Abstract
Detecting and controlling murine fur mites continues to be challenging. Here we compared the efficacy of fur-pluck, cage PCR, and fur PCR testing of mice naturally infested with Myocoptes musculinus and make recommendations regarding the application of these diagnostic strategies in aged or treated mice. We compared all 3 diagnostic methods in groups of infested and noninfested control mice over time. For fur plucks, we used a scoring system to quantitatively compare mite infestations across ages. Mice that were 4 wk old had higher egg and mite scores than did older mice, with average scores at 4 wk corresponding to 40 to 100 individual fur mites and eggs per sample. Furthermore, 15% and 20% of samples from infested mice at 24 and 28 wk of age, respectively, lacked all fur mites and eggs. Cage PCR results varied as mice grew older. Fur PCR testing was the most sensitive and specific assay in untreated infested mice, particularly when mite densities were low. In addition, we compared fur-pluck and fur PCR tests for evaluating the efficacy of selamectin treatment. Two treatments with selamectin eliminated Myocoptes fur-mite infestations. At 8 wk after treatment, all fur-pluck samples were negative, but one-third of treated infested cages remained positive by fur PCR assay; at 16 wk after treatment, all cages were negative by fur PCR assay. Because offspring of infested mice were invariably heavily infested, breeding of suspected infested mice with subsequent testing of offspring was the definitive testing strategy when fur-pluck and PCR results conflicted.
Despite advances in laboratory animal colony management, murine fur mites continue to be challenging to detect and control in laboratory mice, with as many as 40% of facilities self-reporting the presence of fur mites in their rodent colonies.4 Murine acariasis is commonly caused by Myocoptes musculinus, Myobia musculi, and Radfordia affinis. All 3 species frequently are excluded from laboratory mouse colonies because of their deleterious effects on animal health and potential to confound ongoing research. Specifically, fur mite infestations provoke a Th2 immune response, alter inflammatory cytokines, and elevate serum IgE.12,13,18 Pathologic changes due to fur mite infestation include lymphadenopathy, hypergammaglobulinemia, secondary amyloidosis, lymphocytopenia, and splenic hypertrophy.1 Clinical manifestations vary from none to severe dermatitis with ulceration and pyoderma, depending on mouse strain5,6 and the species of mite, with M. musculi more commonly causing clinical disease.1 Interestingly, the severity of lesions does not appear to be directly related to the number of mites,25 but lesions may be more severe in older mice.8 Although clinical conditions may resolve with treatment, immunologic and pathologic changes due to fur mite infestation can persist even after treatment.8,11 Therefore, the existence of fur mites within many laboratory mouse colonies is a cause for concern. Because the failure to detect and treat even a few fur mites represents a source for reinfestation,1 the need for clear recommendations regarding accurate surveillance and effective treatment of fur mite infestations is evident.
Fur mites persist in modern laboratory mouse colonies largely because of difficulties in detection. One source of error is the diagnostic technique. Microscopic examination of impressions, fur plucks, and skin scrapes prepared in mineral oil or on cellophane tape; sticky paper applied postmortem; pooled fecal floats; and direct examination of the pelt of either anesthetized or euthanized mice have all been used.1,3,14,16,20,24,26 However these traditional methods, even when conducted by commercial laboratories, have the potential for false-negative results due to factors including the selection of the wrong test animal, low mite yield, wrong sampling site, hair overlap, and technical error.2,16,20,24 Recently, diagnosis by PCR assay has become commercially available, but information regarding its efficacy compared with that of traditional techniques is limited.26 Fur pluck remains a common diagnostic screening tool at many institutions, likely owing to its low cost, relative technical ease, and minimal deleterious effect on tested subjects. Here we report our investigation regarding whether PCR analysis of fur swabs or environmental cage samples was more sensitive in detecting mice infested with M. musculinus than was the fur-pluck technique customarily used at our institution.
Another potential source of error is the failure to select appropriate animals for testing. Soiled-bedding sentinels frequently are used for colony surveillance, but their effectiveness is highly controversial, with some reports describing successful detection of fur mites and others showing that using sentinels is quite ineffective in this context.14,15,20,24 An alternative strategy is to sample colony mice, but information regarding which animals are most likely to yield positive results is limited. It is known that monoinfestations of both M. musculi and M. musculinus increase rapidly in neonatal mice.6,14 Furthermore, M. musculi populations decrease in numbers concurrent with the development of host immunity and then subsequently equilibrate and then demonstrate cyclical fluctuations (cycle length of 20 to 25 d, presumably related to the 23-d life cycle of mites) in population size.6,7 However, only sparse information on population fluctuations in M. musculinus is available: a single previous report suggested that population densities varied and that group-housed animals were more likely to yield positive results than were than single-housed mice.16 Here we investigated how M. musculinus populations varied with age and determined the most appropriate age of mice for sampling.
Posttreatment testing may be confounded by false-positive results if lingering eggs of unknown viability that are cemented to hair shafts are detected. Indeed, it may take more than 8 mo for the entire fur coat to be replaced completely.20 Similarly, false-positive PCR tests after treatment could result from the persistence of mite DNA in the haircoat. Here we documented how soon diagnostic testing by fur-pluck and by fur PCR analysis correctly identified mice that had been treated successfully with selamectin. Finally, we evaluated whether the mite status of mice with low or undetectable populations of mites could be identified by breeding them and evaluating their offspring.
Materials and Methods
Animals.
Infested mice were wildtype mice on a mixed B6×129 background and donated from a research colony naturally infested with a monoinfection of M. musculinus. Infestation was diagnosed initially by direct microscopic examination of fur-pluck samples and confirmed by microscopic pelt examination and fur-mite PCR analysis with speciation (Charles River, Wilmington, MA). Negative controls were from a breeding colony of FVB/NCrl mice (Charles River) that was maintained at Johns Hopkins University. All animals were maintained on a 14:10-h light:dark cycle in individually ventilated cages within the rodent quarantine facility. Feed consisted of autoclaved rodent chow (2018S, Harlan Teklad, Indianapolis, IN), and all cages contained autoclaved corncob bedding (Harlan) and a cotton enrichment square. Reverse-osmosis–purified water was provided through an automated watering system (Rees Scientific, Trenton, NJ). Cages were changed every 2 wk in a dedicated change station (Lab Products, Seaford, DE) by using a chlorine dioxide disinfectant (Vimoba, Quip Laboratories, Wilmington, DE). Health surveillance was conducted by using a soiled-bedding sentinel system, and mice were considered negative for Sendai virus, pneumonia virus of mice, mouse hepatitis virus, mouse minute virus, mouse parvovirus 1 and 2, mouse encephalomyelitis virus, reovirus, epizootic diarrhea of infant mice, lymphocytic choriomeningitis virus, ectromelia virus, murine adenovirus, murine cytomegalovirus, Mycoplasma pulmonis, and Aspiculuris and Syphacia spp. pinworms. The animal care and use program was accredited by AAALAC International, and all procedures were IACUC-approved. Research was done according to the principles set forth in the Guide for the Care and Use of Laboratory Animals.9
Diagnostic sampling.
