Highlights
-
•
Fluorescent tagging enables detailed analysis of enteroendocrine cell physiology.
-
•
Electrogenic nutrient uptake evokes electrical activity and hormone secretion.
-
•
G protein coupled receptors play key roles in lipid stimulated incretin secretion.
-
•
Targeting enteroendocrine cells is under investigation to treat diabetes and obesity.
Abstract
Incretin peptides (glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP)) are secreted from enteroendocrine cells in the intestinal epithelium, and help to coordinate metabolic responses to food ingestion. A number of molecular mechanisms have recently been defined that underlie carbohydrate, lipid and protein sensing in gut endocrine cells. Knockout mice lacking sodium glucose tranporter-1 (SGLT-1) or the short chain fatty acid sensing receptor FFAR2 (GPR43), for example, have highlighted the importance of these molecules in incretin secretion. This review outlines our current understanding of sensory pathways in incretin secreting cells and highlights the therapeutic potential of targeting them for the development of novel therapies for obesity and diabetes.
Current Opinion in Pharmacology 2013, 13:922–927
This review comes from a themed issue on Endocrine and metabolic diseases
Edited by Frank Reimann and Fiona M Gribble
For a complete overview see the Issue and the Editorial
Available online 10th September 2013
1471-4892/$ – see front matter, © 2013 The Authors. Published by Elsevier Ltd. All rights reserved.
Introduction
The gastrointestinal (GI) tract, in addition to digesting food and absorbing the available nutrients, releases hormones with important physiological roles in regulating plasma glucose levels, gut motility and satiety. Amongst these, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are known as incretins, based on their ability to enhance glucose-stimulated insulin secretion. The possibility of stimulating the release of endogenous incretins as a therapeutic strategy for the treatment of type 2 diabetes (T2DM) and obesity has led to heightened interest in the physiology of enteroendocrine cells and the gut-brain-pancreatic axis. This review will focus on our current understanding of the enteroendocrine cell populations known as K-cells and L-cells, which primarily secrete GIP and GLP-1, respectively.
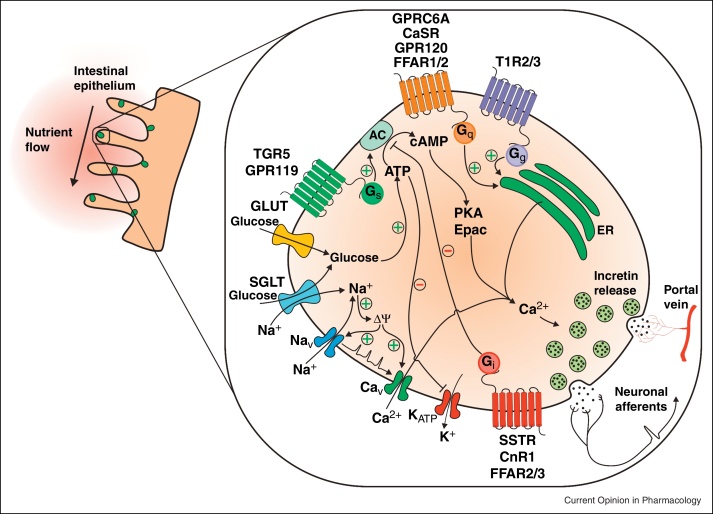
Enteroendocrine cells have historically been difficult to study as they are found scattered within the intestinal epithelium, but recent advances in labelling specific cell populations in transgenic mice with fluorescent reporters have enabled live cell identification and purification. As a consequence, there are increasing numbers of published studies analysing the molecular events underlying stimulus secretion coupling in enteroendocrine cell types. An unexpected outcome of these investigations has been the observation that enteroendocrine cells are more plurihormonal than previously thought. Although L-cells have long been known to produce peptideYY (PYY) in addition to the products of proglucagon processing (GLP-1, GLP-2 and oxyntomodulin), it was surprising to find that most K-cells and L-cells also produce cholecystokinin (CCK) [1,2]. Many mechanisms described here might also therefore play a role in the secretion of other enteroendocrine hormones, although the relative contributions of different signalling pathways likely differ between cells and along the length of the GI tract. Thus, whereas GIP secreting K-cells are predominantly located in the duodenum and are exposed to nutrients soon after food ingestion, GLP-1 is also produced more distally where its secretion may be influenced by slowly digested macronutrients and products of bacterial fermentation (Figure 1).
