Summary
Bisphosphonates are a class of drugs that are widely used to inhibit loss of bone mass in patients. We show here that the administration of clinically relevant doses of bisphosphonates in mice increases antibody responses to live and inactive viruses, proteins, haptens and existing commercial vaccine formulations. Bisphosphonates exert this adjuvant-like activity in the absence of CD4+ and γδ T cells, neutrophils or dendritic cells and their effect does not rely on local macrophage depletion nor does it depend upon Toll-like receptor signaling or the inflammasome. Rather, bisphosphonates target directly B cells and enhance B cell expansion and antibody production upon antigen encounter. These data establish bisphosphonates as a novel class of adjuvants that boost humoral immune responses.
Introduction
Bisphosphonates (BPs) are small-molecule inhibitors of bone resorption that are clinically approved for the treatment of skeletal diseases such as osteoporosis and Paget’s disease of bone (Favus, 2010; Scott and Gershon, 1970); these compounds represent a large family of drugs that include first generation clodronate (CLD) and etidronate (ETD) and nitrogen-containing alendronate (ALD), pamidronate (PMD), zoledronate (ZLD) and neridronate (NRD). Upon liposome encapsulation, BPs like CLD have been widely used to experimentally deplete tissue-resident phagocytes in rodents (Moseman et al., 2012; van Rooijen and Sanders, 1994). A few studies in BP-treated mice unexpectedly noted increased antigen (Ag)-specific humoral immune responses (Gonzalez et al., 2010; Iannacone et al., 2010; Norton et al., 2011); herein, we set out to systematically dissect the mechanistic basis for this activity.
Results and Discussion
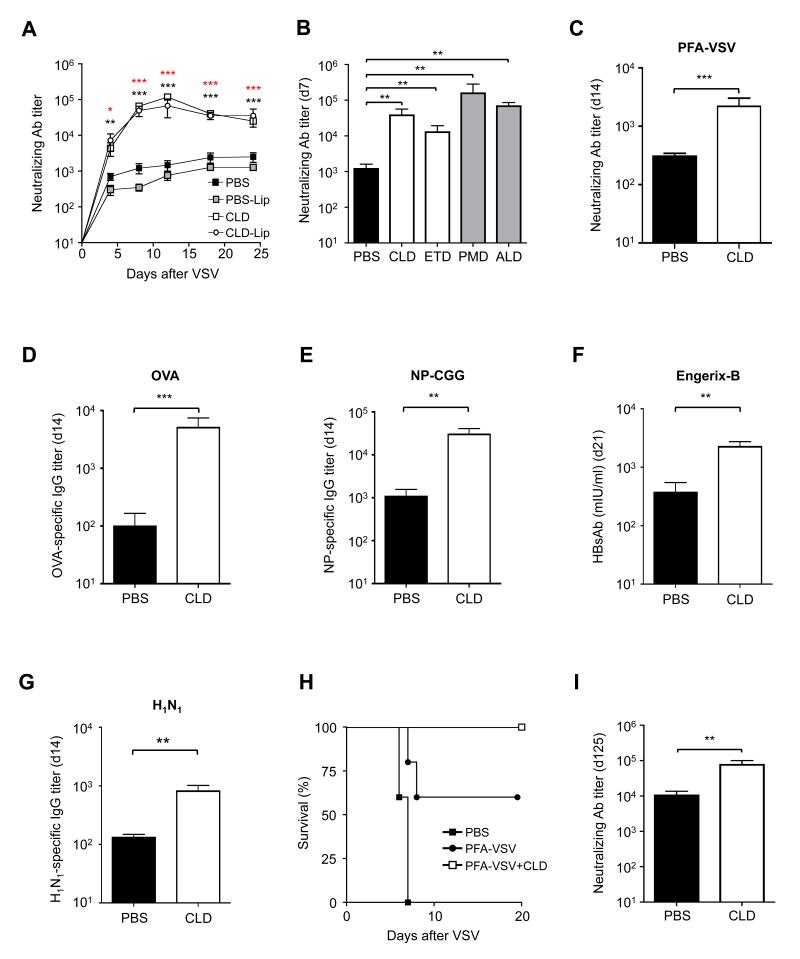
As the majority of the above-mentioned studies utilized subcutaneously administered BP-encapsulated liposomes prior to local viral challenge, we initially chose the same experimental setup to ask whether liposome encapsulation is required to increase antibody (Ab) responses. To this end, footpads of C57BL/6 mice were injected with PBS, PBS liposomes (PBS-Lip), CLD liposomes (CLD-Lip) or CLD prior to infection in the same footpad with vesicular stomatitis virus (VSV), a prototypic cytopathic virus that induces an early T-independent IgM response followed by a T-dependent IgG response (Hangartner et al., 2006). When compared to PBS-injected mice, mice that received CLD exhibited up to 100-fold higher neutralizing antibody (nAb) titers towards VSV (Figure 1A), and this occurred whether CLD was administered prior to or concomitantly with the Ag (Figure S1A). Importantly, free CLD was as effective as CLD-Lip (Figure 1A), it exhibited a dose-dependent effect (Figure S1B) and its adjuvant activity was shared by other BPs that are currently in clinical use, including ETD, PMD and ALD (Figure 1B).
Figure 1. Bisphosphonates Increase Antibody Responses to Live and Inactive Viruses, Proteins, Haptens and Existing Commercial Vaccine Formulations.