Fur-pluck test.
Fur samples were obtained by manual removal of fur from the nape of the neck, posterior dorsum, and ventral inguinal region of each animal; fur was placed on clear cellophane tape and adhered to precleaned glass microscope slides (Fisher Scientific, Waltham, MA). All fur-pluck samples were blinded prior to evaluation by randomly assorting samples and assigning a coded nondescript numerical identification. Fur-pluck samples were considered positive when there was any evidence of mites or eggs (including egg casings or mite parts). Cages were considered positive for fur mite infestation when at least one mouse in the cage was positive for fur mites.
Cage PCR test.
Cages were sampled by using sterile flocked swabs (Puritan Medical Products, Guilford, ME) as recommended by the commercial laboratory for obtaining environmental samples for fur-mite PCR analysis. The flocked end of the swab was passed through cage bedding around the complete periphery of the cage and then crossed through the center of the cage twice. The swab was cut approximately 1 cm above the flocked tip, and the flocked end was placed in a sealed microcentrifuge tube (USA Scientific, Ocala, FL) for shipping to the commercial laboratory (Charles River) for PCR analysis.
Fur PCR test.
Fur swabs were taken by using sterile adhesive swabs (Puritan, Guilford, ME) as recommended by the commercial laboratory for obtaining direct fur samples for fur mite PCR analysis. The pink tip of the swab was used to aggressively massage along the neck, back, and belly of mice in the opposite direction to fur growth. Swab tips were cut from the shaft into microcentrifuge tubes, stored, and shipped in the same manner as for cage PCR samples.
Scoring system.
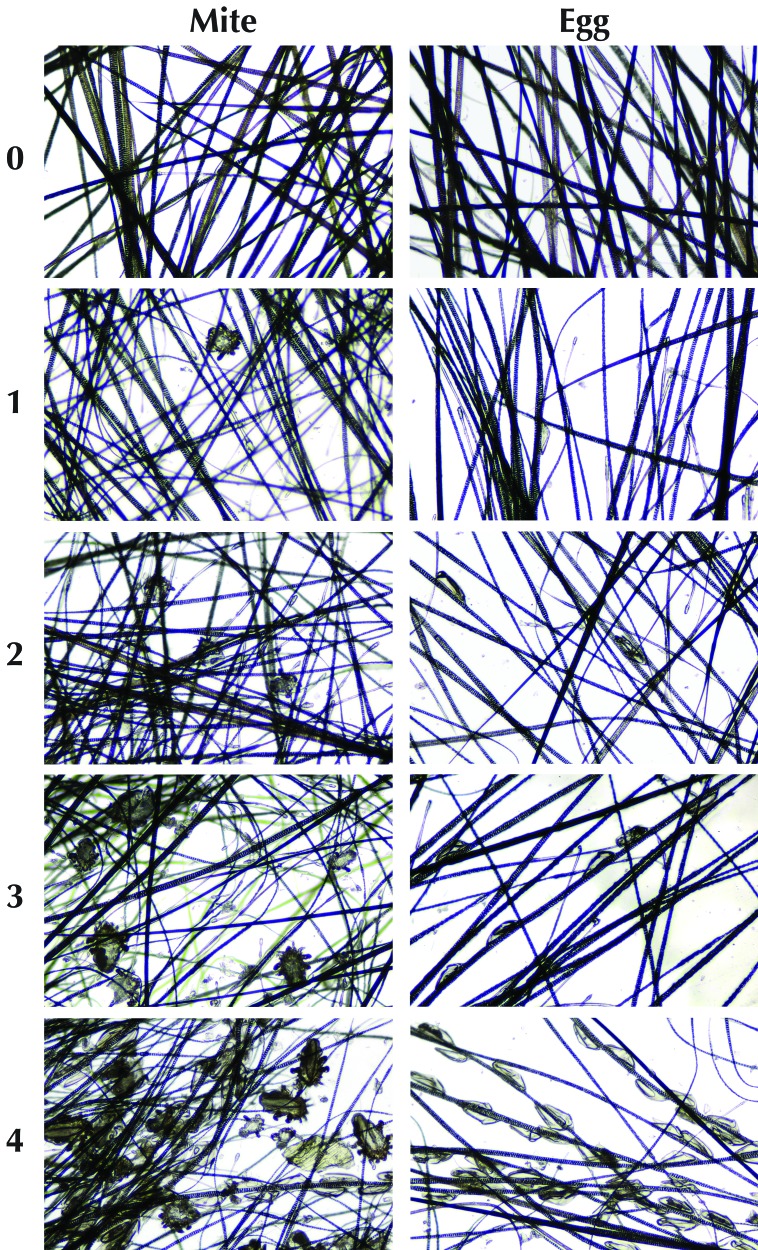
To quantify the density of infestations, a scoring system was developed for microscopic examination of fur-pluck slides. Separate scores were assigned for eggs and mites. All mites or eggs on a slide were counted, and a score between 0 and 4 was assigned (Figure 1), for which 0 represented no mites or eggs per slide and 4 represented more than 50 mites or eggs per slide. Slides were evaluated microscopically by using the 4× objective lens and scored by an experienced technician who was blinded to the source of the slides.
Figure 1.
System for scoring egg and mite burden from fur pluck samples. Score of 0, no eggs or mites; 1, 1 to 5 eggs or mites per slide; 2, 6 to 20 eggs or mites; 3, 21 to 50 eggs or mites; and 4, greater than 50 eggs or mites per slide. Examples are shown as single high-powered field of an individual sample by using the 4× objective.
Aging study.
To evaluate changes in the density of mite infestation over time, fur plucks were examined from a cohort of infested mice at different time points as they aged. A group of 42 mice (21 of each sex) from fur-mite–positive breeding cages was weaned at 3 wk of age and housed 3 per cage in same-sex groups. Fur-pluck samples from mice at the following ages were scored: 4 wk, n = 25; 6 wk, n = 12; 8 wk, n = 9; 24 wk, n = 26; and 28 wk, n = 10. In addition, samples from age-matched negative controls (n = 18) were included, with at least one negative control scored for each time point. The first 100 slides from the mixed, blinded study slides were selected for review, which resulted in unequal numbers of slides for each age group. The results of this study were used in developing the diagnostic methods study. To further quantify the change in fur-mite density in individual infested mice as they aged, the total numbers of individual eggs and fur mites were counted for all fur-pluck samples obtained from male mice in this cohort at 4 wk (n = 21) and 24 wk (n = 21) of age.
Diagnostic methods study.
