Abstract
BACKGROUND AND PURPOSE
Cholinesterase inhibitors such as neostigmine are used for acute colonic pseudo-obstruction, but cardio-bronchial side-effects limit use. To minimize side-effects, lower doses could be combined with a 5-HT4 receptor agonist, which also facilitates intestinal cholinergic activity. However, safety concerns, especially in the elderly, require drugs with good selectivity of action. These include the AChE inhibitor donepezil (used for Alzheimer's disease, with reduced cardio-bronchial liability) and prucalopride, the first selective, clinically available 5-HT4 receptor agonist. This study examined their individual and potential synergistic activities in human colon.
EXPERIMENTAL APPROACH
Neuronally mediated muscle contractions and relaxations of human colon were evoked by electrical field stimulation (EFS) and defined phenotypically as cholinergic, nitrergic or tachykinergic using pharmacological tools; the effects of drugs were determined as changes in ‘area under the curve’.
KEY RESULTS
Prucalopride increased cholinergically mediated contractions (EC50 855 nM; 33% maximum increase), consistent with its ability to stimulate intestinal motility; donepezil (477%) and neostigmine (2326%) had greater efficacy. Concentrations of donepezil (30–100 nM) found in venous plasma after therapeutic doses had minimal ability to enhance cholinergic activity. However, donepezil (30 nM) together with prucalopride (3, 10 μM) markedly increased EFS-evoked contractions compared with prucalopride alone (P = 0.04). For example, the increases observed with donepezil and prucalopride 10 μM together or alone were, respectively, 105 ± 35%, 4 ± 6% and 35 ± 21% (n = 3–7, each concentration).
CONCLUSIONS AND IMPLICATIONS
Potential synergy between prucalopride and donepezil activity calls for exploration of this combination as a safer, more effective treatment of colonic pseudo-obstruction.
Keywords: donepezil, prucalopride, human, colon, pseudo-obstruction, elderly, neostigmine
Introduction
Restoration of normal intestinal motility is a key objective in treatment of a range of acute and chronic digestive motility disorders. In the most common – chronic constipation, laxatives remain the mainstay of pharmacological intervention, although new drugs have recently been introduced. These include prucalopride, a selective 5-HT4 receptor agonist, which facilitates enteric cholinergic and nitrergic activities to promote intestinal motility (Cellek et al., 2006), and lubiprostone and linaclotide, which, respectively, activate chloride type-2 channels and guanylate cyclase type-C receptors to promote defecation primarily by increasing fluid secretion into the lumen (Lembo et al., 2011; Chey et al., 2012). Such breakthroughs have renewed interest in how best to treat other conditions associated with hypomotility and also with acute or chronic small and/or large intestinal dilation. These include post-operative ileus, intestinal pseudo-obstruction (Coulie and Camilleri, 1999), ‘acute colonic pseudo-obstruction’ (Saunders, 2007) and ‘megacolon’ (Bharucha and Phillips, 1999), collectively leading to significant individual morbidity and incident mortality (Coulie and Camilleri, 1999).
Cholinesterase inhibitors such as neostigmine or pyridostigmine are sometimes used to enhance intestinal propulsion in patients with severe hypomotility (Law et al., 2001), especially those with acute colonic pseudo-obstruction in which dilatation of the colon can lead to perforation and death (Turégano-Fuentes et al., 1997; Ponec et al., 1999; Amaro and Rogers, 2000; Trevisani et al., 2000; Loftus et al., 2002; Mehta et al., 2006; Saunders, 2007; McNamara and Mihalakis, 2008; O'Dea et al., 2010; Elsner et al., 2012; Bharucha et al., 2013). The latter can occur in patients of any age after major surgery or trauma, severe illness or during intensive care (Saunders and Kimmey, 2005; Saunders, 2007; Giorgio and Knowles, 2009). However, it most commonly occurs in susceptible elderly patients with extensive co-morbidities (Hyatt, 1987), in whom neostigmine and pyridostigmine may be absolutely contraindicated or hazardous due to cardio-bronchial side effects or renal insufficiency (Saunders, 2007). Further, neostigmine is an unattractive treatment option in any patient due to side effects of abdominal pain, excess salivation, nausea and/or vomiting, bradycardia (necessitating cardiac monitoring; Saunders, 2007; McNamara and Mihalakis, 2008), hypotension and bronchospasm (Turégano-Fuentes et al., 1997; Amaro and Rogers, 2000). For these reasons, the use of cholinesterase inhibitors beyond acute colonic pseudo-obstruction has been limited to anecdotal reports of patients with cancer and opioid-induced chronic constipation (Rubiales et al., 2006; Papa and Turconi, 2010), chronic constipation associated with spinal injury (Singal et al., 2006; Ebert, 2012) and patients with autonomic neuropathies (Bharucha et al., 2008).
In recent years, new cholinesterase inhibitors have been developed for treatment of Alzheimer's disease (Birks, 2006), with improved selectivity for AChE, retaining therapeutic benefit while minimizing adverse events in the elderly (Imbimbo, 2001). These include donepezil, rivastigmine and galantamine. Given their improved safety profile, it is possible that these agents might also be more suitable for treatment of intestinal motility disturbances, replacing the older, often contraindicated cholinesterase inhibitors. The current study therefore investigated the ability of donepezil to enhance cholinergic activity in human isolated colon and compared this activity with the effects of neostigmine and prucalopride when applied individually and for donepezil and prucalopride when applied together. In this model, electrical field stimulation (EFS) evokes neuronally mediated contractions and relaxations of the colonic muscle, defined phenotypically as cholinergic, nitrergic or tachykinergic (Broad et al., 2012). The use of prucalopride is important because in contrast to all other 5-HT4 receptor agonists used in clinical practice, this drug has good selectivity of action, improving clinical safety and simplifying interpretation of 5-HT4 receptor functions (Sanger, 2009).
The results demonstrate a weak ability of therapeutically relevant concentrations of donepezil to facilitate cholinergic activity but when combined with prucalopride, a marked increase in activity occurred, greater than the sum of each activity alone. These results, obtained for the first time using clinically available drugs in human tissues, call for exploration of this combination as a safer and more effective treatment of colonic pseudo-obstruction particularly in the elderly for whom there is a lack of suitable medications. Preliminary results were previously reported to the British Pharmacology Society (Broad et al., 2010).
