Abstract
Since contradictory findings have been reported on potential effects of statins in modulating the inflammatory response, we have analysed the biological activity of lovastatin both in vitro using the Raw 264.7 murine macrophagic cell line and in vivo using BALB/c mice. When added to Raw 264.7 cells in combination with lipopolysaccharide, lovastatin significantly potentiated the release of interleukin-1β, interleukin-6 and interleukin-12 with respect to lipopolysaccharide alone and showed an additive effect on the release of nitric oxide. Similarly, when lovastatin was intraperitoneally administrated to BALB/c mice, it did not induce any pro-inflammatory effect when used alone, but it significantly potentiated the pro-inflammatory activity of lipopolysaccharide, in terms of number of intraperitoneal cells and serum levels of serum amyloid A, interleukin-1β, interleukin-6 and interleukin-12. A potential clinical implication of our study is that lovastatin might exert a pro-inflammatory activity in subjects affected by inflammatory processes, with clinically evident or subclinical infections.
Keywords: Statin, Cytokines, Inflammation, Cholesterol
Introduction
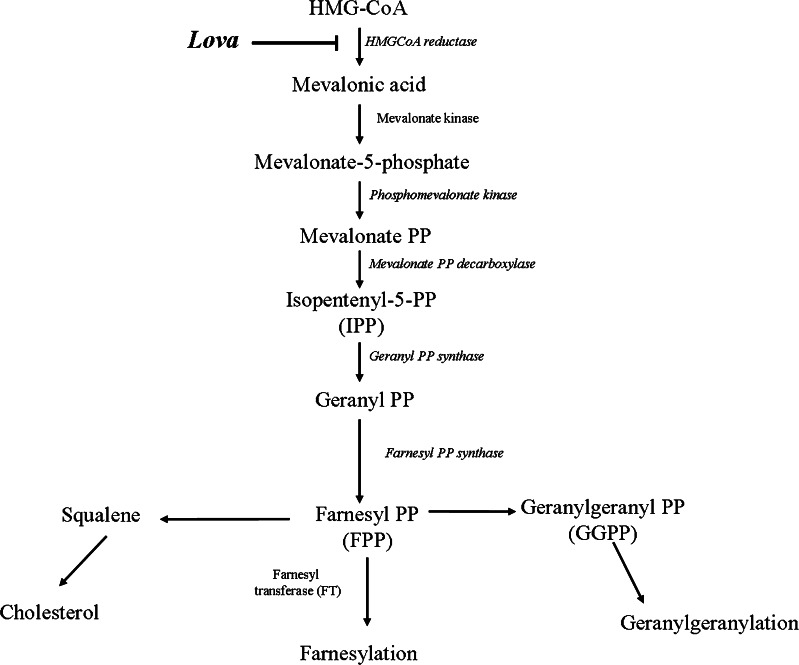
Statins are 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA) inhibitors, which act on the rate-limiting step of the cholesterol pathway in which HMG-CoA is converted to mevalonate [1] (Fig. 1). In clinical practice, statins represent the elective treatment for the reduction of cholesterol blood levels in hyperlipidemic patients [2]. In the last years, numerous pleiotropic properties of statins have been described, beyond their well-known lipid lowering function, such as the improvement of the endothelial function, the capacity to maintain plaque stability and the ability to prevent thrombus formation [3]. These pleiotropic effects are of clinical relevance and may largely depend on the individual patient response [4]. In addition, statins trigger apoptosis in a variety of tumour cells in vitro [5], and this pro-apoptotic activity has been ascribed to the depletion of geranylgeranyl pyrophosphate and, thus, to the consequent inhibition of protein prenylation [6], a biological activity which might have impact on the inflammatory response of these cells. Other groups of investigators reported anti-inflammatory properties of statins, such as the downregulation of leukocyte functional antigen and the inhibition of the release of pro-inflammatory cytokines [7]. Nevertheless, this last issue is controversial since other studies have shown that, under certain circumstances, statins may induce a strong pro-inflammatory response [8–10]. It should be noticed, however, that different drug concentrations and models used to test the effect of statins produced quite discordant results consistent with these conflicting data on the interplay between statins and inflammation, and patients in treatment with statins sometimes showed adverse reactions [11].
Fig. 1.
Mevalonate pathway. Schematic representation of mevalonate pathway. Compounds used in the experiments are indicated along the pathway in bold characters: Lova lovastatin
On these bases, the objective of our study was to investigate both in vitro and in vivo the effect of statins in a well-established cellular model (Raw 264.7), and in an animal model (BALB/c mice) of LPS-induced systemic inflammation.
