Abstract
Rationale
Women are increasingly prescribed selective serotonin reuptake inhibitors (SSRIs) during pregnancy, with potential implications for neurodevelopment. Whether prenatal SSRI exposure has an effect on neurodevelopment and behavior in the offspring is an important area of investigation.
Objectives
The aim of this paper was to review the existing preclinical and clinical literature of prenatal SSRI exposure on serotonin-related behaviors and markers in the offspring. The goal is to determine if there is a signal in the literature that could guide clinical care and/or inform research.
Results
Preclinical studies (n = 4) showed SSRI exposure during development enhanced depression-like behavior. Half of rodent studies examining anxiety-like behavior (n = 13) noted adverse effects with SSRI exposure. A majority of studies of social behavior (n = 4) noted a decrease in sociability in SSRI exposed offspring. Human studies (n = 4) examining anxiety in the offspring showed no adverse effects of prenatal SSRI exposure. The outcome of one study suggested that children with autism were more likely to have a mother who was prescribed an SSRI during pregnancy.
Conclusions
Preclinical findings in rodents exposed to SSRIs during development point to an increase in depression- and anxiety-like behavior and alteration in social behaviors in the offspring, though both the methods used and the findings were not uniform. These data are not robust enough to discourage use of SSRIs during human pregnancy, particularly given the known adverse effects of maternal mental illness on pregnancy outcomes and infant neurodevelopment. Future research should focus on consistent animal models and prospective human studies with larger samples.
Keywords: Perinatal depression, Perinatal SSRI, Serotonin, Anxiety, Social behavior, Pain reactivity, Psychomotor development, S100B, HPA axis
Introduction
Major depressive disorder (MDD) is widespread in women throughout the USA, with a lifetime prevalence of 16.5 % (Kessler et al. 2005). Up to 15 % of women will suffer from depression while pregnant (Evans et al. 2001; Bennet et al. 2004; Gavin et al. 2005; Oberlander et al. 2006). Recent studies report that 3 % of pregnant women take antidepressants for some portion of their pregnancy (Petersen et al. 2011), most commonly selective serotonin reuptake inhibitors (SSRI), and the number of women being treated is increasing (Bakker et al. 2008; Petersen et al. 2011). While antidepressant use during pregnancy has increased in the last decade, reflecting an increase in use by the general population, pregnancy is a major reason many women stop receiving antidepressants (Ververs et al. 2006; Petersen et al. 2011). Most likely, pregnant women terminate antidepressant use due to concerns regarding potential adverse effects on the developing fetus. However, there can be difficulty teasing apart the relative contributions of maternal psychopathology versus the treatment of psychopathology on adverse pregnancy outcomes.
Indeed, maternal anxiety and depression have been implicated in poor pregnancy outcomes, including premature delivery, pre-eclampsia, impaired fetal growth, neonatal complications, and compromised fetoplacental function (Kurki et al. 2000; Bonari et al. 2004; Jablensky et al. 2005; reviewed in Gentile et al. 2010a; Chen et al. 2011). Premature birth itself is linked to neurodevelopmental difficulties including cerebral palsy, fine or gross motor delay, cognitive impairments, and visual and auditory impairments (reviewed in Allen 2008). Therefore, maternal depression should not be thought of as a benign presence. While antidepressant treatment is recommended for moderate to severe major depressive disorder during pregnancy (Yonkers et al. 2009), treatment may also have consequences on pregnancy outcome. Common adverse consequences reported with SSRI use during pregnancy are preterm birth and poor neonatal adaptation syndrome, which is similar to what adults may experience with discontinuation of antidepressant treatment and is characterized by irritability, sleep disturbances, tremor, rigidity, and limpness (Moses-Kolko et al. 2005; Gentile 2010b; El Marroun et al. 2012). A recent study found a relative risk of 2.1 (95 % CI=1.0–4.6) for preterm birth primarily with continuous serotonin reuptake inhibitor use throughout pregnancy (Yonkers et al. 2012). Another recent study found increased risk of hospitalization within the first year of life in children exposed to SSRIs; however, the most frequent cause was bronchiolitis, which has an unclear link to SSRIs themselves and may be associated with other exposures (Colvin et al. 2012). The literature does not support an association between maternal SSRIs as a class and major congenital malformations (Alwan et al. 2007; Louik et al. 2007; Kornum et al. 2010), although there is some evidence that certain SSRIs may increase the risk of cardiac septal defects, as fluoxetine was associated with an increased risk of isolated ventricular septal defects and paroxetine was associated with an increased risk of right ventricular outflow tract abnormalities (Malm et al. 2011).
Serotonin (5-HT) is a broadly distributed neurotransmitter, with a vital role in both the developing and mature brain. Developmentally, 5-HT modulates a variety of processes, acting as a trophic factor overseeing cell division, neuronal migration, and synaptogenesis, among other important processes (reviewed in Gaspar et al. 2003; Bonnin and Levitt 2011). An emerging line of thought is that 5-HT plays a role in response to environmental factors by modulating neuroplasticity. Following this line of reasoning, increased levels of 5-HT could generate a propensity for the fetal central nervous system (CNS) to be influenced by anything that alters the intra-uterine environment. Therefore, individuals with low 5-HT would be less adversely affected by negative experiences; however, they benefit less from enriched environments as well (Kiser et al. 2012). This concept becomes even more intriguing when considering the high level of plasticity during fetal development. It is certainly possible that alterations in 5-HT homeostasis with SSRI during this time period could change an individual’s reactivity to their environment, which depending on the environment could be deleterious or beneficial. In adults, the 5-HT system modulates mood, cognition, attention, arousal, and stress response (Lucki 1997; Anderson 2004). Levels of 5-HT are regulated by the serotonin transporter (SERT), which is the target of SSRIs. It is this increase in intra-synaptic 5-HT that is thought to trigger a chain of events, including a second messenger cascade, increased transcription of trophic factors as well as neurogenesis, which are hypothesized to contribute to behavioral changes, and symptom improvement. In comparison to our unfolding understanding of the mechanism of SSRI efficacy in adults, the molecular underpinnings of their pathway during fetal development are murky. Genetic deletion of SERT leads to a 7–9-fold increase in extra-neuronal 5-HT, as well as an overall decrease in the 5-HT found in tissue (reviewed in Kalueff et al. 2010). Overall, in the presence of a genetic deletion of SERT, the developing fetal brain is likely exposed to alterations in 5-HT receptor function and density due to changes in extraneuronal and tissue 5-HT levels.
It is generally accepted that 5-HT serves a critical role in neurodevelopment. Whether alteration in the 5-HT system by treatment with SSRI during pregnancy leads to adverse behavioral sequelae is of utmost concern, yet poorly understood (Oberlander et al., 2009). SSRIs cross the placenta (Hendrick et al. 2003; Rampono et al. 2009) and therefore have the capability to impact the developing 5-HT system. However, the effect and extent of alterations remains an area of intense research, and it cannot be assumed that developmental perturbations are responsible for adult malfunction. In preclinical studies, there is increasing evidence that alterations in the 5-HT system during neurodevelopment, either by genetic manipulation or exposure to SSRIs, can produce persistent changes in central serotonergic function and behavior into adulthood. However, there is a dearth of studies examining behavior in children exposed to SSRI, and reports to date have not described findings from children beyond 72 months of age. In addition to its role in the CNS, 5-HT also participates in control of gastrointestinal secretion and motility, hemostatic processes, and cardiovascular activity. It is secreted peripherally by parafollicular cells in the thyroid, neuroepithelial cells in the lung, and enterochromaffin cells in the gut. Approximately 95 % of the body’s serotonin is found in the bowel (Berger et al. 2009), making the GI system a prime target for SSRIs. However, the impact of prenatal SSRI exposure on the development and function of these systems has not been investigated in preclinical models, but such studies are underway in human subjects (Bakker et al. 2010; Niejenhus et al. 2012a, b). This review focuses on the emerging animal and human studies examining the impact of SSRI exposure on CNS development and addresses the strength of the signal concerning the effect of SSRI on serotonin-related behaviors and functions in those offspring exposed prenatally.
Serotonin manipulation effects on brain and behavior: preclinical studies
Animal research paradigms
A central difficulty in examining the contribution of prenatal SSRI exposure to the development of behavioral disturbances in human offspring is the need to differentiate the effect of illness from its treatment. Animal models allow for standardization of timing and extent of SSRI exposure or other serotonin system manipulation, providing control that is lacking in human subjects studies. Scientists are able to measure behavioral changes thought to be reflective of depression, anxiety, and social behaviors in mice with a variety of paradigms, though some of these behavioral changes may be better described as sensitive to pharmacological intervention rather than manifestations of the human experience of “depression” per se. They certainly permit examination of the impact of pharmacological and genetic manipulations. For example, increased immobility in the forced swim test (FST) is decreased by administration of acute or chronic antidepressant medications in adult rodents (Lucki et al. 1997). Also in adult rodents, increased latency to feed in the novelty induced hypophagia test (NIH) is reversed by chronic, but not acute, antidepressant treatment (Merali et al. 2003). The latter paradigm has temporal validity, as it reflects the time course needed to observe clinical benefit in humans undergoing SSRI treatment for affective disorders. The open field test and elevated plus maze are both paradigms that rely on a rodents inherent mistrust of open, brighter spaces, and are sensitive to the effects of benzodiazepines, which are used in humans to treat anxiety. The evaluation of social interaction is a less standardized process, with a variety of paradigms being implemented by individual studies. One such paradigm involves placing the mouse in a three-chambered apparatus, and measuring the amount of time it spends with a novel mouse placed in a cylinder, versus a cylinder enclosing an inanimate object (Sankoorikal et al. 2006). Certain mouse strains, as well as certain mouse models of psychiatric disorders (e.g., schizophrenia), will spend less time interacting socially with the novel mouse (Sankoorikal et al. 2006; Halene et al. 2009).
