Abstract
BACKGROUND AND PURPOSE
Cannabis is the most popular drug used in the European Union, closely followed by cocaine. Whereas cannabis impairs neurocognitive function in occasional cannabis users, such impairments appear less prominent in heavy users, possibly as a result of tolerance. The present study was designed to assess whether the impairing effects of Δ9-tetrahydrocannabinol (THC) in heavy cannabis users would present in a wide range of neuropsychological functions or selectively affect specific performance domains. We also assessed the acute effects of cocaine on neurocognitive functions of heavy cannabis users.
EXPERIMENTAL APPROACH
Heavy cannabis users, who had a history of cocaine use (n = 61), participated in a double-blind, placebo-controlled, three-way crossover study. Subjects received single doses of cocaine HCl (300 mg), cannabis (THC μg·kg−1) and placebo, and completed a number of tests measuring impulse control and psychomotor function.
KEY RESULTS
Single doses of cannabis impaired psychomotor function and increased response errors during impulsivity tasks. Single doses of cocaine improved psychomotor function and decreased response time in impulsivity tasks, but increased errors.
CONCLUSIONS AND IMPLICATIONS
Heavy cannabis users display impairments in a broad range of neuropsychological domains during THC intoxication. Impairments observed in psychomotor tasks, but not in impulsivity tasks, appeared smaller in magnitude as compared with those previously reported in occasional cannabis users. Heavy cannabis users were sensitive to the stimulating and inhibitory effects of cocaine on psychomotor function and impulsivity respectively. The reduction in proficiency in impulse control may put drug users at increased risk of repeated drug use and addiction.
Keywords: cannabis, cocaine, tolerance, cross-tolerance, impulsivity, addiction
Introduction
Cannabis is the most frequently used illicit drug in the Western world, with up to 32.2% of adults in the European Union indicating that they have used the drug at least once in their lifetime (EMCDDA, 2011). Data suggest that even though spontaneous cessation of cannabis use occurs, a multitude of cannabis users develop a stable pattern of use, characterized by frequent use of cannabis. In one study, up to 74.2% of occasional cannabis users went on to develop regular use patterns (Perkonigg et al., 1999).
Cannabis consists of several cannabinoids, with Δ9-tetrahydrocannabinol (THC) being the psychoactive compound. Acute administration of cannabis in both animals and humans causes impairment in a number of cognitive domains. Administration of a cannabinoid receptor agonist in rats mainly leads to impairments of memory acquisition and working memory (Zanettini et al., 2011), and low doses of cannabis increased motor impulsivity in rodents (Childers and Breivogel, 1998). Similar findings have been reported in humans as single doses of cannabis impaired memory, attention and motor skills (Solowij, 1998; Gonzalez, 2007). However, several lines of evidence demonstrate that the level of cannabis-induced impairment is inversely related to history of cannabis use. Performance impairments are very prominent in occasional cannabis users (McDonald et al., 2003; Ramaekers et al., 2006a; Crean et al., 2012), but are virtually absent in frequent, heavy users of cannabis (Ramaekers et al., 2009; Hart et al., 2010). The latter has been interpreted as the development of tolerance after repeated cannabis use (Ramaekers et al., 2009; Hart et al., 2010). Studies in frequent cannabis users indicate that single doses of cannabis do not affect a number of cognitive functions such as critical tracking, divided attention or decision making (Ramaekers et al., 2006b; 2009; 2011) when compared with placebo. However, it is not clear whether tolerance to the impairing effects of cannabis extends to every neuropsychological domain. Studies assessing THC effects on impulse control of heavy cannabis users appear to indicate impairment that is not subject to tolerance. This is corroborated by a study in chronic cannabis users who showed impairment on a stop-signal task (SST) at THC levels greater than 10 ng·mL−1, but not when THC blood concentrations were below this level (Ramaekers et al., 2006b; 2009). These findings were also corroborated by Metrik et al. (2012) who demonstrated that single doses of cannabis impaired impulse control in heavy cannabis users and that these users were unable to behaviourally compensate for the intoxication effects. The current literature thus appears to indicate that tolerance to the impairing effect of THC consistently develops in a number of psychomotor domains, but may be absent or less prominent in some specific neuropsychological functions requiring impulse control.
Animal research has indicated that it is likely cross-tolerance occurs between cannabis and cocaine. Pistis et al. (2004) showed that the dopamine neurons in the midbrain of adolescent rats chronically administered the cannabinoid agonist WIN 55212.2 were less responsive to cocaine than those of drug-naive rats. This suggests that after subchronic cannabis administration at a young age (in rats) subsequent responses to other drugs of abuse, including cocaine, may be altered due to neuronal adaptation. The authors further speculated that this adaptation could potentially lead to a decrease in responses to naturally occurring rewarding and motivating stimuli, ultimately leading to increased vulnerability for use of other drugs. In addition, prior exposure to cannabis has been shown to decrease the reinforcing effects and enhance the anxiogenic effects of cocaine (Panlilio et al., 2007), indicating that THC might potentiate some of the effects of cocaine while negating others. Thus, animal studies indicate the possibility of cross-tolerance to the effects of cocaine after chronic cannabinoid administration. It is not unlikely that humans who are tolerant to cannabis will also develop tolerance to the effects of cocaine. If this is the case, it would affect a substantial number of cannabis users as 6–20% of cannabis users in the European Union have used cocaine recently (EMCDDA, 2009).
