Abstract
The common marmoset (Callithrix jacchus), a laboratory nonhuman primate, is a well-known model of several human diseases and conditions, but the nutritional needs of these animals are not fully understood. Here we describe a 4-mo controlled study in which we increased the dietary fat and protein of subadult male common marmosets by using healthy snacks. Six male marmosets received their normal diet (control), and an additional 6 were given their normal diet supplemented daily with a 14-kcal snack. Cashews and waxworms were used as the snack, given their high-fat content. Although body weight did not differ between the 2 groups, only control male marmosets showed increased chest circumferences over the course of the study. Glucoregulatory function remained consistent in the snack-fed marmosets, whereas control animals had progressed toward higher insulin. Other indices of glucoregulation indicated significant differences in adiponectin and the cortisol:cortisone ratio between the 2 groups, but no differences in lipid concentration were detected. Therefore, the most notable difference attributable to the snack feeding was improved glucoregulation. Because the snacks we used had a high proportion of unsaturated compared with saturated fat, we suggest that these healthy high-fat–high-protein snacks provide an important contribution to the nutrition of this laboratory species. This study also demonstrates the utility of marmosets as a model for understanding the implications of dietary fats in humans.
Abbreviation: HMW, high molecular weight
The common marmoset (Callithrix jacchus) has proven to be an excellent model for biomedical and behavioral research in both the United States and Europe for many years (see reference 15 for review). Recently, a particular interest has been in the use of marmosets for modeling human obesity and metabolic syndrome.27 There are many advantages to using marmosets in understanding metabolic diseases. As a primate species, marmosets are similar to humans in fat cell function.33 The marmoset pancreas exhibits the same structure, marker genes, and the presence of the Glut 5 and 9 transporters as does human pancreas.22 In addition, lipogenesis occurs in the adipose tissues of marmosets, as in all primates, rather than in the liver, as in rodents.2 Marmosets are cooperative breeders, showing the family arrangement most often seen in humans. Because marmosets have a short lifespan of approximately 16 y and achieve reproductive maturity by 2 y of age, many studies can be performed in a relatively short time. Furthermore, marmosets generally produce twins, thus affording the opportunity to select subjects for different treatments within the same study. Indicating similar dietary needs to those of humans, the natural diet of marmosets is omnivorous and consists of fruits, plant exudates, nectar, invertebrates, and small vertebrates.26 In captivity, marmosets show diet-dependent early-onset weight gain and obesity.27 Dietary changes can have a pronounced influence on their weight and health.4,30,33
There is a lack of consensus regarding appropriate diets for laboratory-housed marmosets. Little is known about the optimal energy and nutrient requirements of marmosets.18 Commercially available diets for marmosets differ in nutrients and makeup. There is limited knowledge of the fat requirements or the effects of different fat composition on the glucoregulatory system. Only one study has examined the effect of a high-fat diet on regulatory functioning. In that study,33 8 adult marmosets fed a high-fat diet (that is, 20% added saturated fats) for 1 y showed no adverse effects on the levels of glycosylated hemoglobin as an index of blood glucose use but manifested atherosclerosis. Whether the same effects occur with a combination of unsaturated and saturated fats is unknown.
Many teenagers receiving a Western diet consume high amounts of sugars and fats in the form of snacks. Teen obesity has grown to epidemic proportions in the United States, with as many as 17% of 12- to 19-y-olds considered obese.3 Fat consumption and adiposity are significantly associated,4,8,21 but the relationship between BMI and high fat consumption is unclear.1 Fats are important for brain and body functions, including the regulation of circulating lipid levels, neuronal development, cardiovascular health and immune, insulin, and visual functions.7,12,24,28 The discrepancy in dietary fat's influence on body function may be due to the type of fats consumed. The current study aims to provide evidence that increased fats, providing that they comprise appropriately balanced fatty acids (saturated and unsaturated), may be beneficial in adolescents. We monitored the metabolic effects of providing high-fat–high-protein snacks to male marmosets for 3 mo. We hypothesized that this dietary scheme would not lead to detrimental changes.
Materials and Methods
Animals.
Twelve subadult male common marmosets (Callithrix jacchus; age, 18 to 24 mo [equivalent to approximately 15 to 20 y for humans]) from the Wisconsin National Primate Research Center's marmoset colony were randomized equally into 2 treatment groups (control and snack-fed) balancing body weight between the 2 groups. We chose only male marmosets to eliminate sex as a factor in a small sample-size study. There were 4 brother sets in the study, such that one brother was in the control group and the other in the snack-fed group to control for any possible genetic differences. At the start of the study, all of the marmosets were moved into the same room to facilitate supplemental feeding. Each study male was paired with a female and lived in cage sizes of 0.6 × 0.91 × 1.83 m. Female marmosets were prevented from becoming pregnant by using a prostaglandin F2α analog (cloprostenol sodium) to terminate conceptions. Animals were maintained on a 12:12-h lighting schedule (lights on, 0630 to 1830), with ambient temperature at approximately 27 °C and humidity of approximately 50%. Animals were weighed weekly, fed their standard allotment in 2 daily feedings (approximately 0800 and 1300; Mazuri 5MI6, Land O'Lakes, Arden Hills, MN), with browse material daily (fruit, mealworms, vegetables). The diet was offered at 64 kcal/kg body weight as 40 g of diet daily in 2 allotments, provides 1600 kcal/kg of metabolizable energy, and consists of 20% protein and 4.5% fat (less than 3% of the total fat as unsaturated fatty acids). After the first month, supplemental feeding was provided at approximately 0900 to 1000 daily for 3 mo. Each subject in the snack-fed group received an additional approximately 14 kcal/d whereas the marmosets in the control group received maximum of 3 kcal/d additional. The snacks consisted of a grape for the control group and high-fat–high-protein cashews and waxworms (Figure 1) for the snack-fed group. Each marmoset in the snack-fed group received 2 waxworms (2.1 kcal) and 2 cashews (11.48 kcal) daily. The monkeys were handfed and voluntarily accepted their once-daily supplement while in their home cage. This feeding method assured that the marmosets ingested their allotment.