These and other behavioral paradigms can be used to examine both the effect of medications as well as the effect of genetic manipulations on brain function and behavior. Animal studies also enable the examination of the timing of exposure and the impact on behavior across the developmental trajectory of the offspring, which is difficult to do in humans. However, one challenge in interpreting and comparing data both between studies as well as between rodents and humans is variations in the type, duration, and timing of the exposure, as well the type and timing of the behavioral assessment, as seen in Fig. 1. Exposure to SSRI throughout pregnancy and early development may be very different than a limited exposure during late gestation, potentially as is behavioral testing during a juvenile period versus adulthood. Also demonstrated in this figure is the relationship between human time points and rodent time points, i.e., the first 3 weeks of life in rodents is roughly equivalent to the third trimester in humans (Clancy et al. 2001; Nagarajan et al. 2010). It may be noted that other authors suggest that, in terms of hippocampal development, the first week of life in rats is comparable to the third trimester gestational period of humans and the second week of life corresponds roughly to the first year of life in humans (Avishai-Eliner et al. 2002). Overall, these studies underscore the point that the rodent brain is essentially born “premature”, which may have implications in its development. Finally, it is important to keep in mind that while clinical studies are carried out in individuals that are ill, some animal studies are performed in healthy animals. These limitations should not overshadow the tremendous potential of animal studies to contribute to our understanding of the impact of prenatal SSRIs; rather they highlight the need for future studies to take these issues under advisement.
Fig. 1.
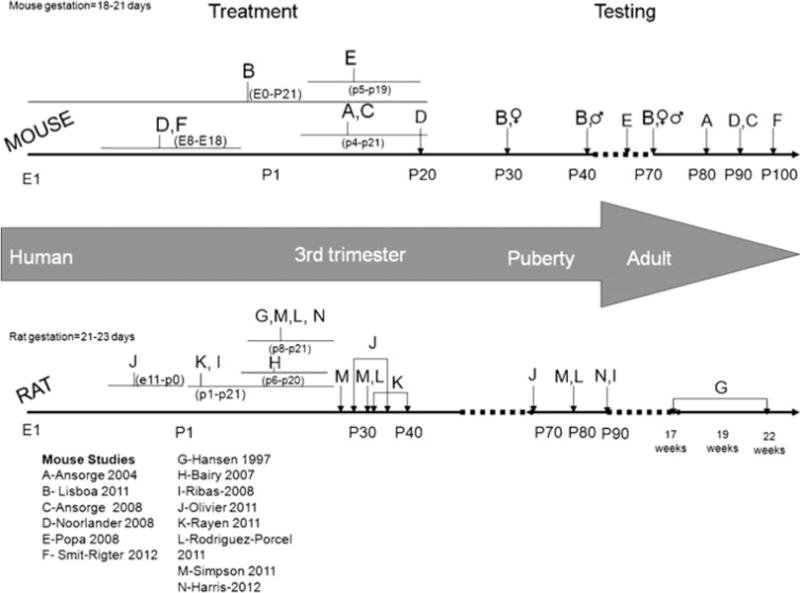
Timeline of gestation and pharmacological treatment and testing in mice and rat. Top line illustrates studies performed in rats. Middle line demonstrates corresponding development in humans. Bottom line illustrates studies performed in rats. Studies are indicated by uppercase letters (A–M). The duration and timing of treatment is shown with flat bars. Timing of testing is indicated by arrows
Impact of genetic and pharmacologic manipulations on depression and anxiety
The consequence of altering 5-HT homeostasis during development by targeting SERT has been accomplished with both genetic deletion and SSRI administration. While in utero administration of SSRIs can be time limited, and provides a temporally refined investigation of the effects of a serotonin manipulation on neurodevelopment and behavior, genetic inhibition of SERT in the knockout mouse model is enduring, from development through adulthood, and leads to worsening performance in paradigms designed to measure depressive-like symptoms (Lira et al. 2003). This may seem paradoxical, as it is inhibition of this very transporter by SSRIs that is thought to initiate the cascade responsible for its positive antidepressant and anxiolytic effects in adult rodents. However, the effect of inhibiting SERT function during development is likely to be different than inhibiting it during adulthood. Indeed, the SERT knockout mice had fewer 5-HT immunopositive neurons, which could account for the behavioral changes seen. It is also worth noting that the offspring are carried by a genetically altered dam, and the maternal alteration could potentially influence both the intrauterine environment during gestation as well as maternal behavior. Indeed, transient inhibition of SERT with SSRI during development was carried out with a variety of treatment paradigms, yielding mixed results, and there is substantial heterogeneity on how this is characterized in the literature (Fig. 1; Table 1).
Table 1.
Preclinical studies examining the effect of developmental SSRI on serotonergic behaviors in the offspring
| Study | Model/sex | Treatment | Testing | Depression | Anxiety | Social interaction |
|---|---|---|---|---|---|---|
| Ansorge et al. 2004 | Mouse: 29s6/Svev: male + female | Fluoxetine: p4–P21 | EPM, OFT, NSF, shock avoidance: 12 weeks | ↑ | ||
| Lisboa et al. 2007 | Mouse: Swiss: male + female | Fluoxetine: el–p21 | EPM, OFT, FST, Intruder Resident Test, Hot Plate Test. Females p30 and p70; males p40 and p70 | ♀↑ | ↔ | |
| Ansorge et al. 2008 | Mouse: 29S6/Svev: male + female | Fluoxetine, Citalopram p4–p21 | EPM, OFT, NSF, NIH, shock avoidance, 3–4 months of age | ↑ | ||
| Noorlander et al. 2008 | Mouse: C57BI/6-Jico: male + female | Fluoxetine or fluvoxamine: E8–E18 | EPM (p20, p90), OFT, NSF (p90) | ↑ | ||
| Popa et al. 2008 | Mouse: Swiss: female | Escitalopram p5–P19 | EPM, FST, TST, sucrose preference, dark–light choice—2-month-old | ↑ | ↔ | |
| Smit-Rigter et al. 2012 | Mouse: C57/BL6J: male + female | Fluoxetine: E8–E18 | NFS—approx. p100 | ↑ | ||
| Hansen et al. 1997 | Rat: Wistar: male | Lu10-134-C (SSRI): P8–P21 | OFT (17–19 weeks), FST (18–20 weeks) | ↑ | ↔ | |
| Bairy et al. 2007 | Rats: albino | Fluoxetine p6–p20 | Geotaxis, open field exploration, rota-rod test, elevated plus-maze and passive avoidance test | ↔ | ||
| Ribas et al. 2008 | Rats: Wistar: male | Fluoxetine 10 mg Pl–p21 | EPM (p90) | ↔ | ||
| Olivier et al. 2011 | Rat: Wistar: male + female | Fluoxetine E11–P0 | Juvenile play (p28–p35), EPM, OFT, NSF, FST, foot shock aversion, sucrose preference, adult social behavior all performed >p65 | ↑ | ↓ | |
| Rayen et al. 2011 | Rats: Sprague–Dawley: male + female | Fluoxetine pl–p21 | OFT, FST (p32–p39) | ↑ | ↔ | |
| Rodriguez-Porcel et al. 2011 | Rats: Long-Evans: male + female | Citalopram, bupropion, fluoxetine P8–p21 | Responsible to novel tone or novel object, juvenile play | ↓ | ||
| Simpson et al. 2011 | Rat: male + female | Citalopram p8–p21 | Novel tone (p25), juvenile play (p32–p34), novel object (p30 and 82) | ↑ | ↓ | |
| Harris et al. 2011 | Rats: Long-Evans: male | Citalopram (5, 10, 15) p8–p21 | EPM | ↔ |
↑ increase, ↔ no change, ↓ decrease, ♀ female
Ansorge et al., using fluoxetine at therapeutically relevant doses during postnatal days 4–21, which is roughly equivalent to the third trimester of pregnancy and early infancy in human development, produced a similar abnormal phenotype in adult mice as that seen with genetic SERT deletion throughout development (Ansorge et al. 2004, 2008). Likewise, several studies, utilizing medications at therapeutically relevant doses, demonstrate that prenatal or early postnatal exposure to an SSRI in mice increases anxiety-like behavior in adulthood (Ansorge et al. 2004, 2008; Noorlander et al. 2008; Olivier et al. 2011; Smit-Rigter et al. 2012). However, several studies using SSRI during development did not result in increased anxiety in adulthood (Hansen et al. 1997; Bairy et al. 2007; Popa et al. 2008; Ribas et al. 2008; Harris et al. 2011; Rayen et al. 2011) (Table 1). These discrepancies may arise from the differences between studies with respect to paradigms, strain of rodents, and type and timing of the SSRI administered. Namely, it is possible that using citalopram, with a shorter half-life than fluoxetine, administered during a different time period, and then testing for an anxiety phenotype in a different paradigm (e.g., the EPM instead of NIH) will lead to divergent results.
Similar to findings with genetic inactivation of SERT, rodent studies have suggested that prenatal or early postnatal exposure to SSRIs results in depressive-like behavior when the rodent is tested during adulthood (Hansen et al. 1997; Lisboa et al. 2007). An additional study of only female mice revealed an increase in anhedonia and behavioral despair following neonatal treatment with escitalopram. Of note, the depression-like symptoms were reversible when the mice were treated with chronic SSRI as adults (Popa et al. 2008). In the FST, prenatal stress exposure led to a reduction in immobility while surprisingly treatment with fluoxetine initiated on postnatal day 1 (equivalent to prenatal administration in humans) reversed this effect of stress, essentially enhancing immobility, a depression-like behavior. On a cellular level, fluoxetine treatment reversed the prenatal stress-induced decrease in neurogenesis, suggesting a disconnection between cellular changes and resulting behaviors (Rayen et al. 2011). Further discrepancies are demonstrated by a study where prenatal and lactational SSRI exposure did not alter behavior in the FST or elevated plus maze in males. However, female pups did demonstrate increased immobility in the FST (Lisboa et al. 2007). There are few animal studies examining sex differences in these behavioral paradigms, which is notable both because prenatal stress does demonstrate sex differences in effects on behavior (Bale 2011) and because depression is more prevalent in females (Fava and Kendler 2000).
Therefore, the preclinical signal with respect to the offspring’s behavior after serotonin manipulation during development is decidedly mixed, in terms of increased risk for anxiety or depressive-like symptoms. While genetic deletion of SERT has a clearer effect, with increased levels of anxiety in the offspring, pharmacologic treatment does not reach this level of consistency. Therefore, developmental exposure to SSRI does not equate to genetic SERT manipulation, and how relevant these data are to human subjects may be limited and is unclear at this time.
