Abstract
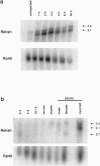
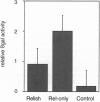
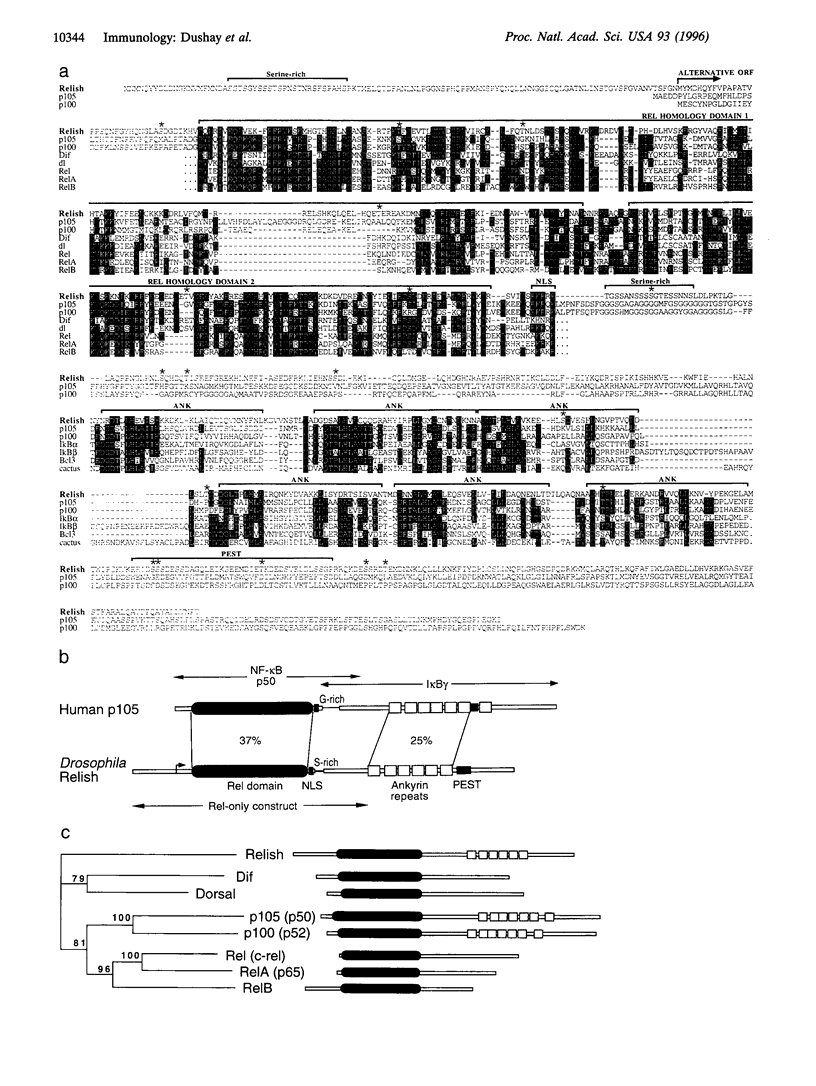
NF-kappa B/Rel transcription factors are central regulators of mammalian immunity and are also implicated in the induction of cecropins and other antibacterial peptides in insects. We identified the gene for Relish, a compound Drosophila protein that, like mammalian p105 and p100, contains both a Rel homology domain and an I kappa B-like domain. Relish is strongly induced in infected flies, and it can activate transcription from the Cecropin A1 promoter. A Relish transcript is also detected in early embryos, suggesting that it acts in both immunity and embryogenesis. The presence of a compound Rel protein in Drosophila indicates that similar proteins were likely present in primordial immune systems and may serve unique signaling functions.
