Abstract
Summary
–The high degree to which plant roots compete with soil microbes for organic forms of nitrogen (N) is becoming increasingly apparent. This has culminated in the finding that plants may consume soil microbes as a source of N, but the functional significance of this process remains unknown.
–We used 15N- and 14C-labelled cultures of soil bacteria to measure rates of acquisition of microbes by sterile wheat roots and plants growing in soil. We compared these rates with acquisition of 15N delivered as nitrate, amino acid monomer (l-alanine) and short peptide (l-tetraalanine), and the rate of decomposition of [14C] microbes by indigenous soil microbiota.
–Acquisition of microbe 15N by both sterile roots and roots growing in soil was one to two orders of magnitude slower than acquisition of all other forms of 15N. Decomposition of microbes was fast enough to account for all 15N recovered, but approximately equal recovery of microbe 14C suggests that microbes entered roots intact.
–Uptake of soil microbes by wheat (Triticum aestivum) roots appears to take place in soil. If wheat is typical, the importance of this process to terrestrial N cycling is probably minor in comparison with fluxes of other forms of soil inorganic and organic N.
Keywords: dissolved organic nitrogen, endocytosis, mineralization, nitrogen cycle, oligopeptide, phagomixotrophy
Introduction
Nitrogen (N) is a key factor in the control of primary productivity in terrestrial systems (Vitousek & Howarth, 47; Liu & Greaver, 26). Consequently, a mechanistic understanding of the processes controlling its availability to photosynthetic organisms is crucial not only to provide a wider understanding of the N cycle, but also to make predictions of carbon (C) residence in the terrestrial biosphere. However, the paradigm of plant–soil N cycling has shifted repeatedly over the last few decades and significant breakthroughs continue to be made.
Historically, it was thought that plants derived all of their N nutrition from the inorganic forms of N, 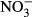 and
and 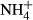 . However, the principal form of N entering soils not receiving additions of inorganic fertilisers is protein derived from dead portions of plants, animals or microbes. Consequently, it was thought that protein must be cleaved by microbial extracellular proteases and taken up by microbes as amino acids before inorganic N could become available to plants. In many soils, soil microbes are C limited and often excrete much of the N acquired in amino acids as inorganic N (Schimel & Bennett, 44; Roberts et al., 41). By contrast, as photosynthetic organisms, plants generally have an abundant supply of C and are frequently limited by N. Thus, if plants acquire N only in inorganic forms, their competition with soil microbes for N-containing compounds is generally low. However, it is now widely accepted that plants are able to compete with soil microbes for more complex N-containing compounds, when proteins are cleaved only to amino acids (Borstlap, 2; Chapin et al., 4; Jones & Darrah, 20; Näsholm et al., 32; Paungfoo-Lonhienne et al., 36). Further, it has recently become apparent that plants and soil microbes are both able to acquire amino acids when proteins have been cleaved only to short peptides (Komarova et al., 24; Hill et al., 14,b,c, 15; Soper et al., 46; Farrell et al., 7). Due probably principally to methodological constraints and variation between ecosystems, the exact importance of organic N to plant N nutrition remains uncertain (Jones et al., 21; Näsholm et al., 32; Hill et al., 14; Paungfoo-Lonhienne et al., 36). Nevertheless, although the limiting element may differ between organisms, it is clear that fierce competition for soil organic N exists between plant roots and soil microbes.
. However, the principal form of N entering soils not receiving additions of inorganic fertilisers is protein derived from dead portions of plants, animals or microbes. Consequently, it was thought that protein must be cleaved by microbial extracellular proteases and taken up by microbes as amino acids before inorganic N could become available to plants. In many soils, soil microbes are C limited and often excrete much of the N acquired in amino acids as inorganic N (Schimel & Bennett, 44; Roberts et al., 41). By contrast, as photosynthetic organisms, plants generally have an abundant supply of C and are frequently limited by N. Thus, if plants acquire N only in inorganic forms, their competition with soil microbes for N-containing compounds is generally low. However, it is now widely accepted that plants are able to compete with soil microbes for more complex N-containing compounds, when proteins are cleaved only to amino acids (Borstlap, 2; Chapin et al., 4; Jones & Darrah, 20; Näsholm et al., 32; Paungfoo-Lonhienne et al., 36). Further, it has recently become apparent that plants and soil microbes are both able to acquire amino acids when proteins have been cleaved only to short peptides (Komarova et al., 24; Hill et al., 14,b,c, 15; Soper et al., 46; Farrell et al., 7). Due probably principally to methodological constraints and variation between ecosystems, the exact importance of organic N to plant N nutrition remains uncertain (Jones et al., 21; Näsholm et al., 32; Hill et al., 14; Paungfoo-Lonhienne et al., 36). Nevertheless, although the limiting element may differ between organisms, it is clear that fierce competition for soil organic N exists between plant roots and soil microbes.
In the quest for adequate N nutrition, some angiosperms have developed specialized structures to acquire nutrients via carnivory. Recent evidence has shown that entry of the prey-derived protein into the carnivorous plant can occur partially by endocytosis (Adlassnig et al., 1). More surprisingly, it has also been shown that intact proteins may be acquired endocytotically by nonspecialist, nonmycorrhizal plant roots (Arabidopsis thaliana and Hakea actites; Paungfoo-Lonhienne et al., 34).
Phagomixotrophic ingestion of intact microorganisms is widespread amongst algae and endocytosis of prokaryotes is the probable origin of photosynthesis in eukaryotes (Raven et al., 38). Although increasing numbers of plant endophytic microbes have been identified in a range of plant tissues including roots, until recently direct uptake and digestion of microbes by angiosperms for nutritional purposes has been unrecognized (Rosenblueth & Martinez-Romero, 42; Hardoim et al., 12; Reinhold-Hurek & Hurek, 39). However, it has recently been demonstrated that microbes are acquired by the roots of angiosperms and that their associated N can be used nutritionally by the plants (Paungfoo-Lonhienne et al., 35). This discovery represents a potential step change in our understanding of competition for resources between soil microbes and plants, and how N is cycled in the terrestrial biosphere. What is not currently clear is what the quantitative significance of this process is when compared with other routes of plant N acquisition from soil.