Impact of genetic and pharmacologic manipulations on social behaviors
In addition to its role in affective states, 5-HT contributes to complex social behaviors in mammals. The neural circuit mediating 5-HT influence on social interaction is a complex one, with contributions from the amygdala, hippocampus, medial prefrontal cortex, sensory cortex, insular cortex, and orbitofrontal cortex each responsible for various components (Olsson and Phelps 2007). Genetic variations in SERT have been found to influence multiple levels of social interactions, from maternal attachment to social play, social hierarchy establishment to aggression. For example, SERT knockout rats with increased extracellular 5-HT demonstrate decreased social play behavior, such as chasing and pouncing. Of note, acute treatment with fluoxetine also decreased social interaction in peri-adolescent wild-type rats in this study (Homberg et al. 2007). An alternative interpretation of these results, as the authors themselves concede, is that the treated rats had increased levels of anxiety, which they did not investigate.
There are several preclinical studies that do demonstrate that juvenile rodents exposed to prenatal or neonatal SSRI show deficits in social interaction (Olivier et al. 2011; Rodriguez-Porcel et al. 2011; Simpson et al. 2011) though this finding was not replicated in all studies (Hansen et al. 1997) (Table 1). Furthermore, Olivier et al. also showed that the citalopram-treated rats had reduced corpus callosum myelin formation, leading to a reduction in callosal connectivity. This is reminiscent of findings in humans, where patients with autism spectrum disorders (ASD) have a reduction in corpus callosum size (reviewed in Brambilla et al. 2003; Egaas et al. 1995; Piven et al. 1998). Rats treated with a 5-HT agonist during development had overreaction to auditory and tactile stimuli as well (Kahne et al. 2002). In terms of aggression, cerebrospinal fluid (CSF) 5-hydroxyindoleacetic acid (5-HIAA; the major metabolite of 5-HT) levels are inversely correlated to aggressive behaviors in rodents, primates, and humans (reviewed in Kiser et al. 2012). A preclinical study in mice demonstrated that prenatal treatment with paroxetine throughout gestation lead to increased male aggression during cage changes (Coleman et al. 1999), suggesting that prenatal SSRI may lead to an overall reduction in 5-HT levels, though this is by no means conclusive.
While on a preclinical level, there is some evidence demonstrating potential contributions of prenatal SSRI treatment to the development of neurochemical and behavioral findings consistent with ASD, such as a reduction of corpus callosum size, the significance of these and other findings to humans needs further elucidation. For instance, prenatal exposure to citalopram leads to increased locus coeruleus (LC) activity in male rats (West et al. 2010; Darling et al. 2011). However, the contribution of an increase in LC activity to behaviors typical of individuals with ASD remains unclear. The majority of the focus on the pathophysiology of ASD has been on 5-HT, not NE. However, one recent study suggests norepinephrine may play a role in some cognitive deficits found in ASD (Bodner et al. 2012).
Impact of pharmacologic intervention on hypothalamic–pituitary–adrenal (HPA) axis regulation
MDD frequently occurs in the context of acute or chronic stress and is often associated with relative hyperactivity of the HPA axis (reviewed in Claes 2009). The relationship becomes more complicated during pregnancy, when the maternal HPA axis is incorporated into a more complex system, where the placenta is in the main source of corticotropin-releasing hormone (CRH) production (McLean et al. 1995). Whereas maternal cortisol exerts a negative effect on the hypothalamus, it has a positive effect on placental CRH (pCRH) synthesis (Marinoni et al. 1998). In addition, circulating pCRH increases production of maternal cortisol, creating a feed-forward maternal–placental HPA system. The HPA axis is a major part of the neuroendocrine system, overseeing many interactions between the brain, glands, and hormones. The establishment of an intact HPA axis is important in regulating an individual’s response to stress as well as homeostatic control mechanisms. Indeed, it is hypothesized that the increased risk of developing depression conferred to children of depressed mothers may be transmitted through suboptimal development of the fetal HPA axis (reviewed in Glover et al. 2010). HPA axis dysfunction could potentially lead to decreased stress tolerance as well as a host of medical problems including metabolic and cardiovascular difficulties (McEwen et al. 2008).
There are major interactions between 5-HT and the HPA axis that are potentially susceptible to prenatal SSRI exposure (reviewed in Andrews and Matthews 2004). First, alterations in 5-HT in the hippocampus during certain developmental stages could lead to permanent changes in hippocampal glucocorticoid receptor expression and consequently its autoregulation. Furthermore, since 5-HT is also involved in regulating neurons releasing CRH in the hippocampus, in theory, prenatal modifications in the fetal 5-HT system could directly alter the development and long-term function of the offspring’s HPA axis. Both of these phenomena could occur independently or in coordination, and it is unclear whether SSRI exposure would preferentially impact one or the other.
Questions have emerged regarding whether prenatal treatment with antidepressants has an impact on the developing HPA axis in infants. Preclinical studies in rats demonstrated that neonatal clomipramine (a tricyclic antidepressant that inhibits 5-HT, NE, and DA transport) exposure not only increased circulating corticosterone levels but reduced suppression of corticosterone secretion in a dexamethasone challenge (Ogawa et al. 1994; Prathiba et al. 1998), reflecting an overall activation of the in HPA axis. In addition, fetal plasma cortisol and ACTH significantly increased after chronic maternal fluoxetine administration in a sheep model (Morrison et al. 2004). However, a study examining adolescent rats exposed to neonatal fluoxetine demonstrated decreased expression of the glucocorticoid receptor in the hippocampus, and decreased circulating corticosterone, primarily in male offspring (Pawluski et al. 2012a). Of note, this effect was found regardless whether the offspring were born to either stressed or non-stressed mothers. Furthermore, when prenatally stressed rats were administered fluoxetine during postnatal weeks 1–3, their corticosterone response to stress was normalized, alongside serotonin turnover in the hippocampus, density of dendritic spines and synapses in the hippocampal CA3 region, and spatial learning (Ishigawa 2005). These studies raise the possibility that prenatal SSRI exposure could reprogram the developing HPA system.
Effects of serotonin manipulation on brain and behavior: clinical studies
Affective behaviors
Unlike preclinical models in which all environmental factors can be controlled, human subjects studies examining the impact of prenatal SSRI exposure on behavioral outcomes are taxed with the burden of considering the impact of the psychiatric disorder for which the drug is being prescribed. One may consider studies in which children were exposed in utero to maternal psychosocial distress without psychotropic medication exposure. However, maternal psychosocial distress frequently continues into the postnatal period, potentially another time of heightened risk for negative effects on infant neurodevelopment (Kingston et al. 2012).
It is therefore important to keep in mind, when examining potential effect of SSRI treatment on development, that long-term behavioral change can occur secondary to antenatal exposure to stress, depression, and anxiety. There are several independent prospective studies that demonstrate that antenatal stress and anxiety contribute to increased anxiety and altered stress response in children (reviewed in Talge et al. 2007; O’Connor et al. 2005, 2012; Davis and Sandman 2012). Of note, the stressors examined in these studies ranged from daily hassles to proximity to the World Trade Center attack on 9/11/2001, and the severity of both anxiety and depression in the mother was not always in the clinical range. This suggests that traumatic levels of stress, leading to clinically significant symptoms, are not required for a behavioral outcome in the offspring.
Generally, several studies have suggested that SSRI exposure during pregnancy does not have a detrimental effect on broad measures of behavioral development as measured by global IQ, language development, or child’s behavior, as assessed by an age-appropriate neurobehavioral scale, such as the Child’s Behavior Checklist or the Bayley Scales of Infant Development (Nulman et al. 1997, 2002, 2012), and even improve language development (Weikum et al. 2012). A more complicated question is whether mothers treated with SSRIs for their depression or anxiety have children with increased or decreased anxiety. Clinically, the correlate to anxiety in children is internalizing behavior. Internalizing behaviors are defined as depression, irritability, anxiety, emotional reactivity, and withdrawal. These are different than typical externalizing behaviors, such as emotional outbursts, non-compliance, and aggressive behavior. There is no evidence that SSRI treatment during pregnancy causes an increase in internalizing behavior in infants or children (Misri et al. 2006; Oberlander et al. 2007, 2010; Nulman et al. 2012) (see Table 2), which does not correlate with some preclinical findings described previously. Maternal depressed mood led to an increase in externalizing behavior at age 3 years, independent of SSRI treatment. In addition, an increase in externalizing behavior at age 3 was associated with maternal anxiety and depression at that time point, independent of prenatal SSRI exposure as well (Oberlander et al. 2010). Finally, a recent study of 4–5-year-olds demonstrated that prenatal antidepressant exposure was not associated with behavioral or emotional problems, and in fact those children exposed to untreated prenatal maternal depression were more likely to have behavioral problems (Pedersen et al. 2013).
Table 2.