Figure 1.
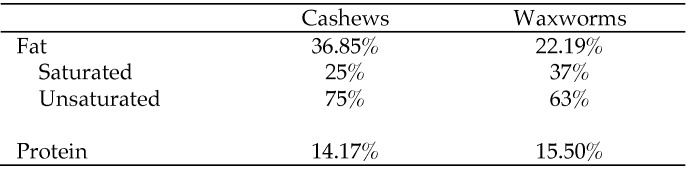
Fat and protein compositions of cashews (Anacardium occidentale)21 and waxworms (Galleria mellonella).4
This study was reviewed and approved by the Graduate School Animal Care and Use Committee of the University of Wisconsin, Madison, and was performed consistent with the USDA.13 The animal care and use program at the University of Wisconsin maintains a Public Health Services Assurance and is fully AAALAC-accredited.
Body composition measures.
Animals were weighed weekly in the morning prior to feeding to determine change over time. Weekly weights were averaged by month for each marmoset. Body composition parameters were collected once each month by manually restraining the marmosets for measurements of abdominal and chest circumferences and crown–rump length. Body mass index was calculated at each monthly time point by using body weight and crown–rump length. All measurements were recorded in centimeters.
Metabolic measures.
Glucoregulatory function was assessed monthly by oral glucose tolerance testing. After a baseline blood sample was obtained, the marmosets each received an oral dose (5 mL/kg) of a 40% sucrose solution. Blood samples were collected at 15, 30, 60, and 120 min after dosing. Insulin was measured by using 50 µL serum in radioimmunoassays according to methods we validated for marmosets;34 the intraassay coefficient of variation was 2.87. Glucose was measured by using a glucometer (Accu-Check Aviva, Roche Diagnostics, Indianapolis, IN). Basal insulin and glucose data were analyzed separately and modeled together by using standard insulin function assessments (homeostatic model assessment of insulin resistance and Matsuda index of insulin sensitivity),19 as is done in humans.
As an index of glucose control, we measured adiponectin (both high molecular weight [HMW] and total) by using a commercial enzyme immunoassay kit (47-ADPHU-E01, ALPCO Diagnostics, Salem, NH) developed for humans but validated for marmosets.34 The kit provides methods for measuring total, HMW, and low-molecular–weight adiponectin, because these variants have different biologic activities.23 Serum samples were diluted 1:2575 and assayed in 10-µL aliquots. For both the HMW and total adiponectin assays, the intraassay coefficient of variation was 1.5.
The cortisol:cortisone ratio, indexed by creatinine, was measured as a marker for increased intracellular cortisol production (a contributor to metabolic dysfunction). Four marmosets from each group were selected for twice-weekly (first morning void) urine collection. Prior to lights-on each morning, each marmoset was ushered individually into a small box within the homecage, where it remained until urination. The urine produced was collected through a funnel under the cage, centrifuged, and stored at −20 °C. For analysis, 500 µL urine underwent solvolysis to break single and complex conjugates, and extraction was performed with 5 mL ethyl acetate, 100 µL saturated NaCl, 50 µL 2.5 M H2SO4 after incubation at 60 °C for 2 h. The glucocorticoids in the samples were separated and quantified by liquid chromatography–mass spectrometry (model 1100, Agilent, Santa Clara, CA). The separation used a mobile phase consisting of 95% acetonitrile in H2O, 0.01% formic acid (B) and 95% H2O, 0.01% formic acid (A). The flow rate was 50 µL/min, and the run time was 15 min. A gradient was performed: 0 to 1 min at 30% B, 1 to 12 min at 60% B, and then 12 to 15 min at 100% B; cortisol and cortisone eluted within the first 10 min. Quality-control urine pools for marmoset urine indicated intraassay coefficients of variation of 10.6 for cortisol and 12.5 for cortisone.
Cardiovascular measures.
Heart rate and blood pressure measurements were collected monthly under manual restraint at the time of body composition assessment. Systolic and diastolic arterial blood pressure and heart rate were measured by using a high-definition oscillometric tail cuff on awake, manually restrained animals. A standard lipid panel (UW-Meriter Hospital Clinics, Madison, WI) was used to measure circulating lipids (cholesterol, triglycerides, HDL, and LDL) in baseline serum samples collected during oral glucose tolerance testing.
