Abstract
BACKGROUND AND PURPOSE
Recent human studies suggest that recreational cannabis strains that are relatively high in cannabidiol (CBD) content produce less cognitive impairment than do strains with negligible CBD and similar Δ9tetrahydrocannabinol (THC) content. Self-selection in such studies means it is impossible to rule out additional variables which may determine both cannabis strain selection and basal cognitive performance level. Controlled laboratory studies can better determine a direct relationship.
EXPERIMENTAL APPROACH
In this study, adult male rhesus monkeys were assessed on visuospatial Paired Associates Learning and Self-Ordered Spatial Search memory tasks, as well as additional tests of motivation and manual dexterity. Subjects were challenged with THC (0.2, 0.5 mg·kg−1, i.m.) in randomized order and evaluated in the presence or absence of 0.5 mg·kg−1 CBD.
KEY RESULTS
CBD attenuated the effects of THC on paired associates learning and a bimanual motor task without affecting the detrimental effects of THC on a Self-Ordered Spatial Search task of working memory. CBD did not significantly reverse THC-induced impairment of a progressive ratio or a rotating turntable task.
CONCLUSIONS AND IMPLICATIONS
This study provides direct evidence that CBD can oppose the cognitive-impairing effects of THC and that it does so in a task-selective manner when administered simultaneously in a 1:1 ratio with THC. The addition of CBD to THC-containing therapeutic products may therefore help to ameliorate unwanted cognitive side-effects.
LINKED ARTICLE
This article is commented on by Mechoulam and Parker, pp 1363–1364 of this issue. To view this commentary visit http://dx.doi.org/10.1111/bph.12400
Keywords: cannabis, marijuana, Macaca mulatta, working memory
Introduction
Studies have shown that acute intoxication with cannabis, or its primary psychoactive constituent Δ9-tetrahydrocannabinol (THC), impairs cognitive function (Braff et al., 1981; Heishman et al., 1989; Wilson et al., 1994; Fant et al., 2010; Kurzthaler et al., 1999). This is even the case when used for medical indications (Corey-Bloom et al., 2012) and may therefore be a significant limitation for medical marijuana. Additional studies suggest that cannabidiol (CBD), a constituent of some cannabis strains, may provide a degree of protection from the acute and lasting cognitive effects of THC. After multiple clinical trials, the combined cannabinoid oral/mucosal spray Sativex, which delivers a 1:1 ratio of CBD : THC, was approved in Canada for spasticity associated with multiple sclerosis; for review see (Oreja-Guevara, 2012). There is some evidence that CBD/THC combinations reduce significant ‘adverse effects’ produced by pure THC, including subjective ratings of intoxication (Robson, 2011; Schoedel et al., 2011), and may reduce cognitive/behavioural impairment (Wade et al., 2004). If so, the moderating effect of CBD might permit the usage of more efficacious THC doses, while reducing adverse events and potentially lasting consequences of THC.
This possibility is echoed by recent studies in humans evaluated after cannabinoid exposure (Juckel et al., 2007; Morgan et al., 2010; 2011). For example, a mixed THC/CBD cannabis extract improved electroencephalographic mismatch negativity activation where THC did not (Juckel et al., 2007) but CBD/THC and THC only treatment both reduced P300 wave amplitude (Roser et al., 2008). More specifically, Morgan et al. showed that smoking CBD-enriched marijuana did not cause the deficits of immediate and delayed prose recall that were caused by CBD-poor cannabis (Morgan et al., 2010) and users habitually exposed to CBD-rich cannabis may have relatively preserved recognition memory versus CBD-poor cannabis users (Morgan et al., 2011).
Such studies cannot, however, rule out the inevitable selection bias associated with individuals who obtain cannabis strains which differ in CBD content through very different quasi-licit or illicit source methods (Burgdorf et al., 2011). Indeed, the subjects studied by Morgan et al. (2010) exhibited group differences in prose recall in the unintoxicated condition. This concern is furthered by a controlled laboratory study which manipulated CBD and THC dose in smoked marijuana and found no effect of CBD on memory impairing effects of THC (Ilan et al., 2005). This study also found a trend for CBD to have differential effects on anxiety self-rating depending on THC dose, thus it may be that CBD : THC ratio is a critical factor in behavioural effects. Together, such observations motivate controlled, preclinical study of CBD to more precisely determine a direct role of this cannabinoid in attenuating THC-induced cognitive disruption.
A prior study from this laboratory (Taffe, 2012b) found that THC impairs monkeys' performance of the visuospatial Paired Associates Learning (vsPAL) and Self-Ordered Spatial Search (SOSS) tasks from the Cambridge Neuropsychological Test Automated Batter (CANTAB); the effects were most specific (task-difficulty dependent) for the vsPAL task. The present study therefore sought to determine if detrimental effects of THC on these tasks could be attenuated by the co-administration of CBD. Additional tests of motor function and motivated responding which are also disrupted by THC were incorporated in the study to determine the potential breadth of behavioural effects of CBD.
Materials and methods
Animals
Ten male rhesus monkeys (Macaca mulatta) were used in these experiments. Animals were of a single birth year and ranged from 12 to 13 years of age during the study. Daily chow [LabDiet® 5038, PMI Nutrition International, Richmond, IN, USA; 3.22 kcal of metabolizable energy (ME) per gram or Teklad® 15% Monkey Diet #8714, Harlan Laboratories, Madison, WI, USA; 3.00 kcal of ME per gram] allocations were supplemented with fruit or vegetables 7 days per week and water was available ad libitum in the home cage. Animals were food restricted with reference to our prior studies to maintain adequate body condition scores and stable behavioural responding; see (Taffe, 2004; Taffe et al., 2004). In some cases, this was accomplished with a fixed daily post-testing feeding amount and in other cases this required adjusting the chow on a daily basis to account for intake of the chow-based reinforcer pellets. Overall, this amounted to daily chow allocations which ranged from 90 to 240 grams; bodyweights at the initiation of the study are provided in Table 1. Animals on this study had previously been immobilized with ketamine (5–20 mg·kg−1) no less than semi-annually for purposes of routine care and some experimental procedures. Animals also had various acute exposures to challenge drugs in additional studies, including THC for a thermoregulatory study (Taffe, 2012a) and additional cognitive investigations (Taffe, 2012b). The United States National Institutes of Health guidelines for laboratory animal care (Clark et al., 1996) were followed and all protocols were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute. These studies are reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010).
