Abstract
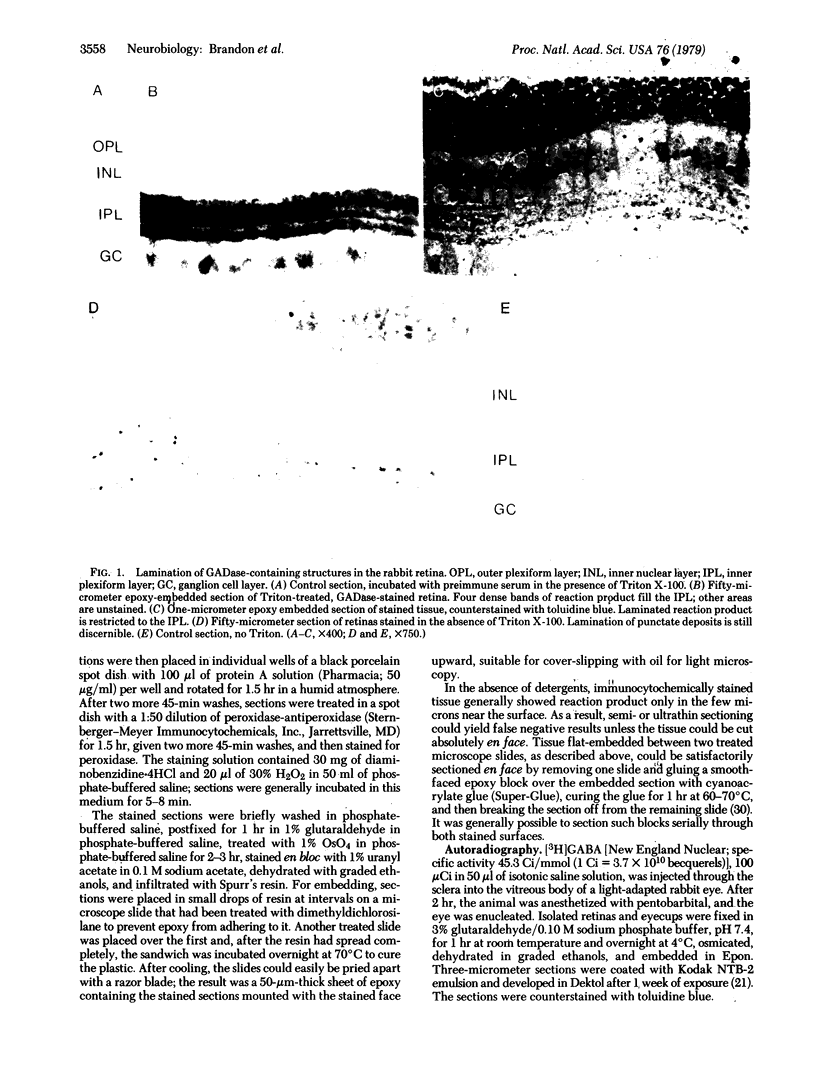
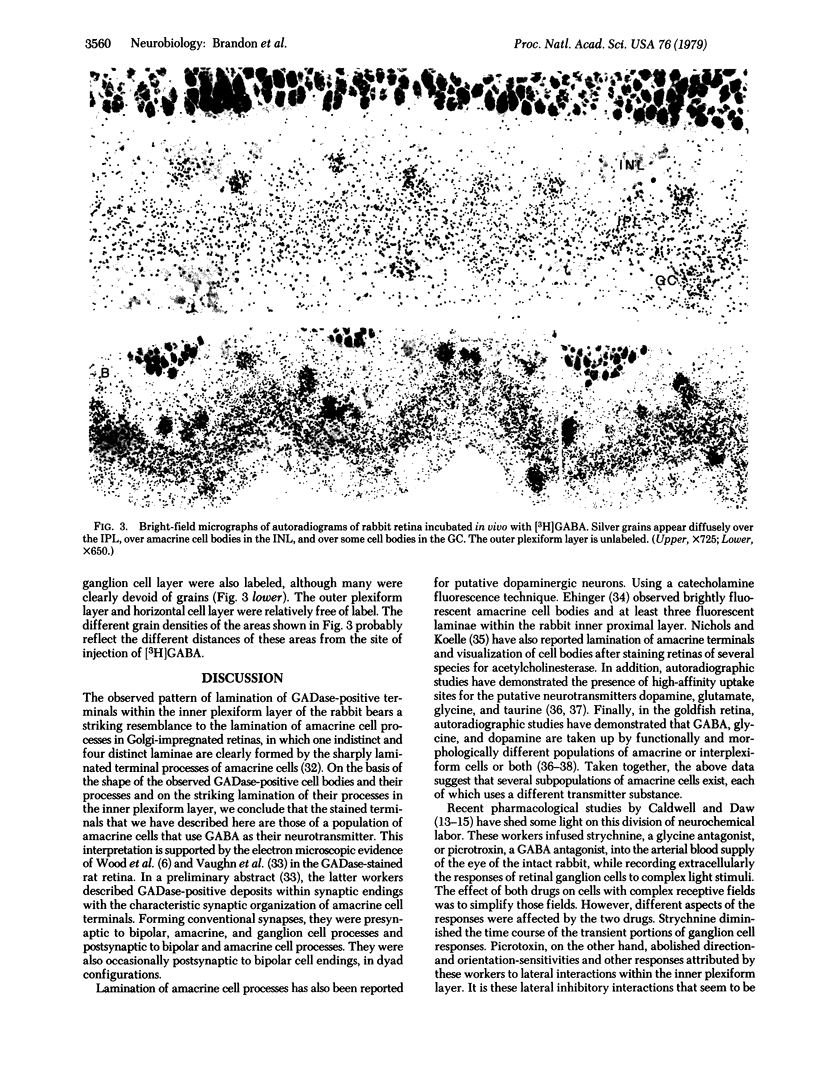
The localization of gamma-aminobutyric acid (GABA) neurons in the rabbit retina has been studied by immunocytochemical localization of the GABA-synthesizing enzyme L-glutamate decarboxylase (L-glutamate I-carboxy-lyase, EC 4.1.1.15) and by [3H]GABA uptake autoradiography. When Triton X-100 was included in immunocytochemical incubations with a modified protein A-peroxidase-antiperoxidase method, reaction product was found in four broad, evenly spaced laminae within the inner plexiform layer. In the absence of the detergent, these laminae were seen to be composed of small, punctate deposits. When colchicine was injected intravitreally before glutamate decarboxylase staining, cell bodies with the characteristic shape and location of amacrine cells were found to be immunochemically labeled. Intravitreally administered [3H]GABA produced a diffuse labeling of the inner plexiform layer and a dense labeling of certain amacrine cell bodies in the inner nuclear layer. Both immunocytochemical and autoradiographic results support the notion that certain, if not all, amacrine cells use GABA as their neurotransmitter.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames A., 3rd, Pollen D. A. Neurotransmission in central nervous tissue: a study of isolated rabbit retina. J Neurophysiol. 1969 May;32(3):424–442. doi: 10.1152/jn.1969.32.3.424. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B., HILL R. M., LEVICK W. R. RETINAL GANGLION CELLS RESPONDING SELECTIVELY TO DIRECTION AND SPEED OF IMAGE MOTION IN THE RABBIT. J Physiol. 1964 Oct;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: changes in centre surround receptive fields. J Physiol. 1978 Mar;276:299–310. doi: 10.1113/jphysiol.1978.sp012234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W. New properties of rabbit retinal ganglion cells. J Physiol. 1978 Mar;276:257–276. doi: 10.1113/jphysiol.1978.sp012232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W., Wyatt H. J. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: lateral interactions for cells with more complex receptive fields. J Physiol. 1978 Mar;276:277–298. doi: 10.1113/jphysiol.1978.sp012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M., McFarland H., McFarlin D. Protein A-peroxidase: a valluable tool for the localization of antigens. J Histochem Cytochem. 1977 Nov;25(11):1201–1206. doi: 10.1177/25.11.199666. [DOI] [PubMed] [Google Scholar]