(a) VSV nAb titers in the serum of C57BL/6 mice that were footpad injected with PBS, PBS-liposomes (PBS-Lip), clodronate (CLD) or clodronate liposomes (CLD-Lip) prior to VSV infection in the same footpad (see Methods for details). n = 5 per group. Black asterisks, PBS versus CLD-Lip; red asterisks PBS versus CLD. Results are representative of >10 independent experiments. (b) VSV nAb titers 7 days p.i. in the serum of C57BL/6 mice that were footpad injected with CLD, etidronate (ETD), pamidronate (PMD) or alendronate (ALD) prior to VSV infection in the same footpad. n = 5 per group; results are representative of 3 independent experiments. (c) VSV nAb titers at day 14 post immunization in the serum of C57BL/6 mice that were footpad injected with PBS or CLD prior to paraformaldehyde-inactivated VSV (PFA-VSV) immunization in the same footpad. n = 10 per group; results are representative of 3 independent experiments. (d) Ovalbumin (OVA)-specific IgG titers at day 14 post immunization in the serum of C57BL/6 mice that were footpad injected with PBS or CLD prior to OVA immunization in the same footpad. n = 4 per group; results are representative of 3 independent experiments. (e) 4-Hydroxy-3-nitrophenylacetyl (NP)-specific IgG titers at day 14 post immunization in the serum of C57BL/6 mice that were footpad injected with PBS or CLD prior to NP-chicken gamma globulin (CGG) immunization in the same footpad. n = 5 per group; results are representative of 2 independent experiments. (f) HBsAb titers (mIU/ml) 21 days p.i. in the serum of C57BL/6 mice that were injected in the footpad with PBS or CLD prior to Engerix-B (an approved vaccine against hepatitis B virus) immunization in the same footpad. n = 5 per group; results are representative of 5 independent experiments. (g) H1N1-specific IgG titers in the serum of C57BL/6 mice that were injected intramuscularly with PBS or CLD prior to intramuscular immunization with H1N1. n = 5 per group; results are representative of 2 independent experiments. (h) Kaplan-Meier survival curves of C57BL/6 mice injected in the right footpad with PBS, 106 pfu equivalents of PFA-inactivated VSV (PFA-VSV) or 106 pfu equivalents of PFA-inactivated VSV and clodronate (PFA-VSV+CLD) 3 weeks prior to infection in the left footpad with 3.5 × 108 pfu of VSV. n = 12 per group; PFA-VSV+CLD versus PFA-VSV: P < 0.05, Log-rank (Mantel-Cox) test. Results are representative of 2 independent experiments. (i) VSV nAb titers 125 days p.i. in the serum of C57BL/6 mice that were footpad injected with PBS or CLD prior to VSV infection in the same footpad. n = 5 per group; results are representative of 2 independent experiments. ***: P < 0.001; **: P < 0.01; *: P < 0.05 See also Figure S1.
Subcutaneously administered CLD also increased Ab titers against inactive VSV, soluble proteins (OVA), haptens (NP-CGG) and the adjuvant-containing formulation Engerix-B (an approved vaccine against hepatitis B virus) (Figure 1C-F); similar results were observed when CLD was administered intramuscularly along with the hemoagglutinin/neuroaminidase subunits of the human influenza virus A/NewCaledonia/20/99 (H1N1, Figure 1G).
CLD treatment increased both neutralizing IgM and IgG responses against VSV (Figure S1C-E) without altering the subtype of Ag-specific IgG induced upon immunization (Figure S1F), and this correlated with the total number of CD138+ plasma cells recovered from draining LNs (Figure S1G). In combination with inactivated VSV, CLD boosted the partial protection afforded by immunization with inactivated virus alone (Figure 1H), and its adjuvant effect lasted for at least 4 months after a single administration (Figure 1I). CLD treatment also increased neutralizing IgM titers upon VSV infection in MHC-II−/− mice (which lack CD4+ T cells) and in CD40L−/− mice, in which T cell help for B cells is known to be compromised (Renshaw et al., 1994) (Figure 2A,B). While these data do not rule out a possible effect of CLD on T cells, they indicate that CLD adjuvant activity can occur independently of CD4+ T cell help and raise mechanistic questions about how this adjuvant effect is mediated. To address this issue, we systematically analyzed cellular and molecular changes induced by BPs at the site of injection and at the level of the draining LN.
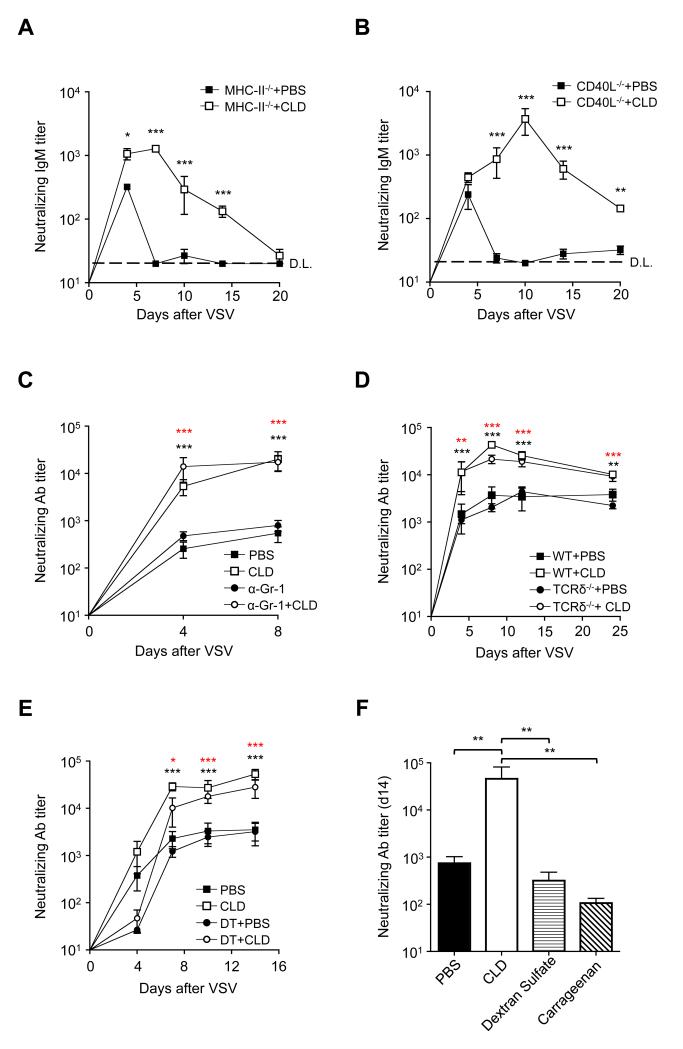
Figure 2. Bisphosphonates Increase Antibody Responses in the Absence of CD4+ and γδ T Cells, Neutrophils or Dendritic Cells and their Effect Does Not Require Local Macrophage Depletion.