Table 1.
Indication of individual subject body weight at start of the study and task participation
| Subject | 530 | 531 | 532 | 533 | 534 | 535 | 537 | 539 | 540 | 541 |
| Wt (kg) | 16.6 | 12.2 | 15.8 | 14.4 | 14.0 | 16.4 | 16.2 | 15.8 | 11.4 | 14.2 |
| BMS | ✓ | ✓ | ✓ | ✓ | ✓ | NT | ✓ | ✓ | ✓ | ✓ |
| PR | ✓ | ✓ | ✓ | NT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| RTT | ✓ | CT5 | ✓ | T5/CT5 | ✓ | CT5 | ✓ | ✓ | ✓ | ✓ |
| SOSS | ✓ | ✓ | ✓ | NT | ✓ | NT | ✓ | NT | NT | ✓ |
| vsPAL | ✓ | ✓ | ✓ | NT | ✓ | ✓ | ✓ | ✓ | NT | ✓ |
NT indicates that the individual was not trained to stable baseline on that task. A check mark indicates the animal participated in all dosing conditions. The T5 and CT5 indicate missing data for the THC 0.5 + Vehicle and THC 0.5 mg·kg−1 + CBD 0.5 mg·kg−1 conditions, respectively. Abbreviations: BMS, Bimanual Motor Skill; NT, Not Trained on the task; PR, Progressive Ratio; RTT, Rotating Turntable; SOSS, Self-Ordered Spatial Search; vsPAL, visuospatial Paired Associates Learning.
Behavioural testing
For behavioural testing, a touch-sensitive computer monitor was placed in front of the caged, unrestrained animal. All subjects had been trained to reach out of the cage to touch the location on the screen at which visual stimuli were presented to obtain a food pellet reward. The computer test battery consisted of three variants of the behavioural tasks included in the non-human primate CANTAB (Cambridge Cognition, Cambridge, UK). General descriptions of the individual tasks and the procedural details have been previously reported (Weed et al., 1999; Taffe et al., 2004; Crean et al., 2011) and the animals participated in daily (Monday–Friday) sessions of about 60 min [extended to 120 min for the bimanual motor skill (BMS) task]. Individual animals only participated for a given task if their baseline levels of performance were stable (ibid) and they completed sufficient trials for analysis under the majority of the dosing conditions.
BMS task
A transparent polycarbonate board (10 cm wide × 25 cm high × 2.75 cm thick) drilled with 15 holes (spaced 13 mm apart in a 3 horizontal × 5 vertical array) was filled with raisins and mounted perpendicular to the door of the transport cage. Subjects acquire a technique wherein they push the raisin out of the hole with one finger before retrieving it with the opposite hand, thus entailing bimanual dexterity. The time elapsed to retrieve all 15 raisins was recorded.
vsPAL
Coloured abstract stimuli were displayed in one of four possible target locations (see Taffe et al., 2002b) and the subject was required to touch this sample stimulus, which then disappeared. The same pattern reappeared during the choice phase in 2, 3 or 4 locations on the screen (the original location plus one or more novel locations) after a 1-s screen blank. The subject was required to touch the stimulus presented in the same location as the sample item to obtain a reinforce delivery. Subjects were allowed up to five additional attempts to successfully complete the set of stimulus-location associations in a given trial, thus measuring incremental learning. Each session consisted of 35 trials in sequential blocks including 5 × 1-stimulus (three choice locations) trials, 10 × 2-stimuli (two choice locations) trials, 10 × 3-stimuli (three choice locations) trials and 10 × 4-stimuli (four choice locations) trials. Performance was measured by percent correct trials on the initial attempt to complete a trial and the percent correct of trials successfully completed within the allowed attempts (repeated-attempt completion).
SOSS
Two or more small coloured rectangles (boxes) were displayed on the screen in positions randomly allocated from 16 possible locations. Subjects were required to select all boxes without revisiting a box once it had been touched for a successful trial completion. A session consisted of 40 trials grouped into eight blocks by trial type as follows: 5 (two boxes), 7 (three boxes), 7 (four boxes), 8 (three boxes), 8 (four boxes), 5 (two boxes). Accuracy scores were calculated for each trial type by dividing the number of correctly completed trials by the number of trials in which there was at least one response.
Progressive-ratio (PR) schedule of reinforcement
Subjects were required to respond to a single coloured rectangle presented in the centre of the screen for pellet reinforcement. The response requirement started at one touch and incremented by arithmetic progression within blocks of eight reinforcers and by geometric progression between blocks of eight (i.e., the first successive eight ratios increase by one, the second successive eight increase by two, the third successive eight increase by four, etc.). The session was terminated after 10 min, or earlier if 3 min elapsed following a response. The primary dependent variable was the number of reinforcers acquired.
Rotating turntable task (RTT)
This test was designed to assess unimanual motor coordination, procedural learning and tracking/targeting of moving objects. A 58 cm opaque white plastic disk containing short radial slots at the edge was mounted to a motor controlled by rheostat. The speed of this turntable was modulated from 0 to 150 rpm. Pellets were placed in the slots and if a monkey successfully retrieved 6 of 10 attempted it was considered to ‘pass’ at a given speed. The speed was then incremented and up to 10 additional pellets are provided. If an animal failed to retrieve or dropped 5 of 10, the trial was considered a ‘fail’ and the speed of the table was reduced for the next attempt. The dependent value for a given session was derived from the speed above which a monkey failed three attempts to reach criterion. That is, the speed changes for a session might go ‘up, up, up… . up, down, up, down, up’ with the speed of the two final ‘down’ changes being recorded as the maximum speed for that session.