This investigation was predicated on the need to evaluate the significance of this new route of N acquisition to plants when growing alone in sterile media and in soil. As our model angiosperm we chose to use the widely investigated and agriculturally important crop plant, wheat (Triticum aestivum). Wheat has previously been shown to utilize N forms ranging in organism or protein decomposition state from short l-peptides to 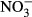 (Näsholm et al., 31; Hill et al., 18). We aimed to make a comparison of the rate of direct plant N acquisition from soil as intact bacteria with that as peptide, amino acid or inorganic N.
(Näsholm et al., 31; Hill et al., 18). We aimed to make a comparison of the rate of direct plant N acquisition from soil as intact bacteria with that as peptide, amino acid or inorganic N.
Materials and Methods
Soil
Agricultural Brown Earth soil (FAO classified as a Eutric Cambisol) was sampled (0–10 cm; n = 4) from Henfaes Agricultural Research Station, Abergwyngregyn, Bangor (53°14′N, 4°01′W). Upon return to the laboratory the soil was sieved to pass 5 mm, removing stones, earthworms and vegetation. Soil solution was collected via centrifugal drainage (Giesler & Lundström, 11) and passed through a 1 kDa ultrafiltration membrane (Millipore). The collected soil solution was analysed for amino acids by the fluorometric method of Jones et al. (22), before and after hydrolysis in 6 M HCl at 105°C for 16 h under N2. Fresh soil was extracted with 0.5 M K2SO4 (1 : 5 w/v) and analysed for 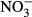 and
and 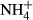 , according to Miranda et al. (29) and Mulvaney (30), respectively, and total extractable C and N using a Shimadzu TOC-V-TN analyser (Shimadzu Corp., Kyoto, Japan). Microbial biomass C and N were determined by measuring total K2SO4-extractable C and N before and after chloroform fumigation according to Voroney et al. (48) (KEC = 0.35; KEN = 0.5). Soil moisture content was determined by oven drying at 105°C. The pH and electrical conductivity of the soil (1 : 5 w/v distilled water extract) were determined using standard electrodes. Soil total C and N were measured in a Carlo Erba NA 1500 Elemental Analyzer (Thermo Fisher Scientific, Milan, Italy).
, according to Miranda et al. (29) and Mulvaney (30), respectively, and total extractable C and N using a Shimadzu TOC-V-TN analyser (Shimadzu Corp., Kyoto, Japan). Microbial biomass C and N were determined by measuring total K2SO4-extractable C and N before and after chloroform fumigation according to Voroney et al. (48) (KEC = 0.35; KEN = 0.5). Soil moisture content was determined by oven drying at 105°C. The pH and electrical conductivity of the soil (1 : 5 w/v distilled water extract) were determined using standard electrodes. Soil total C and N were measured in a Carlo Erba NA 1500 Elemental Analyzer (Thermo Fisher Scientific, Milan, Italy).
Culture of soil microbes
15N-labelled microbes
Soil microbial cultures were obtained by adding c. 2 mg of soil to 50 ml of sterile (0.2 μm filtered) nutrient solution, in a sterile 250 ml Erlenmeyer flask, with a foam stopper. The nutrient solution contained cycloheximide (10 μg ml−1) to minimize growth of hyphal fungi, and the following macronutrients (in mM): KH2PO4, 8.0; MgSO4, 2.0; CaCl2, 0.2; KNO3, 1.0; glycine, 2.0. Glucose (50 mM) was added as a C source and micronutrients were included as Kao and Michayluk vitamin mixture (Sigma-Aldrich, Gillingham, UK) at a concentration of 1 : 100 (v/v). The soil and nutrient solution were incubated aerobically on a rotary shaker at 75 rpm at 20°C. After 3 d, 1 ml of the microbial suspension was added to 50 ml of new nutrient solution, as above, except without cycloheximide. After a further 3 d of incubation, 1 ml of the microbial suspension was sub-sampled and suspended in 50 ml of new media containing K15NO3, in replacement of the unlabelled KNO3, at the same concentration as above. This last step was repeated to ensure that microbial N was effectively all 15N. After the final incubation, the microbial suspension was centrifuged at 4500 g for 10 min. The supernatant was discarded and the pellet was washed with 0.01 M CaCl2. The washing procedure was repeated twice, followed by re-suspension of the pellet in 20 ml of 0.01 M CaCl2. Total organic C and N of the microbial suspension were measured on the Shimadzu TOC-V-TN analyser. The suspension was subsequently diluted in 0.01 M CaCl2 to make a final concentration of 1 mmol N l−1 (c. 6 mmol C l−1). The solution was used immediately following preparation. Haemocytometer counts found microbe numbers in the injected solution to be c. 8 × 106 cells ml−1.
14C-labelled microbes
In order to sensitively measure plant incorporation of microbial C, a second culture of 14C-labelled microbes was prepared. Due to constraints on stable isotope analysis, it was not possible to dual-label microbes with 15N and 14C. Initially the 14C-labelled microbes were prepared in the same way as the 15N-labelled microbes. However, after the second incubation 2 MBq of [U−14C] glucose was added to the nutrient solution. To ensure high 14C incorporation, the unlabelled glucose in the nutrient solution was reduced from 50 mM to 50 μM. After 2 d of incubation the washing procedure described above was carried out. The solution was diluted to 1 mmol N l−1, and the 14C activity in the microbial suspension was determined by liquid scintillation counting in a Wallac 1404 scintillation counter (Perkin-Elmer Life Sciences, Boston, MA, USA) after mixing with Scintisafe scintillation cocktail (Fisher Scientific, Loughborough, UK).
