Abstract
Serotonin (5-HT) modulates the hypothalamic-pituitary-adrenal (HPA) axis response to stress. We examined the effect of chronic restraint stress (CRS; 20 min/day) as compared to control (CTRL) conditions for 14 days, on: 1) restraint-induced ACTH and corticosterone (CORT) secretion in rats pretreated with vehicle or SB-656104 (a 5-HT7 receptor antagonist); 2) 5-HT7 receptor-like immunoreactivity (5-HT7-LI) and protein in the hypothalamic paraventricular nucleus (PVN) and adrenal glands (AG); 3) baseline levels of 5-HT and 5-hydroxyindolacetic acid (5-HIAA), and 5-HIAA/5-HT ratio in PVN and AG; and 4) 5-HT-like immunoreactivity (5-HT-LI) in AG and tryptophan hydroxylase (TPH) protein in PVN and AG. On day 15, animals were subdivided into Treatment and No treatment groups. Treatment animals received an i.p. injection of vehicle or SB-656104; No Treatment animals received no injection. Sixty min later, Treatment animals were either decapitated with no further stress (0 min) or submitted to acute restraint (10, 30, 60 or 120 min); hormone serum levels were measured. No Treatment animals were employed for the rest of measurements. CRS decreased body weight gain and increased adrenal weight. In CTRL animals, acute restraint increased ACTH and CORT secretion in a time of restraint-dependent manner; both responses were inhibited by SB-656104. Exposure to CRS abolished ACTH but magnified CORT responses to restraint as compared to CTRL conditions; SB-656104 had no effect on ACTH levels but significantly inhibited sensitized CORT responses. In CTRL animals, 5-HT7-LI was detected in magnocellular and parvocellular subdivisions of PVN and sparsely in adrenal cortex. Exposure to CRS decreased 5-HT7-LI and protein in the PVN, but increased 5-HT7-LI in the adrenal cortex and protein in whole AG. Higher 5-HT and 5-HIAA levels were detected in PVN and AG from CRS animals but 5-HIAA/5-HT ratio increased in AG only. Finally, whereas 5-HT-LI was sparsely observed in the adrenal cortex of CTRL animals, it strongly increased in the adrenal cortex of CRS animals. No TPH protein was detected in AG from both animal groups. Results suggest that CRS promotes endocrine disruption involving decreased ACTH and sensitized CORT responses to acute restraint. This phenomenon may be associated with increased function and expression of 5-HT7 receptors as well as 5-HT turnover in AG.
Keywords: ACTH, adrenal glands, chronic restraint stress, corticosterone, hypothalamic paraventricular nucleus, 5-HT7 receptors
1. Introduction
It is established that the hypothalamic-pituitary-adrenal (HPA) axis is modulated by the serotonin (5-hydroxytryptamine; 5-HT) system via projections from the dorsal and median raphe nuclei that reach the parvocellular and corticotrophin releasing factor (CRF)-producing neurons in the hypothalamic paraventricular nucleus (PVN) (Larsen et al., 1996; Liposits et al., 1987; Sawchenko et al., 1983). Thus, serotonergic inputs stimulate CRF (Holmes et al., 1982; Jones et al., 1976) and ACTH secretion (Kageyama et al., 1998) via 5-HT1A and 5-HT2A/2C receptors (Jorgensen et al., 1999, 2002; Pan and Gilbert, 1992). In addition, pharmacological evidence has suggested the involvement of 5-HT7 receptors in HPA axis modulation as the 5-HT1A/5-HT7 receptor agonist, 8-OH-DPAT, elicits neuroendocrine responses (Jorgensen et al., 1999; Vicentic et al., 1998) which, in the case of corticosterone (CORT) secretion, are not completely inhibited by the 5-HT1A receptor antagonist, WAY-100635 (Vicentic et al., 1998). Furthermore, expression of 5-HT7 receptors has been documented in human hypothalamus (Varnas et al., 2004), rat PVN (Bonaventure et al., 2004; Muneoka and Takigawa, 2003; Neumaier et al., 2001) and rat adrenal glands (AG) (Contesse et al., 1999).
Hyperactivity of the HPA axis involving high circulating levels of glucocorticoids is a common pathophysiological feature of stress-related diseases, including major depression (Gold et al., 1986; Plotsky et al., 1995), however, the mechanisms underlying this phenomenon remain unknown. In this regard, chronic stress has been proposed as one of the key factors predisposing to endocrine disruption and the development of stress-associated mood disorders (Joëls et al., 2003; Mizoguchi et al., 2008; Ulrich-Lai et al., 2006). Interestingly, there is extensive evidence that chronic stress and glucocorticoids regulate brain 5-HT receptors (Bambico et al., 2009; Czyrak et al., 2002; McKittrick et al., 1995). This raises the possibility that 5-HT receptors involved in HPA axis modulation could be regulated by chronic stress thus resulting in endocrine dysfunction. Clinical studies have indeed demonstrated that HPA axis overdrive and the accompanying psychopathological outcomes in stress-related disorders are improved by drugs that target the brain 5-HT system, such as 5-HT reuptake inhibitors (Blier et al., 2001; Inder et al., 2001). Moreover, the early findings that antidepressant drugs display high affinity for 5-HT7 receptors and that, under chronic treatment, such drugs induce downregulation of these receptors (Sleight et al., 1995; Mullins et al. 1999) have recently drawn the attention as to their potential role in mood disorders (see Hedlund, 2009 for review). Actually, pharmacological blockade of 5-HT7 receptors has recently been demonstrated to produce a fast acting antidepressant-like action in rats (Mnie-Filali et al., 2011). Since dysfunction of the HPA axis might precede psychopathological symptoms in stress-related diseases (see Pariante and Lightman, 2008), we have hypothesized -based upon the above suggestive evidence- that an abnormal function of 5-HT7 receptors in the HPA axis, possibly as a result of chronic stress, could lead to HPA axis dysregulation. The aim of the present study was therefore to investigate the role of 5-HT7 receptors in modulating the HPA system under control and chronic stress conditions in rats.
2. Material and methods
2.1. Animals
Male Wistar rats (200-220 g of body weight; n=240) from our own Institutional inbred facilities were used. Animals (five per cage) were kept at constant temperature (22±1°C) and humidity (50-55%) under a 12:12 h: light/dark cycle (lights on 06:00-18:00 h) with food and water available ad libitum. All the procedures and protocols complied with Federal regulations and were performed in accordance with the National Institutes of Health guide for the Care and Use of Laboratory animals (NIH Publications No. 8023, revised 1978). Efforts were made to minimize unnecessary suffering of the animals and their number.
2.2. Chronic restraint stress
One group of animals was subjected to chronic restraint stress (CRS) and served as the experimental group. Treatment with CRS consisted of 20 min daily sessions of restraint (between 8:00 and 11:00 h) for 14 days, which was induced by placing the animals in well-ventilated adjustable-length cylindrical plexiglass tubes (6.5 cm in internal diameter and 20 cm in length). Another group of animals, which served as the control (CTRL) group, was daily taken from the tail for 20 sec and returned to their home cages for 14 days. Since animals submitted to CRS were placed in the tubes by taking them from the tail, tail lifting was performed in CTRL animals in an attempt to exclude the effect of tail lifting itself on potential chronic restraint-induced changes in the HPA system.
2.3. Experimental design
Animals submitted to CTRL and CRS conditions were assigned to different groups and protocols for performance of neuroendocrine, immunohistochemistry, Western blot and HPLC experiments (Fig. 1). For neuroendocrine assays, in which the animals were exposed to a final restraint stress session (see below), CTRL (n=60) and CRS (n=60) animal groups were each subdivided into vehicle (n=30) and antagonist (n=30) treatment (Treatment groups) (Fig. 1). Other groups of CTRL and CRS animals, which received no vehicle or antagonist treatment (No Treatment groups) and were not exposed to further restraint, were used for determination of body, AG and thymus weights (n=8 per group), as well as 5-HT7 receptor immunohistochemistry (n=6 per group), Western blot for 5-HT7 receptors (n=4 per group, see below) and HPLC assays (n=10 per group) in the PVN and AG (Fig. 1). Additional immunohistochemistry and Western blot assays were subsequently performed in order to determine, respectively, the distribution of 5-HT-like immunoreactivity (5-HT-LI) in the AG (n=4 per group) and tryptophan hydroxylase (TPH) protein content in the PVN and AG (n=3 per group, see below) (Fig. 1).
Figure 1.
Design of the study. Two groups of rats were submitted to either daily 20 sec tail-lifting (TL) and home cage control (CTRL) or chronic restraint stress (CRS) conditions for 14 days. Each group was subdivided into Treatment and No Treatment groups. Treatment animals, which received an i.p. injection of vehicle (VEH; 1 ml/kg) or the selective 5-HT7 receptor antagonist, SB-656104 (SB; 1 mg/kg), were employed exclusively for neuroendocrine assays. No Treatment animals received no injection and were used for determination of body and dry adrenal gland (AG) and thymus weights, immunohistochemistry, Western blot and HPLC assays. Experiments were initiated/completed one day after the end of CTRL and CRS treatments (i.e. on day 15). a Each assay was performed in pulled samples of the hypothalamic paraventricular nucleus (PVN) and AG taken from 8 and 3 animals, respectively. Since the 5-HT7 receptor assay was performed in quadruplicate (n=4), a total of 32 and 12 animals per group were employed, respectively. b Protein samples from the same tissues for 5-HT7 receptor assays were used. The tryptophan hydroxylase (TPH) assays in PVN and AG were performed in triplicate (n=3 per group) for a total of 24 and 9 animals per group, respectively.
2.4. Determination of body, adrenal gland and thymus weights
Body weight was recorded before and after CTRL and CRS treatments. One day after these treatments (i.e. on day 15), animals were euthanized and AG from both sides and thymus were removed and then allowed to dry for determination of dry organ weights. To exclude possible differences in the weight of AG and thymus derived from normal changes in body weight, particularly in chronically stressed animals in which these parameters are reportedly altered (Cruz et al., 2012; Zelena et al., 2004), organ weights were expressed in milligrams per 100 g of the average body weight.
2.5. Neuroendocrine studies
One day after the end of CTRL and CRS periods (i.e. on day 15), animals assigned to the Treatment groups received an i.p. injection of vehicle (polyethylene glycol, dimethyl sulfoxide and 0.9% saline, 1:1:1.6; 1 ml/kg) or the selective 5-HT7 receptor antagonist, SB-656104 (1 mg/kg, prepared at 1 mg/ml in the above vehicle). Sixty min later, animals of each group (i.e. CTRL and CRS) were subdivided into five subgroups for exposure to either no further stress (0 min) or an acute restraint stress session for 10, 30, 60 or 120 min, respectively (see Fig. 1). At the end of each restraint period, animals were immediately decapitated and trunk blood was collected and centrifuged; animals submitted to no acute stress (i.e. 0 min groups) were decapitated before the beginning of restraint sessions. Serum was stored at −80°C until used; concentrations of ACTH and CORT were measured using the Milliplex Multi-Analyte Profiling method (Linco Research by Millipore, Billerica, MA, USA) and a [125I] radioimmunoassay kit (Diagnostic Systems Laboratories, Inc., Texas, USA), respectively. The minimum detection concentration for each assay was 3.2 pg/ml and 2.7 ng/ml, with an intra-assay precision of <7% and 2.6%, respectively.
