Abstract
Humans are considered among the greatest if not the greatest endurance land animals. Over the last 50 years, as the population has become more sedentary, rates of cardiovascular disease and its associated risk factors such as obesity, type 2 diabetes and hypertension have all increased. Aerobic fitness is considered protective for all-cause mortality, cardiovascular disease, a variety of cancers, joint disease and depression. Here, I will review the emerging mechanisms that underlie the response to exercise, focusing on the major target organ the skeletal muscle system. Understanding the mechanisms of action of exercise will allow us to develop new therapies that mimic the protective actions of exercise.
Keywords: exercise, endurance, resistance, nuclear receptors, insulin-like growth factor, myostatin, skeletal muscle
The health benefits of exercise
There is a strong argument that humans have evolved as endurance runners, potentially being among the best endurance land animals taking account size and distance (Bramble and Lieberman, 2004). Evolution has led to a highly efficient thermoregulation system in man, which allows us to travel over long distances while expending energy independent of all but the extreme climatic conditions (Bramble and Lieberman, 2004; Noakes and Spedding, 2012). Pressure for these evolutionary changes have been hypothesized as the need for long distant migration, the need to scavenge over greater distances and the potential also to persistence hunt, chase animals over long distances to exhaustion, or to get close enough to use weapons (Bramble and Lieberman, 2004). Over the last 100-50 years, physical activity in many populations has declined. This decline is also associated with an increase in the incidence of metabolic disorders such as type 2 diabetes, obesity and cardiovascular disease. Our evolutionary pressure to exercise therefore may underlie our susceptibility to these conditions when living a sedentary lifestyle (Noakes and Spedding, 2012).
The World Health Organization has now identified that physical inactivity is the fourth leading risk factor for global mortality just behind hypertension, tobacco use and hyperglycaemia (http://whqlibdoc.who.int/publications/2010/9789241599979_eng.pdf). As such, organizations such as the World Health Organization, the American Heart Association (http://www.heart.org/HEARTORG/GettingHealthy/PhysicalActivity/StartWalking/American-Heart-Association-Guidelines_UCM_307976_Article.jsp) and the British Heart Foundation (http://www.bhf.org.uk/heart-health/prevention/staying-active.aspx) recommend that individuals undertake an exercise regime of the order of at least 30 min of moderate-intensity aerobic activity at least 5 days per week, and moderate-to-high intensity muscle-strengthening activity for least 2 or more days per week for additional health benefits. This benefit of maintaining exercise capacity was highlighted in a recent study of 15 660 US veterans over 23 years, where it was shown that those individuals who could maintain a peak exercise task equivalent to gently running had a reduction in all-cause mortality (including cardiovascular diseases) by approximately 50% (Kokkinos et al., 2008). Exercise level is measured in metabolic equivalents (METs). One MET is defined as the energy expended at rest, equivalent to an oxygen consumption of 3.5 mL·kg−1 body weight per minute. The fitness level was strongly inversely related to mortality in individuals with and without cardiovascular disease, regardless of factors related to socioeconomic strata. Moreover, mortality was 13% lower for every 1-MET increase in exercise capacity up to 7 to 10 METs (Kokkinos et al., 2008). Exercise has also been associated with a reduction in risk of developing diabetes (Wang and Reusch, 2012), obesity (Poirier and Despres, 2001), breast and colon cancer (Newton and Galvao, 2008); it aids in strengthening joints and bone (Nikander et al., 2010), and is considered one of the best treatments for depression (Strohle, 2009). Exercise can however take many forms; at least experimentally it is often divided in to two main forms: resistance or strength type exercises and endurance types. Experimental examples of this are currently knee extensions for resistance exercise, and cycling (often stationary) or treadmill running for endurance. As discussed following, although the vast majority of the associations for health benefits of exercise come with endurance type activity, there is emerging evidence that resistance exercise or programmes such as high intensity training, where short 30 s periods of maximum activity a few times a week are being studied may also have benefits particularly in type 2 diabetes and glucose control (Gibala et al., 2012).
In the context of both resistance and endurance exercise, I will therefore discuss the emerging pathways that underlie the exercise response. Over the last 20 years, there has been an explosion in the interest of the molecular and biochemical processes that mediate the response to exercise. These advances bring with them a host of novel therapeutic avenues for a wide variety of chronic diseases (that exercise is known to be protective in).
Skeletal muscle as the major target for exercise
In this review, I will focus on the role of the skeletal muscle (Figure 1). Skeletal muscle is one of the most abundant tissues in the human body. At rest, skeletal muscle takes approximately 15–20% of cardiac output, during exercise however, it takes 70–85%. Compare this to the heart which takes 4–5% at rest and during exercise respectively (Saltin et al., 1998). Skeletal muscle therefore requires the greatest adaptation to exercise and has one of the greatest adaptive responses to exercise.
Figure 1.
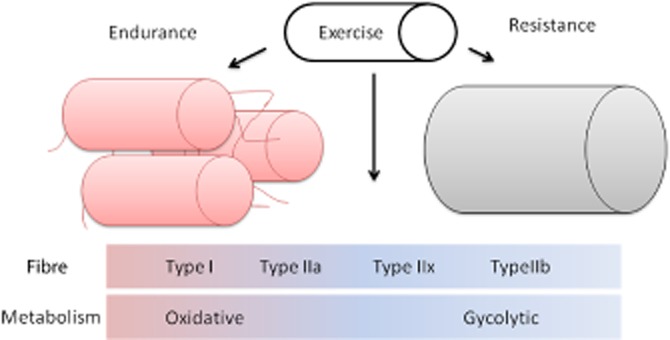
Exercise induces changes to skeletal muscle. Exercise induces metabolic and phenotypic changes to skeletal muscle that gives us major health benefits to whole body physiology. Endurance type exercise favours the growth and expression of type I and type IIa (type IIx) muscle fibres, which have increasing oxidative capacity (from type IIx to type I), are mitochondria rich and have an increased blood supply. Resistance exercise favours the growth and hypertrophy of type IIb muscle fibres, which are fast twitch, have increase glycolytic/anaerobic metabolism and produce power/strength over endurance.
The basic unit of the skeletal muscle is the myocyte. Myocytes are formed from the fusion of precursor myoblasts giving rise to long cylindrical multinucleated myofibres that characterize skeletal muscle. The muscle fibres themselves can then be subdivided into subtypes dependant on their ability to undertake oxidative phosphorylation as well as the presence of different types of myosin (Schiaffino and Reggiani, 2011). Type 1 fibres are slow twitch fibres that utilize oxygen for aerobic oxidative phosphorylation to generate ATP for work. Type 1 fibres are dark/red due to the presence of high levels of the oxygen carrying protein myoglobin, they are capillary dense, contain large amounts of mitochondria and use fats (triglycerides) as fuel (Schiaffino and Reggiani, 2011). Type 1 fibres therefore are strongly associated with endurance over power activity, and link fat utilization to activity. In contrast, type 2 fibres are more variable and are divided into more of a continuum of types IIa, IIx, IIb, with increasing concentration of fast twitch muscle myosin, increasing power production, high glycolytic activity, but decreasing myoglobin, capillary density, oxidative capacity, and resistance to fatigue (as you go from a to x to b). Type IIa fibres use creatinine phosphate and glycogen for energy, while type IIb use ATP and creatinine phosphate (Schiaffino and Reggiani, 2011).
All skeletal muscles have all three types of muscle fibre, but the composition changes with their role in the body and with activity, for exapmle postural muscles have a higher proportion of type I fibres (Eriksson and Thornell, 1983), marathon runners have a high proportion of type I (Luden et al., 2012) while power athletes like sprinters have a high proportion of type II fibres (Fry, 2004). Real world exercise often combines endurance and resistance exercises, and the combination does lead to increase aerobic capacity and muscle size (Lundberg et al., 2013). Since endurance exercise is linked most highly to the protective actions of exercise, it is likely that the beneficial effects of exercise are mostly linked to the muscles oxidative capacity, driven by the extent and activity of the type I and type IIa fibres.
In addition to the differentiated myocytes, small progenitor ‘satellite cells’ surround myofibres between the basal membrane (sarcolemma) and basal membrane (Relaix and Zammit, 2012). These satellite cells are activated upon damage, and have the ability to proliferate, differentiate and fuse in to the existing skeletal muscle, as well as self-regenerate. Moreover, the numbers of satellite cell increase with either resistance or endurance exercise in man (Relaix and Zammit, 2012).
Muscle growth/function can, however, occur by more than one mechanism: hyperplasia, hypertrophy and via new protein synthesis. Central to these processes are a core of transcription factors that drive the myogenic gene programme. The basic helix-loop-helix factor MyoD appears central to myotubule formation during development (Berkes and Tapscott, 2005), while related factors Myf5, Myf6 and myogenic can also drive myotubule differentiation (Berkes and Tapscott, 2005). The development of skeletal muscle relies on the expression of paired homeobox transcription factors (Pax); initially Pax3 to lay the patterning framework for which Pax 7 is then required incorporate tissue for complete muscular development (Ludolph and Konieczny, 1995; Berkes and Tapscott, 2005).
Skeletal muscle represents a major tissue type in the body. Important to whole body physiology therefore is its metabolic capacity, linking diet, both in terms of calories, but also different food groups required for fuel, new protein synthesis, etc. to general health. Skeletal muscle therefore also communicates with other metabolic tissues and reservoirs such as adipose stores and the liver, both through fuelling requirements, but also being the source of a wide variety of secreted muscle cytokines or ‘myokines’ including IL-6, IL-15 and brain-derived neurotrophic factor (Pedersen, 2009b).
As we continue the discussion, a note of caution should also be raised particularly surrounding the interpretation of mechanistic data found using animal, particularly rodent models and their translation in to man. Hypertrophy as opposed to hyperplasia is clearly the major response to exercise in man (Sjostrom et al., 1991; McCall et al., 1996). Rodents have inherently higher rates of muscle turnover and protein synthesis, so experimental effects can be far more dramatic (Phillips et al., 2009). Moreover, although there are some similar gene changes in muscle aging in mice and men such as Rac-1, glucose-6-phosphate isomerase ATP synthase 6/8 and pro-a(III) collagen, there are as least as many differences (Welle et al., 2001).
Muscle pathologies
A sedentary lifestyle or conditions such as long-term immobilization or malnutrition leads to muscle atrophy. For everyone, the process of aging leads to sarcopenia and reduced muscle mass, and of course increases susceptibility to metabolic diseases. A component of aging itself may be a lack of sensitivity to exercise, potentially due to reduced exercised induced muscle satellite cell activation (Leiter et al., 2011). Although beyond the scope of this review to discuss in detail, additionally, there are a number of common chronic conditions, pathologies and physiological processes associated with loss of muscle mass; including heart failure, chronic obstructive pulmonary disease, chronic kidney disease, AIDs, muscular dystrophies, sepsis, autoimmune diseases and cancer lead to cachexia/muscle wasting (Fanzani et al., 2012). In addition to indirect health benefits, exercise may therefore have a direct benefit reversing the decline in these muscle wasting conditions.