Materials and Methods
Reagents
For in vitro and in vivo treatments, the following reagents were used: lipopolysaccharide (LPS; Escherichia coli-serotype 055:B5) and lovastatin-LPS free (Lova), both purchased from Sigma-Aldrich (St. Louis, MO, USA).
In Vitro
The murine macrophagic cell line Raw 264.7 was obtained from Sigma-Aldrich (St Louis, MO, USA). Cells were routinely cultured in DMEM + 10 % FBS and exposed for 48 h to Lova (at concentrations ranging between 0.1 and 80 μM), and consequently analysed for cell viability and evaluation of the levels of cytokines released in the culture supernatants. In additional experiments, after 24 h of treatment with Lova, cells were exposed to LPS (10 μg/mL) for additional 24 h. Time and dose of LPS were decided according to previous studies conducted in our laboratories [12, 13].
Evaluation of Cell Viability
The cytotoxicity of the treatments with Lova, used either alone or in combination with LPS, was monitored by double staining with annexin V-FITC and propidium iodide (Apoptosis Detection Kit, Immunostep, Salamanca, Spain) and flow cytometric analysis, according to manufacturer’s instructions. Fluorescence was acquired with a FACScan Cytometer and CellQuest software (Becton Dickinson, Franklin Lakes, NJ, USA) and then analysed with FlowJo software (version7.6, TreeStar Inc., Ashland, OR, USA). This technique was used to assess the effects of treatments on cells viability. Debris were excluded from the plot based on the scatter (FSC vs. SSC), and apoptotic (annexin V positive (A+), propidium iodide negative (PI−) and positive (PI+)) and necrotic (annexin V negative (A−) and propidium iodide positive (PI+)) cells were characterized based on the fluorescence emitted.
Biochemical Measurements on Raw 264.7 Cell Cultures
Nitric oxide production was assayed on supernatants using Griess Reagent (Sigma-Aldrich). Levels of free cholesterol in total cell lysates were determined with the Amplex red cholesterol assay kit (Molecular Probes, Invitrogen, Carlsbad, CA, USA). The assays were performed following manufacturers’ indications.
In Vivo
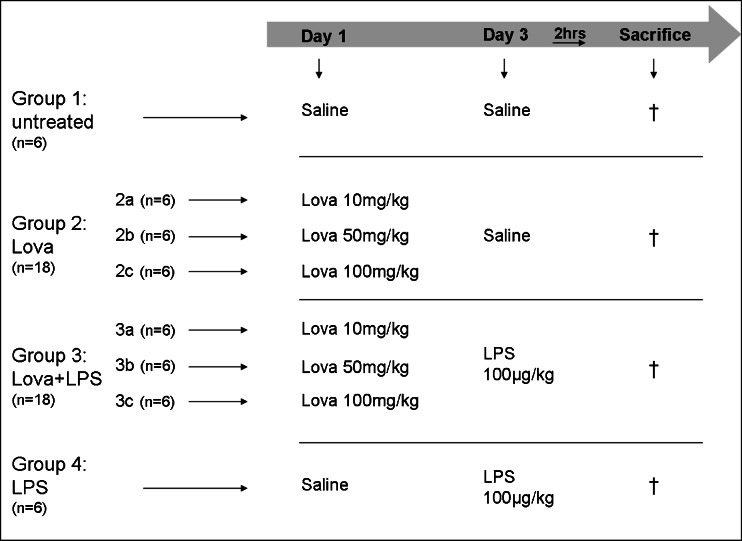
BALB/c male mice (Harlan, Udine, Italy) aged 6–8 weeks and weighing between 25 and 30 g, were used for this study. Mice were housed in standard cages with a 12-h light/dark cycle, with free access to tap water and pellet food. Environmental temperature was constantly maintained at 21 °C and the mice were kept under pathogen-free conditions. Experiments were carried out in accordance with institutional guidelines in compliance with international and Italians laws (EEC Council Directive 86/609, OJL 358, December 1987 and Italian Ministry of Health registration number 62/2000-B, October 6, 2000), upon approval by institutional ethical Committee (permit number 7–2.10.2006). Briefly, mice were randomly divided in groups of six animals each: groups 1, controls (saline); 2, Lova (10–50–100 mg/kg) on day 1; 3, Lova (10–50–100 mg/kg) on day 1 and LPS 100 μg/kg on day 3; 4, LPS 100 μg/kg on day 3 (Fig. 2). All the solutions were administered by intraperitoneal (i.p.) route. After 2 h of LPS stimulation, blood was collected directly into test tubes. Dose and time of LPS treatment were decided according to the literature and to previous studies conducted in our laboratory [14, 15]. Serum was recovered by centrifugation at 2,000×g at 4 °C and then stored at −80 °C until biochemical analysis. Moreover, immediately after the mice were killed, 2 mL of PBS + 0.1 % BSA were injected into the peritoneal cavity, and the cavity was massaged for 4 min. The i.p. fluid was recovered using a syringe and the number of cells was counted by using a Bürker chamber.