Cocaine is a stimulating drug which elevates mood, increases feelings of well-being, energy and alertness and has long been shown to improve performance and reaction speed on measures of information processing, vigilance and divided attention (Burns, 1991; 1992). Most research on the acute effects of cocaine has focused on impulse control (Fillmore et al., 2002; 2005; 2006; Garavan et al., 2008), showing that cocaine in general facilitates inhibitory control while decreasing or not affecting reaction times, thereby improving the speed/accuracy trade-off. In line with previous animal research, it is not unlikely that cross-tolerance to the improvement in motor control induced by cocaine will occur in heavy cannabis users.
The present study was designed to assess whether the impairing effects of THC in heavy cannabis users would present in a wide range of neuropsychological functions or selectively affect specific performance domains. A second aim was to assess the acute effects of cocaine on neurocognitive functions of heavy cannabis users. In order to test these aims, a number of psychomotor and cognitive tests were included that have previously demonstrated THC-induced impairments in occasional but not in heavy users [i.e. divided attention task (DAT), critical tracking task (CTT), Tower of London (TOL)], as well as a number of impulsivity tasks [SST, matching familiar figures task (MFF)] for which THC-induced impairment has been reported in occasional users, but for which presence in heavy users has been disputed (Ramaekers et al., 2006a, b; 2009; 2011). Relative to the latter studies, we increased sample size by a factor 2–3 in order to increase statistical power.
Methods
Subjects
Sixty-one healthy regular cannabis and cocaine users (48 men, 13 women) participated in the study (for demographics, see Table 1).
Table 1.
Mean age, weight, lifetime drug use (total number of times used) and drug use in years of the 61 subjects enrolled in the study
| Mean | SD | Min | Max | Drug use (years) | |
|---|---|---|---|---|---|
| Age (years) | 23.05 | 3.04 | 18.3 | 32.4 | |
| Weight (kg) | 69.34 | 9.98 | 51 | 91 | |
| Cannabis | 1027.43 | 1329.79 | 40 | 6 000 | 6.96 |
| Cocaine | 56.54 | 85.58 | 4 | 450 | 3.31 |
| Alcohol | 1063.00 | 2714.01 | 50 | 19 200 | 8.54 |
| Ecstasy | 26.81 | 30.74 | 0 | 125 | 4.00 |
| Amphetamines | 44.71 | 167.79 | 1 | 1 000 | 2.72 |
| Mushrooms | 7.84 | 14.33 | 0 | 75 | 2.88 |
| LSD | 7.88 | 8.17 | 0 | 20 | 1.64 |
| Other | 17.93 | 37.45 | 0 | 150 | 1.64 |
Max, maximum; Min, minimum.
Subjects were recruited through advertisements in local newspapers and by word of mouth. Before inclusion, subjects were examined by a study physician, who checked for general health and took blood and urine samples for standard chemistry and haematology. Inclusion criteria were age, 18–40 years; regular cannabis use, two or more times per week; cocaine use, more than five times in the previous year; free from psychotropic medication; good physical health; normal weight (body mass index 18–28); and written informed consent. Exclusion criteria were dependence on cocaine according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria; presence or history of psychiatric or neurological disorder as assessed during a clinical interview; pregnancy or lactating; cardiovascular abnormalities as measured by EKG; hypertension; and excessive drinking (>20 U per week) or smoking (>20 cigarettes per day).
This study was part of a larger trial on the association between drug use and impulse control (see Dutch Trial Register, trial number NTR2127) conducted according to the code of ethics on human experimentation established by the Declaration of Helsinki (1964) and amended in Seoul (2008), and was approved by the Medical Ethics Committee of the Academic Hospital of Maastricht and Maastricht University. A permit for obtaining, storing and administering cocaine and cannabis was obtained from the Dutch Drug Enforcement Administration. Subjects were paid for their participation in the study.
Design, doses and administration
Subjects participated in a double-blind, placebo-controlled, three-way crossover study. Treatments were placebo, 300 μg·kg−1 of THC and 300 mg of cocaine HCl. Cannabis was administered through a vaporizer (Volcano) obtained from Storz & Bickel GmbH & Co. (Tuttlingen, Germany) and was used according to the manual provided by the producer. Cannabis inhalation took place in a standardized manner (Hazekamp et al., 2006). The vapour was prepared from batches containing 11% THC, a standard potency for cannabis sold at Dutch pharmacies for medical/medicinal use. Cannabidiol levels in the cannabis breed were less than 1%. Preparation of cannabis and cannabis placebo were similar to previous studies from our group (Toennes et al., 2008; Ramaekers et al., 2009). Cocaine HCl or placebo was administered in an opaque white capsule. Conditions were separated by a minimum washout period of 7 days to avoid cross-condition contamination. Order of conditions was balanced over subjects and sessions.
Procedures
A test day started with a urine drug screen and a breath alcohol screen; female subjects underwent an additional pregnancy test. If negative (except cannabis), subjects could proceed with breakfast, followed by vital sign measurements and blood samples. Immediately hereafter, subjects received a capsule containing either 300 mg of cocaine HCl or placebo orally (T1). Forty-five minutes after capsule administration, subjects inhaled cannabis, 300 μg·kg−1 of body wt, or placebo (T2). Immediately after, vital signs were measured, blood samples were taken and the visual analogue scale (VAS) questionnaire was administered followed by the test battery. The latter was conducted between 15 and 60 min following T2. Between T1 and T2, subjects were allowed to read a book or watch television.
Prior to experimental sessions, subjects were familiarized with the procedures and tasks. Subjects had to refrain from all drugs of abuse (except cannabis) at least a week before the start of the experiment until the end of the study.