Statistical analyses.
Depending on the normality of the data, repeated-measures ANOVA, parametric (t tests), and nonparametric (Kruskal–Wallis) tests were used to determine changes over time. Changes in individual animals across the study were determined by using paired t or Mann–Whitney U tests. Change from baseline by treatment was assessed by using Mann–Whitney U tests.
Results
Weight and body composition.
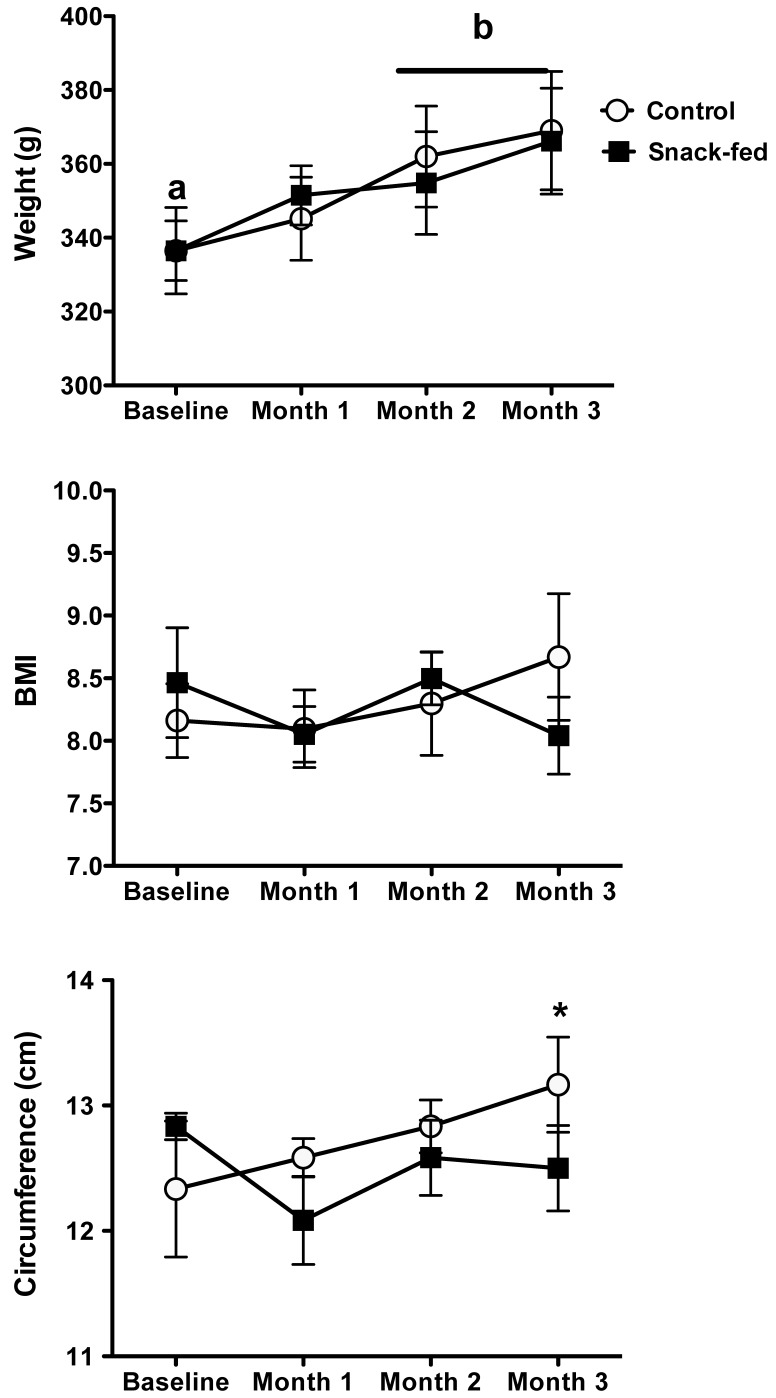
In all animals, body weight increased each month, indicating that the marmosets were still maturing; there were no differences between the 2 groups (f3,40 = 2.31, P = 0.09, Figure 2) . Weights at months 2 and 3 were significantly (Kruskal–Wallis, f = 12.7, P = 0.005, n = 12) increased over baseline values. Chest circumference increased significantly (f3,30 = 3.67, P = 0.02) in controls, with the highest increase during month 3, when it was significantly (U6,6 = 4.0, P = 0.03) greater than that in snack-fed marmosets. Other measurements did not change according to treatment or time.
Figure 2.
Body weight, BMI, and chest circumference (mean ± SEM) of control and snack-fed male subadult common marmosets. No differences were found between groups, and all marmosets showed significant (F = 12.7, P = 0.005, n = 12) increases in weight during months 2 and 3 of the treatment. BMI did not differ significantly between the 2 groups, but chest circumference was significantly (U6,6 = 4.0, P = 0.03) increased in control compared with snack-fed marmosets.
Glucoregulatory function.