Uptake of N forms by plants growing in soil
Seeds of wheat (Triticum aestivum L. var. AC Barrie) were sown singly into rhizotubes (240 mm long; internal diameter 8 mm; Owen & Jones, 33) containing 12 g of field-moist soil (Table 1). The plants were grown at 15°C, 70% relative humidity and 16 h photoperiod (c. 500 μmol photons m−2 s−1 PAR) until the third leaf stage (root weight 0.03 ± 0.002 g DW; mean ± SEM; n = 23). Injections of 0.5 ml of the N-treatments were made into the rhizosphere halfway up the rhizotube, (i.e. at a depth of 12 cm) using sterile 1 ml polypropylene syringes with 18G needles. Rhizotubes allowed injection of solutes and suspended microbes directly into the rhizosphere, thereby reducing possible confounding effects of differing mobility of N forms in the soil. Solutions injected were 1 mM K15NO3, 1 mM [13C15N] l-alanine (C3H7NO2), 250 μM [13C15N] l-tetraalanine (C12H22N4O5) and 14C or 15N labelled microbes (1 mmol N l−1). Four plants, selected randomly, were supplied with each of the N treatments. One hour after 15N injection, plants were removed from rhizotubes and roots carefully washed with c. 1 l distilled water and c. 200 ml 0.01 M CaCl2. After drying (80°C) root and shoot were weighed. 15N-labelled material was ground and analysed in a Thermo Finnigan Delta Plus XL continuous flow mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). 14C-labelled material was combusted in an OX400 Biological Oxidiser (Harvey Instruments Corp., Hillsdale, NJ, USA). Liberated 14CO2 was captured in Oxosol Scintillant (National Diagnostics, Atlanta, GA, USA) and 14C activity measured by liquid scintillation counting.
Basic properties of soil used to fill rhizotubes before growth of plants or culture of microbes
| Soil property | |
|---|---|
| Moisture content (g g−1 DW) | 0.28 ± 0.04 |
| pH | 6.5 ± 0.04 |
| Electrical conductivity (μS cm−1) | 22 ± 1.6 |
| Total C (mg g−1 DW) | 34 ± 3* |
| Total N (mg g−1 DW) | 0.54 ± 0.08* |
| Total soluble C (μmol g−1 DW) | 10.3 ± 0.9 |
| Total soluble N (μmol g−1 DW) | 3.3 ± 0.3 |
| Microbial C (μmol g−1 DW) | 267 ± 42 |
| Microbial N (μmol g−1 DW) | 39 ± 6 |
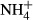 (nmol g−1 DW) (nmol g−1 DW) |
83 ± 12 |
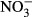 (nmol g−1 DW) (nmol g−1 DW) |
249 ± 7 |
| Soil solution amino acids (μmol N l−1) | 42 ± 9 |
| Soil solution < 1 kDa peptides (μmol N l−1) | 107 ± 34 |
Values are mean ± SEM; n = 4.
Determined for this soil by Farrell et al. (6).
Three plants were retained as 15N and 13C background enrichment controls. Three rhizotubes were injected with 0.5 ml of blue ink to estimate how much root and soil came into contact with the injected solution. The resulting blue section was cut out of the rhizotube and roots were washed, dried (80°C) and weighed. A further four rhizotubes had the same section cut out to determine pre-injection pools of soil soluble N with which injected solutions mixed. Soil from these sections was extracted for 5 min in 6 ml of de-ionized water. Nitrate, amino acids and short peptides (< 1 kDa) in the extract were measured as for the starting soil or soil solution. Roots from rhizotubes used to determine soil N pools were washed, cleared in 10% w/v KOH, stained in 0.25% w/v aniline blue and inspected for mycorrhizal colonization by light microscopy after destaining and mounting in 80% w/v lactic acid.
Uptake of N forms by sterile plants
Seeds were surface sterilized in sodium hypochlorite (10–14% available chlorine) with one drop of Tween-20, for 10 min followed by 80% v/v ethanol for 1 min. Seeds were then washed thoroughly in sterile distilled water. Seeds were placed on agar plates to germinate, following which, they were transferred to 114 × 86 × 102 mm Phytatrays (Sigma-Aldrich). Phytatrays contained 10 g of sterile (autoclaved) perlite, with 60 ml of sterile 50% Long Ashton nutrient solution (containing Na-metasilicate). Plants were kept under the same conditions as the wheat grown in soil. Upon reaching the third leaf stage, the plants were removed from the perlite and rinsed thoroughly with distilled water and 0.01 M CaCl2 as for plants grown in soil. The intact roots of three plants per treatment were submerged individually in sterile containers containing 4 ml of either 1 mM K15NO3, 1 mM [13C15N] l-alanine, 250 μM [13C15N] l-tetraalanine or 15N labelled microbes (1 mmol N l−1). Solutions not containing cultured microbes were sterilized by filtration to 0.2 μm before use. After 1 h under the same conditions as when growing, plants were removed from solutions and washed, dried and weighed as above. Root and shoot were ground together before mass spectrometer analysis.
The diameter of fresh roots (n = 10) of three plants grown in sterile culture was measured using a micrometer. Root length was measured with a ruler. These roots were subsequently dried (80°C) and used to determine the approximate relationship between root length and dry weight.
Consumption of added microbes by the indigenous soil microbial community
In order to evaluate the rate at which microbes added to soil were predated and decomposed by pre-existing soil microbes, 14C-labelled microbes were added to soil and their decomposition to 14CO2 measured according to Hill et al. (13). Briefly, 2 g of soil was placed in each of three 10 ml glass tubes and 83 μl of the washed 14C-labelled microbe suspension were added to the surface (matching the overall ratio of solution to soil in rhizotube injections). Air was drawn over the soil at a rate of c. 100 ml min−1 and 14CO2 was captured in two 3 ml vials of 0.1 M NaOH connected in series (described in detail in Hill et al., 16). Vials of NaOH were changed 1, 5, 10, 20, 40 and 60 min after addition of the microbes to soil. There was no potential for significant loss of 14CO2 during changing of NaOH as pumping was suspended for this period. Captured 14C was measured by liquid scintillation counting after mixing NaOH solution with Scintisafe scintillation cocktail. To account for 14CO2 lost in respiration from the microbial culture alone, the experiment was repeated with 1 ml of culture solution in sterile tubes without soil.
Statistical analysis
Data were analysed by One-way ANOVA with LSD post-hoc test (SPSS v14; SPSS, Chicago, IL, USA) after testing for normality and homogeneity of variance with Shapiro-Wilk and Levene's test, respectively. Data not normally distributed or without homogeneity of variance were log10-transformed before analysis. Following transformation all data were normally distributed and had homogeneity of variance. Statistical differences were accepted at P < 0.05. All presented probabilities are for post hoc comparisons.