To compare the efficacy of different diagnostic methods in diagnosing mite infestation, another cohort of mice was evaluated by 3 different methods: microscopy of fur-pluck samples, environmental cage PCR testing, and direct PCR analysis of animals. Test mice consisted of weanlings from fur-mite–positive breeding cages. All mice were housed in groups of 3 by sex, for a total of 2 cages of infested female mice, 6 cages of infested male mice, and 8 cages of uninfested female age-matched negative control mice.
Mice were evaluated at 4, 6, 8, 10, 12, 14, and 16 wk of age by fur-pluck, cage PCR, and direct fur PCR analysis. Fur plucks were obtained from every mouse in each cage, and a single fur PCR swab was used to sample all 3 mice in each cage. Cage and fur PCR swabs were sent to the commercial laboratory for evaluation. The swab samples were coded so that the diagnostic lab was blinded to the samples submitted. At the testing laboratory, DNA was isolated from sample swabs by automated magnetic isolation and screened for fur-mite DNA by using 2 PCR assays, which were specific for the 18S ribosomal RNA genes of the Myobia and Radfordia genera and the Myocoptes genus. Initial positive results were confirmed by repeat testing. Species-specific 18S rRNA PCR assays for M. musculi, R. affinis, R. ensifera, and M. musculinus were used only during the initial evaluation of the study group to confirm that only Myocoptes was present in the study population. A PCR inhibition control was used to monitor samples for PCR inhibition.19 PCR inhibition was not detected in any samples. An estimate of copy number per microliter of eluted DNA was determined from the cycle threshold value obtained for the 100-copy control.23
Treatment study.
This study was undertaken to evaluate the ability of different diagnostic methods to document the disappearance of fur mites after treatment with selamectin.
Mice.
Test mice consisted of 2 groups: aged adult mice (chronic, sparsely infested) and juvenile mice (postnatal transmission, heavily infested). All mice were housed in same-sex trios as previously described. At the start of the study, adult mice were 28 wk old and juvenile mice were 5 wk old. A total of 8 cages of infested mice (4 male, 4 female) and 6 cages (all female) of negative controls were used for the adult treatment group. For the juvenile treatment group, 8 cages (4 male, 4 female) of infested mice and 8 cages (all female) of negative controls were included.
Treatment.
Six cages of mice (3 of each sex) from each age group were treated to eliminate fur-mite infestations. Two cages of infested mice (1 of each sex) from each age group served as positive controls and did not receive treatment. Treatment comprised topical application of selamectin (10 mg/kg; Revolution, Pfizer New York, NY) to the nape of the neck. Selamectin was applied twice (with 4 wk between applications) to every mouse in treatment cages. Mice in negative control cages were not treated.
Testing.
Mice were tested by individual fur-plucks, cage PCR, and fur PCR analysis at time 0 (immediately before the start of treatment), at 4 wk (immediately prior to the second treatment), and at 12 and 20 wk after the initial treatment. Fur plucks were obtained from every mouse in each cage. A single fur PCR swab was used to sample all 3 mice in each cage. Cage PCR swabs for each cage were obtained as previously described. All fur-pluck samples were blinded prior to evaluation of positive or negative status. PCR samples were blinded and sent to the commercial laboratory for analysis.
Diagnostic breeding.
Because diagnosis by fur pluck is prone to false-negative results and because the validity of PCR testing after treatment is under investigation, we needed an additional method for confirming the presence or absence of infestation after treatment. Our previous experience in breeding and maintaining this mite-infested colony had shown that the offspring of infested but fur-pluck–negative mice had numerous, easily detectable mites. Therefore, to confirm the efficacy of selamectin treatment and the results of posttreatment fur-pluck and fur PCR diagnostic testing, we conducted a diagnostic breeding study. Specifically, this study aimed to confirm that the breeding of suspected fur-mite–positive mice with subsequent testing of offspring could serve as a definitive strategy for the identification of active fur-mite infestations. Mice from the juvenile treatment group were 23 wk old at the time of breeding; those from the adult treatment group were 46 wk of age at breeding. Fur-mite–negative FVB mice (age, approximately 6 wk; Charles River) were bred with the untreated positive controls (4 cages) and selamectin-treated mice (12 cages) at the conclusion of the treatment study. Study cages housing female mice were made into harem breeding cages (3 female, 1 male) by the addition of an FVB male mouse. Male study animals were separated into breeding pairs with FVB female mice. Positive controls consisted of untreated female breeding cages (3 infested female mice and 1 naïve FVB male mouse) and untreated male breeding cages (1 infested male mouse and 1 naïve FVB female mouse). Treatment cages consisted of treated female breeding cages (3 treated female mice and 1 naïve FVB male mouse) and treated male breeding cages (1 treated male mouse and 1 naïve FVB female mouse). Follow-up testing was conducted only on breeding cages that produced litters, and testing occurred when litters reached 2 to 3 wk of age. Every animal in each breeding cage was fur-plucked. Separate fur PCR swabs were obtained from adults and pups. All fur-pluck samples were blinded prior to determination of positive or negative status. PCR samples were blinded and sent to the commercial laboratory for analysis.
Statistical analysis.
All results were evaluated by using a commercial statistical software package (Prism, GraphPad Software, San Diego, CA). For statistical analysis of PCR cycle threshold values, an arbitrary value of 40 was assigned when no fur mite DNA was detected. Because the statistical tests varied with the type of data, the individual tests used are listed with the results for each study segment. For the purposes of analysis with regard to sensitivity and specificity, cages were considered positive when they were positive initially (that is, live mites detected on fur pluck) and remained positive (live mites detected at the end of the study on mice or their offspring by fur pluck).
Results
Aging study.