Inflammation may underlie a number of these pathologies. Low grade inflammation occurs in obese visceral adipose tissue (Yudkin, 2007), while inflammatory processes underlie the pathogenesis of cardiovascular disease and the development of insulin resistance and diabetes (Tabas and Glass, 2013). Common to cancer, metabolic and inflammatory conditions, are high proinflammatory cytokine levels, of which tumour necrosis factor-a, also originally called cachexin due to its wasting properties in cancer is elevated (Aggarwal et al., 2002). Although chronic inflammation impairs exercise ability, exercise reduces the level of low grade inflammation (Michigan et al., 2011; de Lemos et al., 2012) and can even be beneficial in muscle specific inflammation (Nader and Lundberg, 2009).
General mechanisms of exercise
There are a number of pathways that mediate the benefits of exercise in terms of muscle biology, growth and metabolism: these including inputs such as mechanical activation, neurohormonal, steroids, growth factors, cytokines and other local mediators. Inside the cell, these pathways lead to changes in specific transcription factors and their co-regulators, oxidant stress, induction of cytoprotective proteins, muscle specific regulatory proteins, oxygen carry proteins, and proteins involved in intracellular recycling and degradation of proteins and organelles. Inputs include the levels and type of physical exertion, nutrition status, genetic predisposition along with emerging epigenetic mechanisms. Although muscle mass clearly alters with development, particularly in infancy and during puberty through the actions of hormones such as growth hormone and testosterone, it is beyond the scope of the review to go through these pathways in great detail (see Supporting Information Appendix S1 for more detail on these pathways). Rather than the developmental aspects, here we will focus on the emerging pathways directly related predominantly to endurance exercise, and their potential for use for therapy and misuse in doping.
The discovery of exercise pathways have come from multiple scientific directions, from directly studying muscle biology, naturally variations in the population, for example single nucleotide polymorphisms, and more recently from the application of omic' technologies to look for global changes in gene expression and metabolites released.
Health benefits of resistance exercise
Although we will focus on endurance exercise, resistance exercises are important for reversing the loss of muscle function and structure, and joint weakness, associated with age and chronic illnesses. The cardiovascular health benefits of exercise are generally attributed to endurance exercise (Chudyk and Petrella, 2011). Resistance exercise alone is, however, commonly associated with a better glycaemic control in particular (Hurley et al., 2011); by example, in a in a recent study of 100 type 2 diabetic patients, endurance and resistance exercise led to a greater insulin sensitivity and lower triglycerides levels compared to controls. Individuals who just undertook the endurance exercise protocol, also had significant improvements in circulating lipids, blood pressure, inflammation (decreased high-sensitivity C-reactive protein) and a reduction in carotid intima-media thickness progression (Kadoglou et al., 2013). So, although endurance exercise does have major cardiovascular benefits, a number of trials suggest a combination of resistance and endurance exercise may be better than endurance exercise alone for the reduction of cardiovascular risk factors (Chudyk and Petrella, 2011); findings reflected in the exercise recommendations of the World Health Organization (see above).
Endurance exercise
Endurance exercise results in the metabolic switching of the skeletal muscle to a more oxidative phenotype (Figure 2). Underlying this activation are a variety of inputs such as physical activity and intracellular Ca2+ release, the fuel status of the muscle, for example the ratio of ATP to AMP, which when favouring AMP, leads to AMKP activation, the redox status of the cell (NAD/NADH), and the relative hormone/nutrition status of the environment. Training with different nutritional status tends to adaptations, for example a low carbohydrate, tends to increase pathways of carbohydrate uptake and metabolism to compensate (Hawley et al., 2011). In contrast, a high fat diet acutely may aid fatty acid oxidation over when switched back to a more balanced diet, but a continuous high fat diet appears detrimental to exercise performance (Hawley et al., 2011). The first gene identified to have polymorphic influence over exercise, however, was ACE. The mechanism my which it exerts this effect is still not known.
Figure 2.
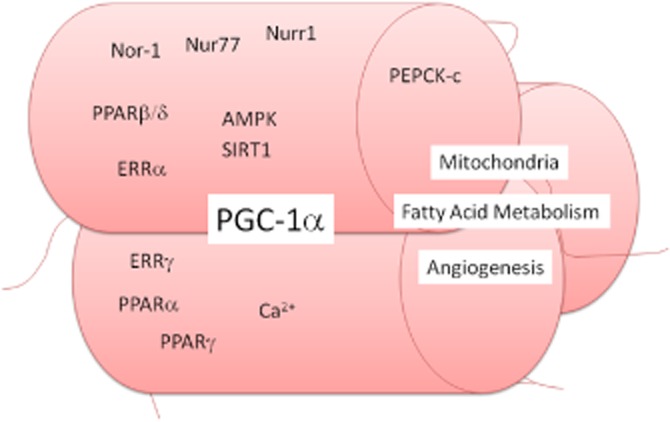
Control of endurance exercise muscle phenotype. The increase in oxidative fatty acid metabolic, fatigue resistance muscle fibre generation seen with endurance exercise are mediated by several signalling processes: (i) activation of the master regulator of mitochondrial biosynthesis the PPARγ coactivator-1 (PGC-1α) appears to be a central process. (ii) Several nuclear receptors have also been implicated in the endurance phenotype, which PGC-1α may co-activate, including the PPARα, PPARγ, but in particular PPARβ/δ; the oestrogen-like receptors (ERR)α and ERRγ, and members of the NR4A family, nor-1, nur77 and nur1. (iii) Like resistance exercise, rises in intracellular Ca2+ induced with work are also important. (iv) Also controlling the activation of PGC-1α are the activation of the AMP kinase (AMPK), which is activation with an increase in the AMP : ATP ratio, and the deactylase sirtuin (SIRT)-1. (v) Downstream, overexpression of cytosolic phosphoenolpyruvate carboxykinase (PEPCK-c), an enzyme key to central gluconeogenesis, induces an extreme endurance phenotype in mice.
ACE
In the late 1990s, polymorphisms in the ACE gene were identified as being linked to exercise performance (Montgomery et al., 1997). ACE is responsible for the conversion of the angiotensin I to the vasoconstrictor peptide angiotensin II, along with the inactivation of the peptide mediator bradykinin (Regoli et al., 2012). In particular, an insertion (I) polymorphism, associated with higher levels of circulating bradykinin (and therefore a reduced ACE activity), was found more prevalent in some endurance athletes including triathletes, experienced mountaineers and elite lightweight rowers (Puthucheary et al., 2011), but not elite cyclists or lightweight rowers (Muniesa et al., 2010). In contrast, individuals with a deletion (D) ACE polymorphism had more of an association with more strength/power resistance exercise including elite sprint swimmers (Puthucheary et al., 2011), and football (soccer) players (Juffer et al., 2009). Similar mutational differences may also exist across species. Alaskan sled dogs divided into endurance dog (1000 + miles in 10 days) or sprinters (30 miles, 18–25 mph), also have a polymorphism within the ACE gene that significantly distinguishes the sprint from endurance phenotypes (Huson et al., 2011). Although these ACE polymorphisms are associated with some of these elite athlete populations, it is also clear that any effect is likely to be due to polygenetic contribution. When examined in healthy volunteers (non-elite), there was no evidence for an association of either polymorphism with baseline strength, but with training there were larger strength gains with individuals with the I/I genotype (Thomis et al., 2004).
ACE inhibitors are front line anti-hypertensive drugs that are commonly used in patients with ongoing cardiovascular diseases. Whether the effects of ACE in exercise are due to the control of blood pressure are not known. ACE inhibitors do appear to reverse the decline in physical performance due to congestive heart failure and slow the decline in muscle strength, particularly in elderly women (Gray et al., 2012), and in hypertensive subjects, result in better exercise performance (Onder et al., 2006). The mechanism of action of ACE in exercise is far from clear. Angiotensin II is angiogenic in the repair process of toxin-induced muscle damage (Bellamy et al., 2010); however, ACE knockout mice also have an increased capillary density around type IIa (oxidative) fibres (Zhang et al., 2005), suggesting the contribution of ACE to vascularization may play a role.
α-Actinin-3 (ACTN3)
The skeletal-muscle actin-binding protein ACTN3 is specifically expressed in fast-twitch myofibres. In 18% of healthy white Australians, and approximately 1 billion individuals worldwide, ACTN3 is absent due to a homozygous stop-codon polymorphism (R577X). Male and female elite sprint athletes express functional ACTN3 compared to controls (Yang et al., 2003; Eynon et al., 2009). Moreover, endurance athletes (Eynon et al., 2009; 2012) and centenarians (Fiuza-Luces et al., 2011) appear to lack ACTN3. So far, there has been no particular association of ACTN3 expression with ironman triathletes (Saunders et al., 2007), elite swimmers (Wang et al., 2013) or professional endurance cyclists (Lucia et al., 2006). One potential mechanism for this endurance phenotype has been suggested a better ability to recover from multiple exercise bouts (Venckunas et al., 2012).
When looking at the muscle fibre localization of ACTN3, individuals homozygous for ACTN3 had higher relative knee power, compared with those lacking ACTN3. At the structural levels, there does not appear to be a significant difference in oxidative capacity or type I or type II muscle fibres with the presence or absence of ACTN3 (Vincent et al., 2012), but as potentially expected, ACTN3 does appear to be type II fibre specific (Ichinoseki-Sekine et al., 2012).
An ACTN3 knockout mouse has been generated and shows, similar to the observations of elite athletes, an endurance phenotype, with increased aerobic oxidative metabolism (MacArthur et al., 2007), increased glycogen content, altered Ca2+ signalling (Quinlan et al., 2010) reduced force generation, and enhanced recovery from fatigue (Chan et al., 2008; MacArthur et al., 2008), but an increased susceptibility to damage (Seto et al., 2011).
Glucocorticoids
The long-term therapeutic use of glucocorticoids is associated with side effects of decreased muscle mass, a suppression of myogenesis and increased skeletal muscle catabolism (Hanaoka et al., 2012). The role of endogenous glucocorticoids or acute use glucocorticoid steroids (used in injury management to decrease inflammation) is less understood. Endogenous glucocorticoids, for example cortisol are, however, increased with exercise, and appears to have a small but significant effect on endurance exercise, and in learning and memory in rodents (Hajisoltani et al., 2011). In man, in a number of studies, there appears little beneficial effect of acute glucocorticoid use. The acute stimulated release of cortisol using the banned synthetic adrenocorticotropic hormone Synacthen, in trained cyclists had no effect on performance over a 20k time trial (Baume et al., 2008). Similarly, 4 weeks of corticosteroid or placebo inhalation in healthy, well-trained athletes did not affect maximal power output in cycle ergometer tests or mood state by questionnaire (Kuipers et al., 2008). In contrast, studies examining submaximal exercise in recreational athletes, 1 week of oral prednisolone dosing, but not acute prednisolone (Arlettaz et al., 2008) did show an increase in cycling endurance and recovery (Arlettaz et al., 2007); whether this effect was due to the population studied (recreational vs. trained athletes) or the different protocols (1 week dosing, submaximal training) is not known.