Results
Plants grown in sterile culture
Uptake of 15N by sterile plant roots when in nonmicrobial forms was 18-, to c. 100-fold faster (tetraalanine, and alanine and nitrate, respectively; P < 0.001; Fig. 1) than when present in a microbial form. Uptake of 15N-nitrate took place at the same rate as 15N-alanine and 15N uptake was approximately five-fold faster (P ≤ 0.003) in both these forms than as tetraalanine. Recovered 15N accounted for 5.1 ± 0.6% of nitrate, 3.1 ± 0.2% of alanine, 0.84 ± 0.1% of tetraalanine and 0.046 ± 0.02% of microbe 15N supplied in the 4 ml of solution. The ratio of the proportion of 13C recovered to the proportion of 15N recovered from organic forms of N was almost identical in plants supplied with alanine and those supplied with tetraalanine (0.63 ± 0.01 and 0.58 ± 0.04, respectively; mean ± SEM; n = 3; Fig. 2).
Figure 1.
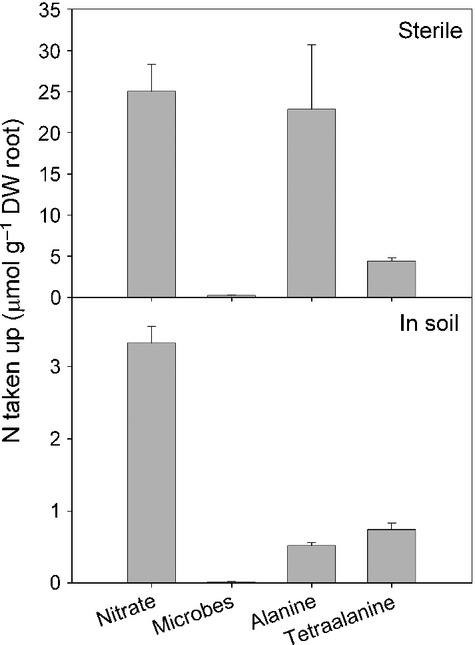
Rate of uptake of 15N supplied as nitrate, l-alanine, l-tetraalanine or 15N-labelled microbial culture to sterile wheat (Triticum aestivum) plant roots and roots of plants growing in soil. Values are mean ± SEM; n = 3 for sterile plants and n = 4 for plants grown in soil. Values for both plants in sterile culture and plants grown in soil assume uptake over the entire root system.
Figure 2.
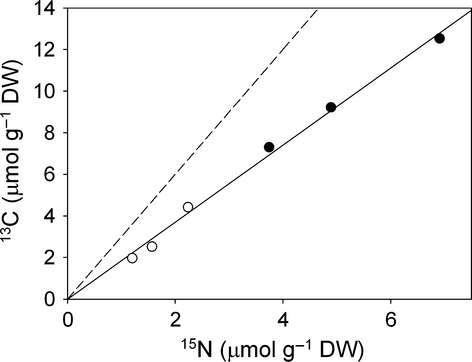
Concentrations of 15N and 13C recovered in tissues of wheat (Triticum aestivum) plants grown in sterile culture after supply of 15N13C-dual-labelled l-alanine or l-tetraalanine. Values are data for individual plants. The solid line is the line of best fit using data for plants supplied with both alanine and tetraalanine (r2 = 0.994; slope = 1.85). The dashed line represents the relationship between 15N and 13C in the compounds supplied to roots. Closed circles, alanine; open circles, tetraalanine.
Roots within the microcosms were predominantly second-order laterals and average root diameter was 0.3 ± 0.03 mm (mean ± SEM; n = 10). The specific root length was 63 ± 8 m g−1 DW.
Plants grown in soil
As in sterile culture, root uptake of 15N added as nitrate, alanine or tetraalanine in plants grown in soil was much greater (P < 0.001; 208-, 32- and 46-fold, respectively; Fig. 1) than when added as the microbial suspension. Uptake of 15N by roots of plants supplied with nitrate was six- and five-fold higher (P < 0.001) than in plants supplied with alanine or tetraalanine, respectively, which were not different from each other. After 1 h 15.8 ± 2% of the added nitrate 15N, 4.5 ± 0.6% of the alanine 15N and 3.9 ± 0.5% of the tetraalanine 15N was recovered in plants, but only 0.079 ± 0.008% of the microbial 15N. Post-uptake partitioning of 15N also differed (P < 0.02) between plants supplied with the different forms of N. The ratio of root 15N to shoot 15N was 1.2 ± 0.1 for plants supplied with microbial N, 2.4 ± 0.6 for those supplied with nitrate, 9.8 ± 1.5 for those supplied with tetraalanine and 18 ± 2.7 for plants supplied with alanine. Ratios for plants receiving nitrate and those receiving microbes were not statistically different.
Data for 13C were much more variable than 15N data in plants receiving dual-labelled compounds. This was especially true of shoots where 13C recovery was only 26 ± 10 or 15 ± 7% (mean ± SEM; n = 3; alanine and tetraalanine, respectively) of that recovered in roots. Recovery of 13C and 15N from dual-labelled compounds in plants grown in soil receiving N as both alanine and tetraalanine were not linearly correlated. The ratio of the proportion of 13C recovered to the proportion of 15N recovered was 0.6 in plants receiving alanine and 0.4 in those receiving tetraalanine, but we suggest that values for the ratio of 13C to 15N for plants grown in soil should be interpreted with caution. Recovered microbial 15N and 14C could not be correlated as they were separate samples. The mean value for recovery of 14C was, however, similar to that for 15N at 0.070 ± 0.02% of that injected.
From injections of ink into rhizotubes it was estimated that added solutes mixed with the soil of a 7 cm section of the rhizotube. These 7 cm sections contained 11.6 ± 0.8 mg root DW (approximately one-third of the total root in the rhizotube), 15.7 ± 9 nmol 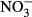 , 47.4 ± 8 nmol free amino acid N and 255 ± 34 nmol short (< 1 kDa) peptide N (mean ± SEM; n = 3 for root DW and n = 4 for solute concentrations). If it is assumed that the root biomass in contact with labelled solutes was a constant 11.6 mg DW, pre-existing soil solutes mixed completely with labelled solutes, and l-alanine and l-tetraalanine are typical of all soil solution amino acids and short peptides, rates of uptake for alanine and tetraalanine remain statistically the same at 2.1 ± 0.3 and 2.5 ± 0.3 μmol N g−1 DW root h−1, respectively. Uptake of nitrate is 7.0 ± 0.8 μmol N g−1 DW root h−1, making it approximately three-fold greater (P < 0.001) than amino acid and peptide N uptake.