Impulsivity tests
MFF task
This task is a measure of reflection impulsivity, which is the tendency to reflect on the validity of problem solving under the special condition of several possible alternatives. Thus, subjects who respond quickly often make mistakes, whereas those who reflect on response alternatives are more often correct (Messer, 1976). For a more detailed description of tasks specifics, see van Wel et al. (2012). Two dependent measures, mean latency to first response and total number of errors, were automatically recorded. Two additional dependent variables can be calculated (Perales et al., 2009): an Impulsivity score (I-score) and an Efficiency score (E-score).
The SST
The SST measures motor impulsivity, which is defined as the inability to inhibit an activated or pre-cued response leading to errors of commission (No-Go errors). The current test has been validated for showing stimulant and sedative drug effects (Ramaekers and Kuypers, 2006). Dependent variables are the proportion of commission errors on stop trials with a maximum of 48, omission errors on go trials and the reaction times on go and stop signal trials (i.e. stop reaction time). Stop reaction time to stop signal trials represents the estimated mean time required to inhibit a response. The method for calculating stop reaction time is taken from the race model of inhibitory control (Logan and Cowan, 1984). The resulting values for each stop signal delay are averaged to yield a single measure of stop reaction time for the test.
Psychomotor tests
CTT
The CTT (Jex et al., 1966) measures the subject's ability to control a displayed error signal in a first-order compensatory tracking task. Error is displayed as a horizontal deviation of a cursor from the midpoint on a horizontal, linear scale. Compensatory joystick movements null the error by returning the cursor to the midpoint. The frequency at which the subject loses the control is the critical frequency or lambda (λc). The test includes five trials of which the lowest and the highest score are removed; the average of the remaining scores is taken as the final λc score.
DAT
The DAT measures the ability to divide attention between two tasks performed simultaneously. Subjects were asked to perform the same tracking task as described earlier, but now at a constant level of difficulty. As a secondary task, the subject was instructed to monitor 24 single digits (0–9) that were presented in the four corners of the computer screen (six digits per corner). These numbers changed asynchronously every 5 s. The subjects were instructed to react to the target number ‘2’ by removing their foot as fast as possible from a pedal switch and return. Inter-target time varied between 5 and 25 s with a maximum of 48 targets. Average tracking error, correct responses and mean reaction time (in ms) to the target are the performance measures in this secondary subtask.
TOL
The TOL is a decision-making task that measures executive function and planning (Shallice, 1982). The current version consists of computer-generated images of initial and target arrangements of three coloured balls on three sticks. The subject decides as quickly as possible, whether the end arrangement can be accomplished in two, three, four or five steps from the initial arrangement by pushing the corresponding coded button. The total number of correct decisions and mean reaction time (in seconds) are the main outcome measures.
Subjective measures
Visual analogue scales
Two VAS (10 cm) were presented to participants where they had to indicate how ‘high’ or how ‘active’ they felt at that moment, compared with the most high or active ever (0 represented not high or active at all, 10 extremely high or active).
Pharmacokinetic assessments
Blood samples to determine drug concentrations were taken at baseline, 50 and 110 min after administration of a cocaine or placebo capsule. Blood samples were centrifuged at 2200 × g, and serum was frozen at −20°C until analysis for pharmacokinetic assessments. For cannabinoid determinations serum was used (Serum-Gel Vacuette system of Greiner Bio-One, Alphen a/d Rijn; The Netherlands); cocaine and metabolites were determined in plasma (glucose FX Vacuette system containing 2.5 mg·mL−1 of sodium fluoride and 2.0 mg·mL−1 of potassium oxalate). The determination of THC, 11-hydroxy-THC, 11-nor-9-carboxy-THC, cocaine, benzoylecgonine and ecgonine methyl ester in plasma was performed in a specialized forensic toxicological laboratory using validated procedures (Toennes et al., 2005; Kauert et al., 2006).
Statistics
All cognitive measures were analysed with Statistical Package for the Social Sciences 18.0 (SPSS, Chicago, IL, USA) using a general linear model (GLM) univariate anova with drug (three levels) as within subject factor. If the sphericity assumption was violated, the Greenhouse–Geisser correction was used. In case of significant main drug effects, separate drug–placebo contrasts were conducted. The univariate model tested for main effects of drug, which was followed by separate drug–placebo contrasts. A sequential Bonferroni procedure was applied to correct for multiple testing. Potential associations between cannabis use history and drug-induced performance impairments were assessed by means of Pearson r correlations between drug use history and drug-induced change scores from placebo.
The α-criterion significance level was set to 0.05.
Results
All subjects for whom complete performance data sets were collected entered the GLM analyses. Due to side effects (nausea) in the cannabis condition, four subjects could only partially complete the test battery. In sum, 59 complete data sets were collected for the MFF20, 58 for the TOL, and 57 for the SST and CTT. Due to technical failures, only 50 complete data sets could be used in the DAT.
Impulsivity tests
Mean (and SEM) performance scores for the MFF and SST are shown in Figures 1 and 2. THC decreased E-score [F(1, 58) = 15.97, P < 0.001] and increased the number of errors [F(1,58) = 7.90, P = 0.007]. Mean latency of first response increased and decreased following THC and cocaine administration, respectively, but these changes just failed to reach significance. THC and cocaine did not affect I-scores. On the SST, subjects under the influence of cocaine or cannabis made significantly more commission errors [F(1, 56) = 8.51, P = 0.005 and F(1,56) = 7.47, P = 0.003 respectively]. Cocaine also decreased reaction time in Go trials [F(1,56) = 10.19, P = 0.002]. Stop reaction time and number of omission errors were not affected by cannabis and cocaine. There was no correlation between history of cannabis use and performance impairments during cannabis or cocaine on any of the variables of the SST.