Full text
PDF
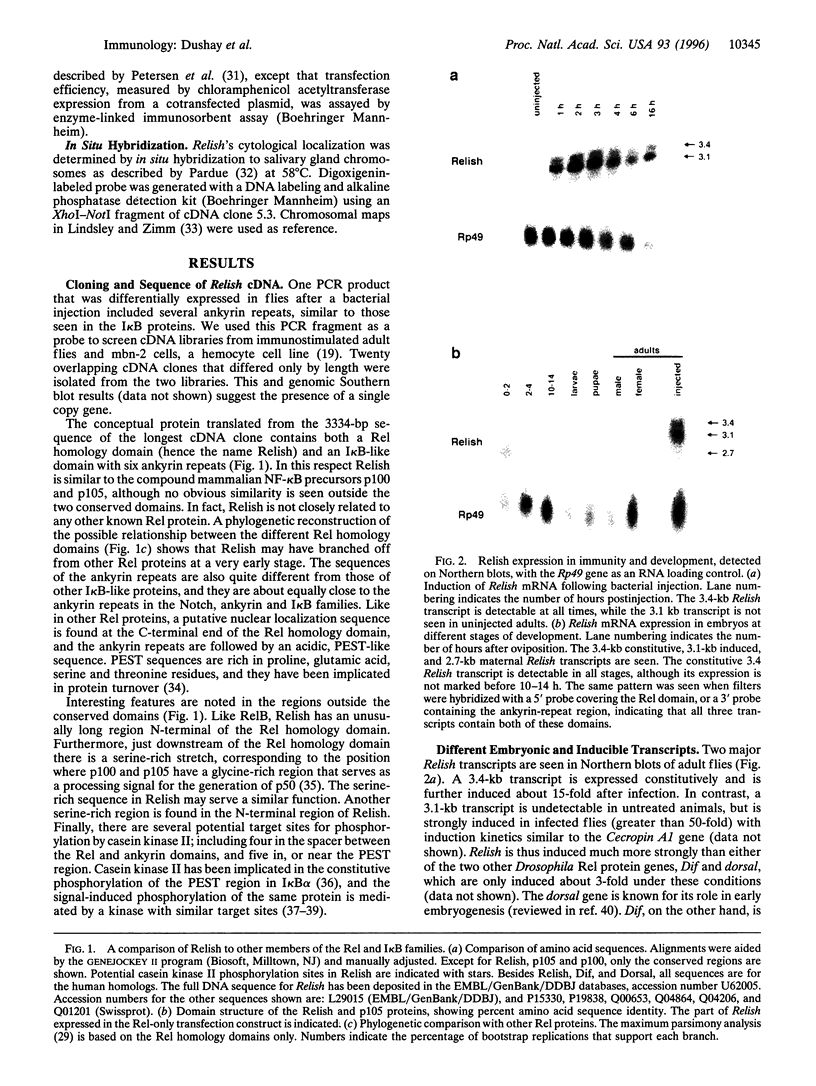
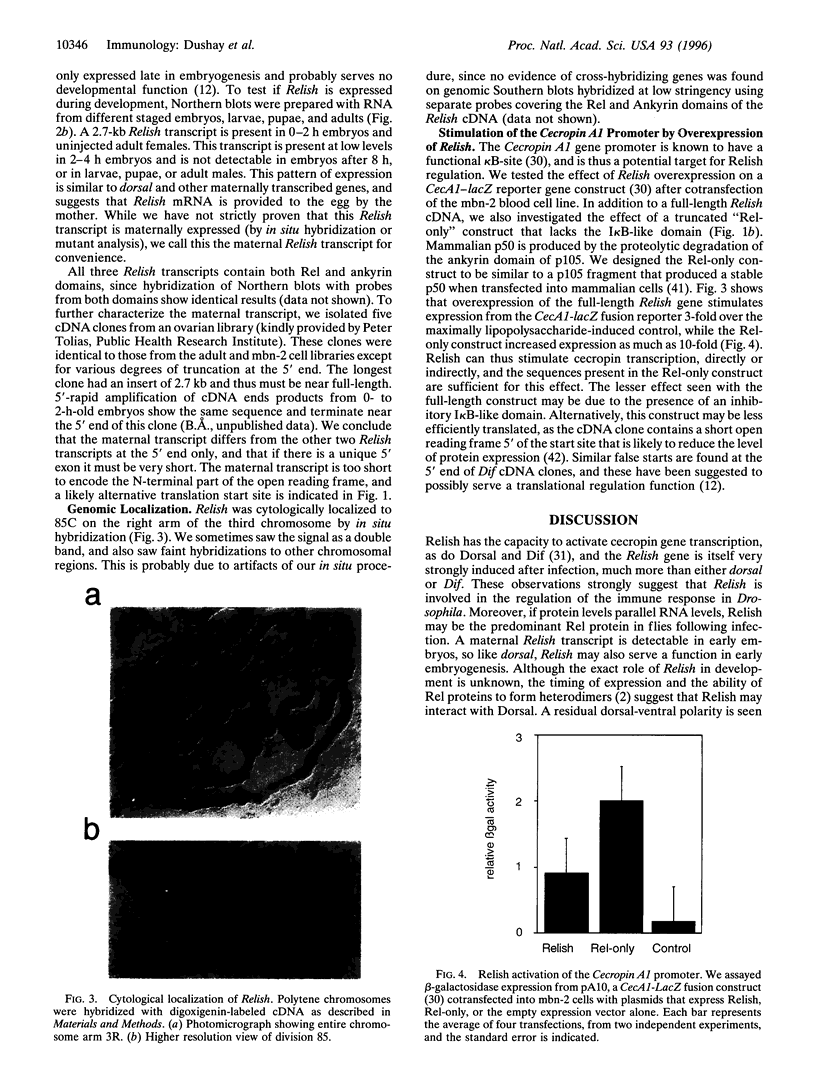

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abastado J. P., Miller P. F., Hinnebusch A. G. A quantitative model for translational control of the GCN4 gene of Saccharomyces cerevisiae. New Biol. 1991 May;3(5):511–524. [PubMed] [Google Scholar]
- Asling B., Dushay M. S., Hultmark D. Identification of early genes in the Drosophila immune response by PCR-based differential display: the Attacin A gene and the evolution of attacin-like proteins. Insect Biochem Mol Biol. 1995 Apr;25(4):511–518. doi: 10.1016/0965-1748(94)00091-c. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Barroga C. F., Stevenson J. K., Schwarz E. M., Verma I. M. Constitutive phosphorylation of I kappa B alpha by casein kinase II. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7637–7641. doi: 10.1073/pnas.92.17.7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours V., Burd P. R., Brown K., Villalobos J., Park S., Ryseck R. P., Bravo R., Kelly K., Siebenlist U. A novel mitogen-inducible gene product related to p50/p105-NF-kappa B participates in transactivation through a kappa B site. Mol Cell Biol. 1992 Feb;12(2):685–695. doi: 10.1128/mcb.12.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours V., Villalobos J., Burd P. R., Kelly K., Siebenlist U. Cloning of a mitogen-inducible gene encoding a kappa B DNA-binding protein with homology to the rel oncogene and to cell-cycle motifs. Nature. 1990 Nov 1;348(6296):76–80. doi: 10.1038/348076a0. [DOI] [PubMed] [Google Scholar]
- Brown K., Gerstberger S., Carlson L., Franzoso G., Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995 Mar 10;267(5203):1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- Chen Z. J., Parent L., Maniatis T. Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity. Cell. 1996 Mar 22;84(6):853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- Cociancich S., Bulet P., Hetru C., Hoffmann J. A. The inducible antibacterial peptides of insects. Parasitol Today. 1994 Apr;10(4):132–139. doi: 10.1016/0169-4758(94)90260-7. [DOI] [PubMed] [Google Scholar]
- Engström Y., Kadalayil L., Sun S. C., Samakovlis C., Hultmark D., Faye I. kappa B-like motifs regulate the induction of immune genes in Drosophila. J Mol Biol. 1993 Jul 20;232(2):327–333. doi: 10.1006/jmbi.1993.1392. [DOI] [PubMed] [Google Scholar]
- Fan C. M., Maniatis T. Generation of p50 subunit of NF-kappa B by processing of p105 through an ATP-dependent pathway. Nature. 1991 Dec 5;354(6352):395–398. doi: 10.1038/354395a0. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Gifford A. M., Riviere L. R., Tempst P., Nolan G. P., Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990 Sep 7;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Govind S., Steward R. Dorsoventral pattern formation in Drosophila: signal transduction and nuclear targeting. Trends Genet. 1991 Apr;7(4):119–125. doi: 10.1016/0168-9525(91)90456-z. [DOI] [PubMed] [Google Scholar]