2.6. Immunohistochemistry assays
One day after CTRL and CRS treatments (i.e. on day 15) animals were anesthetized with sodium pentobarbital (30 mg/kg, i.p.) and perfused via the ascending aorta with 0.1M phosphate-buffered saline (PBS; pH 7.4) followed by 0.1M phosphate-buffered 4% paraformaldehyde (pH 7.4). The brains and AG were removed, post-fixed for 24 h at 4°C, and stored in 0.01M PBS containing 30% sucrose at 4°C until used. Serial coronal sections of the brain across the PVN (30 μm) and AG (35 μm) were obtained using a freezing microtome and collected in culture wells containing 0.01M PBS with 0.2% sodium azide and stored at 4°C. On the day of the assay, sections were incubated in 0.1M PBS for 10 min, washed three times with 0.1M PBS and incubated in 1% hydrogen peroxide with 5% bovine serum albumin (BSA) for 2 h at room temperature. Next, sections were incubated with a primary antibody raised in goat against 5-HT7 receptors (1:200; Santa Cruz Biotechnology Inc., cat. SC-19160, Santa Cruz, CA, USA) for 60 h at 4°C. In subsequent experiments using additional CTRL and CRS animal groups, AG sections were incubated with a rabbit primary antibody against 5-HT (1:1000; ImmunoStar, cat. 20079, Hudson, WI, USA) for 24 h at 4°C. After three washes, sections were incubated with biotin conjugated donkey anti-goat (1:400; Jackson ImmunoResearch, cat. 705-065-003, West Grove, PA, USA) or goat anti-rabbit (1:250; Vector Laboratories, cat. BA-1000, Burlingame, CA, USA) secondary antibodies for 2 h at room temperature. After several washes in 0.1M PBS, sections were incubated with the avidine-biotin-peroxidase complex (VECTASTAIN Elite ABC kit, 1:200; Vector Laboratories, Burlingame, CA, USA) in 0.1M PBS with 0.03% triton X-100 for 2 h at room temperature. After thorough washing with 0.1M PBS, the presence of peroxidase was revealed with 3,3′-diaminobenzidine (DAB Substrate Kit; Vector Laboratories, Burlingame, CA, USA) in Tris-buffer for 5-10 min at room temperature; the reaction product was intensified with nickel. Sections were then washed, mounted on gelatin-coated slides, cover-slipped and photographed using a digital camera.
2.7. Western blot analyses
The effect of CRS on the amount of protein for 5-HT7 receptors and TPH in the PVN and AG was determined. Thus, one day after CTRL and CRS periods, No Treatment animals of both groups were euthanized by decapitation, and brains and AG were removed and frozen with dry ice and liquid nitrogen, respectively. Next, 300 μm-thick coronal sections of the brain were obtained using a motorized vibroslice and the PVN was micro-dissected using hollowed needles as previously described (Palkovits, 1983). Samples of both tissues were homogenized in 10 ml of RIPA solution (Tris HCL-RIPA, 500 μm EDTA, protease inhibitors and 10% sodium dodecyl sulfate; SDS) using a Polytron (Kinematica AG, Luzern, Switzerland). For 5-HT7 receptor assays, samples of the PVN were taken from 8 animals and pulled together for one determination (n=1 per group) whereas 3 AG were randomly selected from the above group of 8 animals and pulled together for one determination (n=1 per group). Since the assays were performed in quadruplicate (n=4), a total of 32 and 12 animals per group, respectively, were employed. For TPH assays, protein samples of the same collected tissues for 5-HT7 receptor determinations were used but, due to the limited amount of sample available, the assays were performed in triplicate (n=3) for a total of 24 and 9 animals per group, respectively. The AG homogenates were centrifuged at 4500 rpm for 15 min at 4°C and then the PVN samples and AG supernatants were ultracentrifuged at 20,000 g. The supernatants were removed, and the pellets were re-suspended in a mixture containing 40 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 10% glycerol, 1% Triton X-100, 5% sodium deoxycholate and 0.2% SDS at pH 7.5, with a complete protease inhibitor cocktail (20 μl/ml; Hoffmann-La Roche, Konzern-Hauptsitz, Switzerland), and phenylmethanesulfonyl fluoride (2 μl/ml; Sigma-Aldrich Corp., St Louis, MO, USA). Finally, samples were sonicated and 5 l aliquots were used for protein determination using the Bradford method (Bradford, 1976). Identical amounts of protein (35 g) of PVN and AG samples from CTRL and CRS rats were loaded per line and separated by SDS-PAGE. Next, the gels were transferred on to polyvinyldiene diflouride membranes (Amersham Pharmacia Biotech, Little Chalfont, UK), which were blocked with 5% non-fat milk for 2 h at room temperature and then incubated with primary antibodies raised in rabbit against either 5-HT7 receptors (1:1000; Novus Biologicals, cat. NB100-56352, Littleton, CO, USA), TPH (1:5000; mouse, rat and human TPH1 specific reactivity; Santa Cruz Biotech., cat. SC-30079, Santa Cruz, CA, USA) or the control protein, the â-1 subunit of Na+/K+-ATPase (1:10,000; Millipore, cat. 05-382, Billerica, MA, USA), overnight at 4°C. This incubation period was followed by a 2 h incubation period with a goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:10,000; Invitrogen, cat. G21234, Carlsbad, CA, USA). The peroxidase activity was revealed by incubating the membranes in a chemoluminescent reaction (General Electric Healthcare Biosciences, Pittsburgh, PA, USA) and then by exposing them on to photographic films (Hyperfilm, Amersham). To estimate the amounts of 5-HT7 receptor and TPH proteins relative to Na+/K+-ATPase β-1 subunit for each sample, a densitometric analysis was performed using the Kodak Molecular Imaging Software (Carestream Health, Rochester, New York, USA). Since the Na+/K+-ATPase β-1 is a subunit insert into the membrane and thus considered a membrane marker (Kinne-Saffran and Kinne, 1989), it was decided to use it as a control protein.
2.8. Measurement of 5-HT and 5-HIAA levels by HPLC
Baseline levels of 5-HT and 5-HIAA in the PVN and AG were determined using HPLC with electrochemical detection (Bioanalytical Systems, Inc., West Lafayette, IN, USA), as previously described (Kempf and Mandel, 1981). Briefly, one day after CTRL and CRS periods (i.e. on day 15), PVN and AG samples were obtained; the adrenals were thoroughly washed in isotonic saline to remove remaining blood. Samples were then sonicated in cold 0.1N perchloric acid and centrifuged; supernatants were collected, filtered (0.22 μm nylon filters, Millipore) and stored at −70°C; protein concentrations were determined using the Bradford method (Bradford, 1976). Samples were then separated using a C-18 reverse-phase column (5 m in diameter, 3.9 mm in width and 150 mm in length; polarity 2.1 × 50 mm, Waters Co, Milford, MA, USA) and eluted with a mobile phase containing 0.1 M monochloroacetic acid, 0.65 mM ethylenediaminetetracetic acid, 0.9 mM sodium octyl sulphate and 4.5% acetonitrile at pH 4.5; flow rate was set at 0.35 ml/min with a pressure of 4500 psi. Indolamines were quantified using the Millennium 32 Software (Waters Co., Milford, MA, USA) and their concentrations calculated from the chromatographic peak heights based on external standards.
2.9. Data presentation and statistical evaluation
All data in the text and Figures are presented as the mean ± SEM of at least three determinations. Differences in body and relative organ weights, relative tissue content of 5-HT7 receptor and TPH protein between CTRL and CRS rats, 5-HT and 5-HIAA concentrations, and 5-HIAA/5-HT ratio, were all compared by paired or unpaired t tests as appropriate. The effect of chronic stress exposure and pharmacological treatment (i.e. vehicle or SB-656104) on acute restraint-induced ACTH and CORT responses was analyzed by a three-way ANOVA, with chronic stress and pharmacological treatment as between-subject independent factors. The ANOVA test was followed by a Student-Newman-Keuls post-hoc test to determine differences. In all cases, the level of significance was set at P ≤ 0.05. Statistical analyses were performed using SigmaStat 3.5 (Systat Software Inc., Chicago, IL, USA).
3. RESULTS
3.1. Effect of chronic stress on somatometric parameters
The physiological parameters reportedly altered subsequent to chronic stress exposure were determined. Animals that received CRS exhibited a significantly lower total body weight as compared to CTRL rats (t(14) = 9.028, P < 0.001), and this was reflected as a 72% loss of body weight gain as compared to body weight gain in CTRL animals (t(14) = 6.972, P < 0.001) (Table 1). Relative AG weight (t(30) = −3.847, P < 0.001), but not relative thymus weight (P = 0.276), was 25% higher in CRS rats as compared to the corresponding parameter in CTRL animals (Table 1).
Table 1.
Effect of chronic restraint stress (CRS), as compared to control tail-lifting and home cage conditions (CTRL), on total body weight before (initial) and after treatments (final), on body weight gain (Gain) and on relative adrenal gland (AG) and thymus weights expressed as mg of tissue per 100 g of body weight. One day after the end of CTRL and CRS treatments, AG from both sides and thymus were removed, allowed to dry, and weighted. Values are the mean ± the standard error of the mean of 8 observations.