(a) VSV neutralizing IgM titers in the serum of MHC-II−/− mice that were footpad injected with PBS or CLD immediately prior to VSV infection in the same footpad. n = 5 per group; results are representative of 3 independent experiments. (b) VSV neutralizing IgM titers in the serum of CD40L−/− mice that were footpad injected with PBS or CLD immediately prior to VSV infection in the same footpad. n = 5 per group; results are representative of 2 independent experiments. (c) VSV nAb titers in the serum of C57BL/6 mice that were injected (or not) with anti-Gr-1 depleting Ab (α–Gr-1, see Methods for details) and were subsequently footpad injected with PBS or CLD immediately prior to VSV infection in the same footpad. n = 6 per group; black asterisks, PBS versus CLD; red asterisks αGr-1+PBS versus αGr-1+CLD; results are representative of 2 independent experiments. (d) VSV nAb titers in the serum of C57BL/6 (WT) or TCRδ−/− mice that were footpad injected with PBS or CLD immediately prior to VSV infection in the same footpad. n = 10 per group; black asterisks, WT+PBS versus WT+CLD; red asterisks TCRδ−/−+PBS versus TCRδ−/−+CLD; results are representative of 3 independent experiments. (e) VSV nAb titers in the serum of CD11c-DTR-GFP mice that were treated (or not) with diphtheria toxin (see Methods for details) and were subsequently footpad injected with PBS or CLD immediately prior to VSV infection in the same footpad. n = 6 per group; black asterisks, PBS versus CLD; red asterisks DT+PBS versus DT+CLD. Results are representative of 3 independent experiments. (f) VSV nAb titers at day 14 p.i. in the serum of C57BL/6 mice that were footpad injected with PBS, CLD, dextran sulfate or carrageenan prior to VSV infection in the same footpad (see Methods for detail). n = 3 per group; results are representative of 3 independent experiments. ***: P < 0.001; **: P < 0.01; *: P < 0.05; D.L.: detection limit. See also Figure S2-4.
First, we examined BP injection site. In agreement with previously published data (Iannacone et al., 2010; Norton et al., 2011), footpad injection of CLD alone induced a local inflammatory infiltrate comprised mostly of Gr-1+ neutrophils and inflammatory monocytes (Figure S2A). Elimination of this infiltrate by systemic anti-Gr-1 treatment (Figure S2A,B), however, did not prevent CLD from increasing nAb titers upon VSV infection (Figure 2C), indicating that CLD adjuvant activity does not require Gr-1+ leukocytes.
Next, we considered the role of two additional footpad-resident cell types: γδ T cells and conventional dendritic cells (DCs). γδ T cells can provide help to B cells (Pao et al., 1996; Wen et al., 1996) and are reportedly activated by CLD (Maeda et al., 2001) or ALD (Thompson et al., 2010). Footpad CLD or ALD injection did not increase the local number of γδ T cells (Figure S2C) and VSV infection of TCRδ−/− mice (Itohara et al., 1993) revealed that CLD and ALD adjuvant activity is maintained in the absence of these cells (Figure 2D and Figure S2D). We then reasoned that BPs might act on DCs because DCs can present Ag to B cells (Qi et al., 2006) and most known adjuvants are thought to function by activating DCs (Coffman et al., 2010). Incubating DCs with CLD in vitro did not alter expression of activation markers, such as CD86 and CD40 (Figure S3A,B), and the systemic elimination of these cells in vivo (Figure S3C) did not significantly inhibit CLD adjuvant activity. Indeed, CLD administration in DC-depleted mice challenged with VSV resulted in nAb titers that, by day 7 post infection (p.i.), were comparable to those observed in DC-competent animals subjected to the same procedures (Figure 2E). It is worth noting that, independently of CLD treatment, DC depletion caused a transient reduction in nAb titers at day 4 p.i., consistent with a role of DC in early B cell activation (Qi et al., 2006; Scandella et al., 2007). Overall, these data indicate that CLD adjuvant activity does not require conventional DCs.
We next analyzed the draining LN. The only quantitative change in LN cellular composition detectable upon CLD treatment was the depletion of CD169+ macrophages lining the subcapsular and medullary sinuses (Figure S4A,B). We found surprising that BPs increase Ab responses despite depleting subcapsular sinus macrophages, since these cells were recently identified as critical Ag-presenting cells for B cell responses (Carrasco and Batista, 2007; Junt et al., 2007; Phan et al., 2007). To clarify these seemingly contradictory observations, we sought alternative strategies to deplete LN macrophages independently of BP administration. These strategies (footpad dextran sulfate or carrageenan injections in WT mice or footpad diphtheria toxin injection in CD11c-DTR mice) effectively depleted LN macrophages (Figure S4C and ref. (Iannacone et al., 2010)) but they failed to increase nAb titers upon VSV infection (Figure 2F and Figure S4D). This indicates that LN macrophage depletion per se does not increase humoral immune responses and suggests that CLD adjuvant activity does not rely on LN macrophages.
To investigate the molecular basis for CLD adjuvant effect, we next performed genome-wide mRNA profile analysis of draining LNs from mice treated with either PBS or CLD and sacrificed before (0h) or 8h after VSV infection (Figure S5 and Table S1). This analysis showed that a relatively small number of genes were regulated by CLD treatment (Figure 3A, Figure S6-10 and Table S1). Most of the down-regulated genes upon CLD treatment were macrophage-specific (Figure S6-8 and Table S1), in line with the observation that CLD treatment depleted LN macrophages (Figure S4). CLD treatment also down-regulated type I interferon (IFN-I) genes induced upon VSV infection (Figure S7,8 and Table S1). This is consistent with previously published data showing that LN subcapsular sinus macrophages are a major source of IFN-I during this infection and their depletion inhibits IFN-I gene expression (Iannacone et al., 2010). Reduced IFN-I gene expression could have also resulted from plasmacytoid DC (pDC) dysfunction, as these cells represent an additional source of IFN-I in this system and they are not depleted upon BP treatment (Iannacone et al., 2010). The CLD-dependent reduced expression of the pDC-specific marker Siglec-H (Blasius et al., 2006) is also suggestive of pDC dysfunction (Figure S6,7 and Table S1). It is noteworthy, however, that depletion of pDCs (which reduced IFN-I by ~50% (Iannacone et al., 2010)) failed to increase Ab responses (Figure S11A), suggesting that CLD adjuvant activity does not rely on pDCs. We also noted that some natural killer (NK) cell-specific transcripts were reduced upon CLD treatment (Figure S6,7 and Table S1). Depleting these cells did not alter the capacity of CLD to increase nAb responses upon VSV infection (Figure S11B), arguing against a role for NK cells in the adjuvant activity of BPs.