Erythropoietin (EPO)
The oxygen carrying capacity of the blood is critically important for endurance exercise. This has led to many elite endurance athletes training at altitude to increase their haematocrit. Although this is common practise, the evidence for a beneficial effect of high altitude training has not been tested in a randomized control trial, and benefits are commonly only ascribed to a subset of responders (Chapman et al., 1998).
EPO is a glycoprotein hormone produced by the kidney in response to hypoxia, including exercised induced hyperoxaemia (Roberts and Smith, 1999). EPO binding its specific EPO receptor stimulates erythrocyte production in the bone marrow (Watowich, 2011). EPO may also act centrally to improve performance independent of effects on haematocrit (Schuler et al., 2012).
Although the benefits of EPO doping for endurance exercise has recently been questioned (Heuberger et al., 2013), anecdotal contemporary reports from elite cyclists such as David Millar and Tyler Hamilton at the time of widespread doping indicated a strong communal belief in long-term endurance performance benefits of EPO and/or blood doping, as evidenced by the number of winners and leading cyclists subsequently found to have doped at the time. Additionally, of particular note is the rare EPO receptor mutation, such as found in the Finnish multi-Olympic cross-country ski champion Eero Mäntyranta (1937-). The EPO receptor mutation exhibited by Mäntyranta results in a greatly increased haematocrit and an increase in oxygen carrying capacity of up to 50% (de la Chapelle et al., 1993).
EPO treatment of mice increases maximal oxygen uptake (VO2 max) but not voluntary running (Kolb et al., 2010), it also increases exercise performance in small scale studies healthy volunteers (Rasmussen et al., 2010), in patients with chronic heart failure (Mancini et al., 2003) and standard bred horses (McKeever et al., 2006). In addition to pharmacological approach, EPO gene-modified mesenchymal stem cells have been implanted to treat anaemic induced by chronic kidney disease. Mice that had chronic renal failure and received these EPO producing cells showed significantly greater haematocrit and swimming exercise capacity (Eliopoulos et al., 2006). Conversely, functional EPO knockout mice exhibit a decrease in the aerobic exercise capacity, correlated with a decreased haematocrit, reflecting poor oxygen supply to the muscles. Moreover, the muscles of EPO receptor knockout mice exhibit gene changes in muscle hypoxia, oxidative stress, glycolysis and mitochondrial oxidative phosphorylation pathways (Mille-Hamard et al., 2012).
EPO is not without side effects. EPO elevates the arterial blood pressure even in healthy subjects (Lundby and Olsen, 2011). Increased haematocrit increases blood viscosity, which can reach levels that not only severely limit exercise capacity (Schuler et al., 2010) but also increase the risk of thrombosis, heart attack, stroke and sudden death (Clyne et al., 1995; Lage et al., 2002). Indeed, it is believed the introduction of EPO in cycling was associated with close to 20 sudden deaths in this period (late 1980s to early 1990s) of cyclists such as Johannes Draaijer.
Metabolic nutrition nuclear receptors and tier co-regulators
There is a strong link between aerobic capacity mitochondria, fatty acid metabolism and the endurance exercise phenotype (Wisloff et al., 2005). Conditions, such as obesity associated insulin resistance may result of the imbalance between beta-oxidation pathway induction (increased lipids) and lack of up-regulation of tricarboxylic acid (TCA) cycle activity (lack of exercise, so no need to generate more ATP). Endurance exercise training enhances mitochondrial performance, favouring tighter coupling between beta-oxidation and the TCA cycle, and concomitantly restores insulin sensitivity in animals fed a chronic high-fat diet. A number of energy-sensing molecules exist that sense changes in nutrition and energy homeostasis to trigger compensatory gene expression. Nuclear receptors such as the PPARs and their co-regulators, AMP-activated protein kinase, and sirtuin (SIRT)1 are all known to play important roles (Freyssenet, 2007). The exercise-activated nuclear receptor transcriptional co-activator, PGC-1α, appears to plays a key role in coordinating metabolic flux through a number of these pathways (Muoio and Koves, 2007).
PPARs
The PPAR subfamily of nuclear receptors has three members: PPARα (NR1C1), PPARβ/δ (NR1C2) and PPARγ (NR1C3), all of which are expressed in skeletal muscle (Bishop-Bailey, 2000). PPARα and PPARβ/δ induce β-oxidation pathways, while PPARγ is critical for adipocyte differentiation programme (Bishop-Bailey, 2000). Since their discovery, PPARs have been shown to be expressed in monocytes/ macrophages, the heart, vascular smooth muscle cells, endothelial cells, and in atherosclerotic lesions, and all experimentally appear to be cardioprotective (Bishop-Bailey, 2000). PPARs can be activated by a vast number of compounds including clinically used drugs, such as clofibrate (PPARα), and the anti-diabetic thiazoldinedione classes (PPARγ), polyunsaturated fatty acids, and a number of eicosanoids, including prostaglandins, lipoxygenase and epoxygenase products, and oxidized low density lipoprotein components (Bishop-Bailey and Wray, 2003). Although there is evidence for a role for all the PPARs, in exercise there is considerable evidence that PPARβ/δ in particular is a critical signalling component of the endurance response.
PPARβ/δ
PPARβ/δ is more highly expressed in type I compared to type II myofibres (Lunde et al., 2007). Endurance exercise (Luquet et al., 2003) or electrical stimulation of slow type I fibres (Lunde et al., 2007) induces PPARβ/δ expression in skeletal muscle. Electrically stimulation mimicking fast/glycolytic IIb activation (Lunde et al., 2007) or age (Giordano et al., 2009) decreases the expression of PPARβ/δ.
Muscle-specific PPARβ/δ (Luquet et al., 2003) or constitutively active PPARβ/δ (Wang et al., 2004) transgenic mice, or overexpression of a constitutively active PPARβ/δ in adult muscle (Lunde et al., 2007) results in a profound hyperplasia and shift to more oxidative fibre (Luquet et al., 2003), accompanied by an increased blood supply due to a rapid angiogenic response (Piqueras et al., 2007; Wagner et al., 2009; Bishop-Bailey, 2011). These changes in muscle are accompanied by a reduction of body fat mass, mainly due to a large reduction of adipose cell size (Luquet et al., 2003), and a dramatic reduction in high fat-diet induced obesity and insulin resistance. Importantly, these PPARβ/δ muscle mice have an endurance phenotype being able to run for twice as long and twice as far as control mice (Wang et al., 2004).
In contrast to PPARβ/δ overexpression, activation of PPARβ/δ by a highly selective agonist (GW501516) did not enhance endurance performance by itself. The PPARβ/δ agonist did, however, synergize with treadmill training to increase oxidative myofibres and running endurance similar to that seen with the transgenic mice (Narkar et al., 2008). Moreover, PPARβ/δ, activation activated adenosine monophosphate kinase (AMPK), and an orally active AMPK agonist AICAR alone could partially mimic the induction of metabolic genes and enhanced running endurance (Narkar et al., 2008). Recently, in the mdx mouse model of muscular dystrophy, a combination of exercise, with pharmacological activation of PPARβ/δ and AMPK over a month showed a significant improvement in exercise performance, compared to training alone (Bueno Junior et al., 2012).
Activation of PPARβ/δ by overexpression or by selective agonists promotes a rapid myonuclear accretion via the calcineurin/NFAT pathway, to induce fusion rather than proliferation (Giordano et al., 2009). In terms of metabolism, not only is fatty acid metabolism optimized, PPARβ/δ in conjunction with AMPK and MEF-2 induce lactate dehydrogenase b which diverts pyruvate into mitochondria for the final steps of glucose oxidation (Gan et al., 2011). The skeletal muscle from PPARβ/δ transgenic mice have high glycogen stores, increased levels of the glucose transporter GLUT4, and augmented capacity for mitochondrial pyruvate oxidation (Gan et al., 2011). Activation of PPARβ/δ therefore appears to effectively re-integrate β-oxidation and the TCA cycle to optimize metabolism that becomes uncoupled by diet induced obesity.
Although there appears to be a gender difference in PPARβ/δ expression, with women expressing higher amounts in the vastus lateralis than men (Maher et al., 2009), PPARβ/δ is readily induced after gently or strenuous exercise independent of nutrition or condition state (Russell et al., 2005). Even relatively low intensity exercise such as walking for greater than 150 min per week over a 4-month period is sufficient to induce PPARβ/δ (Fritz et al., 2006). Induction of PPARβ/δ is even seen in highly trained individuals such as elite cyclists after training bouts (Psilander et al., 2010).
These finding have led to a huge interest in PPARβ/δ activators as exercise mimetics and the illegal use of PPARβ/δ in training regimes in man. The first doping case has now been reported with GW501516, which has also been nicknamed ‘Endurobol’. In April 2013, the European track cycling champion Valery Kaykov was sacked by the Rusvelo team after failing a drugs test for GW501516. GW501516 has been trialled in man for dyslipidaemia for which it effectively elevates high-density lipoprotein (HDL) (Olson et al., 2012). However, GW501516 clinical development has now been stopped for safety reasons, due to long-term carcinogenesis in rodents (Olson et al., 2012), which has led to World Anti-Doping Agency taking the unprecedented step to issue an alert about safety of the compound.
PPARα and PPARγ
PPARα and PPARγ are induced in the adipose (Ruschke et al., 2010) and skeletal muscle (Fatone et al., 2010; Ruschke et al., 2010; Sixt et al., 2010), of type 2 diabetic patients following exercise prescription (Sixt et al., 2010). PPARα and its target genes, hepatocyte nuclear factor-4, carnitine palmitoyltransferase 1, catalase and ABCA1 are also induced by exercise in the liver of diabetic Otsuka Long-Evans Tokushima Fatty rats, again indicating potentially important metabolic targets for these receptors outside the skeletal muscle system (Zhang et al., 2011). Although little is known regarding the direct endurance modulating role of PPARα, single nucleotide polymorphisms (SNPs) in man do indicate a potential role. A G/C polymorphism in intron 7 of the PPARα gene appears to significantly influence athletic performance (Ahmetov et al., 2006). In a large cohort of Russian athletes, the C allele was associated with increasing resistance/strength performance, while the GG genotype were associated with endurance athletes. These GG genotypes had approximately 20% more slow-twitch fibres than CC individuals (Ahmetov et al., 2006).
Similarly, little is known about PPARγ and the exercise phenotype, particularly in skeletal muscle. In a low-intensity exercise (8 weeks walking) study, volunteers had a significant reduction in total serum cholesterol and oxidized low-density lipoprotein, an increase in HDL, and a significant increase in leukocyte PPARγ expression and activation (Butcher et al., 2008). In mice, PPARγ is activated in adipose tissue after of 8 weeks of voluntary wheel running (Petridou et al., 2007), and PPARγ may mediate anti-depressant activity due to exercise (Eissa Ahmed and Al-Rasheed, 2009; Sadaghiani et al., 2011). The Pro12Ala polymorphism in the PPARγ gene, Pro12 was significantly associated with type 2 diabetes in those with low physical activity (Nelson et al., 2007). PPARγ Pro12Ala carriers also experience greater improvements in glucose and insulin metabolism in response to a 20-week endurance training programme (Ruchat et al., 2010).