Clinical studies examining the effect of developmental SSRI on anxiety, autism spectrum disorder (ASD), and the hypothalamic–pituitary–adrenal axis (HPA)
| Study | Subjects | Treatment | Measures used | Outcome examined | Finding |
|---|---|---|---|---|---|
| Misri et al. 2006 | 22 exposed to SSRI, 14 unexposed to SSRI | SERT, FLX, PAROX | Child Behavioral Checklist, Child Teacher Report Form | Anxiety | ↔ |
| Oberlander et al. 2007 | 4-year-olds: 14 unexposed, 22 exposed | SERT, FLX, PAROX | Child Behavioral Checklist | Anxiety | ↔ |
| Oberlander et al. 2010 | 3-year-olds: 42 exposed, 22 exposed | SERT, FLX, FLVX, PAROX, CIT, VEN | Child Behavioral Checklist, observation | Anxiety | ↔ |
| Nulman et al. 2012 | Children: 62 ven exposed, 62 SSRI exposed, 54 depressed unexposed, 62 non-depressed non-exposed | SERT, FLX, FLVX, PAROX, CIT, VEN | Child Behavioral Checklist, Connor Parents Rating Scale Wechsler IQ | Anxiety, IQ | ↔ |
| Pedersen et al. 2013 | Children 4–5 years old: 127 SSRI exposed, 98 depressed unexposed, 723 non-depressed non-exposed | SERT, FLX, FLVX, PAROX, CIT, VEN, TCA | Strengths and Difficulties Questionnaire | Emotion, social behavior, attention | ↔ |
| ASD Croen et al. 2011 | 1,507 healthy children, 298 children with ASD | SSRI, dual uptake antidepressants, tricyclic antidepressant | Diagnosis of autism spectrum disorder | Autism spectrum disorder | ↑ |
| HPA axis | |||||
| Brennan et al. 2008 | 6-month-olds: 105 boys, 84 girls | Psychotropics-antidepressants, antipsychotics, anxiolytics | Salivary Cortisol | Cortisol | ↓ |
| Davidson et al. 2009 | Newborns: 21 exposed, 20 unexposed | CIT, FLX, PAROX | Cord blood hormone level | Cortisol | ↓ |
| Pawluski et al. 2012a, b | Newborn and 3 months old: 19 exposed, 44 unexposed | SSRI | Newborn: Cord CBG, Cortisol, 3months: salivary Cortisol | Cortisol-binding globulin Diurnal Cortisol | ↓ |
| Oberlander et al. 2008a | 3 months old: 31 exposed, 45 unexposed | SERT, FLX, FLVX, PAROX, CIT, VEN | Salivary Cortisol | Cortisol | ↓ |
| Oberlander et al. 2008b | Infants of. treated depressed mothers (n = 33); depressed non-treated mothers (n = 13), non-depressed/non-treated mothers (n = 36) | SERT, FLX, FLVX, PAROX, CIT, VEN | Bisulfite pyrosequencing DNA methylation analysis; salivary Cortisol | Methylation of the glucocorticoid receptor gene | ↑ |
| HPA stress reactivity | ↔ |
↑ increase, ↔ no change, ↓ decrease
SERT=Sertraline; FLX=Fluoxetine; FLVX=Fluvoxamine; PAROX=Paroxetine; CIT=Citalopram; VEN=Venlafaxine; TCA=Tricyclic Antidepressant
Therefore, while preclinical studies suggest that prenatal SSRI treatment or other serotoninergic manipulation lead to behavioral changes reflective of mood symptoms and anxiety in rodent models, this has yet to be observed in a clinical population. Future studies, with larger sample sizes and at later time points in development, are needed to clearly establish the separate effect of maternal mood, and its treatment, on affective disorders in children. This may be particularly important with respect to internalizing behaviors as depression and anxiety disorders are more likely to occur in females, and in both male and females to have their onset after puberty.
Social behaviors
Given the increase in number of children diagnosed with ASDs (Kogan et al. 2009) and its neurodevelopmental trajectory, investigators are rightfully questioning the role of prenatal factors in contributing to risk for ASDs. With 5-HT’s role in complex social behaviors and the aforementioned results from preclinical studies, it is not surprising that the relationship between prenatal SSRI use and ASD has been recently investigated. Croen and colleagues (2011) compared children with ASD with randomly selected healthy controls and reported that children with ASD were twice as likely to have had a mother who was taking an SSRI immediately prior or during pregnancy than children in the comparison group (prevalence 6.7 % vs. 3.3 %) (see Table 2). This risk was not found for children whose mothers had a history of mental illness in the absence of SSRI treatment. They found the strongest effect (3.8 adjusted odds ratio) with treatment during the first trimester. However, there are many caveats to this study, and the authors do not come to the conclusion that SSRIs themselves lead to increased rates of ASD. Namely, maternal psychiatric illness, including depression for which SSRIs are a treatment, is also a risk factor for development of ASD (Larsson et al. 2005; Daniels et al. 2008). The study by Croen et al. relied on medical records to specify medication use, which is fraught with limitations and presence of a sibling affected with ASD was not a factor in the analyses. A clearer interpretation is that a child with ASD is more likely to have a mother who has a psychiatric disorder such as depression or anxiety, requiring SSRI treatment.
In order to answer the questions posed by the preclinical data, and this preliminary clinical result, further prospective studies examining a relationship between treatment with SSRI and development of autism and autism spectrum disorders are needed. As described above, there are emerging data that social behavior in rodents may be negatively affected by prenatal SSRI exposure, with neuroanatomical changes reflecting those found in humans with ASD.
HPA axis
Work conducted in a clinical population demonstrates that prenatal exposure to a SSRI is associated with decreased basal salivary cortisol in infants at 3 and 6 months of age (Oberlander 2008a; Brennan et al. 2008), and decreased cord blood cortisol (Davidson et al. 2009). This mirrors recent preclinical findings (Pawluski 2012a). Furthermore, corticosteroid binding globulin, which has a key role in transporting serum cortisol therefore regulating the amount of “free” cortisol that is able to enter target tissues, was found to be increased in newborns (Pawluski 2012b). Together, these studies suggest that perhaps prenatal exposure to SSRI buffers the infant from increased HPA reactivity and at the very least indicates that the developing HPA axis is indeed influenced by prenatal SSRI exposure (see Table 2). The exact mechanism remains unclear. Of note, maternal mood, not prenatal SSRI exposure, was associated with methylation of the glucocorticoid receptor gene (NRC31) and HPA stress reactivity (Oberlander et al. 2008b). Translational studies, utilizing emerging epigenetic techniques, will be important in teasing out the differential effect of maternal mood and its treatment on outcomes in the offspring.
Psychomotor development
ASD and mood disorders are illnesses that develop over time, making them somewhat more challenging to assess in regards to their link to SSRI treatment in utero. Psychomotor development in newborns and infants, as quantified by well-established tests, provides a more immediate time point to determine whether maternal SSRI treatment has altered infant neurodevelopment. In one study, children born to depressed mothers who were treated with SSRIs during pregnancy demonstrated lower APGAR scores and Bayley psychomotor development scores (controlling for lower APGAR scores) than those born to untreated depressed mothers (Casper et al. 2003). In addition, a follow-up study demonstrated that a longer duration of treatment was associated with a lower score on the psychomotor portion of the Bayley rating scale (Casper et al. 2011). Another study showed that children born to depressed mothers who were treated with SSRI in the second or third trimester sat and walked later than those born to untreated depressed mothers, though they still were within the normal range of development, and by 19 months of age the differences had resolved (Pedersen et al. 2010). Intrauterine assessment of psychomotor development has been performed as well. One study found that standard and high doses of SSRI lead to increased motor activity at the beginning and end of the second trimester, as well as continual body movement, reflective of impaired non-REM sleep, during the third trimester (Mulder et al. 2011). Increased motor activity was also found immediately following birth (Zeskind and Stephens 2004).
However, an additional study prospectively examining mothers treated with fluoxetine or tricyclic antidepressants and comparing them to a control group found no effect on psychomotor outcome as measured by the Bayley scale (Nulman et al. 2002). No significant differences on the Brazelton Neonatal Behavioral Assessment Scale were found between a group of depressed untreated mothers, depressed treated mothers, and a control group either (Suri et al. 2011). A recent study attempted to tease apart the role of maternal depression from exposure to SSRI using neurobehavioral outcomes prior to 3 weeks of age. They found distinct neurobehavioral profiles when comparing infants exposed to maternal depression to those exposed to maternal depression and SSRI. Infants exposed to depression alone had higher quality of movement scores, which is a measurement of the smoothness and maturity of movement as well as the presence of startles and tremors. However, they received lower attention scores than control or depression plus SSRI-exposed infants (Salisbury et al. 2011). Hence, some domains of behavior and neurodevelopment may be adversely affected by SSRI exposure while others are more sensitive to the effects of maternal mental illness (see Table 3). Until replicated in larger samples, these data need to be viewed with caution keeping in mind the multiple detrimental effects of maternal mental illness on pregnancy and infant outcomes.
Table 3.
Clinical studies examining the effect of developmental SSRI on psychomotor development, pain reactivity, and S100B levels
| Study | Subjects | Treatment | Measures used | Outcome examined | Finding |
|---|---|---|---|---|---|
| Casper et al. 2003 | Infants: 13 unexposed, 31 exposed | SERT, FLX, FLVX, PAROX | APGAR, Bayley Psychomotor development | Psychomotor development | ↓ |
| Casper et al. 2011 | Infants: 55 exposed | SERT, FLX, FLVX, PAROX | APGAR, Bayley Psychomotor development | Psychomotor development | ↓ |
| Pederson et al. 2010 | Danish national birth cohort | SSRI | Postnatal interview at 6 and 19 months of age | Psychomotor development 6 months | ↓ |
| 19 months | ↔ | ||||
| Mulder et al. 2011 | Fetuses: 96 exposed, 37 unexposed, 130 controls | SERT, FLX, FLVX, PAROX, CIT, VEN | Ultrasound during pregnancy | Intrauterine motor activity | ↑ |
| Zeskind and Stephen 2004 | Newborn: 17 exposed, 17 unexposed | SERT, FLX, CIT, PAROX | Neonatal Behavioral Assessment Scale | Motor activity at birth | ↑ |
| Nulman et al. 1997 | Children: 80 tricyclic, 55 fluoxetine, 36 unexposed | TCA FLX | Bayley Scales of Infant Development, McCarthy Scales of Children’s Abilities, Reynell Developmental Language Scales | Psychomotor development | ↔ |
| Nulman et al. 2002 | Children: 46 tricyclic, 40 fluoxetine; 16 unexposed, 15 control | TCA FLX | Bayley Scales of Infant Development, McCarthy Scales of Children’s Abilities, Reynell Developmental Language Scales | Psychomotor development | ↔ |
| Suri et al. 2011 | Infants: 33 exposed, 16 unexposed, 15 control | FLX, SERT | Brazelton Neonatal Behavioral Assessment Scale | Psychomotor development | ↔ |
| Salisbury et al. 2011 | Infants: 36 exposed, 20 unexposed, 56 control | SERT, FLX, FLVX, PAROX, ESCIT, VEN | NICU Network Neurobehavioral Scale | Quality of movement | ↑ |
| Attention | ↓ | ||||
| Oberlander et al. 2002 | Infants: 22 SSRI, 16 SSRI + BDZ, 23 unexposed | SERT, FLX, PAROX | Parasympathetic response, facial response | Pain reactivity | |
| Oberlander et al. 2005 | Infants: 11 prenatal SSRI, 30 pre-/postnatal SSRI, 22 unexposed | SERT, FLX, PAROX | Parasympathetic response, facial response | Pain reactivity—prenatal | ↓ |
| Pre-/postnatal | ↑ | ||||
| Pawluski et al. 2009 | Infants: 15 exposed, 37 unexposed | SERT, FLX, PAROX, CIT, VEN | Cord serum levels | S100B | ↓ |
↑ increase, ↔ no change, ↓ decrease
SERT=Sertraline; FLX=Fluoxetine; FLVX=Fluvoxamine; PAROX=Paroxetine; CIT=Citalopram; VEN=Venlafaxine; TCA=Tricyclic Antidepressant
Pain reactivity
5-HT has an inhibitory role in nociception at many levels, from skin through the spinal cord, to the thalamus and cortex. Preclinically, exposure to fluoxetine during pregnancy and lactation did not alter nociception in a rodent model (Lisboa et al. 2007). Oberlander and colleagues (2002, 2005) investigated whether alteration in 5-HT secondary to prenatal SSRI exposure leads to increased inhibition of a pain response. An initial study in newborns suggested that infants exposed to SSRI demonstrated both a blunted facial response as well as increased parasympathetic response, as measured by cardiac modulation, when compared with unexposed infants (Oberlander et al. 2002). A follow-up study further characterized these findings by examining 2-month-olds with the hypothesis that they would also demonstrate blunted facial response, alongside an increased parasympathetic response, in response to a noxious event. While blunted facial-action responses were observed in infants with prenatal SSRI exposure alone, those infants with both pre- and postnatal exposure (through breast milk) did not demonstrate a blunted response. Furthermore, both prenatal and postnatal SSRI exposure was associated with reduced parasympathetic withdrawal and increased parasympathetic cardiac modulation during recovery, in contrast to the initial hypothesis (Oberlander et al. 2005). Of note, levels of SSRIs in infant serum were very low, making it difficult to discern possible mechanisms through which it could contribute to these findings. The concern regarding these findings as a whole is that neonates exposed to SSRIs could have altered ability to react to external stimuli. However, the long-term clinical implications of these findings remain unclear. At this time, alterations in pain reactivity and psychomotor development are limited to serving as early markers of potential changes in neurodevelopment, without clear clinical implications or impact. Moreover, it is not yet clear whether these observed effects of prenatal SSRI exposure on pain reactivity endure past the first few months of life (see Table 3).
