Abstract
Scope
Our aim was to investigate whether dietary wolfberry altered carotenoid metabolic gene expression and enhanced mitochondrial biogenesis in the retina of diabetic mice.
Methods and Results
Six-week-old male db/db and wild type mice were fed the control or wolfberry diets for 8 weeks. At study termination, liver and retinal tissues were collected for analysis by transmission electron microscopy, real-time PCR, immunoprecipitation, Western blot, and HPLC. Wolfberry elevated zeaxanthin and lutein levels in the liver and retinal tissues and stimulated expression of retinal scavenger receptor class B type I, glutathione S-transferase Pi 1, and β,β-carotene 9’,10’-oxygenase 2, and induced activation and nuclear enrichment of retinal AMP-activated protein kinase α2 (AMPKα2). Furthermore, wolfberry attenuated hypoxia and mitochondrial stress as demonstrated by declined expression of hypoxia-inducible factor-1α, vascular endothelial growth factor, and heat shock protein 60. Wolfberry enhanced retinal mitochondrial biogenesis in diabetic retinas as demonstrated by reversed mitochondrial dispersion in the retinal pigment epithelium, increased mitochondrial copy number, elevated citrate synthase activity, and up-regulated expression of peroxisome proliferator-activated receptor γ co-activator 1 α, nuclear respiratory factor 1, and mitochondrial transcription factor A.
Conclusion
Consumption of dietary wolfberry could be beneficial to retinoprotection through reversal of mitochondrial function in diabetic mice.
Keywords: Carotenoids, Diabetic retinopathy, Hypoxia, Mitochondrial biogenesis, Wolfberry
INTRODUCTION
Epidemiological studies suggest that the levels of zeaxanthin and lutein in the retina and circulating system are inversely associated with risk of diabetic retinopathy, the most common diabetic eye disease and a leading cause of blindness in American adults [1–5]. However, the causal direction of this association remains unknown. Zeaxanthin and lutein are carotenoids specifically accumulated in the retina which is believed to protect the retina from light and other environmental and/or pathological stimuli-induced damage [4–8]. Uptake, transport, and metabolism of zeaxanthin and lutein in healthy humans have been documented recently [9–12]. Some key proteins regulating homeostasis of zeaxanthin and lutein have recently been identified. For instance, scavenger receptor class B type I (SR-BI) is one of the uptake proteins [13]. Glutathione S-transferase Pi 1 (GSTP1) mediates the transport of carotenoid within the retinal cells [14]. β,β-carotene 15’,15’-monooxygenase 1 (BCMO1) and β,β-carotene 9’,10’-oxygenase 2 (BCO2), are two enzymes to cleave carotenoids [15–19]. BCO2 cleaves lutein and zeaxanthin in the mitochondrion of the liver cells [18]. However, expression of those genes in the retina has not been profiled in a mouse model. No retinal measurements of lutein and zeaxanthin and their cleavage enzymes have been reported. Thus, it would be great interesting to investigate the potential roles of those proteins in carotenoid metabolism, mitochondrial function, and retinoprotection in diabetic mice.
Two finely ordered vasculature systems provide blood supply to the highly oxygen-consuming retina: 1) retinal and 2) choroidal vasculatures. The retinal vascular system provides nutrients and oxygen to the inner retina; the choroidal vasculature supplies the outer retina [20,21]. In diabetes, elevated blood glucose and decrease in blood flow result in hyperglycemia and hypoxia in the retina [21,22]. Interaction of hyperglycemia and hypoxia is implicated in pathogenesis of diabetic retinopathy [23–25]. The occurrence of hypoxia can be confirmed by determining induction of the hypoxia-inducible factor (HIF) and activation of vascular endothelial growth factor (VEGF) [26–28]. Inhibiting VEGF signaling has been clinically applied to maintain visual acuity and delay the progression of proliferative diabetic retinopathy [29].
A mitochondrion is a primary target of hyperglycemia [30]. Retinal mitochondrial apoptosis and mitochondrial DNA (mt DNA) damage are associated with changes in the retinal blood vessels and breakdown of the blood-retinal barrier at the late stage of diabetic retinopathy [31]. We and others reported that early changes in the structure and function of the retina occur before observation of clinical retinopathy [32–38], including damage of retinal photoreceptor and inner nuclear layers, and loss of ganglion cells. Understanding whether alteration of mitochondrial function mediates retinal cell damage at the early stage of diabetes is critical to the development of retinoprotective strategies.
Wolfberry is a fruit traditionally consumed in China that is now available in US grocery stores. Wolfberry is unique because it has high amounts of bioactive components, including diester forms of lutein and zeaxanthin, polysaccharides, betaine, and taurine [32,39,40]. We recently reported that application of 1 % (kcal) wolfberry for 8 weeks increased overall activity of AMP-activated protein kinase (AMPK), enhanced expression of manganese superoxide dismutase and thioredoxin, and attenuated endoplasmic reticulum (ER) stress. This application prevented retinal degeneration in db/db mice at the early stage of diabetes, without leaving any systemic effects on the hyperglycemia and hyperinsulinemia [32]. In this paper we reported the underlying molecular mechanism on how dietary wolfberry up-regulated carotenoid metabolic gene expression, attenuated hypoxia, and enhanced mitochondrial biogenesis in the retina, which would result in reversal of mitochondrial function and subsequent retinoprotection in db/db diabetic mice.
MATERIALS AND METHODS
Animals, diets, and fasting glucose test
Sixty three male wild type (C57BLKS/J, abbreviated WT) and 63 male db/db leptin receptor deficient type 2 diabetic mice (abbreviated db/db) at 5 weeks of age were purchased from the Jackson Laboratory (Bar Harbor, MA, USA), and housed in our animal facility on a regular chow diet (the control diet (CD), for formulation see Supporting Information Table S1) for a week prior to experimental treatments.
Then, db/db and WT mice were equally divided into 3 experimental groups (21 mice per group): the first group was euthanized immediately at 6 weeks of age, as a control group of the dietary treatments at time zero; the second group was fed a CD for additional 8 weeks; and the third group was fed a wolfberry diet (WD, CD with addition of 1% (kcal) wolfberry, for formulation see Table S1) for 8 weeks. Wolfberry and diet formulation were the same as our previous report [32].
Mice were group-housed (3/cage) in a controlled environment with a 12-h light/dark cycle at a constant room temperature. All animals had free access to water and food throughout the study. Food consumption, body weight, and blood glucose were monitored on a weekly basis. All experiments conformed to the Association for Research in Vision and Ophthalmology Statement for Use of Animals in Ophthalmic and Vision Research and were performed under an institutionally approved animal protocol (IACUC #2782). At termination of experiments (for all 3 groups), animals were fasted for 6 hours prior to sacrifice by CO2 according to the approved protocols. Blood, liver tissues and eyeballs were collected for experiments.
Six hours fasting blood glucose was tested by the Precision Xtraw blood glucose monitoring system (Alameda, CA, USA) as previously reported [32].
Real-time PCR
Eyeballs were preserved in RNAlater (Life Technologies, Grand Island, NY). Retinal tissues were dissected and total RNA was extracted using the RNeasy total RNA isolation kit (Qiagen, Valencia, CA). Total RNA quality and quantity were measured by the Nanodrop (Thermo Scientific, Wilmington, DE) and the Bioanalyzer (Agilent Technologies, Santa Clara, CA). cDNA was synthezed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Gene expression of SR-BI, GSTP1, BCMO1, BCO2, peroxisome proliferator-activated receptor γ co-activator-1α (PGC-1α), and nuclear respiratory factor 1 (NRF1) in the mouse retina was quantified by real-time PCR using the SYBR green assay (Bio-Rad) with gene specific primers (synthesized by IDT, Coralville, IW). β–actin was used as an internal control. Changes of mRNA abundance were calculated using the ΔΔCt method according to the manufacturer’s instruction (C1000 Real Time PCR unit, Bio-Rad. Data were presented as % of β-actin. The primer sequences were:
BCO2 left primer: 5’-aacatggggaacagctatgg-3’, the right primer: 5’-ggcaatggaacatagcacct-3’:
GSTP1 left primer: 5’-tgccaccatacaccattgtc-3’, the right primer: 5’-caagccttgcatccaggtat-3’;
SR-BI left primer: 5’-aagtggtcaacccaaacgag-3’, the right primer: 5’-acggtgtcgttgtcattgaa-3’;
NRF1 left primer: 5’-ccacgttggatgagtacacg-3’, the right primer: 5’-gcaccacattctccaaaggt-3’;
PGC1α left primer: 5’-aaggtccccaggcagtagat-3’, the right primer: 5’-ggctgtagggtgaccttgaa-3’;
β-actin left primer: 5’-gggaatgggtcagaaggact-3’, the right primer: 5’-cttctccatgtcgtcccagt-3’.
Mitochondrial copy number and mass
Mitochondrial DNA copy number was determined by genomic DNA real-time PCR expressed as abundance ratio of cytochrome b (Cyt b) to β-actin as previously described [41]. Cyt b and β– actin are markers of mitochondrial and genomic DNA, respectively. Retinal genomic DNA was isolated using DNeasy mini kit (Qiagen, Valencia, CA). Both markers were quantified by real-time PCR using the SYBR green assay (Bio-Rad) with gene specific primers.
Cyt b left primer: 5’-gcaaccttgacccgattcttcgc-3’, the right primer: 5’-tgaacgattgctagggccgcg-3’;
β-actin left primer: 5’-ggactcctatgtgggtgacg-3’, the right primer: 5’-aggtgtggtgccagatcttc-3’.
Mitochondrial mass was semi-quantitated by measuring relative protein expression levels of cytochrome c oxidase subunit IV (Cox IV) to β-actin by Western blot [42].