| Body weight (g) |
Relative organ weight (mg/100 g body weight) |
||||
|---|---|---|---|---|---|
| Initial | Final | Gain | AG | Thymus | |
| CTRL | 226.4±1.3 | 293.8±5 a | 67.4±5 | 3.03±0.08 | 44.2±2.2 |
| CRS | 227.3± 4 | 245.8±2 b | 18.5±4.8 c | 3.8±0.18 d | 40.3±2.6 |
P < 0.001 vs Initial CTRL;
P < 0.01 vs Initial CRS;
P < 0.001 vs Gain in CTRL animals;
P < 0.001 vs CTRL AG
3.2. Restraint-induced ACTH and CORT secretion in control and chronically stressed animals: effect of 5-HT7 receptor blockade
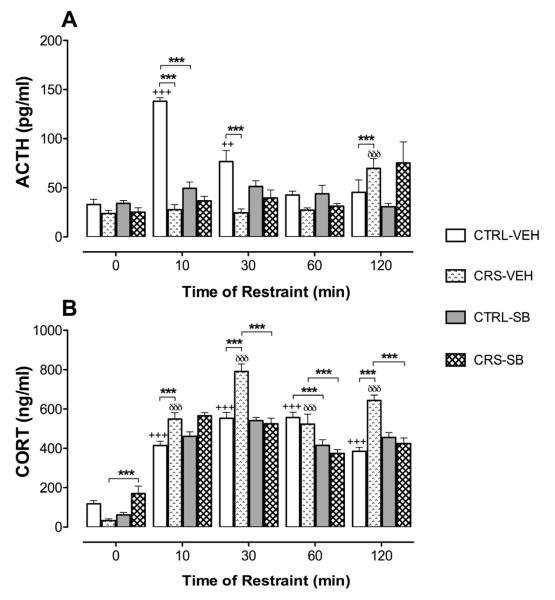
Figure 2 shows ACTH (A) and CORT (B) responses induced by acute restraint in CTRL and CRS rats that received vehicle or the 5-HT7 receptor antagonist, SB-656104, previous to the acute restraint sessions. Thus, exposure to acute restraint resulted in higher ACTH concentrations as compared to baseline levels (i.e. 0 min), with restraint duration having a significant effect on ACTH levels (F4,100 = 11.314, P < 0.001). On the other hand, a significant interaction between restraint duration and animal groups was revealed (F4,100= 17.902, P < 0.001) thus indicating that CTRL and CRS animals respond with a different pattern of restraint-induced ACTH release. Accordingly, Student Newman-Keuls post hoc tests showed that, whereas ACTH concentrations were significantly higher after 10 and 30 min of restraint in vehicle-treated CTRL animals, significantly higher ACTH levels were detected only after 120 min of restraint in the corresponding CRS animal group (P < 0.001; Fig. 2A). Then, exposure to CRS induced significant blunting of ACTH responses at 10 and 30 min of restraint, concomitant with magnified ACTH responses after 120 min of restraint, as compared to the corresponding responses in animals submitted to CTRL conditions (Fig. 2A).
Figure 2.
The secretion of ACTH (pg/ml; panel A) and corticosterone (CORT; ng/ml; panel B) on resting conditions (0 min) and after acute restraint stress (10, 30, 60 or 120 min) in rats previously submitted to daily 20 sec tail-lifting and home cage control conditions (CTRL) or chronic restraint stress (CRS; 20 min/day) for 14 days. On day 15, animals from both groups received an i.p. injection of vehicle (VEH; 1 ml/kg) or the 5-HT7 receptor antagonist, SB-656104 (SB; 1 mg/kg) 60 min before decapitation (0 min) or exposure to acute restraint stress sessions. Bars represent the mean and vertical lines denote the standard error of the mean of 6 observations. +++ P < 0.001 vs baseline (0 min); ++ P < 0.01 vs baseline (0 min); äää P < 0.001 vs baseline (0 min); *** P < 0.001.
Acute restraint on the other hand increased CORT serum concentrations as compared to baseline levels (i.e. 0 min), with restraint duration having a significant effect on CORT levels (F4,100 = 203.011, P < 0.001). In addition, a significant interaction between restraint duration and animal groups was revealed (F4,100= 7.056, P < 0.001). In close resemblance to ACTH results, these data indicate that CTRL and CRS animals respond with a different pattern of restraint-induced CORT secretion. Then, Student Newman-Keuls post hoc tests revealed that, whereas CORT levels measured after 10, 30, 60 and 120 min of restraint were significantly higher than the baseline levels (i.e. 0 min) in both vehicle-treated animal groups (P < 0.001; Fig. 2B), the CORT levels achieved after 10, 30 and 120 min of restraint in CRS were significantly higher than those measured in CTRL animals (Fig. 2B), thus indicating that CRS exposure magnified restraint-induced CORT responses. It should be highlighted that a closely similar pattern of restraint-induced hormonal responses was observed in a group of CTRL and CRS animals that received no injection before acute restraint exposure (data not shown). This suggests that injection (i.e. puncture-induced stress) and/or vehicle per se had no remarkable effects on restraint-induced ACTH and CORT responses in the Treatment animal groups.
As far as the effect of SB-656104 on restraint-induced ACTH responses is concerned, a significant interaction between restraint duration, animal groups and pharmacological treatment was detected (F4,100 = 6.324, P < 0.001). Furthermore, Student Newman-Keuls post hoc tests showed that SB-656104 pre-treatment produced significant blockade of the ACTH response after 10 min of restraint in CTRL rats but had no effect on restraint-induced ACTH levels in CRS animals (Fig. 2A). Regarding the effect of the antagonist on CORT secretion, a significant interaction between restraint duration, animal groups and pharmacological treatment was detected (F4,100 = 13.468, P < 0.001). Student Newman-Keuls post hoc tests revealed that this interaction was driven by the detection of significantly lower CORT levels after 60 min of restraint in antagonist-treated CTRL animals (P < 0.001; Fig. 2B), and significantly lower CORT levels after 30, 60 and 120 min of restraint in antagonist-treated CRS animals with respect to the corresponding vehicle-treated groups (P < 0.001; Fig. 2B). Unexpectedly, baseline (i.e. 0 min) CORT levels were significantly higher in antagonist- as compared to vehicle-treated CRS animals (Fig. 2B).
3.3. Effect of chronic restraint stress on 5-HT7 receptor-like immunoreactivity and protein in the PVN and adrenal glands
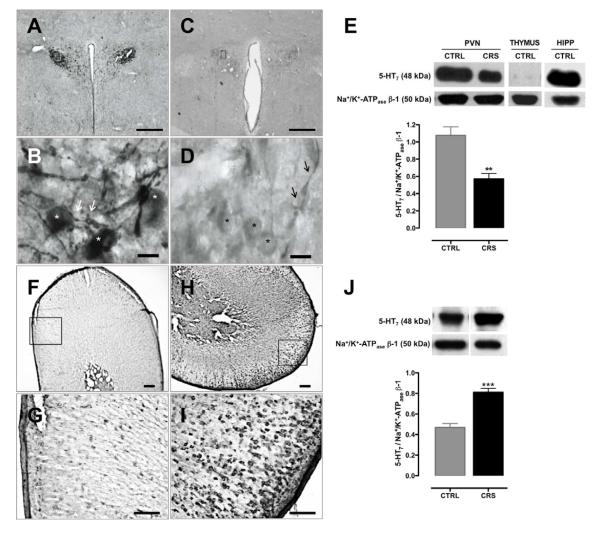
Intense 5-HT7-LI was observed in neurons of the magnocellular and parvocellular subdivisions of the PVN from CTRL rats (Fig. 3A and 3B), with immunostaining predominately localized in cell bodies and dendrites (stars and arrows, respectively, in Fig. 3B). In contrast, 5-HT7-LI in the PVN from CRS animals was remarkably less intense (Fig. 3C and 3D) although immunopositive signals also predominated in cell bodies and dendrites (stars and arrows, respectively, in Fig. 3D). On the other hand, 5-HT7-LI in AG markedly differed from that in the PVN with regard to animal groups. Thus, in AG from CTRL rats, modest 5-HT7-LI was sparsely located in the cortex including areas corresponding to the zona glomerulosa and zona fasciculata (Figs. 3F and 3G). Nevertheless, in AG from CRS animals, 5-HT7-LI was clearly stronger and much more abundant in cortical areas that comprised predominantly the zona glomerulosa and the outer zona fasciculata; immunostaining was observed as largely disseminated clusters (Figs. 3H and 3I). Consistent with the above observations, Western blot analyses revealed that CRS rats exhibited a 47% lower relative content of 5-HT7 receptor protein in the PVN (t(8) = 4.269, P < 0.01; Fig. 3E) concomitant with a 72% higher relative protein content in whole adrenals (t(10) = −6.399, P < 0.001; Fig. 3J) as compared to the corresponding tissues in CTRL animals.
Figure 3.
Effect of chronic restraint stress (CRS; 20 min/day) as compared to daily 20 sec tail-lifting and home cage control (CTRL) conditions for 14 days on 5-HT7 receptor-like immunoreactivity (5-HT7-LI) and protein in the hypothalamic paraventricular nucleus (PVN) and adrenal glands (AG). In the PVN from CTRL animals, neurons of magnocellular and parvocellular subdivisions exhibited intense 5-HT7-LI (A and B) that was predominantly located within the cell bodies (stars in B); numerous dendrites of magnocellular neurons also showed 5-HT7-LI (arrows in B). In the PVN from CRS rats 5-HT7-LI decreased drastically (C and D) although the remaining signal persisted in the cell bodies and dendrites (stars and arrows in D, respectively). In the AG, whereas 5-HT7-LI was scarcely and sparsely observed in the cortex of CTRL tissues (F and G), strong 5-HT7-LI was observed in the outer cortical areas of CRS tissues H and I). In agreement with the above, the content of the 5-HT7 receptor protein with respect to Na+/K+ ATPase β-1, as determined by Western blot analysis, significantly decreased in the PVN of CRS as compared to CTRL animals (E) whereas an opposite change occurred in whole adrenal samples with CRS inducing a significant increase of 5-HT7 receptor protein as compared to CTRL tissues (J). Negative (thymus; E) and positive (hippocampus; HIPP; E) controls for expression of the 5-HT7 receptor protein were carried out. ** P < 0.01 vs CTRL; *** P < 0.001 vs CTRL. Bars in A and C = 500 ìm; bars in B and D = 20 ìm; bars in F and H = 200 ìm; bars in G and I = 100 ìm. Sections of PVN are from −1.85 to −1.90 mm relative to Bregma.
3.4. Effect of chronic stress on 5-HT availability in the PVN and adrenal glands
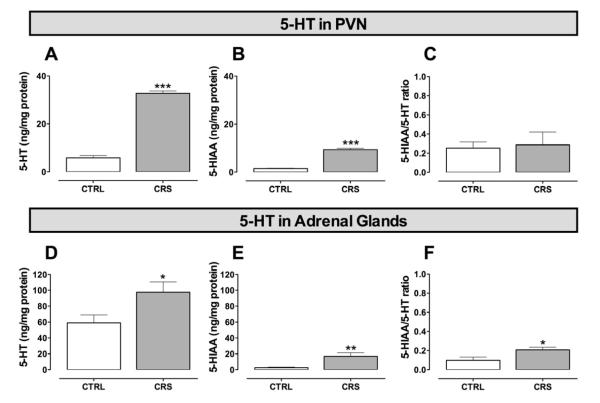
Consistent with a potential involvement of the serotonergic system in chronic stress-induced changes in the HPA axis, exposure to CRS led to four-fold higher concentrations of 5-HT (t(10)= −19.206, P < 0.001; Fig. 4A) and 5-HIAA (t(10) = −13.280, P < 0.001; Fig. 4B) in the PVN with respect to those measured in the corresponding tissue samples from CTRL animals. Nonetheless, the 5-HIAA/5-HT ratio was similar in both animal groups thus suggesting that 5-HT turnover in the PVN was unaffected by CRS (Fig. 4C). By contrast, in the AG, whereas significantly greater concentrations of 5-HT (t(19) = −2.310, P < 0.05; Fig. 4D) and 5-HIAA (t(11) = −4.441, P < 0.001; Fig. 4E) were also detected in CRS animals, the 5-HIAA/5-HT ratio was significantly higher (t(10) = −2.367, P < 0.05; Fig. 4F) thereby implying that 5-HT turnover was increased in CRS adrenals with respect to that in CTRL tissues. It should be pointed out that, concentrations of 5-HIAA below the detection limit (probably because of degradation) occurred in 5 AG samples from CTRL animals so in these cases the 5-HIAA/5-HT ratio could not be calculated.