Figure 1.
Model of the molecular mechanisms involved in the secretion of incretin peptides from enteroendocrine cells. Stimulation of nutrient and non-nutrient pathways ultimately leads to an increase in intracellular calcium via G protein coupled pathways or membrane depolarisation, facilitating the release of incretin peptides. The effect of incretin peptides on orchestrating the physiological response to nutrient intake, such as potentiating glucose-dependent insulin secretion, is facilitated through peptide uptake into blood vessels of the portal vein and/or through direct activation of neighbouring neuronal afferents.
L-cells and K-cells, like many of the enteroendocrine family, consist of an apical pole with microvilli facing the gut lumen and a broader base where peptides are released, and it is believed that peptide secretion is a result of direct sensing of nutrients in the lumen [3]. Recent work has begun to elucidate the mechanisms underlying nutrient sensing and peptide release, and in vitro studies suggest that L-cells are electrically active, exhibiting action potentials and calcium transients in response to glucose [4]. Whilst minor differences in glucose sensing by K-cells have been described [5], many molecular mechanisms described below are active in GIP and GLP-1 secreting cells and are likely also to be involved in the release of other enteroendocrine hormones. However, to what extent the different identified sensory pathways contribute to nutrient detection in vivo remains to be fully established.
Nutrient signalling
Carbohydrate sensing
Glucose is a robust stimulant of incretin release and several pathways of carbohydrate sensing have been proposed. In the upper small intestine, there is good evidence that GLP-1 and GIP release are triggered by Na+ coupled glucose uptake mediated by the brush border sodium glucose cotransporter (SGLT1). Small transporter-associated currents appear sufficient to drive membrane depolarisation, in turn triggering electrical activity, voltage-gated calcium entry and peptide release [6•]. This idea is supported by the demonstration that glucose-dependent GLP-1 and GIP secretion in vitro are prevented by pharmacological SGLT1 inhibitors [7•] and that SGLT1 knockout mice have impaired GIP and GLP-1 release early after glucose gavage [8]. By contrast, plasma GLP-1 levels measured later after glucose administration appeared markedly elevated in mice lacking SGLT1, suggesting that attenuated glucose absorption in the upper small intestine results in increased delivery to the L-cell richer distal gut, where alternative sensing pathways may be recruited [9]. One hypothesis is that an increased distal glucose load facilitates microbial fermentation and the production of short chain fatty acids, which in turn activate L-cells via alternative signalling pathways [10]. SGLT1 independent glucose-sensing pathways have, however, been identified in enteroendocrine cells, and may contribute to the delayed elevation of GLP-1 levels in SGLT1 knockout mice. L-cells and K-cells express glucokinase and ATP sensitive potassium (KATP) channel subunits, providing the machinery to couple electrical activity to glucose metabolism. This pathway seems not to be responsible for the peak incretin levels detected early after glucose ingestion, which were unaffected in humans treated with KATP channel inhibitors [11]. Measurement of L-cell glucose concentrations suggests that although the SGLT1-mediated Na+ flux is large enough to drive membrane depolarisation, the accompanying monosaccharide flux is insufficient to alter intracellular glucose concentrations. L-cells have a high phloretin-sensitive glucose flux, however, suggesting that facilitative glucose transporters could equilibrate cytoplasmic with basolateral glucose levels, and that L-cell metabolism would predominantly be influenced by glucose arriving from the plasma rather than luminal glucose direction [7•]. The interplay between plasma glucose, metabolism and GLP-1 secretion remains, however, an enigma. A third, and highly controversial pathway links enteroendocrine secretion to activation of sweet taste receptors. This pathway utilises a G protein coupled receptor heterodimer (T1R2/T1R3) as the detector of glucose and other sweeteners and couples through the G protein α-gustducin. α-gustducin and T1R3 have been detected in the gut, and in some studies were found colocalised with GLP-1 and GIP [12–14], but several findings suggest they may not themselves act as the L-cell glucose sensor. Convincing arguments against an important role of sweet taste receptors are the multiple demonstrations that ingestion of glucose, but not artificial sweeteners, triggers elevation of plasma incretin levels in rodents [15] and humans [16].