Fig. 2.
In vivo experimental design. Forty-eight male Balb/c were divided into the experimental groups. Untreated: saline on days 1 and 3 (group 1, n = 6); Lova: lovastatin on day 1—10 (group 2a, n = 6), 50 (group 2b, n = 6) and 100 mg/kg (group 2c, n = 6) and saline on day 3; Lova + LPS: lovastatin on day 1—10 (group 3a, n = 6), 50 (group 3b, n = 6) and 100 mg/kg (group 3c, n = 6) and LPS—100 μg/kg on day 3; LPS: saline on day 1 and LPS—100 μg/kg on day 3 (group 4, n = 6). All compounds have been injected via intraperitoneal route
Biochemical Measurements on BALB/c Mouse Sera
The serum amyloid A (SAA) was assayed using a specific ELISA kit (Biosource, Camarillo, CA). Serum total cholesterol was analysed by an enzymatic colorimetric method (Chol-DAP; Roche Diagnostic, GmbH, Mannheim, Germany). All samples were assessed in duplicate and according to the manufacturers’ instructions.
Measurement of Cytokines in Cell Culture Supernatants and in Mice Sera
Cytokine levels were measured both in Raw 264.7 cell culture supernatants and in BALB/c mouse serum samples by performing a bead-based multiplex immunoassay (23 mouse-Bio-Plex assay; BioRad Laboratories, Milan, Italy), following manufacturer’s instructions. Samples were assessed in duplicate, using the Bio-Plex 200 reader (Bio-Rad, Hercules, CA, USA) equipped with the Bioplex Manager software, using a five-parameter nonlinear regression formula to compute sample concentrations from standard curves.
Statistical Analyses
Results are expressed as the mean ± standard deviation (SD). Statistical significance was calculated using one-way analysis of variance (ANOVA), followed by the Bonferroni multiple comparison test. Statistical analysis was performed using GraphPad Prism software, version 5 (GraphPad Software, San Diego, CA). Statistical significance was defined as p < 0.05.
Results
Lova Inhibits Cholesterol Synthesis in Raw 264.7 Macrophagic Cells in a Range of Concentrations, Showing Limited In Vitro Cytotoxicity
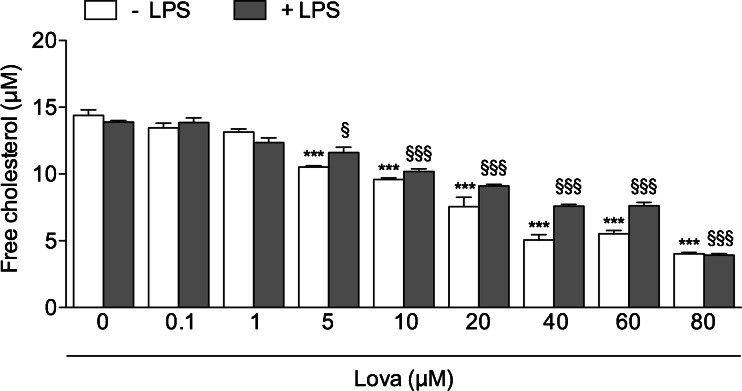
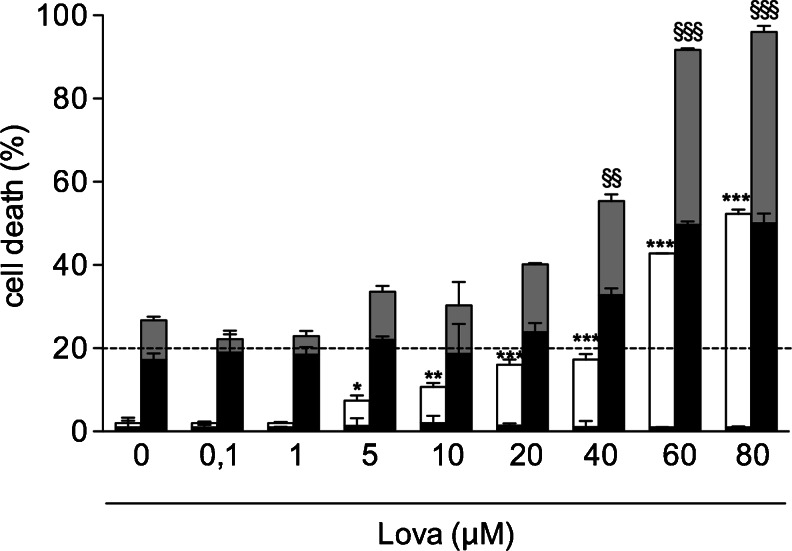
The effect of the exposure to a wide range of Lova concentrations (0.1–1–5–10–20–40–60–80 μM) was initially tested in the Raw 264.7 murine macrophage cell line to find the optimal concentrations able to significantly decrease the levels of free cholesterol, which represents the major therapeutic target of statins. As shown in Fig. 3, Lova induced a significant decrease in free cholesterol, starting from concentrations of 5 μM onwards. For the purpose of this study, it is noteworthy that the ability of Lova to lower the level of free cholesterol was observed also when Raw 264.7 cells were simultaneously treated with Lova plus LPS (Fig. 3). Conversely, significant cytotoxic effects of Lova, evaluated