Methods
Nomenclature
The receptor nomenclature described by Alexander et al. (2011) has been used throughout.
Human tissues
The study was approved by the local ethics committee (REC 10/H0703/71) and written informed consent was obtained from all patients. Full thickness human ascending and descending colonic tissue segments (4 × 2 cm strips) were obtained from patients undergoing surgery mostly for bowel cancer (tissues were taken from macroscopically normal areas, at least 10 cm from the tumour); the details are shown in Table 1 and for individual patients, in Supporting Information Table S1. The segments were transferred to the research laboratory within 2 h after resection in Krebs solution (mmol·L−1: NaCl 121.5, CaCl2 2.5, KH2PO4 1.2, KCl 4.7, MgSO4 1.2, NaHCO3 25, glucose 5.6) at room temperature, equilibrated with 5% CO2 and 95% O2.
Table 1.
Patients and tissues used in functional studies
| Region (patients; strips) | Gender (M : F) | Age (median, range) | Response (number of strips) | Recovery time (minutes: median, range) |
|---|---|---|---|---|
| Ascending (19; 146) | 1:1.1 | 67 (39–88) | Contract (25) | 137 (92–195) if used on day of surgery (3 patients) or 238 (175–299) if stored overnight (16 patients) |
| Contract-AC (81) | ||||
| Relax (5) | ||||
| Relax-AC (35) | ||||
| Descending (34; 314) | 1:0.4 | 61 (35–82) | Contract (30) | 159 (120–220) if used on day of surgery (8 patients) or 218 (110–330) if stored overnight (25 patients) |
| Contract-AC (213) | ||||
| Relax (10) | ||||
| Relax-AC (61) |
The phenotype of responses to 5 Hz EFS studied on the day of surgery or after overnight storage of the tissue were similar and data obtained using these two groups of tissues are grouped together. Gender- or age-related differences in responses to 5 Hz EFS were not apparent in this study and data were pooled together. There was a tendency for tissues stored overnight to take longer to recover (defined as the time to achieve consistent responses to 5 Hz EFS following suspension in tissue baths). Data are expressed as medians (ranges in parenthesis). For one patient, these details were not collected.
AC = after-contraction observed on termination of EFS.
Experimental protocol
Immediately on arrival in the laboratory, the mucosa was removed by blunt dissection and discarded. Strips (∼5 mm wide, 12 mm long) were cut parallel to the circular muscle fibres from the intertaenial region of each colon and these were used immediately or after overnight storage at 4°C in fresh, oxygenated Krebs solution. The method used to evoke neuromuscular contractions and relaxations of human isolated gastrointestinal (GI) tissues by EFS has previously been described (Broad et al., 2012; Broad and Sanger, 2013). In brief, for each experiment, strips (4–16 from each patient) were mounted in tissue baths (10 mL) containing Krebs solution at 37°C and gassed with 5% CO2 in O2. Changes in muscle tension were recorded using isometric force transducers (AD Instruments, Chalgrove, UK) on a data acquisition system (Biopac Systems Inc., Goleta, CA, USA). The strips were given 2 g tension (enabling maximum tension to be developed in response to 5 Hz EFS: data not shown) and allowed to recover for 60 min during which time the bath solutions were changed every 15 min. Thereafter, strips were stimulated via two parallel platinum ring electrodes connected to a stimulator (STG2008, Scientifica, Uckfield, UK). The stimulation parameters were 50 V (c. 200 mA), 0.5 ms bipolar pulse duration, 5 Hz (unless otherwise specified), given for 10 s, every 1 min and the bathing solution changed every 15 min until consistency of responses to EFS was achieved. Drugs were then applied non-cumulatively or in some experiments frequency-response curves were established using 1, 2, 5, 10, 15 and 20 Hz EFS, each delivered for 10 s every 1 min.
Pharmacological characterization of responses evoked by EFS was achieved using tetrodotoxin (TTX), atropine and the NOS inhibitor L-NAME (Nω-nitro-L-arginine methyl ester hydrochloride). Each was applied for 30 min before measuring any effect on the EFS-evoked responses. The actions of donepezil, neostigmine and prucalopride were investigated using either single concentrations added to each strip, or single concentrations of two drugs administered together. Due to the often biphasic response to EFS (contractions or relaxations during EFS, usually followed by an ‘after-contraction’ on termination of EFS), the effects of EFS were measured as the total change in muscle movement [area under the curve (AUC)] over the 30 s period following initiation of EFS. Drug-induced changes in AUC were determined as a percentage of the mean of at least three pre-drug EFS-induced responses. To investigate the effects of the drugs on contractions evoked by the muscarinic receptor agonist carbachol, the establishment of a concentration-effect curve (data not shown) identified 1 μM carbachol as the concentration, which evoked a contraction approximately 50% of maximum. This concentration was then used to obtain consistent contractions (5 min contact time followed by washout, repeated at 15 min intervals). Fifteen minutes before the last application, the drugs were added to the bath. Drug-induced changes in response to carbachol were determined as a percentage of the mean of at least three pre-drug responses.
Data analysis and statistical procedures
Data were expressed as medians and ranges or as the mean ± SEM with n values indicating the number of patients. Differences between the medians were determined using the Mann–Whitney U-test for unpaired observations. EC50 and Emax (maximum response to agonist) values were obtained from three parameter agonist-response curves plotted using GraphPad Prism 5.0; Emax values are reported as mean ± standard error. anova models were used to analyse independent or interdependent effects of drugs, followed by Bonferroni post test analysis to investigate effects at individual concentrations. P < 0.05 was considered to represent statistical significance.
Drugs and chemical reagents used
All drugs were freshly prepared prior to use. Donepezil HCl (Molekula, Gillingham, UK), neostigmine bromide (Sigma, Gillingham, UK), prucalopride succinate (Shire-Movetis, Turnhout, Belgium), carbachol, atropine, L-NAME (each from Sigma) and TTX (Tocris, Abingdon, UK) were each dissolved in distilled water (dH2O).