Drug challenges
Monkeys were administered acute intramuscular doses (0.2, 0.5 mg·kg−1) of THC, 0.5 mg·kg−1 CBD or vehicle prior to behavioural testing. For injection, CBD or THC was suspended in a vehicle of 95% ethanol, Cremophor EL and saline in a 1:1:18 ratio. Active drug challenges were conducted no more frequently than twice per week at 3–4 day intervals. Since the 0.5 CBD + THC conditions were administered in two injections (different muscular location) all single-compound days included a second vehicle injection and the vehicle day comprised two injections. THC was administered 30 min before the start of behavioural sessions and CBD was administered either 30 min prior to THC (for the BMS task, see below) or concurrently, in two separate injections. Treatment order was pseudo-randomized across individuals such that each animal trained on a given task received each dose condition, but in a different order. The THC was provided by the US National Institute on Drug Abuse and the CBD was purchased from Cayman Chemical (Ann Arbor, MI, USA).
Data analysis
Analysis of the behavioural data employed randomized block anova with a consistent within-subjects factor of drug treatment condition. Analysis of the BMS data employed an additional repeated-measures factor of time post-injection. Two factor repeated-measures analysis of the vsPAL and SOSS trial completion data were necessary to include a factor of trial difficulty in addition to treatment condition. Post hoc analyses of any significant main effects in the multifactor anovas were conducted using the Neuman–Keuls procedure [Fisher's least significant difference (LSD) for the initial pilot Bimanual task study] and the criterion for significance in all tests was P < 0.05. Analyses were conducted with GB-STATv7.0; Dynamic Microsystems, Silver Spring MD, USA.
Results
BMS task
An initial study was conducted using the BMS procedure to verify the necessary pretreatment intervals. For this purpose, animals (n = 9) were assessed on the BMS task immediately prior to the first (0.5 mg·kg−1 CBD or vehicle) injection, 30 min later, immediately prior to the second injection (vehicle or 0.5 mg·kg−1 THC), and then repeatedly at 30, 60, 90 and 120 min after the second injection (Figure 1). The anova confirmed that raisin retrieval speed was altered by treatment condition [F2,16 = 5.21; P < 0.05] and time of determination within the session [F5,40 = 14.21; P < 0.0001]. The Fisher's LSD post hoc analysis did not confirm any differences between the first two task determinations, thus there was no effect of the pretreatment with CBD or vehicle. All subsequent post hoc comparisons focused on the changes after the second injection compared with the BMS evaluation just prior to that treatment. The post hoc test confirmed that raisin retrieval was slowed after the Vehicle + 0.5 mg·kg−1 THC condition (30–120 min after the second injection) compared with both the pre-THC baseline and the Vehicle + Vehicle condition. Retrieval speed was slower after the administration of 0.5 CBD + 0.5 mg·kg−1 THC compared with the pre-THC baseline (90, 120 min after the second injection) and the Vehicle + Vehicle conditions (120 min only). Finally, the retrieval speed differed significantly between the Vehicle + 0.5 mg·kg−1 THC and 0.5 CBD + 0.5 mg·kg−1 THC conditions 60 min after the second injection.
Figure 1.
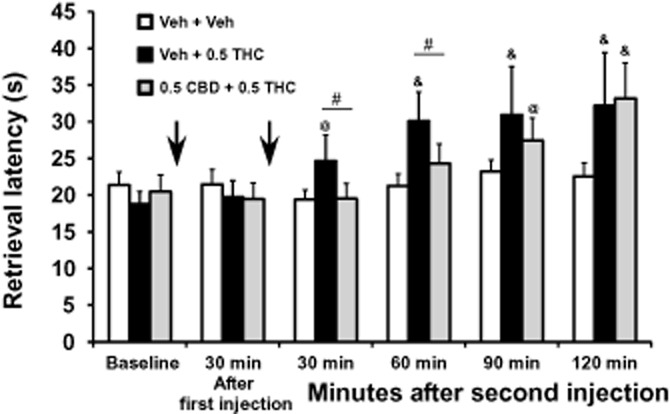
Bimanual Motor Skill Task: The mean (n = 9; ± SEM) latency to retrieve 15 raisins in the Bimanual Motor Skill task is depicted. A significant difference from the baseline and the respective timepoint after vehicle treatment is depicted with the &, from the baseline with @ and between THC and THC/CBD conditions by #. CBD, cannabidiol; THC, Δ9tetrahydrocannabinol. 0.5 = intramuscular dose in mg·kg−1 bodyweight.
These results suggested that protective behavioural effects of CBD would be observed along a similar timecourse as the detrimental effects of THC and therefore all subsequent experiments were conducted with the two injections made in quick succession for a simultaneous treatment interval.
vsPAL task
The administration of THC impaired overall trial completion accuracy, and the percent of trials completed on the vsPAL task, in a dose and trial-difficulty dependent manner as is illustrated in Figure 2. The concurrent administration of 0.5 mg·kg−1 CBD attenuated the deficits induced by THC on the most-difficult, four-stimulus, trials (Figure 3). The design for this task included evaluation of each THC dose (0, 0.2, 0.5 mg·kg−1) with either vehicle or 0.5 CBD co-injection, thus the co-injection was analysed as a third factor in the anova. The analysis confirmed that overall completion accuracy depended on THC treatment condition [F2,14 = 7.35; P < 0.01] and trial difficulty [F3,21 = 12.78; P < 0.0001]. The Neuman–Keuls post hoc procedure confirmed that completion accuracy was lower for three-stimuli trials after Vehicle + 0.5 mg·kg−1 THC compared with the Vehicle + Vehicle condition and after Vehicle + 0.2 mg·kg−1 THC compared with the 0.5 mg·kg−1 CBD + Vehicle condition (Figure 2). Completion accuracy for four-stimuli trials was likewise lower after Vehicle + 0.2 and Vehicle + 0.5 mg·kg−1 THC when compared with both the Vehicle + Vehicle and 0.5 mg·kg−1 CBD + Vehicle conditions.
Figure 2.