in terms of both apoptosis and necrosis over the background level, were observed only at the highest concentrations used (60–80 μM) (Fig. 4). Moreover, LPS maintained the effects of lovastatin on the cellular cytotoxicity consistent, even if at higher levels, compared with lovastatin treatment alone. Indeed, the two groups follow the same increasing trend at all the different concentrations (Fig. 4). Therefore, most of the following experiments were carried out using a range of Lova concentrations (5–10–20–40 μM), which significantly inhibited cholesterol synthesis with minimal (<20 %) cytotoxicity in Raw 264.7 cells.
Fig. 3.
Cholesterol levels after lovastatin treatment on Raw 264.7-cells. Raw 264.7 cells (murine monocyte/macrophage cell line) were incubated with (0/0.1/1/5/10/20/40/60/80 μM) lovastatin for 20 h alone or with the addition of 10 μg/mL LPS for further 24 h. The bars represent the means of three independent experiments ± SD. Analyses were performed with one-way ANOVA and Bonferroni post-test. *p < 0.05; **p < 0.01; ***p < 0.001 vs. 0 μM Lova (white bars); §p < 0.05; §§p < 0.01, §§§p < 0.001 vs. LPS + 0 μM Lova (grey bars)
Fig. 4.
Apoptosis and necrosis in Raw 264.7 lovastatin-treated cells. Raw 264.7 cells (murine monocyte/macrophage cell line) were incubated with (0/0.1/1/5/10/20/40/60/80 μM) lovastatin for 20 h alone or with the addition of 10 μg/mL LPS for further 24 h. Apoptosis and necrosis were measured as reported in the “Materials and Methods”. The bars represent the means of three independent experiments ± SD. Percentage of necrotic cells are represented by the black portion of each bar. Analyses were performed with one-way ANOVA and Bonferroni post-test. *p < 0.05; **p < 0.01; ***p < 0.001 vs. 0 μM Lova (white bars); §p < 0.05; §§p < 0.01; §§§p < 0.001 vs. LPS + 0 μM Lova (grey bars)
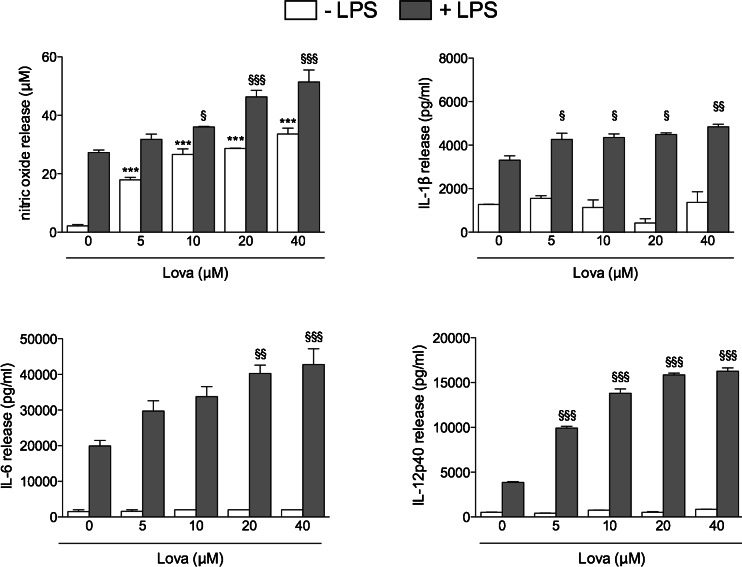
Lova Alone Induces Nitric Oxide Release But Does Not Affect Pro-inflammatory Cytokines, While It Potentiates the LPS-Mediated Release of Nitric Oxide and of IL-6, IL-12p40 and IL-1β by Raw 264.7 Cells
To evaluate the effect of lovastatin on inflammatory mediators, we evaluated the effect of Lova used alone or in association with LPS on several markers of inflammation, such as nitric oxide (NO) production, and on the secretion of IL-6, IL-12p40 and IL-1β. Lova alone dose-dependently increased NO production by Raw 264.7 cells (p < 0.05), whereas it showed negligible effects on the release of pro-inflammatory cytokines at any concentration investigated (Fig. 5). When used in association with LPS, Lova significantly (p < 0.05) and dose-dependently potentiated the ability of LPS to promote the release not only of nitric oxide, but also of all the pro-inflammatory cytokines investigated (Fig. 5).