It is important to note, that PPAR response overall may, however, be dependent on the muscle type being studied, and the type of training programme. In different muscles, either glycolytic (plantaris; type II fibre dominant) or oxidative (soleus- type I fibre dominant) in the rat, PPARα and PPARγ mRNAs were expressed to a greater extent in the soleus muscle than in the plantaris muscle, while PPARβ/δ was equally expressed. PPARγ was the least abundantly expressed PPAR. After 12 weeks of training, only PPARγ mRNA expression increased in the soleus muscle, while PPARβ/δ and gamma mRNA levels increased in the plantaris muscle (Spangenburg et al., 2009).
PGC-1α
The PPARγ co-activator (PGC) family are central transcriptional co-activators for induction of mitochondrial biogenesis (Spiegelman, 2007). Although, originally identified as a cold-activated co-activator of PPARγ, PGC-1α co-activates a number of nuclear receptors (Spiegelman, 2007). PGC-1α and can be up-regulated and/or activated by a number of pathways that induce skeletal muscle exercise phenotype including: β2-adrenoceptor activation (Miura et al., 2007), Akt signalling, cAMP-cAMP response element binding protein activation or intracellular coupling of Ca2+/calmodulin-dependent protein kinase in a p38-depdendent manner (Wright et al., 2007), or as the case of adiponectin via a Ca2+/calmodulin-dependent protein kinase beta-AMPK-SIRT pathway (Iwabu et al., 2010), or oxidant stress via a similar AMPK-SIRT dependent pathway. Antioxidants such as vitamin C or E supplementation may therefore blunt the response to exercise training (Ristow et al., 2009).
The capacity of muscle mitochondria to fully oxidize fatty acid correlates with the extent of type I fibres expression (Koves et al., 2005). PGC-1α is activated by exercise, and its expression alone is sufficient to produce the endurance phenotype (Baar, 2004) and lipid β-oxidation in vitro (Koves et al., 2005). Muscle-specific PGC-1α transgenic mice have increased mitochondrial gene expression, mitochondrial DNA, and mitochondrial enzyme activity and improved exercise performance and peak oxidative capacity (Calvo et al., 2008). Increased mitochondrial biogenesis, capillaries (Chinsomboon et al., 2009) and fatty acid transporters all contribute to this improved exercise capacity via an increase in fatty acid utilization (Tadaishi et al., 2011). Angiogenesis is particularly important for supplying oxygen to oxidative type I (and type IIa) fibres. One of the most important recent findings in vascular biology is that PGC-1α induces VEGF and angiogenesis in a hypoxia inducible factor (HIF-1)-independent manner [via co-activation or oestrogen-like receptor (ERR)α] (Arany et al., 2008). HIF-2α (endothelial PAS domain-containing protein 1) is a hypoxia inducible gene, which is transcriptionally activated by the PGC-1α-ERRα complex. The PGC-1α-mediated switch to slow, oxidative fibres in vitro and in vivo is dependent on HIF-2α activation (Rasbach et al., 2010).
Activation of muscle PGC-1α by exercise may also direct benefit metabolism in adipose depots by the inducing myokine production. A recent report showed that exercised induced PGC-1α induces the expression FNDC5, a membrane protein that is cleaved and secreted as a newly identified myokine termed irisin. Circulating irisin binds white adipose tissue and stimulates uncoupling protein-1 expression and a broad programme of brown fat-like development, increasing energy expenditure without increasing activity or food intake (Bostrom et al., 2012).
Interestingly, a novel splice variant of PGC-1α, which results from alternative promoter usage and splicing, has been recently identified and called PGC-1α4 (Ruas et al., 2012). PGC-1 α4 is highly expressed in resistance exercised muscle but unlike expected for canonical PGC-1α activation, PGC-1α4 induces insulin-like growth factor (IGF-1) and represses myostatin leading to skeletal muscle hypertrophy. Skeletal muscle-specific PGC-1α4 transgenic mice in contrast to PGC-1α show increased muscle mass and strength, and a dramatic resistance to muscle wasting induced by cancer cachexia (Ruas et al., 2012).
Other nuclear receptors and co-regulators
ERRγ (NR3B3)
Like ERRα, the sister orphan nuclear receptor ERRγ is highly expressed in type I skeletal muscle. ERRγ when overexpressed in anaerobic type II muscles induces fatty acid metabolism, mitochondrial respiration (Rangwala et al., 2010), and a type I fibre gene programme (Narkar et al., 2011), along with angiogenesis to support the increased oxidative requirements (Narkar et al., 2011). Consequently, similar to PPARβ/δ transgenic mice, ERRγ transgenic mice have a doubling in running endurance. Interestingly, these fibre switching actions of ERRγ were independent of PGC-1α, but were still partially dependent on AMPK activation (Narkar et al., 2011). Mice lacking ERRγ in contrast showed decreased mitochondria and reduced exercise capacity (Rangwala et al., 2010).
Neuron-derived orphan receptor 1 (Nor-1; NR4A3)
Nor-1 is a commonly found as an exercised induced nuclear receptor by transcriptomic analysis of endurance exercise in man (see below). Nor-1 is also induced by β2-adrenoceptor activation and is up-regulated after exercise and down-regulation with limb immobilization in mice (Kawasaki et al., 2009). siRNA targeted knockdown of Nor-1 in skeletal muscle cells in vitro reduced oxidative metabolism. Skeletal muscle specific constitutively-active Nor-1 transgenic mice have been generated (Pearen et al., 2012). In the skeletal muscle, active Nor-1 resulted in induction of myoglobin, mitochondria, oxidative enzymes and various subunits of electron transport chain complexes, leading to type IIa and IIx fibres switching from type IIb fibres, and increased MEF-2 and phospho-histone deacetylase 5 and cytoplasmic HDAC5 staining (Pearen et al., 2012). Indicative of this fibre-switching phenotype, Nor-1 skeletal muscle transgenic mice had significantly improved glucose tolerance, oxygen consumption and running endurance (Pearen et al., 2012).
Nerve growth factor IB (Nur77; NR4A1)
Nur77 is decreased in the skeletal muscle from obese individuals. In vitro, Nur77 overexpression in skeletal myoblasts induces glucose oxidation and glycogen synthesis, GLUT4 and glycogenin and increased hexokinase and phosphofructokinase activity (Kanzleiter et al., 2010). Endurance exercise training of rats (Kawasaki et al., 2009) or man (Lewis et al., 2010) induces skeletal muscle Nur77. Nur77 is also particularly sensitive to induction by the β2-adreoceptor–cAMP/PKA pathway, but not AMPK (Kanzleiter et al., 2009).
Rev-erbβ (NR1D2)-retinoic acid receptor-related orphan receptor alpha (RORα; NR1F1)
Rev-erbβ is an orphan nuclear receptor that functionally antagonizes the RORα nuclear receptor. Rev-erbβ and RORα are both expressed in skeletal muscle. Dominant negative Rev-erbβ expression in skeletal muscle cells in vitro decreased the expression of fatty acid transporter CD36, and the fatty acid binding proteins-3 and -4, and resulted in a dramatic repression (>20-fold) of myostatin mRNA (Ramakrishnan et al., 2005). Whether, this effect of Rev-erbβ is RORα dependent is not known, nor whether this effect is seen in vivo or in man.
Nuclear receptor co-repressor 1 (NCoR1)
The nuclear receptor ro-repressor is a common co-repressor regulator of a wide range of nuclear receptors. Muscle-specific deletion NCoR1 in mice enhanced exercise endurance which is associated with increased muscle mass mitochondrial number and the activation of transcriptional regulators of muscle function: MEF2, PPARβ/δ and ERRs (Yamamoto et al., 2011).
Other metabolic pathways: the phosphoenolpyruvate carboxykinase (PEPCK)-cytosolic muscle transgenic mice
Clearly altering metabolism is critical to changing the function and phenotype of skeletal muscle, which then impacts also on whole body health. Endurance exercise increases concentrations of the hexose phosphates and phosphoenolpyruvate and depresses those of fructose 1,6-bisphosphate, triose phosphates in the liver (Dohm and Newsholme, 1983). These changes are associated with were increased maximal activity of PEPCK, which increases with exercise (Dohm et al., 1985). PEPCK is strongly up-regulated by glucocorticoids, agents that elevated cAMP (Friedman, 1994; Nizielski et al., 1996). Skeletal muscle-specific PEPCK-c transgenic mice were subsequently generated and found to have probably the most extreme endurance phenotype yet described (Hakimi et al., 2007). PEPCK-c muscle transgenic mice were found to be seven times more active than control mice, and the male mice extremely aggressive. With treadmill testing, the PEPCK-c transgenic mice ran up to 6 km at a speed of 20 m·min−1, whereas control mice stopped at 0.2 km. The PEPCK-c transgenic mice ate 60% more than controls but had half the body weight and 10% the body fat as determined by magnetic resonance imaging, while the skeletal muscle were greatly enriched with mitochondria, store up to five times more triglyceride than control mice, and have a greatly extended life span (females are reproductively active up to 35 months), and up to an age of 2.5 years ran twice as fast as 6–12-month-old control animals (Hakimi et al., 2007; Hanson and Hakimi, 2008).
Myokines and local mediators
Skeletal muscle release several cytokines, including IL-6, IL-8 and IL-15. IL-6 and IL-8 are induced upon muscle contractions. IL-6, in particular, increases insulin-stimulated glucose disposal and fatty acid oxidation in man, whereas IL-8 is only released in small amounts locally from contracting muscle, suggesting that IL-8 may exert its effects locally in the muscle that is by exerting a pro-angiogenic effect (Nielsen and Pedersen, 2007). The role of IL-6 in muscle, although one of the best characterized myokines, is poorly understood. IL-6 is a mediator of muscle wasting in models of cancer cachexia (Baltgalvis et al., 2009) and heart failure (Janssen et al., 2005), but also appears to be an important regulator of satellite cell mediated muscle hypertrophy (Serrano et al., 2008). IL-15 is also expressed at high levels by skeletal muscle. IL-15 appears to have little effect on myoblast proliferation or differentiation in vitro. However, IL-15 does appear to have anabolic activity, by increasing myosin heavy chain and myotubes hypertrophy, and synergizing with IGF-1 to increase myosin heavy chain (Quinn et al., 1995).