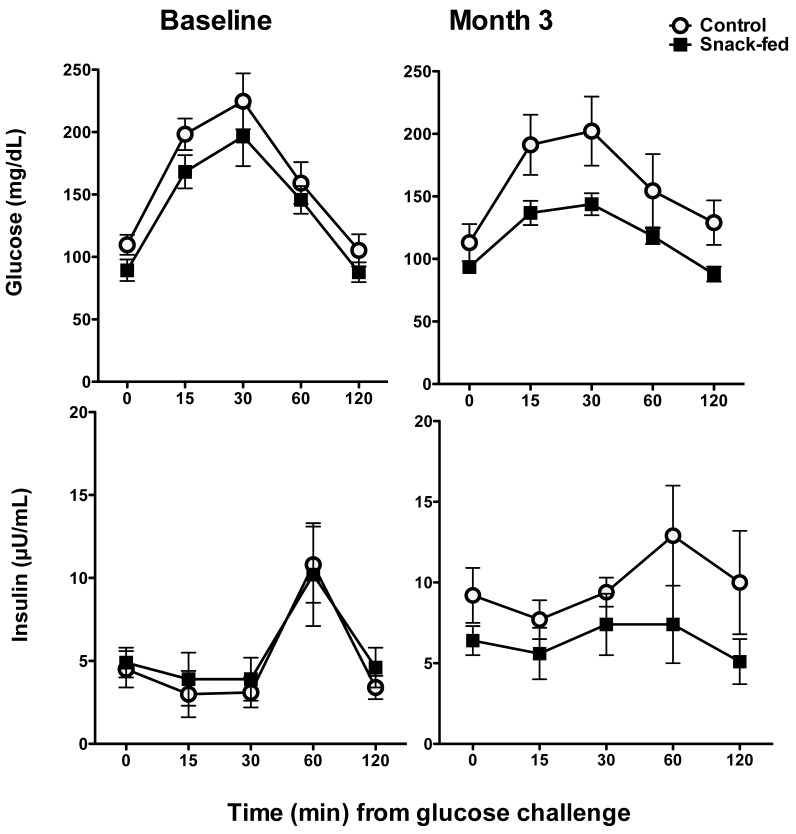
At baseline, all marmosets exhibited normal serum glucose levels and insulin response to glucose (Figure 3). However, by the third month of treatment, significant differences in mean glucose (f1,4 = 14.63, P = 0.0004) and insulin (f1,4 = 7.54, P = 0.008) levels had developed between groups. During the final month of treatment, insulin levels at 0 and 120 min were higher (0 min: U6,6 = 3.0, P = 0.02; 120 min: U6,6 = 6.0, P = 0.03) in controls than in snack-fed marmosets, and glucose did not return to baseline levels in the controls. Insulin sensitivity and resistance did not differ between groups.
Figure 3.
Glucocorticoid responses (mean ± SEM) of control and snack-fed males at baseline and after 3 mo of snack feeding. All marmosets showed similar baseline values of glucose and insulin (left graphs). At the third month of snack feeding, the groups differed significantly in both glucose and insulin at 0 and 120 min during an oral glucose tolerance test (0 min: U6,6 = 3.0, P = 0.02; 120 min: U6,6 = 6.0, P = 0.03).
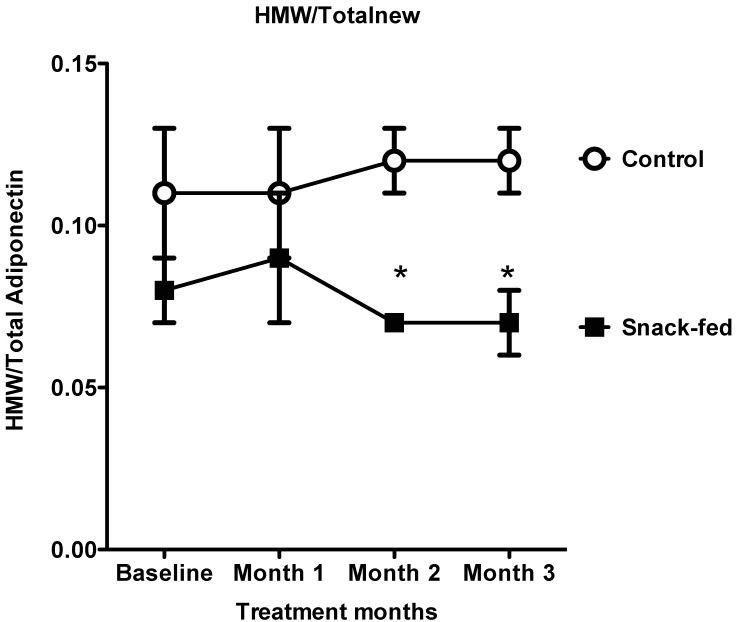
HMW and the ratio of HMW:total adiponectin both differed by treatment, whereas total adiponectin did not differ between the control and snack-fed conditions. HMW:total adiponectin differed significantly (f1,3 = 13.36, P = 0.001) between differences, such that HMW:total values remained consistent across the study in control animals but declined in snack-fed marmosets. The ratio of HMW:total adiponectin differed significantly between groups at months 2 (t8 = 3.75, P = 0.006, Figure 4) and 3 (t8 = 4.81, P = 0.001).
Figure 4.