Figure 4.
The impact of chronic restraint stress (CRS), as compared to daily 20 sec tail-lifting and home cage control (CTRL) conditions, on baseline levels of 5-HT and 5-HIAA, and the 5-HIAA/5-HT ratio in the hypothalamic paraventricular nucleus (PVN; A, B and C, respectively) and adrenal glands (D, E and F, respectively). In the PVN, the CRS paradigm induced a marked increase of 5-HT and 5-HIAA levels (A and B, respectively) with no change in the 5-HIAA/5-HT ratio (C) with respect to CTRL tissues. In adrenal glands, however, whereas CRS similarly induced a significant increase of 5-HT and 5-HIAA concentrations (D and E, respectively), it evoked a significant increase in the 5-HIAA/5-HT ratio (F) with respect to CTRL tissues thus suggesting increased 5-HT turnover. Bars represent the mean and vertical lines denote the standard error of the mean of 10 observations. * P < 0.05 vs CTRL; ** P < 0.01 vs CTRL; *** P < 0.001 vs CTRL.
3.5. Immunoreactivity to 5-HT in adrenal glands and TPH protein in the PVN and adrenals: impact of chronic stress
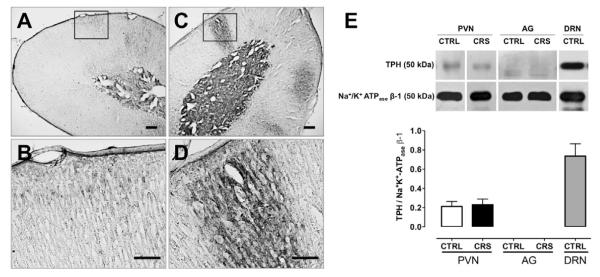
On the basis of the above findings suggesting higher expression of 5-HT7 receptors in adrenocortical cells, along with a higher serotonergic drive in AG as a result of CRS exposure, it was decided to further evaluate the role of the 5-HT system in this tissue by performing additional 5-HT immunohistochemistry assays. In addition, in an attempt to determine whether the increased availability of 5-HT in AG could be due to increased synthesis, a Western blot assay for TPH was performed; available protein samples of PVN were also included in the assay. Indeed, the increase of 5-HT (Fig. 4D) and 5-HIAA levels (Fig. 4E) as well as 5-HT turnover (Fig. 4F) in whole AG does not allow to know the level at which such increase has taken place (e.g. the cortex and/or the medulla or both) and, consequently, the potential significance of such change in terms of hormone secretion mechanisms. Thus, whereas 5-HT-LI was thinly observed in the adrenal cortex of CTRL rats (Figs. 5A and 5B), large clusters of 5-HT-LI were clearly noticed in adrenocortical areas corresponding predominantly to the zona fasciculata of CRS adrenals (Figs. 5C and 5D). Consistent also with the higher content of 5-HT in AG from CRS animals (Fig. 4D), a more intense immunostaining was evident in the adrenal medulla of CRS (Fig. 5C) as compared to CTRL animals (Fig. 5A). That the increase of 5-HT-LI in the adrenal cortex of CRS rats (and the concomitant increase of 5-HT turnover) might be accounted for an increased 5-HT synthesis, could not be demonstrated as no TPH protein, relative to the ubiquitous Na+/K+-ATPase β-1 subunit, was detected in AG (Fig. 5E); the amount of TPH protein in the PVN did not change as a result of CRS (Fig. 5E). A control assay in dorsal raphe nucleus samples from CTRL animals confirmed the efficacy of the Western blot assay (Fig. 5E).
Figure 5.
Effect of chronic restraint stress (CRS; 20 min/day) as compared to daily 20 sec tail-lifting and home cage control (CTRL) conditions for 14 days on 5-HT-like immunoreactivity (5-HT-LI) in adrenal glands (AG) and tryptophan hydroxylase (TPH) protein in the hypothalamic paraventricular nucleus (PVN) and adrenal glands (AG). In AG from CTRL animals, scarce 5-HT-LI was sparsely observed in outer cortical areas. Interestingly, in AG from CRS animals, 5-HT-LI strongly increased in the form of large clusters clearly and predominantly observed in outer cortical areas corresponding to the zona glomerulosa and the zona fasciculata. No TPH protein was detected in whole adrenals from both animals groups (E). Immunoblotting of TPH occurred in the PVN but this was unaffected by CRS (E). A positive control for TPH protein detection employed dorsal raphe nucleus (DRN) samples from CTRL animals (E). Bars in A and C = 200 ìm; bars in B and D = 100ìm.
4. DISCUSSION
4.1 General
The major finding of the present study was that restraint-induced CORT secretion in rats subjected to CRS for two weeks is sensitized relative to that in CTRL animals through a mechanism involving activation of 5-HT7 receptors and, apparently, dissociation from ACTH secretion. Since 5-HT7-LI and protein in the PVN decreased, whereas 5-HT7-LI in the adrenal cortex and 5-HT7 receptor protein in whole AG increased as a result of CRS treatment, the hypothesis that sensitized CORT responses to restraint under conditions of chronic stress may be mediated by a mechanism involving adrenal 5-HT7 receptors is supported. This notion seems strengthened by the finding that baseline 5-HT turnover in AG from CRS rats increased with respect to that in CTRL tissues, whereas no change in 5-HT turnover occurred in the PVN. In addition, 5-HT-LI in the adrenal cortex, but not TPH protein in AG, was clearly magnified as a result of the CRS experience. Apart from the implications discussed below, the present data suggest an interesting association between chronic stress and 5-HT7 receptors in the development of HPA axis overdrive, which is a frequently observed phenomenon in stress-related diseases.
4.2 Chronic restraint as a model of chronic stress
It is established that the HPA system, through secretion of glucocorticoids, regulates a number of functions including food intake, metabolic rate and immune function (Kim et al., 2006; Marti et al., 1994), and that high circulating levels of glucocorticoids during prolonged periods of stress lead to AG hypertrophy and hyperplasia (Ulrich-Lai et al., 2006), decreased rate of body weight gain (Harris et al., 1998) and thymus involution (Choi et al., 2008), all of which are used as reliable physiologic markers of chronic stress. In the present study, AG hypertrophy and decreased body weight gain, but not thymus involution, were detected in rats submitted to CRS. The absence of thymus involution might suggest that longer-lasting daily stress sessions or periods of chronic stress are required to induce suppression of immune function and measurable decreases in thymus weight, as suggested by previous observations using restraint (Zelena et al., 2004) and other stressors (Figueiredo et al., 2003). Then, despite the absence of thymus involution, the above changes are consistent with the development of chronic stress in CRS rats.
4.3 Effect of chronic stress on baseline and acute restraint-induced hormonal levels
The absence of a significant change in baseline ACTH levels between CTRL and CRS animals resembles previous findings in which no elevated basal levels of ACTH were detected after chronic stress (Tauchi et al., 2008; Malkesman et al., 2006). In the case of baseline CORT levels, a trend towards a decline was noticed but the difference was not significant (P = 0.027; Fig. 2B). Decreases in baseline CORT levels in animal models of chronic stress have been less frequently observed. Thus, whereas baseline CORT levels reportedly increase and return to control values during the course of chronic stress (Silberman et al., 2002; Zardooz et al., 2006), with a trend towards a decrease in some cases (see Azpiroz et al., 1999; Mizoguchi et al., 2008), significant declines in baseline CORT levels have also been noticed (Malkesman et al., 2006; Sanchez et al., 1998). The reason for the above discrepancies is unknown but it is likely to reflect the large variability of chronic stress protocols and the use of different animal strains.
Acute restraint stress has consistently been found to increase plasma ACTH and CORT in rats (Barnum et al., 2007; Girotti et al., 2006; Gray et al., 2010). The present experiments in CTRL animals showed that exposure to acute restraint for 10, 30, 60 and 120 min increased serum levels of ACTH and CORT in a time of restraint-dependent manner with peaks at 10 and 30 min of restraint, respectively. Remarkably, a radically different situation was observed in animals that had received CRS for two weeks, as ACTH responses after 10 and 30 min of restraint were virtually abolished, whereas those after 120 min of restraint increased significantly (see Fig. 2A). In addition, the magnitude of restraint-induced CORT responses was significantly higher (Fig. 2B) thus raising the possibility that glucocorticoid secretion might have become dissociated from ACTH in CRS animals (see below). The sensitized restraint-induced CORT secretion in CRS rats is in striking contrast to earlier observations showing that a previous comparable experience of chronic stress (20-60 min/day) for periods between 5 and 14 consecutive days leads to adaptation of the HPA axis response to further stress exposure, which translates into a decrease in (and/or a faster recovery of) the CORT response to homotypic stressors, including restraint (Barnum et al., 2007; Bhatnagar et al., 2002; Girotti et al., 2006; Gray et al., 2010; Ma and Lightman, 1998; Ma et al., 1999; Stamp and Herbert, 1999), noise (Armario et al., 1986), water immersion (De Boer et al., 1990), cage confinement (Barnum et al., 2007), forced swimming (Dal-Zotto et al., 2000), low intensity subcutaneous electric shocks (Pitman et al., 1990) and immobilization (García et al., 2000). The reason for the discrepancy between the present data and the above studies, particularly with those using a 14 day chronic restraint stress treatment (Ma and Lightman, 1998; Ma et al., 1999; Stamp and Herbert, 1999), is not known but it is likely to relate to the animal strain as Wistar rats were used here whereas Sprague-Dawley rats were employed in the above studies. There is evidence in the literature that different rat strains may exhibit variable susceptibility not only to the same stressor but also to identical chronic stress treatments (see Dhabhar et al., 1997).
In contrast to the aforementioned observations, repeated exposure to more severe homotypic stressors may not only fail to induce habituation of the CORT response to stress re-exposure but instead promote sensitization. For example, in rats exposed to repeated tailshocks (15 min/day), CORT levels in plasma collected on days 2, 3, 4 and 5 were higher than the hormone levels following tailshock on day 1, whereas increased CORT levels occurred in rats restrained from the tail (15 min/day) for 4 and 5 days as compared to day 1 (Orr et al., 1990). Likewise, chronic predator stress (i.e. cat exposure) in rats (60 min/day) for 6 and 13 days led to increased CORT secretion (as measured 60, 120 and 180 min after stress exposure) with respect to that in animals stressed for 1 day (Figueiredo et al., 2003). Similarly, plasma CORT responses in rats chronically given high (but not low) intensity electric shocks failed to habituate and instead, exhibited increased responses indicative of sensitization over the course of 1 week (Pitman et al., 1990). Thus, in addition to sensitization of CORT responses to novel stress exposure subsequent to a period of chronic homotypic stress (Chen et al., 2008; Harris et al., 2004; Ma et al., 1999), the above studies suggest that sensitization of CORT secretion to acute homotypic stressors may also occur after periods of chronic stress, as we actually observed in the present study.