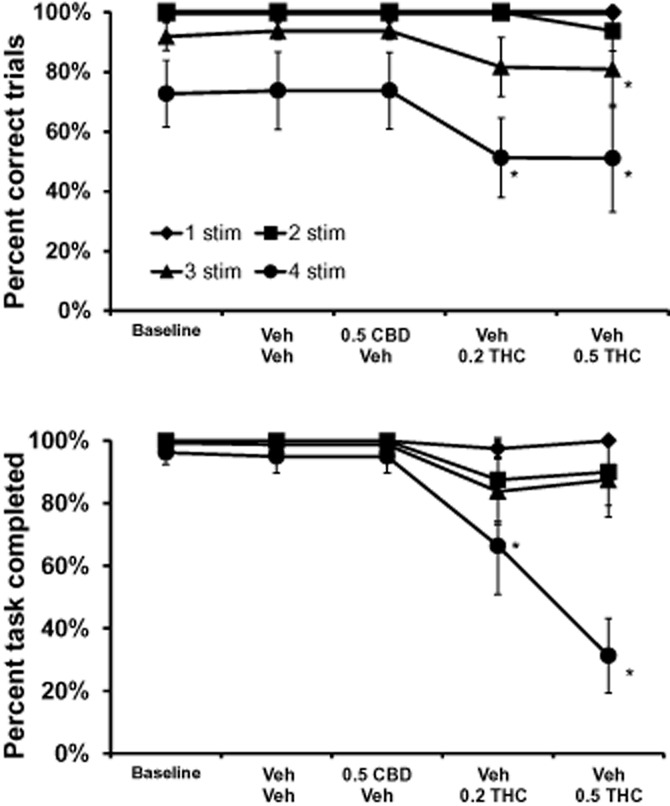
Visuospatial Paired Associates Learning Task: The mean (n = 8; ± SEM) overall task completion accuracy and percent task completed is presented for the four trial difficulty conditions of the visuospatial Paired Associates Learning task. This figure illustrates that the effect of THC administered alone is most pronounced on the most-difficult trial types. A significant difference from the vehicle condition is depicted with *. CBD, cannabidiol; THC, Δ9tetrahydrocannabinol. 0.2, 0.5 = intramuscular dose in mg·kg−1 bodyweight.
Figure 3.
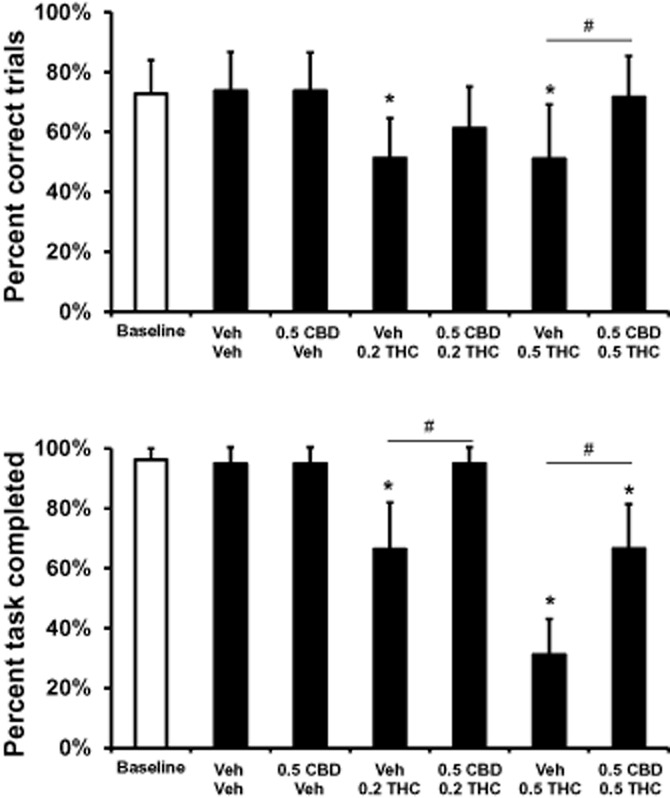
Cannabidiol reverses the THC effect on the vsPAL task: The effect of CBD on the THC-induced impairment of mean (n = 8; ± SEM) overall task completion accuracy and percent task completed is presented for the four-stimulus trials of the visuospatial Paired Associates Learning task. A significant difference from the vehicle condition is depicted with * and differences associated with the presence/absence of CBD with #. CBD, cannabidiol; THC, Δ9tetrahydrocannabinol. 0.2, 0.5 = intramuscular dose in mg·kg−1 bodyweight; vsPAL, visuospatial paired associated learning.
The percent of the task completed (trials on which at least the observing response was made to the sample stimulus) was significantly affected by trial difficulty [F3,21 = 15.44; P < 0.0001] as well as by the interaction of trial difficulty with THC condition [F6,42 = 6.88; P < 0.0001], the interaction of trial difficulty with the pretreatment [F3,21 = 6.81; P < 0.01] as well as by the three-way interaction [F6,42 = 2.72; P < 0.05]. The post hoc test confirmed that the percent of trials completed was significantly lower after 0.2 and 0.5 mg·kg−1 THC compared with the Vehicle + Vehicle or 0.5 CBD + Vehicle conditions.
The post hoc test also confirmed protective effects of 0.5 CBD when administered along with THC (Figure 3). The trial completion accuracy was significantly higher for four-stimuli trials following the administration of 0.5 mg·kg−1 CBD + 0.5 mg·kg−1 THC compared with Vehicle + 0.5 mg·kg−1 THC. In addition, the percent of the task completed was significantly higher for four-stimuli trials when 0.5 CBD was co-administered with either dose of THC.
SOSS task
Trial completion in the SOSS procedure depended on trial difficulty and was impaired by the administration of THC (Figure 4). The overall anova confirmed that overall completion accuracy depended on THC treatment condition [F5,25 = 3.87; P < 0.01] and trial difficulty [F2,10 = 25.52; P < 0.0005]. The Neuman–Keuls post hoc test confirmed that performance of each trial difficulty level differed significantly from each of the other levels within each drug treatment condition. The post hoc test further confirmed that performance was lower than that of the baseline or Vehicle + Vehicle conditions for each trial type after the administration of 0.5 mg·kg−1 THC in either the presence or absence of concurrently administered 0.5 mg·kg−1 CBD co-administration. There were also no differences in performance after 0.5 mg·kg−1 THC treatment associated with whether or not CBD was co-administered.