Figure 1.
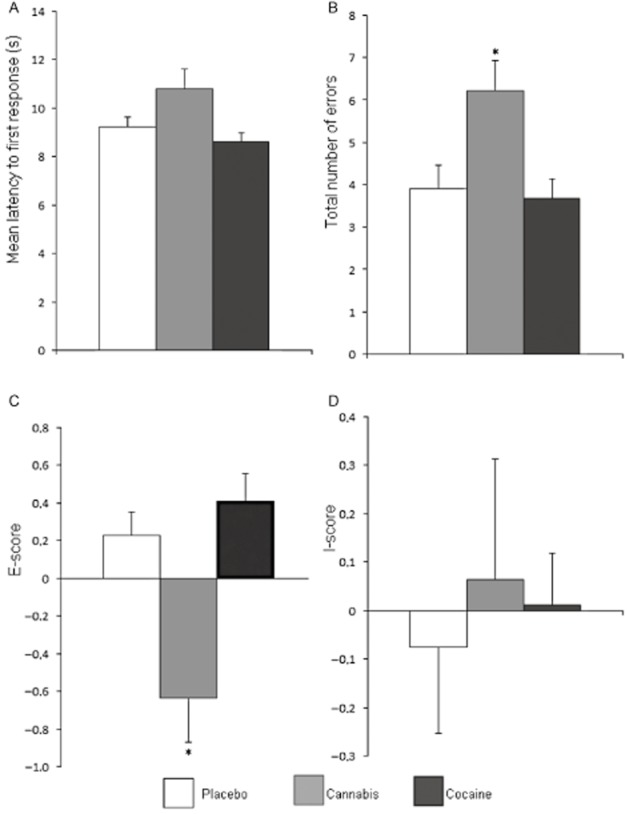
Mean (and SEM) performance in the matching familiar figures (MFF20) test during cannabis or cocaine intoxication or placebo. Performance parameters include (A) mean latency to first response, (B) total number of errors, (C) E-score and (D) I-score. *Significant differences between drug and placebo groups with P < 0.05.
Figure 2.
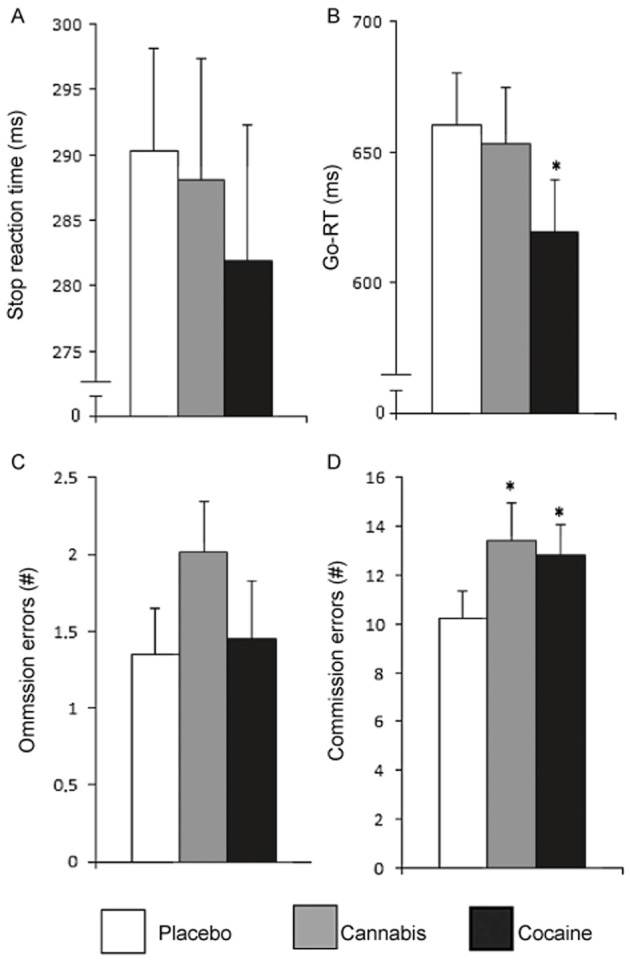
Mean (and SEM) performance in the SST during cannabis or cocaine intoxication or placebo. Task parameters include (A) stop reaction time (B) Go-reaction time (in ms), (C) number of omission errors and (D) number of commission errors. *Significant differences between drug and placebo groups with P < 0.05.
Psychomotor tests
In the CTT, performance during cocaine improved relative to placebo as indicated by an increase in λc [F(1,56) = 28.75, P < 0.001], whereas THC had the opposite effect [F(1,56) = 4.97, P = 0.03]. Performance on the DAT was significantly affected by cocaine. Cocaine significantly decreased average tracking error [F(1,49) = 28.42, P < 0.001], reaction time [F(1,49) = 19.22, P < 0.001] and control losses [F(1,49) = 13.68, P = 0.001], and increased the correct responses [F(1,49) = 12.68, P = 0.001] when compared with placebo. THC significantly increased the number of false alarms [F(1,49) = 5.87, P = 0.02]. For mean (SE) scores on the CTT and DAT, see Figure 3. Accuracy of TOL performance was significantly affected by THC [F(1,57) = 19.95, P < 0.001] (see Figure4). No effect of either drug on reaction time was found. There was no correlation between history of cannabis use and THC or cocaine intoxication on any of the variables of the CTT, DAT and TOL.