Further to the strength of the stressor as a factor to promote, under repeated exposure, sensitization of the CORT response to acute stress, the length of the chronic stress period also seems relevant. For instance, exposure of naturally aggressive mice to 4 and 8 sessions of social defeat led to blunted (i.e. habituated) CORT responses evoked by the presence of an aggressive intruder in a partition cage (i.e. anticipatory stress), whereas exaggerated CORT responses were detected in mice that had been submitted to 12 sessions of social defeat thereby suggesting the activation of a ‘switch’ that occurred sometime between the 8th and the 12th session, leading to disruption of HPA axis adaptation to chronic stress and high secretion of CORT (Keeney et al., 2006). This and the aforementioned studies are in agreement with the present results showing disruption of HPA axis habituation and increased restraint-induced CORT secretion subsequent to CRS. We believe the findings of the present study provide insight into the nature of the ‘switch’ referred to above, the occurrence of which seems to be key for endocrine disruption to happen (see below).
4.4 Possible dissociation of restraint-induced CORT responses from ACTH secretion in chronically restrained animals
This is the first work showing a virtually abolished acute stress-induced ACTH response subsequent to chronic stress exposure. Earlier studies have demonstrated that stress-induced ACTH secretion decreases in animals subjected to chronic stress for 5 and 8 days, and that stress-induced CORT responses do not decrease in the proportion that ACTH responses do (Bhatnagar et al., 2002; Girotti et al., 2006). Similarly, a recent study in rats submitted to chronic restraint (30 min/day for 10 days and 180 min/day for 5 days) reported a strongly decreased ACTH response to acute restraint along with modest CORT habituation (Gray et al., 2010). Since a longer-lasting (i.e. 14 days) chronic stress paradigm was employed in the present study, the possibility arises that the extent to which acute stress-induced ACTH and CORT responses become dissociated may be dependent upon the duration of the chronic stress experience. In support of this notion, an increasing number of preclinical and clinical studies report dissociation of ACTH and cortisol levels in critical illness, inflammation and mental disorders including chronic depression (see Parker et al., 2003 for review). In fact, there is evidence that ACTH responses to stress do not always parallel CORT responses (Bhatnagar et al., 2004) and that ACTH-independent activation of adrenal CORT secretion through neural or humoral factors may occur (Bornstein and Chrousos, 1999; Ehrhart-Bornstein et al., 1998). These factors may also act in concert to modulate adrenocortical sensitivity to ACTH and be stressor specific, and include for instance sympathetic (e.g. splanchnic nerve) activity (Jasper and Engeland, 1997), and locally produced peptides and other factors that may have a paracrine function, including 5-HT (Ehrhart-Bornstein et al., 1998). Further studies are required to elucidate the nature of the changes in ACTH secretion that develop in longer-lasting chronic stress paradigms, such as the one employed here.
4.5 Involvement of 5-HT7 receptors in restraint-induced endocrine responses: impact of chronic stress
Limited evidence has suggested a role of 5-HT7 receptors in HPA axis function. Thus, the non-selective 5-HT7 receptor agonist, 5-carboxamidotryptamine (5-CT), was reported to increase CORT secretion in rats, and this response was blocked by the 5-HT2/5-HT7 receptor antagonist, methysergide (Jorgensen et al., 1999). On the other hand, an immunohistochemical study reported expression of 5-HT7 receptors in the periventricular area of the PVN of developing rat brains (Muneoka and Takigawa, 2003), whereas a 5-HT7 receptor-directed antisense oligonucleotide had no effect on baseline CORT levels in rats (Clemett et al., 1998). The present work notwithstanding is the first to show a functional role of 5-HT7 receptors in stress-induced HPA axis activation, as demonstrated by the ability of the selective 5-HT7 receptor antagonist, SB-656104 (Forbes et al., 2002), to inhibit significantly restraint-induced ACTH and CORT secretion in CTRL rats. Since magnified restraint-induced CORT responses in CRS animals were restored to CTRL values by SB-656104, they are most likely accounted for increased function and/or expression of 5-HT7 receptors in the HPA axis. This of course does not exclude the involvement of other mechanisms in CRS animals (e.g. 5-HT1A, 5-HT2A and 5-HT2C receptors; Jorgensen et al., 2002), the elucidation of which requires further investigation.
Admittedly, given the use of a systemic route (i.p.) for SB-656104 administration, we cannot be certain about the specific site at which the antagonist could have exerted its blocking effects on restraint-induced hormonal responses. Based upon our immunohistochemical observations, we hypothesize that the drug target is most likely located in the adrenal glands (see below), but this requires confirmation experimentally (e.g. with site-specific pharmacology). Regarding the antagonist dose employed (1 mg/kg), this is half of the ED50 oral dose of 2 mg/kg previously determined to antagonize 5-CT-induced hypothermia in guinea pigs with maximal inhibition achieved 2 hours after administration (Forbes et al., 2002). The reason for selecting this lower dose was to prevent potential interactions of SB-656104 with other 5-HT receptor mechanisms as the compound exhibits 12-, 31-, 45- and 91-fold higher affinity for 5-HT7 receptors (pKi = 8.7) with respect to 5-HT1D (pKi = 7.6), 5-HT2A (pKi = 7.2), 5-HT2B (pKi = 7.04), and 5-HT5A receptors (pKi = 6.74), respectively (Forbes et al., 2002). Since the time gap after administration (i.e. 60 min) is well within the reported half-life of SB-656104 (between 1.4 and 2 h; Thomas et al., 2003 and Forbes et al., 2002, respectively) the inhibitory effects of the drug are most likely accounted for antagonism of 5-HT7 receptors. Finally, the reason why an increase in baseline CORT secretion occurred in CRS animals that had received SB-656104 remains to be determined.
4.6 Effect of chronic stress on 5-HT7 receptor-like immunoreactivity and protein in the hypothalamic paraventricular nucleus and adrenal glands
This is the first study showing 5-HT7-LI in various aspects of the PVN, including the posterior magnocellular and medial parvocellular subdivisions, and in the AG. The presence of 5-HT7 receptor protein in both tissues was confirmed by Western blot analysis. The strong immunostaining in the magnocellular subdivision of the PVN is suggestive of an involvement of 5-HT7 receptors in the serotonergic modulation of the neurohypophysial release of argininevasopressin and oxytocin (Bagdy and Kalogeras, 1993; Saydoff et al., 1993), which is consistent with the presence of 5-HT-LI in the magnocellular subdivision of the rat PVN (Ju et al., 1988). In addition, the modest 5-HT7-LI in the parvocellular subdivision of the PVN, in which CRF-producing neurons are predominantly located (Sawchenko et al., 1984; Swanson et al., 1983), parallels the reportedly low density of 5-HT-immunoreactive nerve fibers in this area (Sawchenko et al., 1983) and is consistent with a likely role of the receptor in stress-induced activation of the HPA axis and hormone secretion, as the present neuroendocrine results suggest. It remains to be determined notwithstanding whether 5-HT7-LI may be located on CRF-immunoreactive cell bodies with which 5-HT immunoreactive nerve terms are known to form axo-dendritic and axo-somatic synapses within the medial parvocellular PVN (Liposits et al., 1987). Although it is unclear whether the reduction of 5-HT7 receptor availability in the PVN has any functional implications, the observation that ACTH release appears insensitive to an acute episode of restraint in CRS rats suggests a decreased role of the receptor in the serotonergic control of ACTH secretion via CRF release in the hypothalamus (Holmes et al., 1982; Jones et al., 1976). Whether the 5-HT7 receptor changes that occur in the PVN of CRS animals might underlie similar alterations in pituitary CRF release, which could in turn represent a potential mechanism of dissociation between CORT and ACTH secretion, requires further investigation.
Consistent with the present observations in AG using immunohistochemistry, a previous study reported expression of 5-HT7 receptor mRNA in rat adrenal glands and the presence of 5-HT7 receptor protein in the adrenocortical zona glomerulosa and zona fasciculata/reticulata, but not in the medulla (Contesse et al., 1999). On the basis of pharmacological criteria, it was suggested that adrenal 5-HT7 receptors mediate aldosterone secretion, which agrees with their presence in zona glomerulosa cells (Contesse et al., 1999; present study); such study however did not screen for a potential role of 5-HT7 receptors in glucocorticoid secretion. In the present study, 5-HT7-LI was sparsely detected in the adrenal cortex of CTRL animals with immunostaining observed not only in the zona glomerulosa but also in the zona fasciculata (Figs. 3F and 3G). These observations, which are in agreement with the aforementioned findings, suggest a potential role of 5-HT7 receptors in glucocorticoid secretion. They also provide a mechanism for sensitization of restraint-induced CORT responses involving increased expression of 5-HT7 receptors in AG, concomitant with an opposite change in the PVN.
Similar to the present observations in the PVN and consistent with 5-HT7 receptor downregulation by glucocorticoids, increased expression of 5-HT7 receptor mRNA in the hippocampal CA3 subregion subsequent to adrenalectomy was reported in rats (Le Corre et al., 1997; Yau et al., 1997) whereas CORT replacement prevented this effect (Yau et al., 1997). In accordance with this, dexamethasone treatment was found to decrease transcription of 5-HT7 receptors in cultured frontocortical astrocytes (Shimizu et al., 1997). In contrast to the above observations, however, studies suggesting 5-HT7 receptor upregulation by stress have also been reported. For instance, acute stress was shown to increase 5-HT7 receptor mRNA in the rat hippocampus (Yau et al, 2001), whereas chronic unpredictable mild stress exposure increased it both in the hippocampus and the hypothalamus (Li et al., 2009). Furthermore, repetitive CORT administration (i.e. for 7 and 21 days) increased the reactivity of the hippocampal circuitry to 5-HT7 receptor activation by 5-CT, although no significant effect of the treatment on hippocampal 5-HT7 receptor binding was detected (Tokarski et al., 2009). Overall, these observations imply that the effects of chronic stress and glucocorticoids on 5-HT7 receptor function and expression are rather complex, with both decreases and increases being possible. In the case of the PVN from CRS animals, the decline of 5-HT7-LI and protein suggests downregulation of 5-HT7 receptors, whereas the increase of 5-HT7-LI in the adrenal cortex and 5-HT7 receptor protein in whole adrenals suggests upregulation of 5-HT7 receptors. Presently, we do not have an explanation for these contrasting changes in the PVN and AG from CRS animals. It is interesting, nevertheless, that clinical observations have shown that illegitimate membrane receptors, including 5-HT7 receptors, are involved in cortisol production in both adrenal adenomas and ACTH-independent macronodular adrenal hyperplasias (AIMAHs) causing Cushing’s syndrome (Louiset et al. 2006). Although the mechanisms leading to this abnormal expression of receptors in human tissues remain unknown, the present results in AG suggest an interesting association between chronic stress, increased expression of 5-HT7 receptors in adrenocortical cells involved in steroidogenesis, and magnified CORT secretion. It seems further remarkable that in human adrenal adenomas and AIMAHs, 5-HT was reported to stimulate cortisol secretion with increased sensitivity and/or efficacy (Contesse et al. 2005) probably accounted for abnormal expression of 5-HT7 receptors in adrenocortical cells involved in glucocorticoid production (Louiset et al. 2006).