Citrate synthase activity assay
Citrate synthase activity was used as a parameter of mitochondrial function integrity as previously described [41, 42]. The citrate synthase activity in retinal mitochondria was measured by reduction rate of 5,5-dithio-bis (2-nitrobenzoic acid) (DTNB)(Sigma, St Louis, MO). The reaction mixture contained 10 µg isolated retinal mitochondrial proteins, 100 µM DTNB, 300 µM acetyl coenzyme A, and 50 mM Tris-HCl (pH 8.0). The reaction was initiated by adding 500 µM oxaloacetate, and was followed at 412 nm for 3 min. The extinction co-efficiency E equals 13.6 mM/cm. The enzyme activity was expressed as nmol/mg protein/min.
Western blot, mitochondrial and nuclear fractionation, and AMPK immunoprecipitation
Whole retinal tissues, and/or nuclear or mitochondrial fractions were lysed on ice with cell lysis buffer followed by homogenization and sonication. The cell lysis buffer contained 20 mM Tris-HCl (pH 7.5), 0.5 mM EDTA, 0.5 mM EGTA, 0.5% Triton X-100, 0.1% protease inhibitor cocktail and 0.1 % phosphatase inhibitors cocktail (Sigma, St Louis, MO). After centrifugation at 10,000 g for 15 min, the supernatants were collected as protein samples and used for Western blotting as described previously [32]. Immunoreactive bands were detected by chemiluminescence (ECL, Thermo Scientific Pierce, Rockford, IL) and visualized by the FluorChem 8800 advanced image system (Alpha Innotech, San Leandro, CA). Total pixel intensity of each protein band was normalized to a loading control as indicated in the figures and was used for graphing and statistical analysis.
A mitochondrial fractionation kit (Active Motif, Carlsbad, CA) was used to isolated mitochondria from whole retinal tissues as described in the manufacturer’s protocol. Separation of the nuclear and cytoplasmic fractions was carried out using the nuclear extract kit from Active Motif (Carlsbad, CA). Mitochondrial, nuclear, and cytoplasmic proteins were extracted as above and used for Western blot and/or immunoprecipitation (IP).
For IP, 75 µg mitochondrial and/or nuclear proteins were pre-cleaned by Protein A/G PLUS-Agarose according to the manufacturer’s instruction (Santa Cruz Biotech, Santa Cruz, CA). AMPKα1 and/or α2 proteins were pulled down using their specific antibodies. Their phosphorylation forms were detected afterward by Western blotting using phospho-Thr172-AMPK (pThr172-AMPK) antibody.
For AMPK nuclear enrichment experiment, the nuclear and cytoplasmic fractions were prepared as above, then isolated protein samples from both fractions were subjected to Western blot to determine expression levels of AMPKα1 and α2 in the nuclei and cytosols. β–actin was a loading control.
Antibodies against AMPKα1 and AMPKα2 were purchased from Bethyl Laboratories (Montgomery, TX). Antibodies against pThr172-AMPK, Cox IV, β-actin, and HIF-1α were purchased from Cell Signaling (Danvers, MA). Anti-SR-BI was from Thermo Scientific Pierce (Rockford, IL). Antibodies against GSTP1, BCO2, transcription factor A, mitochondrial (TFAM), and NRF1 were ordered from Proteintech (Chicago, IL). BCMO1 and prohibitin antisera were purchased from Santa Cruz Biotech (Santa Cruz, CA). Anti-PGC-1α came from Abcam (Cambridge, MA). VEGF and HSP60 antibodies were provided by Enzo (Plymouth Meeting, PA).
Lutein and zeaxanthin contents by HPLC
Lutein and zeaxanthin contents in the liver and retinal tissues were measured as previously described [32]. Due to limited amount of retinal tissues, each retinal sample was pooled from 8 eyeballs. The contents of lutein and/or zeaxanthin were expressed as ng/g fresh tissues.
Electron microscopy
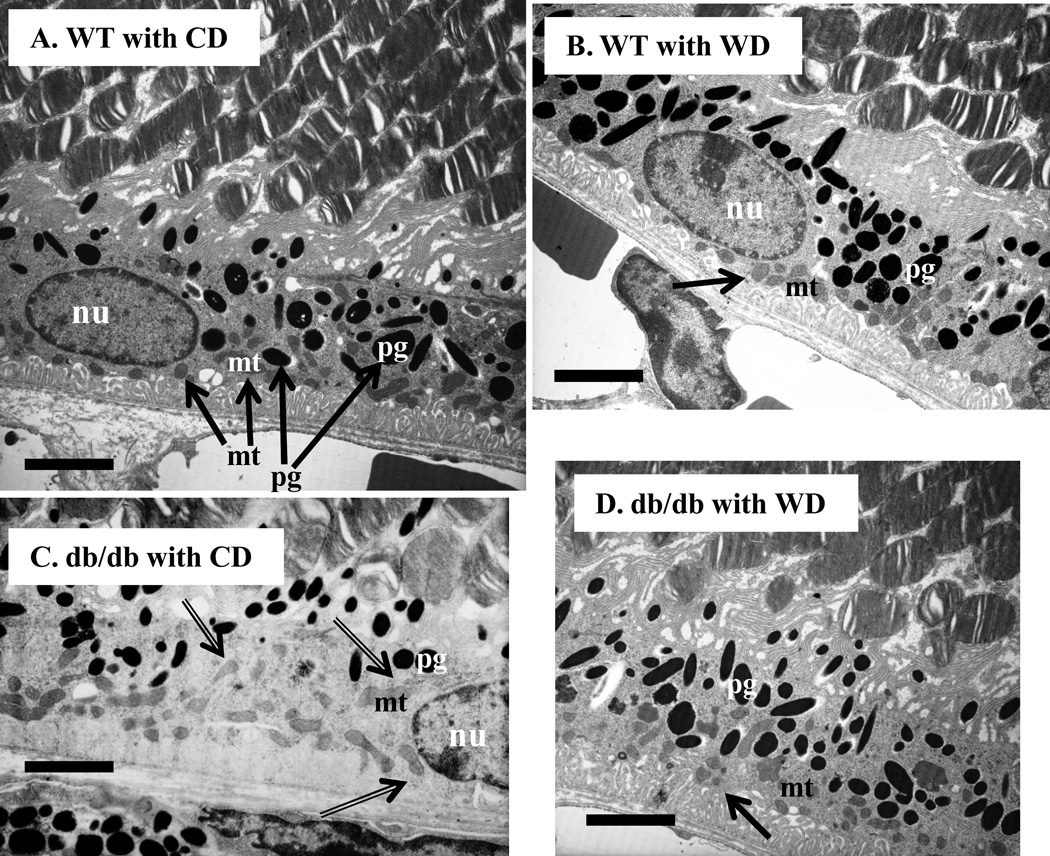
Mouse retinal micro-sections were prepared as previously described [32]. Briefly, eyeballs were briefly fixed in a fixative solution containing 2% paraformaldehyde, 2.5% glutaraldehyde (Sigma-Aldrich, St Louis, MO, USA) and 0.1mol/L cacodylate, and postfixed with osmium tetroxide (Electron Microscopy Sciences, Fort Washington, PA, USA), dehydrated in ethanol, and embedded in Epon LX112 (Electron Microscopy Sciences). Sections were taken perpendicular to the optic nerve so layers of the retina could be observed. Thin sections (0.5 or 1 µm) were obtained and viewed under a transmission electron microscope. The retinal pigment epithelium (RPE) and surrounding areas were photographed at a site about 300 µm away from the center of the optic nerves (defined as a central retinal area [32]). Eight serial sections were viewed and photographed in each sample. To quantify mitochondrial distribution, we drew a dot line to divide the RPE layer into half, the blood side half (close to the choroidal vasculature) and the photoreceptor side half, as shown in Figure 4 A. The number of mitochondria in each half-layer was counted and the ratio was analyzed by strain and diet. In the RPE layer of wild type mice fed the control diet (WT with CD, Fig. 4A), more than 80% mitochondria were localized in the blood side half. Thus, we set 80% as a gate, the lower percentage of mitochondria in the blood side half, the greater mitochondrial dispersion. The number of pigment granules was counted per 20µm area of the RPE layer, and the distribution was then compared by mouse strain and diet.
Figure 4. Wolfberry ameliorates dispersion of mitochondria and increases pigment granules in RPE cells of db/db diabetic retina.
Whole eyeballs were fixed and subjected to transmission electron microscopy focusing on the retinal pigment epithelium (RPE). Distribution of mitochondria (mt) and pigment granules (pg) in RPE cells was shown. Mitochondrial dispersion in diabetic RPE was indicated by double line arrows (C). n=5. A, WT with CD; B, WT with WD; C, db/db with CD; D, db/db with WD. mt, mitochondria; pg, pigment granules; nu, nucleus. CD, the control diet; WD, the wolfberry diet (CD with 1 % (kcal) wolfberry); WT, the wild type mouse; db/db, the db/db diabetic mouse. The dot line was used for mitochondrial distribution analysis as described in the section of Materials and Methods. Scale bar, 5 µm
Statistical analysis
Due to limited amount of retinal tissues, sample size (or the number of mice, n) in the individual experimental group varied from 5 to 9, although the initial number of mice in the dietary treatment was 21 per group. All results were expressed as mean ± SD. Differences in measured variables corresponding to dietary treatments (control and wolfberry) and strains (WT and db/db mice) were tested by two-way ANOVA using SAS 9.1 (SAS Institute, Cary, NC). The D’Agostino-Pearson omnibus test was used for data normality test. AMPK nuclear enrichment data were analyzed by the student’s t-test. Representative images of Western blot and electron microscopy on RPE structure were shown. Significance was set at p < 0.05 and/or p <0.01.