Results
Characterization of tissues used
Colon was obtained from 53 patients. Their age, gender and region of colon are summarized in Table 1 (see also, Supporting Information Table S1 for more details on most of these patients), as well as the times taken to recover from the surgery and preparation of the tissues. Before evaluating the actions of prucalopride and the cholinesterase inhibitors, in a subset of experiments, EFS was applied at different frequencies in order to select the most suitable stimulation parameters. The results showed that small muscle relaxations and larger contractions were evoked in approximately equal numbers during low frequencies of EFS (1–2 Hz; 44% relaxed; n = 37; 296 strips), whereas with increasing frequencies of stimulation the proportion of relaxations decreased (5 Hz: 24%, n = 53, 460 strips, see Table 1 Hz: 8%, n = 37, 296 strips). Each response was usually followed by an ‘after-contraction’ on termination of EFS, the occurrence of which also tended to be frequency dependent (1–2 Hz 76%, n = 37, 296 strips; 5 Hz 85%, n = 53, 460 strips; 10–20 Hz, 90%, n = 37, 296 strips; Figure 1A). Subsequently, all experiments that examined the actions of drugs were conducted using EFS at 5 Hz. Thus, stimulation at this frequency evoked clear, apparently submaximal responses, which represented each of the relaxation and contraction phenotypes observed throughout the range of frequencies studied.
Figure 1.
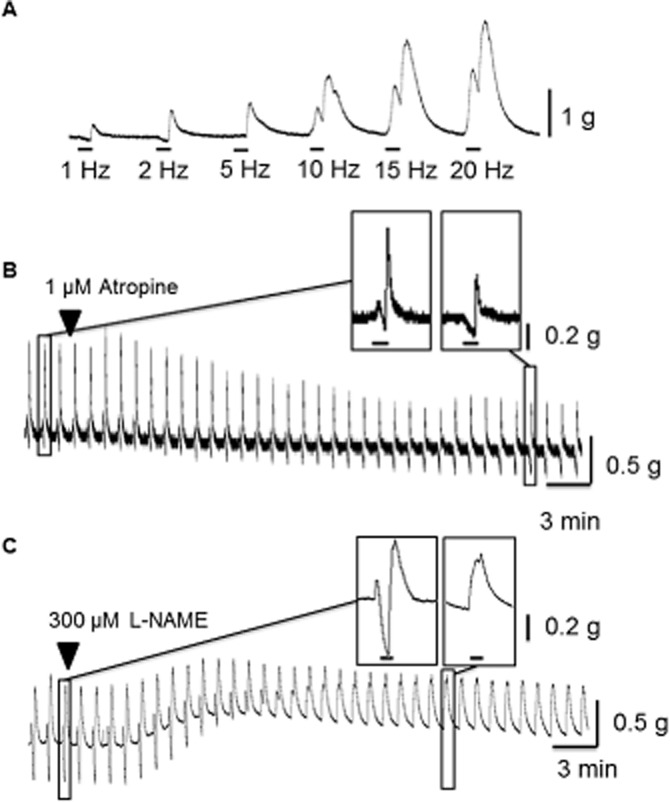
Representative traces illustrating muscle relaxations and contractions to EFS in circular muscle from human isolated colon. (A) Trace showing effects of different frequencies of EFS (1, 2, 5, 10, 15 and 20 Hz, 50 V, 0.5 ms bipolar pulse duration) applied for 10 s every 1 min. The horizontal bar indicates the period of EFS, with the response generated during this time usually followed by an ‘after-contraction’ on termination of EFS. Contractions and relaxations during 5 Hz EFS were prevented, respectively, by (B) 1 μM atropine and (C) by 300 μM L-NAME. The enlarged sections of tracing show the ability of atropine to prevent contractions during EFS (revealing muscle relaxation) and greatly reduce the large after-contractions, which followed termination of EFS. Similarly, muscle relaxation was prevented by L-NAME and the resultant contraction became monophasic.
All responses to 5 Hz EFS were prevented by application of TTX 1 μM (n = 4; data not shown). Contractions during EFS were prevented by atropine 1 μM, revealing EFS-evoked muscle relaxation (n = 8). Similarly, relaxations during EFS were prevented by L-NAME 300 μM (n = 11). Further, the presence of L-NAME 300 μM often resulted in the EFS-evoked contractions becoming monophasic and the distinct after-contractions were no longer apparent (in 39/75 strips, n = 28 patients; Figure 1C). In the experiments with atropine 1 μM, the after-contractions were also attenuated (−49 ± 11%, n = 7; Figure 1B) and these contractions were further reduced by application of a neurokinin (NK) NK1,2,3 receptor antagonist combination of L732138 1 μM, GR 159897 0.1 μM and SB235375 0.1 μM (by −34 ± 19%, n = 3).
Actions of donepezil and neostigmine
Donepezil 0.03–1 μM facilitated contractions evoked both during and after EFS in a concentration-dependent manner with baseline muscle tension unaffected (Figure 2; Supporting Information Table S1 shows the data matched to individual patients). Overall, the maximum increase in EFS-evoked AUC was 477 ± 129%, with an EC50 value of 124 nM; n = 3–4 each concentration (Figure 2). Donepezil 1 μM did not affect the amplitude of contractions evoked by carbachol 1 μM (change of 4 ± 15% from control; n = 3).
Figure 2.