S100B: a potential biomarker of risk?
The development of the 5-HT system occurs through autoregulation (Whitaker-Azmitia and Azmitia 1994; Whitaker-Azmitia 2001). Current theory holds that it does so in conjunction with the protein S100B, an astroglia-specific calcium-binding protein (Whitaker-Azmitia and Azmitia 1994). In low (nanomolar) concentrations in tissue culture, S100B promotes neuronal outgrowth and survival (Winningham-Major et al. 1989; Selinfreund et al. 1991). However, higher (micromolar) levels of S100B can be cytotoxic (Fano et al. 1993). In rodent models, decreased levels of S100B are found following several prenatal manipulations including stress (Van den Hove et al. 2006) and cocaine (Akbari et al. 1994) exposure. Such prenatal exposures, and concomitant S100B deficits, are associated with altered central serotonin levels, supporting the theory that increased extracellular serotonin levels (e.g., following SSRI exposure) in the developing brain may hinder the growth of 5-HT neurons via autoregulation. This may subsequently impede astroglial maturation and thereby delay the release of S100B. However, mice with a genetic deletion of S100B demonstrated normal development of the 5-HT system, suggesting at the very least that there are compensatory mechanisms in existence (Nishiyama et al. 2002). Clinically, infants exposed to prenatal SSRI demonstrated decreased cord blood serum S100B levels, controlling for prenatal maternal mood. Also of note, S100B levels did not correlate to behavioral characteristics in infants. Furthermore, prenatal SSRI had the opposite effect on maternal S100B levels, perhaps reflecting a difference on the effect of SSRI on the mature versus developing brain (Pawluski et al. 2009). One caveat is that it is unclear how much peripheral S100B levels reflect central nervous system levels of that protein. Overall, these findings support the theory that decreased level of S100B, a biomarker, following prenatal SSRI exposure could potentially reflect an alteration in the developing nervous system. However, these cohorts were not examined at later time points to evaluate when S100B levels normalized or if there were any long-lasting behavioral differences between the two groups. Therefore, it remains to be determined if there is a long-term developmental risk and whether S100B is a potential useful biomarker of this risk (see Table 3).
Conclusions
Whether or not a pregnant woman with depression should be started on an antidepressant is a highly personal choice, to be made by the mother and her significant other, with the guidance of her physician. Unfortunately, confusion remains over whether the choice to seek pharmacologic treatment to alleviate illness has untoward, negative outcomes for fetal and child development. This uncertainty prevents women from seeking treatment and may add a burden of stress and guilt to women who require medications for psychological stability. With increasing numbers of women on SSRI medications during pregnancy, increased awareness of psychiatric illness in children, and recent litigations regarding birth defects in exposed infants, the onus is upon us as investigators to determine the safety profile of these medications with respect to fetal development and pregnancy outcome. Currently, we are presented with the thorny issue of reconciling the preclinical evidence suggesting that alterations in the developing 5-HT system caused by SSRI exposure may lead to dysfunction in some 5-HT-dependent behaviors with the clinical literature largely supporting the safety of prenatal SSRI exposure with respect to infant neurodevelopment. Caveats to this discussion include the possibility that we are not looking at the appropriate behavioral outcomes, at the appropriate time points, and that current sample sizes are insufficient.
Reliance on animal models to predict both the safety of prenatal drug exposure has a mixed and unfortunate history, thalidomide being one notable example. While thalidomide teratogenicity is well documented in humans, rats did not demonstrate physical deformities mirroring human results and therefore were of limited use in predicting outcomes for the offspring (Greek et al. 2011). There is some evidence for increased rates of autism and mental retardation in children exposed to thalidomide, and one study has demonstrated that rats exposed to prenatal thalidomide had deficits in the Cincinnati water maze, a test of sequential learning. Of note, they did not demonstrate deficits on a wide variety of other behavioral paradigms, providing further demonstration of the divergence between rodent models and humans (Voorhees et al. 2001). Preclinical and clinical studies of prenatal benzodiazepines, another class of medications frequently prescribed during pregnancy, provide yet another example of such a discrepancy. Initial studies in rodents demonstrated an increased risk of cleft palate following prenatal benzodiazepine exposure (Miller and Becker, 1975; Wee and Zimmerman, 1983), and genetic manipulation of the GABAergic system, the target of benzodiazepines, produced cleft palates as well (Culiat et al. 1995; Condie et al. 1997). However, a case–control study and a meta-analysis of human studies did not find an increased risk of cleft palate following prenatal benzodiazepine exposure (Rosenberg et al. 1983; Dolovich et al. 1998). Animal studies are only one component of the rubric that guides pharmacologic treatment of prenatal psychiatric disorders; there is a great need for translational studies that effectively bridge the divide between preclinical and clinical findings. For example, looking at biomarkers and imaging data in children exposed to developmental SSRI and contrasting them with findings in rodent models would help confirm or discard certain hypotheses. This would expedite the vetting process for prenatal medication safety and determine whether there are indeed long-term effects in the offspring.
Certainly, prenatal exposures to other stimuli, such as maternal diet, smoking, and maternal drug use, have a long-lasting impact on the offspring. Maternal obesity has been linked to an increased rate of obesity in the offspring (Schack-Nielsen et al. 2010) as prenatal and early postnatal environments play an important role via epigenetic processes (reviewed in Lillycrop and Burdge 2011). Maternal obesity can have more widespread outcomes than obesity in the offspring. For example, preclinical studies have shown that maternal obesity altered dopamine transporter and μ-opioid receptor levels in key brain regions in progeny, who also showed an increased preference for high-fat diet (Vucetic et al. 2010). In terms of maternal tobacco use, which is more common in pregnant women with depression, there are both epidemiological studies and animal studies that demonstrate smoking during pregnancy has negative effects on fetal development. There are also deleterious long-term consequences on postnatal development including low birth weight, increased risk of sudden infant death syndrome, and behavioral disorders including attention deficit hyperactivity disorder, as well as externalizing and internalizing behavioral problems (reviewed in Abbot and Winzer-Serhan 2012). Prenatal exposure to drugs of abuse also has a long-term impact. Prenatal cocaine use, of particular relevance because it targets monoamine reuptake, leads to alterations in attention, social behavior, stress response, and aggression in the offspring (reviewed in Williams et al. 2011). Of note, these exposures have clearer clinical outcomes in the offspring, unlike SSRIs. This further demonstrates the need to pinpoint whether the signal is not there following SSRI exposure or whether it is being missed due to the current approaches being taken.
What are the next steps in moving the investigation of prenatal SSRI treatment down the translational path? Quantitative outcomes, such as biomarkers, would certainly be useful to guide treatment practices. As seen above, S100B has the potential to reflect changes to the 5-HT system caused by prenatal SSRIs; however, it did not correspond to behavioral changes in infants. Brain-derived neurotrophic factor (BDNF) has reliably been shown to respond to chronic anti-depressant treatment in adult animal models, is altered by stress, responsive to changes in ovarian hormones, and demonstrates changes across development (Epperson and Bale 2012); however, studies have not examined its role in preclinical or clinical prenatal SSRI treatment to date. Additional studies of changes in gene regulation following SSRI exposure, such as the one carried out by Oberlander and colleagues implicating the human glucocorticoid receptor, are also merited. Towards this end, a recent study examining cord blood demonstrated that prenatal exposure to SSRIs did not lead to large-scale changes in methylation in infants, and further studies such as these are warranted (Schroeder et al. 2012). Finally, the debate over the safety of prenatal SSRI serves as a good illustration of the challenges encountered at the junction of psychiatry and neuroscience. While there is a real clinical need for treatment modalities for patients struggling with depression and anxiety, the preclinical investigations measuring the effects of these interventions are still underway. Treatment should not be withheld, but rather investigations should intensify and results incorporated into daily practice.
Acknowledgments
Supported by R25 MH060490, Clinical Research Scholars Program in Psychiatry (TLG) and K24DA030301 (CNE), P50MH099910 (CNE, DRK), and K23 MH092399 (DK). Potential conflicts of interest (CEN): Pfizer-Consultant; Shire-Research Grant.
Contributor Information
Tamar L. Gur, Penn Center for Women’s Behavioral Wellness, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania Philadelphia, 3535 Market St, 3rd Floor, Philadelphia, PA 19104, USA
Deborah R. Kim, Penn Center for Women’s Behavioral Wellness, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania Philadelphia, 3535 Market St, 3rd Floor, Philadelphia, PA 19104, USA Penn Center for the Study of Sex and Gender in Behavioral Health, Philadelphia, PA, USA.