Figure 4.
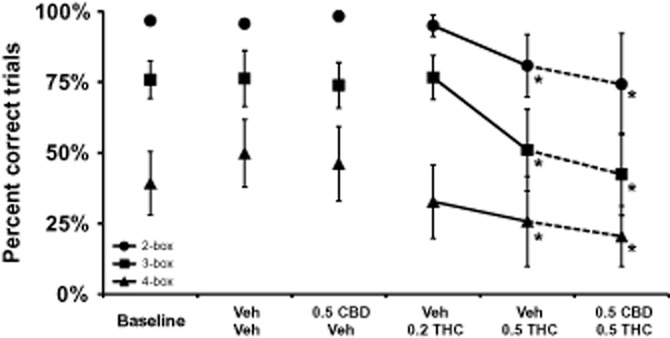
Self-Ordered Spatial Search Task: The mean (n = 6; ± SEM) trial completion accuracy is presented for the three trial types within the Self-Ordered Spatial Search task. A significant difference from the vehicle condition within trial type is depicted with *. CBD, cannabidiol; THC, Δ9tetrahydrocannabinol. 0.2, 0.5 = intramuscular dose in mg·kg−1 bodyweight.
PR task
The number of reinforcers acquired [F5,40 = 4.60; P < 0.01] and the time of last response [F5,40 = 4.11; P < 0.01] were significantly affected by the drug treatment condition (Figure 5). The Neuman–Keuls post hoc tests confirmed that the number of reinforcers acquired was significantly lower than either baseline or vehicle conditions when 0.5 mg·kg−1 THC was administered in either the presence or absence of concurrently administered 0.5 mg·kg−1 CBD. The time of last response differed significantly from baseline and vehicle conditions only after 0.5 mg·kg−1 THC administered with vehicle; the 0.5 mg·kg−1 CBD + 0.5 mg·kg−1 THC condition did not differ significantly.
Figure 5.
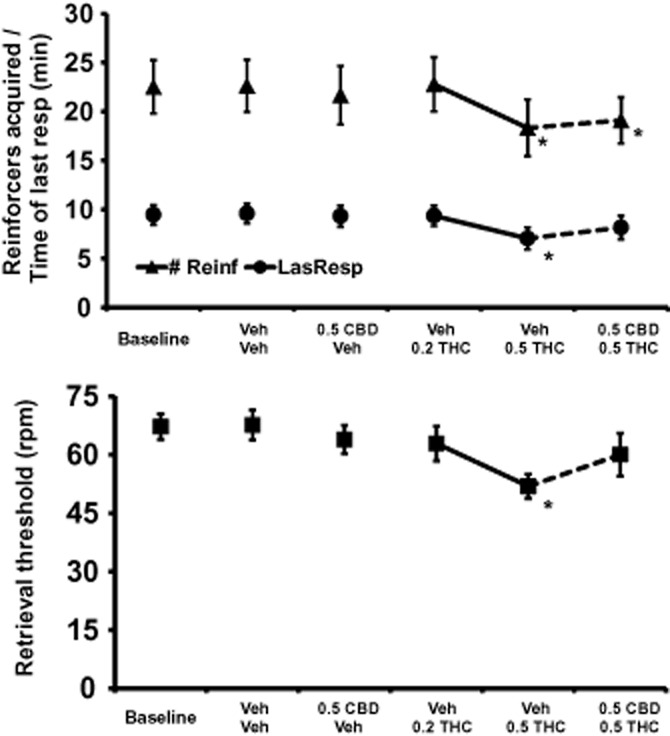
Progressive Ratio and Rotating Turntable Tasks: The mean reinforcers acquired and time of last response in the Progressive Ratio (n = 9; ± SEM) task (upper panel) and the mean retrieval threshold for the Rotating Turntable (n = 10; ± SEM) task (lower panel) are presented. A significant difference from the vehicle condition is depicted with *. CBD, cannabidiol; THC, Δ9tetrahydrocannabinol. 0.2, 0.5 = intramuscular dose in mg·kg−1 bodyweight.
RTT task
The threshold retrieval speed on the RTT task was significantly affected by the treatment condition [F5,45 = 5.14; P < 0.001] were significantly affected by the drug treatment condition (Figure 5) and the Neuman–Keuls post hoc tests confirmed that performance differed from both vehicle and baseline conditions (as well as the concurrent 0.5 mg·kg−1 CBD condition) following 0.5 mg·kg−1 THC. The Neuman–Keuls post hoc test did not confirm any distinction between retrieval speed after treatment with 0.5 mg·kg−1 THC + 0.5 mg·kg−1 CBD and retrieval speed under any other condition.
Discussion
This study provides direct evidence that CBD attenuates some of the cognitive-impairing effects of the primary psychoactive constituent of cannabis, THC, in non-human primates. As with a prior study from this group (Taffe, 2012b), we have confirmed that THC itself impairs behavioural performance in monkeys and that visuospatial associative learning and memory seems to be most specifically vulnerable. These detrimental effects were dependent on task-difficulty in the vsPAL task, thereby showing that THC effects on cognition are not just due to a general sedating or disrupting effect. Although the SOSS task of spatial working memory was impaired by THC, the effects were similar across trial-difficulty conditions and were not reversed by CBD co-administration. This replicates and reinforces a prior observation and further underlines the specificity of low-dose and selective mnemonic effects of THC exposure, as previously discussed (Taffe, 2012b). CBD by itself did not have any behavioural effects, consistent with a prior demonstration of CBD to have no effect on repeated acquisition accuracy in macaques over a range of 0.32–3.2 mg·kg−1, i.m. (Winsauer et al., 1999).
The co-administration of CBD attenuated performance impairments in the vsPAL task produced by THC, but did not restore THC-associated performance deficits in a motivated responding task (PR task) or in a motor coordination and tracking task (Turntable). This outcome suggests that the non-specific motor and motivational effects of THC may be less amenable to reversal with CBD. If so, this puts the Percent Task Completed measure of the vsPAL task in a somewhat different light. It may be most parsimonious to infer that for that task, a failure to respond in a trial may reflect mnemonic uncertainty to some extent and not merely the willingness to respond in the task.