RESULTS
Wolfberry alleviates hypoxia and protects against mitochondrial stress in the diabetic retina
Before the dietary wolfberry treatment, fasting blood glucose concentration was 164.7±15.3 mg/dL in db/db mice at 6 weeks of age, and 89.5±6.1 mg/dL in age-matched WT litters. During the dietary treatment (for 8 weeks), fasting blood glucose concentrations were constant in WT mice (reaching 97.6 ± 10.3 mg/dL at 14 weeks of age). However, in db/db mice, fasting blood glucose concentrations were gradually increased; it reached 300 mg/dL at 10 weeks of age, and 368.5 ± 43.6 mg/dL at 14 weeks of age, indicating the development of hyperglycemia and onset of diabetes in db/db mice at 10 weeks of age. Adding wolfberry did not lower the fasting blood glucose level in db/db mice and no difference was observed by diet (CD vs WD). This result suggests that wolfberry at 1% (kcal) did not systemically improve hyperglycemia, which is in consistent with our previous observation (32).
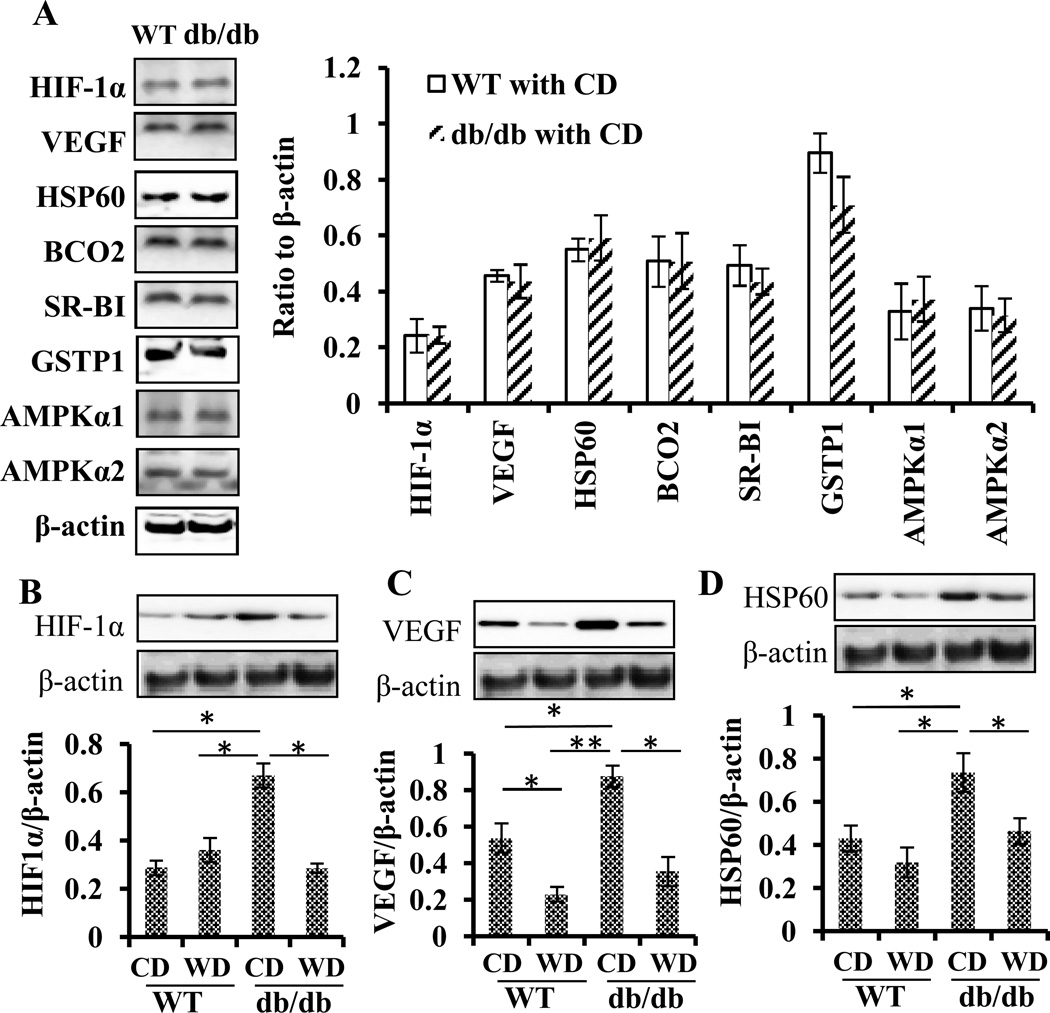
Hypoxia biomarkers HIF-1α and VEGF and a mitochondrial stress biomarker HSP60 were evaluated before and after the dietary wolfberry treatment. Before the treatment (in the groups of time zero), expression of retinal HIF-1α, VEGF, and HSP60 proteins did not differ by mouse strain at 6 weeks of age (db/db vs WT) (Fig. 1A). However, retinal HIF-1α, VEGF, and HSP60 protein levels were significantly higher in db/db than in WT mice at 14 weeks of age (Fig. 1B–D), which suggests that db/db mice suffered from hypoxia and mitochondrial stress starting sometime between 6 weeks and 14 weeks of age. Applying wolfberry reversed those protein expression patterns in the retina of db/db mice (Fig. 1B, HIF-1α; 1C, VEGF; and 1D, HSP60), suggesting attenuation of hypoxia and mitochondrial stress by wolfberry. No elevation of retinal HIF-1α and HSP60 protein levels occurred in WT mice fed the wolfberry diet. But significantly lowered EVGF protein level was found in the retina of WT mice fed wolfberry, as the result of an unknown mechanism.
Figure 1. Wolfberry alleviates hypoxia and protects against mitochondrial stress in the diabetic retina.
Whole retina tissues were isolated and the proteins were subjected to Western blot analysis. A: Retinal protein expression in WT and db/db mice at 6 weeks of age (before the dietary wolfberry treatment). B—D: Protein expression profiles after dietary treatments for 8 weeks: B. hypoxia-inducible factor 1 α (HIF-1α); C. vascular endothelial growth factor (VEGF); and D. heat shock protein 60 (HSP60). Representative images were shown. β-actin was used as an equal loading control to normalize each protein expression. Relative band intensity of each protein to β-actin was used for graphing and statistical analysis. n=9 in A; n=6 in B, C, and D. The statistical significance was at p<0.05 (*) and/or p<0.01 (**). BCO2, β, β-carotene 9’,10’-oxygenase 2; GSTP1, glutathione S-transferase Pi 1; SR-BI, scavenger receptor class B type I; AMPKα1, AMP-activated protein kinase α 1; AMPKα2, AMP-activated protein kinase α 2. CD, the control diet; WD, the wolfberry diet (CD with 1 % (kcal) wolfberry); WT, the wild type mouse; db/db, the db/db diabetic mouse.
Wolfberry induces accumulation of zeaxanthin and lutein in the liver and retina of mice
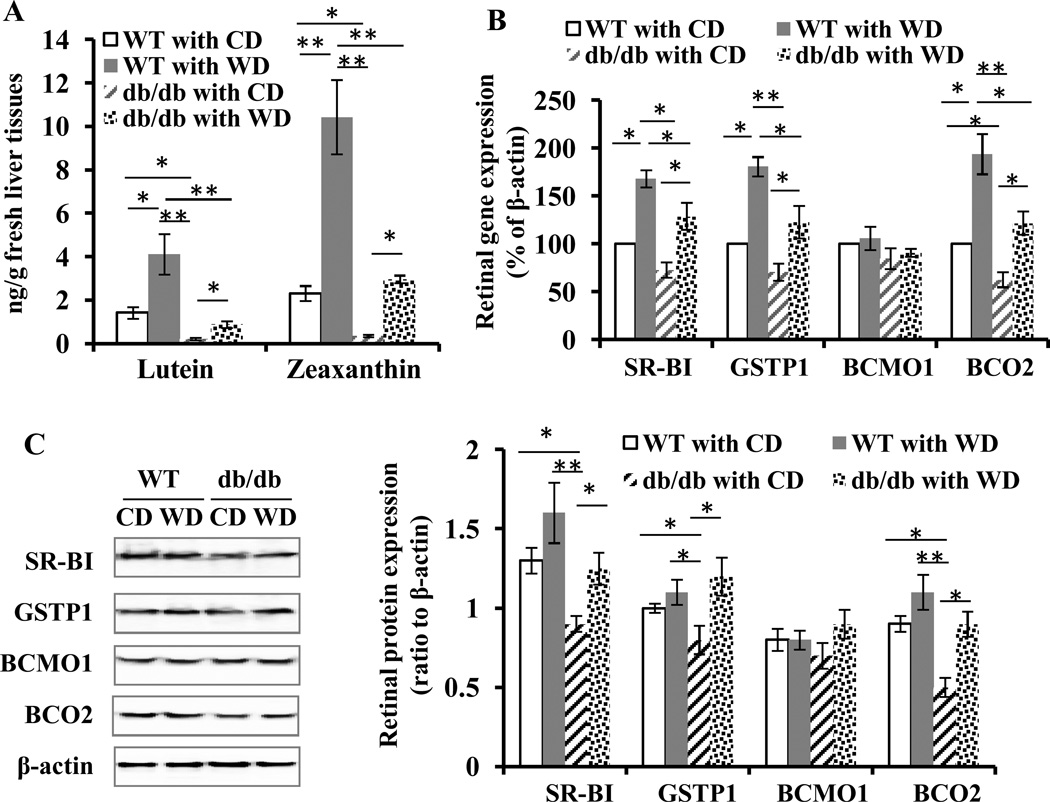
HPLC results showed that at 14 weeks of age, hepatic lutein and zeaxanthin levels were significantly lower in diabetic db/db mice than in age-matched WT litters (Fig. 2A). Applying wolfberry caused significant accumulation of hepatic lutein and zeaxanthin in both db/db and WT mice. There was no difference in hepatic lutein and zeaxanthin contents between the groups of the db/db with WD and the WT with CD, which indicates wolfberry-induced lutein and zeaxanthin accumulation in the liver of db/db mice.