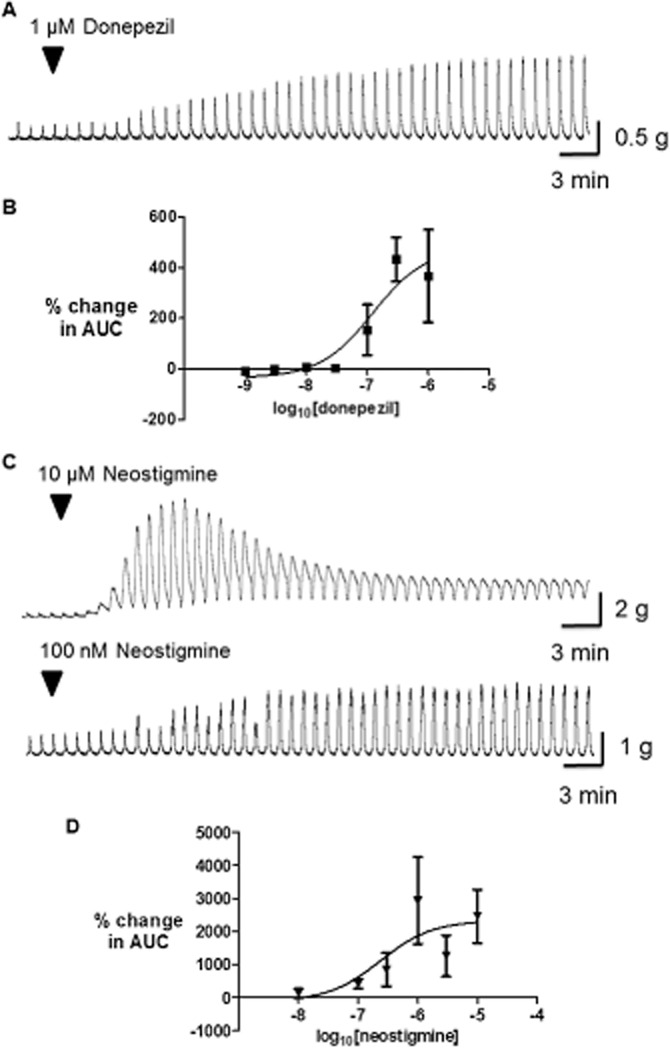
Effects of donepezil and neostigmine on cholinergically mediated contractions evoked by (EFS of human isolated colon circular muscle. Typical traces are shown in the upper panels (A, C), with the graphs below each trace showing the mean (±SEM) increase in EFS-evoked AUC at increasing concentrations of donepezil (B) and neostigmine (D) (n = 3–4 and 4–5 patients for each data point, respectively); note the different g-tension scales for the traces shown. EFS (50 V, 0.5 ms bipolar pulse duration, 5 Hz) was given for 10 s, every 1 min.
Neostigmine (0.1–10 μM) evoked large increases in the amplitude of contractions evoked during and after EFS (Figure 2; Supporting Information Table S1). At the higher concentrations the increased contractions appeared to merge together to become monophasic, and baseline muscle tension was increased (observed at 1–10 μM in 10/15 strips tested); the magnitude of the latter was variable (e.g. the increase in muscle tension at 10 μM was 725 ± 621% EFS). This increase in baseline tension made it difficult to define the maximally effective concentration, which increased EFS-evoked contractions; however, the calculated Emax was 2326 ± 624% and EC50 value was 235 nM; n = 4–5 (Figure 2).
Actions of prucalopride
The interactions between prucalopride and the responses to EFS were more complex. In summary, prucalopride (1 μM or above) usually increased the amplitude of contractions during EFS whilst exerting smaller increases in the amplitude of the after-contractions (Figure 3). Overall, the EFS-evoked AUC increased in an approximately concentration-dependent manner (Emax 33 ± 14%; EC50 = 855 nM; n = 3–7 each concentration; Figure 3). In this action, the AUC increased in most (but not all) of the tissues exposed to the higher concentrations of prucalopride, with males and females of different ages appearing to be similarly affected (Supporting Information Table S1); it should be noted, however, that the study was not powered to provide statistically-meaningful data on potential gender- and age-related variations. Finally, 10–30 μM prucalopride caused a small, but consistent reduction in baseline muscle tension.
Figure 3.
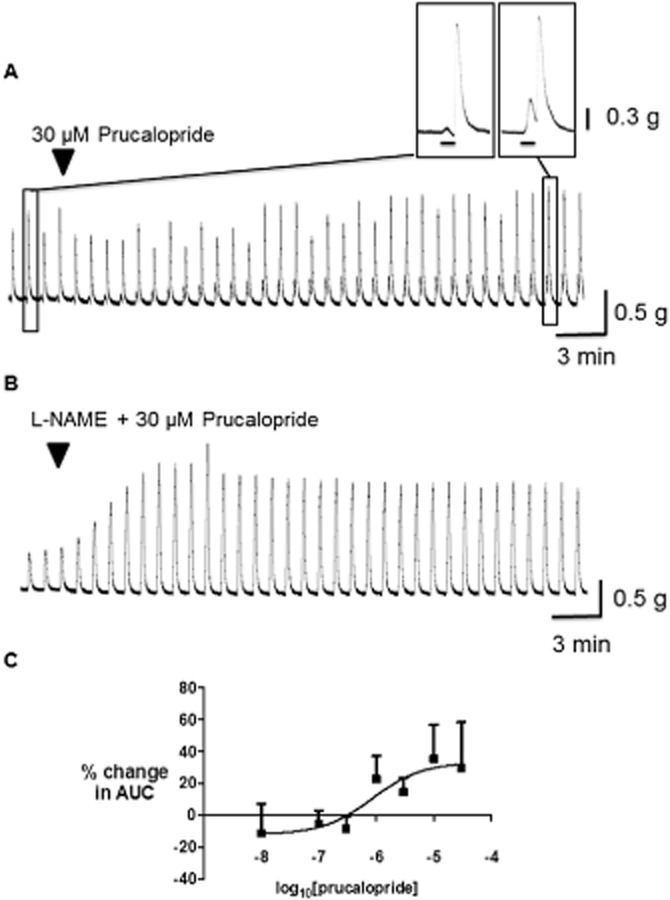
Effects of prucalopride on cholinergically mediated contractions evoked by EFS of the human isolated colon circular muscle. Typical traces are shown in the upper panels in the absence (A) and presence (B) of L-NAME 300 μM; the enlarged section of tracing obtained in the absence of L-NAME shows the ability of prucalopride to act mostly by increasing muscle contractions during EFS. The graph below (C) shows the concentration-response curve for prucalopride in the absence of L-NAME, measuring the mean (±SEM) increase in EFS-evoked AUC. EFS (50 V, 0.5 ms bipolar pulse duration, 5 Hz) was given for 10 s, every 1 min (n = 3–6 patients for each data point).