Lipid sensing
Products of fat ingestion have not been reported to alter L-cell electrical activity, but are rather thought to be sensed by G protein coupled receptors (GPCRs). In L-cells, several GPCRs have been implicated in lipid signalling, such as GPR120 [17], GPR119 [18,19] and FFAR1 (GPR40) [20]. FFAR1 and GPR120 respond to long-chain and medium-chain fatty acids, and are thought to be Gq-coupled, activating phospholipase C and thereby triggering IP3 mediated Ca2+ release and secretion of peptides [21•]. Other lipids that rise in concentration in the intestine postprandially include 2-monoacylglycerols, which are produced from triglycerides by lipases and act as ligands for GPR119 [22], which is expressed both in K-cells and L-cells [4,5,19]. GPR119 is preferentially Gs-coupled and ligand binding results in activation of adenylyl cyclase, an increase in cAMP levels and enhanced L-cell secretion [19,23]. Alternative lipid sensing pathways underlying GLP-1 secretion, involving uptake by fatty-acid transport protein (FATP4) [24] and activation of atypical protein kinase C [25] have also been described.
SCFA are produced in the colon during bacterial fermentation of dietary fibre or, less usually, of non-absorbed carbohydrate. They may provide one link between fibre content, the gut microbiome and L-cells [26,27], acting through two GPCRs, FFAR2 and FFAR3. FFAR2 can couple to Gq-signalling pathway and Gi/o-signalling pathway, whilst FFAR3 seems to lack a Gq component [28•]. A role for FFAR2 in GLP-1 secretion was suggested by the findings that SCFA triggered Ca2+ transients in primary L-cells and that circulating GLP-1 levels and SCFA-dependent GLP-1 release in vitro were impaired in mice lacking FFAR2 [29].
Protein sensing
Although protein digestion is an effective stimulus of GLP-1 release, the optimal size of the digestion products for triggering secretion remains uncertain. Several amino acids have been shown to stimulate GLP-1 release in vitro [30,31]. The effectiveness of L-Gln has been attributed to its ability both to trigger membrane depolarisation via electrogenic Na+-dependent amino acid uptake, and to elevate cytoplasmic cAMP concentrations, perhaps though activation of an unidentified Gs-coupled GPCR [30,31]. Ingestion of L-Gln also stimulates GLP-1 release in healthy and obese and diabetic humans [32]. GPCRs have been linked to the sensing of other luminal amino acids by enteroendocrine cells: GPRC6A to ornithine [33] and the CaSR to phenylalanine [34]. The sensing of larger protein digestion products simulated by, for example meat hydrolysate has been linked to activation of mitogen-activated protein (MAP) kinases, but the receptors involved are less clear [35,36].