The finding of a selective effect of THC on spatial memory is consistent with reports of relatively high density of the CB1 endocannabinoid receptor in the dentate gyrus and cornu ammonis fields of the hippocampus and the entorhinal cortex of the monkey brain (Eggan and Lewis, 2010). As has been previously discussed at greater length in the context of the vsPAL task (Taffe et al., 2002b; Taffe, 2004; Taffe, 2012b), the dominant contribution to pattern/spatial associative memory in the macaque monkey brain appears to be in the temporal lobe systems (Malkova and Mishkin, 2003; Browning and Gaffan, 2008). Similarly, a functional magnetic resonance imaging (fMRI) study conducted in humans with diagnosed mild cognitive impairment found activity differences in hippocampal areas, but not frontal cortex, during the performance of a human analogue of vsPAL (de Rover et al., 2011). Medial temporal fMRI responses to cognitive tasks are affected in opposite directions by THC and CBD (Bhattacharyya et al., 2009; 2010). Consequently, the present results are highly consistent with task-specific effects of THC on the temporal lobe memory system.
The present study was focused on a demonstration of specific behavioural efficacy of CBD in a 1:1 ration with THC and since additional pharmacological challenges were not performed, it is not warranted to speculate at great length on the mechanisms underlying the behavioural antagonism. It is the case that some effects of CBD may be mediated by interactions with serotonin 1A (5HT1A) receptors (Resstel et al., 2009; Magen et al., 2010; Gomes et al., 2012; Stern et al., 2012) in rodents and there is additional evidence that human cognitive effects of CBD may not be CB1 mediated (Stadelmann et al., 2011). Given the pharmacological promiscuity of CBD (Pertwee, 2009; De Petrocellis and Di Marzo, 2010; Campos et al., 2012), it is probably premature to draw firm conclusions about the mechanism of action.
The timecourse results in the initial bimanual study, and the success of the resulting decision to administer CBD and THC simultaneously, have important practical implications. Existing medications such as Sativex® and the natural product recreational drug (cannabis) result in simultaneous exposure. Thus, the present data do not have to be qualified in translational application on the basis of pretreatment intervals. Likewise, these data suggest that future products would not have to consider separate administration of CBD in advance of any THC-based pharmacotherapy.
This is important to observe, although CBD can reverse a conditioned place aversion produced by 10 mg·kg−1 THC in rats (Vann et al., 2008), a recent paper reported that CBD potentiates the anxiogenic and locomotor suppressant effects of THC in rats treated chronically (Klein et al., 2011). As Zuardi et al. (2012) noted, CBD/THC interactions may depend on the pretreatment offset, at least in rodents. When CBD is administered 30 min (or up to 24 h) prior to THC in rats or mice, a potentiation can be observed whereas simultaneous administration results in blockade or amelioration of THC effects. The picture may be complicated even further by a suggestion that CBD/THC ratios on the order of eight are necessary for antagonistic properties and a ratio of only 1.8 for the potentiation of detrimental THC effects in rodents (Zuardi et al., 1984). An early study reported that 30 mg·kg−1 CBD blocked THC-induced reductions in fixed-interval responding for food when administered 60 min prior to 0.3 mg·kg−1 THC in macaques, but had no effect prior to 1.0 mg·kg−1 THC (Brady and Balster, 1980). However, this study also reported a performance impairment following 30 mg·kg−1, but not 10 mg·kg−1, CBD i.m. when administered without any other drug.
The data from the present study suggest that a CBD/THC ratio that is no greater than 1:1 may be sufficient in primates, thereby reinforcing the studies by Morgan et al. (2010) and Morgan et al. (2011) since their reported CBD/THC ratios from street cannabis were generally less than 1:1. It is similarly important that the overall CBD dose was low and caused no deficits by itself, in contrast to the results of Brady and Balster (1980). One prior human study which manipulated THC and CBD ratios and found no overall effect on cognition used a 2:1 CBD : THC ratio for the high dose THC condition and noted that in human studies a lack of strong dose-dependent effects of THC may be due to subject expectancy (Ilan et al., 2005). The present data suggest that future attempts to verify the findings of Morgan et al. in repeated-measures human experimental studies should use a range of CBD : THC ratios. Similarly, it should be noted that one limitation of this study was the use of only one dose of CBD; additional studies using a broader range of doses would be of significant interest.
One important technical outcome of the present study was the stability of the behaviour and the effect of THC on behaviour over the course of the repeated-measures design. This was critical because repeated dosing with THC can often produce profound tolerance. As with many of our prior studies (Taffe et al., 1999; 2002a; Von Huben et al., 2006; Taffe, 2012b; Wright et al., 2012), the behavioural baselines under untreated conditions (i.e. during non-challenge days of the week) were stable across the entire study interval. Perhaps more importantly, the dose range of THC that was effective at altering performance was consistent across the entire study. This was the case when data were inspected at the individual level and reflected, for example, in the vsPAL and SOSS tasks which were run on different days thus the effect of a given dosing condition could be compared.
This study may also support the development of high-CBD strains of cannabis for medical marijuana purposes. A recent study confirmed that smoked cannabis [National Institute on Drug Abuse products with ∼4% THC and less than 0.01% CBD; (RTI_International, 2012)] can alleviate spasticity in multiple sclerosis but noted that unwanted cognitive and subjective effects might limit use (Corey-Bloom et al., 2012). It is possible that use of strains that are relatively high in CBD may attenute side effects. One additional intriguing question raised by the present study, and the findings of Morgan et al. (2010), relates to recent demonstration that cannabis users who start in adolescence may experience essentially permanent decrements of full-scale IQ (Meier et al., 2012). It may be the case that the degree of lasting impairment could be modulated by CBD content. Additional study using preclinical models of complex cognition are necessary to further explore the potential for high-CBD strains of cannabis to constitute an improved product for the rapidly expanding medical marijuana market and possibly a less harmful recreational product.