In the presence of L-NAME 300 μM, during which monophasic contractions were now often observed in response to EFS, prucalopride caused a larger, more consistent increase in the responses to EFS (Figure 3); the maximum observed increase in AUC was 115 ± 39% at 30 μM, n = 4). Small decreases in baseline muscle tension continued to be observed at 30 μM. At 10 μM, prucalopride did not affect the amplitude of contractions evoked by the submaximally effective concentration of carbachol 1 μM; contractions were changed by −9 ± 9% from control (n = 3).
Actions of donepezil together with prucalopride
An approximately threshold concentration of donepezil (30 nM) was added together with 3 or 10 μM prucalopride; these experiments were conducted in the absence of L-NAME, when the excitatory actions of prucalopride on cholinergic function were sometimes less clear (see Figure 3). Addition of donepezil markedly increased the response to prucalopride. Thus, the increases observed during the presence of donepezil and prucalopride 10 μM together or alone were, respectively, 105 ± 35% (n = 8), 4 ± 6% (n = 3) and 35 ± 22% (n = 7). Similarly, at the lower concentration of 3 μM, the increases in AUC for prucalopride together with donepezil or when given alone were 50 ± 22% (n = 6) and 11 ± 10% (n = 6) respectively. Individually, the increased responses to prucalopride in the presence of donepezil did not reach statistical significance (P > 0.05; Bonferroni post test; cholinergic activity was not increased by prucalopride alone in tissues from two patients) but when the effects of donepezil on both concentrations of prucalopride were assessed together, statistical significance was achieved (Figure 4; P = 0.04; two-way anova).
Figure 4.
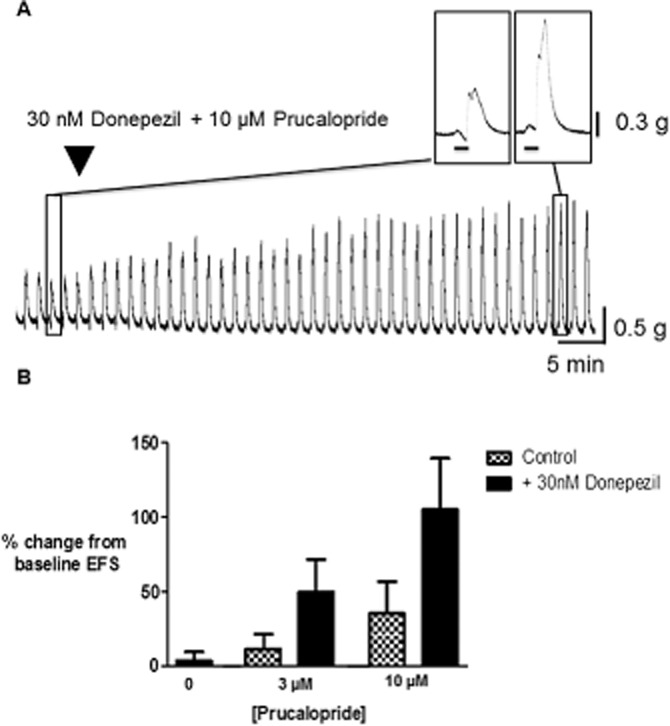
Facilitation by donepezil, in the absence and presence of prucalopride, of cholinergically mediated contractions evoked by EFS in human colonic circular muscle. (A) Trace showing the large increase in EFS-evoked contractions after application of donepezil together with prucalopride. (B) Bars showing the mean (±SEM) facilitation of EFS-evoked AUC caused by donepezil 30 nM alone (n = 3) and in the presence of prucalopride 3 and 10 μM (respectively, n = 6 each for 3 μM in the absence and presence of donepezil, and 7 and 8 for 10 μM). EFS (50 V, 0.5 ms bipolar pulse duration, 5 Hz) was given for 10 s, every 1 min (n = 3–7 patients for each data point). P = 0.04 (two-way anova) when the effects of donepezil on both concentrations of prucalopride were assessed together.
Discussion and conclusions
The main findings of this study were (i) that donepezil facilitated cholinergic activity in human isolated colon, but at therapeutically relevant concentrations, this activity was at or just above threshold levels; (ii) neostigmine and prucalopride also facilitated cholinergic activity in human colon with a rank-order of efficacy: neostigmine >> donepezil > prucalopride; (iii) the combination of a low, therapeutically relevant concentration of donepezil with prucalopride facilitated cholinergic activity, the combined activity appearing greater than the sum of each drug alone.
To reach these conclusions, considerable care was taken to ensure that reproducible data could be obtained with human isolated tissues, which unlike tissues from laboratory animals, do not originate from a homogeneous source; procedures for minimizing potential variations by attention to surgical techniques, tissue storage conditions and recovery times (Sanger et al., 2013) were adhered to in the present studies. In brief, EFS evoked cholinergically mediated contractions (prevented by atropine 1 μM) or nitrergically mediated relaxations (prevented by L-NAME 300 μM), usually followed by a large ‘after-contraction’ on termination of EFS. The latter were abolished or reduced by a combination of atropine plus NK1, NK2 and NK3 receptor antagonists, suggesting predominant cholinergic and tachykinergic involvement. These results are consistent with structural data indicating high numbers of cholinergic and nitrergic motor and interneurones in human colon (Porter et al., 2002). Interestingly, the lack of obvious gender-related differences in responses to EFS appears to contrast with Maselli et al. (2011), who suggested that contractions evoked by EFS were more pronounced in tissues from elderly males. Perhaps the discrepancy can be explained by the fact that in the study of Maselli et al. the number of observations represented the number of muscle strips studied and not the number of patients. Since certain patients generated more muscle strips than others, the potential to artificially skew the data must therefore be high. In the present study, all n values refer to numbers of patients studied.
Prucalopride usually increased (but not always) the amplitude of the cholinergically mediated contractions of the colon, an action greatly enhanced and made more consistent by the presence of the NOS inhibitor L-NAME; small reductions in baseline muscle tension were also observed. These observations are consistent with previous reports of an ability of prucalopride to activate the 5-HT4 receptor and increase cholinergic and nitrergic activity in human isolated colon circular muscle (Cellek et al., 2006), increase cholinergic activity in human taenia coli (Prins et al., 2000a) and cause a small reduction in baseline muscle tension of the human colon (Prins et al., 2000b). The importance of ACh as the major motor neurotransmitter in the colon, together with the known ability of prucalopride to promote colonic motility and defecation (Coremans, 2008) therefore support the translational value of the present model.