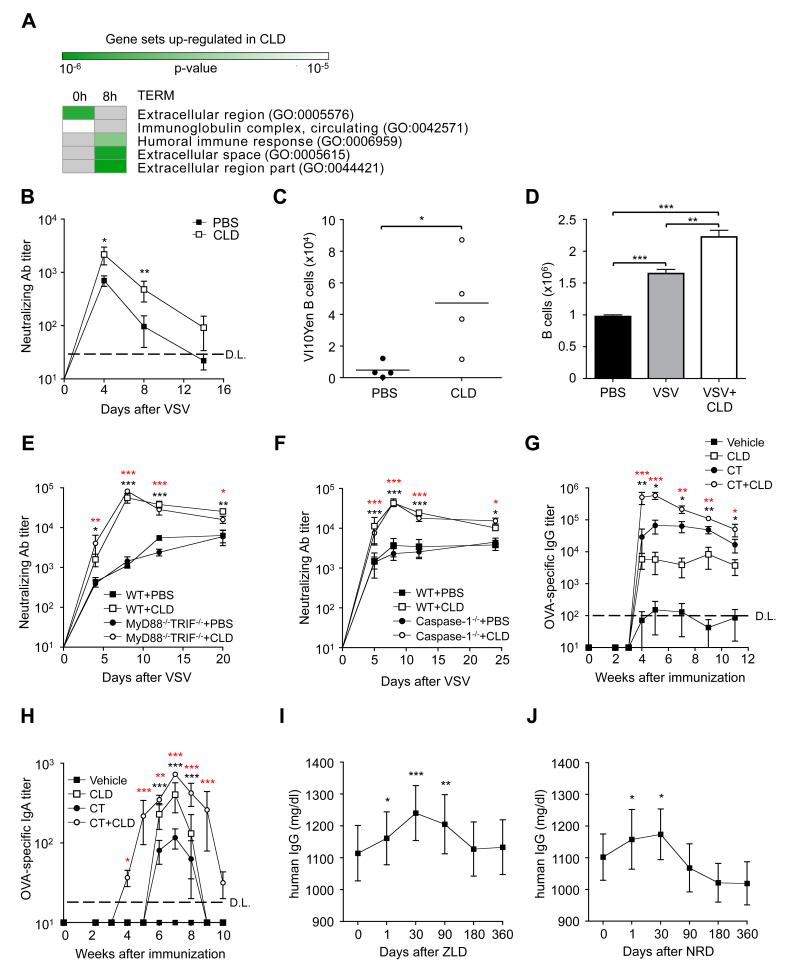
Figure 3. Bisphosphonates Directly Target B Cells and Enhance B Cell Expansion and Antibody Production upon Antigen Encounter Independently of Toll-Like Receptor Signaling or the Inflammasome.
(a) Gene enrichment analysis of clodronate-dependent genes from microarray measurements of whole lymph node. Shown are the Gene Ontology (GO) terms (rows) statistically enriched among the 156 genes with at least a 2-fold increase in expression at 0 and 8 hours (columns) after VSV infection in clodronate (CLD)-treated mice compared to control (PBS). Enriched GO term categories include BP: biological process, and CC: cellular component. Green: p value < 10-6; white: p value < 10-4; grey: no enrichment. See Methods for details. (b) VSV nAb titers in the serum of DHLMP2A mice that were intravenously transferred with PBS- or CLD-treated VI10Yen B cells 24 hours prior to footpad VSV infection. n = 6 per group; results are representative of 2 independent experiments. (c) Quantification of the total number of VI10Yen B cells recovered from the spleen of the mice described in Figure 3B, 8 days after VSV. (d) Quantification of the total number of cells recovered 48h upon in vitro culture of purified VI10Yen B cells with PBS, VSV or 10nM clodronate and VSV. (e) VSV nAb titers in the serum of C57BL/6 (WT) or MyD88−/−/Trif -/- mice that were footpad injected with PBS or CLD immediately prior to VSV infection in the same footpad. n = 4 per group; black asterisks, WT + PBS versus WT + CLD; red asterisks MyD88−/−/Trif−/−+PBS versus MyD88−/−/Trif−/−+CLD; results are representative of 3 independent experiments. (f) VSV nAb titers in the serum of C57BL/6 (WT) or Caspase-1−/− mice that were injected in the footpad with PBS or CLD immediately prior to VSV infection in the same footpad. n = 10 per group; black asterisks, WT+PBS versus WT+CLD; red asterisks Caspase-1−/−+PBS versus Caspase-1−/−+CLD; results are representative of 3 independent experiments. (g) Ovalbumin (OVA)-specific IgG titers in the serum of C57BL/6 mice that were immunized orally with OVA alone (vehicle), OVA + CLD (CLD), OVA + cholera toxin (CT) or OVA + cholera toxin + CLD (CT + CLD). n = 5 per group; results are representative of 2 independent experiments. (h) OVA-specific IgA titers in the feces of the mice described in Figure 3G. (i) Total IgG in the sera of patients immediately prior to or at the indicated time points after a single intravenous infusion of zoledronate (ZLD). n = 11; see Suppl. Table 2 for patients characteristics. (j) Total IgG in the sera of patients immediately prior to or at the indicated time points after a single intravenous infusion of neridronate (NRD). n = 9; see Suppl. Table 2 for patients characteristics.