Fig. 5.
Inflammation marker in Raw 264.7-treated cells. Cells were incubated with (0/5/10/20/40 μM) lovastatin for 20 h alone or with the addition of 10 μg/mL LPS for supplementary 24 h. Bars represent the mean values of NO (in micromolars), IL-1β, IL-6 and IL-12p40 (pg/ml) of three independent experiments ± SD. Analyses were performed with one-way ANOVA and Bonferroni post-test. *p < 0.05; **p < 0.01; ***p < 0.001 vs. 0 μM Lova (white bars); §p < 0.05; §§p < 0.01; §§§p < 0.001 vs. LPS + 0 μM Lova (grey bars)
Lova Enhances the Pro-inflammatory Activity of LPS Also In Vivo, in BALB/c Mice
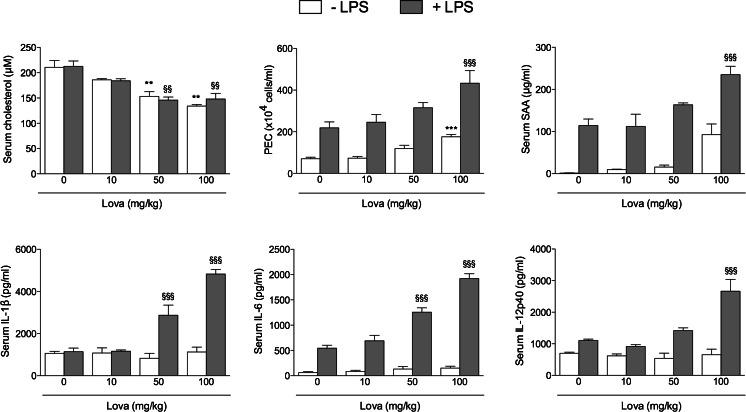
In the next group of experiments, we sought to investigate whether the data obtained in vitro on the Raw 264.7 cell model could have a correspondence in a more relevant in vivo inflammation model. For this purpose, BALB/c mice were treated for three days with Lova, LPS or with a combination of Lova + LPS. As expected, the range of Lova concentrations used in these in vivo experiments dose-dependently lowered the amount of free serum cholesterol, and the simultaneous addition of LPS did not hamper the effect of Lova (Lova vs. Lova + LPS p > 0.05 at all concentrations) (Fig. 6). As markers of inflammation, we analysed the number of cells in the peritoneal exudate on the third day, a time required to obtain the maximum increase of inflammation. In parallel, we measured the serum levels of SAA, IL-6, IL-12p40 and IL-1β. As expected, both the number of peritoneal cells and the serum markers of inflammation significantly increased in response to i.p. injection of LPS (Fig. 6). Although Lova alone did not significantly modulate the levels of any inflammatory marker, Lova + LPS showed a greater induction of SAA as compared with LPS alone. Finally, all the three cytokines with higher levels in the culture supernatants of Raw 264.7 cells (IL-1β, IL-6 and IL-12p40) also had increased levels in the serum of mice treated with LPS alone. Again, the combination of Lova + LPS was superior in increasing the circulating levels of all cytokines if compared to LPS alone (Fig. 6).
Fig. 6.