Figure 3.
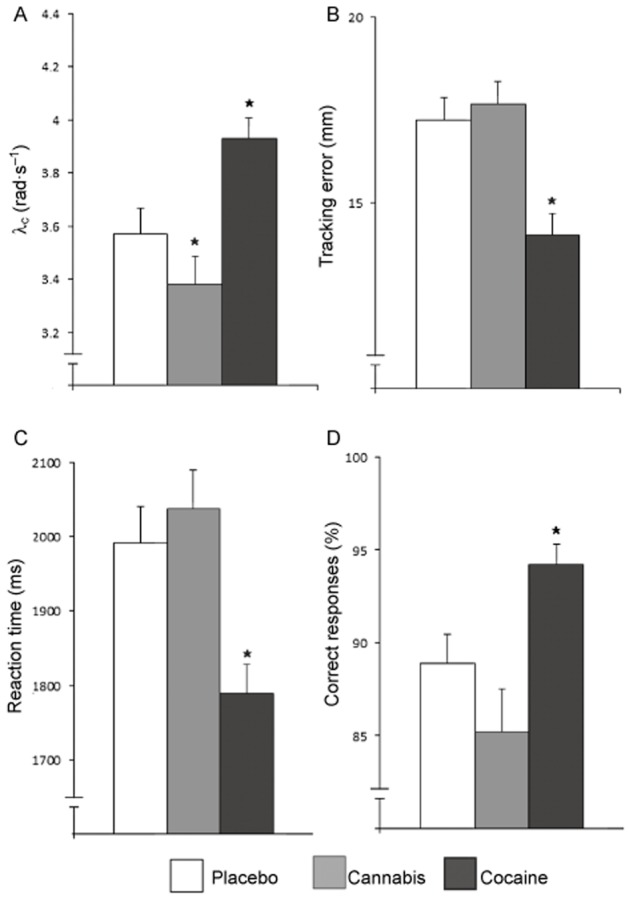
Mean (and SEM) performance in the CTT (A) and DAT (B–D) during cannabis or cocaine intoxication or placebo. Performance parameters include (A) λc (rad·s−1), (B) tracking error (in mm), (C) reaction time (in ms) and (D) correct responses. *Significant differences between drug and placebo groups with P < 0.05.
Figure 4.
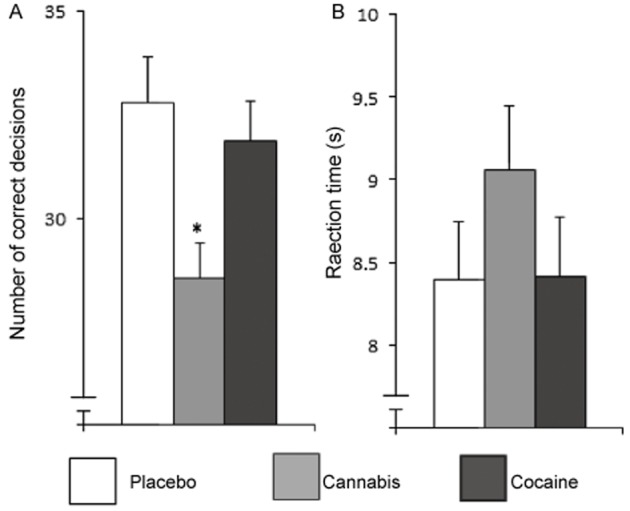
Mean (and SEM) performance on the TOL during cannabis or cocaine intoxication or placebo. Performance parameters include (A) number of correct decisions and (B) reaction time (in s). *Significant differences between drug and placebo groups with P < 0.05.
Subjective measures
VAS
Mean (SEM) subjective ratings of ‘feeling high’ during placebo, THC and cocaine treatment were 1.84 (0.19), 5.75 (0.40) and 3.53 (0.37) respectively. Mean (SEM) ratings of ‘feeling active’ during placebo, THC and cocaine treatment were 4.44 (0.33), 4.47 (0.36) and 6.57 (0.36) respectively. Cocaine [F(1,60) = 21.5, P < 0.001] and THC [F(1,60) = 96.19, P < 0.001] significantly increased feelings of high relative to placebo. In addition, subjects also felt more active under the influence of cocaine [F(1,60) = 26.68, P < 0.001], but not during THC intoxication. History of cannabis use and ratings of activity after cocaine administration was positively correlated (r = 0.36, P = 0.004). No correlation between ratings of high and history of cannabis use was found in any of the drug conditions.
Pharmacokinetics
Mean concentration of THC, cocaine and their metabolites are given in Table 2. The cocaine concentrations observed 50 min after ingestion are in accordance with previous publications (Van Dyke et al., 1976; Wilkinson et al., 1980) where the large inter-individual variability can be explained by variations in the absorption process. Also the cannabinoid concentrations are in the range that has been observed in previous studies with similar THC doses (Toennes et al., 2008; 2011). In some of the conditions a few of the blood samples are missing, for example, due to some subjects completing the conditions only partially or the inability to draw blood (see Table 2).
Table 2.