- Ehinger B. Autoradiographic identification of rabbit retinal neurons that take up GABA. Experientia. 1970 Oct 15;26(10):1063–1064. doi: 10.1007/BF02112673. [DOI] [PubMed] [Google Scholar]
- Ehinger B. Cellular location of the uptake of some amino acids into the rabbit retina. Brain Res. 1972 Nov 13;46:297–311. doi: 10.1016/0006-8993(72)90021-2. [DOI] [PubMed] [Google Scholar]
- Ehinger B., Falck B. Autoradiography of some suspected neurotransmitter substances: GABA glycine, glutamic acid, histamine, dopamine, and L-dopa. Brain Res. 1971 Oct 8;33(1):157–172. doi: 10.1016/0006-8993(71)90314-3. [DOI] [PubMed] [Google Scholar]
- Graham L. T. Intraretinal distribution of GABA content and GAD activity. Brain Res. 1972 Jan 28;36(2):476–479. doi: 10.1016/0006-8993(72)90759-7. [DOI] [PubMed] [Google Scholar]
- Johnson P. C. A rapidly setting glue for resectioning and remounting epoxy embedded tissue. Stain Technol. 1976 Sep;51(5):275–276. doi: 10.3109/10520297609116719. [DOI] [PubMed] [Google Scholar]
- Kuriyama K., Sisken B., Haber B., Roberts E. The gamma-aminobutyric acid system in rabbit retina. Brain Res. 1968 Jun;9(1):165–168. doi: 10.1016/0006-8993(68)90269-2. [DOI] [PubMed] [Google Scholar]
- Lam D. M., Steinman L. The uptake of ( - 3 H) aminobutyric acid in the goldfish retina. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2777–2781. doi: 10.1073/pnas.68.11.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M., Su Y. Y., Swain L., Marc R. E., Brandon C., Wu J. Y. Immunocytochemical localisation of L-glutamic acid decarboxylase in the goldfish retina. Nature. 1979 Apr 5;278(5704):565–567. doi: 10.1038/278565a0. [DOI] [PubMed] [Google Scholar]
- Marc R. E., Stell W. K., Bok D., Lam D. M. GABA-ergic pathways in the goldfish retina. J Comp Neurol. 1978 Nov 15;182(2):221–244. doi: 10.1002/cne.901820204. [DOI] [PubMed] [Google Scholar]
- Marshall J., Voaden M. An autoradiographic study of the cells accumulating 3H gamma-aminobutyric acid in the isolated retinas of pigeons and chickens. Invest Ophthalmol. 1974 Aug;13(8):602–607. [PubMed] [Google Scholar]
- Marshall J., Voaden M. An investigation of the cells incorporating (3H)GABA and (3H)glycine in the isolated retina of the rat. Exp Eye Res. 1974 Apr;18(4):367–370. doi: 10.1016/0014-4835(74)90113-4. [DOI] [PubMed] [Google Scholar]
- Marshall J., Voaden M. Autoradiographic identification of the cells accumulating 3H gamma-aminobutyric acid in mammalian retinae: a species comparison. Vision Res. 1975 Mar;15(3):459–461. doi: 10.1016/0042-6989(75)90102-9. [DOI] [PubMed] [Google Scholar]