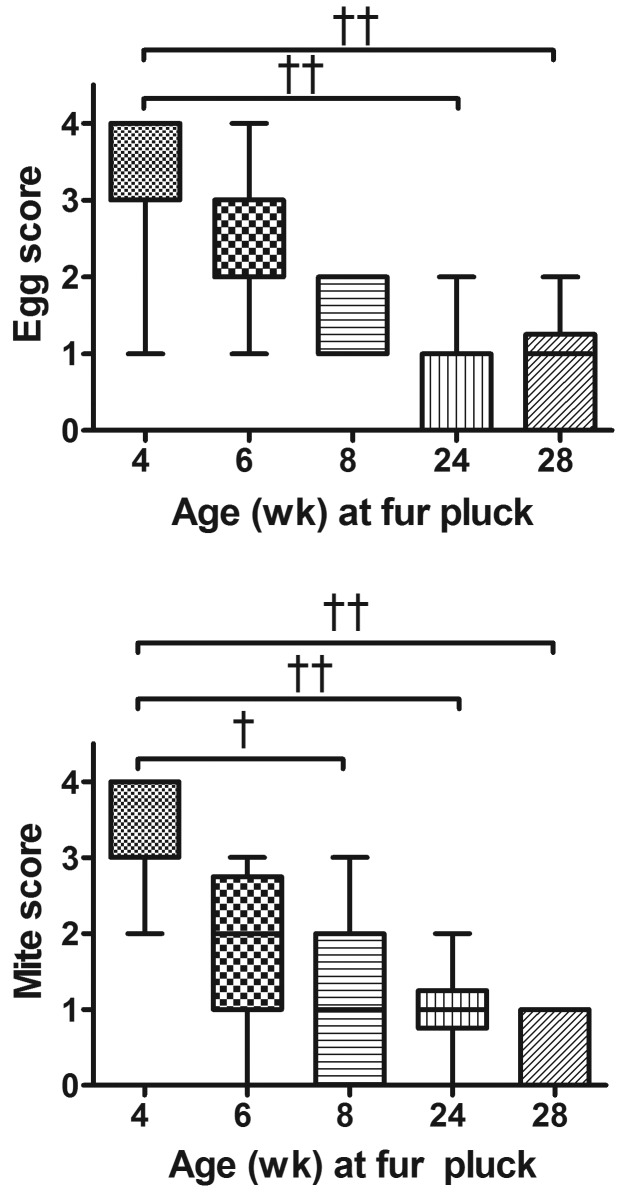
Mite and egg scores for all time points are shown in Figure 2. Egg scores from infested mice were significantly higher in samples obtained when mice were 4 wk of age than in those from infested mice at 24 or 28 wk of age (Kruskal–Wallis, P < 0.0001; posthoc Dunn multiple-comparison test, P < 0.001 for both). Similarly, mite scores for samples from infested mice at 4 wk of age were significantly higher than those of samples from infested mice at 8, 24, or 28 wk of age (Kruskal–Wallis, P < 0.0001; posthoc Dunn multiple-comparison test: P < 0.01, P < 0.001, and P < 0.001, respectively). The average mite and egg scores of fur-pluck samples from mice at 4 wk of age were greater than 3, indicating an average of 40 to 100 individual fur mites and eggs per slide. Mite and egg scores both decreased with the age of the mice sampled, such that by 28 wk, average scores were less than l, indicating fewer than 1 to 5 fur mites or eggs per slide. Overall, chronically infested adult mice were sparsely infested (combined mite and egg score of 3 or less), and postnatally infested juvenile mice were heavily infested (combined mite and egg score of 4 or greater). Furthermore, 15% and 20% of fur-pluck samples from mice at 24 and 28 wk of age, respectively, revealed no fur mites or eggs at all. All mice were confirmed to be positive at 24 and 28 wk by fur-mite PCR testing. Blinded negative control samples (n = 18) at all time points received mite and egg scores of 0 and were negative by PCR. The total number of individual mites or eggs on each fur-pluck sample from male infested animals at 4 wk of age were significantly higher than corresponding those from samples from the same infested animals at 24 wk of age (Mann–Whitney: P < 0.0001 for eggs, P < 0.0001 for mites; Figure 3). Fur-pluck samples from 4 wk old infested male mice had an average of 130.5 individual eggs and 68.1 individual mites per slide. Fur-pluck samples from these same mice at 24 wk of age had an average total of 0.7 individual eggs and 1.1 individual mites per slide.
Figure 2.
(A) Eggs scores for samples from infested mice at 4 wk of age were significantly higher than samples from infested mice at 24 wk and 28 wk of age (Kruskal–Wallis: P < 0.0001, followed by posthoc Dunn multiple-comparison test: ‡, P < 0.001 for both). (B) Mite scores for samples from infested mice at 4 wk of age were significantly higher than those of samples from infested mice at 8, 24, and 28 wk of age (Kruskal–Wallis: P < 0.01; P < 0.0001; followed by posthoc Dunn multiple-comparison test: †, P < 0.01, ‡, P < 0.001, and P < 0.001, respectively).
Figure 3.
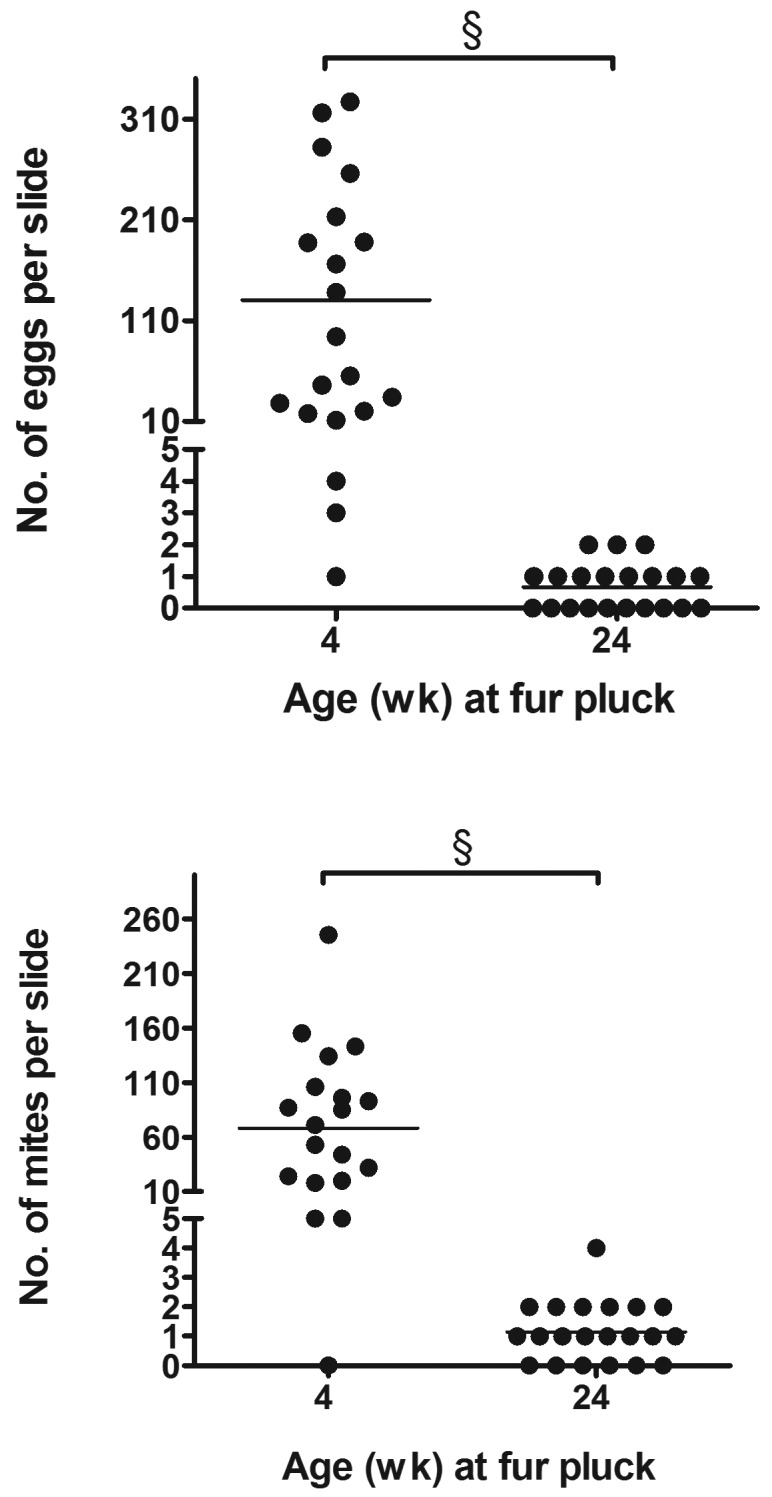
The total numbers of individual mites or eggs per fur pluck assay were significantly higher for samples from 4-wk-old mice than for samples from the same mice at 24 wk of age (Mann–Whitney: §, P < 0.0001 for both eggs and mites).