, 47.4 ± 8 nmol free amino acid N and 255 ± 34 nmol short (< 1 kDa) peptide N (mean ± SEM; n = 3 for root DW and n = 4 for solute concentrations). If it is assumed that the root biomass in contact with labelled solutes was a constant 11.6 mg DW, pre-existing soil solutes mixed completely with labelled solutes, and l-alanine and l-tetraalanine are typical of all soil solution amino acids and short peptides, rates of uptake for alanine and tetraalanine remain statistically the same at 2.1 ± 0.3 and 2.5 ± 0.3 μmol N g−1 DW root h−1, respectively. Uptake of nitrate is 7.0 ± 0.8 μmol N g−1 DW root h−1, making it approximately three-fold greater (P < 0.001) than amino acid and peptide N uptake.
No evidence of mycorrhizal infection could be found on roots.
Over the hour of incubation, 3.1 ± 0.2% of the added microbial 14C was mineralized to 14CO2 after accounting for that lost in respiration from the added microbes themselves (0.3 ± 0.06%).
Discussion
Acquisition of dissolved forms of N
In sterile hydroponic culture, nitrate 15N was taken up at the same rate as alanine 15N and five times faster than tetraalanine 15N. In contrast to the equal rate of nitrate and amino acid acquisition in sterile culture, competition from microbes caused a large reduction in acquisition of alanine 15N relative to that of nitrate 15N when the plants were grown in nonsterile soil. Previous investigations have suggested some form of preference of soil microbes for l-peptides over the amino acid monomer (Farrell et al., 8, 7; Hill et al., 17, 15). Consequently, it was surprising that while 15N uptake of alanine was considerably reduced in soil, the rate of acquisition of 15N as tetraalanine relative to nitrate by roots in soil was similar to that in sterile solution. Further, what evidence there is suggests that soil solution concentrations of amino acids bound in short peptides are generally higher than those of free amino acids (Farrell et al., 6; Hill et al., 14,b,c; Table 1). This may indicate that acquisition of N as short l-peptides by wheat growing in agricultural soil exceeds that as l-amino acid monomers.
Realistic evaluation of the importance of different forms of soil N to plant nutrition presents formidable experimental problems, especially for organic forms. Even when attempting to determine relative fluxes, it is necessary to consider a range of potential caveats in the interpretation of data. We found no statistically significant correlation between recovered 13C and 15N for plants grown in soil. This may principally be a consequence of variation caused by the dilution of the 13C taken up as intact alanine or tetraalanine in a c. 15 000-fold larger pool of plant carbon with a variable 13C content. Some support for this view is provided by the much larger recovery of 13C in roots than in shoots and the fact that rates of recovery of 13C in plants grown in soil did not depart dramatically from those which would be expected from recovery of 15N after losses in respiration (Hill et al., 18). Nevertheless, we cannot exclude the possibility that some or even all of the amino acid and/or peptide 15N was acquired by plants following prior mineralization by soil microbes. Taking account of isotopic pool dilution in pre-existing pools of soil N also relies on various assumptions. Even if it is clear what proportion of pre-existing soil soluble N is mixed with, differing flux rates through soil pools for the different forms of N add uncertainty. For instance, the residence time of l-amino acids and short l-peptides in soil is probably only of the order of a few minutes (Hill et al., 15). Thus, if acquired intact, the flux of N into plants as amino acid or peptide relative to that of forms of N which are less desirable to most soil microbes, such as nitrate, is probably underestimated with a chase period of an hour (Jones et al., 21). If amino acid and peptide N are only acquired following mineralization, recovery of 15N in plants would be likely to increase with the chase period. In this case, the actual flux of N derived from mineralization of organic forms would be underestimated due to dilution by pre-existing inorganic N and this dilution would probably increase with residence time. Many uncertainties relating to solute production sites and mobility in soil may also be of considerable importance in the design of experiments and interpretation of experimental data. Further, we know little about the composition of individual peptides in soil solution. For instance, 160 000 possible tetrapeptides may be formed from 20 common protein amino acids. To date, the availability to plants of very few peptides has been investigated. Consequently, after correction for pool dilution fluxes of, particularly peptide, N from soil to plant must be interpreted with some caution.
Acquisition of N as microbes
Nitrogen delivered to plant roots as a microbial culture was acquired both by plants with sterile roots and those growing in soil. In both cases the nitrate, amino acid monomer and tetrapeptide forms of N were taken up more than an order of magnitude faster than the microbial suspension. If all of the measured microbial 15N acquired by plants was acquired as intact microbes, our results suggest that sterile roots of plants ingested c. 2 × 106 microbial cells g−1 root DW h−1. This suggests that microbes were ingested at a rate of c. 32 000 cells m−1 root length h−1 or 35 cells mm−2 root surface area h−1, although this does not take account of fine root structure such as root hairs, which may have a role in microbe acquisition (Paungfoo-Lonhienne et al., 35; Mercado-Blanco & Prieto, 27). To match the rate of N uptake as nitrate or alanine from sterile solution, plants would need to ingest c. 2 × 108 cells g−1 root DW h−1 and c. 4 × 107 to match N uptake as tetraalanine. When growing in soil, 15N recovery suggests that plants ingested c. 390 000 cells g−1 root DW h−1; c. 6 cells mm−2 root surface area h−1 (assuming a constant 11.6 mg of root in a 7 cm section of rhizotube was accessed by injected microbes). If previous estimates of numbers of bacteria on wheat roots are typical and it is assumed that labelled microbes mixed homogeneously with existing rhizoplane microbes, this suggests that rates of uptake were c. 975 000 cells g−1 root DW h−1: c. 15 cells mm−2 root surface area h−1; c. 4.5% of the standing rhizoplane bacterial biomass d−1 (Liljeroth, 25). If it is assumed that injected cells mixed with all bacteria on the rhizoplane and in the rhizo-sphere, this value rises to c. 106 cells g−1 root DW h−1 or c. 16 cells mm−2 root surface area h−1. Nevertheless, this flux of N into roots still represents only a maximum of c. 6% of the flux of other forms of N when they are similarly corrected for pool dilution. There are uncertainties in the dilution of amino acid, peptide and nitrate in pre-existing soil pools. However, very poor understanding of the process of direct microbe uptake by roots means that the size of the pool with which added microbes mixed is very difficult to establish. Consequently, our estimates of uptake of N as microbes are probably subject to the greatest uncertainty.