***: P < 0.001; **: P < 0.01; *: P < 0.05; D.L.: detection limit.
See also Figure S5-11.
Next, we examined genes that were up-regulated by CLD treatment. CLD induced several neutrophil-specific transcripts (Figure 3A, Figure S9,10 and Table S1), in keeping with the ability of BPs to recruit and activate these cells (Norton et al., 2011; 2012). Data previously described in Figure 2C, however, ruled out a role for neutrophils in BP adjuvant activity. Of note, CLD up-regulated a number of B cell-specific transcripts and gene sets associated with B cell function (Figure 3A, Figure S9,10 and Table S1). When compared to controls, these B cell-related transcripts were higher in CLD-treated animals before (Figure S9) and after (Figure S10) VSV infection, suggesting that CLD might directly impact B cells. To directly test this possibility, we isolated B cells from VI10Yen mice (which express a VSV-specific B cell receptor), and treated them with PBS or CLD in vitro prior to adoptive transfer into DHLMP2A mice (which are devoid of surface-expressed and secreted Abs). In this experimental setup - where Abs can be produced only by the transferred B cells – VSV infection led to nAb titers that were higher in mice that received CLD-treated, rather than PBS-treated, VI10Yen B cells (Figure 3B); this correlated with the capacity of CLD to promote Ag-specific B cell expansion both in vivo and in vitro (Figure 3C,D). Taken together, these results indicate that CLD directly targets B cells to enhance their expansion and their Ab production upon Ag encounter. Although the molecular pathways by which CLD and other BPs impact B cell function remain to be determined, it is worth noting that CLD adjuvanticity did not involve the two known signaling pathways through which most other adjuvants are thought to act (Coffman et al., 2010) (the Toll-like receptors and the inflammasome pathway, which rely on Myd88/TRIF and Caspase 1 for signaling, respectively). Indeed, VSV nAb titers in CLD-treated Myd88−/− /TRIF−/− and Caspase 1−/− mice were indistinguishable from those of similarly treated WT mice (Figure 3E,F).
Since BPs are often taken orally by patients, we next sought to evaluate whether these small molecules act as adjuvants upon oral immunization in mice. As expected (Pierre et al., 1992), the oral administration of OVA alone failed to induce a detectable OVA-specific Ab response (Figure 3G,H). By contrast, the concomitant oral administration of OVA and CLD induced OVA-specific serum IgG and fecal IgA responses (Figure 3G,H). Furthermore, CLD was able to increase the OVA-specific Ab response afforded when OVA was administered together with the mucosal adjuvant cholera toxin (Pierre et al., 1992) (Figure 3G,H). These data indicate that CLD is an effective oral adjuvant capable of increasing both systemic and mucosal Ab responses.
Finally, we tested whether the nitrogen-containing BPs ZLD and NRD increase Ab responses in a cohort of patients affected by osteoporosis or Paget’s disease of bone (Table S2). Notably, a transient but significant increase in total serum IgG levels was detected for up to 3 months upon a single intravenous infusion of BPs (Figure 3I,J). Although the impact of BPs on Ag-specific Ab responses remains to be determined, this observation suggests that these drugs enhance B cell responses in humans.
In conclusion, these data establish BPs as novel adjuvants that target B cells to increase humoral immune responses. Translational evaluation of these compounds in humans should be relatively straightforward because BPs, unlike all other adjuvants, are already widely used in the clinic as stand-alone drugs with excellent safety profiles. Thus, BPs could be readily combined with both existing and newly developed vaccines, especially in settings where immune responses to a vaccine alone are weak or where Ags are in short supply.
Experimental Procedures
Mice
C57BL/6 and Balb/c mice were purchased from Charles River or The Jackson Laboratory. CD11c-GFP-DTR (B6.FVB-Tg(Itgax-DTR/EGFP)57Lan/J) mice were purchased from The Jackson Laboratory. MHC-II−/− (B6.129-H2-Ab1tm1Doi/DoiOrl) mice were provided by P. Dellabona (San Raffaele Scientific Institute). TCRδ−/− (B6.129P2-Tcrdtm1Mom/J) mice were provided by W. Havran (The Scripps Research Institute). MyD88−/−/TrifLps2/Lps2 mice were provided by B. Beutler (The Scripps Research Institute). Caspase-1−/− (B6.129S2-Casp1tm1sesh) and CD40L−/− (B6.129S2-Cd40tm1Imx/J) mice were obtained through the Swiss Immunological Mutant Mouse Repository (Zurich, Switzerland). DHLMP2A mice (Casola et al., 2004) were originally provided by K. Rajewsky (Harvard Medical School) and bred 5 generations against C57BL/6 mice. VI10Yen mice (Hangartner et al., 2003) were originally provided by R. M. Zinkernagel and H. Hengartner. Bone marrow chimeras were generated by irradiation of C57BL/6 mice with 1300 rad in split doses and reconstitution with CD11c-DTR-GFP bone marrow; mice were allowed to reconstitute for at least 8 weeks prior to use. Mice were housed under specific pathogen-free conditions and used at 6-8 weeks of age. All experimental animal procedures were approved by the Institutional Animal Committees of San Raffaele Scientific Institute, Harvard Medical School and The Scripps Research Institute.
Bisphosphonate treatment
Clodronate (CLD), Alendronate (ALD), Pamidronate (PMD) and Etidronate (ETD) were obtained from Sigma, dissolved in phosphate-buffered saline (PBS) and injected in the footpad in a volume of 20μl. The injected amount for each bisphosphonate was 2 mg, unless otherwise indicated. In the experiments described in Figure 1A, 30 μl of clodronate liposomes (CLD-Lip) or PBS liposomes (PBS-Lip, both provided by N. Van Rooijen) were injected in the footpad 7 days before antigen administration.