Donepezil (0.03–1 μM) and neostigmine (0.1–10 μM) markedly facilitated cholinergic activity in the human colon. Indeed, at the higher concentrations of neostigmine, but not donepezil, the baseline muscle tension was also increased. These actions of donepezil and neostigmine have not previously been described using human isolated colon although similar activities have been reported using rat isolated stomach (Jarvie et al., 2008). The reasons for the difference in efficacy between donepezil and neostigmine are not clear and likely to be complex. Different AChE inhibitors may, for example, interact in different ways with different isoforms of the enzyme or perhaps the additional ability of neostigmine to inhibit butyrylcholinesterase may contribute to its greater efficacy, relative to donepezil (see Jarvie et al., 2008, for references). Notably, however, the plasma concentrations of donepezil found in healthy volunteers receiving donepezil [e.g. Cmax (peak concentration of drug measured in blood after a single dose) values around 11 ng·mL−1 (equivalent to 26 nM) after a single oral dose of 6 mg (Rogers and Friedhoff, 1998) or 34–60 ng·mL−1 (82–144 nM) after repeat dosing in Alzheimer patients with 5 or 10 mg; Tiseo et al., 1998] correspond to the lower regions of the concentration-response curve in the present experiments. This apparently ‘threshold activity’ of donepezil is consistent with the findings of Farlow et al. (2010), who found a 5.5% incidence of diarrhoea as a treatment-related adverse event in Alzheimer's patients receiving 10 mg of donepezil per day, the incidence rising (along with a larger increase in nausea and vomiting) with higher doses of the drug. Diarrhoea was also the most common adverse event (15.6%) in an open-label study in patients with amnesic mild cognitive impairment receiving 10–20 mg daily (Doody et al., 2010).
The combination of a low concentration of donepezil (30 nM) together with different concentrations of prucalopride facilitated cholinergic activity in the human colon in a manner which appeared to be greater than the activities of either drug given alone. These data are consistent with the previously reported synergy between neostigmine and prucalopride, or between neostigmine and tegaserod (Cellek et al., 2008; Campbell-Dittmeyer et al., 2009). However, this is the first report of similar activity in the human intestine with donepezil in combination with prucalopride. The fact that both of these drugs are readily available in many countries for clinical use now offers the potential for improved efficacy over prucalopride alone and improved cardiovascular and bronchial safety over the use of physostigmine. Thus, when compared with neostigmine, donepezil is safer to use, especially in the elderly (Birks, 2006; Lockhart et al., 2009) who are more likely to suffer from acute colonic pseudo-obstruction (Hyatt, 1987). Further, prucalopride has been shown to have no cardiac safety liability in elderly patients treated for constipation (Camilleri et al., 2009).
In summary, our experiments have provided an effective and translationally relevant model of human colonic motility in vitro. Using this model, therapeutically relevant concentrations of donepezil have been shown to facilitate the increase in colonic cholinergic activity caused by prucalopride in an apparently synergistic manner. These experiments raise the possibility of using donepezil (at current therapeutic doses or lower) in combination with prucalopride to treat significant digestive motility disorders. Obvious first targets for this approach would be those conditions currently treated with neostigmine, for example, acute colonic pseudo-obstruction; however, other populations could also benefit such those with severe chronic constipation, prolonged ileus or intestinal pseudo-obstruction syndromes. Given the specificity of action of prucalopride for the GI system (Coremans, 2008; Camilleri et al., 2009), an increase in non-GI adverse events is not expected. Nevertheless, it remains a possibility that in other systems where 5-HT4 receptors are expressed by cholinergic neurones (e.g. the urinary bladder and the brain; Johnson et al., 2012; Kullmann et al., 2013), similar interactions will occur and adverse events could be noted. Further preclinical and clinical studies are therefore now required to test the efficacy and safety of this combination of drugs.
Acknowledgments
We thank the consultant colorectal surgeons at Barts Health: Mohammed Thaha, Shafi Ahmed and Chris Chan, for assisting with identification of patients for recruitment of tissue samples. This study was supported by a skills-gap award from the MRC (G. J. S.), by Shire-Movetis and the Bowel and Cancer research charity. V. W. S. K. is supported by the research into ageing fund, set up and managed by AgeUK.
Glossary
- AUC
area under the curve
- Cmax
peak concentration of drug measured in blood after a single dose
- EFS
electrical field stimulation
- Emax
maximum response to agonist
- GI
gastrointestinal
- L-NAME
Nω-nitro-L-arginine methyl ester
- NK
neurokinin
- TTX
tetrodotoxin
Conflict of interest
G. J. S. has received funding from GlaxoSmithKline, Shire Pharmaceuticals and Protexin, and in the past 2 years, consulted for Novartis, Theravance and Proximagen. J. H. D.M. is a previous employee of Shire-Movetis. Q. A. has received a small research grant from Shire and participated on advisory panels for both Shire and Almirall. C. H K. has received payment for participating in a Shire-sponsored meeting. The remaining authors have no competing interests.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
http://dx.doi.org/10.1111/bph.12397