Three percent of the 14C added to soil as microbes was mineralized to 14CO2 within an hour. This was around a fifth of the 14C likely to be mineralized to 14CO2 if added to soil of this type as glucose (Hill et al., 13). Nevertheless, respired 14CO2 was an almost 40-fold greater proportion of the microbial biomass 14C than the proportion of the microbial 15N which was recovered in plants (0.08%). Similarly, the 0.3% of the microbial 14C respired by the living microbes alone was a six times greater proportion of microbial 14C than the 0.05% of microbial 15N recovered in sterile plants. This may indicate that in both soil and sterile solutions, the microbial 15N recovered in plants was taken up as inorganic 15N after microbial mineralization. However, although there is some uncertainty inherent in the measurement of 14C and 15N in separate plants, the close agreement between values for recovery of microbial 15N and 14C in plants growing in soil strongly suggests that 15N was not acquired only in inorganic forms. We cannot completely exclude the possibility that both N and C were taken up as organic forms of N following prior lysis of microbes. In our opinion this seems unlikely to account for the entire flux of 14C and 15N, as that would necessitate the maintenance of the overall microbial 14C to 15N ratio in the organic forms of N taken up after any losses of C in respiration. Nevertheless, post-lysis, or even post-mineralization, plant uptake probably accounts for part of the flux of microbial N into roots.
Many living endophytes exist in plants, although our knowledge of how widespread the ability to survive within plants is amongst soil microbes is largely restricted to studies on a few species (Hardoim et al., 12; Ryan et al., 43; Reinhold-Hurek & Hurek, 39). Our measurements of incorporation of microbial 15N and 14C cannot distinguish between microbes internalized and metabolized within root cells and those continuing to survive within the plant, that is within the apoplast or root wounds (Gantar, 10; Hardoim et al., 12; Paungfoo-Lonhienne et al., 35). The close agreement between the 15N and 14C recovery from the microbial cells contrasts with the at least 40% of amino acid and peptide 13C which was rapidly metabolized and lost in respiration. This may also indicate that microbes were not metabolized by the plant after uptake. Further, in some cases, endophytes move from root to shoot within the plant without apparent attack or digestion by the host with bacterial movement from the root epidermis to the stele occurring via the apoplast (Reinhold-Hurek et al., 40; Rosenblueth & Martinez-Romero, 42; Deering et al., 5). Consequently, 15N recovery in the shoot cannot be unequivocally attributed to degradation of microbes in the root, with subsequent transport of 15N to the shoot (Paungfoo-Lonhienne et al., 35). However, even if plant degradation of microbes took place more slowly than other forms of organic N and too slowly to be very obvious over the hour of experiment duration, eventual death and decomposition of some microbes within the plant does seem likely.
Assuming that bacteria can be internalized within root cells, it raises questions about the mechanism of cytophagocytosis and to what extent this is under direct control of the plant (Hardoim et al., 12; Paungfoo-Lonhienne et al., 35). Due to the small pore size of the cell wall (< 10 nm) relative to the size of bacterial cells (c. 1000 nm), internalization can only occur by loosening/digestion of the cell wall in mature root regions or possibly at weak points in actively growing cells (Reinhold-Hurek et al., 40; Miralles et al., 28). If the plant is actively undertaking this process to acquire N, strong selection for nonharmful bacteria is expected, bypassing myriad host-defence processes (Kogel et al., 23; Rosenblueth & Martinez-Romero, 42; Hückelhoven, 19). Perhaps more probable is that many bacterial cells are taken up passively as has been demonstrated for a range of inert micro- and nano-particulates (Solomon & Matthews, 45; Miralles et al., 28). However, whilst it is clear from many studies that live bacteria can rapidly enter and survive in the endorhizosphere, little evidence exists for the passive uptake of dead cells into the apoplast (Quadt-Hallmann et al., 37; Hardoim et al., 12).
Although considerable uncertainty surrounding mechanisms remains, our results suggest that acquisition of microbes from soil by wheat roots, with subsequent translocation of N, does take place. Thus, if this is actively undertaken, all three plant species investigated to date, Arabidopsis thaliana, Solanum lycopersicum and Triticum aestivum, appear to have this capacity (Paungfoo-Lonhienne et al., 35). However, if wheat proves to be typical, low rates of uptake of N as intact microbial cells in comparison with uptake of common inorganic and organic forms of soil N suggest that the importance of this process to overall plant N nutrition is minor. Use of other forms of organic N is often considered to be most important in environments where N mineralization is slow (Chapin et al., 4; Schimel & Bennett, 44; Näsholm et al., 32; Hill et al., 14). Similarly, wider investigation may establish that uptake and digestion of soil microbes has high functional importance in some ecosystems; perhaps when free-living diazotrophs are abundant in an N-limited rhizosphere. In these respects, the use of microbes may be more significant in some angiosperms than it is in a highly-bred agricultural plant such as wheat. Of course, it may also be that microbes are primarily consumed as a source of some nutrient other than N, or that their consumption is only of transient importance.
Although each form of N differs in its mobility in soil, strong sorption of microbial cells to soil particles results in a slow rate of diffusion to the root surface (Foppen et al., 9). Consequently, most microbes available to plants live and reproduce very close to roots and derive much of their nutrition from roots, for example, as exudates (Liljeroth, 25; Brimecombe et al., 3). This may mean that active consumption of these microbes primarily represents a mechanism by which plants recover lost nutrients, as has been proposed for some organic solutes (Jones et al., 21).