Diagnostic methods study.
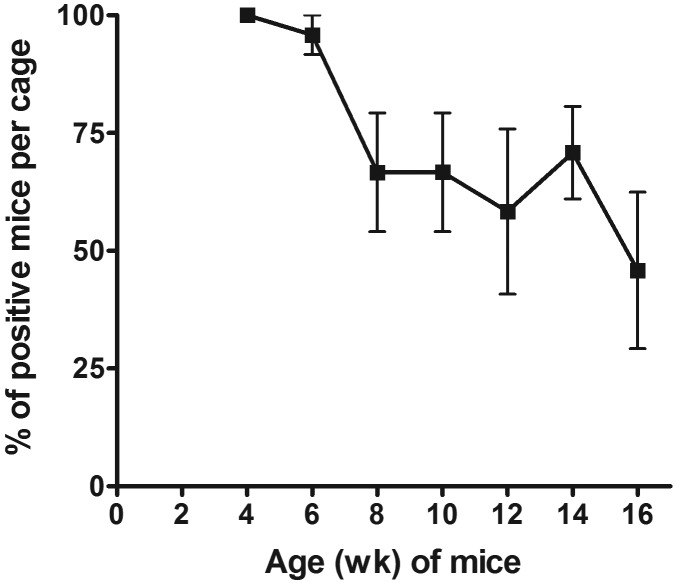
The sensitivity and specificity were determined for each diagnostic method (8 infested cages, 8 uninfested controls) at every time point. The specificity was 100% for fur pluck at all time points. The specificity was 100% for cage PCR and fur PCR testing, except at 8 and 6 wk, respectively, for which it was 88%. The sensitivities are given in Figure 4. Some variation was noted for all 3 diagnostic methods, with fur PCR analysis being the most consistent method and cage PCR testing being the least consistent strategy. All methods were the least sensitive when the mice were 12 wk old (Figure 4). Fur-pluck analysis was 100% sensitive at 4, 6, and 8 wk but was less sensitive at subsequent time points. The percentage of mice in each cage that was positive by fur pluck (n = 8) decreased significantly (Friedman test, P = 0.0025) as the mice aged (Figure 5); therefore we assume that the sensitivity would have been lower if all mice in the cage had not been sampled. The sensitivity of fur PCR testing remained 100% for every time point except 12 wk, and fur PCR testing detected significantly more positive infested cages than did cage PCR when all time points are considered together (Friedman test, P = 0.0084; posthoc Dunn multiple-comparison test, P < 0.05). Furthermore, cycle threshold values were lower, indicating higher copy number, for fur PCR when compared with cage PCR at 4 wk (2-way repeated-measures ANOVA, P = 0.0345; posthoc Bonferroni multiple comparison, P < 0.01).
Figure 4.
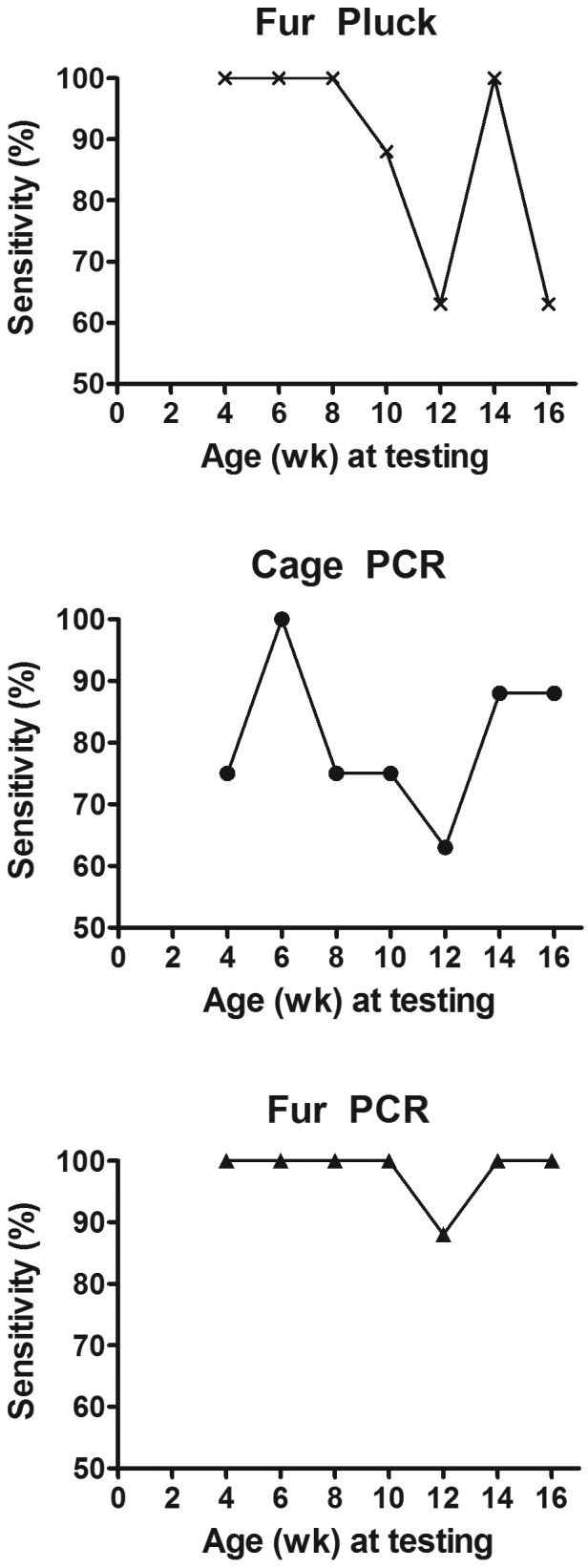
The sensitivities of fur pluck, cage PCR, and fur PCR assays of infested mice and noninfested control mice over time. When all time points were considered together, fur PCR detected significantly more positive cages than did cage PCR (Friedman test, P = 0.0084; posthoc Dunn multiple-comparison test, P < 0.05).