IL-15
IL-15 serum levels negatively correlates with obesity in man (Nielsen and Pedersen, 2007). Plasma IL-15 protein is significantly elevated in man immediately after acute resistance exercise, but did not appear to change with further resistance training, and a SNP in exon 7 of IL-15 receptor is strongly associated with muscle hypertrophy (Riechman et al., 2004). Due to its anabolic activity in myofibres, the initial studies with IL-15 focused on resistance training; however, recent evidence to a greater role mediating the endurance response. A 30-min treadmill running protocol at resulted in a significant increase in circulating IL-15 level in untrained healthy young men (Tamura et al., 2011). However, the role for IL-15 in endurance exercise is far from clear, as both IL-15 receptor knockout and IL-15 overexpression both lead to an endurance phenotype. Whether there is an alternative IL-15 receptor that could account for this is not known. In mice lacking the IL-15 receptor, there is a fibre switch to an oxidative phenotype including markers of mitochondrial biogenesis and increasing resistance to fatigue and increased exercise capacity (Pistilli et al., 2011; 2013). These IL-15 receptor knockouts have greater running endurance and greater ambulatory activity. Consistent with a role in man, a genetic association was found between a SNP in the IL-15 receptor gene and endurance in athletes stratified by sport (Pistilli et al., 2011). In contrast to the receptor knockout, IL-15 transgenic mice, with elevated skeletal muscle and circulating IL-15 levels also have an endurance phenotype, being able to run twice the distance as control mice (Quinn et al., 2013). The muscles of these IL-15 transgenic mice had increased markers for oxidative metabolism, including SIRT1, PGC-1α and PGC-1β, and preferentially metabolized fatty acids, while still maintaining higher levels of myosin heavy chain and troponin I (Quinn et al., 2013).
Brain-derived neurotrophic factor (BDNF)
Exercise clearly has not only effects on muscle but central effects. β-endorphins are released upon exercise and provide effective analgesia for a number of painful conditions (Nijs et al., 2012). In addition, the ability to tolerate pain is clearly also associated with exercise training and performance. Exercise also leads to central adaptations. Of particular importance appears to be an increase in BDNF which via its receptor TrkB leads to long-term potentiation and an increase in both excitatory and inhibitory synapses, new nerve cell growth and an increase in respiration and mitochondrial activity particularly in the hippocampus (Mattson, 2012; Spedding and Spedding, 2008) and learning (Rothman et al., 2012). BDNF levels in the brain and plasma are elevated after exercise (Spedding and Spedding, 2008), and in the brain at least, this appears secondary to IGF-1 (Chen and Russo-Neustadt, 2007); see Supporting Information Appendix S1. Conversely, circulating BDNF levels are reduced in individuals with obesity, type 2 diabetes, cardiovascular disease, depression and with diagnosed Alzheimer's disease (Pedersen, 2009a). BDNF is a critical central regulator of food intake (Rothman et al., 2012). However, BNDF treatment improved insulin resistance in obese (ob/ob) mice independent of effects on food intake suggesting additional peripheral actions (Pedersen, 2009a). BDNF has also now been recently identified as a myokine, being increased in human skeletal muscle after exercise, or in electrically stimulated muscle cells (Matthews et al., 2009). This induced BDNF via TrkB induced an increase in β-oxidation via the activation of AMPK and Akt dependent pathways (Matthews et al., 2009). At this stage, however, it is not known how much BDNF contributes to endurance phenotype of exercising muscle.
Summary and conclusions
Exercise is a vastly underused therapy. No pharmaceutical intervention has the overall benefit on mortality and reduction in risk factors as aerobic fitness. Understanding the underlying mechanisms that mediate exercise will gives us new therapeutic leads to mimic some of these important health benefits. Such therapies could provide novel treatments for life-long health in an increasingly aging population, as well as for muscle specific pathologies, where chronic disease or immobilization invariably leads to muscle wasting. The overall benefit of exercise in its entirety is not going to be replaced by a pill, but the pathways described do does allow multiple opportunities for pharmacologists to test and develop new exercise mimetic therapies.
Acknowledgments
David Bishop-Bailey is funded by the British Heart Foundation (PG/11/39/28890).
Glossary
- ACTN3
α-actinin-3
- AMPK
adenosine monophosphate kinase
- EPO
erythropoietin
- ERR
oestrogen-like receptor
- HIF
hypoxia inducible factor
- IGF-1
insulin-like growth factor
- MET
metabolic equivalent
- Pax
paired homeobox transcription factor
- PEPCK-c
cytosolic phosphoenolpyruvate carboxykinase
- PGC-1α
PPARγ coactivator-1α; SIRT, sirtuin
Conflict of interest
No conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
http://dx.doi.org/10.1111/bph.12399
Appendix S1 Supporting information text.
Figure S1 Signalling of muscle hypertrophy with resistance/strength type exercises.
References
- Aggarwal BB, Shishodia S, Ashikawa K, Bharti AC. The role of TNF and its family members in inflammation and cancer: lessons from gene deletion. Curr Drug Targets Inflamm Allergy. 2002;1:327–341. doi: 10.2174/1568010023344571. [DOI] [PubMed] [Google Scholar]
- Ahmetov II, Mozhayskaya IA, Flavell DM, Astratenkova IV, Komkova AI, Lyubaeva EV, et al. PPARalpha gene variation and physical performance in Russian athletes. Eur J Appl Physiol. 2006;97:103–108. doi: 10.1007/s00421-006-0154-4. [DOI] [PubMed] [Google Scholar]
- Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- Arlettaz A, Portier H, Lecoq AM, Rieth N, De Ceaurriz J, Collomp K. Effects of short-term prednisolone intake during submaximal exercise. Med Sci Sports Exerc. 2007;39:1672–1678. doi: 10.1249/mss.0b013e3180dc992c. [DOI] [PubMed] [Google Scholar]
- Arlettaz A, Collomp K, Portier H, Lecoq AM, Rieth N, Le Panse B, et al. Effects of acute prednisolone administration on exercise endurance and metabolism. Br J Sports Med. 2008;42:250–254. doi: 10.1136/bjsm.2007.039040. discussion 254. [DOI] [PubMed] [Google Scholar]
- Baar K. Involvement of PPAR gamma co-activator-1, nuclear respiratory factors 1 and 2, and PPAR alpha in the adaptive response to endurance exercise. Proc Nutr Soc. 2004;63:269–273. doi: 10.1079/PNS2004334. [DOI] [PubMed] [Google Scholar]
- Baltgalvis KA, Berger FG, Pena MM, Davis JM, White JP, Carson JA. Muscle wasting and interleukin-6-induced atrogin-I expression in the cachectic Apc (Min/+) mouse. Pflugers Arch. 2009;457:989–1001. doi: 10.1007/s00424-008-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baume N, Steel G, Edwards T, Thorstensen E, Miller BF. No variation of physical performance and perceived exertion after adrenal gland stimulation by synthetic ACTH (Synacthen) in cyclists. Eur J Appl Physiol. 2008;104:589–600. doi: 10.1007/s00421-008-0802-y. [DOI] [PubMed] [Google Scholar]
- Bellamy LM, Johnston AP, De Lisio M, Parise G. Skeletal muscle-endothelial cell cross talk through angiotensin II. Am J Physiol Cell Physiol. 2010;299:C1402–C1408. doi: 10.1152/ajpcell.00306.2010. [DOI] [PubMed] [Google Scholar]
- Berkes CA, Tapscott SJ. MyoD and the transcriptional control of myogenesis. Semin Cell Dev Biol. 2005;16:585–595. doi: 10.1016/j.semcdb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Bishop-Bailey D. Peroxisome proliferator-activated receptors in the cardiovascular system. Br J Pharmacol. 2000;129:823–834. doi: 10.1038/sj.bjp.0703149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop-Bailey D. PPARs and angiogenesis. Biochem Soc Trans. 2011;39:1601–1605. doi: 10.1042/BST20110643. [DOI] [PubMed] [Google Scholar]
- Bishop-Bailey D, Wray J. Peroxisome proliferator-activated receptors: a critical review on endogenous pathways for ligand generation. Prostaglandins Other Lipid Mediat. 2003;71:1–22. doi: 10.1016/s0090-6980(03)00003-0. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345–352. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- Bueno Junior CR, Pantaleao LC, Voltarelli VA, Bozi LH, Brum PC, Zatz M. Combined effect of AMPK/PPAR agonists and exercise training in mdx mice functional performance. PLoS ONE. 2012;7:e45699. doi: 10.1371/journal.pone.0045699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher LR, Thomas A, Backx K, Roberts A, Webb R, Morris K. Low-intensity exercise exerts beneficial effects on plasma lipids via PPARgamma. Med Sci Sports Exerc. 2008;40:1263–1270. doi: 10.1249/MSS.0b013e31816c091d. [DOI] [PubMed] [Google Scholar]
- Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, et al. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol. 2008;104:1304–1312. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- Chan S, Seto JT, MacArthur DG, Yang N, North KN, Head SI. A gene for speed: contractile properties of isolated whole EDL muscle from an alpha-actinin-3 knockout mouse. Am J Physiol Cell Physiol. 2008;295:C897–C904. doi: 10.1152/ajpcell.00179.2008. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A, Traskelin AL, Juvonen E. Truncated erythropoietin receptor causes dominantly inherited benign human erythrocytosis. Proc Natl Acad Sci U S A. 1993;90:4495–4499. doi: 10.1073/pnas.90.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RF, Stray-Gundersen J, Levine BD. Individual variation in response to altitude training. J Appl Physiol. 1998;85:1448–1456. doi: 10.1152/jappl.1998.85.4.1448. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Running exercise- and antidepressant-induced increases in growth and survival-associated signaling molecules are IGF-dependent. Growth Factors. 2007;25:118–131. doi: 10.1080/08977190701602329. [DOI] [PubMed] [Google Scholar]
- Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, et al. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci U S A. 2009;106:21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudyk A, Petrella RJ. Effects of exercise on cardiovascular risk factors in type 2 diabetes: a meta-analysis. Diabetes Care. 2011;34:1228–1237. doi: 10.2337/dc10-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne N, Berglund B, Egberg N. Treatment with recombinant human erythropoietin induces a moderate rise in hematocrit and thrombin antithrombin in healthy subjects. Thromb Res. 1995;79:125–129. doi: 10.1016/0049-3848(95)91520-u. [DOI] [PubMed] [Google Scholar]
