781–790. doi: 10.1016/j.phytochem.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Cavin C, Delannoy M, Malnoe A, Debefve E, Touche A, Courtois D, et al. Biochemical and Biophysical Research Communications. 2005;327:742–749. doi: 10.1016/j.bbrc.2004.12.061. [DOI] [PubMed] [Google Scholar]
- 9.Ripoll C, Schmidt BM, Ilic N, Poulev A, Dey M, Kurmukov AG, et al. Natural Product Communications. 2007;2:717–722. [Google Scholar]
- 10.Wesołowska A, Nikiforuk A, Michalska K, Kisiel W, Chojnacka-Wójcik E. Journal of Ethnopharmacology. 2006;107:254–258. doi: 10.1016/j.jep.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Ohnishi M, Morishita H, Iwahashi H, Toda S, Shirataki Y, Kimura M, et al. Phytochemistry. 1994;36:579–583. doi: 10.1016/S0031-9422(00)89778-2. [DOI] [Google Scholar]
- 12.Kim HJ, Lee YS. Planta Medica. 2005;71:871–876. doi: 10.1055/s-2005-873115. [DOI] [PubMed] [Google Scholar]
- 13.Olmos A, Giner RM, Recio MC, Ríos JL, Gil-Benso R, Máñez S. Archives of Biochemistry and Biophysics. 2008;475:66–71. doi: 10.1016/j.abb.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Maffei Facino R, Carini M, Aldini G, Saibene L, Pietta P, Mauri P. Planta Medica. 1995;61:510–514. doi: 10.1055/s-2006-959359. [DOI] [PubMed] [Google Scholar]
- 15.Kitagawa S, Yoshii K, Morita SY, Teraoka R. Chemical and Pharmaceutical Bulletin. 2011;59:793–796. doi: 10.1248/cpb.59.793. [DOI] [PubMed] [Google Scholar]
- 16.Robinson WE, Jr, Reinecke MG, Abdel-Malek S, Jia Q, Chow SA. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6326–6331. doi: 10.1073/pnas.93.13.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDougall B, King PJ, Wu BW, Hostomsky Z, Reinecke MG, Robinson WE., Jr Antimicrobial Agents and Chemotherapy. 1998;42:140–146. doi: 10.1128/aac.42.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KH, Kim YH, Lee KR. Bioorganic & Medicinal Chemistry Letters. 2007;17:6739–6743. doi: 10.1016/j.bmcl.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 19.Kodoma M, Wada H, Otani H, Kohmoto K, Kimura Y. Phytochemistry. 1998;47:371–373. doi: 10.1016/S0031-9422(97)00621-3. [DOI] [Google Scholar]
- 20.Leiss KA, Maltese F, Choi YH, Verpoorte R, Klinkhamer PGL. Plant Physiology. 2009;150:1567–1575. doi: 10.1104/pp.109.138131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono NN, Tian L. Plant Science. 2011;180:439–446. doi: 10.1016/j.plantsci.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Malarz J, Stojakowska A, Kisiel W. Zeitschrift fur Naturforschung. 2002;57c:994–997. doi: 10.1515/znc-2002-11-1207. [DOI] [PubMed] [Google Scholar]
- 23.Malarz J, Stojakowska A, Szneler E, Kisiel W. Phytochemistry Letters. 2013;6:59–61. doi: 10.1016/j.phytol.2012.10.011. [DOI] [Google Scholar]
- 24.Stojakowska A, Malarz J, Szewczyk A, Kisiel W. Acta Physiologiae Plantarum. 2012;34:291–298. doi: 10.1007/s11738-011-0827-4. [DOI] [Google Scholar]
- 25.Kisiel W, Gromek D. Phytochemistry. 1993;34:1644–1646. doi: 10.1016/S0031-9422(00)90864-1. [DOI] [Google Scholar]
- 26.Kisiel W, Barszcz B, Szneler E. Phytochemistry. 1997;45:365–368. doi: 10.1016/S0031-9422(96)00820-5. [DOI] [Google Scholar]