- Masland R. H., Ames A., 3rd Dissociation of field potential from neuronal activity in the isolated retina: failure of the b-wave with normal ganglion cell response. J Neurobiol. 1975 May;6(3):305–312. doi: 10.1002/neu.480060306. [DOI] [PubMed] [Google Scholar]
- Masland R. H., Livingstone C. J. Effect of stimulation with light on synthesis and release of acetylcholine by an isolated mammalian retina. J Neurophysiol. 1976 Nov;39(6):1210–1219. doi: 10.1152/jn.1976.39.6.1210. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Neal M. J., Iversen L. L. Autoradiographic localization of 3 H-GABA in rat retina. Nat New Biol. 1972 Feb 16;235(59):217–218. doi: 10.1038/newbio235217a0. [DOI] [PubMed] [Google Scholar]
- Nichols C. W., Koelle G. B. Comparison of the localization of acetylcholinesterase and non-specific cholinesterase activities in mammalian and avian retinas. J Comp Neurol. 1968 May;133(1):1–16. doi: 10.1002/cne.901330102. [DOI] [PubMed] [Google Scholar]
- Parks J. M., Ames A., 3rd, Nesbett F. B. Protein synthesis in central nervous tissue: studies on retina in vitro. J Neurochem. 1976 Nov;27(5):987–997. doi: 10.1111/j.1471-4159.1976.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Petrali J. P., Hinton D. M., Moriarty G. C., Sternberger L. A. The unlabeled antibody enzyme method of immunocytochemistry. Quantitative comparison of sensitivities with and without peroxidase-antiperoxidase complex. J Histochem Cytochem. 1974 Aug;22(8):782–801. doi: 10.1177/22.8.782. [DOI] [PubMed] [Google Scholar]
- Ribak C. E., Vaughn J. E., Saito K. Immunocytochemical localization of glutamic acid decarboxylase in neuronal somata following colchicine inhibition of axonal transport. Brain Res. 1978 Jan 27;140(2):315–332. doi: 10.1016/0006-8993(78)90463-8. [DOI] [PubMed] [Google Scholar]
- Saito K., Wu J. Y., Matsuda T., Roberts E. Immunochemical comparisons of vertebrate glutamic acid decarboxylase. Brain Res. 1974 Jan 11;65(2):277–285. doi: 10.1016/0006-8993(74)90039-0. [DOI] [PubMed] [Google Scholar]
- Sarthy P. V., Lam D. M. The uptake and release of [3H]dopamine in the goldfish retina. J Neurochem. 1979 Apr;32(4):1269–1277. doi: 10.1111/j.1471-4159.1979.tb11054.x. [DOI] [PubMed] [Google Scholar]
- Voaden M. J., Marshall J., Murani N. The uptake of [3H]gamma-amino butyric acid and [3H]glycine by the isolated retina of the frog. Brain Res. 1974 Feb 15;67(1):115–132. doi: 10.1016/0006-8993(74)90302-3. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Matsuda T., Roberts E. Purification and characterization of glutamate decarboxylase from mouse brain. J Biol Chem. 1973 May 10;248(9):3029–3034. [PubMed] [Google Scholar]