4.7 Is adrenal 5-HT responsible for increased restraint-induced CORT secretion in animals with chronic stress?
The increase in baseline 5-HT and 5-HIAA levels, and 5-HIAA/5-HT ratio in AG suggest the involvement of a local 5-HT system in CORT secretion under chronic stress conditions. This putative serotonergic system acting via adrenocortical 5-HT7 receptors (the amount of which actually increased in AG from chronically stressed rats) would explain the magnified SB-656104-sensitive restraint-induced CORT responses in CRS animals. These data suggest that 5-HT may be produced locally in adrenocortical cells independently from chromaffin (Holzwarth and Brownfield, 1985; Verhofstad and Jonsson, 1983) and mast cells (Hinson et al., 1989), which were previously thought of as the exclusive source of 5-HT in the rat AG, and play a role in glucocorticoid synthesis and secretion. It should be highlighted however that mast cells modulate corticosteroid secretion via vascular or cell-cell interactions in rat AG (Hinson et al., 1989) and that they degranulate in response to acute restraint stress, at least in the dura mater and skin (Singh et al., 1999; Theoharides et al., 1995). Accordingly, pharmacological degranulation of AG mast cells increases serum 5-HT and plasma CORT levels (Kaida et al., 2010). Although the present experiments do not allow to exclude a possible role of mast cell-derived 5-HT in restraint-induced CORT secretion, it must be recalled that immunohistochemistry (as well as HPLC) assays for 5-HT were performed under no acute restraint stress conditions (i.e. one day after CTRL and CRS treatments) thus making the above possibility unlikely.
Given the obvious increase of 5-HT-LI in the cortex (and apparently also in the medulla) of CRS adrenals, a key issue to be clarified is the mechanism underlying this change. A previous investigation showed the ability of chromaffin cells to synthesize 5-HT from L-tryptophan in frog AG (Delarue et al., 1992), whereas another study reported the failure of L-tryptophan and the TPH inhibitor, H22/54, to modify the levels of adrenal 5-HT (Verhofstad and Jonsson, 1983). However, L-tryptophan administration promoted a markedly increased 5-HT-LI in the adrenal medulla of reserpine-pretreated animals thus suggesting that TPH-like activity is actually operative in rat AG (Holzwarth and Brownfield, 1985). Then, our negative TPH Western blot results in AG suggest that the enzyme is not present in this tissue or, alternatively, that the methodology employed was incapable of detecting it. In this regard, since TPH has two molecular forms i.e. TPH1 and TPH2, located predominantly in the periphery and the central nervous system, respectively (Walther et al., 2003), it is worth pointing out that the primary antibody employed in the assays displays TPH1 specificity. Although this isoform, which may be expected to express in AG, has been reported to be upregulated by stress (Abumaria et al., 2008), the possibility remains that TPH2 might also be present -and thus regulated by chronic stress- in AG. Further experiments using specific antibodies against each isoform will be required to explore this possibility in more detail. Alternatively, it is also possible that 5-HT could be synthesized within the adrenal cortex via decarboxylation of 5-hydroxytryptophan (Verhofstad and Jonsson, 1983) as the enzyme, L-amino acid decarboxylase, has been detected by immunocytochemistry in the adrenal cortex and the medulla (Burns et al., 1996). Whatever the mechanism involved, the present results suggest an interesting relationship between chronic stress, increased adrenocortical 5-HT availability and sensitized restraint-induced CORT secretion. Remarkably, a similar relationship between increased 5-HT and cortisol secretion has been observed in a subpopulation of glucocorticoid-producing cells in tumoral human adrenal tissues (Contesse et al. 2005).
4.8 Conclusions
The results of the present study have revealed an interesting association between CRS and endocrine dysregulation involving sensitization of CORT and blunting of ACTH secretory responses to restraint. This endocrine alteration seems to be accounted, at least in part, for an increased expression/function of adrenocortical 5-HT7 receptors along with higher availability/turnover of 5-HT in AG. It is to be highlighted notwithstanding that, whereas neuroendocrine studies were conducted in acutely stressed CTRL and CRS animals, the other studies were conducted in the absence of acute stress. Then, the possibility that the reported changes in hormone secretion might not be directly linked to the effects of CRS on increased function or expression of 5-HT7 receptors cannot be categorically excluded. Therefore, caution is needed in interpreting these findings. Results with SB-656104 suggest that pharmacological blockade of adrenocortical 5-HT7 receptors, possibly upregulated as a result of chronic stress exposure, could be of therapeutic benefit to overcome endocrine disruption in stress-related diseases. These data also invite the -yet speculative- interpretation that the faster-acting antidepressant action of 5-HT7 receptor blockade as compared to 5-HT uptake inhibition (Mnie-Filali et al., 2011) might partly involve an antagonist action on adrenocortical 5-HT7 receptors putatively involved in cortisol hypersecretion. Finally, the increased 5-HT turnover in AG as a result of CRS exposure implies a potential relationship between chronic stress and disruption of the 5-HT system involving an increased production and/or release of the indolamine, at least in the adrenals. Elucidation of the nature of this relationship, which does not seem to involve CRS-induced expression of TPH1 in adrenals, requires further investigation. The parallel of the present findings in CRS animals with clinically relevant reports in humans (Contesse et al. 2005; Louiset et el., 2006) is certainly promising as to the potential usefulness of 5-HT7 receptor blockade in the treatment of the endocrine alterations commonly observed in stress-related diseases.
Highlights.
Chronic restraint led to sensitization of restraint-induced corticosterone secretion.
Dissociation of corticosterone from ACTH may develop in chronically restrained rats.
Corticosterone hypersecretion under chronic stress is blocked by a 5-HT7 receptor antagonist.
The mechanism may involve an increase in adrenal 5-HT7 receptors and 5-HT turnover.
Chronic restraint in rats partly parallels pathological adrenal mechanisms in humans.
Acknowledgements
Authors are in debt with Dr. Benjamín Florán (Departamento de Fisiología, Biofísica y Neurociencias, CINVESTAV-IPN) and Dr. Erick Escartín (Laboratorio de Neurobiología de la Alimentación, FES Iztacala, UNAM) for providing guidance, assistance and unlimited access to the HPLC equipment for 5-HT and 5-HIAA measurements, and with Dr. Eduardo Ramírez-San Juan (Departamento de Fisiología, ENCB del IPN) for his critical and helpful comments on the statistical analyses. Authors also thank Glaxo-SmithKline (Harlow, U.K.) for providing the sample of SB-656104. The skillful technical assistance of Carolina Sánchez-Maldonado is greatly appreciated. This work was supported by the National Institutes of Health (NIH Grant 5R01TW006622).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Abumaria N, Ribic A, Anacker C, Fuchs E, Flügge G. Stress upregulates TPH1 but not TPH2 mRNA in the rat dorsal raphe nucleus: identification of two TPH2 mRNA splice variants. Cell Mol. Neurobiol. 2008;28(3):331–342. doi: 10.1007/s10571-007-9259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armario A, Lopez-Calderon A, Jolin T, Balasch J. Response of anterior pituitary hormones to chronic stress. The specificity of adaptation. Neurosci. Biobehav. Rev. 1986;10(3):245–250. doi: 10.1016/0149-7634(86)90011-4. [DOI] [PubMed] [Google Scholar]
- Azpiroz A, Fano E, Garmendia L, Arregi A, Cacho R, Beitia G, Brain PF. Effects of chronic mild stress (CMS) and imipramine administration, on spleen mononuclear cell proliferative response, serum corticosterone level and brain norepinephrine content in male mice. Psychoneuroendocrinology. 1999;24(3):345–361. doi: 10.1016/s0306-4530(98)00084-5. [DOI] [PubMed] [Google Scholar]
- Bagdy G, Kalogeras KT. Stimulation of 5-HT1A and 5-HT2/5-HT1C receptors induce oxytocin release in the male rat. Brain Res. 1993;611(2):330–332. doi: 10.1016/0006-8993(93)90521-n. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Nguyen NT, Gobbi G. Decline in serotonergic firing activity and desensitization of 5-HT1A autoreceptors after chronic unpredictable stress. Eur. Neuropsychopharmacology. 2009;19:215–228. doi: 10.1016/j.euroneuro.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Barnum CJ, Blandino P, Jr., Deak T. Adaptation in the corticosterone and hyperthermic responses to stress following repeated stressor exposure. J. Neuroendocrinol. 2007;19(8):632–642. doi: 10.1111/j.1365-2826.2007.01571.x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J. Neuroendocrinol. 2002;14:403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Sun LM, Raber J, Manes S, Julius DMF, Dallman MF. Changes in anxiety-related behavior and hypothalamic pituitary adrenal activity in mice lacking the 5-HT3A receptor. Physiol. Behav. 2004;81:545–555. doi: 10.1016/j.physbeh.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Blier P, Haddjeri N, Szabo ST, Dong J. Enhancement of serotoninergic function -a sometimes insufficient cause of antidepressant action. Hum. Psychopharmacol. 2001;16:23–27. doi: 10.1002/hup.179. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Nepomuceno D, Hein L, Sutcliffe JG, Lovenberg T, Hedlund PB. Radioligand binding analysis of knockout mice reveals 5-hydroxytryptamine(7) receptor distribution and uncovers 8-hydroxy-2-(di-n-propylamino) tetralin interaction with alpha(2) adrenergic receptors. Neuroscience. 2004;124(4):901–911. doi: 10.1016/j.neuroscience.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Chrousos GP. Clinical review 104: Adrenocorticotropin (ACTH)- and non-ACTH-mediated regulation of the adrenal cortex: neural and immune inputs. J. Clin. Endocrinol. Metab. 1999;84:1729–1736. doi: 10.1210/jcem.84.5.5631. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burns N, Brett L, Olverman HJ, Nagatsu T, Lee MR, Williams BC. The role of L-aromatic amino acid decarboxylase in serotonin-stimulated aldosterone secretion in response to salt intake. Endocr. Res. 1996;22(4):577–578. doi: 10.1080/07435809609043749. [DOI] [PubMed] [Google Scholar]