Non-nutrient pathways
GLP-1 secretion is not only stimulated by nutrients, but also by other luminal components. Enteral progesterone has been implicated in incretin secretion through activation of plasma membrane receptors [37]. Bile acids are also involved in the integration of metabolic signals and have been implicated in fibroblast growth factor 15/19 secretion from the distal gut downstream of the well-characterised nuclear hormone receptor FXR [38]. In L-cells, however, bile acids appear to stimulate GLP-1 secretion through activation of the predominantly Gs coupled receptor TGR5 (GPBAR) [39,40]. Recently, administration of bile acids has been shown to have positive effects on glucose homeostasis and plasma GLP-1 levels in human volunteers [41,42]. Interestingly plasma bile acid levels increase after bariatric surgery, and bile acid stimulated GLP-1 secretion might contribute to the associated improvements in metabolic control [43].
Inhibitory pathways
In addition to stimulatory pathways enhancing incretin secretion, enteroendocrine cells also express GPCRs coupled to Gi proteins with inhibitory functions. K-cells and L-cells, for example, express Gi-coupled somatostatin receptors. Somatostatin impairs the release of GLP-1 and GIP, and in enteroendocrine cell lines inhibits forskolin-stimulated cAMP transients, consistent with the recruitment of a Gi coupled signalling pathway [44,45]. Enhanced somatostatin release is a likely pathway underlying the observed inhibition of GLP-1 secretion in patients treated with GLP-1 mimetics [46]. The Gi-coupled endocannabinoid receptor Cnr1 has also been linked to modulation of incretin hormone secretion. Cnr1 is expressed at higher levels in K-cells than L-cells and preferentially inhibits secretion of GIP rather than GLP-1 [44].
It is becoming clear that enteroendocrine cells receive signals from multiple inputs, both from ingested nutrients and components in the gut lumen, and also from other enteroendocrine cells and tissues. These inputs involve on the one hand electrogenic pathways, through co-transport of Na+, and the other hand GPCRs and classical downstream G protein coupled pathways. It is hoped that a greater understanding of the physiological signalling pathways employed by enteroendocrine cells in vivo could be exploited to target the cells for the treatment of T2DM and obesity.
Therapeutic potential and future possibilities
On the basis of the fundamental importance of incretins for glucose homeostasis, therapies based on activating the incretin axis have proved highly effective in treating T2DM. Stable injectable GLP-1 mimetics and inhibitors of dipeptidyl peptidase 4 (DPP4), which rapidly inactivates circulating GLP-1, are licensed for the treatment of T2DM and, in the case of GLP-1 mimetics, offer additional benefits such as weight loss and cardioprotection [47,48]. Therapies based on targeting endogenous enteroendocrine cells, however, could potentially offer the benefits of releasing more than one peptide, thus activating appetite suppressants as well as insulinotropic pathways. Several candidate enteroendocrine targets, including FFAR1, GPR119 and GPR120, are currently under investigation. Amongst these, the FFAR1 agonist TAK-875 targets pancreatic β-cells as well as L-cells and exhibits favourable glycaemic effects in patients with T2DM [49], although the relative contribution of GLP-1 may be minor. Agonists for GPR119 showed encouraging efficacy in animal models, but had limited glucose lowering and incretin activity in humans, for reasons that remain to be established [50,51]. Somewhat surprisingly, a dual acting SGLT1/2 inhibitor has recently been shown to cause elevation in GLP-1 and PYY levels in rodents and human patients with type 2 diabetes [9,52], thus mimicking the effect of SGLT1 knockout, perhaps by feeding the gut microbiome and enhancing SCFA production in the L-cell rich distal gut [10]. It seems likely that the markedly elevated post-prandial GLP-1 and PYY levels that follow some forms of bariatric surgery [53] are similarly linked to increased delivery of nutrients to the distal gut, and have dramatic effects on appetite and T2DM resolution [54,55,56••,57,58]. Mimicking the effects of bariatric surgery by medical interventions would be a major therapeutic breakthrough.