Serum concentrations (ng·mL−1) of THC, THC-COOH, THC-OH and plasma concentrations (ng·mL−1) of benzoylecgonine, ecgonine methyl ester, cocaine before (T0) and after drug administration
| Time after cocaine/placebo | −5 min | 50 min | ||
|---|---|---|---|---|
| Drug condition | n | Time after THC/placebo | −45 min | 5 min |
| Placebo condition | ||||
| THC | 60 | 1.4 ± 3.9 | 1.8 ± 3.2 | |
| THC-OH | 60 | 3.9 ± 0.6 | 3.2 ± 0.6 | |
| THC-COOH | 60 | 19.8 ± 37.5 | 17.4 ± 32.7 | |
| Cannabis condition | ||||
| THC | 56 | 1.7 ± 3.4 | 55.3 ± 29.5 | |
| THC-OH | 56 | 0.8 ± 1.7 | 6.9 ± 4.8 | |
| THC-COOH | 56 | 24.1 ± 39.2 | 41.9 ± 33.9 | |
| Cocaine condition | ||||
| THC | 60 | 1.2 ± 2.4 | 1.7 ± 3.4 | |
| THC-OH | 60 | 0.6 ± 1.3 | 0.7 ± 1.7 | |
| THC-COOH | 60 | 24.0 ± 55.8 | 24.6 ± 54.0 | |
| Cocaine | 57 | 7.2 ± 53.7 | 284 ± 198 | |
| Benzoylecgonine | 57 | 40.0 ± 269.7 | 509 ± 226 | |
| Ecgonine methyl ester | 57 | 10.3 ± 77.2 | 162 ± 124 |
Results are shown as mean±SD. THC-COOH, 11-nor-9-carboxy-THC; THC-OH, 11-hydroxy-THC.
Discussion and conclusions
The present study aimed to clarify whether the impairing effects of THC in regular cannabis users apply to neuropsychological functions in general or develop selectively in specific performance domains. Furthermore, the study aimed to assess the acute effects of cocaine in regular cannabis users who were expected to be tolerant to the impairing effects of single doses of cannabis.
Results from this study indicate that THC-induced impairments were present in all neuropsychological domains, rather than in some specific domains only. None of the performance tasks were completely unaffected by cannabis inhalation. This contrasts with previous results showing that single doses of THC impair critical tracking and divided attention performance in occasional cannabis users but not in heavy, frequent cannabis users (Ramaekers et al., 2009; 2011); these results were obtained using the same psychomotor tests and procedures and comparable THC doses as in the present study. However, the magnitude of THC-induced impairments during critical tracking and divided attention was about a factor 2–3 less in magnitude compared with THC-induced impairments in occasional cannabis users as reported in other studies (Ramaekers et al., 2006b; Ramaekers et al., 2009). Although this paper does not compare cannabis effects in occasional and heavy smokers directly, the latter finding seems to support the general notion that heavy users develop some tolerance to the impairing effects of cannabis on psychomotor function, albeit not complete. The sensitivity in the present study to detect mildly impairing effects of THC may be related to the large sample size that increased statistical power to significantly detect even the smallest changes in performance.
Data revealed that THC significantly affected performance in the impulsivity tests (MFF, SST). THC decreased general efficiency in the MFF task and increased the number of commission errors in No-Go trials of the SST. Efficiency is a composite measure that takes mean response latency and the number of incorrect responses into account. In the present study, subjects generally responded more slowly in the MFF although this change just failed to reach statistical significance. The loss of accuracy, however, was highly significant during THC intoxication. This may indicate that subjects made more errors even though they took more time to reflect on the presented problems. Subjects also made more commission errors during No-Go trials in the SST after THC intoxication. The increase in errors, however, was unrelated to the general speed of responding in Go and No-Go trials (i.e. stop reaction time) that remained unaffected during THC when compared with placebo. The same effect was seen in the TOL, where subjects made more errors after THC administration, without affecting reaction time. These data point to the notion that subjects during THC intoxication were unable to alter their first response selection when demanded.
Heavy cannabis users also displayed sensitivity for the stimulant effects of cocaine. Cocaine significantly improved psychomotor performance in the CTT and DAT on a number of performance parameters. It improved tracking performance and choice and speed of responding. Cocaine also produced ‘stimulating’ effects on the impulsivity tasks. It decreased response time in the MFF and in the SST and increased the number of commission errors in the same test. However, cocaine did not affect the composite measures of impulsivity and efficiency in the MFF.
Both THC and cocaine altered the subjective experiences of subjects significantly. After cannabis administration, subjects indicated feeling significantly more high. After cocaine, subjects not only reported feeling high, but they also felt more active. The current results confirm previous findings (Ramaekers et al., 2009; Hart et al., 2010), showing that heavy cannabis users do not develop pharmacological tolerance to the subjective effects of the drug.
The presence of performance impairment during THC intoxication in the present subject sample did not result from inexperience with drug use. All subjects were regular cannabis users who on average reported cannabis drug use in over 1000 occasions. Correlational analysis also demonstrated that THC-induced impairments were broadly present in the whole subject sample and were not related to cannabis use history. Likewise, cocaine-induced changes in performance were generally not related to cannabis use history of the present set. Only one parameter (subjective rating of activity) was significantly correlated to cannabis use history. This correlation, however, was fairly moderate and not necessarily meaningful.