Figure 2. Wolfberry induces accumulation of zeaxanthin and lutein in the liver and up-regulates carotenoid metabolic genes in the retina of db/db diabetic mice.
The lutein and zeaxanthin contents in mouse liver tissues at study termination were analyzed by HPLC and expressed as ng/g fresh liver tissues (A). Whole retinal tissues were isolated and subjected to real-time PCR (B) or Western blot (C). The values of PCR products were normalized and expressed as % to β –actin in each sample (B). Representative images of Western blot were shown (C). Relative band intensity of each protein was normalized to β-actin in each sample for graphing and statistical analysis. n=6. The statistical significance was at p<0.05 (*) and/or p<0.01 (**). BCMO1, β, β-carotene 15’,15’-monooxygenase 1; BCO2, β, β-carotene 9’,10’-oxygenase 2; GSTP1, glutathione S-transferase Pi 1; SR-BI, scavenger receptor class B type I. CD, the control diet; WD, the wolfberry diet (CD with 1% (kcal) wolfberry); WT, the wild type mouse; db/db, the db/db diabetic mouse.
Furthermore, we determined retinal zeaxanthin and lutein concentrations. In the mouse retina, we were able to detect lutein, zeaxanthin and cis-lutein/zeaxanthin. The results indicated that retinal lutein and zeaxanthin levels were significant lower in db/db than in WT mice (Table 1, db/db with CD vs WT with CD). Applying wolfberry slightly increased total concentration of lutein and zeaxanthin by about 10 % in the retina of WT mice at 14 weeks of age. Of interest, application of wolfberry significantly induced accumulation of lutein and zeaxanthin in the retina of db/db mice at 14 weeks of age, although the total concentration was still lower than those in WT mice fed CD (Table 1). The results might suggest that wolfberry, to some extent, reversed the lutein and zeaxanthin concentration in the retina of db/db diabetic mice.
Table 1. Contents of retinal lutein and zeaxanthin in mice fed CD or WD for 8 weeks.
Values were expressed as ng/g fresh retinal tissues, different letters showing statistic significance (p < 0.05). CD, the control diet (10 kCal % fat); WD, the wolfberry diet (the control diet with 1 % (kCal) wolfberry); WT, wild type mice; db/db, db/db diabetic mice; Lute, lutein; Zeax, zeaxanthin; cis-Lute/Zeax, cis-lutein/zeaxanthin; nd, not detected (<0.1 ng/g fresh retinal tissues)
| Animal with diet | Lute | Zeax | cis-Lute/Zeax | Total |
|---|---|---|---|---|
| WT with CD | 20.3±4.1a | 4.9±0.7a,b | 6.4±1.0a | 31.6±4.9a |
| WT with WD | 20.9±2.8a | 6.8±1.1a | 7.1±0.5a | 34.8±5.3a |
| db/db with CD | 8.5±0.9b | nd | nd | ~8.5±0.9 |
| db/db with WD | 14.8±1.2c | 3.2±0.5b | 1.6±0.4b | 19.6±2.4b |
Wolfberry up-regulated carotenoid metabolic genes in the retina of mice at 14 weeks of age
Expression of genes involved in metabolism of lutein and zeaxanthin in the retina of mice after dietary treatments was analyzed by quantitative real-time PCR (Fig. 2B) and Western blot (Fig. 2C). Real-time PCR results demonstrated no significant transcriptional down-regulation of SR-BI and GSTP1 in the retina of db/db mice, compared with the WT group at 14 weeks of age. Wolfberry up-regulated transcriptional expression of SR-BI and GSTP1 in the retina of both db/db and WT mice. No difference was observed by strain and diet in retinal BCMO1 mRNA abundance, but retinal BCO2 mRNA abundance in db/db was significantly lower than that of WT mice fed CD at 14 weeks of age. Applying wolfberry tremendously up-regulated retinal BCO2 mRNA expression in both animal groups; moreover, the BCO2 mRNA level in db/db with WD did not differ from that of WT with CD.
Protein expression data in mice at 14 weeks of age are presented in Fig. 2C. BCMO1 protein expression was not altered by either strain (db/db vs WT) or diet (CD vs WD), suggesting that BCMO1 was not involved in wolfberry-altered carotenoid metabolism in the retina of db/db and WT mice. Lowered expression of retinal SR-BI, GSTP1 and BCO2 proteins was observed in db/db mice at 14 weeks of age after onset of diabetes. Apparently wolfberry elevated those protein levels back to those of WT with CD (Fig. 2C).
Furthermore, prior to the onset of diabetes, protein levels of retinal BCO2, SR-BI, and GSTP1 in db/db mice (6 weeks of age) did not differ from those of age-matched WT mice (Fig. 1A). mRNA and protein expression data indicated that SR-BI, GSTP1 and BCO2 levels were altered at the onset of diabetes, and BCO2 expression might be regulated through a different mechanism from SR-BI and GSTP1 in the retina of db/db mice.
Wolfberry stimulates AMPKα2 activation and nuclear enrichment in the diabetic retina
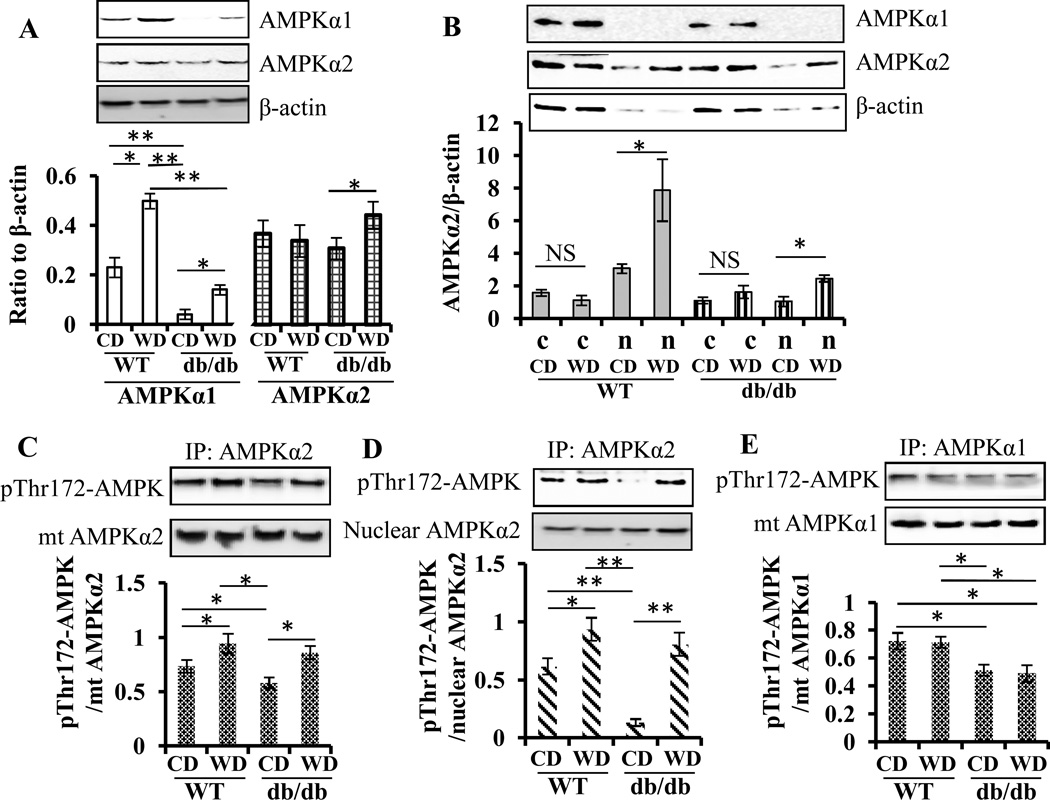
Then, we determined whether dietary wolfberry activated AMPK, a sensor of cellular energy homeostasis, in the retina of db/db mice. First, we studied the differential responses of retinal AMPK catalytic subunits AMPKα1 and α2 to wolfberry and found that total protein levels of retinal AMPKα1 but not α2 declined in db/db mice compared with WT fed CD at 14 weeks of age (Fig. 3A). Applying wolfberry for 8 weeks elevated protein levels of AMPKα1 in both db/db and WT mice. AMPKα2 protein expression was stimulated by wolfberry in db/db mice (Fig. 3A). On the other hand, no changes occurred at protein levels of either AMPKα1 or AMPKα2 between db/db and WT fed CD at 6 weeks of age (Fig. 1A). The data suggest that the differential expression of AMPKα1 and/or AMPKα2 could be caused by the onset of diabetes in db/db mice.
Figure 3. Wolfberry stimulates AMPKα2 activation and nuclear enrichment in the retina of db/db diabetic mice.