Infections and immunizations
Mice were infected with 104 plaque-forming units (pfu) of VSV serotype Indiana (VSV-IND), 104 pfu of VSV serotype New Jersey (VSV-NJ), or 106 pfu of VSVeGFP (Iannacone et al., 2010). Alternatively, mice were immunized with 106 pfu equivalent of PFA-inactivated VSV (Bachmann et al., 1993). Viruses were dissolved in 20μl of PBS and injected into the footpad. In survival experiments, mice were challenged with 3.5 × 108 pfu of VSV in the left footpad 3 weeks after PFA-VSV immunization in the right footpad. Other antigens used for footpad immunization experiments included: 4-hydroxy-3-nitrophenyl-chicken gamma globulin (NP-CGG, 50μg/dose, Biosearch Technologies), ovalbulmin (OVA, 100μg/dose, Sigma), Engerix-B ® (20 μl/dose given twice two weeks apart, GlaxoSmithKline). Hemoagglutinin/neuroaminidase subunits from the human influenza virus A/NewCaledonia/20/99 (H1N1, 3 μg/dose, provided by P. Dellabona) was injected in the anterior tibialis muscle.
For experiments involving oral immunization, mice were given vehicle or 10 mg of CLD 24 hours prior to receiving 1 mg of OVA with or without 10 μg of cholera toxin. All treatments were administered via gavage in 0.2 ml of antacid buffer (3% sodium bicarbonate) at day 0, 7 and 21. For the measurement of mucosal IgA, fresh fecal pellets were collected and immediately frozen at -20°C. Before analysis, fecal pellets were weighed and dissolved in protease inhibitor solution at a concentration of 200 μg/ml, as described (Lauterslager et al., 2001).
All infectious work was performed in designated BL-2 workspaces, in accordance with institutional guidelines. Mice were retro-orbitally bled at the indicated time points for antigen-specific antibodies, measured by endpoint ELISA (Galli et al., 2007; Tonti et al., 2012), by VSV neutralization assay (Iannacone et al., 2010) or with an HBsAb detection kit (Diagnostic Bioprobes), in accordance with the manufacturer’s instructions. For determination of VSV-neutralizing IgG titers, sera were incubated with equal volumes of 0.1 M 2-mercaptoethanol in PBS for 1 h at room temperature before dilution (Scott and Gershon, 1970).
Confocal microscopy
Confocal microscopy analysis of popliteal LNs was performed as described (Moseman et al., 2012). Sections were stained with eFluor450-conjugated anti-B220 (RA3-6B2, eBioscience), FITC-conjugated anti-CD169 (3D6.112, AbD Serotec), Alexa Fluor 647-conjugated anti-TCRβ (H57-597, BioLegend).
In vivo depletion of neutrophils, DCs, LN macrophages, pDCs and NK cells
Neutrophils were depleted by intraperitoneal injection of 100μg of anti-Gr-1 antibodies (RB6-8C5, BioXCell) every 2 days, beginning 3 days prior to immunization.
DCs were depleted from CD11c-GFP-DTR → C57BL/6 bone marrow chimeric mice by injecting diphtheria toxin (DT, Sigma) in the footpad (50ng) and in the peritoneum (500ng) every 2 days, starting the day prior to immunization.
Popliteal LN macrophages were depleted by injecting 2 mg of carrageenan (Sigma) in the footpad every 2 days, starting 5 days prior to immunization. Alternatively, LN macrophages were depleted by footpad injection of 1 mg of dextran sulfate (Sigma) 5 days prior to immunization. Serum from CD11c-DTR-GFP mice (described in Figure S3 of ref. (Iannacone et al., 2010)), where LN macrophages are depleted by a single footpad injection of DT (4ng) 6 days before VSV infection, were assessed for VSV neutralizing Ab titers 4 days after VSV infection.
Serum from the mice described in Figure 3J and Figure S8b of ref. (Iannacone et al., 2010) (where pDCs were depleted by intravenous injection of 500 μg of anti-PDCA-1 depleting antibody (JF05-1C2.4.1; Miltenyi Biotec) 24 hours prior to VSV infection) were assessed for VSV neutralizing Ab titers 7 days after VSV infection.
NK cells were depleted by intravenous injection of anti-Asialo-GM1 Abs (Cedarlane) every 2 days beginning 3 days prior to VSV infection, as described (Tonti et al., 2012).
All depletions were confirmed by flow cytometry and/or confocal microscopy.
Tissue digestion and flow cytometry
Single-cell suspensions of LNs, spleens and footpads were generated as described (Iannacone et al., 2010). All flow cytometry analyses were performed in FACS buffer containing PBS with 2 mM EDTA and 2% FBS on a FACS CANTO (BD Pharmingen) and analysed with FlowJo software (Treestar Inc.). Antibodies used included FITC-conjugated anti-CD169 (3D6.112, AbD Serotec), Alexa Fluor 488- and PE-Cy7-conjugated anti-B220 (RA3-6B2, BioLegend), PE-conjugated anti-TCRδ (GL3, BioLegend) and anti-CD86 (GL-1, BioLegend), PE-Cy7-conjugated anti-CD45 (30-F11, BioLegend), PB-conjugated anti-Ly6C (HK1.4, BioLegend), APC-Cy7-conjugated anti-F4/80 (BM8, BioLegend), Alexa Fluor 647-conjugated anti-CD8 (53-6.7, BioLegend), biotinylated anti-CD11b (M1/70, BioLegend), PE-conjugated anti-CD138 (281-2, BioLegend), PE-Cy7-conjugated anti-CD3 (145-2C11, BioLegend), PE-Cy5-conjugated anti-NK1.1 (PK136, BioLegend), PerCP-Cy5.5-conjugated anti-Gr1 (RB6-8C5), APC-conjugated anti-CD40 (HM40-3, BD Pharmingen), Alexa Fluor 488-conjugated anti-IgG (A-11023, Invitrogen), PE-Cy7-conjugated anti-CD11c (N418, eBioscience), eFluor450-conjugated anti-CD4 (RM4-5, eBioscience). The anti-idiotypic antibody 35.61 for detection of the VI10 BCR in VI10Yen mice (Hangartner et al., 2003) was produced from hybridoma supernatants in accordance with standard methods.