- Dohm GL, Newsholme EA. Metabolic control of hepatic gluconeogenesis during exercise. Biochem J. 1983;212:633–639. doi: 10.1042/bj2120633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohm GL, Kasperek GJ, Barakat HA. Time course of changes in gluconeogenic enzyme activities during exercise and recovery. Am J Physiol. 1985;249(1 Pt 1):E6–11. doi: 10.1152/ajpendo.1985.249.1.E6. [DOI] [PubMed] [Google Scholar]
- Eissa Ahmed AA, Al-Rasheed NM. Antidepressant-like effects of rosiglitazone, a PPARgamma agonist, in the rat forced swim and mouse tail suspension tests. Behav Pharmacol. 2009;20:635–642. doi: 10.1097/FBP.0b013e328331b9bf. [DOI] [PubMed] [Google Scholar]
- Eliopoulos N, Gagnon RF, Francois M, Galipeau J. Erythropoietin delivery by genetically engineered bone marrow stromal cells for correction of anemia in mice with chronic renal failure. J Am Soc Nephrol. 2006;17:1576–1584. doi: 10.1681/ASN.2005101035. [DOI] [PubMed] [Google Scholar]
- Eriksson PO, Thornell LE. Histochemical and morphological muscle-fibre characteristics of the human masseter, the medial pterygoid and the temporal muscles. Arch Oral Biol. 1983;28:781–795. doi: 10.1016/0003-9969(83)90034-1. [DOI] [PubMed] [Google Scholar]
- Eynon N, Duarte JA, Oliveira J, Sagiv M, Yamin C, Meckel Y, et al. ACTN3 R577X polymorphism and Israeli top-level athletes. Int J Sports Med. 2009;30:695–698. doi: 10.1055/s-0029-1220731. [DOI] [PubMed] [Google Scholar]
- Eynon N, Ruiz JR, Femia P, Pushkarev VP, Cieszczyk P, Maciejewska-Karlowska A, et al. The ACTN3 R577X polymorphism across three groups of elite male European athletes. PLoS ONE. 2012;7:e43132. doi: 10.1371/journal.pone.0043132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanzani A, Conraads VM, Penna F, Martinet W. Molecular and cellular mechanisms of skeletal muscle atrophy: an update. J Cachexia Sarcopenia Muscle. 2012;3:163–179. doi: 10.1007/s13539-012-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatone C, Guescini M, Balducci S, Battistoni S, Settequattrini A, Pippi R, et al. Two weekly sessions of combined aerobic and resistance exercise are sufficient to provide beneficial effects in subjects with Type 2 diabetes mellitus and metabolic syndrome. J Endocrinol Invest. 2010;33:489–495. doi: 10.1007/BF03346630. [DOI] [PubMed] [Google Scholar]
- Fiuza-Luces C, Ruiz JR, Rodriguez-Romo G, Santiago C, Gomez-Gallego F, Yvert T, et al. Are ‘endurance’ alleles ‘survival’ alleles? Insights from the ACTN3 R577X polymorphism. PLoS ONE. 2011;6:e17558. doi: 10.1371/journal.pone.0017558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyssenet D. Energy sensing and regulation of gene expression in skeletal muscle. J Appl Physiol. 2007;102:529–540. doi: 10.1152/japplphysiol.01126.2005. [DOI] [PubMed] [Google Scholar]
- Friedman JE. Role of glucocorticoids in activation of hepatic PEPCK gene transcription during exercise. Am J Physiol. 1994;266(4 Pt 1):E560–E566. doi: 10.1152/ajpendo.1994.266.4.E560. [DOI] [PubMed] [Google Scholar]
- Fritz T, Kramer DK, Karlsson HK, Galuska D, Engfeldt P, Zierath JR, et al. Low-intensity exercise increases skeletal muscle protein expression of PPARdelta and UCP3 in type 2 diabetic patients. Diabetes Metab Res Rev. 2006;22:492–498. doi: 10.1002/dmrr.656. [DOI] [PubMed] [Google Scholar]
- Fry AC. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med. 2004;34:663–679. doi: 10.2165/00007256-200434100-00004. [DOI] [PubMed] [Google Scholar]
- Gan Z, Burkart-Hartman EM, Han DH, Finck B, Leone TC, Smith EY, et al. The nuclear receptor PPARbeta/delta programs muscle glucose metabolism in cooperation with AMPK and MEF2. Genes Dev. 2011;25:2619–2630. doi: 10.1101/gad.178434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590(Pt 5):1077–1084. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano C, Rousseau AS, Wagner N, Gaudel C, Murdaca J, Jehl-Pietri C, et al. Peroxisome proliferator-activated receptor beta activation promotes myonuclear accretion in skeletal muscle of adult and aged mice. Pflugers Arch. 2009;458:901–913. doi: 10.1007/s00424-009-0676-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SL, Aragaki AK, LaMonte MJ, Cochrane BB, Kooperberg C, Robinson JG, et al. Statins, angiotensin-converting enzyme inhibitors, and physical performance in older women. J Am Geriatr Soc. 2012;60:2206–2214. doi: 10.1111/jgs.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajisoltani R, Rashidy-Pour A, Vafaei AA, Ghaderdoost B, Bandegi AR, Motamedi F. The glucocorticoid system is required for the voluntary exercise-induced enhancement of learning and memory in rats. Behav Brain Res. 2011;219:75–81. doi: 10.1016/j.bbr.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Hakimi P, Yang J, Casadesus G, Massillon D, Tolentino-Silva F, Nye CK, et al. Overexpression of the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) in skeletal muscle repatterns energy metabolism in the mouse. J Biol Chem. 2007;282:32844–32855. doi: 10.1074/jbc.M706127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka BY, Peterson CA, Crofford LJ. Glucocorticoid effects on skeletal muscle: benefit and risk in patients with autoimmune inflammatory rheumatoid diseases. Expert Rev Clin Immunol. 2012;8:695–697. doi: 10.1586/eci.12.76. [DOI] [PubMed] [Google Scholar]
- Hanson RW, Hakimi P. Born to run; the story of the PEPCK-Cmus mouse. Biochimie. 2008;90:838–842. doi: 10.1016/j.biochi.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley JA, Burke LM, Phillips SM, Spriet LL. Nutritional modulation of training-induced skeletal muscle adaptations. J Appl Physiol. 2011;110:834–845. doi: 10.1152/japplphysiol.00949.2010. [DOI] [PubMed] [Google Scholar]
- Heuberger JA, Cohen Tervaert JM, Schepers FM, Vliegenthart AD, Rotmans JI, Daniels JM, et al. Erythropoietin doping in cycling: lack of evidence for efficacy and a negative risk-benefit. Br J Clin Pharmacol. 2013;75:1406–1421. doi: 10.1111/bcp.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley BF, Hanson ED, Sheaff AK. Strength training as a countermeasure to aging muscle and chronic disease. Sports Med. 2011;41:289–306. doi: 10.2165/11585920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Huson HJ, Byers AM, Runstadler J, Ostrander EA. An SNP within the angiotensin-converting enzyme distinguishes between sprint and distance performing Alaskan sled dogs in a candidate gene analysis. J Hered. 2011;102(Suppl 1):S19–S27. doi: 10.1093/jhered/esr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinoseki-Sekine N, Yoshihara T, Kakigi R, Ogura Y, Sugiura T, Naito H. Fiber-type specific expression of alpha-actinin isoforms in rat skeletal muscle. Biochem Biophys Res Commun. 2012;419:401–404. doi: 10.1016/j.bbrc.2012.02.034. [DOI] [PubMed] [Google Scholar]
- Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- Janssen SP, Gayan-Ramirez G, Van den Bergh A, Herijgers P, Maes K, Verbeken E, et al. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation. 2005;111:996–1005. doi: 10.1161/01.CIR.0000156469.96135.0D. [DOI] [PubMed] [Google Scholar]
- Juffer P, Furrer R, Gonzalez-Freire M, Santiago C, Verde Z, Serratosa L, et al. Genotype distributions in top-level soccer players: a role for ACE? Int J Sports Med. 2009;30:387–392. doi: 10.1055/s-0028-1105931. [DOI] [PubMed] [Google Scholar]
- Kadoglou NP, Fotiadis G, Kapelouzou A, Kostakis A, Liapis CD, Vrabas IS. The differential anti-inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with Type 2 diabetes. Diabet Med. 2013;30:e41–e50. doi: 10.1111/dme.12055. [DOI] [PubMed] [Google Scholar]
- Kanzleiter T, Wilks D, Preston E, Ye J, Frangioudakis G, Cooney GJ. Regulation of the nuclear hormone receptor nur77 in muscle: influence of exercise-activated pathways in vitro and obesity in vivo. Biochim Biophys Acta. 2009;1792:777–782. doi: 10.1016/j.bbadis.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Kanzleiter T, Preston E, Wilks D, Ho B, Benrick A, Reznick J, et al. Overexpression of the orphan receptor Nur77 alters glucose metabolism in rat muscle cells and rat muscle in vivo. Diabetologia. 2010;53:1174–1183. doi: 10.1007/s00125-010-1703-2. [DOI] [PubMed] [Google Scholar]
- Kawasaki E, Hokari F, Sasaki M, Sakai A, Koshinaka K, Kawanaka K. Role of local muscle contractile activity in the exercise-induced increase in NR4A receptor mRNA expression. J Appl Physiol. 2009;106:1826–1831. doi: 10.1152/japplphysiol.90923.2008. [DOI] [PubMed] [Google Scholar]
- Kokkinos P, Myers J, Kokkinos JP, Pittaras A, Narayan P, Manolis A, et al. Exercise capacity and mortality in black and white men. Circulation. 2008;117:614–622. doi: 10.1161/CIRCULATIONAHA.107.734764. [DOI] [PubMed] [Google Scholar]
- Kolb EM, Kelly SA, Middleton KM, Sermsakdi LS, Chappell MA, Garland T., Jr Erythropoietin elevates VO2,max but not voluntary wheel running in mice. J Exp Biol. 2010;213:510–519. doi: 10.1242/jeb.029074. [DOI] [PubMed] [Google Scholar]
- Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, et al. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280:33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- Kuipers H, Van't Hullenaar GA, Pluim BM, Overbeek SE, De Hon O, Van Breda EJ, et al. Four weeks' corticosteroid inhalation does not augment maximal power output in endurance athletes. Br J Sports Med. 2008;42:868–871. doi: 10.1136/bjsm.2007.042572. [DOI] [PubMed] [Google Scholar]
- Lage JM, Panizo C, Masdeu J, Rocha E. Cyclist's doping associated with cerebral sinus thrombosis. Neurology. 2002;58:665. doi: 10.1212/wnl.58.4.665. [DOI] [PubMed] [Google Scholar]
- Leiter JR, Peeler J, Anderson JE. Exercise-induced muscle growth is muscle-specific and age-dependent. Muscle Nerve. 2011;43:828–838. doi: 10.1002/mus.21965. [DOI] [PubMed] [Google Scholar]
- de Lemos ET, Oliveira J, Pinheiro JP, Reis F. Regular physical exercise as a strategy to improve antioxidant and anti-inflammatory status: benefits in type 2 diabetes mellitus. Oxid Med Cell Longev. 2012;2012:741545. doi: 10.1155/2012/741545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GD, Farrell L, Wood MJ, Martinovic M, Arany Z, Rowe GC, et al. Metabolic signatures of exercise in human plasma. Sci Transl Med. 2010;2:33ra37. doi: 10.1126/scitranslmed.3001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucia A, Gomez-Gallego F, Santiago C, Bandres F, Earnest C, Rabadan M, et al. ACTN3 genotype in professional endurance cyclists. Int J Sports Med. 2006;27:880–884. doi: 10.1055/s-2006-923862. [DOI] [PubMed] [Google Scholar]
