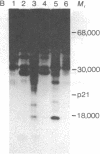
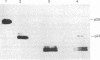
Abstract
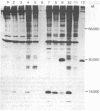
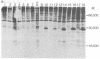
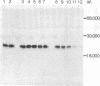
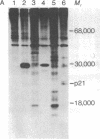
Kirsten sarcoma virus (Ki-MSV) and Harvey sarcoma virus (Ha-MSV) are mouse-rat recombinant viruses that were originally isolated by experimental inoculation of rats with helper-independent mouse type C viruses. We have recently identified in cells transformed by Ki-MSV or Ha-MSV, a phosphoprotein, p21, coded for by Ki-MSV and Ha-MSV [Shih, T.Y., Weeks, M.O., Young, H.A. & Scolnick, E.M. (1979) Virology 95, in press]. The p21, which is not a virion structural protein, was identified with antisera prepared by transplantation in rats of syngeneic Ha-MSV- or Ki-MSV-transformed nonproducer cells. In this study, we have applied the same methodology to examine a purely rat sarcoma virus (RaSV), which was isolated in cell culture by using helper-independent rat type C viruses [Rasheed, S., Gardner, M.B. & Huebner, R.J. (1978) Proc. Natl. Acad. Sci. USA 75, 2972-2976]. We report here that this new, purely rat sarcoma virus apparently codes for a p29, which shares immunological determinants and common V-8 protease-generated peptides with the p21 of Ha-MSV. The data suggest that the RaSV has acquired genetic information with similar coding capacity to some rat genetic information with similar coding combinant viruses, Ki-MSV and Ha-MSV. Based on data obtained on the p21 of a mutant of Ki-MSV temperature-sensitive for the maintenance of transformation, we suggest that the gene in RaSV that codes for the p29 is also required for the maintenance of RaSV-induced fibroblast transformation.
Full text
PDF

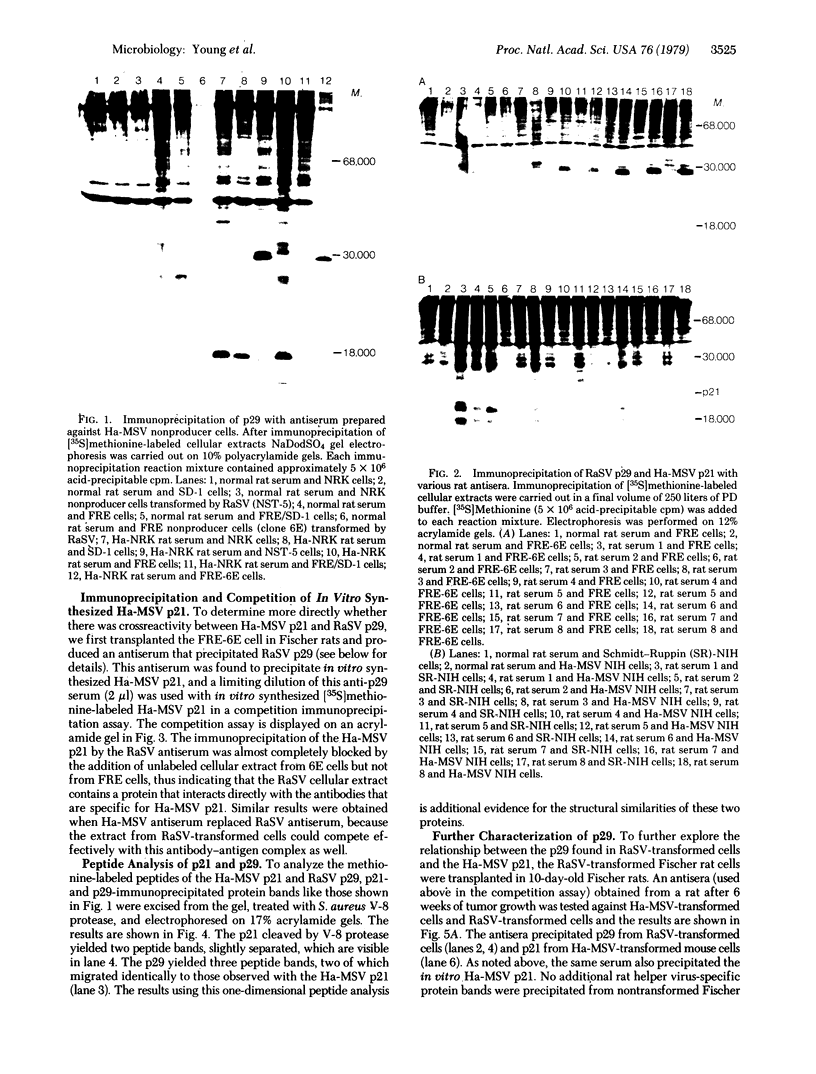

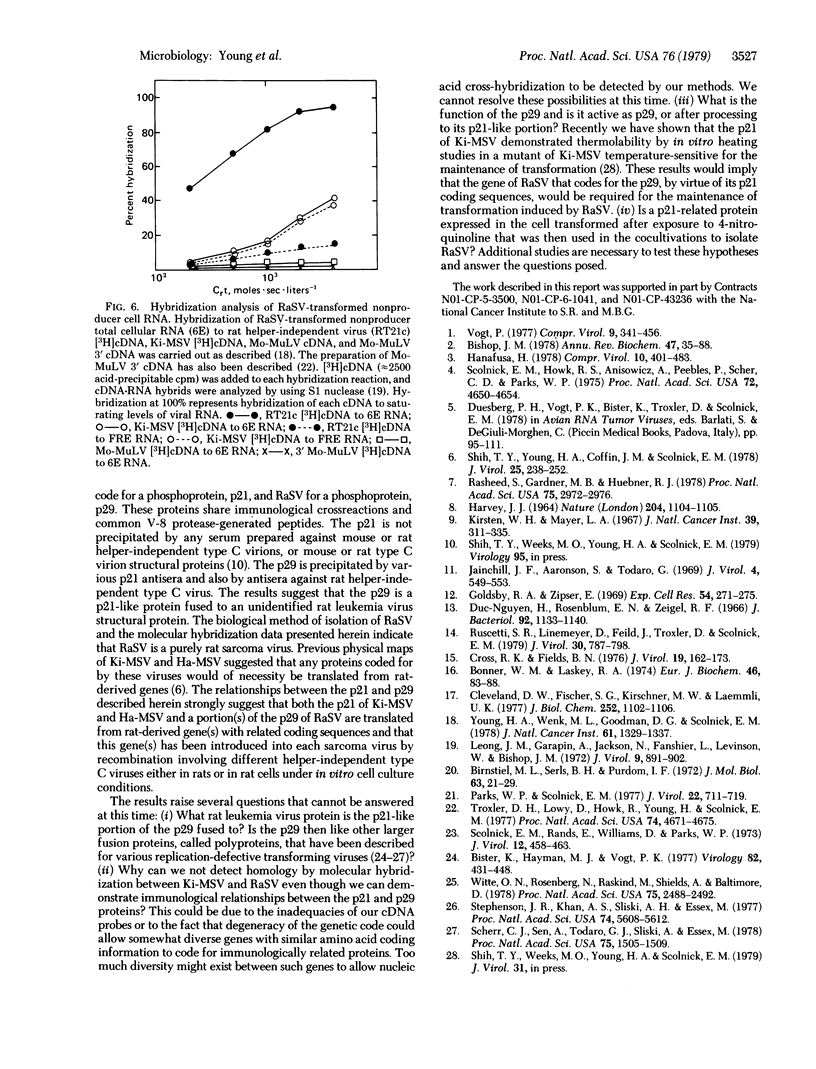
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnstiel M. L., Sells B. H., Purdom I. F. Kinetic complexity of RNA molecules. J Mol Biol. 1972 Jan 14;63(1):21–39. doi: 10.1016/0022-2836(72)90519-0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cross R. K., Fields B. N. Reovirus-specific polypeptides: analysis using discontinuous gel electrophoresis. J Virol. 1976 Jul;19(1):162–173. doi: 10.1128/jvi.19.1.162-173.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsby R. A., Zipser E. The isolation and replica plating of mammalian cell clones. Exp Cell Res. 1969 Feb;54(2):271–275. doi: 10.1016/0014-4827(69)90250-x. [DOI] [PubMed] [Google Scholar]