Lovastatin improves the inflammation markers in BALB/c mice. BALB/c mice were randomly divided in groups of six animals as reported in “Materials and Methods”. Values of cholesterol concentration (in micromolars), PEC (×104cells/mL), and SAA (in microgrammes per millilitre), IL-1β, IL-6, IL-12p40 (in picogrammes per millilitre) are reported as means of three independent experiments ± SD. Analysis was carried out with one-way ANOVA and Bonferroni post-test. *p < 0.05; **p < 0.01; ***p < 0.001 vs. mice 0 mg/kg Lova (white bars); §p < 0.05; §§p < 0.01; §§§p < 0.001 vs. LPS (100 μg/kg) + 0 mg/kg Lova (grey bars)
Discussion
Statins are a class of hyperlipidemic blockbuster drugs routinely used for reducing serum cholesterol in the treatment and prevention of hypercholesterolemia and other cardiovascular conditions [2]. Beyond this main effect, several other effects of statins have been described. In particular a controversial role of statins in modulating the inflammatory response has been reported [7–10]. In this study, we first established a range of concentrations for which Lova was able to lower the production of free cholesterol in Raw 264.7 cells, whereas promoting minimal cytotoxicity (5 to 40 μM). When used alone, Lova promoted the release of nitric oxide by Raw 264.7 cells but it did not significantly induce cytokine release. When associated with LPS, Lova showed a synergistic effect on the release of IL-6, IL-12p40 and IL-1β and an additive effect on the release of nitric oxide in the culture supernatants.
Using the same experimental conditions described in a previous paper by our group [16] with BALB/c male mice [17], the combination of LPS + Lova increased different markers of inflammation, such as SAA and PEC in vivo, and increased the serum levels of the same cytokines (IL-6, IL-12p40 and IL-1β) found to be increased in the culture supernatants of Raw 264.7 cells. Concerning the potential mechanism implicated in the ability of lovastatin to promote nitric oxide release, previous studies have shown that statins up-regulate the expression of inducible NOS inhibiting small G proteins of the Rho family in various cell types [18, 19]. In addition, inhibition of isoprenoid production by statins has been shown to increase NOS expression and activity in cultures, while the restoration of the mevalonate pathway with exogenous isoprenoids leads to a decrease in NO production [13].
With respect to the ability of lovastatin to potentiate LPS in promoting the release of IL-6, our current data are in contrast with a previous study describing the ability of statins to reduce the amount of IL-6 [20]. Even though we are not able at present to explain these discrepancies, it is of interest to note that IL-6 seems to be up-regulated in the liver and it could be suggested that an increased production of this cytokine might contribute to explain the liver dysfunction [21] and the deep vein inflammation [22], which can occur in patients under prolonged statin therapy. With respect to the increase of IL-12p40 observed both in vitro and in vivo in response to the Lova + LPS combination, it is remarkable that IL-12 is a key cytokine involved in the inflammatory process [23, 24], and results higher in patients with heart failure. Thus, the IL-12 increase might contribute to explain the major adverse reaction to statins, represented by rhabdomyolysis [25]. Our data on IL-12p40 production in response to Lova + LPS are in line with the data of other authors [26] which attributed the production of pro-inflammatory cytokines (IL-12p40 and IL-6) to the statin-mediated repression/activation of proto-oncogene c-Fos/c-Jun respectively, whose repression induce an increase of pro-inflammatory cytokine production. Moreover, in the same study, Matsumoto et al. have highlighted the role of binding site of IL-12p40 promoter (Ap-1) to increase the cytokine production simvastatin–mediated [26].
The last cytokine analysed, IL-1β, is an important protein that mediates many of the damaging consequences of inflammatory process synergies with other pro-inflammatory cytokines such as IL-6 and IL-12, allowing the adaptive immune response to feed-back and amplify innate immunity [27]. In this respect, it has been shown that pharmacological inhibitors of the mevalonate pathway activate pro-IL-1β processing and IL-1β release by human monocytes [28].
Statins act on the first enzyme of the mevalonate pathway, leading to a decrease of intracellular cholesterol and lack of mevalonate-derived isoprenoids. By inhibiting the biosynthesis pathway of isoprenoids, the levels of several regulatory proteins, essential for the prenylation, decrease. Since in vitro studies [29] reported the ability of natural isoprenoid compounds to induce anti-inflammatory effects when the mevalonate pathway is blocked, it will be interesting to investigate in future studies the role of exogenous isoprenoids in restoring the mevalonate pathway in the presence of statins.
At present no theories have been proposed about the pro-inflammatory mechanism generated by statins. Our aim was to identify the role of isoprenoids in this mechanism, as the lack of these compounds contributes to a higher susceptibility to the inflammatory phenotype.
As controversial data are present in the literature concerning the safety of statin therapy in septic patients [30, 31], our current data add a cautionary note on the use of lovastatin in septic experimental conditions, both in vitro and in vivo, owing to the potential increase of the pro-inflammatory activity of statins in the presence of high levels of endotoxin. In conclusion, we describe evidences for a dose-dependent pro-inflammatory role of Lova in association with LPS on cellular and animal models. Further experiments, even if preliminary, using simvastatin and atorvastatin are currently ongoing with promising results.