- Hultmark D. Immune reactions in Drosophila and other insects: a model for innate immunity. Trends Genet. 1993 May;9(5):178–183. doi: 10.1016/0168-9525(93)90165-e. [DOI] [PubMed] [Google Scholar]
- Hultmark D. Insect immunology. Ancient relationships. Nature. 1994 Jan 13;367(6459):116–117. doi: 10.1038/367116a0. [DOI] [PubMed] [Google Scholar]
- Inoue J., Kerr L. D., Kakizuka A., Verma I. M. I kappa B gamma, a 70 kd protein identical to the C-terminal half of p110 NF-kappa B: a new member of the I kappa B family. Cell. 1992 Mar 20;68(6):1109–1120. doi: 10.1016/0092-8674(92)90082-n. [DOI] [PubMed] [Google Scholar]
- Ip Y. T., Reach M., Engstrom Y., Kadalayil L., Cai H., González-Crespo S., Tatei K., Levine M. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell. 1993 Nov 19;75(4):753–763. doi: 10.1016/0092-8674(93)90495-c. [DOI] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Klemenz R., Hultmark D., Gehring W. J. Selective translation of heat shock mRNA in Drosophila melanogaster depends on sequence information in the leader. EMBO J. 1985 Aug;4(8):2053–2060. doi: 10.1002/j.1460-2075.1985.tb03891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnow M. A., Saffman E. E., Kornfeld K., Hogness D. S. Transcriptional activation and repression by Ultrabithorax proteins in cultured Drosophila cells. Cell. 1989 Jun 16;57(6):1031–1043. doi: 10.1016/0092-8674(89)90341-3. [DOI] [PubMed] [Google Scholar]
- Kylsten P., Samakovlis C., Hultmark D. The cecropin locus in Drosophila; a compact gene cluster involved in the response to infection. EMBO J. 1990 Jan;9(1):217–224. doi: 10.1002/j.1460-2075.1990.tb08098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Averboukh L., Pardee A. B. Distribution and cloning of eukaryotic mRNAs by means of differential display: refinements and optimization. Nucleic Acids Res. 1993 Jul 11;21(14):3269–3275. doi: 10.1093/nar/21.14.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Lin L., Ghosh S. A glycine-rich region in NF-kappaB p105 functions as a processing signal for the generation of the p50 subunit. Mol Cell Biol. 1996 May;16(5):2248–2254. doi: 10.1128/mcb.16.5.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio F., Didonato J., Rosette C., Karin M. Molecular cloning and characterization of a novel Rel/NF-kappa B family member displaying structural and functional homology to NF-kappa B p50/p105. DNA Cell Biol. 1992 Sep;11(7):523–537. doi: 10.1089/dna.1992.11.523. [DOI] [PubMed] [Google Scholar]