High-molecular–weight (HMW):total adiponectin ratio in control and snack-fed preadult male marmosets. Compared with control animals, snack-fed marmosets had significantly lower HMW:total adiponectin ratios at months 2 (t8 = 3.75, P = 0.006) and 3 (t8 = 4.81, P = 0.001).
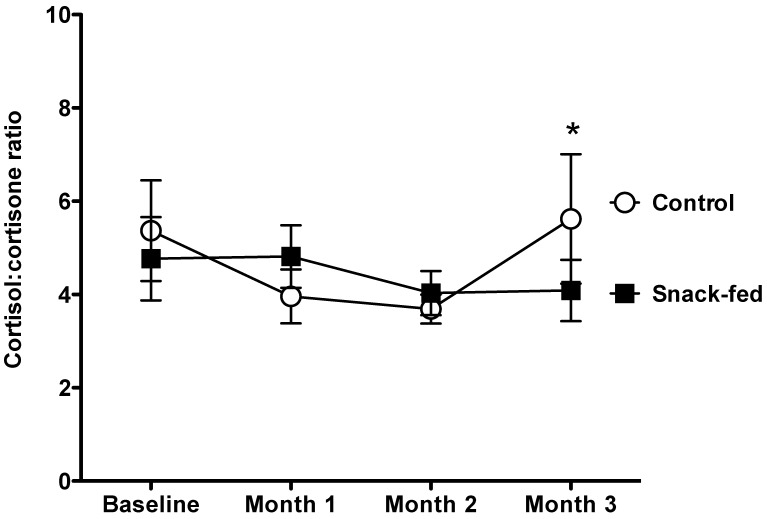
The ratio of urinary cortisol:cortisone was compared according to the 2 conditions (Figure 5). The snack-fed marmosets showed a consistent mean level of the ratio of cortisol:cortisone across the months of the study, but the control animals had a significantly increased ratio during the final month of the study (U5,5 = 4.0, P = 0.04).
Figure 5.
Cortisol:cortisone ratio (mean ± SEM) in the first void urine control and snack-fed male marmosets. Control males showed significantly (U5,5 = 4.0, P = 0.04) higher cortisol:cortisone ratios during month 3.
Cardiovascular measures.
Total cholesterol, triglycerides, HDL, LDL, and total cholesterol:HDL ratios did not change throughout the study (Table 1). The only difference noted was a trend toward a decline in LDL levels (P = 0.08, t5 = 2.09) in control marmosets. No differences were detected in measurements of heart rate or blood pressure.
Table 1.
Lipid measurements (mean ± SEM) during 3 mo of snack-feeding in subadult male marmosets
| Baseline | Month 3 | ||
| Cholesterol | Control | 174.5 ± 10.2 | 180.7 ± 18.1 |
| Snack-fed | 167.8 ± 15.0 | 191.8 ± 9.7 | |
| Triglycerides | Control | 97.3 ± 14.8 | 139.3 ± 27.9 |
| Snack-fed | 84.3 ± 14.4 | 92.0 ± 14.8 | |
| HDL | Control | 90.3 ± 12.1 | 99.0 ± 9.2 |
| Snack-fed | 91.5 ± 10.8 | 105.2 ± 6.2 | |
| LDL | Control | 76.8 ± 13.7 | 53.7 ± 14.1a |
| Snack-fed | 56.3 ± 4.3 | 68.3 ± 7.6 | |
| Cholesterol:HDL | Control | 2.1 ± 0.3 | 1.8 ± 0.1 |
| Snack-fed | 1.9 ± 0.1 | 1.8 ± 0.1 |
Value significantly different (Mann–Whitney U test: P = 0.08, t = 2.09, df = 5) from that at baseline.
Discussion
Our findings support our prediction that feeding high-fat–high-protein snacks to adolescent male marmosets for 3 mo would not lead to detrimental changes. Importantly, snack-fed animals had improved glucoregulatory function in the absence of weight gain compared with that in controls. Our findings suggest that the types of fats or the saturated:unsaturated ratio may be relevant to the controversy regarding the role of fats in the diet.
Cashews are high in unsaturated compared with saturated fats,25 in a 1:2:1 ratio of saturated: monounsaturated:polyunsaturated fats, the optimal ratio for healthy fat consumption.28 In addition, cashews provide protein and essential minerals.25 Waxworms have a high fat content— 60% as dry matter (22% as is)—but the majority of the fats are unsaturated, consisting of oleic, linoleic, and linolenic acids.5 The only other study concerning fats in marmosets29 used a diet supplemented with saturated fats only. Although the sample size was small in the cited study, the number of marmosets that developed atherosclerotic lesions was increased significantly after saturated fat supplementation, indicating the contribution of saturated fats to heart disease. We did not euthanize the marmosets in our study and therefore were unable to determine vessel condition, but heart rate and blood pressure—indicators of cardiovascular function—did not differ between control and snack-fed marmosets.