C. Neill Epperson, Email: cepp@mail.med.upenn.edu, Penn Center for Women’s Behavioral Wellness, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania Philadelphia, 3535 Market St, 3rd Floor, Philadelphia, PA 19104, USA; Penn Center for the Study of Sex and Gender in Behavioral Health, Philadelphia, PA, USA; Department of Obstetrics and Gynecology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
References
- Abbott LC, Winzer-Serhan UH. Smoking during pregnancy: lessons learned from epidemiological studies and experimental studies using animal models. Crit Rev Toxicol. 2012;42:279–303. doi: 10.3109/10408444.2012.658506. [DOI] [PubMed] [Google Scholar]
- Akbari HM, Whitaker-Azmitia PM, Azmitia EC. Prenatal cocaine decreases the trophic factor S-100β and induced microcephaly: reversal by postnatal 5-HT1A receptor agonist. Neurosci Lett. 1994;170:141–144. doi: 10.1016/0304-3940(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Allen MC. Neurodevelopmental outcomes of preterm infants. Curr Opin Neurol. 2008;21:123–128. doi: 10.1097/WCO.0b013e3282f88bb4. [DOI] [PubMed] [Google Scholar]
- Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356:2684–2692. doi: 10.1056/NEJMoa066584. [DOI] [PubMed] [Google Scholar]
- Anderson GM. Peripheral and central neurochemical effects of the selective serotonin reuptake inhibitors (SSRIs) in humans and nonhuman primates: assessing bioeffect and mechanisms of action. Int J Dev Neurosci. 2004;22:397–404. doi: 10.1016/j.ijdevneu.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Andrews MH, Matthews SG. Programming of the hypothalamo–pituitary–adrenal axis: serotonergic involvement. Stress. 2004;7:15–27. doi: 10.1080/10253890310001650277. [DOI] [PubMed] [Google Scholar]
- Ansorge MS. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairy KL, Madhyastha S, Ashok KP, Bairy I, Malini S. Developmental and behavioral consequences of prenatal fluoxetine. Pharmacology. 2007;79:1–11. doi: 10.1159/000096645. [DOI] [PubMed] [Google Scholar]
- Bakker MK, Kölling P, van den Berg PB, de Walle HEK, de Jong van den B, Lolkje TW. Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population-based cohort study from the Netherlands. Br J Clin Pharmacol. 2008;65:600–606. doi: 10.1111/j.1365-2125.2007.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker MK, De Walle HEK, Wilffert B, de Jong-Van den Berg LTW. Fluoxetine and infantile hypertrophic pylorus stenosis: a signal from a birth defects-drug exposure surveillance study. Pharmacoepidemiol Drug Saf. 2010;19:808–813. doi: 10.1002/pds.1964. [DOI] [PubMed] [Google Scholar]
- Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress. 2011;14:348–356. doi: 10.3109/10253890.2011.586447. [DOI] [PubMed] [Google Scholar]
- Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103:698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodner KE, Beversdorf DQ, Saklayen SS, Christ SE. Noradrenergic moderation of working memory impairments in adults with autism spectrum disorder. J Int Neuropsychol Soc. 2012;18:556–564. doi: 10.1017/S1355617712000070. [DOI] [PubMed] [Google Scholar]
- Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49:726–735. doi: 10.1177/070674370404901103. [DOI] [PubMed] [Google Scholar]
- Bonnin A, Levitt P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience. 2011;197:1–7. doi: 10.1016/j.neuroscience.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P, Hardan A, di Nemi SU, Perez J, Soares JC, Barale F. Brain anatomy and development in autism: review of structural MRI studies. Brain Res Bull. 2003;61:557–569. doi: 10.1016/j.brainresbull.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Pargas R, Walker EF, Green P, Newport DJ, Stowe Z. Maternal depression and infant cortisol: influences of timing, comorbidity and treatment. J Child Psychol Psychiatry. 2008;49:1099–1107. doi: 10.1111/j.1469-7610.2008.01914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A, Hoyme HE. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142:402–408. doi: 10.1067/mpd.2003.139. [DOI] [PubMed] [Google Scholar]
- Casper RC, Gilles AA, Fleisher BE, Baran J, Enns G, Lazzeroni LC. Length of prenatal exposure to selective serotonin reuptake inhibitor (SSRI) antidepressants: effects on neonatal adaptation and psychomotor development. Psychopharmacol (Berl) 2011;217:211–219. doi: 10.1007/s00213-011-2270-z. [DOI] [PubMed] [Google Scholar]
- Chen C, Lin H. Prenatal care and adverse pregnancy outcomes among women with depression: a nationwide population-based study. Can J Psychiatry. 2011;56:273–280. doi: 10.1177/070674371105600506. [DOI] [PubMed] [Google Scholar]
- Claes S. Glucocorticoid receptor polymorphisms in major depression. Ann N Y Acad Sci. 2009;1179:216–228. doi: 10.1111/j.1749-6632.2009.05012.x. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Coleman FH, Christensen HD, Gonzalez CL, Rayburn WF. Behavioral changes in developing mice after prenatal exposure to paroxetine (Paxil) Am J Obstet Gynecol. 1999;181:1166–1171. doi: 10.1016/s0002-9378(99)70102-x. [DOI] [PubMed] [Google Scholar]
- Colvin L, Slack-Smith L, Stanley FJ, Bower C. Early morbidity and mortality following in utero exposure to selective serotonin reuptake inhibitors: a population-based study in Western Australia. CNS Drugs. 2012;26:e1–e14. doi: 10.2165/11634190-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condie BG, Bain G, Gottlieb DI, Capecchi MR. Cleft palate in mice with a targeted mutation in the gamma-aminobutyric acid-producing enzyme glutamic acid decarboxylase 67. Proc Natl Acad Sci. 1997;94:11451–11455. doi: 10.1073/pnas.94.21.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68:1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- Culiat CT, Stubbs LJ, Woychik RP, Russell LB, Johnson DK, Rinchik EM. Deficiency of the beta 3 subunit of the type A gamma-aminobutyric acid receptor causes cleft palate in mice. Nat Genet. 1995;11:344–346. doi: 10.1038/ng1195-344. [DOI] [PubMed] [Google Scholar]
- Daniels JL, Forssen U, Hultman CM, Cnattingius S, Savitz DA, Feychting M, Sparen P. Parental psychiatric disorders associated with autism spectrum disorders in the offspring. Pediatrics. 2008;121:e1357–e1362. e1357–1362. doi: 10.1542/peds.2007-2296. [DOI] [PubMed] [Google Scholar]
- Darling RD, Alzghoul L, Zhang J, Khatri N, Paul IA, Simpson KL, Lin RCS. Perinatal citalopram exposure selectively increases locus ceruleus circuit function in male rats. J Neurosci. 2011;31:16709–16715. doi: 10.1523/JNEUROSCI.3736-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Prokonov D, Taler M, Maayan R, Harell D, Gilad I, Weizman A. Effect of exposure to selective serotonin reuptake inhibitors in utero on fetal growth: potential role for the IGF-I and HPA axes. Pediatr Res. 2009;65:236–241. doi: 10.1203/PDR.0b013e318193594a. [DOI] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology. 2012;37:1224–1233. doi: 10.1016/j.psyneuen.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolovich LR, Addis A, Vaillancourt JM, Power JD, Koren G, Einarson TR. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case–control studies. BMJ. 1998;317:839–843. doi: 10.1136/bmj.317.7162.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egaas B, Courchesne E, Saitoh O. Reduced size of corpus callosum in autism. Arch Neurol. 1995;52:794–801. doi: 10.1001/archneur.1995.00540320070014. [DOI] [PubMed] [Google Scholar]
- El Marroun H, Jaddoe VV, Hudziak JJ, et al. Maternal use of selective serotonin reuptake inhibitors, fetal growth, and risk of adverse birth outcomes. Arch Gen Psych. 2012;69:706–714. doi: 10.1001/archgenpsychiatry.2011.2333. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Bale TL. BDNF Val66Met polymorphism and brain-derived neurotrophic factor levels across the female life span: implications for the sex bias in affective disorders. Biol Psychiatry. 2012;72:434–436. doi: 10.1016/j.biopsych.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ. 2001;323:257–260. doi: 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanò G, Mariggiò MA, Angelella P, et al. The S-100 protein causes an increase of intracellular calcium and death of PC12 cells. Neuroscience. 1993;53:919–925. doi: 10.1016/0306-4522(93)90477-w. [DOI] [PubMed] [Google Scholar]
- Fava M, Kendler KS. Major depressive disorder. Neuron. 2000;28:335–341. doi: 10.1016/s0896-6273(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Gentile S. Neurodevelopmental effects of prenatal exposure to psychotropic medications. Depress Anxiety. 2010a;27:675–686. doi: 10.1002/da.20706. [DOI] [PubMed] [Google Scholar]
- Gentile S. On categorizing gestational, birth, and neonatal complications following late pregnancy exposure to antidepressants: the prenatal antidepressant exposure syndrome. CNS Spectr. 2010b;15:167–185. doi: 10.1017/s1092852900027449. [DOI] [PubMed] [Google Scholar]
- Glover V, O’Connor TG, O’Donnell K. Prenatal stress and the programming of the HPA axis. Neurosci Biobehav Rev. 2010;35:17–22. doi: 10.1016/j.neubiorev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Greek R, Shanks N, Marks MJ. The history and implications of testing thalidomide on animals. J Philos Sci Law. 2011 Oct11 [Google Scholar]
- Halene TB, Ehrlichman RS, Liang Y, Christian EP, Jonak GJ, Gur TL, Blendy JA, Dow HC, Brodkin ES, Schneider F, Gur RC, Siegel SJ. Assessment of NMDA receptor NR1 subunit hypofunction in mice as a model for schizophrenia. Genes Brain Behav. 2009;8:661–675. doi: 10.1111/j.1601-183X.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen HH, Sánchez C, Meier E. Neonatal administration of the selective serotonin reuptake inhibitor Lu 10-134-C increases forced swimming-induced immobility in adult rats: a putative animal model of depression? J Pharmacol Exp Ther. 1997;283:1333–1341. [PubMed] [Google Scholar]