Conclusion
Uncertainty still surrounds the extent to which plants can acquire different forms of organic N from soil. Previous studies undertaken in sterile hydroponic culture have clearly demonstrated the potential for plant roots to take up intact microbial cells, but the functional significance of this process in soil environments remains unknown. The results presented here for wheat plants grown in both hydroponics and in soil strongly suggest that the rate of uptake of N as intact microbial cells is very low in comparison with uptake of common inorganic and organic forms of soil N. Although wider investigation is needed and other functions cannot be excluded, this relatively low incorporation of microbial N suggests that digestion of soil microbes probably represents only a small component of overall plant N acquisition.
Acknowledgments
This research was funded by the UK Natural Environment Research Council Grant no. NE/IO12303/1.
References
- Adlassnig W, Koller-Peroutka M, Bauer S, Koshkin E, Lendl T, Lichtscheidl IK. Endocytotic uptake of nutrients in carnivorous plants. Plant Journal. 2012;71:303–313. doi: 10.1111/j.1365-313X.2012.04997.x. [DOI] [PubMed] [Google Scholar]
- Borstlap AC. The use of model-fitting in the interpretation of ‘dual’ uptake isotherms. Plant, Cell & Environment. 1983;6:407–416. [Google Scholar]
- Brimecombe MJ, De Leij FA, Lynch JM. The effect of root exudates on rhizosphere microbial populations. In: Pinton R, Varanini Z, Nannipieri P, editors. The rhizosphere. New York, NY, USA: Marcel Dekker; 2001. pp. 95–140. [Google Scholar]
- Chapin FS, Moilanen L, Kielland K. Preferential use of organic nitrogen for growth by a non-mycorrhizal arctic sedge. Nature. 1993;361:150–153. [Google Scholar]
- Deering AJ, Pruitt RE, Mauer LJ, Reuhs BL. Identification of the cellular location of internalized Escherichia coli O157:H7 in mung bean, Vigna radiata by immunocytochemical techniques. Journal of Food Protection. 2011;74:1224–1230. doi: 10.4315/0362-028X.JFP-11-015. [DOI] [PubMed] [Google Scholar]
- Farrell M, Hill PW, Farrar JF, Bardgett RD, Jones DL. Seasonal variation in soluble soil carbon and nitrogen across a grassland productivity gradient. Soil Biology & Biochemistry. 2011a;43:835–844. [Google Scholar]
- Farrell M, Hill PW, Farrar J, DeLuca TH, Roberts P, Kielland K, Dahlgren R, Murphy DV, Hobbs PJ, Bardgett RD. Oligopeptides represent a preferred source of organic N uptake: a global phenomenon? Ecosystems. 2012;16:133–145. [Google Scholar]
- Farrell M, Hill PW, Wanniarachchi SD, Farrar JF, Bardgett RD, Jones DL. Rapid peptide metabolism: a major component of soil nitrogen cycling? Global Biogeochemical Cycles. 2011b;25:GB3014. [Google Scholar]
- Foppen JWA, Mporokoso A, Schijven JF. Determining straining of Escherichia coli from breakthrough curves. Journal of Contaminant Hydrology. 2005;76:191–210. doi: 10.1016/j.jconhyd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Gantar M. Mechanical damage of roots provides enhanced colonization of the wheat endorhizosphere by the dinitrogen-fixing cyanobacterium Nostoc sp strain 2S9B. Biology and Fertility of Soils. 2000;32:250–255. [Google Scholar]
- Giesler R, Lundström US. Soil solution chemistry: effects of bulking soil samples. Soil Science Society of America Journal. 1993;57:1283–1288. [Google Scholar]
- Hardoim PR, van Overbeek LS, van Elsas JD. Properties of bacterial endophytes and their proposed role in plant growth. Trends in Microbiology. 2008;16:463–471. doi: 10.1016/j.tim.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Hill PW, Farrar JF, Jones DL. Decoupling of microbial glucose uptake and mineralization in soil. Soil Biology & Biochemistry. 2008;40:616–624. [Google Scholar]
- Hill PW, Farrar J, Roberts P, Farrell M, Grant H, Newsham KK, Hopkins DW, Bardgett RD, Jones DL. Vascular plant success in a warming Antarctic may be due to efficient nitrogen acquisition. Nature Climate Change. 2011a;1:50–53. [Google Scholar]
- Hill PW, Farrell M, Jones DL. Bigger may be better in soil N cycling: does rapid acquisition of small L-peptides by soil microbes dominate fluxes of protein-derived N in soil? Soil Biology & Biochemistry. 2012;48:106–112. [Google Scholar]
- Hill PW, Kuzyakov Y, Jones D, Farrar J. Response of root respiration and root exudation to alterations in root C supply and demand in wheat. Plant and Soil. 2007;291:131–141. [Google Scholar]
- Hill PW, Farrell M, Roberts P, Farrar J, Grant H, Newsham KK, Hopkins DW, Bardgett RD, Jones DL. Soil- and enantiomer-specific metabolism of amino acids and their peptides by Antarctic soil microorganisms. Soil Biology & Biochemistry. 2011b;43:2410–2416. [Google Scholar]
- Hill PW, Quilliam RS, DeLuca TH, Farrar JF, Farrell M, Roberts P, Newsham KK, Hopkins DW, Bardgett RD, Jones DL. Acquisition and assimilation of nitrogen as peptide-bound and D-enantiomers of amino acids by wheat. PLoS ONE. 2011c;6:e19220. doi: 10.1371/journal.pone.0019220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hückelhoven R. Transport and secretion in plant–microbe interactions. Current Opinion in Plant Biology. 2007;10:573–579. doi: 10.1016/j.pbi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Jones DL, Darrah PR. Amino-acid influx at the soil–root interface of Zea mays L. and its implications in the rhizosphere. Plant and Soil. 1994;163:1–12. [Google Scholar]
- Jones DL, Healey JR, Willett VB, Farrar JF, Hodge A. Dissolved organic nitrogen uptake by plants – an important N uptake pathway? Soil Biology & Biochemistry. 2005;37:413–423. [Google Scholar]