Among the important functions of dietary fats is their role in metabolic function. In humans, saturated fat can worsen insulin resistance, whereas monounsaturated and polyunsaturated fatty acids improve insulin sensitivity.24 Increasing the amount of ω3 fatty acids reduces diastolic blood pressure and improves cardiac function.28 Substituting unsaturated for saturated fats reduces both triglycerides and LDL cholesterol.12 Fats are also important for brain development and functioning.7,32 Evidence in nonhuman primates is sparse, but one would expect findings similar to those in humans. Fats in combination with other important substances such as fiber, vitamins, minerals, and protein facilitate improved metabolic activity.
The subadult male marmosets in our study were still growing, as evidenced by their increasing weight over the course of the study. By 2 y of age, male marmosets reach adult height, as indicated by crown–rump measurements, but they continue to gain weight as they mature. Given that body weight did not differ between the 2 groups over the course of the study, food intake likely did not greatly differ. Chest circumference increased in the control marmosets but not in the snack-fed animals, likely reflecting an increase in the axillary fat pads typically displayed around the nipple area in both female and male marmosets. These fat stores are available for periods of high-energy need, such as when they are carrying infants—among common marmosets, the male is the primary infant caretaker.26,35
Glucoregulatory function differed between our 2 groups of marmosets. All animals in the study underwent oral glucose tolerance testing to assess insulin response to glucose. At the beginning of the study (baseline), glucose and insulin curves throughout testing were similar between the 2 study groups. Both groups reached peak glucose by 30 min and peak insulin by 60 min after glucose challenge. However, by the third month, the snack-fed marmosets showed lower overall and peak levels of glucose than those in the controls. Insulin levels in the snack-fed marmosets did rise but consistently returned to baseline by 120 min after glucose challenge. The control marmosets showed an insulin peak by 60 min, but insulin levels did not return to baseline, indicating decreased insulin responsiveness over the study period. Marmosets in both conditions were normal and healthy in weight and appearance. However, it appears that under the present diet, glucose metabolism was not regulated as tightly in the controls as in the snack-fed animals. The dietary supplementation achieved improved regulation of glucose metabolism.
Although there were no differences in total adiponectin between the 2 groups, the HMW form was significantly lower in the snack-fed marmosets. Adiponectin, which is secreted from fat cells, circulates in levels inversely correlated with obesity and insulin resistance in adult humans.10,11 The correlation may be mediated by altered secretion of adipokines by the adipose tissue, because increased adiposity downregulates the secretion of adiponectin.16 However, the roles of adiponectin and its multiple forms in prepubertal and adolescent humans are still unclear.15,31 When adiponectin levels in obese and normal-weight children and adolescents are compared, there is a correlation between adiponectin and waist circumference, such that higher levels of adiponectin are associated with smaller waist circumference.9 This association indicates that the more abdominal fat, the lower the adiponectin level. In addition, in prepubertal children, HMW adiponectin differs according to weight and BMI in girls but not in boys.20 Less is known about the relationship of HMW adiponectin to body fat and metabolism in normal healthy adolescents, for which there are no obese subjects for comparison. Among our male marmosets, we saw no differences in abdominal circumference between our 2 conditions, and abdominal circumference did not correlate with HMW adiponectin (r = 0.24, P = 0.49). However, chest circumference did differ between the 2 groups, with the larger chest circumference occurring in our control animals. In addition, chest circumference was positively correlated with adiponectin (r = 0.77, P = 0.02). This finding may be unexpected, and little is known about the fat pads that exist in the chest area of marmosets. Additional research is required to understand what levels of adiponectin are appropriate in healthy, normal weight, adolescent marmosets.
The urinary cortisol:cortisone ratio has been established as a means to determine the enzymatic activity of 11β-hydroxysteroid dehydrogenase type 1, which converts inactive cortisone to active cortisol in cells; the lower the cortisone, the higher the cortisol in the ratio. The additional cortisol works to amplify the action of local glucocorticoids in adipose and liver tissue, where they can reduce insulin sensitivity and action.29 The urinary cortisol:cortisone can provide additional information regarding the regulation of glucose metabolism, although 11 β-hydroxysteroid dehydrogenase does not seem to differ between healthy and type 2 diabetic subjects.14 Our snack-fed marmosets showed no significant change in the cortisol:cortisone ratio, whereas the control marmosets showed a higher cortisol:cortisone ratio, suggesting that more cortisone is converted to cortisol within the cells and could possibly affect insulin action. Additional research into the role of an increased cellular cortisol in adolescents is needed to increase our understanding regarding this relationship.
Although the proportion of unsaturated to saturated fats was the likely cause of the improved glucoregulation in our marmosets, an alternative source of regulation may have been the protein added to the diet from the cashews and waxworms. In addition, both cashews and waxworms have other bionutrients, such as minerals, fiber, and other bioactive compounds, that may have a positive effect on metabolism.25 Increased protein may have led more rapidly to satiety in the snack-fed marmosets, whereas the control animals may have eaten more to increase their caloric intake. However, without direct information on consumption, this hypothesis is unclear. If so, this mechanism may explain the similar weight between the 2 groups and the increased chest circumference (larger fat pads) in control compared with the snack-fed animals. Alternatively, if the amount of calories consumed between the 2 groups did not differ, then perhaps components in the snacks altered (limited) fat accumulations in the fat pads.