We believe such evidence might be useful in clinical practice, for the use of statins in patients affected by clinically evident or subclinical infections.
Acknowledgements
This study was supported by a grant from the Institute for Maternal and Child Health-IRCCS “Burlo Garofolo”-Trieste, Italy (RC 42/2011).
Footnotes
Valentina Zanin, Annalisa Marcuzzi. The first two authors equally contributed to this study.
References
- 1.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 2.Biasucci LM, Biasillo G, Stefanelli A. Inflammatory markers, cholesterol and statins: pathophysiological role and clinical importance. Clinical Chemistry and Laboratory Medicine: CCLM/FESCC. 2010;48(12):1685–1691. doi: 10.1515/CCLM.2010.277. [DOI] [PubMed] [Google Scholar]
- 3.Ray KK, Cannon CP. The potential relevance of the multiple lipid-independent (pleiotropic) effects of statins in the management of acute coronary syndromes. Journal of the American College of Cardiology. 2005;46(8):1425–1433. doi: 10.1016/j.jacc.2005.05.086. [DOI] [PubMed] [Google Scholar]
- 4.Endres M. Statins: potential new indications in inflammatory conditions. Atherosclerosis Supplements. 2006;7(1):31–35. doi: 10.1016/j.atherosclerosissup.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Paoletti R, Corsini A, Bellosta S. Pharmacological interactions of statins. Atherosclerosis Supplements. 2002;3(1):35–40. doi: 10.1016/S1567-5688(02)00002-8. [DOI] [PubMed] [Google Scholar]
- 6.Cordle A, Koenigsknecht-Talboo J, Wilkinson B, Limpert A, Landreth G. Mechanisms of statin-mediated inhibition of small G-protein function. The Journal of Biological Chemistry. 2005;280(40):34202–34209. doi: 10.1074/jbc.M505268200. [DOI] [PubMed] [Google Scholar]
- 7.Ehrenstein MR, Jury EC, Mauri C. Statins for atherosclerosis—as good as it gets? The New England Journal of Medicine. 2005;352(1):73–75. doi: 10.1056/NEJMe048326. [DOI] [PubMed] [Google Scholar]
- 8.Kuijk LM, Mandey SH, Schellens I, et al. Statin synergizes with LPS to induce IL-1beta release by THP-1 cells through activation of caspase-1. Molecular Immunology. 2008;45(8):2158–2165. doi: 10.1016/j.molimm.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Coward WR, Marei A, Yang A, Vasa-Nicotera MM, Chow SC. Statin-induced proinflammatory response in mitogen-activated peripheral blood mononuclear cells through the activation of caspase-1 and IL-18 secretion in monocytes. Journal of Immunology. 2006;176(9):5284–5292. doi: 10.4049/jimmunol.176.9.5284. [DOI] [PubMed] [Google Scholar]
- 10.Monick MM, Powers LS, Butler NS, Hunninghake GW. Inhibition of Rho family GTPases results in increased TNF-alpha production after lipopolysaccharide exposure. Journal of Immunology. 2003;171(5):2625–2630. doi: 10.4049/jimmunol.171.5.2625. [DOI] [PubMed] [Google Scholar]
- 11.Bellosta S, Corsini A. Statin drug interactions and related adverse reactions. Expert Opinion on Drug Safety. 2012;11(6):933–946. doi: 10.1517/14740338.2012.712959. [DOI] [PubMed] [Google Scholar]
- 12.Marcuzzi A, De Leo L, Decorti G, Crovella S, Tommasini A, Pontillo A. The farnesyltransferase inhibitors tipifarnib and lonafarnib inhibit cytokines secretion in a cellular model of mevalonate kinase deficiency. Pediatric Research. 2011;70(1):78–82. doi: 10.1203/PDR.0b013e31821b581c. [DOI] [PubMed] [Google Scholar]
- 13.Marcuzzi A, Tommasini A, Crovella S, Pontillo A. Natural isoprenoids inhibit LPS-induced-production of cytokines and nitric oxide in aminobisphosphonate-treated monocytes. International Immunopharmacology. 2010;10(6):639–642. doi: 10.1016/j.intimp.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Deng X, Yu Z, Funayama H, et al. Mutual augmentation of the induction of the histamine-forming enzyme, histidine decarboxylase, between alendronate and immuno-stimulants (IL-1, TNF, and LPS), and its prevention by clodronate. Toxicology and Applied Pharmacology. 2006;213(1):64–73. doi: 10.1016/j.taap.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Marcuzzi A, Secchiero P, Crovella S, Zauli G. TRAIL administration down-modulated the acute systemic inflammatory response induced in a mouse model by muramyldipeptide or lipopolysaccharide. Cytokine. 2012;60(1):43–46. doi: 10.1016/j.cyto.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Marcuzzi A, Pontillo A, De Leo L, et al. Natural isoprenoids are able to reduce inflammation in a mouse model of mevalonate kinase deficiency. Pediatric Research. 2008;64(2):177–182. doi: 10.1203/PDR.0b013e3181761870. [DOI] [PubMed] [Google Scholar]