Table S1 Characteristics of the patients used in this study. The Table provides pertinent details of the patients used, alongside the experimental data obtained using the tissues from these patients. AUC = Area under the curve (see Methods for details); NA = Not Applicable.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edn. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaro R, Rogers AI. Neostigmine infusion: new standard of care for acute colonic pseudo-obstruction? Am J Gastroenterol. 2000;95:304–305. doi: 10.1111/j.1572-0241.2000.01737.x. [DOI] [PubMed] [Google Scholar]
- Bharucha AE, Phillips SF. Megacolon: acute, toxic, and chronic. Curr Treat Options Gastroenterol. 1999;2:517–523. doi: 10.1007/s11938-999-0055-9. [DOI] [PubMed] [Google Scholar]
- Bharucha AE, Low PA, Camilleri M, Burton D, Gehrking TL, Zinsmeister AR. Pilot study of pyridostigmine in constipated patients with autonomic neuropathy. Clin Auton Res. 2008;18:194–202. doi: 10.1007/s10286-008-0476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharucha AE, Low P, Camilleri M, Veil E, Burton D, Kudva Y, et al. A randomised controlled study of the effect of cholinesterase inhibition on colon function in patients with diabetes mellitus and constipation. Gut. 2013;62:708–715. doi: 10.1136/gutjnl-2012-302483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev. 2006;(1) doi: 10.1002/14651858.CD005593. CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad J, Sanger GJ. The antibiotic azithromycin is a motilin receptor agonist in human stomach: comparison with erythromycin. Br J Pharmacol. 2013;168:1859–1867. doi: 10.1111/bph.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad J, Mukherjee S, Boundouki G, Knowles C, Sanger GJ. The prokinetic-like activity of the 5-HT4 receptor agonist prucalopride and the cholinesterase inhibitor donepezil as prokinetic agents in human isolated colon. Proc Br Pharmacol Soc. 2010 Available at: http://www.pA2online.org/abstracts/Vol8Issue1abst071P.pdf (accessed 3/10/2013) [Google Scholar]
- Broad J, Mukherjee S, Samadi M, Martin JE, Dukes GE, Sanger GJ. Regional- and agonist-dependent facilitation of human neurogastrointestinal functions by motilin receptor agonists. Br J Pharmacol. 2012;167:763–774. doi: 10.1111/j.1476-5381.2012.02009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M, Beyens G, Kerstens R, Robinson P, Vandeplassche L. Safety assessment of prucalopride in elderly patients with constipation: a double-blind, placebo-controlled study. Neurogastroenterol Motil. 2009;21:1256–e117. doi: 10.1111/j.1365-2982.2009.01398.x. [DOI] [PubMed] [Google Scholar]
- Campbell-Dittmeyer K, Hicks GA, Earnest DL, Greenwood-Van Meerveld B. Increased colonic transit in rats produced by a combination of a cholinesterase inhibitor with a 5-HT4 receptor agonist. Neurogastroenterol Motil. 2009;21:1197–e108. doi: 10.1111/j.1365-2982.2008.01238.x. [DOI] [PubMed] [Google Scholar]
- Cellek S, John AK, Thangiah R, Dass NB, Bassil AK, Jarvie EM, et al. 5-HT4 receptor agonists enhance both cholinergic and nitrergic activity in human isolated colon circular muscle. Neurogastroenterol Motil. 2006;18:853–861. doi: 10.1111/j.1365-2982.2006.00810.x. [DOI] [PubMed] [Google Scholar]
- Cellek S, Thangiah R, Jarvie EM, Vivekanandan S, Lalude O, Sanger GJ. Synergy between 5-HT4 receptor activation and acetylcholinesterase inhibition in human colon and rat forestomach. Neurogastroenterol Motil. 2008;20:539–545. doi: 10.1111/j.1365-2982.2007.01062.x. [DOI] [PubMed] [Google Scholar]
- Chey WD, Drossman DA, Johanson JF, Scott C, Panas RM, Ueno R. Safety and patient outcomes with lubiprostone for up to 52 weeks in patients with irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2012;35:587–599. doi: 10.1111/j.1365-2036.2011.04983.x. [DOI] [PubMed] [Google Scholar]
- Coremans G. Prucalopride: the evidence for its use in the treatment of chronic constipation. Core Evid. 2008;3:45–54. doi: 10.3355/ce.2008.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulie B, Camilleri M. Intestinal pseudo-obstruction. Annu Rev Med. 1999;50:37–55. doi: 10.1146/annurev.med.50.1.37. [DOI] [PubMed] [Google Scholar]
- Doody RS, Ferris S, Salloway S, Yijun S, Goldman R, Yikang X, et al. Safety and tolerability of donepezil in mild cognitive impairment: open-label extension study. Am J Alzheimers Dis Other Demen. 2010;25:155–159. doi: 10.1177/1533317509352334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert E. Gastrointestinal involvement in spinal cord injury: a clinical perspective. J Gastrointestin Liver Dis. 2012;21:75–82. [PubMed] [Google Scholar]
- Elsner JL, Smith JM, Ensor CR. Intravenous neostigmine for postoperative acute colonic pseudo-obstruction. Ann Pharmacother. 2012;46:430–435. doi: 10.1345/aph.1Q515. [DOI] [PubMed] [Google Scholar]
- Farlow MR, Salloway S, Tariot PN, Yardley J, Moline ML, Wang Q, et al. Effectiveness and tolerability of high-dose (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer's disease: a 24-week, randomized, double-blind study. Clin Ther. 2010;32:1234–1251. doi: 10.1016/j.clinthera.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio R, Knowles CH. Acute colonic pseudo-obstruction. Br J Surg. 2009;96:229–239. doi: 10.1002/bjs.6480. [DOI] [PubMed] [Google Scholar]
- Hyatt R. Colonic pseudo-obstruction: an important complication in hospitalized elderly patients. Age Ageing. 1987;16:145–152. doi: 10.1093/ageing/16.3.145. [DOI] [PubMed] [Google Scholar]
- Imbimbo BP. Pharmacodynamic-tolerability relationships of cholinesterase inhibitors for Alzheimer's disease. CNS Drugs. 2001;15:375–390. doi: 10.2165/00023210-200115050-00004. [DOI] [PubMed] [Google Scholar]
- Jarvie EM, Cellek S, Sanger GJ. Potentiation by cholinesterase inhibitors of cholinergic activity in rat isolated stomach. Pharmacol Res. 2008;58:297–301. doi: 10.1016/j.phrs.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Drummond E, Grimwood S, Sawant-Basak A, Miller E, Tseng E, et al. The 5-hydroxytryptamine4 receptor agonists prucalopride and PRX-03140 increase acetylcholine and histamine levels in the rat prefrontal cortex and the power of stimulated hippocampal θ oscillations. J Pharmacol Exp Ther. 2012;341:681–691. doi: 10.1124/jpet.112.192351. [DOI] [PubMed] [Google Scholar]