A. Whole retinal lysates were isolated, and differential expression of AMPKα1 and α2 were determined by Western blot. n=6. B. The retinal nuclear and cytoplasmic fractions were archived. Enrichment of AMPKα1 and α2 in nuclei and cytosols was determined by Western blot. Relative band intensity of each protein was normalized to β-actin in each sample for graphing and statistical analysis (A and B). c, the cytosol, n, the nuclei. Mitochondrial fractions were isolated, then the proteins were subjected to immunoprecipitation (IP) by anti-AMPKα2 (C) or AMPKα1 antibodies (E), and immunoblotted with anti-phospho-Thr172 AMPK (anti-pThr172 AMPK) antibody. Nuclear proteins were immumoprecipitated by anti-AMPKα2 and immunoblotted by anti-pThr172 AMPK (D). Activation of the α1 and α2 AMPK subunits was shown as ratio of band intensity of pThr172 AMPK to that of AMPKα1 or α2, respectively. Representative images were shown. n=9 (in B-E). The statistical significance was at p<0.05 (*) and/or p<0.01 (**). AMPK, AMP-activated protein kinase; mt, mitochondria; CD, the control diet; WD, the wolfberry diet (CD with 1 % (kcal) wolfberry); WT, the wild type mouse; db/db, the db/db diabetic mouse; NS, no significant difference
Next, we determined the subcellular distribution of two AMPKα subunits by nuclear fractionation and Western blot in the retina of mice at 14 weeks of age. As shown in Fig. 3B, AMPKα2 was enriched in nuclei of the retina of both db/db and WT mice after wolfberry treatment. No significant AMPKα1 nuclear enrichment was observed in both mouse strains fed either CD or WD.
We also tested activation of AMPKα1 and α2 by immunoprecipitation and Western blot using anti-pThr172-AMPK antibody. Phosphorylation of AMPKα subunits on Thr172 indicates activation of AMPK (43). Diabetes caused inactivation of AMPKα2 in both fractions of nuclei and mitochondria, which was reversed by wolfberry (Fig. 3C and D). AMPKα1 activity was inhibited in db/db but not in WT mice at 14 weeks of age, which did not differ by diet (CD vs WD) (Fig. 3E), suggesting that wolfberry induced activation and nuclear enrichment of AMPKα2, which, in turn might trigger regulation of gene expression associated to mitochondrial biogenesis, such as PGC-1α and NRF1 [41,42].
Wolfberry ameliorates dispersion of mitochondria and increases pigment granules in RPE of db/db diabetic mice
As expected, mitochondria were enriched to the blood side of RPE cells (Fig. 4A, arrowed) (close to the choroidal vasculature), and wolfberry did not affect mitochondrial distribution in the RPE of WT retina as determined by transmission electron microscopy (Fig. 4B). No difference existed in overall cell structure and distribution of mitochondria and pigment granules in the RPE of db/db and WT mice at 6 weeks of age (data not shown); however, at the early stage of diabetes, no apoptotic but dispersed mitochondria were found throughout the RPE cells and the RPE was still intact in db/db mice at 14 weeks of age (Fig. 4C, arrowed). Decreased numbers of retinal pigment granules (Fig. 4C) might suggest exposure of RPE cells to light-induced damage in the diabetic retina [44]. Importantly, applying wolfberry, to some extent, ameliorated the mitochondrial dispersion, relocated mitochondria back to the blood side, and increased pigment granules in RPE (Fig. 4D) of db/db diabetic mice, suggesting that wolfberry preserved RPE structure in diabetes.
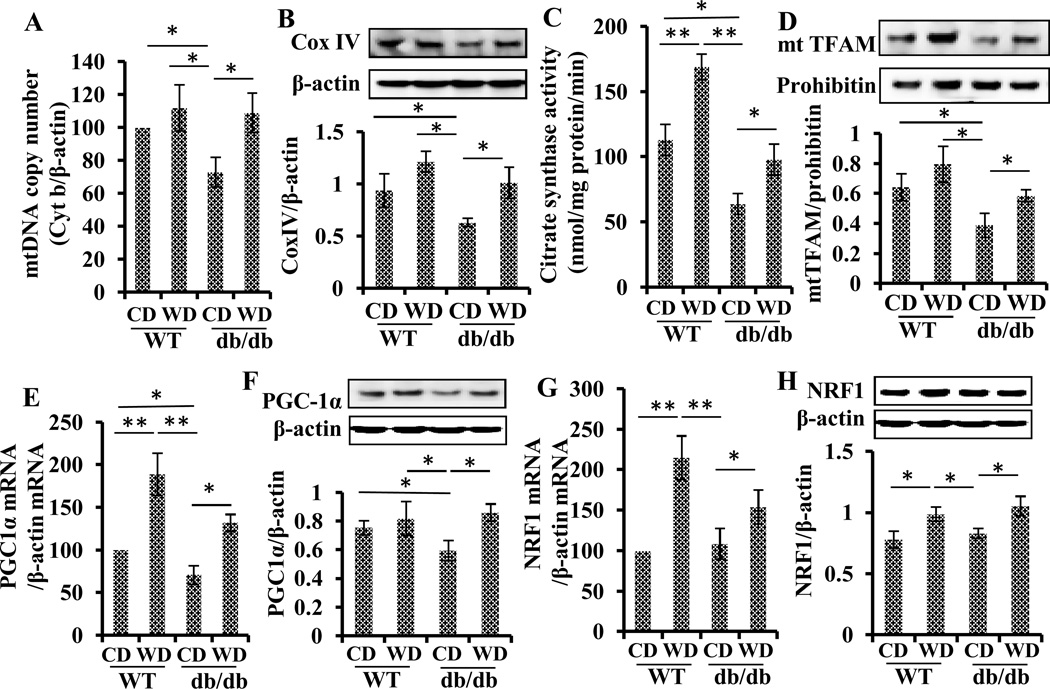
Wolfberry enhances mitochondrial biogenesis in the diabetic retina
As shown in Fig. 5A and B, the mitochondrial DNA copy number (Cyt b/β-actin) and mitochondrial mass (Cox IV/ β-actin) were significantly decreased in the retina of db/db diabetic mice compared with WT mice at 14 weeks of age. In db/db diabetic mice, mitochondrial function was also significantly impaired as evidenced by decreased activity of citrate synthase (Fig. 5C). Mitochondrial transcription factor A (TFAM) protein expression in mitochondria was inhibited by about 50 % (Fig. 5D), indicating mitochondrial dysfunction in the retina of diabetes. Applying wolfberry for 8 weeks in db/db mice reversed these parameters back to the levels similar to those in WT mice fed CD.
Figure 5. Wolfberry enhances mitochondrial biogenesis in the retina of db/db diabetic mice.
DNA copy numbers of cytochrome b (Cyt b) and β-actin were determined by real-time PCR using retinal genomic DNA and designed gene specific primers. Mitochondrial DNA copy number was expressed as Cyt b/β-actin (A). Mitochondrial mass in the retina was also determined by Western blot using antibody against Cox IV, a mitochondrial protein. The Cox IV protein level was normalized to β-actin in each sample (B). Retinal mitochondrial function integrity was tested by citrate synthase activity assay expressed as nmol/mg protein/min (C). Protein levels of TFAM in mitochondria were monitored by Western blot using anti-TFAM. Prohibitin was used as a loading control to normalize the TFAM values (D). Transcriptional and translational expression of transcription factors PGC-1α and NRF1 was analyzed by real time PCR (E, PGC-1α mRNA; G, NRF1 mRNA) and Western blot (F, PGC-1α protein; H, NRF1 protein). Data were normalized to β-actin. Representative Western blot images were shown. n=6. The statistical significance was at p<0.05 (*) and/or p<0.01 (**). mt DNA, mitochondrial DNA; Cyt b, cytochrome b; Cox IV, cytochrome c oxidase subunit IV; mt TFAM, mitochondrial transcription factor in mitochondria; NRF1, nuclear respiratory factor 1; PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1α.
Fig. 5E and F showed that expression of PGC-1α was significantly inhibited by the onset of diabetes at both transcriptional and translational levels, which were significantly reversed by wolfberry in the retina of db/db mice. The expression of NRF1 mRNA and protein did not differ by animal strain (WT vs db/db), but wolfberry stimulated expression of NRF1 at both mRNA and protein levels (Fig. 5G and 5H). Taken together, wolfberry enhanced mitochondrial biogenesis in the retina of db/db diabetic mice.
DISCUSSION
Retinal degeneration is a progressive, neural disorder, currently with no cure. Because diabetic retinopathy is associated with carotenoid metabolism in humans [1–5], understanding its underlying mechanism of pathogenesis at the very early stage of diabetes is critical to developing preventive strategies focused on diet and nutrition. In the current study, we demonstrated that disruption of lutein and zeaxanthin metabolic gene expression and AMPK activation, induction of hypoxia, and impairment of mitochondrial biogenesis occurred during the development of diabetes. Dietary wolfberry at 1% (kcal) alleviated the pathological changes that might eventually cause retinal neuroprotection in db/db diabetic mice.
db/db mice are genetically programmed diabetic animals, with no retinal disorder at birth [45]. The animal develops hyperglycemia (fasting blood glucose concentration > 300 mg/dL) at 10 weeks of age; retinal hyperinsulinemia [32], hyperglycemia, and hypoxia between 6 and14 weeks of age (also see Fig. 1A, 1B, 1D), and clinical retinopathy at about 25 weeks of age [46–49]. The db/db mouse at 6–14 weeks of age is therefore an ideal animal model to study the effects of hyperglycemia and hypoxia on the development of diabetic retinopathy.
Hypoxia is a secondary insult closely linked to inner retinal damage in diabetes [50]. VEGF is required for the maintenance of capillaries of retinal and choroidal vessels after birth, but elevated expression of VEGF induced by hypoxia is the key stimulus to abnormal vessel growth in the very late stage of diabetic retinopathy [50]. Blocking VEGF signaling by using VEGF inhibitors, neutralizing the anti-VEGF antibody, or solubilizing VEGF receptor −1 has been shown to improve vision in diabetic retinopathy patients [29]. Attenuation of hypoxia and inhibition of VEGF signaling by wolfberry would help protect against diabetic retinal degeneration.