Isolation and activation of DCs and B cells
Splenic DCs were isolated from C57BL/6 mice that were injected subcutaneously 11-14 days earlier with 4×106 Flt3-ligand-secreting B16 tumor cells, as described (Cavanagh et al., 2005). CD11c+ DCs were purified by positive selection with anti-CD11c-microbeads (>95% CD11c+; Miltenyi) and cultured in the presence of 1 μg/ml LPS (E.coli 0.26:B6; Sigma) or with the indicated concentrations of CLD for 48 h prior to flow cytometry analysis.
Naive B cells from spleens of VI10Yen mice were negatively selected by magnetic isolation with CD43 beads (Miltenyi), as described (Junt et al., 2007). The purity was 98% as determined by CD19 surface staining. B cells (106 cells/ml) were cultured in RPMI 1640 media (Lonza) supplemented with 10% FBS, 50 mM 2-ME (Sigma), 10 mM HEPES (Lonza), 2 mM L-glutamine (Lonza), 100U/ml Pen/Strep with or without 10 nM CLD. After 4 hours, VI10Yen B cells were harvested, washed 3 times and either intravenously injected into DH LMP2A recipients (107 cells/mouse) 24 hours prior to footpad VSV infection (Figure 3B,C) or cultured for 48 h in the presence of VSV at a MOI of 1 (Figure 3D).
Gene expression profiling
Whole lymph nodes were lysed in 600 μL of QIAzol (Qiagen) reagent using the TissueLyser II. Total RNA was extracted following the miRNeasy kit’s procedure (Qiagen), and sample quality was tested on a 2100 Bioanalyzer (Agilent). RNA was reverse transcribed with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). Real time quantitative PCR reactions were performed on the LightCycler 480 system (Roche) with FastStart Universal SYBR Green Master Mix (Roche). Every reaction was run in triplicate and GAPDH levels were used as an endogenous control for normalization. For oligonucleotide microarray hybridization, 1 μg of RNA were labeled, fragmented, and hybridized to an Affymetrix Mouse Genome 430A 2.0 Array. After scanning, the expression value for each gene was calculated with RMA (Robust Multi-Array) normalization using R. The average intensity difference values were normalized across the sample set. Probe sets that were absent in all samples according to Affymetrix flags were removed. All values below 40 were floored to 40. Only probe sets that changed in two biological duplicates by 2 fold or more were analyzed further in this study: (1) regulated genes were hierarchically clustered using the software Gene-E (http://www.broadinstitute.org/cancer/software/GENE-E/), and (2) functional enrichment of GO terms (biological processes, BP; cellular components, CC) and KEGG pathway enrichment analysis were performed using DAVID (http://david.abcc.ncifcrf.gov) (Huang et al., 2009).
Patients
Total serum IgG levels were assessed in 20 patients affected by osteoporosis or Paget’s disease of bone (age range 52-78 years, see Suppl. Table 2) that received a single intravenous injection of 5 mg zoledronate (Aclasta, Novartis Pharmaceuticals) or 200 mg neridronate (Nerixia, Abiogen Pharma). Serum samples were obtained before and 1, 30, 90, 180 and 360 days after BP infusion. IgG levels were quantified from stored samples by an immunodiffusion technique using NOR Partigen immunoplates purchased from Siemens (Siemens Healthcare Diagnostics S.r.l., Milan, Italy).
Statistical analyses
Results are expressed as mean ± SEM. All statistical analyses were performed in Prism (GraphPad Software). Means between two groups were compared with two-tailed t test. Means among three or more groups were compared with one-way or two-way analysis of variance with Bonferroni’s post-test. Kaplan-Meier survival curves were compared with the Log-rank (Mantel-Cox) test.
Supplementary Material
Highlights.
Bisphosphonates are a novel class of adjuvants that boost humoral immune responses
Bisphosphonate activity is independent of DCs, TLR signaling or the inflammasome
Bisphosphonates directly target B cells to enhance antibody production
Acknowledgements
We thank T. Cataudella, A. Fiocchi, B. Fiore, D. Covarello, M. Mainetti for technical support; R. Serra for secretarial assistance; P. Dellabona (San Raffaele Scientific Institute) for providing MHC-II−/− mice; W. Havran (The Scripps Research Institute) for providing TCRδ−/− mice; B. Beutler (The Scripps Research Institute) for providing MyD88−/−/TrifLps2/Lps2 mice; S. Whelan (Harvard Medical School) for providing VSV and VSVeGFP; N. van Rooijen for providing clodronate liposomes; F. Benvenuti (International Centre for Genetic Engineering and Biotechnology) for providing Flt3-ligand-secreting B16 tumor cells; S. Cenci, C. Scielzo, P. Ghia and the members of the Iannacone, Guidotti and von Andrian laboratories for helpful discussions; F.V. Chisari, A. Mondino, P. Dellabona, R. Pardi and Z.M. Ruggeri for critical reading of the manuscript. This work was supported by ERC grants 281648 (to M.I.) and 250219 (to L.G.G); NIH grants AI40696 (to L.G.G) and AI078897, AI069259 (to U.H.v.A.); and a Career Development Award from the Giovanni Armenise-Harvard Foundation (to M.I.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession numbers
The microarray data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE50403 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE50403).
Author contribution: E.T., L.G.G., U.H.v.A. and M.I. designed the study; E.T., N.J.d.O, G.G., E.A.M., P.D.L., A.A., S.S., M.D.G., L.S. and M.I. performed experiments; E.T. and M.I. analyzed the data; N.C. performed and analyzed the microarray data; L.G. performed measurements in human subjects; G.S. gave conceptual advice; E.T., L.G.G., U.H.v.A. and M.I. wrote the manuscript.