- Luden N, Hayes E, Minchev K, Louis E, Raue U, Conley T, et al. Skeletal muscle plasticity with marathon training in novice runners. Scand J Med Sci Sports. 2012;22:662–670. doi: 10.1111/j.1600-0838.2011.01305.x. [DOI] [PubMed] [Google Scholar]
- Ludolph DC, Konieczny SF. Transcription factor families: muscling in on the myogenic program. FASEB J. 1995;9:1595–1604. doi: 10.1096/fasebj.9.15.8529839. [DOI] [PubMed] [Google Scholar]
- Lundberg TR, Fernandez-Gonzalo R, Gustafsson T, Tesch PA. Aerobic exercise does not compromise muscle hypertrophy response to short-term resistance training. J Appl Physiol. 2013;114:81–89. doi: 10.1152/japplphysiol.01013.2012. [DOI] [PubMed] [Google Scholar]
- Lundby C, Olsen NV. Effects of recombinant human erythropoietin in normal humans. J Physiol. 2011;589(Pt 6):1265–1271. doi: 10.1113/jphysiol.2010.195917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde IG, Ekmark M, Rana ZA, Buonanno A, Gundersen K. PPARdelta expression is influenced by muscle activity and induces slow muscle properties in adult rat muscles after somatic gene transfer. J Physiol. 2007;582(Pt 3):1277–1287. doi: 10.1113/jphysiol.2007.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Lopez-Soriano J, Holst D, Fredenrich A, Melki J, Rassoulzadegan M, et al. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J. 2003;17:2299–2301. doi: 10.1096/fj.03-0269fje. [DOI] [PubMed] [Google Scholar]
- MacArthur DG, Seto JT, Raftery JM, Quinlan KG, Huttley GA, Hook JW, et al. Loss of ACTN3 gene function alters mouse muscle metabolism and shows evidence of positive selection in humans. Nat Genet. 2007;39:1261–1265. doi: 10.1038/ng2122. [DOI] [PubMed] [Google Scholar]
- MacArthur DG, Seto JT, Chan S, Quinlan KG, Raftery JM, Turner N, et al. An Actn3 knockout mouse provides mechanistic insights into the association between alpha-actinin-3 deficiency and human athletic performance. Hum Mol Genet. 2008;17:1076–1086. doi: 10.1093/hmg/ddm380. [DOI] [PubMed] [Google Scholar]
- McCall GE, Byrnes WC, Dickinson A, Pattany PM, Fleck SJ. Muscle fiber hypertrophy, hyperplasia, and capillary density in college men after resistance training. J Appl Physiol. 1996;81:2004–2012. doi: 10.1152/jappl.1996.81.5.2004. [DOI] [PubMed] [Google Scholar]
- McKeever KH, Agans JM, Geiser S, Lorimer PJ, Maylin GA. Low dose exogenous erythropoietin elicits an ergogenic effect in standardbred horses. Equine Vet J Suppl. 2006;36:233–238. doi: 10.1111/j.2042-3306.2006.tb05545.x. [DOI] [PubMed] [Google Scholar]
- Maher AC, Fu MH, Isfort RJ, Varbanov AR, Qu XA, Tarnopolsky MA. Sex differences in global mRNA content of human skeletal muscle. PLoS ONE. 2009;4:e6335. doi: 10.1371/journal.pone.0006335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini DM, Katz SD, Lang CC, LaManca J, Hudaihed A, Androne AS. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003;107:294–299. doi: 10.1161/01.cir.0000044914.42696.6a. [DOI] [PubMed] [Google Scholar]
- Matthews VB, Astrom MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Evolutionary aspects of human exercise – born to run purposefully. Ageing Res Rev. 2012;11:347–352. doi: 10.1016/j.arr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michigan A, Johnson TV, Master VA. Review of the relationship between C-reactive protein and exercise. Mol Diagn Ther. 2011;15:265–275. doi: 10.1007/BF03256418. [DOI] [PubMed] [Google Scholar]
- Mille-Hamard L, Billat VL, Henry E, Bonnamy B, Joly F, Benech P, et al. Skeletal muscle alterations and exercise performance decrease in erythropoietin-deficient mice: a comparative study. BMC Med Genomics. 2012;5:29. doi: 10.1186/1755-8794-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, Kawanaka K, Kai Y, Tamura M, Goto M, Shiuchi T, et al. An increase in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to exercise is mediated by beta-adrenergic receptor activation. Endocrinology. 2007;148:3441–3448. doi: 10.1210/en.2006-1646. [DOI] [PubMed] [Google Scholar]
- Montgomery HE, Clarkson P, Dollery CM, Prasad K, Losi MA, Hemingway H, et al. Association of angiotensin-converting enzyme gene I/D polymorphism with change in left ventricular mass in response to physical training. Circulation. 1997;96:741–747. doi: 10.1161/01.cir.96.3.741. [DOI] [PubMed] [Google Scholar]
- Muniesa CA, Gonzalez-Freire M, Santiago C, Lao JI, Buxens A, Rubio JC, et al. World-class performance in lightweight rowing: is it genetically influenced? A comparison with cyclists, runners and non-athletes. Br J Sports Med. 2010;44:898–901. doi: 10.1136/bjsm.2008.051680. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Koves TR. Skeletal muscle adaptation to fatty acid depends on coordinated actions of the PPARs and PGC1 alpha: implications for metabolic disease. Appl Physiol Nutr Metab. 2007;32:874–883. doi: 10.1139/H07-083. [DOI] [PubMed] [Google Scholar]
- Nader GA, Lundberg IE. Exercise as an anti-inflammatory intervention to combat inflammatory diseases of muscle. Curr Opin Rheumatol. 2009;21:599–603. doi: 10.1097/BOR.0b013e3283319d53. [DOI] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkar VA, Fan W, Downes M, Yu RT, Jonker JW, Alaynick WA, et al. Exercise and PGC-1alpha-independent synchronization of type I muscle metabolism and vasculature by ERRgamma. Cell Metab. 2011;13:283–293. doi: 10.1016/j.cmet.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TL, Fingerlin TE, Moss LK, Barmada MM, Ferrell RE, Norris JM. Association of the peroxisome proliferator-activated receptor gamma gene with type 2 diabetes mellitus varies by physical activity among non-Hispanic whites from Colorado. Metabolism. 2007;56:388–393. doi: 10.1016/j.metabol.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Newton RU, Galvao DA. Exercise in prevention and management of cancer. Curr Treat Options Oncol. 2008;9:135–146. doi: 10.1007/s11864-008-0065-1. [DOI] [PubMed] [Google Scholar]
- Nielsen AR, Pedersen BK. The biological roles of exercise-induced cytokines: IL-6, IL-8, and IL-15. Appl Physiol Nutr Metab. 2007;32:833–839. doi: 10.1139/H07-054. [DOI] [PubMed] [Google Scholar]
- Nijs J, Kosek E, Van Oosterwijck J, Meeus M. Dysfunctional endogenous analgesia during exercise in patients with chronic pain: to exercise or not to exercise? Pain Physician. 2012;15(3 Suppl):ES205–ES213. [PubMed] [Google Scholar]
- Nikander R, Sievanen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P. Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010;8:47. doi: 10.1186/1741-7015-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizielski SE, Arizmendi C, Shteyngarts AR, Farrell CJ, Friedman JE. Involvement of transcription factor C/EBP-beta in stimulation of PEPCK gene expression during exercise. Am J Physiol. 1996;270(5 Pt 2):R1005–R1012. doi: 10.1152/ajpregu.1996.270.5.R1005. [DOI] [PubMed] [Google Scholar]
- Noakes T, Spedding M. Olympics: run for your life. Nature. 2012;487:295–296. doi: 10.1038/487295a. [DOI] [PubMed] [Google Scholar]
- Olson EJ, Pearce GL, Jones NP, Sprecher DL. Lipid effects of peroxisome proliferator-activated receptor-delta agonist GW501516 in subjects with low high-density lipoprotein cholesterol: characteristics of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2012;32:2289–2294. doi: 10.1161/ATVBAHA.112.247890. [DOI] [PubMed] [Google Scholar]
- Onder G, Vedova CD, Pahor M. Effects of ACE inhibitors on skeletal muscle. Curr Pharm Des. 2006;12:2057–2064. doi: 10.2174/138161206777442137. [DOI] [PubMed] [Google Scholar]
- Pearen MA, Eriksson NA, Fitzsimmons RL, Goode JM, Martel N, Andrikopoulos S, et al. The nuclear receptor, Nor-1, markedly increases type II oxidative muscle fibers and resistance to fatigue. Mol Endocrinol. 2012;26:372–384. doi: 10.1210/me.2011-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK. The diseasome of physical inactivity – and the role of myokines in muscle – fat cross talk. J Physiol. 2009a;587(Pt 23):5559–5568. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK. Edward F. Adolph distinguished lecture: muscle as an endocrine organ: IL-6 and other myokines. J Appl Physiol. 2009b;107:1006–1014. doi: 10.1152/japplphysiol.00734.2009. [DOI] [PubMed] [Google Scholar]
- Petridou A, Tsalouhidou S, Tsalis G, Schulz T, Michna H, Mougios V. Long-term exercise increases the DNA binding activity of peroxisome proliferator-activated receptor gamma in rat adipose tissue. Metabolism. 2007;56:1029–1036. doi: 10.1016/j.metabol.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Glover EI, Rennie MJ. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J Appl Physiol. 2009;107:645–654. doi: 10.1152/japplphysiol.00452.2009. [DOI] [PubMed] [Google Scholar]
- Piqueras L, Reynolds AR, Hodivala-Dilke KM, Alfranca A, Redondo JM, Hatae T, et al. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:63–69. doi: 10.1161/01.ATV.0000250972.83623.61. [DOI] [PubMed] [Google Scholar]
- Pistilli EE, Bogdanovich S, Garton F, Yang N, Gulbin JP, Conner JD, et al. Loss of IL-15 receptor alpha alters the endurance, fatigability, and metabolic characteristics of mouse fast skeletal muscles. J Clin Invest. 2011;121:3120–3132. doi: 10.1172/JCI44945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistilli EE, Guo G, Stauber WT. IL-15Ralpha deficiency leads to mitochondrial and myofiber differences in fast mouse muscles. Cytokine. 2013;61:41–45. doi: 10.1016/j.cyto.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier P, Despres JP. Exercise in weight management of obesity. Cardiol Clin. 2001;19:459–470. doi: 10.1016/s0733-8651(05)70229-0. [DOI] [PubMed] [Google Scholar]
- Psilander N, Wang L, Westergren J, Tonkonogi M, Sahlin K. Mitochondrial gene expression in elite cyclists: effects of high-intensity interval exercise. Eur J Appl Physiol. 2010;110:597–606. doi: 10.1007/s00421-010-1544-1. [DOI] [PubMed] [Google Scholar]
- Puthucheary Z, Skipworth JR, Rawal J, Loosemore M, Van Someren K, Montgomery HE. The ACE gene and human performance: 12 years on. Sports Med. 2011;41:433–448. doi: 10.2165/11588720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Quinlan KG, Seto JT, Turner N, Vandebrouck A, Floetenmeyer M, Macarthur DG, et al. Alpha-actinin-3 deficiency results in reduced glycogen phosphorylase activity and altered calcium handling in skeletal muscle. Hum Mol Genet. 2010;19:1335–1346. doi: 10.1093/hmg/ddq010. [DOI] [PubMed] [Google Scholar]
- Quinn LS, Haugk KL, Grabstein KH. Interleukin-15: a novel anabolic cytokine for skeletal muscle. Endocrinology. 1995;136:3669–3672. doi: 10.1210/endo.136.8.7628408. [DOI] [PubMed] [Google Scholar]