Both THC and cocaine decreased proficiency of impulse control, but in apparently opposing ways. Results indicate that subjects during THC and cocaine intoxication were unable to inhibit a response when requested and that such errors occurred because subjects generally responded too fast during the cocaine condition and too slow during THC intoxication. These differential effects of THC and cocaine on impulse control may be related to differential pharmacological actions of both drugs in the limbic circuit associated with impulse control and addiction. The circuit has been broadly described to consist of a reflective system located in the medial prefrontal cortex and reactive system located in the striatum. The reactive system generates impulses and reward following dopaminergic stimulation of the nucleus accumbens, whereas the reflective systems exacerbates glutamatergic control over impulses generated in the striatum (Bechara, 2005; Kalivas and Volkow, 2005; Faure et al., 2010). Cocaine is known to increase dopamine levels in parts of the striatum that innervate limbic and motor circuits (Breiter et al., 1997). This may account for the finding that subjects respond faster and more impulsive during cocaine. The effects of single doses of THC are not as clear, indicating either an increase (Bossong et al., 2009) or decrease of dopamine levels in the striatum (Barkus et al., 2011; Kuepper et al., 2013). Recent studies indicate that THC decreases dopamine levels throughout the brain after chronic exposure (Pistis et al., 2004; Urban et al., 2012). Subjects in the present study were all heavy users who had been using for an average of 7 years. The reduction in proficiency of impulse control during THC intoxication is thus less likely to result from overactivation of the ‘reactive’ part of the ‘reward’ circuit and more likely to result from a deactivation of the ‘reflective’ part of the reward system. More research is needed to confirm differential activations of the frontal cortex and limbic system after THC and cocaine administration, particularly in the context of repeated drug use or addiction. Reduction in proficiency of impulse control during cannabis and cocaine intoxication stimulates repeated drug use, and may as such even play an important role in the neuropathology and development of drug addiction.
In sum, the present study demonstrated that heavy cannabis users showed impairment in a broad range of neuropsychological domains during THC intoxication. Impairments observed in psychomotor tasks, however, appeared smaller in magnitude as compared with those previously reported in occasional cannabis users. Administration of THC also affected performance of heavy users in tests of impulse control. Heavy cannabis users were sensitive to the stimulating effects of cocaine. Cocaine increased psychomotor function and decreased response time on an impulsivity task at the cost of making more errors. It was speculated that the reduction in proficiency of impulse control observed during THC and cocaine intoxication may put drug users at risk of repeated drug use and addiction.
Acknowledgments
This study was funded by a research grant from ZonMw (grant number 31160206), The Netherlands, awarded to J.G.R. and R.J.V.
Glossary
- CTT
critical tracking task
- DAT
divided attention task
- MFF20
matching familiar figures
- SST
stop-signal task
- THC
Δ9-tetrahydrocannabinol
- TOL
Tower of London
- VAS
visual analogue scale
Conflict of interest
The authors report no financial interests or potential conflicts of interest.
References
- Barkus E, Morrison PD, Vuletic D, Dickson JC, Ell PJ, Pilowsky LS, et al. Does intravenous Δ9-tetrahydrocannabinol increase dopamine release? A SPET study. J Psychopharmacol. 2011;25:1462–1468. doi: 10.1177/0269881110382465. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bossong MG, Berckel BNM, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, et al. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34:759–766. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Burns M. Pharmacology of cocaine and its relationship to behavior impairment. 1991. In The effects of drugs on human performance and behavior: drugs and driving/drugs in the workplace.
- Burns M. Cocaine effects on performance. 1992. pp. 613–619. In International Conference on Alcohol, Drugs and Traffic Safety.
- Childers SR, Breivogel CS. Cannabis and endogenous cannabinoid systems. Drug Alcohol Depend. 1998;51:173–187. doi: 10.1016/s0376-8716(98)00075-1. [DOI] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2012;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMCDDA. 2009. Polydrug use: patterns and responses (Luxembourg)
- EMCDDA. 2011. Annual drug update: 2011 in review.
- Faure A, Richard JM, Berridge KC. Desire and dread from the nucleus accumbens: cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat. PLoS ONE. 2010;5:e11223. doi: 10.1371/journal.pone.0011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Hays L. Acute effects of oral cocaine on inhibitory control of behavior in humans. Drug Alcohol Depend. 2002;67:157–167. doi: 10.1016/s0376-8716(02)00062-5. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Hays L. Cocaine improves inhibitory control in a human model of response conflict. Exp Clin Psychopharmacol. 2005;13:327–336. doi: 10.1037/1064-1297.13.4.327. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Hays L. Acute effects of cocaine in two models of inhibitory control: implications of non-linear dose effects. Addiction. 2006;101:1323–1332. doi: 10.1111/j.1360-0443.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Kaufman JN, Hester R. Acute effects of cocaine on the neurobiology of cognitive control. Philos Trans R Soc Lond B Biol Sci. 2008;363:3267–3276. doi: 10.1098/rstb.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R. Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychol Rev. 2007;17:347–361. doi: 10.1007/s11065-007-9036-8. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ilan AB, Gevins A, Gunderson EW, Role K, Colley J, et al. Neurophysiological and cognitive effects of smoked marijuana in frequent users. Pharmacol Biochem Behav. 2010;96:333–341. doi: 10.1016/j.pbb.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazekamp A, Ruhaak R, Zuurman L, Gerven JVA, Verpoorte ROB. Evaluation of a vaporizing device (Volcano 1) for the pulmonary administration of tetrahydrocannabinol. J Pharm Sci. 2006;95:1308–1317. doi: 10.1002/jps.20574. [DOI] [PubMed] [Google Scholar]