- 27.Kisiel W, Michalska K. Fitoterapia. 2008;71:86–87. doi: 10.1016/S0367-326X(99)00112-4. [DOI] [Google Scholar]
- 28.Malarz J, Stojakowska A, Szneler E, Kisiel W. Plant Cell Reports. 2005;24:246–249. doi: 10.1007/s00299-005-0953-9. [DOI] [PubMed] [Google Scholar]
- 29.Murashige T, Skoog F. Physiologia Plantarum. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 30.Spitaler R, Schlorhaufer PD, Ellmerer EP, Merfort I, Bortenschleger S, Stuppner H, et al. Phytochemistry. 2006;67:409–417. doi: 10.1016/j.phytochem.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Grass S, Zidorn C, Blattner FR, Stuppner H. Phytochemistry. 2006;67:122–131. doi: 10.1016/j.phytochem.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Molyneux P. Songklanakarin J Science and Technology. 2004;26:211–219. [Google Scholar]
- 33.Velioglu YS, Mazza G, Gao L, Oomah BD. Journal of Agricultural and Food Chemistry. 1998;46:4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- 34.Huang D, Ou B, Prior RL. Journal of Agricultural and Food Chemistry. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 35.Nicolle C, Carnat A, Fraisse D, Lamaison JL, Rock E, Michel H, et al. Journal of the Science of Food and Agriculture. 2004;84:2061–2069. doi: 10.1002/jsfa.1916. [DOI] [Google Scholar]
- 36.Rossetto M, Lante A, Vanzani P, Spettoli P, Scarpa M, Rigo A. Journal of Agricultural and Food Chemistry. 2005;53:8169–8175. doi: 10.1021/jf051116n. [DOI] [PubMed] [Google Scholar]
- 37.Tamura H, Akioka T, Ueno K, Chujyo T, Okazaki KI, King PJ, et al. Molecular Nutrition & Food Research. 2006;50:396–400. doi: 10.1002/mnfr.200500216. [DOI] [PubMed] [Google Scholar]
- 38.Basnet P, Matsushige K, Hase K, Kadota S, Namba T. Biological and Pharmaceutical Bulletin. 1996;19:1479–1484. doi: 10.1248/bpb.19.1479. [DOI] [PubMed] [Google Scholar]
- 39.An RB, Sohn DH, Jeong GS, Kim YC. Archives of Pharmacal Research. 2008;31:594–597. doi: 10.1007/s12272-001-1198-1. [DOI] [PubMed] [Google Scholar]
- 40.Zafar R, Ali SM. Journal of Ethnopharmacology. 1998;63:227–231. doi: 10.1016/S0378-8741(98)00087-7. [DOI] [PubMed] [Google Scholar]
- 41.Bais HP, Sudha G, Ravishankar GA. Journal of Plant Growth Regulation. 1999;18:159–165. doi: 10.1007/PL00007064. [DOI] [PubMed] [Google Scholar]
- 42.Tamaki H, Robinson RW, Anderson JL, Stoewsand GS. Journal of Agricultural and Food Chemistry. 1995;43:6–8. doi: 10.1021/jf00049a002. [DOI] [Google Scholar]
- 43.Foster JG, Clapham WM, Belesky DP, Labreveux M, Hall MH, Sanderson MA. Journal of Agricultural and Food Chemistry. 2006;54:1772–1778. doi: 10.1021/jf052546g. [DOI] [PubMed] [Google Scholar]
- 44.Ferioli F, D'Antuono LF. Food Chemistry. 2012;135:243–250. doi: 10.1016/j.foodchem.2012.04.079. [DOI] [Google Scholar]
- 45.Peters AM, van Amerongen A. Z. Lebensmittel Untersuch Forschung A. 1997;204:189–193. doi: 10.1007/s002170050060. [DOI] [Google Scholar]
- 46.Łuczkiewicz M, Zárate R, Dembińska-Migas W, Migas P, Verpoorte R. Plant Science. 2002;163:91–100. doi: 10.1016/S0168-9452(02)00065-1. [DOI] [Google Scholar]