- Quinn LS, Anderson BG, Conner JD, Wolden-Hanson T. IL-15 overexpression promotes endurance, oxidative energy metabolism, and muscle PPARdelta, SIRT1, PGC-1alpha, and PGC-1beta expression in male mice. Endocrinology. 2013;154:232–245. doi: 10.1210/en.2012-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan SN, Lau P, Burke LJ, Muscat GE. Rev-erbbeta regulates the expression of genes involved in lipid absorption in skeletal muscle cells: evidence for cross-talk between orphan nuclear receptors and myokines. J Biol Chem. 2005;280:8651–8659. doi: 10.1074/jbc.M413949200. [DOI] [PubMed] [Google Scholar]
- Rangwala SM, Wang X, Calvo JA, Lindsley L, Zhang Y, Deyneko G, et al. Estrogen-related receptor gamma is a key regulator of muscle mitochondrial activity and oxidative capacity. J Biol Chem. 2010;285:22619–22629. doi: 10.1074/jbc.M110.125401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasbach KA, Gupta RK, Ruas JL, Wu J, Naseri E, Estall JL, et al. PGC-1alpha regulates a HIF2alpha-dependent switch in skeletal muscle fiber types. Proc Natl Acad Sci U S A. 2010;107:21866–21871. doi: 10.1073/pnas.1016089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen P, Foged EM, Krogh-Madsen R, Nielsen J, Nielsen TR, Olsen NV, et al. Effects of erythropoietin administration on cerebral metabolism and exercise capacity in men. J Appl Physiol. 2010;109:476–483. doi: 10.1152/japplphysiol.00234.2010. [DOI] [PubMed] [Google Scholar]
- Regoli D, Plante GE, Gobeil F., Jr Impact of kinins in the treatment of cardiovascular diseases. Pharmacol Ther. 2012;135:94–111. doi: 10.1016/j.pharmthera.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development. 2012;139:2845–2856. doi: 10.1242/dev.069088. [DOI] [PubMed] [Google Scholar]
- Riechman SE, Balasekaran G, Roth SM, Ferrell RE. Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J Appl Physiol. 2004;97:2214–2219. doi: 10.1152/japplphysiol.00491.2004. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D, Smith DJ. Erythropoietin concentration and arterial haemoglobin saturation with supramaximal exercise. J Sports Sci. 1999;17:485–493. doi: 10.1080/026404199365795. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Griffioen KJ, Wan R, Mattson MP. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann N Y Acad Sci. 2012;1264:49–63. doi: 10.1111/j.1749-6632.2012.06525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, et al. A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151:1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchat SM, Rankinen T, Weisnagel SJ, Rice T, Rao DC, Bergman RN, et al. Improvements in glucose homeostasis in response to regular exercise are influenced by the PPARG Pro12Ala variant: results from the HERITAGE Family Study. Diabetologia. 2010;53:679–689. doi: 10.1007/s00125-009-1630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruschke K, Fishbein L, Dietrich A, Kloting N, Tonjes A, Oberbach A, et al. Gene expression of PPARgamma and PGC-1alpha in human omental and subcutaneous adipose tissues is related to insulin resistance markers and mediates beneficial effects of physical training. Eur J Endocrinol. 2010;162:515–523. doi: 10.1530/EJE-09-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AP, Hesselink MK, Lo SK, Schrauwen P. Regulation of metabolic transcriptional co-activators and transcription factors with acute exercise. FASEB J. 2005;19:986–988. doi: 10.1096/fj.04-3168fje. [DOI] [PubMed] [Google Scholar]
- Sadaghiani MS, Javadi-Paydar M, Gharedaghi MH, Fard YY, Dehpour AR. Antidepressant-like effect of pioglitazone in the forced swimming test in mice: the role of PPAR-gamma receptor and nitric oxide pathway. Behav Brain Res. 2011;224:336–343. doi: 10.1016/j.bbr.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- Saunders CJ, September AV, Xenophontos SL, Cariolou MA, Anastassiades LC, Noakes TD, et al. No association of the ACTN3 gene R577X polymorphism with endurance performance in Ironman Triathlons. Ann Hum Genet. 2007;71(Pt 6):777–781. doi: 10.1111/j.1469-1809.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- Schuler B, Arras M, Keller S, Rettich A, Lundby C, Vogel J, et al. Optimal hematocrit for maximal exercise performance in acute and chronic erythropoietin-treated mice. Proc Natl Acad Sci U S A. 2010;107:419–423. doi: 10.1073/pnas.0912924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler B, Vogel J, Grenacher B, Jacobs RA, Arras M, Gassmann M. Acute and chronic elevation of erythropoietin in the brain improves exercise performance in mice without inducing erythropoiesis. FASEB J. 2012;26:3884–3890. doi: 10.1096/fj.11-191197. [DOI] [PubMed] [Google Scholar]
- Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, Munoz-Canoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008;7:33–44. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Seto JT, Lek M, Quinlan KG, Houweling PJ, Zheng XF, Garton F, et al. Deficiency of alpha-actinin-3 is associated with increased susceptibility to contraction-induced damage and skeletal muscle remodeling. Hum Mol Genet. 2011;20:2914–2927. doi: 10.1093/hmg/ddr196. [DOI] [PubMed] [Google Scholar]
- Sixt S, Beer S, Bluher M, Korff N, Peschel T, Sonnabend M, et al. Long- but not short-term multifactorial intervention with focus on exercise training improves coronary endothelial dysfunction in diabetes mellitus type 2 and coronary artery disease. Eur Heart J. 2010;31:112–119. doi: 10.1093/eurheartj/ehp398. [DOI] [PubMed] [Google Scholar]
- Sjostrom M, Lexell J, Eriksson A, Taylor CC. Evidence of fibre hyperplasia in human skeletal muscles from healthy young men? A left-right comparison of the fibre number in whole anterior tibialis muscles. Eur J Appl Physiol Occup Physiol. 1991;62:301–304. doi: 10.1007/BF00634963. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Brown DA, Johnson MS, Moore RL. Alterations in peroxisome proliferator-activated receptor mRNA expression in skeletal muscle after acute and repeated bouts of exercise. Mol Cell Biochem. 2009;332:225–231. doi: 10.1007/s11010-009-0195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spedding M, Spedding C. Drugs in sport: a scientist-athlete's perspective: from ambition to neurochemistry. Br J Pharmacol. 2008;154:496–501. doi: 10.1038/bjp.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman BM. Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. Novartis Found Symp. 2007;287:60–63. discussion 63-69. [PubMed] [Google Scholar]
- Strohle A. Physical activity, exercise, depression and anxiety disorders. J Neural Transm. 2009;116:777–784. doi: 10.1007/s00702-008-0092-x. [DOI] [PubMed] [Google Scholar]
- Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadaishi M, Miura S, Kai Y, Kano Y, Oishi Y, Ezaki O. Skeletal muscle-specific expression of PGC-1alpha-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PLoS ONE. 2011;6:e28290. doi: 10.1371/journal.pone.0028290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Watanabe K, Kantani T, Hayashi J, Ishida N, Kaneki M. Upregulation of circulating IL-15 by treadmill running in healthy individuals: is IL-15 an endocrine mediator of the beneficial effects of endurance exercise? Endocr J. 2011;58:211–215. doi: 10.1507/endocrj.k10e-400. [DOI] [PubMed] [Google Scholar]
- Thomis MA, Huygens W, Heuninckx S, Chagnon M, Maes HH, Claessens AL, et al. Exploration of myostatin polymorphisms and the angiotensin-converting enzyme insertion/deletion genotype in responses of human muscle to strength training. Eur J Appl Physiol. 2004;92:267–274. doi: 10.1007/s00421-004-1093-6. [DOI] [PubMed] [Google Scholar]
- Venckunas T, Skurvydas A, Brazaitis M, Kamandulis S, Snieckus A, Moran CN. Human alpha-actinin-3 genotype association with exercise-induced muscle damage and the repeated-bout effect. Appl Physiol Nutr Metab. 2012;37:1038–1046. doi: 10.1139/h2012-087. [DOI] [PubMed] [Google Scholar]
- Vincent B, Windelinckx A, Van Proeyen K, Masschelein E, Nielens H, Ramaekers M, et al. Alpha-actinin-3 deficiency does not significantly alter oxidative enzyme activity in fast human muscle fibres. Acta Physiol (Oxf) 2012;204:555–561. doi: 10.1111/j.1748-1716.2011.02366.x. [DOI] [PubMed] [Google Scholar]
- Wagner N, Jehl-Pietri C, Lopez P, Murdaca J, Giordano C, Schwartz C, et al. Peroxisome proliferator-activated receptor beta stimulation induces rapid cardiac growth and angiogenesis via direct activation of calcineurin. Cardiovasc Res. 2009;83:61–71. doi: 10.1093/cvr/cvp106. [DOI] [PubMed] [Google Scholar]
- Wang CC, Reusch JE. Diabetes and cardiovascular disease: changing the focus from glycemic control to improving long-term survival. Am J Cardiol. 2012;110(9 Suppl):58B–68B. doi: 10.1016/j.amjcard.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Mikami E, Chiu LL, de Perini A, Deason M, Fuku N, et al. Association analysis of ACE and ACTN3 in Elite Caucasian and East Asian Swimmers. Med Sci Sports Exerc. 2013;45:892–900. doi: 10.1249/MSS.0b013e31827c501f. [DOI] [PubMed] [Google Scholar]
- Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, et al. Regulation of muscle fiber type and running endurance by PPARdelta. Plos Biology. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watowich SS. The erythropoietin receptor: molecular structure and hematopoietic signaling pathways. J Investig Med. 2011;59:1067–1072. doi: 10.231/JIM.0b013e31820fb28c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle S, Brooks A, Thornton CA. Senescence-related changes in gene expression in muscle: similarities and differences between mice and men. Physiol Genomics. 2001;5:67–73. doi: 10.1152/physiolgenomics.2001.5.2.67. [DOI] [PubMed] [Google Scholar]
- Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Williams EG, Mouchiroud L, Canto C, Fan W, Downes M, et al. NCoR1 is a conserved physiological modulator of muscle mass and oxidative function. Cell. 2011;147:827–839. doi: 10.1016/j.cell.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, MacArthur DG, Gulbin JP, Hahn AG, Beggs AH, Easteal S, et al. ACTN3 genotype is associated with human elite athletic performance. Am J Hum Genet. 2003;73:627–631. doi: 10.1086/377590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin JS. Inflammation, obesity, and the metabolic syndrome. Horm Metab Res. 2007;39:707–709. doi: 10.1055/s-2007-985898. [DOI] [PubMed] [Google Scholar]
- Zhang B, Shono N, Fan P, Ando S, Xu H, Jimi S, et al. Histochemical characteristics of soleus muscle in angiotensin-converting enzyme gene knockout mice. Hypertens Res. 2005;28:681–688. doi: 10.1291/hypres.28.681. [DOI] [PubMed] [Google Scholar]
- Zhang S, Liu Y, Li Q, Dong X, Hu H, Hu R, et al. Exercise improved rat metabolism by raising PPAR-alpha. Int J Sports Med. 2011;32:568–573. doi: 10.1055/s-0031-1271755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