Figure 5.
The percentage of fur-pluck–positive mice in each cage decreased significantly (Friedman test: P = 0.0025) over time.
Treatment study.
Treatment.
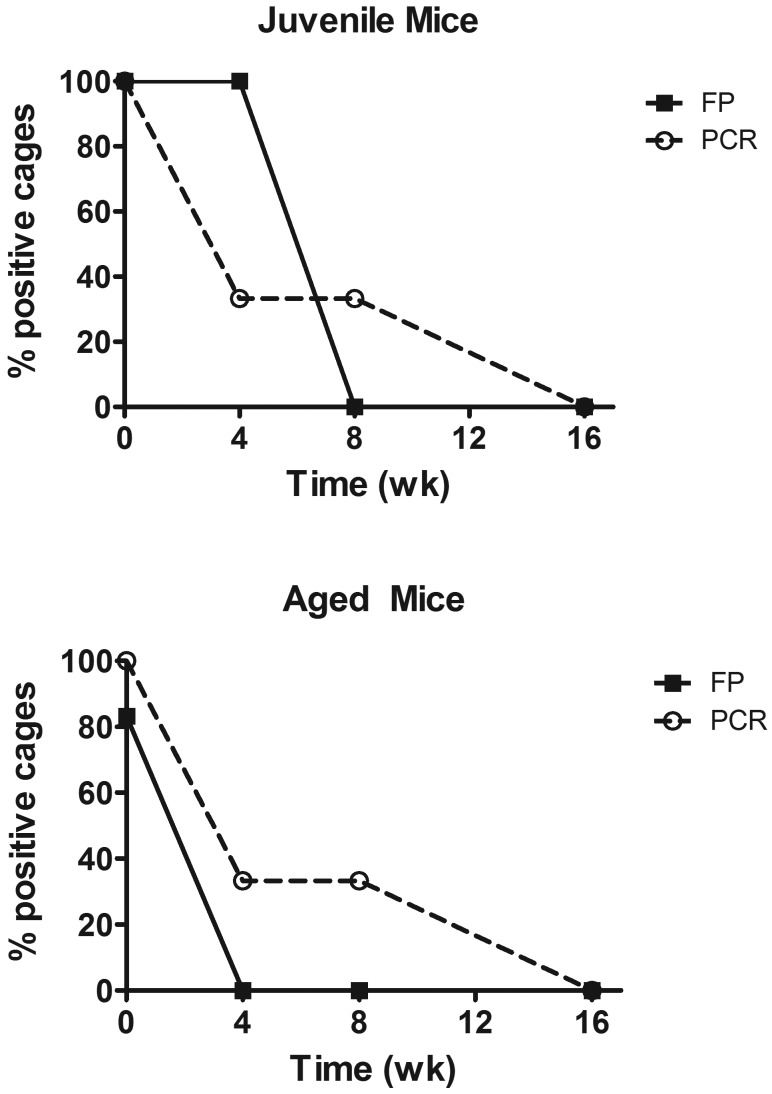
The percentages of juvenile and adult mice that individually tested positive by fur pluck and fur PCR at 0, 4, 8 and 16 wk after selamectin treatment are listed in Table 1. Prior to treatment, fur pluck detected all juvenile (heavily infested) mice but failed to detect some sparsely infested adults. Fur PCR detected all infested cages. At the 4-wk retreatment, both fur-pluck and fur PCR tests still detected evidence of fur mites, although results differed between juveniles and adults (Figure 6). Particularly, all of the treated juvenile cages (n = 6) remained positive by fur-pluck analysis. Because mice were treated again at this time point, the success of the initial treatment—and thus the accuracy of the 4-wk test results—was not known. At 8 wk after the first treatment, all fur-pluck tests for both juvenile and adult mice (n = 36) were negative, but one third of the fur PCR tests (4 of 12) were still positive (Figure 6). The individual cages identified as fur-mite positive by fur PCR testing after treatment differed at 4 and 8 wk; that is, the 2 positive cages at 4 wk were then negative at 8 wk and vice versa. By 16 wk, all fur-pluck (n = 36) and fur PCR (n = 12) tests were negative from all treated cages (juvenile, n = 6; aged, n = 6). Subsequent breeding (see following) confirmed that mites had been eliminated from the treated mice. Fur-pluck samples from negative control cages (juvenile, n = 8; aged, n = 6) were negative at all time points, but 3 false-positive results were obtained among the 54 fur PCR tests conducted on negative control cages.
Table 1.
Number of cages that tested positive by fur pluck (FP) and fur PCR assays among treated infested, untreated infested positive control, and uninfested negative control mice
| Time (wk) past initial treatment | |||||||||
| 0 | 4 | 8 | 16 | ||||||
| FP | PCR | FP | PCR | FP | PCR | FP | PCR | ||
| Juvenile mice | Treated (6 cages) | 6 | 6 | 6 | 2 | 0 | 2 | 0 | 0 |
| Positive controls (2 cages) | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | |
| Negative controls (8 cages) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Adult mice | Treated (6 cages) | 5 | 6 | 0 | 2 | 0 | 2 | 0 | 0 |
| Positive controls (2 cages) | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | |
| Negative controls (6 cages) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
Juvenile mice were 5 wk old at the start of the study; adult mice were 28 wk old. Selamectin was administered to treated cages at 0 (initial treatment) and 4 wk.
Figure 6.
The percentages of positive cages as determined by fur pluck (FP) and fur PCR assay among infested cages of juvenile mice (n = 6) and aged mice (n = 6) after 2 treatments with topical selamectin (that is, at 0 and 4 wk).
Breeding.
Not all breeding cages resulted in successful matings. Litter sizes ranged from 3 to 12 pups. Study cages consisted of 7 positive control cages (2 litters from untreated female mice and 5 litters from untreated male mice) and 20 treatment cages (5 litters from treated female mice and 15 litters from treated male mice).
Fur-pluck testing of breeding cages.
All positive control cages (female, n = 2; male, n = 5) were positive by fur-pluck testing. In female positive control cages, all mice (pups and adults) were individually positive by fur pluck. In male positive control cages, all pups were positive in 2 of 5 cages; in the other 3 cages, at least 3 pups in each litter were positive by fur pluck. Only 3 of the 10 adults from male positive control cages were positive by fur-pluck analysis. All adults and pups (n = 213) from treated female cages (n = 5) and treated male cages (n = 15) were negative by fur pluck.
Fur PCR of breeding cages.