Conclusion
The enteroendocrine system plays a fundamental role in orchestrating post-prandial physiology, and is central to the regulation of glucose homeostasis and satiety. The success of current GLP-1-based therapies and the dramatic effects of bariatric surgery on insulin secretion and appetite greatly support the future development of therapeutic strategies that exploit targets upstream of enteroendocrine secretion as novel treatments for T2DM and obesity. Despite the notable progress made to date in dissecting the mechanisms of stimulation-coupled enteroendocrine secretion, there are currently no drugs clinically approved that directly target endogenous enteroendocrine cells. The unexpected success of bariatric surgery in treating T2DM, however, highlights the benefits that could be achieved through a gut-based therapeutic approach.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
Research in the Gribble and Reimann laboratories is supported by grants from the Wellcome Trust (WT088357/Z/09/Z and WT084210/Z/07/Z), the MRC-Metabolic Diseases Unit (Cambridge) and EU FP7 (full4health, grant agreement n° 266408).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Egerod K.L., Engelstoft M.S., Grunddal K.V., Nohr M.K., Secher A., Sakata I. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153:5782–5795. doi: 10.1210/en.2012-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Habib A.M., Richards P., Cairns L.S., Rogers G.J., Bannon C.A.M., Parker H.E. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153:3054–3065. doi: 10.1210/en.2011-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker H.E., Reimann F., Gribble F.M. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev Mol Med. 2010;12:e1. doi: 10.1017/S146239940900132X. [DOI] [PubMed] [Google Scholar]
- 4.Reimann F., Habib A.M., Tolhurst G., Parker H.E., Rogers G.J., Gribble F.M. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker H.E., Habib A.M., Rogers G.J., Gribble F.M., Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia. 2009;52:289–298. doi: 10.1007/s00125-008-1202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6•.Gribble F.M., Williams L., Simpson A.K., Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003;52:1147–1154. doi: 10.2337/diabetes.52.5.1147. [DOI] [PubMed] [Google Scholar]; This study uses pharmacological and genetic approaches to show that SGLT1 is required for glucose-stimulated GLP-1 secretion and Ca2+ transients in L-cells. The study suggests that glucose-mediated GLP-1 secretion is mediated by electrogenic Na+-coupled pathways rather than metabolic pathways
- 7•.Parker H.E., Adriaenssens A., Rogers G., Richards P., Koepsell H., Reimann F. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia. 2012;55:2445–2455. doi: 10.1007/s00125-012-2585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study characterises the role of SGLT1 in glucose absorption and incretin release using Sglt1−/− mice. The study shows that SGLT1 is expressed in K-cells and L-cells that SGLT1 is required for glucose-stimulated incretin release, confirming the role of electrogenic pathways in incretin secretion
- 8.Gorboulev V., Schurmann A., Vallon V., Kipp H., Jaschke A., Klessen D. Na(+)-d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61:187–196. doi: 10.2337/db11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell D.R., Smith M., Greer J., Harris A., Zhao S., DaCosta C. LX4211 increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)-mediated absorption of intestinal glucose. J Pharmacol Exp Ther. 2013;345:250–259. doi: 10.1124/jpet.113.203364. [DOI] [PubMed] [Google Scholar]
- 10.Powell D.R., DaCosta C.M., Gay J., Ding Z.M., Smith M., Greer J. Improved glycemic control in mice lacking Sglt1 and Sglt2. Am J Physiol Endocrinol Metab. 2013;304:E117–E130. doi: 10.1152/ajpendo.00439.2012. [DOI] [PubMed] [Google Scholar]