- Meyer R., Hatada E. N., Hohmann H. P., Haiker M., Bartsch C., Röthlisberger U., Lahm H. W., Schlaeger E. J., van Loon A. P., Scheidereit C. Cloning of the DNA-binding subunit of human nuclear factor kappa B: the level of its mRNA is strongly regulated by phorbol ester or tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):966–970. doi: 10.1073/pnas.88.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri A., Chang C. C., Lombardi L., Salina M., Corradini P., Maiolo A. T., Chaganti R. S., Dalla-Favera R. B cell lymphoma-associated chromosomal translocation involves candidate oncogene lyt-10, homologous to NF-kappa B p50. Cell. 1991 Dec 20;67(6):1075–1087. doi: 10.1016/0092-8674(91)90285-7. [DOI] [PubMed] [Google Scholar]
- O'Connell P. O., Rosbash M. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 1984 Jul 11;12(13):5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen U. M., Björklund G., Ip Y. T., Engström Y. The dorsal-related immunity factor, Dif, is a sequence-specific trans-activator of Drosophila Cecropin gene expression. EMBO J. 1995 Jul 3;14(13):3146–3158. doi: 10.1002/j.1460-2075.1995.tb07317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichhart J. M., Georgel P., Meister M., Lemaitre B., Kappler C., Hoffmann J. A. Expression and nuclear translocation of the rel/NF-kappa B-related morphogen dorsal during the immune response of Drosophila. C R Acad Sci III. 1993 Oct;316(10):1218–1224. [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Roth S., Hiromi Y., Godt D., Nüsslein-Volhard C. cactus, a maternal gene required for proper formation of the dorsoventral morphogen gradient in Drosophila embryos. Development. 1991 Jun;112(2):371–388. doi: 10.1242/dev.112.2.371. [DOI] [PubMed] [Google Scholar]
- Samakovlis C., Kimbrell D. A., Kylsten P., Engström A., Hultmark D. The immune response in Drosophila: pattern of cecropin expression and biological activity. EMBO J. 1990 Sep;9(9):2969–2976. doi: 10.1002/j.1460-2075.1990.tb07489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid R. M., Perkins N. D., Duckett C. S., Andrews P. C., Nabel G. J. Cloning of an NF-kappa B subunit which stimulates HIV transcription in synergy with p65. Nature. 1991 Aug 22;352(6337):733–736. doi: 10.1038/352733a0. [DOI] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Franzoso G., Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- Steward R. Dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogene, c-rel. Science. 1987 Oct 30;238(4827):692–694. doi: 10.1126/science.3118464. [DOI] [PubMed] [Google Scholar]
- Traenckner E. B., Pahl H. L., Henkel T., Schmidt K. N., Wilk S., Baeuerle P. A. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995 Jun 15;14(12):2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]