All positive control cages (female, n = 2; male, n = 5) were positive according to fur PCR testing of pups. Fur PCR analysis of adults detected 5 of 7 positive control cages; the 2 undetected cages were cages of untreated male mice and represented both age groups. Adults in these 2 cages also were negative by fur pluck. All fur PCR tests for adults from female (n = 5) and male (n = 15) treated cages were negative for fur mites. Fur PCR analysis of pups was negative for 18 of 20 selamectin-treated cages; in the 2 cages that tested positive, adults had previously tested negative by fur-pluck and fur PCR testing before breeding and remained negative by both testing methods. Because all pups in these 2 cages were fur-pluck–negative, we therefore assumed that these 2 positive results were false positives.
Discussion
This study showed that mite burdens for M. musculinus-infected cages are extremely high by 4 wk of age, drop precipitously after 6 wk of age, and continue to remain low in mice 24 and 28 wk of age. In our study, infested adult male mice in breeding cages were more likely to test negative than were female mice in breeding cages, but whether this difference was due to the smaller numbers of mice in our male study cages than in our female cages is unknown—it has been shown that single-housed mice are more likely to test negative than group-housed mice.16 The mechanism for the reduction in mite population with increasing age is unknown; however, it is likely mediated at least in part by IgE.13,17,18 Elevations in serum IgE in fur-mite–infested mice were reported to return to normal after elimination of the mites.21 Cyclical fluctuations in mite populations such as those reported for M. musculi did not occur in the current study, but given the shorter life cycle (14 d compared with 23 d) of M. musculinus, our sampling strategy was not sufficiently frequent to detect fluctuations related to the mite life cycle. We did document a reduction in positive test results for all strategies at 12 wk of age but did not identify a cause.
Regarding diagnostic testing of neonatally infested mice, there was a clear distinction between young mice up to 6 wk of age and those 8 wk and older. The large numbers of mites and eggs present on the younger mice resulted in sensitivities of 100% for both fur PCR and fur-pluck analysis, whereas in the older age group, only fur PCR testing approached 100% sensitivity. Cage PCR sensitivity was usually less than 100% in both age groups and similar to that of fur-pluck testing in the older age group. However, it should be noted that the fur-pluck technique we used involved intensive testing—every mouse in the cage was sampled from 3 sites, and slides were reviewed by an experienced technician. Sampling from fewer mice per cage or fewer sites or by less experienced personnel would likely have identified fewer fur-pluck–positive mice in the older age group. Cage PCR testing, although not 100% sensitive, might therefore be useful if time constraints prevent extensive testing by fur pluck or if colony mice are unavailable for testing by fur PCR, such as when manipulation of the mice would interfere with the research project. Our study found that fur PCR testing was much more sensitive than was described in a previous report.26 Differences may have resulted from the technique used to collect the DNA or the outside laboratory used for testing. In our study, a special sticky swab (provided by the laboratory) was used in an aggressive massaging technique over the whole body of the mouse to maximize the DNA collected. In addition, DNA copy numbers detected in our study were generally low, ranging from 10 to 100 in fur PCR swabs, so another possible explanation for our differing results is that copy numbers may have been below the test threshold of detection in the prior study. Regardless, the occasional false-positive fur PCR test in our study underscores the need to confirm PCR-positives by microscopic detection of mites or eggs.
The negative controls in the current study were all white FVB mice; however all samples were mixed and blinded before analysis, and there were both positive and negative white-colored fur pluck samples (the breeding study yielded positive white fur-pluck slides from in-contact mice and from offspring). Coat color was not a factor for DNA analysis. No microscopic evidence of mites was found on any of the negative control fur-pluck samples throughout the study, confirming lack of transmission between cages. This finding was as we expected, because the mice were housed in ventilated racks with HEPA filtration of both incoming and exhausted air; in any case, transmission between cages has not been proven.
Our results showing that not all mice from infested cages test positive by fur-pluck analysis confirms a previous report16 and suggests that to maximize the chance of detection, all mice in the cage should be tested, particularly of adult animals that harbor few mites. For fur PCR testing, we used a single swab to test all the mice in the cage, so results for individual mice were not available. However, given the relatively low copy numbers found on the PCR tests, it seems prudent to collect from all mice in each cage.
Posttreatment testing should not be conducted too soon after treatment if an incorrect positive diagnosis is to be avoided. In our study, one third of treated cages still tested positive by fur PCR analysis at 8 wk after the first treatment regardless of the initial infestation status (heavy or sparse), and juvenile, heavily infested cages remained positive by fur pluck at 4 wk after treatment. In general, these positive fur-pluck findings consisted of mite parts, eggs and egg casings, which were numerous in some samples. It was not possible to determine whether these findings were associated with active infestation until later testing confirmed a negative status. In theory, retained mite material in treated mice could affect diagnosis for even longer: a previous report showed that fur may be retained for more than 8 mo.20
We classified several other PCR results in our studies as false-positives because subsequent tests by PCR, fur pluck, and breeding were all negative. In these cases, we presumed that the false-positive findings were due to cross-contamination at some point in the collection or testing process. The high proportion of positive PCR samples in this study may have resulted in cross-contamination during PCR testing. In any case, the occurrence of false-positive PCR results in the current study highlights the need for confirmatory testing. When a definitive diagnosis is unavailable (for example, if a positive PCR cannot be substantiated by detection of live mites), breeding the suspect mice and testing the offspring at weaning will confirm or refute the diagnosis.
The question still remains regarding the most efficient means to detect the presence of mites in laboratory mouse colonies. Sentinel mice are evaluated routinely, but whether they are sufficiently sensitive to detect the presence of mites under ‘real world’ conditions is unknown.2,15,22,24 PCR analysis of rack exhaust samples shows promise,10 but its efficacy on a large scale has yet to be tested. Regardless, because sentinel evaluation and rack PCR are both retrospective, any positive results must be confirmed by testing of the colony animals on the rack. We here provide definitive information regarding the optimum age of animals to test and the most appropriate testing technique for Myocoptes spp. mites. Although we did not evaluate Radfordia and Myobia spp., previous reports suggest that Myobia spp. undergo a similar pattern of rapid colonization of offspring followed by a reduction in mite populations.1 Because the life cycle of these other species is approximately 9 d longer than that of Myocoptes, it is likely that the population peak and subsequent reduction occur later than those in Myocoptes.1,7
We therefore have the following suggestions regarding testing to maximize the detection of fur mites in mice. The population most likely to support heavy infestations is female group-housed mice that are 4 to 6 wk old. In heavily infested mice, both fur PCR and fur-pluck testing are likely to detect positive mice, but if the extent of infestation is likely to be low, as in older or single-housed mice, then fur PCR analysis is most likely to detect positive animals. All mice in the cage should be tested, regardless of test used. Testing after selamectin application may falsely indicate an active infestation if performed sooner than 8 wk (fur-pluck testing) or 16 wk (fur PCR analysis) after treatment. Testing environmental cage and bedding samples by PCR is not 100% sensitive but may be useful, particularly in cases in which the mice themselves cannot be tested.