- 11.Stephens J.W., Bodvarsdottir T.B., Wareham K., Prior S.L., Bracken R.M., Lowe G.D. Effects of short-term therapy with glibenclamide and repaglinide on incretin hormones and oxidative damage associated with postprandial hyperglycaemia in people with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2011;94:199–206. doi: 10.1016/j.diabres.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Jang H.J., Kokrashvili Z., Theodorakis M.J., Carlson O.D., Kim B.J., Zhou J. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margolskee R.F., Dyer J., Kokrashvili Z., Salmon K.S., Ilegems E., Daly K. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young R.L., Chia B., Isaacs N.J., Ma J., Khoo J., Wu T. Disordered control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes. Diabetes. 2013;62:3532–3541. doi: 10.2337/db13-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita Y., Wideman R.D., Speck M., Asadi A., King D.S., Webber T.D. Incretin release from gut is acutely enhanced by sugar but not by sweeteners in vivo. Am J Physiol Endocrinol Metab. 2009;296:E473–E479. doi: 10.1152/ajpendo.90636.2008. [DOI] [PubMed] [Google Scholar]
- 16.Ma J., Bellon M., Wishart J.M., Young R., Blackshaw L.A., Jones K.L. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2009;296:G735–G739. doi: 10.1152/ajpgi.90708.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 18.Lauffer L.M., Iakoubov R., Brubaker P.L. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 2009;58:1058–1066. doi: 10.2337/db08-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu Z.L., Carroll C., Alfonso J., Gutierrez V., He H., Lucman A. A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like Peptide-1 and glucose-dependent insulinotropic Peptide release. Endocrinology. 2008;149:2038–2047. doi: 10.1210/en.2007-0966. [DOI] [PubMed] [Google Scholar]
- 20.Edfalk S., Steneberg P., Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Hara T., Hirasawa A., Ichimura A., Kimura I., Tsujimoto G. Free fatty acid receptors FFAR1 and GPR120 as novel therapeutic targets for metabolic disorders. J Pharm Sci. 2011;100:3594–3601. doi: 10.1002/jps.22639. [DOI] [PubMed] [Google Scholar]; First demonstration that GPR119 is activated by monoacyl-glycerol, thus providing a possible direct link between triglyceride digestion and GLP-1 secretion independent of free fatty acids
- 22.Hansen K.B., Rosenkilde M.M., Knop F.K., Wellner N., Diep T.A., Rehfeld J.F. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab. 2011;96:E1409–E1417. doi: 10.1210/jc.2011-0647. [DOI] [PubMed] [Google Scholar]
- 23.Cox H.M., Tough I.R., Woolston A.M., Zhang L., Nguyen A.D., Sainsbury A. Peptide YY is critical for acylethanolamine receptor Gpr119-induced activation of gastrointestinal mucosal responses. Cell Metab. 2010;11:532–542. doi: 10.1016/j.cmet.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poreba M.A., Dong C.X., Li S.K., Stahl A., Miner J.H., Brubaker P.L. Role of fatty acid transport protein 4 in oleic acid-induced glucagon-like peptide-1 secretion from murine intestinal L cells. Am J Physiol Endocrinol Metab. 2012;303:E899–E907. doi: 10.1152/ajpendo.00116.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iakoubov R., Ahmed A., Lauffer L.M., Bazinet R.P., Brubaker P.L. Essential role for protein kinase Cζ in oleic acid-induced glucagon-like peptide-1 secretion in vivo in the rat. Endocrinology. 2011;152:1244–1252. doi: 10.1210/en.2010-1352. [DOI] [PubMed] [Google Scholar]
- 26.Freeland K.R., Wilson C., Wolever T.M. Adaptation of colonic fermentation and glucagon-like peptide-1 secretion with increased wheat fibre intake for 1 year in hyperinsulinaemic human subjects. Br J Nutr. 2010;103:82–90. doi: 10.1017/S0007114509991462. [DOI] [PubMed] [Google Scholar]