- Jones DL, Owen AG, Farrar JF. Simple method to enable the high resolution determination of total free amino acids in soil solutions and soil extracts. Soil Biology & Biochemistry. 2002;34:1893–1902. [Google Scholar]
- Kogel K-H, Franken P, Hückelhoven R. Endophyte or parasite-what decides? Current Opinion in Plant Biology. 2006;9:358–363. doi: 10.1016/j.pbi.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Komarova NY, Thor K, Gubler A, Meier S, Dietrich D, Weichert A, Suter-Grotemeyer M, Tegeder M, Rentsch D. AtPTR1 and AtPTR5 transport dipeptides in planta. Plant Physiology. 2008;148:856–869. doi: 10.1104/pp.108.123844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeroth E. Microorganisms in the rhizosphere of barley and wheat: the effects of variety and nitrogen fertilization. Svalöv, Sweden: Swedish University of Agricultural Sciences; 1990. PhD thesis. [Google Scholar]
- Liu LL, Greaver TL. A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecology Letters. 2010;13:819–828. doi: 10.1111/j.1461-0248.2010.01482.x. [DOI] [PubMed] [Google Scholar]
- Mercado-Blanco J, Prieto P. Bacterial endophytes and root hairs. Plant and Soil. 2012;361:301–306. [Google Scholar]
- Miralles P, Church TL, Harris AT. Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environmental Science & Technology. 2012;46:9224–9239. doi: 10.1021/es202995d. [DOI] [PubMed] [Google Scholar]
- Miranda KM, Espey MG, Wink DA. A rapid, simple, spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- Mulvaney RL. Nitrogen—inorganic forms. In: Sparks DL, editor. Methods of soil analysis. Part 3. Madison, WI, USA: Soil Science Society of America Inc; 1996. pp. 1123–1184. [Google Scholar]
- Näsholm T, Huss-Danell K, Högberg P. Uptake of glycine by field grown wheat. New Phytologist. 2001;150:59–63. [Google Scholar]
- Näsholm T, Kielland K, Ganeteg U. Uptake of organic nitrogen by plants. New Phytologist. 2009;182:31–48. doi: 10.1111/j.1469-8137.2008.02751.x. [DOI] [PubMed] [Google Scholar]
- Owen AG, Jones DL. Competition for amino acids between wheat roots and rhizosphere microorganisms and the role of amino acids in plant N acquisition. Soil Biology & Biochemistry. 2001;33:651–657. [Google Scholar]
- Paungfoo-Lonhienne C, Lonhienne TGA, Rentsch D, Robinson N, Christie M, Webb RI, Gamage HK, Carroll BJ, Schenk PM, Schmidt S. Plants can use protein as a nitrogen source without assistance from other organisms. Proceedings of the National Academy of Sciences, USA. 2008;105:4524–4529. doi: 10.1073/pnas.0712078105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paungfoo-Lonhienne C, Rentsch D, Robatzek S, Webb R, Sagulenko E, Näsholm T, Schmidt S, Lonhienne T. Turning the table: plants consume microbes as a source of nutrients. PLoS ONE. 2010;5:e11915. doi: 10.1371/journal.pone.0011915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paungfoo-Lonhienne C, Visser J, Lonhienne TGA, Schmidt S. Past, present and future of organic nutrients. Plant and Soil. 2012;359:1–18. [Google Scholar]
- Quadt-Hallmann A, Benhamou N, Kloepper JW. Bacterial endophytes in cotton: mechanisms of entering the plant. Canadian Journal of Microbiology. 1997;43:577–582. [Google Scholar]
- Raven JA, Beardall J, Flynn KJ, Maberly SC. Phagotrophy in the origins of photosynthesis in eukaryotes and as a complementary mode of nutrition in phototrophs: relation to Darwin's insectivorous plants. Journal of Experimental Botany. 2009;60:3975–3987. doi: 10.1093/jxb/erp282. [DOI] [PubMed] [Google Scholar]
- Reinhold-Hurek B, Hurek T. Living inside plants: bacterial endophytes. Current Opinion in Plant Biology. 2011;14:435–443. doi: 10.1016/j.pbi.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Reinhold-Hurek B, Maes T, Gemmer S, van Montagu M, Hurek T. An endoglucanase is involved in infection of rice roots by the not-cellulose-metabolizing endophyte Azoarcus sp. Strain BH72. Molecular Plant-Microbe Interactions. 2006;19:181–188. doi: 10.1094/MPMI-19-0181. [DOI] [PubMed] [Google Scholar]
- Roberts P, Stockdale R, Khalid M, Iqbal Z, Jones DL. Carbon-to-nitrogen ratio is a poor predictor of low molecular weight organic nitrogen mineralization in soil. Soil Biology & Biochemistry. 2009;41:1750–1752. [Google Scholar]
- Rosenblueth M, Martinez-Romero E. Bacterial endophytes and their interactions with hosts. Molecular Plant-Microbe Interactions. 2006;19:827–837. doi: 10.1094/MPMI-19-0827. [DOI] [PubMed] [Google Scholar]
- Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN. Bacterial endophytes: recent developments and applications. FEMS Microbiology Letters. 2008;278:1–9. doi: 10.1111/j.1574-6968.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- Schimel JP, Bennett J. Nitrogen mineralization: challenges of a changing paradigm. Ecology. 2004;85:591–602. [Google Scholar]
- Solomon EB, Matthews KR. Use of fluorescent microspheres as a tool to investigate bacterial interactions with growing plants. Journal of Food Protection. 2005;68:870–873. doi: 10.4315/0362-028x-68.4.870. [DOI] [PubMed] [Google Scholar]
- Soper FM, Paungfoo-Lonhienne C, Brackin R, Rentsch D, Schmidt S, Robinson N. Arabidopsis and Lobelia anceps access small peptides as a nitrogen source for growth. Functional Plant Biology. 2011;38:788–796. doi: 10.1071/FP11077. [DOI] [PubMed] [Google Scholar]
- Vitousek PM, Howarth RW. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry. 1991;13:87–115. [Google Scholar]
- Voroney RP, Brookes PC, Beyaert RP. Soil microbial biomass C, N, P, and S. In: Carter MR, Gregorich EG, editors. Soil sampling and methods of analysis. 2nd edn. Boca Raton, FL, USA: CRC Press; 2008. pp. 637–651. [Google Scholar]