Acknowledgments
This work was supported by NIH R25 OD010913, NIH T32 OD011089 and Johns Hopkins University Research Animal Resources. Charles River Laboratories provided support for testing blinded PCR samples: results were analyzed by Johns Hopkins University.
References
- 1.Baker DG. 2007. Flynn's parasites of laboratory animals. Ames (IA): Blackwell Publishing [Google Scholar]
- 2.Brzezenski L, Rice K, Watson J. 2012. Methods for detecting mites on fur pluck samples from mice sparsely infested with Myocoptes musculinus. J Am Assoc Lab Anim Sci 51:698 [Google Scholar]
- 3.Burdett EC, Heckmann RA, Ochoa R. 1997. Evaluation of five treatment regimens and five diagnostic methods for murine mites (Myocoptes musculinus and Myobia musculi). Contemp Top Lab Anim Sci 36:73–76 [PubMed] [Google Scholar]
- 4.Carty AJ. 2008. Opportunistic infections of mice and rats: Jacoby and Lindsey revisited. ILAR J 49:272–276 [DOI] [PubMed] [Google Scholar]
- 5.Dawson DV, Whitmore SP, Bresnahan JF. 1986. Genetic control of susceptibility to mite-associated ulcerative dermatitis. Lab Anim Sci 36:262–267 [PubMed] [Google Scholar]
- 6.Friedman S, Weisbroth SH. 1975. The parasitic ecology of the rodent mite Myobia musculi. II. Genetic factors. Lab Anim Sci 25:440–445 [PubMed] [Google Scholar]
- 7.Friedman S, Weisbroth SH. 1977. The parasitic ecology of the rodent mite, Myobia musculi. IV. Life cycle. Lab Anim Sci 27:34–37 [PubMed] [Google Scholar]
- 8.Galton M. 1963. Myobic mange in the mouse leading to skin ulceration and amyloidosis. Am J Pathol 43:855–865 [PMC free article] [PubMed] [Google Scholar]
- 9.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press [Google Scholar]
- 10.Jensen ES, Allen KP, Henderson KS, Szabo A, Thulin J. 2013. PCR testing of a ventilated caging system to detect murine fur mites. J Am Assoc Lab Anim Sci 52:28–33 [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston NA, Trammell RA, Ball-Kell S, Verhulst S, Toth LA. 2009. Assessment of immune activation in mice before and after eradication of mite infestation. J Am Assoc Lab Anim Sci 48:371–377 [PMC free article] [PubMed] [Google Scholar]
- 12.Jungmann P, Freitas A, Bandeira A, Nobrega A, Coutinho A, Marcos MA, Minoprio P. 1996. Murine acariasis. II. Immunological dysfunction and evidence for chronic activation of Th-2 lymphocytes. Scand J Immunol 43:604–612 [DOI] [PubMed] [Google Scholar]
- 13.Laltoo H, Van Zoost T, Kind LS. 1979. IgE antibody response to mite antigens in mite infested mice. Immunol Commun 8:1–9 [DOI] [PubMed] [Google Scholar]
- 14. Letscher R. 1970. Observations concerning the life cycle and biology of Myobia musculi (Schrank) and Myocoptes musculinus (Koch). [MS thesis] College Station (TX): Texas A and M University.
- 15.Lindstrom KE, Carbone LG, Kellar DE, Mayorga MS, Wilkerson JD. 2011. Soiled bedding sentinels for the detection of fur mites in mice. J Am Assoc Lab Anim Sci 50:54–60 [PMC free article] [PubMed] [Google Scholar]
- 16.Metcalf Pate KA, Rice KA, Wrighten R, Watson J. 2011. Effect of sampling strategy on the detection of fur mites within a naturally infested colony of mice (Mus musculus). J Am Assoc Lab Anim Sci 50:337–343 [PMC free article] [PubMed] [Google Scholar]
- 17.Morita E, Kaneko S, Hiragun T, Shindo H, Tanaka T, Furukawa T, Nobukiyo A, Yamamoto S. 1999. Fur mites induce dermatitis associated with IgE hyperproduction in an inbred strain of mice, NC/Kuj. J Dermatol Sci 19:37–43 [DOI] [PubMed] [Google Scholar]
- 18.Pochanke V, Hatak S, Hengartner H, Zinkernagel RM, McCoy KD. 2006. Induction of IgE and allergic-type responses in fur mite-infested mice. Eur J Immunol 36:2434–2445 [DOI] [PubMed] [Google Scholar]
- 19.Pritt S, Henderson KS, Shek WR. 2010. Evaluation of available diagnostic methods for Clostridium piliforme in laboratory rabbits (Oryctolagus cuniculus). Lab Anim 44:14–19 [DOI] [PubMed] [Google Scholar]
- 20.Ricart Arbona RJ, Lipman NS, Wolf FR. 2010. Treatment and eradication of murine fur mites: II. Diagnostic considerations. J Am Assoc Lab Anim Sci 49:583–587 [PMC free article] [PubMed] [Google Scholar]
- 21.Roble GS, Boteler W, Riedel E, Lipman NS. 2012. Total IgE as a serodiagnostic marker to aid murine fur mite detection. J Am Assoc Lab Anim Sci 51:199–208 [PMC free article] [PubMed] [Google Scholar]
- 22.Thigpen JE, Lebetkin EH, Dawes ML, Amyx HL, Caviness GF, Sawyer BA, Blackmore DE. 1989. The use of dirty bedding for detection of murine pathogens in sentinel mice. Lab Anim Sci 39:324–327 [PubMed] [Google Scholar]
- 23.Villegas EN, Augustine SA, Villegas LF, Ware MW, See MJ, Lindquist HD, Schaefer FW, 3rd, Dubey JP. 2010. Using quantitative reverse transcriptase PCR and cell culture plaque assays to determine resistance of Toxoplasma gondii oocysts to chemical sanitizers. J Microbiol Methods 81:219–225 [DOI] [PubMed] [Google Scholar]
- 24.Watson J. 2012. Pooled fecal floats from colony cages detect Aspiculuris tetraptera and fur mites. J Am Assoc Lab Anim Sci 51:675 [Google Scholar]
- 25.Weisbroth SH, Friedman S, Scher S. 1976. The parasitic ecology of the rodent mite, Myobia musculi. III. Lesions in certain host strains. Lab Anim Sci 26:725–735 [PubMed] [Google Scholar]
- 26.Weiss EE, Evans KD, Griffey SM. 2012. Comparison of a fur mite PCR assay and the tape test for initial and posttreatment diagnosis during a natural infection. J Am Assoc Lab Anim Sci 51:574–578 [PMC free article] [PubMed] [Google Scholar]