The glucoregulatory differences between the 2 groups of marmosets were pronounced, but there are limitations to this study. Our methods did not include measuring food intake to determine the total amount of calories consumed per subject for the 2 treatments. The addition of snack foods could have reduced the intake of normal chow, although we noted no differences in the amount of leftover chow. In addition, because the snacks were not weighed, the exact amount of supplements per animal cannot be ascertained. Without determining the amount the marmosets were eating each day, we cannot determine the proportions of nutrients provided by the snack foods and whether the control animals had consumed more or fewer calories. Finally, because we fed the high-fat snack food for only 3 mo, we are unable to ascertain whether the differences between the 2 groups would persist. These data, however, do suggest that increasing the composition of balanced fatty acids in the diet may lead to improvements in health.
Both controls and snack-fed marmosets were normal, healthy, and appropriate in weight for their age. Although changes in metabolic function were apparent from the beginning of the study to the last month, it appears that the high-fat–high-protein snacks facilitated a stabilization of the metabolic system that didn't occur in the control marmosets. Longer-term studies of high-fat–high-protein additions to the diet of these small primates likely would provide important additional information regarding longitudinal outcomes of balanced fats in complete food.
Acknowledgments
We thank Maisie Buntin and Lara Bowers for assisting with snack feeding. We thank Fritz Wegner and Dan Wittwer for performing insulin and adiponectin assays and Dr Amita Kapoor for the mass spectrometric analyses of cortisol and cortisone. The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427, and by the WNPRC through the NIH Office of Research Infrastructure Programs (ORIP), base grant RR000167.
References
- 1.Bourre JM. 2004. Roles of unsaturated fatty acids (especially ω3 fatty acids) in the brain at various ages and during ageing. J Nutr Health Aging 8:163–174 [PubMed] [Google Scholar]
- 2.Bremer AA, Stanhope KL, Graham JL, Cummings BP, Wang W, Saville BR, Havel PJ. 2011. Fructose-fed rhesus monkeys: a nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin Transl Sci 4:243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. [Internet] 2012. National health and nutrition examination survey. [Cited 13 March 2013]. Available at: http://www.cdc.gov/nchs/nhanes.htm.
- 4.Dehghan M, Akhtar-Danesh N, Merchant AT. 2005. Childhood obesity: prevalence and prevention. Nutr J 4:24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finke MD. 2002. Complete nutrient composition of commercially raised invertebrates used as a food for insectivores. Zoo Biol 21:269–285 [Google Scholar]
- 6.Fong TM. 2004. Targeting metabolic syndrome. Expert Opin Investig Drugs 13:1203–1206 [DOI] [PubMed] [Google Scholar]
- 7.Frensham LJ, Bryan J, Parletta N. 2012. Influences of micronutrient and ω3-fatty acid supplementation on cognition, learning, and behavior: methodological considerations and implications for children and adolescents in developed societies. Nutr Rev 70:594–610 [DOI] [PubMed] [Google Scholar]
- 8.Gazzaniga JM, Burns TL. 1993. Relationship between diet composition and body fatness, with adjustment for resting energy expenditure and physical activity, in preadolescent children. Am J Clin Nutr 58:21–28 [DOI] [PubMed] [Google Scholar]
- 9.Gherlan I, Vladoiu S, Alexiu F, Giurcaneanu M, Oros S, Brehar A, Procopiuc C, Dumitrache C. 2012. Adipocytokine profile and insulin resistance in childhood obesity. Maedica (Buchar) 7:205–213 [PMC free article] [PubMed] [Google Scholar]
- 10.Hivert MF, Sullivan LM, Fox CS, Nathan DM, D'Agostino RB, Sr, Wilson PW, Meigs JB. 2008. Associations of adiponectin, resistin, and tumor necrosis factor α with insulin resistance. J Clin Endocrinol Metab 93:3165–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hivert MF, Sun Q, Shrader P, Mantzoros CS, Meigs JB, Hu FB. 2011. Higher adiponectin levels predict greater weight gain in healthy women in the Nurses’ Health Study. Obesity (Silver Spring) 19:409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooper L, Summerbell CD, Thompson R, Sills D, Roberts FG, Moore H, Davey Smith G. 2011. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev 7:CD002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press [Google Scholar]
- 14.Kerstens MN, Riemens SC, Sluiter WJ, Pratt JJ, Wolthers BG, Dullaart RP. 2000. Lack of relationship between 11β-hydroxysteroid dehydrogenase setpoint and insulin sensitivity in the basal state and after 24 h of insulin infusion in healthy subjects and type 2 diabetic patients. Clin Endocrinol (Oxf) 52:403–411 [DOI] [PubMed] [Google Scholar]