Acknowledgments
The authors are grateful to the other principal investigators of the Scripps Center for Cannabis Addiction Neurobiology, Drs. Barbara J. Mason, Loren H. Parsons and Susan F. Tapert for many helpful discussions. This work was supported by US Public Health Service/National Institutes of Health grant DA024194; those bodies had no further role in the design, conduct or analysis of the study. Dr. Wright was supported by training grant T32 AA007456. The authors do not have any conflicts of interest to declare for this work. This is publication #21931 from The Scripps Research Institute.
Glossary
- BMS
bimanual motor skill
- CANTAB
Cambridge Neuropsychological Test Automated Battery
- CBD
cannabidiol
- PR
progressive ratio
- RTT
rotating turntable
- SOSS
Self-Ordered Spatial Search
- THC
Δ9tetrahydrocannabinol
- vsPAL
visuospatial paired associate learning
Conflict of interest
The authors declare no conflict of interest.
References
- Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O′Carroll C, et al. Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch Gen Psychiatry. 2009;66:442–451. doi: 10.1001/archgenpsychiatry.2009.17. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Balster RL. The effects of delta 9-tetrahydrocannabinol alone and in combination with cannabidiol on fixed-interval performance in rhesus monkeys. Psychopharmacology (Berl) 1980;72:21–26. doi: 10.1007/BF00433803. [DOI] [PubMed] [Google Scholar]
- Braff DL, Silverton L, Saccuzzo DP, Janowsky DS. Impaired speed of visual information processing in marijuana intoxication. Am J Psychiatry. 1981;138:613–617. doi: 10.1176/ajp.138.5.613. [DOI] [PubMed] [Google Scholar]
- Browning PG, Gaffan D. Impairment in object-in-place scene learning after uncinate fascicle section in macaque monkeys. Behav Neurosci. 2008;122:477–482. doi: 10.1037/0735-7044.122.2.477. [DOI] [PubMed] [Google Scholar]
- Burgdorf JR, Kilmer B, Pacula RL. Heterogeneity in the composition of marijuana seized in California. Drug Alcohol Depend. 2011;117:59–61. doi: 10.1016/j.drugalcdep.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimaraes FS. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc Lond B Biol Sci. 2012;367:3364–3378. doi: 10.1098/rstb.2011.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JD, Baldwin RL, Bayne KA, Brown MJ, Gebhart GF, Gonder JC, et al. Guide for the Care and Use of Laboratory Animals. Washington, DC: Institute of Laboratory Animal Resources, National Research Council; 1996. [Google Scholar]
- Corey-Bloom J, Wolfson T, Gamst A, Jin S, Marcotte TD, Bentley H, et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ. 2012;184:1143–1150. doi: 10.1503/cmaj.110837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Vandewater SA, Katner SN, Huitron-Resendiz S, Taffe MA. Chronic alcohol consumption impairs visuo-spatial associative memory in periadolescent rhesus monkeys. Drug Alcohol Depend. 2011;114:31–40. doi: 10.1016/j.drugalcdep.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Di Marzo V. Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G-protein-coupled receptors and transient receptor potential channels. J Neuroimmune Pharmacol. 2010;5:103–121. doi: 10.1007/s11481-009-9177-z. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Fant RV, Heishman SJ, Bunker EB, Pickworth WB. Acute and residual effects of marijuana in humans. Pharmacol Biochem Behav. 1998;60:777–784. doi: 10.1016/s0091-3057(97)00386-9. [DOI] [PubMed] [Google Scholar]
- Gomes FV, Reis DG, Alves FH, Correa FM, Guimaraes FS, Resstel LB. Cannabidiol injected into the bed nucleus of the stria terminalis reduces the expression of contextual fear conditioning via 5-HT1A receptors. J Psychopharmacol. 2012;26:104–113. doi: 10.1177/0269881110389095. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Yingling JE. Effects of tetrahydrocannabinol content on marijuana smoking behavior, subjective reports, and performance. Pharmacol Biochem Behav. 1989;34:173–179. doi: 10.1016/0091-3057(89)90369-9. [DOI] [PubMed] [Google Scholar]
- Ilan AB, Gevins A, Coleman M, ElSohly MA, de Wit H. Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behav Pharmacol. 2005;16:487–496. doi: 10.1097/00008877-200509000-00023. [DOI] [PubMed] [Google Scholar]
- Juckel G, Roser P, Nadulski T, Stadelmann AM, Gallinat J. Acute effects of Delta9-tetrahydrocannabinol and standardized cannabis extract on the auditory evoked mismatch negativity. Schizophr Res. 2007;97:109–117. doi: 10.1016/j.schres.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, et al. Cannabidiol potentiates Delta(9)-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology (Berl) 2011;218:443–457. doi: 10.1007/s00213-011-2342-0. [DOI] [PubMed] [Google Scholar]
- Kurzthaler I, Hummer M, Miller C, Sperner-Unterweger B, Gunther V, Wechdorn H, et al. Effect of cannabis use on cognitive functions and driving ability. J Clin Psychiatry. 1999;60:395–399. doi: 10.4088/jcp.v60n0609. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magen I, Avraham Y, Ackerman Z, Vorobiev L, Mechoulam R, Berry EM. Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br J Pharmacol. 2010;159:950–957. doi: 10.1111/j.1476-5381.2009.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova L, Mishkin M. One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J Neurosci. 2003;23:1956–1965. doi: 10.1523/JNEUROSCI.23-05-01956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109:E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [corrected] Br J Psychiatry. 2010;197:285–290. doi: 10.1192/bjp.bp.110.077503. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Gardener C, Schafer G, Swan S, Demarchi C, Freeman TP, et al. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol Med. 2011;29:1–10. doi: 10.1017/S0033291711001322. [DOI] [PubMed] [Google Scholar]
- Oreja-Guevara C. Clinical efficacy and effectiveness of Sativex, a combined cannabinoid medicine, in multiple sclerosis-related spasticity. Expert Review of Neurotherapeutics. 2012;12(4 Suppl):3–8. doi: 10.1586/ern.12.11. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol. 2009;156:397–411. doi: 10.1111/j.1476-5381.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Tavares RF, Lisboa SF, Joca SR, Correa FM, Guimaraes FS. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br J Pharmacol. 2009;156:181–188. doi: 10.1111/j.1476-5381.2008.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson P. Abuse potential and psychoactive effects of delta-9-tetrahydrocannabinol and cannabidiol oromucosal spray (Sativex), a new cannabinoid medicine. Expert Opin Drug Saf. 2011;10:675–685. doi: 10.1517/14740338.2011.575778. [DOI] [PubMed] [Google Scholar]
- Roser P, Juckel G, Rentzsch J, Nadulski T, Gallinat J, Stadelmann AM. Effects of acute oral Delta9-tetrahydrocannabinol and standardized cannabis extract on the auditory P300 event-related potential in healthy volunteers. Eur Neuropsychopharmacol. 2008;18:569–577. doi: 10.1016/j.euroneuro.2008.04.008. [DOI] [PubMed] [Google Scholar]
- de Rover M, Pironti VA, McCabe JA, Acosta-Cabronero J, Arana FS, Morein-Zamir S, et al. Hippocampal dysfunction in patients with mild cognitive impairment: a functional neuroimaging study of a visuospatial paired associates learning task. Neuropsychologia. 2011;49:2060–2070. doi: 10.1016/j.neuropsychologia.2011.03.037. [DOI] [PubMed] [Google Scholar]
- RTI_International. 2012. Data Sheet: Marijuana Cigarettes Vol. 12792-0109-120, 99 1–2: RTI International.