- Jex HR, McDonnell JD, Phatak AV. A ‘critical’ tracking task for manual control research. IEEE. 1966;7:138–145. [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction? A pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kauert GF, Iwersen-Bergmann S, Toennes SW. Assay of delta9-tetrahydrocannabinol (THC) in oral fluid-evaluation of the OraSure oral specimen collection device. J Anal Toxicol. 2006;30:274–277. doi: 10.1093/jat/30.4.274. [DOI] [PubMed] [Google Scholar]
- Kuepper R, Ceccarini J, Lataster J, van Os J, van Kroonenburgh M, van Gerven JM, et al. Delta-9-tetrahydrocannabinol-induced dopamine release as a function of psychosis risk: 18F-fallypride positron emission tomography study. PLoS One. 2013;8:e70378. doi: 10.1371/journal.pone.0070378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- Messer SB. Reflection-impulsivity: a review. Psychol Bull. 1976;83:1026–1052. [Google Scholar]
- Metrik J, Kahler CW, Reynolds B, McGeary JE, Monti PM, Haney M, et al. Balanced placebo design with marijuana: pharmacological and expectancy effects on impulsivity and risk taking. Psychopharmacology (Berl) 2012;223:489–499. doi: 10.1007/s00213-012-2740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Solinas M, Matthews S, Goldberg SR. Previous exposure to THC alters the reinforcing efficacy and anxiety-related effects of cocaine in rats. Neuropsychopharmacology. 2007;32:646–657. doi: 10.1038/sj.npp.1301109. [DOI] [PubMed] [Google Scholar]
- Perales JC, Verdejo-Garcia A, Moya M, Lozano O, Perez-Garcia M. Bright and dark sides of impulsivity: performance of women with high and low trait impulsivity on neuropsychological tasks. J Clin Exp Neuropsychol. 2009;31:927–944. doi: 10.1080/13803390902758793. [DOI] [PubMed] [Google Scholar]
- Perkonigg A, Lieb R, Höfler M, Schuster P, Sonntag H, Wittchen HU. Patterns of cannabis use, abuse and dependence over time: incidence, progression and stability in a sample of 1228 adolescents. Addiction. 1999;94:1663–1678. doi: 10.1046/j.1360-0443.1999.941116635.x. [DOI] [PubMed] [Google Scholar]
- Pistis M, Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biol Psychiatry. 2004;56:86–94. doi: 10.1016/j.biopsych.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kuypers KPC. Acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on behavioral measures of impulsivity: alone and in combination with alcohol. Neuropsychopharmacology. 2006;31:1048–1055. doi: 10.1038/sj.npp.1300894. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006a;31:2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Moeller MR, Ruitenbeek P, Theunissen EL, Schneider E, Kauert G. Cognition and motor control as a function of Delta9-THC concentration in serum and oral fluid: limits of impairment. Drug Alcohol Depend. 2006b;85:114–122. doi: 10.1016/j.drugalcdep.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Theunissen EL, Toennes SW, Moeller MR. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol. 2009;23:266–277. doi: 10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Theunissen EL, Brouwer M, Toennes SW, Moeller MR, Kauert G. Tolerance and cross-tolerance to neurocognitive effects of THC and alcohol in heavy cannabis users. Psychopharmacology (Berl) 2011;214:391–401. doi: 10.1007/s00213-010-2042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philos Trans R Soc Lond. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Solowij N. Cannabis and Cognitive Functioning. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- Toennes SW, Steinmeyer S, Maurer H-J, Moeller MR, Kauert GF. Screening for drugs of abuse in oral fluid – correlation of analysis results with serum in forensic cases. J Anal Toxicol. 2005;29:22–27. doi: 10.1093/jat/29.1.22. [DOI] [PubMed] [Google Scholar]
- Toennes SW, Ramaekers JG, Theunissen EL, Moeller MR, Kauert GF. Comparison of cannabinoid pharmacokinetic properties in occasional and heavy users smoking a marijuana or placebo joint. J Anal Toxicol. 2008;32:470–477. doi: 10.1093/jat/32.7.470. [DOI] [PubMed] [Google Scholar]
- Toennes SW, Schneider K, Kauert GF, Wunder C, Moeller MR, Theunissen EL, et al. Influence of ethanol on cannabinoid pharmacokinetic parameters in chronic users. Anal Bioanal Chem. 2011;400:145–152. doi: 10.1007/s00216-010-4449-2. [DOI] [PubMed] [Google Scholar]
- Urban NBL, Slifstein M, Thompson JL, Xu X, Girgis RR, Raheja S, et al. Dopamine release in chronic cannabis users: a [11c]raclopride positron emission tomography study. Biol Psychiatry. 2012;71:677–683. doi: 10.1016/j.biopsych.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke CV, Barash P, Jatlow P, Byck R. Cocaine: plasma concentrations after intranasal application in man. Science. 1976;191:859–861. doi: 10.1126/science.56036. [DOI] [PubMed] [Google Scholar]
- van Wel JHP, Kuypers KPC, Theunissen EL, Bosker WM, Bakker K, Ramaekers JG. Effects of acute MDMA intoxication on mood and impulsivity: role of the 5-HT2 and 5-HT1 receptors. PLoS ONE. 2012;7:e40187. doi: 10.1371/journal.pone.0040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P, Dyke CV, Jatlow P, Barash P, Byck R. Intranasal and oral cocaine kinetics. Clin Pharmacol Ther. 1980;27:386–394. doi: 10.1038/clpt.1980.52. [DOI] [PubMed] [Google Scholar]
- Zanettini C, Panlilio LV, Alicki M, Goldberg SR, Haller J, Yasar S. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci. 2011;5:1–21. doi: 10.3389/fnbeh.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]