- Chen J, Young S, Subburaju S, Sheppard J, Kiss A, Atkinson H, Wood S, Lightman S, Serradeil-Le Gal C, Aguilera G. Vasopressin does not mediate hypersensitivity of the hypothalamic pituitary adrenal axis during chronic stress. Ann. NY Acad. Sci. 2008;1148:349–359. doi: 10.1196/annals.1410.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Evanson NK, Furay AR, Ulrich-Lai YM, Ostrander MM, Herman JP. The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress. Endocrinology. 2008;149:818–826. doi: 10.1210/en.2007-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemett DA, Cockett MI, Marsden CA, Fone KC. Antisense oligonucleotide-induced reduction in 5-hydroxytryptamine7 receptors in the rat hypothalamus without alteration in exploratory behaviour or neuroendocrine function. J. Neurochem. 1998;71(3):1271–1279. doi: 10.1046/j.1471-4159.1998.71031271.x. [DOI] [PubMed] [Google Scholar]
- Contesse V, Lenglet S, Grumolato L, Anouar Y, Lihrmann I, Lefebvre H, Delarue C, Vaudry H. Pharmacological and molecular characterization of 5-hydroxytryptamine(7) receptors in the rat adrenal gland. Mol. Pharmacol. 1999;56:552–561. doi: 10.1124/mol.56.3.552. [DOI] [PubMed] [Google Scholar]
- Contesse V, Reznik Y, Louiset E, Duparc C, Cartier D, Sicard F, Laquerriere A, Parmentier F, Kuhn JM, Vaudry H, Lefebvre H. Abnormal sensitivity of cortisol-producing adrenocortical adenomas to serotonin: in vivo and in vitro studies. J. Clin. Endocrinol. Metab. 2005;90(5):2843–2850. doi: 10.1210/jc.2004-2476. [DOI] [PubMed] [Google Scholar]
- Cruz FC, Marin MT, Leao RM, Planeta CS. Behavioral and neuroendocrine effects of the exposure to chronic restraint or variable stress in early adolescent rats. Int. J. Dev. Neurosci. 2012;30:19–23. doi: 10.1016/j.ijdevneu.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Czyrak A, Maćkowiak M, Chocyk A, Fijał K, Tokarski K, Bijak M, Wedzony K. Prolonged corticosterone treatment alters the responsiveness of 5-HT1A receptors to 8-OHDPAT in rat CA1 hippocampal neurons. Naunyn-Schmiedebergs Arch. Pharmacol. 2002;366(4):357–367. doi: 10.1007/s00210-002-0586-2. [DOI] [PubMed] [Google Scholar]
- Dal-Zotto S, Martí O, Armario A. Influence of single or repeated experience of rats with forced swimming on behavioural and physiological responses to the stressor. Behav. Brain Res. 2000;114(1-2):175–181. doi: 10.1016/s0166-4328(00)00220-5. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koopmans SJ, Slangen JL, Van der Gugten J. Plasma catecholamine, corticosterone and glucose responses to repeated stress in rats: effect of interstressor interval length. Physiol. Behav. 1990;47(6):1117–1124. doi: 10.1016/0031-9384(90)90361-7. [DOI] [PubMed] [Google Scholar]
- Delarue C, Becquet D, Idres S, Hery F, Vaudry H. Serotonin synthesis in adrenochromaffin cells. Neuroscience. 1992;46(2):495–500. doi: 10.1016/0306-4522(92)90069-e. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65(5):360–368. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- Ehrhart-Bornstein M, Hinson JP, Bornstein SF, Scherbaum WA, Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr. Rev. 1998;19:101–143. doi: 10.1210/edrv.19.2.0326. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144(12):5249–5258. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- Forbes IT, Douglas S, Gribble AD, Ife RJ, Lightfoot AP, Garner AE, Riley GJ, Jeffrey P, Stevens AJ, Stean TO, Thomas DR. SB-656104-A: A novel 5-HT(7) receptor antagonist with improved in vivo properties. Bioorg. Med. Chem. Lett. 2002;12:3341–3344. doi: 10.1016/s0960-894x(02)00690-x. [DOI] [PubMed] [Google Scholar]
- García A, Martí O, Vallés A, Dal-Zotto S, Armario A. Recovery of the hypothalamic-pituitary-adrenal response to stress. Effect of stress intensity, stress duration and previous stress exposure. Neuroendocrinology. 2000;72(2):114–125. doi: 10.1159/000054578. [DOI] [PubMed] [Google Scholar]
- Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Gold PW, Loriaux DL, Roy A, Kling MA, Calabrese JR, Kellner CH, Nieman LK, Post RM, Pickar D, Gallucci W, Averginos P, Paul S, Oldfield EH, Cutler GB, Chrousos GP. Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing’s disease. N. Engl. J. Med. 1986;314:1329–1335. doi: 10.1056/NEJM198605223142101. [DOI] [PubMed] [Google Scholar]
- Gray M, Bingham B, Viau V. A comparison of two repeated restraint stress paradigms on hypothalamic-pituitary-adrenal axis habituation, gonadal status and central neuropeptide expression in adult male rats. J. Neuroendocrinol. 2010;22:92–101. doi: 10.1111/j.1365-2826.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- Harris RB, Zhou J, Youngblood BD, Rybkin II, Smagin GN, Ryan DH. Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. Am. J. Physiol. 1998;275(6 Pt 2):R1928–R1938. doi: 10.1152/ajpregu.1998.275.6.R1928. [DOI] [PubMed] [Google Scholar]
- Harris RB, Gu H, Mitchell TD, Endale L, Russo M, Ryan DH. Increased glucocorticoid response to a novel stress in rats that have been restrained. Physiol. Behav. 2004;81:557–568. doi: 10.1016/j.physbeh.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Hedlund PB. The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology (Berl) 2009;206(3):345–354. doi: 10.1007/s00213-009-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JP, Vinson GP, Pudney J, Whitehouse BJ. Adrenal mast cells modulate vascular and secretory responses in the intact adrenal gland of the rat. J. Endocrinol. 1989;121:253–260. doi: 10.1677/joe.0.1210253. [DOI] [PubMed] [Google Scholar]
- Holmes MC, Di RG, Beckford U, Gillham B, Jones MT. Role of serotonin in the control of secretion of corticotrophin releasing factor. J. Endocrinol. 1982;93:151–160. doi: 10.1677/joe.0.0930151. [DOI] [PubMed] [Google Scholar]
- Holzwarth MA, Brownfield MS. Serotonin coexists with epinephrine in rat adrenal medullary cells. Neuroendocrinology. 1985;41:230–236. doi: 10.1159/000124182. [DOI] [PubMed] [Google Scholar]
- Inder WJ, Prickett TC, Mulder RT, Donald RA, Joyce PR. Reduction in basal afternoon plasma ACTH during early treatment of depression with fluoxetine. Psychopharmacology (Berl) 2001;156:73–78. doi: 10.1007/s002130100737. [DOI] [PubMed] [Google Scholar]
- Jasper MS, Engeland WC. Splanchnicotomy increases adrenal sensitivity to ACTH in nonstressed rats. Am. J. Physiol. Endocrinol. Metab. 1997;273(2 Pt 1):E363–E368. doi: 10.1152/ajpendo.1997.273.2.E363. [DOI] [PubMed] [Google Scholar]
- Joëls M, Verkuyl JM, Van Riel E. Hippocampal and hypothalamic function after chronic stress. Ann. NY Acad. Sci. 2003;1007:367–378. doi: 10.1196/annals.1286.036. [DOI] [PubMed] [Google Scholar]
- Jones MT, Hillhouse EW, Burden J. Effect on various putative neurotransmitters on the secretion of corticotrophin-releasing hormone from the rat hypothalamus in vitro -a model of the neurotransmitters involved. J. Endocrinol. 1976;69:1–10. doi: 10.1677/joe.0.0690001. [DOI] [PubMed] [Google Scholar]
- Jorgensen H, Knigge U, Kjaer A, Warberg J. Adrenocorticotropic Hormone secretion in rats induced by stimulation with serotonergic compounds. J. Neuroendocrinol. 1999;11:283–290. doi: 10.1046/j.1365-2826.1999.00328.x. [DOI] [PubMed] [Google Scholar]
- Jorgensen H, Knigge U, Kjaer A, Moller M, Warberg J. Serotonergic stimulation of corticotropin-releasing hormone and pro-opiomelanocortin gene expression. J. Neuroendocrinol. 2002;14:788–795. doi: 10.1046/j.1365-2826.2002.00839.x. [DOI] [PubMed] [Google Scholar]
- Ju G, Duan XJ, Zhang X. Serotonin-like immunoreactive fibers in the medial dorsal accessory group and the anterior fornical nucleus of the magnocellular neurosecretory system in the rat. Neurosci. Lett. 1988;93(1):19–22. doi: 10.1016/0304-3940(88)90005-5. [DOI] [PubMed] [Google Scholar]
- Kageyama K, Tozawa F, Horiba N, Watanobe H, Suda T. Serotonin stimulates corticotropin-releasing factor gene expression in the hypothalamic paraventricular nucleus of conscious rats. Neurosci. Lett. 1998;243:17–20. doi: 10.1016/s0304-3940(98)00097-4. [DOI] [PubMed] [Google Scholar]
- Kaida S, Ohta Y, Imai Y, Ohashi K, Kawanishi M. Compound 48/80 causes oxidative stress in the adrenal gland of rats through mast cell degranulation. Free Radic. Res. 2010;44:171–180. doi: 10.3109/10715760903380466. [DOI] [PubMed] [Google Scholar]
- Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary -adrenal axis function and hippocampal serotonin release in mice. J. Neuroendocrinol. 2006;18(5):330–338. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- Kempf E, Mandel P. Reverse-phase high-performance liquid chromatographic separation and electrochemical detection of norepinephrine, dopamine, serotonin, and related major metabolites. Anal. Biochem. 1981;112(2):223–231. doi: 10.1016/0003-2697(81)90285-2. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Park SH, Choi SH, Moon BH, Lee KJ, Kang SW, Lee MS, Choi SH, Chun BG, Shin KH. Effects of repeated tianeptine treatment on CRF mRNA expression in non-stressed and chronic mild stress-exposed rats. Neuropharmacology. 2006;50:824–833. doi: 10.1016/j.neuropharm.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Kinne-Saffran E, Kinne RKH. Membrane Isolation: Strategy, Techniques, Markers. In: Fleischer S, Fleischer B, editors. Methods in Enzymology. vol 172. Academic press Inc.; San Diego California: 1989. pp. 15–16. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Hay-Schmidt A, Vrang N, Mikkelsen JD. Origin of projections from the midbrain raphe nuclei to the hypothalamic paraventricular nucleus in the rat: a combined retrograde and anterograde tracing study. Neuroscience. 1996;70:963–988. doi: 10.1016/0306-4522(95)00415-7. [DOI] [PubMed] [Google Scholar]
- Le Corre S, Sharp T, Young AH, Harrison PJ. Increase of 5-HT7 (serotonin-7) and 5-HT1A (serotonin-1A) receptor mRNA expression in rat hippocampus after adrenalectomy. Psychopharmacology (Berl) 1997;130:368–374. doi: 10.1007/s002130050252. [DOI] [PubMed] [Google Scholar]