- 17.Ni W, Egashira K, Kataoka C, et al. Antiinflammatory and antiarteriosclerotic actions of HMG-CoA reductase inhibitors in a rat model of chronic inhibition of nitric oxide synthesis. Circulation Research. 2001;89(5):415–421. doi: 10.1161/hh1701.096614. [DOI] [PubMed] [Google Scholar]
- 18.Muniyappa R, Xu R, Ram JL, Sowers JR. Inhibition of Rho protein stimulates iNOS expression in rat vascular smooth muscle cells. American Journal of PHYSIOLOGY HEART and Circulatory Physiology. 2000;278(6):H1762–H1768. doi: 10.1152/ajpheart.2000.278.6.H1762. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda U, Shimada K. Pleiotropic effects of statins on the vascular tissue. Current Drug Targets Cardiovascular & Haematological Disorders. 2001;1(1):51–58. doi: 10.2174/1568006013338187. [DOI] [PubMed] [Google Scholar]
- 20.Mantuano E, Santi S, Filippi C, et al. Simvastatin and fluvastatin reduce interleukin-6 and interleukin-8 lipopolysaccharide (LPS) stimulated production by isolated human monocytes from chronic kidney disease patients. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2007;61(6):360–365. doi: 10.1016/j.biopha.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Collins GS, Altman DG. Predicting the adverse risk of statin treatment: an independent and external validation of Qstatin risk scores in the UK. Heart. 2012;98(14):1091–1097. doi: 10.1136/heartjnl-2012-302014. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez AL, Wojcik BM, Wrobleski SK, Myers DD, Jr, Wakefield TW, Diaz JA. Statins, inflammation and deep vein thrombosis: a systematic review. Journal of Thrombosis and Thrombolysis. 2012;33(4):371–382. doi: 10.1007/s11239-012-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O’Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunological Reviews. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson NG, Szabo SJ, Weber-Nordt RM, et al. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. The Journal of Experimental Medicine. 1995;181(5):1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satoh S, Oyama J, Suematsu N, et al. Increased productivity of tumor necrosis factor-alpha in helper T cells in patients with systolic heart failure. International Journal of Cardiology. 2006;111(3):405–412. doi: 10.1016/j.ijcard.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto M, Einhaus D, Gold ES, Aderem A. Simvastatin augments lipopolysaccharide-induced proinflammatory responses in macrophages by differential regulation of the c-Fos and c-Jun transcription factors. Journal of Immunology. 2004;172(12):7377–7384. doi: 10.4049/jimmunol.172.12.7377. [DOI] [PubMed] [Google Scholar]
- 27.Taylor JJ. Cytokine regulation of immune responses to Porphyromonas gingivalis. Periodontology 2000. 2010;54(1):160–194. doi: 10.1111/j.1600-0757.2009.00344.x. [DOI] [PubMed] [Google Scholar]
- 28.Massonnet B, Normand S, Moschitz R, et al. Pharmacological inhibitors of the mevalonate pathway activate pro-IL-1 processing and IL-1 release by human monocytes. European cytokine network. 2009;20(3):112–120. doi: 10.1684/ecn.2009.0162. [DOI] [PubMed] [Google Scholar]
- 29.Marcuzzi A, Piscianz E, Girardelli M, Crovella S, Pontillo A. Defect in mevalonate pathway induces pyroptosis in Raw 264.7 murine monocytes. Apoptosis: an International Journal on Programmed Cell Death. 2011;16(9):882–888. doi: 10.1007/s10495-011-0621-1. [DOI] [PubMed] [Google Scholar]
- 30.Almog Y, Shefer A, Novack V, et al. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110(7):880–885. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- 31.Mekontso Dessap A, Ouanes I, Rana N, et al. Effects of discontinuing or continuing ongoing statin therapy in severe sepsis and septic shock: a retrospective cohort study. Critical Care. 2011;15(4):R171. doi: 10.1186/cc10317. [DOI] [PMC free article] [PubMed] [Google Scholar]