- 27.Everard A., Lazarevic V., Derrien M., Girard M., Muccioli G.G., Muccioli G.M. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60:2775–2786. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Brown A.J., Goldsworthy S.M., Barnes A.A., Eilert M.M., Tcheang L., Daniels D. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]; This study shows that SCFA stimulate GLP-1 release through the GPCR FFAR2 and triggers elevations in intracellular Ca2+ levels in L-cells. This report reveals a mechanism by which gut microbiota may influence food intake and metabolism
- 29.Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M., Diakogiannaki E. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reimann F., Williams L., da Silva Xavier G., Rutter G.A., Gribble F.M. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia. 2004;47:1592–1601. doi: 10.1007/s00125-004-1498-0. [DOI] [PubMed] [Google Scholar]
- 31.Tolhurst G., Zheng Y., Parker H.E., Habib A.M., Reimann F., Gribble F.M. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology. 2011;152:405–413. doi: 10.1210/en.2010-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenfield J.R., Farooqi I.S., Keogh J.M., Henning E., Habib A.M., Blackwood A. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am J Clin Nutr. 2009;89:106–113. doi: 10.3945/ajcn.2008.26362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oya M., Kitaguchi T., Pais R., Reimann F., Gribble F., Tsuboi T. The G protein-coupled receptor family C Group 6 Subtype A (GPRC6A) receptor is involved in amino acid-induced glucagon-like Peptide-1 secretion from GLUTag cells. J Biol Chem. 2013;288:4513–4521. doi: 10.1074/jbc.M112.402677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mace O.J., Schindler M., Patel S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J Physiol. 2012;590:2917–2936. doi: 10.1113/jphysiol.2011.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reimer R.A., Darimont C., Gremlich S., Nicolas-Metral V., Ruegg U.T., Mace K. A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. Endocrinology. 2001;142:4522–4528. doi: 10.1210/endo.142.10.8415. [DOI] [PubMed] [Google Scholar]
- 36.Cordier-Bussat M., Bernard C., Levenez F., Klages N., Laser-Ritz B., Philippe J. Peptones stimulate both the secretion of the incretin hormone glucagon-like peptide 1 and the transcription of the proglucagon gene. Diabetes. 1998;47:1038–1045. doi: 10.2337/diabetes.47.7.1038. [DOI] [PubMed] [Google Scholar]
- 37.Flock G.B., Cao X., Maziarz M., Drucker D.J. Activation of enteroendocrine membrane progesterone receptors promotes incretin secretion and improves glucose tolerance in mice. Diabetes. 2013;62:283–290. doi: 10.2337/db12-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inagaki T., Choi M., Moschetta A., Peng L., Cummins C.L., McDonald J.G. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Parker H.E., Wallis K., le Roux C.W., Wong K.Y., Reimann F., Gribble F.M. Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br J Pharmacol. 2012;165:414–423. doi: 10.1111/j.1476-5381.2011.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas C., Gioiello A., Noriega L., Strehle A., Oury J., Rizzo G. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu T., Bound M.J., Standfield S.D., Jones K.L., Horowitz M., Rayner C.K. Effects of taurocholic acid on glycemic, glucagon-like peptide-1, and insulin responses to small intestinal glucose infusion in healthy humans. J Clin Endocrinol Metab. 2013;98:E718–E722. doi: 10.1210/jc.2012-3961. [DOI] [PubMed] [Google Scholar]
- 42.Adrian T.E., Gariballa S., Parekh K.A., Thomas S.A., Saadi H., Al Kaabi J. Rectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia. 2012;55:2343–2347. doi: 10.1007/s00125-012-2593-2. [DOI] [PubMed] [Google Scholar]