Carotenoid metabolism and functions in health and diseases are not well understood. Difficulties in measuring lutein and zeaxanthin levels in the mouse retina retard the progression of carotenoid function discovery in retinal neuroprotection. Some key genes participating in lutein and zeaxanthin metabolism have been identified and characterized. Knockout studies show that a deficiency of SR-BI and/or GSTP1 causes retinal abnormality and altered carotenoid homeostasis in mice [13, 14]. Compared with BCO2, BCMO1 expression is low in the retina of mice [17]. Only BCO2, not BCMO1, cleaves lutein and zeaxanthin in the liver [17]. BCO2 knockout causes hepatic mitochondrial dysfunction and increased hepatic cell susceptibility to oxidative stress, which suggests that BCO2 may also be a stress responsive gene in mice [17,18]. In this study, we monitored changes of lutein and zeaxanthin levels and simultaneously investigated regulation of the genes involved in retinal lutein and zeaxanthin metabolism in db/db mice (Fig. 2B and C). Our data suggest that the onset of diabetes (as marked by hyperglycemia, hyperinsulinemia, and hypoxia) would cause inhibition of lutein and zeaxanthin uptake, binding and transport, and degradation within the retinal cells as indicated by lowered retinal lutein and zeaxnathin contents and inhibition of SR-BI, GSTP1 and BCO2 in mice. These findings agree with the epidemiologic observation of decreased retinal pigment density in diabetic patients [4].
Wolfberry is popularly consumed as a functional food, not just a traditional herbal medicine due to its numerous health benefits, including (but not limited to) induction of T-cell activation and immune response, adjustment of energy homeostasis, prevention of liver dysfunction, and improvement of vision [32, 51–53]. We previously reported that wolfberry attenuates hyperglycemia-induced oxidative and endoplasmic reticulum (ER) stress [32]. Here we showed that wolfberry alleviated hypoxia and mitochondrial stress (Fig. 1 B–D) and suggested that its preventive effects on hypoxia and/or mitochondrial stress would be through up-regulation of lutein and zeaxanthin metabolic genes in the retina of db/db diabetic mice.
BCO2 is a mitochondrial protein asymmetrically cleaving lutein and zeaxanthin at the 9',10' double bond [17]. BCO2 acts as not only a carotenoid scavenger but also a gatekeeper for mitochondrial apoptosis [18]. Before the onset of diabetes (at 6 weeks of age), the BCO2 protein level in db/db mice was similar to that of WT mice (Fig. 1A). BCO2 was inhibited at both transcriptional and translational levels (Fig. 2B and 2C) when the db/db mouse developed diabetes as demonstrated by hyperglycemia and hypoxia. We reported previously that ER stress occurs in db/db mice before 14 weeks of age [32]. Investigating how post-translational regulation such as protein misfolding alters BCO2 protein expression and whether BCO2 mediates retinal mitochondrial function in diabetic mice would be interesting.
AMPK exists as a heterotrimeric serine/threonine kinase comprising a catalytic α subunit and regulatory β and γ subunits. The AMPKα has two major isoforms; α1 and α2. AMPKα1 is primarily expressed in the cytoplasm (including mitochondria); AMPKα2 is located throughout the cell but predominantly in nuclei [54]. AMPK in the nucleus targets several transcription factors and cofactors, including FOXO3, PGC1α, NRF1, sirtuin 1, and p53 [32, 55–57]. The α1 and α2 AMPK may play distinct roles in neurodegeneration. Nuclear translocation of AMPKα1 potentiates striatal neurodegeneration in Huntington’s disease [55]. AMPKα2, but not α1, mediates oxidative stress-induced inhibition of RPE cell phagocytosis of photoreceptor outer segments and regulates RPE function and viability [58]. In the retina of diabetic mice, total AMPKα1 protein decreased and did not respond to wolfberry stimulation (Fig. 3A and E). Conversely, AMPKα2 protein had a significant response to wolfberry, including activation and nuclear enrichment. Wolfberry protects the retina from caspase-3 dependent apoptosis in db/db mice [32], suggesting that AMPKα2 could mediate retinoprotection by wolfberry via regulating expression of genes related to retinal cell survival and function in db/db diabetic mice.
PGC-1α and NRF1 activation of TFAM controls mitochondrial biogenesis, which plays a central role in the progression of diabetic retinopathy [59,60]. At the early stage of diabetes in db/db mice, expression of PGC-1α and mitochondrial TFAM proteins was inhibited in the retina, indicating impaired mitochondria biogenesis, which was also evidenced by decreased citrate synthase activity and number of mitochondria (Fig. 5 A—D, F). Wolfberry up-regulated PGC-1α and NRF1 and subsequently reversed TFAM, which in turn alleviated diabetes-induced mitochondrial impairment and potentiated mitochondrial biogenesis. Data suggested that protecting mitochondrial biogenesis could have a marked role in attenuating retinal damage in db/db diabetic mice.
Mitochondrial distribution is coordinated with metabolic function. Redistribution of mitochondria under stress also can be considered as a type of mitochondrial impairment [61], and dispersion of mitochondria may reflect the increase of ATP/ADP ratio and changes in metabolic function [62,63]. RPE is a monolayer of pigmented cells that provides a fundamental support in maintaining functions of the entire retina (especially photoreceptors), mainly by control of nutrients/waste product exchange [63]. Mitochondria in RPE of WT mice were enriched to the side of RPE cells closer to the blood supply (or the choroidal vasculature), but were dispersed with no apparent apoptosis in the RPE cells of db/db diabetic mice at 14 weeks of age (Fig. 4C). Most studies have focused on mitochondrial apoptosis in diabetes, a late stage of diabetic retinopathy. Here we found that, at the early stage of diabetes, hyperglycemia and/or hypoxia-caused stress insults could induce a set of profound physiological responses in RPE, leading to mitochondrial dispersion without initiation of apoptosis.
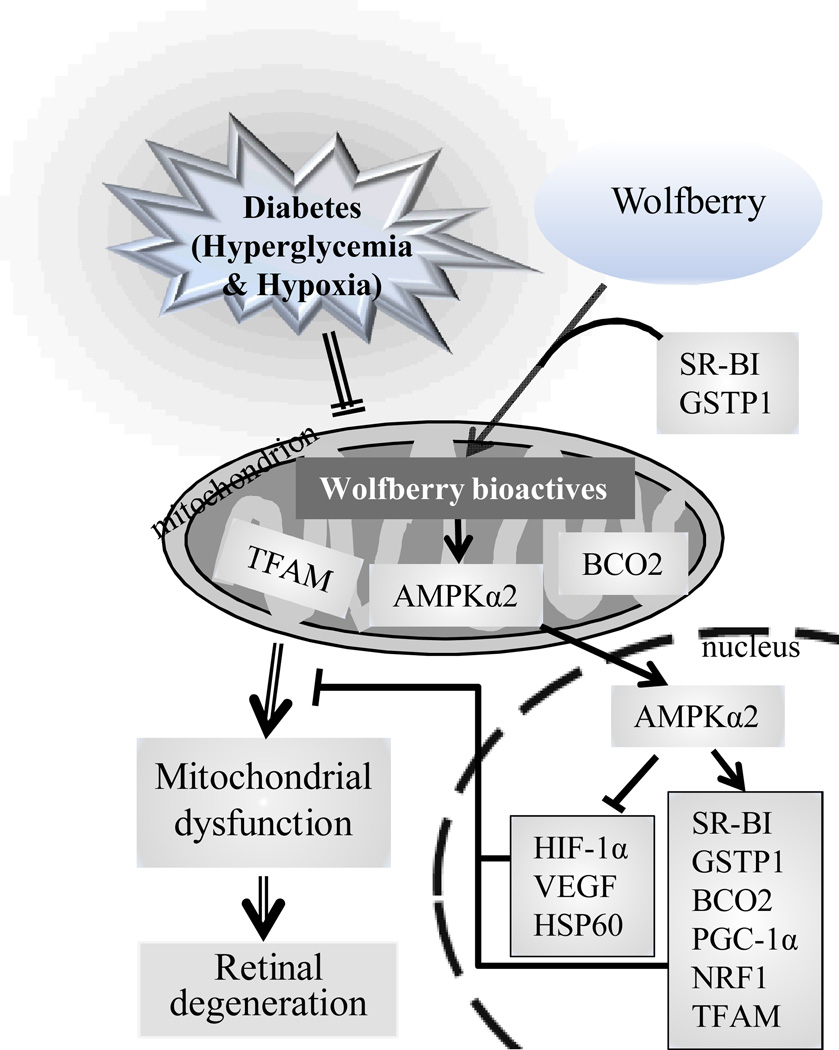
In summary, hyperglycemia and subsequent hypoxia were causative factors initiating changes in lutein and zeaxnathin metabolic homeostasis through inhibiting the metabolic gene expression. This resulted in inhibition of AMPK, decreases in mt TFAM, and mitochondrial dysfunction, and eventually led to alteration of retinal structure and retinodegeneration in db/db diabetic mice (Fig. 6, marked with double lines). Wolfberry primarily activated AMPKα2 in mitochondria and nuclei, which in turn triggered expression of genes related to metabolic homeostasis of lutein and zeaxanthin (SR-BI, GSTP1, and BCO2), mitochondrial biogenesis (PGC-1α, NRF1, and TFAM), and cell stress responses (HIF-1α, VEGF, and HSP60), which reversed mitochondrial function and caused retinoprotection in the retina of db/db diabetic mice (Fig. 6, marked with single lines). Thus, wolfberry and/or its bioactive components, at least including zeaxanthin and lutein, play neuroprotective roles via regulating gene expression by activation of AMPKα2 in db/db mice, although other bioactive components cannot be excluded.