- Harris SS, Maciag D, Simpson KL, Lin RC, Paul IA. Dose-dependent effects of neonatal SSRI exposure on adult behavior in the rat. Brain Res. 2011;1429:52–60. doi: 10.1016/j.brainres.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick V, Stowe ZN, Altshuler LL, Hwang S, Lee E, Haynes D. Placental passage of antidepressant medications. Am J Psychiatry. 2003;160:993–996. doi: 10.1176/appi.ajp.160.5.993. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Schiepers OJG, Schoffelmeer ANM, Cuppen E, Vanderschuren LJMJ. Acute and constitutive increases in central serotonin levels reduce social play behaviour in peri-adolescent rats. Psychopharmacol (Berl) 2007;195:175–182. doi: 10.1007/s00213-007-0895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata H, Shiga T, Okado N. Selective serotonin reuptake inhibitor treatment of early postnatal mice reverses their prenatal stress induced brain dysfunction. Neuroscience. 2005;133:893–901. doi: 10.1016/j.neuroscience.2005.03.048. [DOI] [PubMed] [Google Scholar]
- Jablensky AV, Morgan V, Zubrick SR, Bower C, Yellachich L. Pregnancy, delivery, and neonatal complications in a population cohort of women with schizophrenia and major affective disorders. Am J Psychiatry. 2005;162:79–91. doi: 10.1176/appi.ajp.162.1.79. [DOI] [PubMed] [Google Scholar]
- Kahne D, Tudorica A, Borella A, Shapiro L, Johnstone F, Huang W, Whitaker-Azmitia PM. Behavioral and magnetic resonance spectroscopic studies in the rat hyperserotonemic model of autism. Physiol Behav. 2002;75:403–410. doi: 10.1016/s0031-9384(01)00673-4. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Olivier JD, Nonkes LJ, Homberg JR. Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci Biobehav Rev. 2010;34:373–386. doi: 10.1016/j.neubiorev.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Kessler R, Berglund P, Demler O, Jin R, Walters E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R) Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kingston D, Sword W, Krueger P, Hanna S, Markle-Reid M. Life course pathways to prenatal maternal stress. J Obstetric Gynecol Neonatal Nurs. 2012;41:609–626. doi: 10.1111/j.1552-6909.2012.01381.x. [DOI] [PubMed] [Google Scholar]
- Kiser D, Steemers B, Branchi I, Homberg JR. The reciprocal interaction between serotonin and social behaviour. Neurosci Biobehav Rev. 2012;36:786–798. doi: 10.1016/j.neubiorev.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Kogan MD, Blumberg SJ, Schieve LA, Boyle CA, Perrin JM, Ghandour RM, Singh GK, Strickland BB, Trevathan E, van Dyck PC. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 2009;124:1395–1403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- Kornum JB, Nielsen RB, Pedersen L, Mortensen PB, Nørgaard M. Use of selective serotonin-reuptake inhibitors during early pregnancy and risk of congenital malformations: updated analysis. Clin Epidemiol. 2010;2:29–36. doi: 10.2147/clep.s9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurki T, Hiilesmaa V, Raitasalo R, Mattila H, Ylikorkala O. Depression and anxiety in early pregnancy and risk for pre-eclampsia. Obstet Gynecol. 2000;95:487–490. doi: 10.1016/s0029-7844(99)00602-x. [DOI] [PubMed] [Google Scholar]
- Larsson HJ, Eaton WW, Madsen KM, Vestergaard M, Olesen AV, Agerbo E, Schendel D, Thorsen P, Mortensen PB. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol. 2005;161:916–925. doi: 10.1093/aje/kwi123. discussion 926–928; 916–925; discussion 926–928. [DOI] [PubMed] [Google Scholar]
- Lillycrop KA, Burdge GC. The effect of nutrition during early life on the epigenetic regulation of transcription and implications for human diseases. J Nutrigenet Nutrigenomics. 2011;4:248–260. doi: 10.1159/000334857. [DOI] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, Kung HF, Hofer MA, Hen R, Gingrich JA. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Lisboa SFS, Oliveira PE, Costa LC, Venâncio EJ, Moreira EG. Behavioral evaluation of male and female mice pups exposed to fluoxetine during pregnancy and lactation. Pharmacology. 2007;80:49–56. doi: 10.1159/000103097. [DOI] [PubMed] [Google Scholar]
- Louik C, Lin AE, Werler MM, Hernández-Díaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med. 2007;356:2675–2683. doi: 10.1056/NEJMoa067407. [DOI] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Malm H, Artama M, Gissler M, Ritvanen A selective serotonin reuptake inhibitors and risk for major congenital anomalies. Obstet Gynecol. 2011;118:111–120. doi: 10.1097/AOG.0b013e318220edcc. [DOI] [PubMed] [Google Scholar]
- Marinoni E, Korebrits C, Di Iorio R, Cosmi EV, Challis JR. Effect of betamethasone in vivo on placental corticotropin-releasing hormone in human pregnancy. Am J Obstet Gynecol. 1998;178:770–778. doi: 10.1016/s0002-9378(98)70490-9. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1:460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- Merali Z, Levac C, Anisman H. Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biol Psychiatry. 2003;54:552–565. doi: 10.1016/s0006-3223(02)01827-9. [DOI] [PubMed] [Google Scholar]
- Miller RP, Becker BA. Teratogenicity of oral diazepam and diphenylhydantoin in mice. Toxicol Appl Pharmacol. 1975;32:53–61. doi: 10.1016/0041-008x(75)90194-5. [DOI] [PubMed] [Google Scholar]
- Misri S, Reebye P, Kendrick K, Carter D, Ryan D, Grunau RE, Oberlander TF. Internalizing behaviors in 4-year-old children exposed in utero to psychotropic medications. Am J Psychiatry. 2006;163:1026–1032. doi: 10.1176/ajp.2006.163.6.1026. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Riggs KW, Chien C, Gruber N, McMillen IC, Rurak DW. Chronic maternal fluoxetine infusion in pregnant sheep: effects on the maternal and fetal hypothalamic–pituitary–adrenal axes. Pediatr Res. 2004;56:40–46. doi: 10.1203/01.PDR.0000128981.38670.28. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Bogen D, Perel J, Bregar A, Uhl K, Levin B, Wisner KL. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA. 2005;293:2372–2383. doi: 10.1001/jama.293.19.2372. [DOI] [PubMed] [Google Scholar]
- Mulder EJH, Ververs FF, de Heus R, Visser GHA. Selective serotonin reuptake inhibitors affect neurobehavioral development in the human fetus. Neuropsychopharmacology. 2011;36:1961–1971. doi: 10.1038/npp.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan R, Darlington RB, Finlay BL, Clancy B. ttime: an R package for translating the timing of brain development across mammalian species. Neuroinformatics. 2010;8:201–205. doi: 10.1007/s12021-010-9081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijenhuis CM, ter Horst PGJ, van Rein N, Wilffert B, Jong-van den Berg LTW. Disturbed development of the enteric nervous system after in utero exposure of selective serotonin re-uptake inhibitors and tricyclic antidepressants. Part 1: literature review. Br J Clin Pharmacol. 2012a;73:16–26. doi: 10.1111/j.1365-2125.2011.04075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijenhuis CM, ter Horst PG, van Rein N, Wilffert B, Jong-van den Berg LT. Disturbed development of the enteric nervous system after in utero exposure of selective serotonin re-uptake inhibitors and tricyclic antidepressants. Part 2: testing the hypotheses. Br J Clin Pharmacol. 2012b;73:126–134. doi: 10.1111/j.1365-2125.2011.04081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama H, Takemura M, Takeda T, Itohara S. Normal development of serotonergic neurons in mice lacking S100B. Neurosci Lett. 2002;321:49–52. doi: 10.1016/s0304-3940(01)02549-6. [DOI] [PubMed] [Google Scholar]
- Noorlander CW, Ververs FFT, Nikkels PGJ, van Echteld CJA, Visser GHA, Smidt MP. Modulation of serotonin transporter function during fetal development causes dilated heart cardiomyopathy and lifelong behavioral abnormalities. PLoS One. 2008;3:e2782–e2782. doi: 10.1371/journal.pone.0002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nulman I, Rovet J, Stewart DE, Wolpin J, Gardner HA, Theis JG, Kulin N, Koren G. Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med. 1997;336:258–262. doi: 10.1056/NEJM199701233360404. [DOI] [PubMed] [Google Scholar]
- Nulman I, Rovet J, Stewart DE, Wolpin J, Pace-Asciak P, Shuhaiber S, Koren G. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry. 2002;159:1889–1895. doi: 10.1176/appi.ajp.159.11.1889. [DOI] [PubMed] [Google Scholar]
- Nulman I, Koren G, Rovet J, Barrera M, Pulver A, Streiner D, Feldman B. Neurodevelopment of children following prenatal exposure to venlafaxine, selective serotonin reuptake inhibitors, or untreated maternal depression. Am J Psychiatry. 2012;169:1165–1174. doi: 10.1176/appi.ajp.2012.11111721. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Eckstein Grunau R, Fitzgerald C, Ellwood AL, Misri S, Rurak D, Riggs KW. Prolonged prenatal psychotropic medication exposure alters neonatal acute pain response. Pediatr Res. 2002;51:443–453. doi: 10.1203/00006450-200204000-00008. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Grunau RE, Fitzgerald C, Papsdorf M, Rurak D, Riggs W. Pain reactivity in 2-month-old infants after prenatal and postnatal serotonin reuptake inhibitor medication exposure. Pediatrics. 2005;115:411–425. doi: 10.1542/peds.2004-0420. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63:898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Reebye P, Misri S, Papsdorf M, Kim J, Grunau RE. Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med. 2007;161:22–29. doi: 10.1001/archpedi.161.1.22. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Grunau R, Mayes L, Riggs W, Rurak D, Papsdorf M, Misri S, Weinberg J. Hypothalamic–pituitary–adrenal (HPA) axis function in 3-month old infants with prenatal selective serotonin reuptake inhibitor (SSRI) antidepressant exposure. Early Hum Dev. 2008a;84:689–697. doi: 10.1016/j.earlhumdev.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008b;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Gingrich JA, Ansorge MS. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clin Pharmacol Ther. 2009;86:672–677. doi: 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Papsdorf M, Brain UM, Misri S, Ross C, Grunau RE. Prenatal effects of selective serotonin reuptake inhibitor antidepressants, serotonin transporter promoter genotype (SLC6A4), and maternal mood on child behavior at 3 years of age. Arch Pediatr Adolesc Med. 2010;164:444–451. doi: 10.1001/archpediatrics.2010.51. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Bergman K, Sarkar P, Glover V. Prenatal cortisol exposure predicts infant cortisol response to acute stress. Dev Psychobiol. 2012 doi: 10.1002/dev.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58:211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Mikuni M, Kuroda Y, Muneoka K, Mori KJ, Takahashi K. Effects of the altered serotonergic signalling by neonatal treatment with 5,7-dihydroxytryptamine, ritanserin or clomipramine on the adrenocortical stress response and the glucocorticoid receptor binding in the hippocampus in adult rats. J Neural Transm Gen Sect. 1994;96:113–123. doi: 10.1007/BF01277933. [DOI] [PubMed] [Google Scholar]