- 15.Ley SH, Harris SB, Connelly PW, Mamakeesick M, Gittelsohn J. 2008. Adipokines and incident type 2 diabetes in an Aboriginal Canadian population: the Sandy Lake Health and Diabetes Project. Diabetes Care 31:1410–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Shin HJ, Ding EL, van Dam RM. 2009. Adiponectin levels and risk of type 2 diabetes: a systematic review and metaanalysis. JAMA 302: 179–188 [DOI] [PubMed] [Google Scholar]
- 17.Mansfield K. 2003. Marmoset models commonly used in biomedical research. Comp Med 53:383–392 [PubMed] [Google Scholar]
- 18.Mitura A, Liebert F, Schlumbohm C, Fuchs E. 2012. Improving the energy and nutrient supply for common marmoset monkeys fed under long-term laboratory conditions. J Med Primatol 41:82–88 [DOI] [PubMed] [Google Scholar]
- 19.Muniyappa R, Lee S, Chen H, Quon MJ. 2008. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 294:E15–E26 [DOI] [PubMed] [Google Scholar]
- 20.Murdolo G, Nowotny B, Celi G, Donati M, Bini V, Papi F, Gornitzka G, Castellani S, Roden M, Falorni A, Herder C, Falorni A. 2011. Inflammatory adipokines, high-molecular–weight adiponectin, and insulin resistance: a population-based survey in prepubertal schoolchildren. PLoS ONE 6:e17264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obarzanek E, Schreiber GB, Crawford PB, Goldman SR, Barrier PM, Frederick MM, Lakatos E. 1994. Energy intake and physical activity in relation to indexes of body fat: the National Heart, Lung, and Blood Institute Growth and Health Study. Am J Clin Nutr 60:15–22 [DOI] [PubMed] [Google Scholar]
- 22.Plentz RRPV, Wiedemann A, Diekmann U, Glage S, Naujok O, Jörns A, Müller T. 2012. Islet microarchitecture and glucose transporter expression of the pancreas of the marmoset monkey display similarities to the human. Islets 4:123–129 [DOI] [PubMed] [Google Scholar]
- 23.Rizza S, Clementi F, Porzio O, Cardellini M, Savo A, Serino M, Chiricolo G, Romeo F, Lauro R, Federici M. 2009. Adiponectin isoforms are not associated with the severity of coronary atherosclerosis but with undiagnosed diabetes in patients affected by stable CAD. Nutr Metab Cardiovasc Dis 19:54–60 [DOI] [PubMed] [Google Scholar]
- 24.Roche HM. 2005. Fatty acids and the metabolic syndrome. Proc Nutr Soc 64:23–29 [DOI] [PubMed] [Google Scholar]
- 25.Ryan E, Galvin K, O'Connor TP, Maguire AR, O'Brien NM. 2006. Fatty acid profile: tocopherol, squalene, and phytosterol content of Brazil, pecan, pine, pistachio, and cashew nuts. Int J Food Sci Nutr 57:219–228 [DOI] [PubMed] [Google Scholar]
- 26.Stevenson MF. 1977. The common marmoset (Callithirx jacchus jacchus) as a model of ethological research. Lab Anim Sci 27:895–900 [PubMed] [Google Scholar]
- 27.Tardif SD, Power ML, Ross CN, Rutherford JN, Layne-Colon DG, Paulik MA. 2009. Characterization of obese phenotypes in a small nonhuman primate, the common marmoset (Callithrix jacchus). Obesity (Silver Spring) 17:1499–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomsen C, Rasmussen O, Christiansen C, Pedersen E, Vesterlund M, Storm H, Ingerslev J, Hermansen K. 1999. Comparison of the effects of monounsaturated fat diet and high carbohydrate diet on cardiovascular risk factors in first-degree relatives to type 2 diabetic subjects. Eur J Clin Nutr 53:818–823 [DOI] [PubMed] [Google Scholar]
- 29.Tomlinson JW, Finney J, Hughes BA, Hughes SV, Stewart PM. 2008. Reduced glucocorticoid production rate, decreased 5α reductase activity, and adipose tissue insulin sensitization after weight loss. Diabetes 57:1536–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.United States Department of Agriculture. [Internet] 2012. Dietary reference intakes: macronutrients. [Cited 13 March 2013]. Available at: http://fnicnalusdagov/dietary-guidance/dietary-reference-intakes/dri-tables.
- 31.Valle M, Gascon F, Martos R, Bermudo F, Ceballos P. 2003. Relationship between high plasma leptin concentration and metabolic syndrome in obese prepubertal children. Int J Obes Relat Metab Disord 27:13–18 [DOI] [PubMed] [Google Scholar]
- 32.van de Rest O, van Hooijdonk LWA, Doets E, Schiepers OJG, Eilander A, de Groot LGPM. 2012. B vitamins and n3 fatty acids for brain development and function: review of human studies. Ann Nutr Metab 60:272–292 [DOI] [PubMed] [Google Scholar]
- 33.Wachtman LM, Kramer J, Miller A, Hachey A, Curran E, Mansfield K. 2011. Differential contribution of dietary fat and monosaccharide to metabolic syndrome in the common marmoset (Callithrix jacchus). Obesity (Silver Spring) 19:1145–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziegler TE, Colman RJ, Tardif SD, Sosa ME, Wegner FH, Wittwer DJ, Shrestha H. 2013. Development of metabolic function biomarkers in the common marmoset, Callithirx jacchus. Am J Primatol 75:500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziegler TE, Prudom SL, Zahed SR, Parlow AF, Wegner F. 2009. Prolactin's mediative role in male parenting in parentally experienced marmosets (Callithrix jacchus). Horm Behav 56:436–443 [DOI] [PMC free article] [PubMed] [Google Scholar]