- 43.Patti M.E., Houten S.M., Bianco A.C., Bernier R., Larsen P.R., Holst J.J. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moss C.E., Marsh W.J., Parker H.E., Ogunnowo-Bada E., Riches C.H., Habib A.M. Somatostatin receptor 5 and cannabinoid receptor 1 activation inhibit secretion of glucose-dependent insulinotropic polypeptide from intestinal K cells in rodents. Diabetologia. 2012;55:3094–3103. doi: 10.1007/s00125-012-2663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chisholm C., Greenberg G.R. Somatostatin-28 regulates GLP-1 secretion via somatostatin receptor subtype 5 in rat intestinal cultures. Am J Physiol Endocrinol Metab. 2002;283:E311–E317. doi: 10.1152/ajpendo.00434.2001. [DOI] [PubMed] [Google Scholar]
- 46.Reimann F., Tolhurst G., Gribble F.M. G-protein-coupled receptors in intestinal chemosensation. Cell Metab. 2012;15:421–431. doi: 10.1016/j.cmet.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 47.Buse J.B., Nauck M., Forst T., Sheu W.H., Shenouda S.K., Heilmann C.R. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381:117–124. doi: 10.1016/S0140-6736(12)61267-7. [DOI] [PubMed] [Google Scholar]
- 48.Gejl M., Sondergaard H.M., Stecher C., Bibby B.M., Moller N., Botker H.E. Exenatide alters myocardial glucose transport and uptake depending on insulin resistance and increases myocardial blood flow in patients with type 2 diabetes. J Clin Endocrinol Metab. 2012;97:E1165–E1169. doi: 10.1210/jc.2011-3456. [DOI] [PubMed] [Google Scholar]
- 49.Araki T., Hirayama M., Hiroi S., Kaku K. GPR40-induced insulin secretion by the novel agonist TAK-875: first clinical findings in patients with type 2 diabetes. Diabetes Obesity Metab. 2012;14:271–278. doi: 10.1111/j.1463-1326.2011.01525.x. [DOI] [PubMed] [Google Scholar]
- 50.Habib A.M., Richards P., Rogers G.J., Reimann F., Gribble F.M. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia. 2013;56:1413–1416. doi: 10.1007/s00125-013-2887-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katz L.B., Gambale J.J., Rothenberg P.L., Vanapalli S.R., Vaccaro N., Xi L. Effects of JNJ-38431055, a novel GPR119 receptor agonist, in randomized, double-blind, placebo-controlled studies in subjects with type 2 diabetes. Diabetes, Obesity Metab. 2012;14:709–716. doi: 10.1111/j.1463-1326.2012.01587.x. [DOI] [PubMed] [Google Scholar]
- 52.Zambrowicz B., Freiman J., Brown P.M., Frazier K.S., Turnage A., Bronner J. LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo-controlled trial. Clin Pharmacol Ther. 2012;92:158–169. doi: 10.1038/clpt.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dirksen C., Hansen D.L., Madsbad S., Hvolris L.E., Naver L.S., Holst J.J. Postprandial diabetic glucose tolerance is normalized by gastric bypass feeding as opposed to gastric feeding and is associated with exaggerated GLP-1 secretion: a case report. Diabetes Care. 2010;33:375–377. doi: 10.2337/dc09-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buchwald H., Estok R., Fahrbach K., Banel D., Jensen M.D., Pories W.J. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256. doi: 10.1016/j.amjmed.2008.09.041. e5. [DOI] [PubMed] [Google Scholar]
- 55.Falken Y., Hellstrom P.M., Holst J.J., Naslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96:2227–2235. doi: 10.1210/jc.2010-2876. [DOI] [PubMed] [Google Scholar]
- 56••.Jimenez A., Casamitjana R., Flores L., Viaplana J., Corcelles R., Lacy A. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012;256:1023–1029. doi: 10.1097/SLA.0b013e318262ee6b. [DOI] [PubMed] [Google Scholar]; Demonstration that elevation of postprandial GLP-1 is a major contributor to the improved insulin secretion after bariatric surgery
- 57.Rizzello M., Abbatini F., Casella G., Alessandri G., Fantini A., Leonetti F. Early postoperative insulin-resistance changes after sleeve gastrectomy. Obes Surg. 2010;20:50–55. doi: 10.1007/s11695-009-0017-2. [DOI] [PubMed] [Google Scholar]
- 58.Jørgensen N.B., Dirksen C., Bojsen-Møller K.N., Jacobsen S.H., Worm D., Hansen D.L. The exaggerated glucagon-like peptide-1 response is important for the improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013 doi: 10.2337/db13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]