- Olivier JDA, Vallès A, van Heesch F, Afrasiab-Middelman A, Roelofs JJPM, Jonkers M, Peeters EJ, Korte-Bouws GAH, Dederen JP, Kiliaan AJ, Martens GJ, Schubert D, Homberg JR. Fluoxetine administration to pregnant rats increases anxiety-related behavior in the offspring. Psychopharmacol (Berl) 2011;217:419–432. doi: 10.1007/s00213-011-2299-z. [DOI] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Social learning of fear. Nat Neurosci. 2007;10:1095–1102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Galea LA, Brain U, Papsdorf M, Oberlander TF. Neonatal S100B protein levels after prenatal exposure to selective serotonin reuptake inhibitors. Pediatrics. 2009;124:662–670. doi: 10.1542/peds.2009-0442. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Rayen I, Niessen NA, Kristensen S, van Donkelaar EL, Balthazart J, Steinbusch HW, Charlier TD. Developmental fluoxetine exposure differentially alters central and peripheral measures of the HPA system in adolescent male and female offspring. Neuroscience. 2012a;220:131–141. doi: 10.1016/j.neuroscience.2012.06.034. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Brain UM, Underhill CM, Hammond GL, Oberlander TF. Prenatal SSRI exposure alters neonatal corticosteroid binding globulin, infant cortisol levels, and emerging HPA function. Psychoneuroendocrinology. 2012b;37:1019–1028. doi: 10.1016/j.psyneuen.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Pedersen LH, Henriksen TB, Olsen J. Fetal exposure to antide-pressants and normal milestone development at 6 and 19 months of age. Pediatrics. 2010;125:600–608. doi: 10.1542/peds.2008-3655. [DOI] [PubMed] [Google Scholar]
- Pedersen LH, Henriksen TB, Bech BH, Licht RW, Kjaer D, Olsen J. Prenatal antidepressant exposure and behavioral problems in early childhood—a cohort study. Acta Psychiatr Scand. 2013;127:126–135. doi: 10.1111/acps.12032. [DOI] [PubMed] [Google Scholar]
- Petersen I, Gilbert RE, Evans SJW, Man S, Nazareth I. Pregnancy as a major determinant for discontinuation of antide-pressants: an analysis of data from The Health Improvement Network. J Clin Psychiatry. 2011;72:979–985. doi: 10.4088/JCP.10m06090blu. [DOI] [PubMed] [Google Scholar]
- Piven J, Bailey J, Ranson BJ, Arndt S. No difference in hippocampus volume detected on magnetic resonance imaging in autistic individuals. J Autism Dev Disord. 1998;28:105–110. doi: 10.1023/a:1026084430649. [DOI] [PubMed] [Google Scholar]
- Popa D, Léna C, Alexandre C, Adrien J. Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior. J Neurosci. 2008;28:3546–3554. doi: 10.1523/JNEUROSCI.4006-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prathiba J, Kumar KB, Karanth KS. Hyperactivity of hypothalamic pituitary axis in neonatal clomipramine model of depression. J Neural Transm. 1998;105:1335–1339. doi: 10.1007/s007020050135. [DOI] [PubMed] [Google Scholar]
- Rampono J, Simmer K, Ilett KF, Hackett LP, Doherty DA, Elliot R, Kok CH, Coenen A, Forman T. Placental transfer of SSRI and SNRI antidepressants and effects on the neonate. Pharmacopsychiatry. 2009;42:95–100. doi: 10.1055/s-0028-1103296. [DOI] [PubMed] [Google Scholar]
- Rayen I, van den Hove DL, Prickaerts J, Steinbusch HW, Pawluski JL. Fluoxetine during development reverses the effects of prenatal stress on depressive-like behavior and hippocampal neurogenesis in adolescence. PLoS One. 2011;6:e24003–e24003. doi: 10.1371/journal.pone.0024003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas, et al. Neonatal administration of fluoxetine did not alter the anxiety indicators, but decreased the locomotor activity in adult rats in the elevated plus-maze. Arq Neuropsiquiatr. 2008;66:844–847. doi: 10.1590/s0004-282x2008000600013. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Porcel F, Green D, Khatri N, Harris SS, May WL, Lin RC, Paul IA. Neonatal exposure of rats to antidepressants affects behavioral reactions to novelty and social interactions in a manner analogous to autistic spectrum disorders. Anat Rec (Hoboken) 2011;294:1726–1735. doi: 10.1002/ar.21402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L, Mitchell AA, Parsells JL, Pashayan H, Louik C, Shapiro S. Lack of relation of oral clefts to diazepam use during pregnancy. N Engl J Med. 1983:3091282–5. doi: 10.1056/NEJM198311243092103. [DOI] [PubMed] [Google Scholar]
- Salisbury AL, Wisner KL, Pearlstein T, Battle CL, Stroud L, Lester BM. Newborn neurobehavioral patterns are differentially related to prenatal maternal major depressive disorder and serotonin reuptake inhibitor treatment. Depress Anxiety. 2011;28:1008–1019. doi: 10.1002/da.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoorikal GMV, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Schack-Nielsen L, Michaelsen KF, Gamborg M, Mortensen EL, Sørensen TIA. Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. Int J Obes (Lond) 2010;34:67–74. doi: 10.1038/ijo.2009.206. [DOI] [PubMed] [Google Scholar]
- Schroeder JW, Smith AK, Brennan PA, Conneely KN, Kilaru V, Knight BT, Newport DJ, Cubells JF, Stowe ZN. DNA methylation in neonates born to women receiving psychiatric care. Epigenetics. 2012;7:409–414. doi: 10.4161/epi.19551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinfreund RH, Barger SW, Pledger WJ, Van Eldik LJ. Neurotrophic protein S100β stimulates glial cell proliferation. Proc Natl Acad Sci. 1991;88:3554–3558. doi: 10.1073/pnas.88.9.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson KL, Weaver KJ, de Villers-Sidani E, Lu JY, Cai Z, Pang Y, Rodriguez-Porcel F, Paul IA, Merzenich M, Lin RCS. Perinatal antidepressant exposure alters cortical network function in rodents. Proc Natl Acad Sci USA. 2011;108:18465–18470. doi: 10.1073/pnas.1109353108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit-Rigter LA, Noorlander CW, von Oerthel L, Chameau P, Smidt MP, van Hooft JA. Prenatal fluoxetine exposure induces life-long serotonin 5-HT receptor-dependent cortical abnormalities and anxiety-like behaviour. Neuropharmacology. 2012;62:865–870. doi: 10.1016/j.neuropharm.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Suri R, Hellemann G, Stowe ZN, Cohen LS, Aquino A, Altshuler LL. A prospective, naturalistic, blinded study of early neurobehavioral outcomes for infants following prenatal antide-pressant exposure. J Clin Psychiatry. 2011;72:1002–1007. doi: 10.4088/JCP.10m06135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hove DL, Steinbusch HW, Bruschettini M, et al. Prenatal stress reduces S100B in the neonatal rat hippocampus. Neuroreport. 2006;17:1077–1080. doi: 10.1097/01.wnr.0000223391.74575.c9. [DOI] [PubMed] [Google Scholar]
- Ververs T, Kaasenbrood H, Visser G, Schobben F, Jong-van dB, Egberts T. Prevalence and patterns of antidepressant drug use during pregnancy. Eur J Clin Pharmacol. 2006;62:863–870. doi: 10.1007/s00228-006-0177-0. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Weisenburger WP, Minck DR. Neurobehavioral teratogenic effects of thalidomide in rats. Neurotoxicol Teratol. 2001;23:255–264. doi: 10.1016/s0892-0362(01)00140-4. [DOI] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee EL, Zimmerman EF. Involvement of GABA in palate morphogenesis and its relation to diazepam teratogenesis in two mouse strains. Teratology. 1983;28:15–22. doi: 10.1002/tera.1420280104. [DOI] [PubMed] [Google Scholar]
- West CHK, Ritchie JC, Weiss JM. Paroxetine-induced increase in activity of locus coeruleus neurons in adolescent rats: implication of a countertherapeutic effect of an antidepressant. Neuropsychopharmacology. 2010;35:1653–1663. doi: 10.1038/npp.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikum WM, Oberlander TF, Hensch TK, Werker JF. Prenatal exposure to antidepressants and depressed maternal mood alter trajectory of infant speech perception. Proc Natl Acad Sci. 2012;109:17221–17227. doi: 10.1073/pnas.1121263109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Azmitia EC. Astroglial 5-HT1a receptors and S-100 beta in development and plasticity. Perspect Dev Neurobiol. 1994;2:233–238. [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56:479–485. doi: 10.1016/s0361-9230(01)00615-3. [DOI] [PubMed] [Google Scholar]
- Williams SK, Lauder JM, Johns JM. Prenatal cocaine disrupts serotonin signaling-dependent behaviors: implications for sex differences, early stress and prenatal SSRI exposure. Curr Neuropharmacol. 2011;9:478–511. doi: 10.2174/157015911796557957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winningham-Major F, Staecker JL, Barger SW, Coats S, Vaneldik LJ. Neurite extension and neuronal survival activities of recombinant S100β proteins that differ in the content and position of cysteine residues. J Cell Biol. 1989;109:3063–3071. doi: 10.1083/jcb.109.6.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, Ramin S, Chaudron L, Lockwood C. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol. 2009;114:703–713. doi: 10.1097/AOG.0b013e3181ba0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Norwitz ER, Smith MV, Lockwood CJ, Gotman N, Luchansky E, Lin H, Belanger K. Depression and serotonin reuptake inhibitor treatment as risk factors for preterm birth. Epidemiology. 2012;23:677–685. doi: 10.1097/EDE.0b013e31825838e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeskind PS, Stephens LE. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics. 2004;113:368–375. doi: 10.1542/peds.113.2.368. [DOI] [PubMed] [Google Scholar]