- Kullmann FA, Kurihara R, Ye L, Wells GI, McKenna DG, Burgard EC, et al. Effects of the 5-HT4 receptor agonist, cisapride, on neuronally evoked responses in human bladder, urethra, and ileum. Auton Neurosci. 2013;176:70–77. doi: 10.1016/j.autneu.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Law N-M, Bjarucha AE, Undale AS, Zinsmeister AR. Cholinergic stimulation enhances colonic motor activity, transit and sensation in humans. Am J Physiol. 2001;281:G1228–G1237. doi: 10.1152/ajpgi.2001.281.5.G1228. [DOI] [PubMed] [Google Scholar]
- Lembo AJ, Schneier HA, Shiff SJ, Kurtz CB, MacDougall JE, Jia XD, et al. Two randomized trials of linaclotide for chronic constipation. N Engl J Med. 2011;365:527–536. doi: 10.1056/NEJMoa1010863. [DOI] [PubMed] [Google Scholar]
- Lockhart IA, Mitchell SA, Kelly S. Safety and tolerability of donepezil, rivastigmine and galantamine for patients with Alzheimer's disease: systematic review of the ‘real-world’ evidence. Dement Geriatr Cogn Disord. 2009;28:389–403. doi: 10.1159/000255578. [DOI] [PubMed] [Google Scholar]
- Loftus CG, Harewood GC, Baron TH. Assessment of predictors of response to neostigmine for acute colonic pseudo-obstruction. Am J Gastroenterol. 2002;97:3118–3122. doi: 10.1111/j.1572-0241.2002.07108.x. [DOI] [PubMed] [Google Scholar]
- McNamara R, Mihalakis MJ. Acute colonic pseudo-obstruction: rapid correction with neostigmine in the emergency department. J Emerg Med. 2008;35:167–170. doi: 10.1016/j.jemermed.2007.06.043. [DOI] [PubMed] [Google Scholar]
- Maselli MA, Trisolini P, Demma I, Pezzolla F, De Ponti F. Gender- and age-related differences in muscular and nerve-mediated responses in human colon. Dig Dis Sci. 2011;56:352–358. doi: 10.1007/s10620-010-1324-0. [DOI] [PubMed] [Google Scholar]
- Mehta R, John A, Nair P, Raj VV, Mustafa CP, Suvarna D, et al. Factors predicting successful outcome following neostigmine therapy in acute colonic pseudo-obstruction: a prospective study. J Gastroenterol Hepatol. 2006;21:459–461. doi: 10.1111/j.1440-1746.2005.03994.x. [DOI] [PubMed] [Google Scholar]
- O'Dea CJ, Brookes JH, Wattchow DA. The efficacy of treatment of patients with severe constipation or recurrent pseudo-obstruction with pyridostigmine. Colorectal Dis. 2010;12:540–548. doi: 10.1111/j.1463-1318.2009.01838.x. [DOI] [PubMed] [Google Scholar]
- Papa P, Turconi L. Neostigmine for the treatment of gastrointestinal atony: a report of one case. J Palliat Med. 2010;14:1–4. doi: 10.1089/jpm.2010.0390. [DOI] [PubMed] [Google Scholar]
- Ponec RJ, Saunders MD, Kimmey MB. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med. 1999;341:137–141. doi: 10.1056/NEJM199907153410301. [DOI] [PubMed] [Google Scholar]
- Porter AJ, Wattchow DA, Brookes SJH, Costa M. Cholinergic and nitrergic interneurons in the myenteric plexus of the human colon. Gut. 2002;51:70–75. doi: 10.1136/gut.51.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins NH, Akkermans LM, Lefebvre RA, Schuurkes JA. 5-HT4 receptors on cholinergic nerves involved in contractility of canine and human large intestine longitudinal muscle. Br J Pharmacol. 2000a;131:927–932. doi: 10.1038/sj.bjp.0703615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins NH, Shankley NP, Welsh NJ, Briejer MR, Lefebvre RA, Akkermans LMA, et al. An improved in vitro bioassay for the study of 5-HT4 receptors in the human isolated large intestinal circular muscle. Br J Pharmacol. 2000b;129:1601–1608. doi: 10.1038/sj.bjp.0703254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SL, Friedhoff LT. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following single oral doses. Br J Clin Pharmacol. 1998;46:1–6. doi: 10.1046/j.1365-2125.1998.0460s1001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubiales AS, Hernansanz S, Gutierrez C, Del Valle ML, Flores LA. Neostigmine for refractory constipation in advanced cancer patients. J Pain Symptom Manage. 2006;32:204–205. doi: 10.1016/j.jpainsymman.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Sanger GJ. Translating 5-HT4 receptor pharmacology. Neurogastroenterol Motil. 2009;21:1235–1238. doi: 10.1111/j.1365-2982.2009.01425.x. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Broad J, Kung V, Knowles CH. Translational neuropharmacology: the use of human isolated gastrointestinal tissues. Br J Pharmacol. 2013;168:28–43. doi: 10.1111/j.1476-5381.2012.02198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders MD. Acute colonic pseudo-obstruction. Best Pract Res Clin Gastroenterol. 2007;21:671–687. doi: 10.1016/j.bpg.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Saunders MD, Kimmey MB. Systematic review: acute colonic pseudoobstruction. Aliment Pharmacol Ther. 2005;22:917–925. doi: 10.1111/j.1365-2036.2005.02668.x. [DOI] [PubMed] [Google Scholar]
- Singal AK, Rosman AS, Bauman WA, Korsten MA. Recent concepts in the management of bowel problems after spinal cord injury. Adv Med Sci. 2006;51:15–22. [PubMed] [Google Scholar]
- Tiseo PJ, Rogers SL, Friedhoff LT. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following evening administration. Br J Clin Pharmacol. 1998;46:13–18. doi: 10.1046/j.1365-2125.1998.0460s1013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisani GY, Hyman NH, Church JM. Neostigmine: safe and effective treatment for acute colonic pseudo-obstruction. Dis Colon Rectum. 2000;43:599–603. doi: 10.1007/BF02235569. [DOI] [PubMed] [Google Scholar]
- Turégano-Fuentes F, Muňoz-Jiménez F, Del Valle-Hernández E, Perez-Diaz D, Calvo-Serrano M, De Tomás J, et al. Early resolution of Ogilvie's syndrome with intravenous neostigmine: a simple, effective treatment. Dis Colon Rectum. 1997;40:1353–1357. doi: 10.1007/BF02050822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