- HARVEY J. J. AN UNIDENTIFIED VIRUS WHICH CAUSES THE RAPID PRODUCTION OF TUMOURS IN MICE. Nature. 1964 Dec 12;204:1104–1105. doi: 10.1038/2041104b0. [DOI] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M. In vitro translation of Harvey murine sarcoma virus RNA. J Virol. 1977 Jun;22(3):711–719. doi: 10.1128/jvi.22.3.711-719.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Gardner M. B., Huebner R. J. In vitro isolation of stable rat sarcoma viruses. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2972–2976. doi: 10.1073/pnas.75.6.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S. K., Linemeyer D., Feild J., Troxler D., Scolnick E. M. Characterization of a protein found in cells infected with the spleen focus-forming virus that shares immunological cross-reactivity with the gp70 found in mink cell focus-inducing virus particles. J Virol. 1979 Jun;30(3):787–798. doi: 10.1128/jvi.30.3.787-798.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Howk R. S., Anisowicz A., Peebles P. T., Scher C. D., Parks W. P. Separation of sarcoma virus-specific and leukemia virus-specific genetic sequences of Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4650–4654. doi: 10.1073/pnas.72.11.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Rands E., Williams D., Parks W. P. Studies on the nucleic acid sequences of Kirsten sarcoma virus: a model for formation of a mammalian RNA-containing sarcoma virus. J Virol. 1973 Sep;12(3):458–463. doi: 10.1128/jvi.12.3.458-463.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Sen A., Todaro G. J., Sliski A., Essex M. Pseudotypes of feline sarcoma virus contain an 85,000-dalton protein with feline oncornavirus-associated cell membrane antigen (FOCMA) activity. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1505–1509. doi: 10.1073/pnas.75.3.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Young H. A., Coffin J. M., Scolnick E. M. Physical map of the Kirsten sarcoma virus genome as determined by fingerprinting RNase T1-resistant oligonucleotides. J Virol. 1978 Jan;25(1):238–252. doi: 10.1128/jvi.25.1.238-252.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Khan A. S., Sliski A. H., Essex M. Feline oncornavirus-associated cell membrane antigen: evidence for an immunologically crossreactive feline sarcoma virus-coded protein. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5608–5612. doi: 10.1073/pnas.74.12.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Lowy D., Howk R., Young H., Scolnick E. M. Friend strain of spleen focus-forming virus is a recombinant between ecotropic murine type C virus and the env gene region of xenotropic type C virus. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4671–4675. doi: 10.1073/pnas.74.10.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H. A., Wenk M. L., Goodman D. G., Scolnick E. M. Expression of RNA of an endogenous replication-defective retrovirus in rat mammary adenocarcinomas induced by 7,12-dimethylbenz[a]anthracene. J Natl Cancer Inst. 1978 Nov;61(5):1329–1337. doi: 10.1093/jnci/61.5.1329. [DOI] [PubMed] [Google Scholar]