- Li YC, Wang FM, Pan Y, Qiang LQ, Cheng G, Zhang WY, Kong LD. Antidepressant-like effects of curcumin on serotonergic receptor-coupled AC-cAMP pathway in chronic unpredictable mild stress of rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33(3):435–449. doi: 10.1016/j.pnpbp.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Liposits Z, Phelix C, Paull WK. Synaptic interaction of serotonergic axons and corticotropin releasing factor (CRF) synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A light and electron microscopic immunocytochemical study. Histochemistry. 1987;86:541–549. doi: 10.1007/BF00489545. [DOI] [PubMed] [Google Scholar]
- Louiset E, Contesse V, Groussin L, Cartier D, Duparc C, Barrande G, Bertherat J, Vaudry H, Lefebvre H. Expression of serotonin7 receptor and coupling of ectopic receptors to protein kinase A and ionic currents in adrenocorticotropin-independent macronodular adrenal hyperplasia causing Cushing’s syndrome. J. Clin. Endocrinol. Metab. 2006;91:4578–4586. doi: 10.1210/jc.2006-0538. [DOI] [PubMed] [Google Scholar]
- Ma XM, Lightman SL. The arginine vasopressin and corticotrophin-releasing hormone gene transcription responses to varied frequencies of repeated stress in rats. J. Physiol. 1998;510(Pt 2):605–614. doi: 10.1111/j.1469-7793.1998.605bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 1999;140:3623–3632. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Maayan R, Weizman A, Weller A. Aggressive behavior and HPA axis hormones after social isolation in adult rats of two different genetic animal models for depression. Behav. Brain Res. 2006;175(2):408–414. doi: 10.1016/j.bbr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Marti O, Marti J, Armario A. Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol. Behav. 1994;55:747–753. doi: 10.1016/0031-9384(94)90055-8. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. Biol. Psychiatry. 1995;37:383–393. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Shoji H, Ikeda R, Tanaka Y, Tabira T. Persistent depressive state after chronic stress in rats is accompanied by HPA axis dysregulation and reduced prefrontal dopaminergic neurotransmission. Pharmacol. Biochem. Behav. 2008;91(1):170–175. doi: 10.1016/j.pbb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Mnie-Filali O, Faure C, Lambás-Señas L, El Mansari M, Belblidia H, Gondard E, Etiévant A, Scarna H, Didier A, Berod A, Blier P, Haddjeri N. Pharmacological blockade of 5-HT7 receptors as a putative fast acting antidepressant strategy. Neuropsychopharmacology. 2011;36(6):1275–1288. doi: 10.1038/npp.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins UL, Gianutsos G, Eison AS. Effects of antidepressants on 5-HT7 receptor regulation in the rat hypothalamus. Neuropsychopharmacology. 1999;21:352–367. doi: 10.1016/S0893-133X(99)00041-X. [DOI] [PubMed] [Google Scholar]
- Muneoka KT, Takigawa M. 5-Hydroxytryptamine7 (5-HT7) receptor immunoreactivity-positive ‘stigmoid body’-like structure in developing rat brains. Int. J. Dev. Neurosci. 2003;21:133–143. doi: 10.1016/s0736-5748(03)00029-7. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Sexton TJ, Yracheta J, Diaz AM, Brownfield M. Localization of 5-HT(7) receptors in rat brain by immunocytochemistry, in situ hybridization, and agonist stimulated cFos expression. J. Chem. Neuroanat. 2001;21:63–73. doi: 10.1016/s0891-0618(00)00092-2. [DOI] [PubMed] [Google Scholar]
- Orr TE, Meyerhoff JL, Mougey EH, Bunnell BN. Hyperresponsiveness of the rat neuroendocrine system due to repeated exposure to stress. Psychoneuroendocrinology. 1990;15(5-6):317–328. doi: 10.1016/0306-4530(90)90057-g. [DOI] [PubMed] [Google Scholar]
- Palkovits M. Punch sampling biopsy technique. Methods Enzymol. 1983;103:368–376. doi: 10.1016/s0076-6879(83)03025-6. [DOI] [PubMed] [Google Scholar]
- Pan L, Gilbert F. Activation of 5-HT1A receptor subtype in the paraventricular nuclei of the hypothalamus induces CRH and ACTH release in the rat. Neuroendocrinology. 1992;56:797–802. doi: 10.1159/000126332. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm. Behav. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Pitman DL, Ottenweller JE, Natelson BH. Effect of stressor intensity on habituation and sensitization of glucocorticoid responses in rats. Behav. Neurosci. 1990;104(1):28–36. doi: 10.1037//0735-7044.104.1.28. [DOI] [PubMed] [Google Scholar]
- Plotsky P, Owens MJ, Nemeroff CB. Neuropeptide alterations in affective disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 971–981. [Google Scholar]
- Sánchez MM, Aguado F, Sánchez-Toscano F, Saphier D. Neuroendocrine and immunocytochemical demonstrations of decreased hypothalamo-pituitary-adrenal axis responsiveness to restraint stress after long-term social isolation. Endocrinology. 1998;139(2):579–587. doi: 10.1210/endo.139.2.5720. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Steinbusch HW, Verhofstad AA. The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Res. 1983;277:355–360. doi: 10.1016/0006-8993(83)90945-9. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Vale WW. Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proc. Natl. Acad. Sci. U S A. 1984;81(6):1883–1887. doi: 10.1073/pnas.81.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saydoff JA, Carnes M, Brownfield MS. The role of serotonergic neurons in intravenous hypertonic saline-induced secretion of vasopressin, oxytocin, and ACTH. Brain Res. Bull. 1993;32:567–572. doi: 10.1016/0361-9230(93)90156-6. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Nishida A, Zensho H, Miyata M, Yamawaki S. Down-regulation of 5-hydroxytryptamine7 receptors by dexamethasone in rat frontocortical astrocytes. J. Neurochem. 1997;68:2604–2609. doi: 10.1046/j.1471-4159.1997.68062604.x. [DOI] [PubMed] [Google Scholar]
- Silberman DM, Wald M, Genaro AM. Effects of chronic mild stress on lymphocyte proliferative response. Participation of serum thyroid hormones and corticosterone. Int. Immunopharmacol. 2002;2(4):487–497. doi: 10.1016/s1567-5769(01)00190-4. [DOI] [PubMed] [Google Scholar]
- Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC. Acute immobilization stress triggers skin mast cell degranulation via corticotropin releasing hormone, neurotensin, and substance P: A link to neurogenic skin disorders. Brain Behav. Immun. 1999;13:225–239. doi: 10.1006/brbi.1998.0541. [DOI] [PubMed] [Google Scholar]
- Sleight AJ, Carolo C, Petit N, Zwingelstein C, Bourson A. Identification of 5-hydroxytryptamine7 receptor binding sites in rat hypothalamus: sensitivity to chronic antidepressant treatment. Mol. Pharmacol. 1995;47(1):99–103. [PubMed] [Google Scholar]
- Stamp JA, Herbert J. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience. 1999;94(4):1313–1322. doi: 10.1016/s0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36(3):165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Tauchi M, Zhang R, D’Alessio DA, Seeley RJ, Herman JP. Role of central glucagon-like peptide-1 in hypothalamo-pituitary-adrenocortical facilitation following chronic stress. Exp. Neurol. 2008;210(2):458–466. doi: 10.1016/j.expneurol.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Spanos C, Pang X, Alferes L, Ligris K, Letourneau R, Rozniecki JJ, Webster E, Chrousos GP. Stress-induced intracranial mast cell degranulation: a corticotropin-releasing hormone-mediated effect. Endocrinology. 1995;136:5745–5750. doi: 10.1210/endo.136.12.7588332. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Melotto S, Massagrande M, Gribble AD, Jeffrey P, Stevens AJ, Deeks NJ, Eddershaw PJ, Fenwick SH, Riley G, Stean T, Scott CM, Hill MJ, Middlemiss DN, Hagan JJ, Price GW, Forbes IT. SB-656104-A, a novel selective 5-HT7 receptor antagonist, modulates REM sleep in rats. Br. J. Pharmacol. 2003;139(4):705–714. doi: 10.1038/sj.bjp.0705290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarski K, Pitra P, Duszynska B, Hess G. Imipramine counteracts corticosterone-induced alterations in the effects of the activation of 5-HT(7) receptors in rat hippocampus. J. Physiol. Pharmacol. 2009;60(2):83–88. [PubMed] [Google Scholar]
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am. J. Physiol. Endocrinol. Metab. 2006;291:E965–E973. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- Varnas K, Thomas DR, Tupala E, Tiihonen J, Hall H. Distribution of 5-HT7 receptors in the human brain: a preliminary autoradiographic study using [3H]SB-269970. Neurosci. Lett. 2004;367:313–316. doi: 10.1016/j.neulet.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Verhofstad AA, Jonsson G. Immunohistochemical and neurochemical evidence for the presence of serotonin in the adrenal medulla of the rat. Neuroscience. 1983;10(4):1443–1453. doi: 10.1016/0306-4522(83)90125-2. [DOI] [PubMed] [Google Scholar]
- Vicentic A, Li Q, Battaglia G, Van de Kar LD. WAY-100635 inhibits 8-OH-DPAT-stimulated oxytocin, ACTH and corticosterone, but not prolactin secretion. Eur. J. Pharmacol. 1998;346:261–266. doi: 10.1016/s0014-2999(97)01607-5. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Yau JL, Noble J, Widdowson J, Seckl JR. Impact of adrenalectomy on 5-HT6 and 5-HT7 receptor gene expression in the rat hippocampus. Mol. Brain Res. 1997;45(1):182–186. doi: 10.1016/s0169-328x(97)00026-0. [DOI] [PubMed] [Google Scholar]
- Yau JL, Noble J, Seckl JR. Acute restraint stress increases 5-HT7 receptor mRNA expression in the rat hippocampus. Neurosci. Lett. 2001;309(3):141–144. doi: 10.1016/s0304-3940(01)02054-7. [DOI] [PubMed] [Google Scholar]
- Zardooz H, Zahedi Asl S, Gharib Naseri MK, Hedayati M. Effect of chronic restraint stress on carbohydrate metabolism in rat. Physiol. Behav. 2006;89(3):373–378. doi: 10.1016/j.physbeh.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Zelena D, Foldes A, Mergl Z, Barna I, Kovacs KJ, Makara GB. Effects of repeated restraint stress on hypothalamo-pituitary-adrenocortical function in vasopressin deficient Brattleboro rats. Brain Res. Bull. 2004;63:521–530. doi: 10.1016/j.brainresbull.2004.04.007. [DOI] [PubMed] [Google Scholar]