References
- Bachmann MF, Kündig TM, Kalberer CP, Hengartner H, Zinkernagel RM. Formalin inactivation of vesicular stomatitis virus impairs T-cell- but not T-help-independent B-cell responses. J Virol. 1993;67:3917–3922. doi: 10.1128/jvi.67.7.3917-3922.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–2476. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, Rajewsky K. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- Cavanagh LL, Bonasio R, Mazo IB, Halin C, Cheng G, van der Velden AWM, Cariappa A, Chase C, Russell P, Starnbach MN, Koni PA, Pillai S, Weninger W, Andrian, von UH. Activation of bone marrow-resident memory T cells by circulating, antigen-bearing dendritic cells. Nat Immunol. 2005;6:1029–1037. doi: 10.1038/ni1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favus MJ. Bisphosphonates for Osteoporosis. N Engl J Med. 2010;363:2027–2035. doi: 10.1056/NEJMct1004903. [DOI] [PubMed] [Google Scholar]
- Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, Maione D, Volpini G, Finco O, Nuti S, Tavarini S, Dellabona P, Rappuoli R, Casorati G, Abrignani S. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci USA. 2007;104:3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez SF, Lukacs-Kornek V, Kuligowski MP, Pitcher LA, Degn SE, Kim Y-A, Cloninger MJ, Martinez-Pomares L, Gordon S, Turley SJ, Carroll MC. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat Immunol. 2010 doi: 10.1038/ni.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangartner L, Senn BM, Ledermann B, Kalinke U, Seiler P, Bucher E, Zellweger RM, Fink K, Odermatt B, Bürki K, Zinkernagel RM, Hengartner H. Antiviral immune responses in gene-targeted mice expressing the immunoglobulin heavy chain of virus-neutralizing antibodies. Proc Natl Acad Sci USA. 2003;100:12883–12888. doi: 10.1073/pnas.2135542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006;6:231–243. doi: 10.1038/nri1783. [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Iannacone M, Moseman EA, Tonti E, Bosurgi L, Junt T, Henrickson SE, Whelan SP, Guidotti LG, Andrian, von UH. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature. 2010;465:1079–1083. doi: 10.1038/nature09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, Clarke AR, Hooper ML, Farr A, Tonegawa S. T cell receptor δ gene mutant mice: Independent generation of αβ T cells and programmed rearrangements of γδ TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, van Rooijen N, Mempel TR, Whelan SP, Andrian, von UH. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Lauterslager TG, Florack DE, van der Wal TJ, Molthoff JW, Langeveld JP, Bosch D, Boersma WJ, Hilgers LA. Oral immunisation of naive and primed animals with transgenic potato tubers expressing LT-B. Vaccine. 2001;19:2749–2755. doi: 10.1016/s0264-410x(00)00513-2. [DOI] [PubMed] [Google Scholar]
- Maeda T, Wakasawa T, Shima Y, Tsuboi I, Aizawa S, Tamai I. Induction of proliferation and augmented cytotoxicity of gammadelta T lymphocytes by bisphosphonate clodronate. Blood. 2001;97:2917–2918. doi: 10.1182/blood.v97.9.2917. [DOI] [PubMed] [Google Scholar]
- Moseman EA, Iannacone M, Bosurgi L, Tonti E, Chevrier N, Tumanov A, Fu Y-X, Hacohen N, Andrian, von UH. B Cell Maintenance of Subcapsular Sinus Macrophages Protects against a Fatal Viral Infection Independent of Adaptive Immunity. Immunity. 2012;36:415–426. doi: 10.1016/j.immuni.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton JT, Hayashi T, Crain B, Cho JS, Miller LS, Corr M, Carson DA. Cutting Edge: Nitrogen Bisphosphonate-Induced Inflammation Is Dependent upon Mast Cells and IL-1. The Journal of Immunology. 2012 doi: 10.4049/jimmunol.1100830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton JT, Hayashi T, Crain B, Corr M, Carson DA. Role of IL-1 receptor-associated kinase-M (IRAK-M) in priming of immune and inflammatory responses by nitrogen bisphosphonates. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1107899108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao W, Wen L, Smith AL, Gulbranson-Judge A, Zheng B, Kelsoe G, MacLennan ICM, Owen MJ, Hayday AC. γδ T cell help of B cells is induced by repeated parasitic infection, in the absence of other T cells. Immunity. 1996;6:1317–1325. doi: 10.1016/s0960-9822(02)70718-5. [DOI] [PubMed] [Google Scholar]
- Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- Pierre P, Denis O, Bazin H, Mbongolo Mbella E, Vaerman JP. Modulation of oral tolerance to ovalbumin by cholera toxin and its B subunit. Eur J Immunol. 1992;22:3179–3182. doi: 10.1002/eji.1830221223. [DOI] [PubMed] [Google Scholar]
- Qi H, Egen JG, Huang AYC, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- Renshaw BR, Fanslow WC, Armitage RJ, Campbell KA, Liggitt D, Wright B, Davison BL, Maliszewski CR. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandella E, Fink K, Junt T, Senn B, Lattmann E, Forster R, Hengartner H, Ludewig B. Dendritic Cell-Independent B Cell Activation During Acute Virus Infection: A Role for Early CCR7-Driven B-T Helper Cell Collaboration. The Journal of Immunology. 2007;178:1468. doi: 10.4049/jimmunol.178.3.1468. [DOI] [PubMed] [Google Scholar]
- Scott DW, Gershon RK. Determination of total and merecaptothanol-resistant antibody in the same serum sample. Clin Exp Immunol. 1970;6:313–316. [PMC free article] [PubMed] [Google Scholar]
- Thompson K, Roelofs AJ, Jauhiainen M, Mönkkönen H, Mönkkönen J, Rogers MJ. Activation of γδ T cells by bisphosphonates. Adv. Exp. Med. Biol. 2010;658:11–20. doi: 10.1007/978-1-4419-1050-9_2. [DOI] [PubMed] [Google Scholar]
- Tonti E, Fedeli M, Napolitano A, Iannacone M, Andrian, von UH, Guidotti LG, Abrignani S, Casorati G, Dellabona P. Follicular Helper NKT Cells Induce Limited B Cell Responses and Germinal Center Formation in the Absence of CD4+ T Cell Help. The Journal of Immunology. 2012;188:3217–3222. doi: 10.4049/jimmunol.1103501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Wen L, Pao W, Wong FS, Peng Q, Craft J, Zheng B, Kelsoe G, Dianda L, Owen MJ, Hayday AC. Germinal center formation, immunoglobulin class switching, and autoantibody production driven by “non alpha/beta” T cells. J Exp Med. 1996;183:2271–2282. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.