Figure 6. Schematic diagram showing wolfberry and/or its bioactive components-modulated retinoprotection in db/db mice at the early stage of diabetes.
Hyperglycemia and/or subsequent hypoxia in db/db diabetic mice causes inhibition of lutein and zeaxnathin metabolic gene expression and decreases in protein levels of BCO2, AMPKα2, and TFAM in mitochondria, which leads to mitochondrial dysfunction and subsequent retinal degeneration in diabetes (marked with double lines). Dietary wolfberry and/or the bioactive components (wolfberry bioactives) primarily activates AMPKα2 in mitochondria and nuclei, which then triggers increased expression of genes related to lutein and zeaxanthin metabolism (SR-BI, GSTP1, and BCO2) and mitochondrial biogenesis (PGC-1α, NRF1, and TFAM), and decreases in cell stress responses (HIF-1α, VEGF, and HSP60), resulting in attenuation of hypoxia, enhancement of mitochondrial function, and subsequent retinal neuroprotection in db/db diabetic mice (marked with single lines).
Supplementary Material
ACKNOWLEDGEMENTS
The authors express their gratitude to the staff of the Comparative Medicine Group at Kansas State University (K-State) for animal care. The work was supported by the K-State NIH NCRR Grant P20-RR-017686 and the NIH K-INBRE Major Starter Grant P20-RR-016475 (to DL), and the K-INBRE Summer Scholarship, the K-State Cancer Center Award and the McNair Scholarship (to LW). Contribution no. 13-092-J from the Kansas Agricultural Experiment Station
Abbreviations
- AMPK
AMP-activated protein kinase
- BCMO1
β,β-carotene 15’,15’-monooxygenase 1
- BCO2
β,β-carotene 9’,10’-oxygenase 2
- Cyt b
cytochrome b
- Cox IV
cytochrome c oxidase subunit IV
- ER
endoplasmic reticulum
- GSTP1
glutathione S-transferase Pi 1
- HIF-1α
hypoxia-inducible factor 1α
- HSP60
heat shock protein 60
- NRF1
nuclear respiratory factor 1
- PGC-1α
peroxisome proliferator-activated receptor γ co-activator-1α
- RPE
retinal pigment epithelium
- SR-BI
scavenger receptor class B type I
- TFAM
transcription factor A, mitochondrial
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflict of interest
REFERENCES
- 1.Brazionis L, Rowley K, Itsiopoulos C, O'Dea K. Plasma carotenoids and diabetic retinopathy. Br J Nutr. 2009;101:270–277. doi: 10.1017/S0007114508006545. [DOI] [PubMed] [Google Scholar]
- 2.Coyne T, Ibiebele TI, Baade PD, Dobson A, McClintock C, Dunn S, Leonard D, Shaw J. Diabetes mellitus and serum carotenoids: findings of a population-based study in Queensland, Australia. Am J Clin Nutr. 2005;82:685–693. doi: 10.1093/ajcn.82.3.685. [DOI] [PubMed] [Google Scholar]
- 3.Polidori MC, Mecocci P, Stahl W, Parente B, Cecchetti R, Cherubini A, Cao P, Sies H, Senin U. Plasma levels of lipophilic antioxidants in very old patients with type 2 diabetes. Diabetes Metab Res Rev. 2000;16:15–19. doi: 10.1002/(sici)1520-7560(200001/02)16:1<15::aid-dmrr71>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Thurnham DI. Macular zeaxanthins and lutein -- a review of dietary sources and bioavailability and some relationships with macular pigment optical density and age-related macular disease. Nutr Res Rev. 2007;20:163–179. doi: 10.1017/S0954422407842235. [DOI] [PubMed] [Google Scholar]
- 5.Chiu CJ, Taylor A. Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog Retin Eye Res. 2011;30:18–53. doi: 10.1016/j.preteyeres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chucair AJ, Rotstein NP, Sangiovanni JP, During A, Chew EY, Politi LE. Lutein and zeaxanthin protect photoreceptors from apoptosis induced by oxidative stress: relation with docosahexaenoic acid. Invest Ophthalmol Vis Sci. 2007;48:5168–5177. doi: 10.1167/iovs.07-0037. [DOI] [PubMed] [Google Scholar]
- 7.Kijlstra A, Tian Y, Kelly ER, Berendschot TT. Lutein: more than just a filter for blue light. Prog Retin Eye Res. 2012;31:303–3115. doi: 10.1016/j.preteyeres.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Ozawa Y, Sasaki M, Takahashi N, Kamoshita M, Miyake S, Tsubota K. Neuroprotective effects of lutein in the retina. Curr Pharm Des. 2012;18:51–56. doi: 10.2174/138161212798919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borel P. Genetic variations involved in interindividual variability in carotenoid status. Mol Nutr Food Res. 2012;56:228–240. doi: 10.1002/mnfr.201100322. [DOI] [PubMed] [Google Scholar]
- 10.Lietz G, Oxley A, Boesch-Saadatmandi C, Kobayashi D. Importance of β,β-carotene 15,15'-monooxygenase 1 (BCMO1) and β,β-carotene 9',10'-dioxygenase 2 (BCDO2) in nutrition and health. Mol Nutr Food Res. 2012;56:241–250. doi: 10.1002/mnfr.201100387. [DOI] [PubMed] [Google Scholar]
- 11.Ferrucci L, Perry JR, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C, Fried LP, Albanes D, Corsi AM, Cherubini A, Guralnik J, Bandinelli S, Singleton A, Virtamo J, Walston J, Semba RD, Frayling TM. Common variation in the beta-carotene 15,15'-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet. 2009;84:123–133. doi: 10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borel P, de Edelenyi FS, Vincent-Baudry S, Malezet-Desmoulin C, Margotat A, Lyan B, Gorrand JM, Meunier N, Drouault-Holowacz S, Bieuvelet S. Genetic variants in BCMO1 and CD36 are associated with plasma lutein concentrations and macular pigment optical density in humans. Ann Med. 2011;43:47–59. doi: 10.3109/07853890.2010.531757. [DOI] [PubMed] [Google Scholar]
- 13.Provost AC, Vede L, Bigot K, Keller N, Tailleux A, Jaïs JP, Savoldelli M, Ameqrane I, Lacassagne E, Legeais JM, Staels B, Menasche M, Mallat Z, Behar-Cohen F, Abitbol M. Morphologic and electroretinographic phenotype of SR-BI knockout mice after a long-term atherogenic diet. Invest Ophthalmol Vis Sci. 2009;50:3931–3942. doi: 10.1167/iovs.08-2527. [DOI] [PubMed] [Google Scholar]
- 14.Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J Biol Chem. 2004;279:49447–49454. doi: 10.1074/jbc.M405334200. [DOI] [PubMed] [Google Scholar]
- 15.Bhatti RA, Yu S, Boulanger A, Fariss RN, Guo Y, Bernstein SL, Gentleman S, Redmond TM. Expression of beta-carotene 15,15' monooxygenase in retina and RPE-choroid. Invest Ophthalmol Vis Sci. 2003;44:44–49. doi: 10.1167/iovs.02-0167. [DOI] [PubMed] [Google Scholar]
- 16.Morinobu T, Tamai H. Beta-carotene 15,15'-dioxygenase activity in streptozotocin-induced diabetic rats. J Nutr Sci Vitaminol (Tokyo) 2000;46:263–265. doi: 10.3177/jnsv.46.263. [DOI] [PubMed] [Google Scholar]
- 17.Amengual J, Lobo GP, Golczak M, Li HN, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2011;25:948–959. doi: 10.1096/fj.10-173906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobo GP, Isken A, Hoff S, Babino D, von Lintig J. BCDO2 acts as a carotenoid scavenger and gatekeeper for the mitochondrial apoptotic pathway. Development. 2012;139:2966–2967. doi: 10.1242/dev.079632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276:14110–14116. doi: 10.1074/jbc.M011510200. [DOI] [PubMed] [Google Scholar]
- 20.Funk RH. Blood supply of the retina. Ophthalmic Res. 1997;29:320–325. doi: 10.1159/000268030. [DOI] [PubMed] [Google Scholar]
- 21.Campochiaro PA. Retinal and choroidal neovascularization. J Cell Physiol. 2000;184:301–310. doi: 10.1002/1097-4652(200009)184:3<301::AID-JCP3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 22.Neely KA, Quillen DA, Schachat AP, Gardner TW, Blankenship GW. Diabetic retinopathy. Med Clin North Am. 1998;82:847–876. doi: 10.1016/s0025-7125(05)70027-4. [DOI] [PubMed] [Google Scholar]
- 23.Arden GB, Sivaprasad S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev. 2011;7:291–304. doi: 10.2174/157339911797415620. [DOI] [PubMed] [Google Scholar]
- 24.Nyengaard JR, Ido Y, Kilo C, Williamson JR. Interactions between hyperglycemia and hypoxia: implications for diabetic retinopathy. Diabetes. 2004;53:2931–2938. doi: 10.2337/diabetes.53.11.2931. [DOI] [PubMed] [Google Scholar]
- 25.Wong A, Merritt S, Butt AN, Williams A, Swaminathan R. Effect of hypoxia on circulating levels of retina-specific messenger RNA in type 2 diabetes mellitus. Ann N Y Acad Sci. 2008;1137:243–252. doi: 10.1196/annals.1448.001. [DOI] [PubMed] [Google Scholar]