- Schoedel KA, Chen N, Hilliard A, White L, Stott C, Russo E, et al. A randomized, double-blind, placebo-controlled, crossover study to evaluate the subjective abuse potential and cognitive effects of nabiximols oromucosal spray in subjects with a history of recreational cannabis use. Hum Psychopharmacol. 2011;26:224–236. doi: 10.1002/hup.1196. [DOI] [PubMed] [Google Scholar]
- Stadelmann AM, Juckel G, Arning L, Gallinat J, Epplen JT, Roser P. Association between a cannabinoid receptor gene (CNR1) polymorphism and cannabinoid-induced alterations of the auditory event-related P300 potential. Neurosci Lett. 2011;496:60–64. doi: 10.1016/j.neulet.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Stern CA, Gazarini L, Takahashi RN, Guimaraes FS, Bertoglio LJ. On disruption of fear memory by reconsolidation blockade: evidence from cannabidiol treatment. Neuropsychopharmacology. 2012;37:2132–2142. doi: 10.1038/npp.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA. Effects of parametric feeding manipulations on behavioral performance in macaques. Physiol Behav. 2004;81:59–70. doi: 10.1016/j.physbeh.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Taffe MA. Delta9-Tetrahydrocannabinol attenuates MDMA-induced hyperthermia in rhesus monkeys. Neuroscience. 2012a;201:125–133. doi: 10.1016/j.neuroscience.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA. Delta(9)Tetrahydrocannabinol impairs visuo-spatial associative learning and spatial working memory in rhesus macaques. J Psychopharmacol. 2012b;26:1299–1306. doi: 10.1177/0269881112443743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gold LH. Scopolamine alters rhesus monkey performance on a novel neuropsychological test battery. Brain Res Cogn Brain Res. 1999;8:203–212. doi: 10.1016/s0926-6410(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Davis SD, Gutierrez T, Gold LH. Ketamine impairs multiple cognitive domains in rhesus monkeys. Drug Alcohol Depend. 2002a;68:175–187. doi: 10.1016/s0376-8716(02)00194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Differential muscarinic and NMDA contributions to visuo-spatial paired-associate learning in rhesus monkeys. Psychopharmacology (Berl) 2002b;160:253–262. doi: 10.1007/s00213-001-0954-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Modeling a task that is sensitive to dementia of the Alzheimer's type: individual differences in acquisition of a visuo-spatial paired-associate learning task in rhesus monkeys. Behav Brain Res. 2004;149:123–133. doi: 10.1016/s0166-4328(03)00214-6. [DOI] [PubMed] [Google Scholar]
- Vann RE, Gamage TF, Warner JA, Marshall EM, Taylor NL, Martin BR, et al. Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Delta(9)-tetrahydrocannabinol. Drug Alcohol Depend. 2008;94:191–198. doi: 10.1016/j.drugalcdep.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Huben SN, Davis SA, Lay CC, Katner SN, Crean RD, Taffe MA. Differential contributions of dopaminergic D1- and D2-like receptors to cognitive function in rhesus monkeys. Psychopharmacology (Berl) 2006;188:586–596. doi: 10.1007/s00213-006-0347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004;10:434–441. doi: 10.1191/1352458504ms1082oa. [DOI] [PubMed] [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, et al. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Brain Res Cogn Brain Res. 1999;8:185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Wilson WH, Ellinwood EH, Mathew RJ, Johnson K. Effects of marijuana on performance of a computerized cognitive- neuromotor test battery. Psychiatry Res. 1994;51:115–125. doi: 10.1016/0165-1781(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Lambert P, Moerschbaecher JM. Cannabinoid ligands and their effects on learning and performance in rhesus monkeys. Behav Pharmacol. 1999;10:497–511. doi: 10.1097/00008877-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Jr, Vandewater SA, Angrish D, Dickerson TJ, Taffe MA. Mephedrone (4-methylmethcathinone) and d-methamphetamine improve visuospatial associative memory, but not spatial working memory, in rhesus macaques. Br J Pharmacol. 2012;167:1342–1352. doi: 10.1111/j.1476-5381.2012.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, Teixeira NA, Karniol IC. Pharmacological interaction of the effects of delta 9-trans-tetrahydrocannabinol and cannabidiol on serum corticosterone levels in rats. Arch Int Pharmacodyn Ther. 1984;269:12–19. [PubMed] [Google Scholar]
- Zuardi AW, Hallak JE, Crippa JA. Interaction between cannabidiol (CBD) and (9)-tetrahydrocannabinol (THC): influence of administration interval and dose ratio between the cannabinoids. Psychopharmacology (Berl) 2012;219:247–249. doi: 10.1007/s00213-011-2495-x. [DOI] [PubMed] [Google Scholar]