- 26.Lin M, Hu Y, Chen Y, Zhou KK, Jin J, Zhu M, Le YZ, Ge J, Ma JX. Impacts of hypoxia-inducible factor-1 knockout in the retinal pigment epithelium on choroidal neovascularization. Invest Ophthalmol Vis Sci. 2012;53:6197–6206. doi: 10.1167/iovs.11-8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han XX, Guo CM, Li Y, Hui YN. Effects of bevacizumab on the neovascular membrane of proliferative diabetic retinopathy: reduction of endothelial cells and expressions of VEGF and HIF-1α. Mol Vis. 2012;18:1–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Dong X, Wang YS, Dou GR, Hou HY, Shi YY, Zhang R, Ma K, Wu L, Yao LB, Cai Y, Zhang J. Influence of Dll4 via HIF-1α-VEGF signaling on the angiogenesis of choroidal neovascularization under hypoxic conditions. PLoS One. 2011;6:e18481. doi: 10.1371/journal.pone.0018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan GJ. New pharmacologic approaches to treating diabetic retinopathy. Am J Health Syst Pharm. 2007;64:S15–S21. doi: 10.2146/ajhp070332. [DOI] [PubMed] [Google Scholar]
- 30.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010;12:537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowluru RA, Mohammad G, dos Santos JM, Zhong Q. Abrogation of MMP-9 gene protects against the development of retinopathy in diabetic mice by preventing mitochondrial damage. Diabetes. 2011;60:3023–3033. doi: 10.2337/db11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang L, Zhang Y, Jiang Y, Willard L, Ortiz E, Wark L, Medeiros D, Lin D. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp Biol Med (Maywood) 2011;236:1051–1063. doi: 10.1258/ebm.2011.010400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arden GB, Sivaprasad S. The pathogenesis of early retinal changes of diabetic retinopathy. Doc Ophthalmol. 2012;124:15–26. doi: 10.1007/s10633-011-9305-y. [DOI] [PubMed] [Google Scholar]
- 34.Lieth E, Gardner TW, Barber AJ, Antonetti DA. Retinal neurodegeneration: early pathology in diabetes. Clin Experiment Ophthalmol. 2000;28:3–8. doi: 10.1046/j.1442-9071.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 35.Ly A, Yee P, Vessey KA, Phipps JA, Jobling AI, Fletcher EL. Early inner retinal astrocyte dysfunction during diabetes and development of hypoxia, retinal stress, and neuronal functional loss. Invest Ophthalmol Vis Sci. 2011;52:9316–9326. doi: 10.1167/iovs.11-7879. [DOI] [PubMed] [Google Scholar]
- 36.Obrosova IG, Drel VR, Kumagai AK, Szábo C, Pacher P, Stevens MJ. Early diabetes-induced biochemical changes in the retina: comparison of rat and mouse models. Diabetologia. 2006;49:2525–2533. doi: 10.1007/s00125-006-0356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cukiernik M, Hileeto D, Evans T, Mukherjee S, Downey D, Chakrabarti S. Vascular endothelial growth factor in diabetes induced early retinal abnormalities. Diabetes Res Clin Pract. 2004;65:197–208. doi: 10.1016/j.diabres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Li Q, Zemel E, Miller B, Perlman I. Early retinal damage in experimental diabetes: electroretinographical and morphological observations. Exp Eye Res. 2002;74:615–625. doi: 10.1006/exer.2002.1170. [DOI] [PubMed] [Google Scholar]
- 39.Inbaraj BS, Lu H, Hung CF, Wu WB, Lin CL, Chen BH. Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC-DAD-APCI-MS. J Pharm Biomed Anal. 2008;47:812–818. doi: 10.1016/j.jpba.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Ji H, He H, Lin D. Dietary wolfberry to protect against retinal degeneration. In: Preedy V, editor. Nutrition, Diet, the Eye and Vision. Elsevier; 2013. (in press) [Google Scholar]
- 41.Santos JM, Kowluru RA. Role of mitochondria biogenesis in the metabolic memory associated with the continued progression of diabetic retinopathy and its regulation by lipoic acid. Invest Ophthalmol Vis Sci. 2011;52:8791–8798. doi: 10.1167/iovs.11-8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santos JM, Tewari S, Goldberg AF, Kowluru RA. Mitochondrial biogenesis and the development of diabetic retinopathy. Free Radic Biol Med. 2011;51:1849–1860. doi: 10.1016/j.freeradbiomed.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 44.Dontsov AE, Glickman RD, Ostrovsky MA. Retinal pigment epithelium pigment granules stimulate the photo-oxidation of unsaturated fatty acids. Free Radic Biol Med. 1999;26:1436–1446. doi: 10.1016/s0891-5849(99)00003-9. [DOI] [PubMed] [Google Scholar]
- 45.Midena E, Segato T, Radin S, di Giorgio G, Meneghini F, Piermarocchi S, Belloni AS. Studies on the retina of the diabetic db/db mouse. I. Endothelial cell-pericyte ratio. Ophthalmic Res. 1989;21:106–111. doi: 10.1159/000266787. [DOI] [PubMed] [Google Scholar]
- 46.Clements RS, Jr, Robison WG, Jr, Cohen MP. Anti-glycated albumin therapy ameliorates early retinal microvascular pathology in db/db mice. J Diabetes Complications. 1998;12:28–33. doi: 10.1016/s1056-8727(97)00051-2. [DOI] [PubMed] [Google Scholar]
- 47.Barile GR, Pachydaki SI, Tari SR, Lee SE, Donmoyer CM, Ma W, Rong LL, Buciarelli LG, Wendt T, Horig H, Hudson BI, Qu W, Weinberg AD, Yan SF, Schmidt AM. The RAGE axis in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005;46:2916–2924. doi: 10.1167/iovs.04-1409. [DOI] [PubMed] [Google Scholar]
- 48.Cheung AK, Fung MK, Lo AC, Lam TT, So KF, Chung SS, Chung SK. Aldose reductase deficiency prevents diabetes-induced blood–retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes. 2005;54:3119–3125. doi: 10.2337/diabetes.54.11.3119. [DOI] [PubMed] [Google Scholar]
- 49.Li J, Wang JJ, Chen D, Mott R, Yu Q, Ma JX, Zhang SX. Systemic administration of HMG-CoA reductase inhibitor protects the blood – retinal barrier and ameliorates retinal inflammation in type 2 diabetes. Exp Eye Res. 2009;89:71–78. doi: 10.1016/j.exer.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arden GB, Sivaprasad S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev. 2011;7:291–304. doi: 10.2174/157339911797415620. [DOI] [PubMed] [Google Scholar]
- 51.Reeve VE, Allanson M, Arun SJ, Domanski D, Painter N. Mice drinking goji berry juice (Lycium barbarum) are protected from UV radiation-induced skin damage via antioxidant pathways. Photochem Photobiol Sci. 2010;9:601–607. doi: 10.1039/b9pp00177h. [DOI] [PubMed] [Google Scholar]
- 52.Amagase H, Sun B, Borek C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr Res. 2009;29:19–25. doi: 10.1016/j.nutres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Ren Z, Na L, Xu Y, Rozati M, Wang J, Xu J, Sun C, Vidal K, Wu D, Meydani SN. Dietary supplementation with lacto-wolfberry enhances the immune response and reduces pathogenesis to influenza infection in mice. J Nutr. 2012;142:1596–1602. doi: 10.3945/jn.112.159467. [DOI] [PubMed] [Google Scholar]
- 54.Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem J. 1998;334:177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ju TC, Chen HM, Lin JT, Chang CP, Chang WC, Kang JJ, Sun CP, Tao MH, Tu PH, Chang C, Dickson DW, Chern Y. Nuclear translocation of AMPK-alpha1 potentiates striatal neurodegeneration in Huntington's disease. J Cell Biol. 2011;194:209–227. doi: 10.1083/jcb.201105010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu L, Yang SJ. AMP-activated protein kinase mediates activity-dependent regulation of peroxisome proliferator-activated receptor gamma coactivator-1alpha and nuclear respiratory factor 1 expression in rat visual cortical neurons. Neuroscience. 2010;169:23–38. doi: 10.1016/j.neuroscience.2010.04.063. [DOI] [PubMed] [Google Scholar]
- 57.Okoshi R, Ozaki T, Yamamoto H, Ando K, Koida N, Ono S, Koda T, Kamijo T, Nakagawara A, Kizaki H. Activation of AMP-activated protein kinase induces p53-dependent apoptotic cell death in response to energetic stress. J Biol Chem. 2008;283:3979–3987. doi: 10.1074/jbc.M705232200. [DOI] [PubMed] [Google Scholar]
- 58.Qin S, De Vries GW. Alpha2 but not alpha1 AMP-activated protein kinase mediates oxidative stress-induced inhibition of retinal pigment epithelium cell phagocytosis of photoreceptor outer segments. J Biol Chem. 2008;283:6744–6751. doi: 10.1074/jbc.M708848200. [DOI] [PubMed] [Google Scholar]
- 59.Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23:459–466. doi: 10.1016/j.tem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piantadosi CA, Suliman HB. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J Biol Chem. 2006;281:324–333. doi: 10.1074/jbc.M508805200. [DOI] [PubMed] [Google Scholar]
- 61.Corrado M, Scorrano L, Campello S. Mitochondrial dynamics in cancer and neurodegenerative and neuroinflammatory diseases. Int J Cell Biol. 2012;2012:729290. doi: 10.1155/2012/729290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Overly CC, Rieff HI, Hollenbeck PJ. Organelle motility and metabolism in axons vs dendrites of cultured hippocampal neurons. J Cell Sci. 1996;109:971–980. doi: 10.1242/jcs.109.5.971. [DOI] [PubMed] [Google Scholar]
- 63.Sparrow JR, Hicks D, Hamel CP. The retinal pigment epithelium in health and disease. Curr Mol Med. 2010;10:802–823. doi: 10.2174/156652410793937813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.